1. Introduction
Fasciolosis is an emerging neglected tropical disease that has a significant detrimental impact on ruminant livestock production [
1,
2]. Fasciolosis is typically diagnosed coproscopically such as fecal sedimentation to detect the
Fasciola eggs. However, the
Fasciola eggs can only be detected during patent infection period which is from 15 weeks after ingesting
Fasciola metacercariae [
1]. Other diagnostic techniques includes serological assays of host IgG antibody response, which is less accurate to diagnose active infection as the IgG remains detected for months even after successful treatment. Abattoir examinations are conducted post-mortem, which have limitations in early detection as infections are identified only after the animal has been slaughtered. This delay enables the
Fasciola to persist and spread within the herd before preventive measures can be implemented. However, the abattoir examinations can still provide crucial insights into the extent of the infection, tissue damage, and other pathological changes [
2].
Coproantigen enzyme-linked immunosorbent assay (cELISA) has emerged as a diagnostic method for detecting fasciolosis in ruminants over the past decade. This cELISA captures metabolic antigens produced by newly excysted juvenile (NEJ) and adult
Fasciola [
1], which has demonstrated the effectiveness by offering a high sensitivity and specificity for fasciolosis diagnosis in sheep and cattle [
3]. The primary antigen released by
Fasciola is cathepsins-L, which, upon interaction with the host's digestive enzymes and acids, can be circulated and subsequently retained in the biliary system [
1,
3] Additionally, cathepsin-L can be detected even during the pre-patent period with a low fluke burden, as low as two flukes [
1].
Fasciolosis surveillance utilizing cELISA is widely employed for detecting
Fasciola hepatica in temperate regions [
3,
4,
5,
6], while its application for detecting
F. gigantica is comparatively limited [
4]. Generally, the sensitivity of detection period for both
Fasciola species via cELISA is similar, occurring approximately four to seven weeks before eggs become detectable in sheep (six to nine weeks post-infection (WPI) for
F. hepatica and seven to eleven WPI for
F. gigantica) [
4]. During the patent infection, the eggs passed to the environment upon defaecation by
Fasciola-infected animals and sustain the fasciolosis occurrences within the area. Therefore, early and accurate detection of fasciolosis in ruminant livestock is crucial for controlling the spread and reducing the economic loss due to the infection [
7]. Hence, the aim of the present study was to compare the sensitivity between fecal sedimentation and fecal antigen ELISA (cELISA) for the diagnosis of fasciolosis in free-grazing cattle within an area endemic to
F. gigantica.
2. Materials and Methods
2.1. Fecal sample
Detailed description on the sample collection work is described in a previous study [
8]. Briefly, ruminant fecal samples were collected for fasciolosis surveillance in farms in Taiping, Malaysia. The sampling was conducted from February to August 2020. To ensure precision, the inclusion criteria for the present analysis were exclusively tailored to encompass samples from cattle. This selection was guided by adherence to the manufacturer's protocol, as the commercial cELISA kit utilized in this study had been specifically optimized for fecal samples from only sheep and cattle.
A total of 92 fecal samples were randomly selected through an Excel datasheet and included in the present study. The samples were categorized into two study groups based on Fasciola positivity, determined through Flukefinder® fecal sedimentation. Group FFEC+ve comprised 46 samples where Fasciola eggs were detected, while FFEC-ve group included 46 samples where Fasciola eggs were not detected.
2.2. Preparation of fecal supernatant for cELISA
Fecal supernatants were prepared at a ratio of 1:1 in ProClin® 300 (Sigma-Aldrich, CAS#55965-84-9), using 2 g of each cattle fecal sample. To ensure homogeneity and prevent clumping, fecal samples were thoroughly vortexed before incubating overnight at 4°C. Following incubation, the suspension was thawed to room temperature for 30 minutes, vortexed, and subsequently centrifuged for 10 minutes at 1,000 x g (RCF) to yield the supernatant. A volume of 500 μL of the supernatant from all sample were aliquoted and stored at -20°C for future use. The prepared supernatant remains viable for
Fasciola coproantigen detection for up to six weeks [
5].
2.3. Coproantigen ELISA analysis
In the present study, a commercial semi-quantitative coproantigen ELISA kit (MM3-COPRO-BIOK 201, Bio-X Diagnostics, Jemelle, Belgium) was employed. The analysis was performed in accordance with the manufacturer's instructions. This indirect sandwich cELISA kit utilizes two distinct coatings on alternate strips, consisting of monoclonal and polyclonal antibodies, to minimize false-positive results. The kit provided an avidin-peroxidase conjugate, a highly sensitive agent for detecting
Fasciola coproantigen, higher sensitivity of identifying one NEJ or adult
Fasciola at minimum [
9]. The second conjugate in this cELISA was the MM3 monoclonal antibody (mAb), recognized as the most sensitive and specific among available mAbs for binding with
Fasciola coproantigen [
1]. A volume of 100 μL of the supernatant was loaded into both monoclonal and polyclonal antibody-coated wells, with samples duplicated for result averaging. The provided reference
Fasciola antigen was used as a positive control, while deionized distilled water served as the blank control to assess the extent of nonspecific binding in the procedure.
Optical densities (OD) were measured at a wavelength of 450 nm, and the cut-off for positivity value was determined using the quality control (QC) datasheet supplied with the kit. An OD exceeding 8 was considered as a positive result.
2.4. Statistical analysis
The Kolmogorov-Smirnov test was applied to analyze the raw data, indicating a non-normal distribution of values. The obtained OD results for the tested samples underwent one-way ANOVA analysis to determine the total variance and coefficient of variation (CV) for each sample, with the results expressed as a percent CV. The potential association between the OD values obtained with cELISA and the Fasciola fecal egg count (FFEC) was assessed through Spearman’s rank correlation. The association is considered statistically significant when the p-value < 0.01. All statistical analyses were carried out using R statistical software (version 1.3.1073).
3. Results
The cELISA optical density (OD) for the blank controls and polyclonal antibody coated wells in all microplates in the present study remained below the cut-off value. In contrast, positive controls using the crude Fasciola antigen exhibit above the cut-off value indicating that the OD readings analyzed in this study were validated.
The range of Fasciola eggs from the FFEC+ve samples in the present study varied from 1 egg per 2 grams (0.5 egg per gram, epg) to 113 eggs per 2 grams (56.5 epg). The median value of 2 epg was observed, indicating a skewed distribution of epg in the samples obtained from free-grazing cattle in the present study.
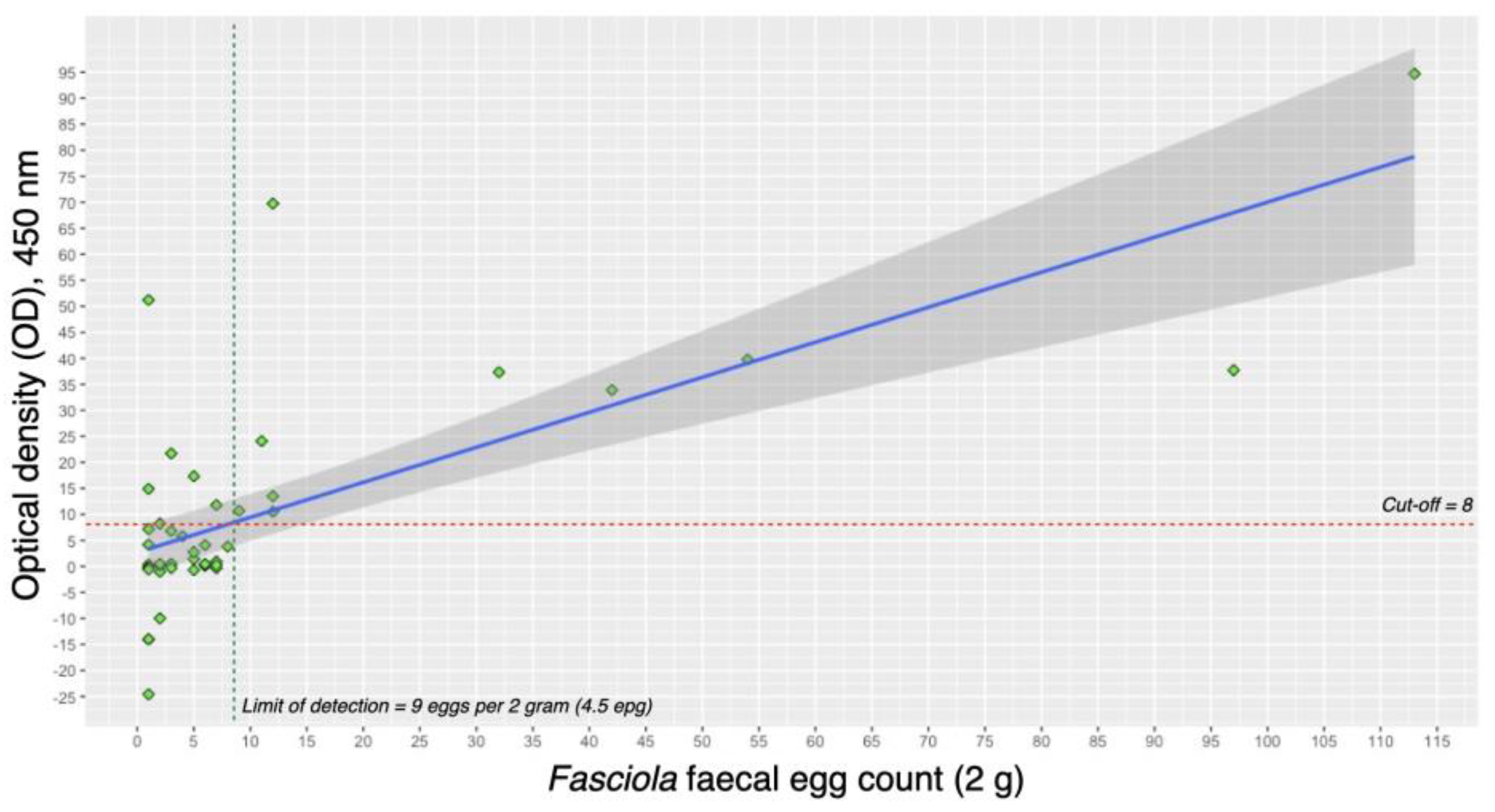
Figure 1 illustrates the linear correlation between
Fasciola fecal egg count (FFEC) and the concentration of
Fasciola coproantigen, as measured by the optical density (OD) readings in the cELISA. A statistically significant moderate positive correlation (r²=0.716, p-value < 0.01) was observed, indicating that higher egg counts are associated with higher OD values. The FFEC+ve group demonstrated a lower limit of detection (LoD) for the cELISA, set at approximately 4.5 epg. Samples with more than 4.5 epg consistently exhibited a 100% positivity rate. The highest observed OD value corresponded to the highest egg count in this study, reaching 113 eggs per 2 grams (56.5 epg). This finding emphasizes the sensitivity of the cELISA method in detecting
Fasciola antigens, particularly in samples with higher egg counts.
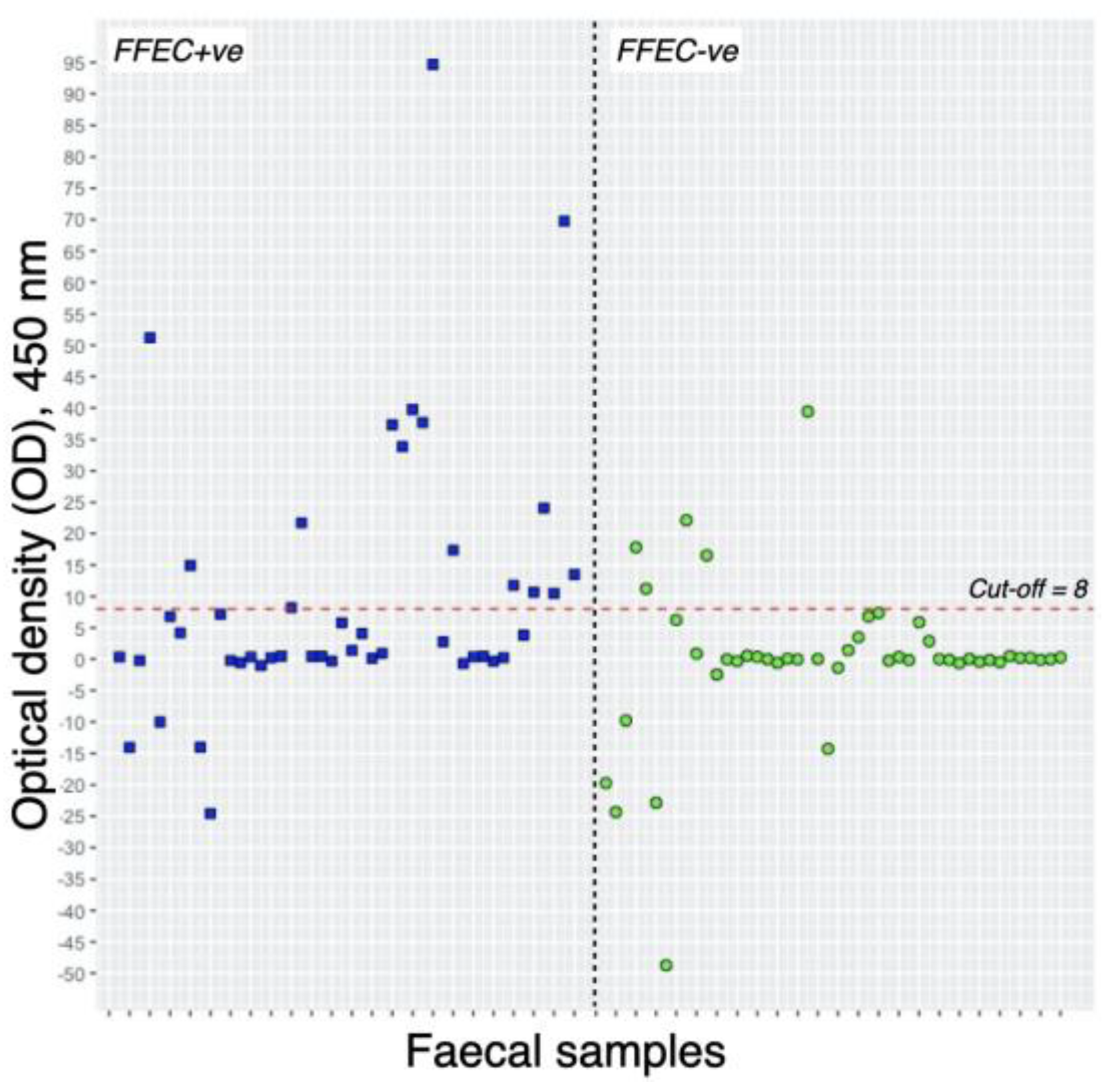
Descriptive overview of the Flukefinder® and cELISA results is presented in
Table 1 and
Figure 2. Out of 46 samples from the FFEC+ve group, 36 exhibited 4.5 epg or fewer, while 10 had more than 4.5 epg. Among the samples with 4.5 epg or fewer, only 6 (16.7%) tested positive through cELISA. In contrast, all samples with more than 4.5 epg showed a positive result with cELISA. The range of optical density (OD) values from cELISA-positive samples varied from 11 to 94, with a median OD value of 34.
Within the FFEC-ve group, 5 samples (10.87%) tested positive for Fasciola coproantigen. Notably, the range of coproantigen, as indicated by the optical density (OD) readings, in these 5 samples varied from 11 to 39 OD values, with a median OD value of 16.
4. Discussion
Early detection of fasciolosis in ruminant livestock is crucial for effective parasite control and management [
9,
10]. The present study aimed to compare the sensitivity of two diagnostic methods, fecal sedimentation and fecal antigen ELISA (cELISA) for the diagnosis of fasciolosis in free-grazing cattle within an area endemic to
F. gigantica. The exploration of this correlation has the potential to enhance the understanding of the applicability of coproantigen ELISA in monitoring parasite egg counts. This information is vital for preventing the spread of fasciolosis, as untreated animals contribute to continuous environmental contamination with
Fasciola eggs, especially in free-ranging animals with a higher prevalence [
8].
Out of the total FFEC+ve group, only 16 samples tested positive in cELISA. The present study delves into the distribution of
Fasciola eggs per gram (epg), revealing that samples with more than 4.5 epg demonstrated a 100% positivity rate compared to those with fewer epg. The 30 fecal samples undetected with
Fasciola coproantigen, despite the presence of
Fasciola eggs, could be attributed to the absence of active infection of newly emerged juveniles (NEJ) and adult flukes [
1]. Additionally, the retention of eggs after parasite elimination through treatment is common, leading to false positives in coproscopic examinations such as Flukefinder® [
10]. Coproantigen ELISA proves to be a more sensitive diagnostic method compared to coproscopic examination [
1,
4], detecting antigens from
Fasciola during active infection. With the lowest detection limit observed at 4.5 epg in the present study, it is noteworthy that these 30 samples were only documented with less than 4 epg.
Other possible justification is that due to the skewed distribution of egg counts, a phenomenon commonly observed in naturally infected animals. In naturally infected populations, there is often a wide range of egg counts among individual animals [
10,
11,
12], with some having very low counts and others having significantly higher counts. This variability can be influenced by several factors, including the stage of infection, host immune response, and individual variations in susceptibility [
10,
11]. The skewed distribution implies that animals in the sample population may have low egg counts, falling below the detection limit of the coproantigen test. The sensitivity of cELISA is limited in detecting very low-level infections, especially when the concentration of antigen in the feces is below the assay's threshold [
1]. Thus, this natural variability in egg distribution highlights the complexity of diagnosing parasitic infections in field settings. It underscores the importance of considering the limitations of diagnostic methods and interpreting results in the context of the specific characteristics of the study population. Future research could explore alternative or complementary diagnostic approaches to improve sensitivity, especially in situations where egg distribution is skewed.
As the moderate positive correlation between the diagnostic tests, the estimation of
Fasciola coproantigen concentration through cELISA proves to be a reliable predictor of egg counts, aligning with previous findings [
1], which is consistent with the correlation observed in the present study. Furthermore, the elevation of
Fasciola coproantigen correlates with egg counts in naturally infected animals [
5]. The examination of the odds ratio between
Fasciola fecal egg count (FFEC) and the optical density (OD) correlation yielded a value of 1.96 (CI: 1.61 ± 2.38, p-value < 0.01). This indicates that for every single unit increase in FFEC, there is a 96% likelihood of obtaining a higher OD reading from the coproantigen ELISA test. The odds ratio underscores that
Fasciola coproantigen in the samples can serve as an estimate for the egg count.
5. Conclusions
The present study reveals a moderate positive correlation between Fasciola coproantigen concentration and fecal egg count (p-value < 0.01), offering a promising diagnostic tool for cattle fasciolosis. The establishment of a lowest detection limit of at least 4.5 eggs per gram in cELISA resulted in 100% positivity, providing a reliable threshold for identifying active Fasciola infection. This finding suggests that the application of cELISA holds potential for monitoring the distribution of parasite eggs and identifying cattle with a high parasite egg burden. Such targeted identification can facilitate precise treatment strategies, thereby contributing to the effective control and limitation of the spread of fasciolosis.
Author Contributions
Conceptualization, NCK and NMI; methodology, NCK; software, NCK; validation, NMI; formal analysis, NCK; investigation, NCK; resources, NMI; data curation, NCK; writing—original draft preparation, NCK; writing—review and editing, NMI; visualization, NCK; supervision, NMI; project administration, NCK and NMI; funding acquisition, NMI. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universiti Putra Malaysia (UPM), Geran Putra IPS grant number 9736700.
Institutional Review Board Statement
The work described in this study involved the use of non-experimental animal and non-invasive sampling. The fecal samples collected were non-invasive and non-painful procedures following clearance number UPM/IACUC/AUP-007/2019.
Data Availability Statement
The raw datasets supporting this article’s findings are available from NCK upon written request. Please send a request to naimck@um.edu.my to request access to the data.
Acknowledgments
The authors would like to thank colleagues from Universiti Malaya (UM) and Universiti Putra Malaysia (UPM) for internal peer-reviews of this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mezo, M.; González-Warleta, M.; Carro, C.; Ubeira, F. M. An Ultrasensitive Capture ELISA for Detection of Fasciola Hepatica Coproantigens in Sheep and Cattle Using a New Monoclonal Antibody (MM3). J Parasitol 2004, 90, 845–852. [Google Scholar] [CrossRef]
- Alvarez Rojas, C. A.; Jex, A. R.; Gasser, R. B.; Scheerlinck, J. P. Y. Techniques for the Diagnosis of Fasciola Infections in Animals: Room for Improvement. Adv Parasitol 2014, 85, 65–107. [Google Scholar] [CrossRef]
- Kajugu, P. E.; Hanna, R. E. B.; Edgar, H. W.; McMahon, C.; Cooper, M.; Gordon, A.; Barley, J. P.; Malone, F. E.; Brennan, G. P.; Fairweather, I. Fasciola Hepatica: Specificity of a Coproantigen ELISA Test for Diagnosis of Fasciolosis in Faecal Samples from Cattle and Sheep Concurrently Infected with Gastrointestinal Nematodes, Coccidians and/or Rumen Flukes (Paramphistomes), under Field Conditions. Vet Parasitol 2015, 212, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Valero, M. A.; Ubeira, F. M.; Khoubbane, M.; Artigas, P.; Muiño, L.; Mezo, M.; Pérez-Crespo, I.; Periago, M. V.; Mas-Coma, S. MM3-ELISA Evaluation of Coproantigen Release and Serum Antibody Production in Sheep Experimentally Infected with Fasciola Hepatica and F. Gigantica. Vet Parasitol 2009, 159, 77–81. [Google Scholar] [CrossRef]
- Gordon, D. K.; Zadoks, R. N.; Stevenson, H.; Sargison, N. D.; Skuce, P. J. On Farm Evaluation of the Coproantigen ELISA and Coproantigen Reduction Test in Scottish Sheep Naturally Infected with Fasciola Hepatica. Vet Parasitol 2012, 187, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Brockwell, Y. M.; Spithill, T. W.; Anderson, G. R.; Grillo, V.; Sangster, N. C. Comparative Kinetics of Serological and Coproantigen ELISA and Faecal Egg Count in Cattle Experimentally Infected with Fasciola Hepatica and Following Treatment with Triclabendazole. Vet Parasitol 2013, 196, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Bishop, S. C. Possibilities to Breed for Resistance to Nematode Parasite Infections in Small Ruminants in Tropical Production Systems. Animal 2012, 6, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Che-Kamaruddin, N.; Hamid, N. F. S.; Idris, L. H.; Yusuff, F. M.; Ashaari, Z. H.; Yahaya, H.; Sahimin, N.; Isa, N. M. M. Prevalence and Risk Factors of Fasciolosis in a Bovine Population from Farms in Taiping, Malaysia. Vet Parasitol Reg Stud Reports 2024, 49, 100998. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sernández, V.; Orbegozo-Medina, R. A.; González-Warleta, M.; Mezo, M.; Ubeira, F. M. Rapid Enhanced MM3-COPRO ELISA for Detection of Fasciola Coproantigens. PLoS Negl Trop Dis 2016, 10. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, A.; Edgar, H. W. J.; Gordon, A.; Hanna, R. E. B.; Brennan, G. P.; Fairweather, I. Comparison of Two Assays, a Faecal Egg Count Reduction Test (FECRT) and a Coproantigen Reduction Test (CRT), for the Diagnosis of Resistance to Triclabendazole in Fasciola Hepatica in Sheep. Vet Parasitol 2011, 176, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E. R.; Aziz, N. A. A.; Blanchard, A.; Charlier, J.; Charvet, C.; Claerebout, E.; Geldhof, P.; Greer, A. W.; Hertzberg, H.; Hodgkinson, J.; Höglund, J.; Hoste, H.; Kaplan, R. M.; Martínez-Valladares, M.; Mitchell, S.; Ploeger, H. W.; Rinaldi, L.; von Samson-Himmelstjerna, G.; Sotiraki, S.; Schnyder, M.; Skuce, P.; Bartley, D.; Kenyon, F.; Thamsborg, S. M.; Vineer, H. R.; de Waal, T.; Williams, A. R.; van Wyk, J. A.; Vercruysse, J. 100 Questions in Livestock Helminthology Research. Trends Parasitol 2019, 35, 52–71. [Google Scholar] [CrossRef] [PubMed]
- Che-Kamaruddin, N.; Isa, N. M. M. Assessment of Fasciola and Paramphistomes Co-Infection in Large Ruminants through Faecal Egg Counts around Taiping, Malaysia. Trop Biomed 2023, 40, 344–350. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).