Submitted:
12 February 2024
Posted:
12 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. Cell Preparation and Spiking
2.3. Rare Cell Enrichment
2.4. Image Acquisition and Analysis
2.5. Statistical Analysis
3. Results
3.1. Reliability
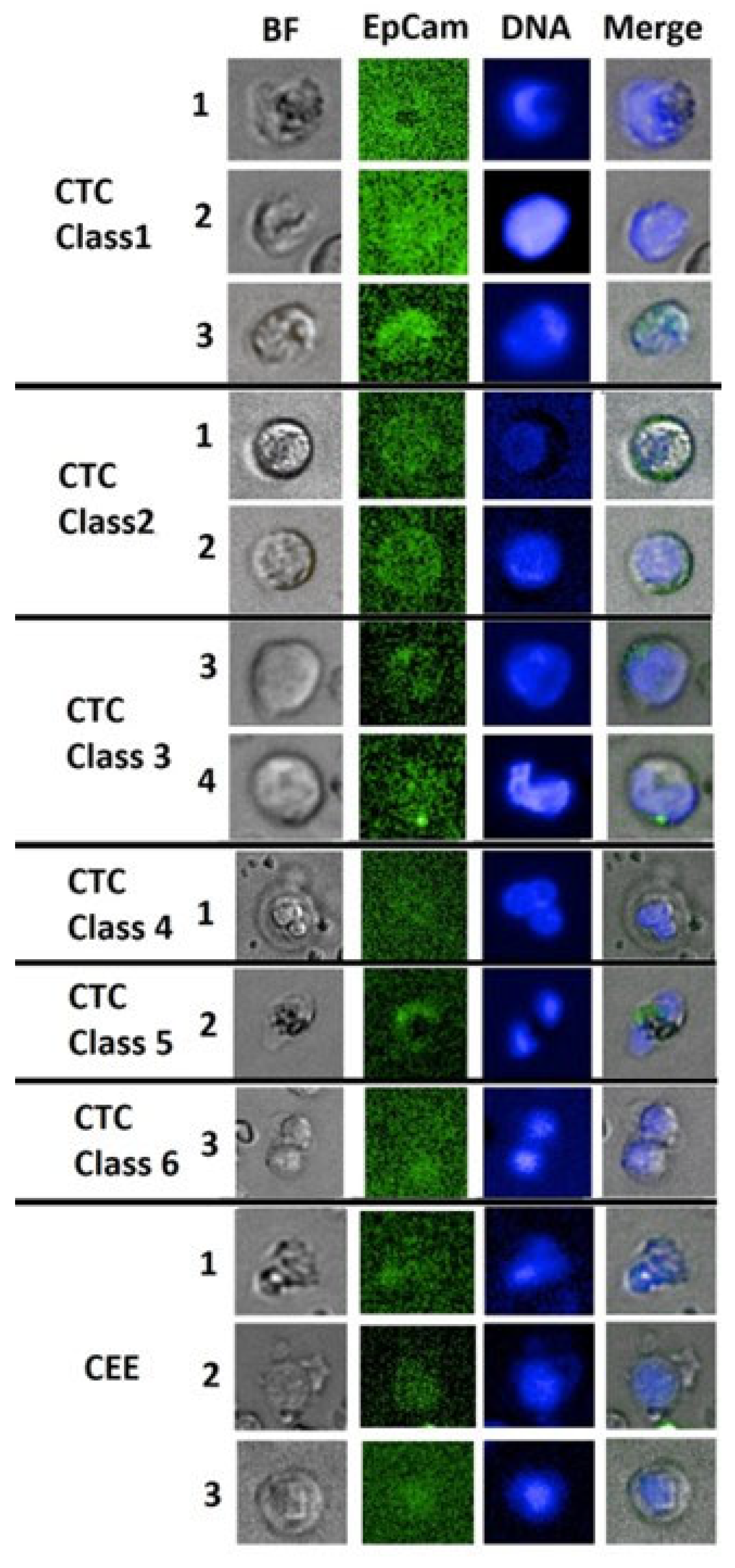
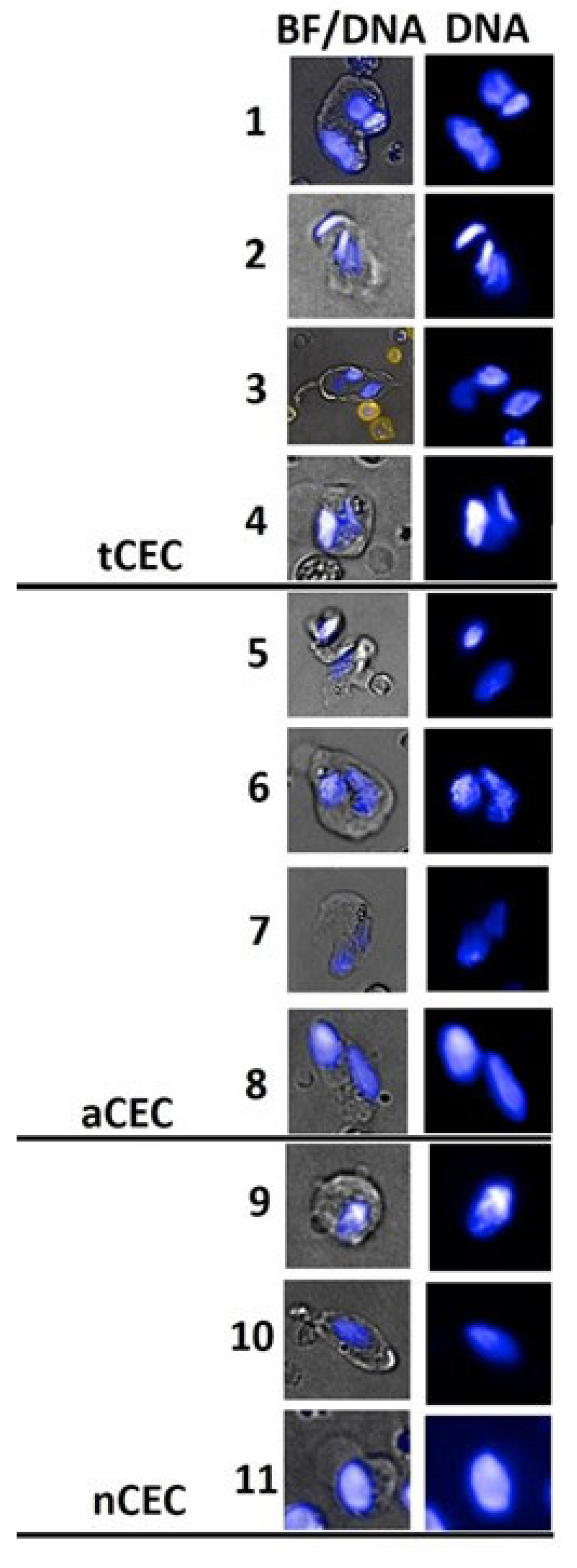
3.2. CASA Panel
| Class 1 | Class 2 | Class 3 | Class 4 | Class 5 | Class 6 | Non-CTC and CTC in suspicion |
|
|---|---|---|---|---|---|---|---|
| Cell feature | Fully nucleated regular large | Round cell (Classic CTC) | Leukocyte-like | Extreme large cells | Multi-nucleation | Cell pairs/ Clusters |
CEE |
| Cell Shape | oval or roundish | roundish to absolute round | Variable | Oval to round | Variable | Oval to round | All shapes, cell budding |
| BF appearance | Clear outer membrane rim with convex cell body, no inner or second membrane, heterogeneous texture |
Strong membrane rim, heterogeneous texture |
Strong inner or outer membrane rim heterogeneous or segmented texture |
heterogeneous or segmented texture | Heterogeneous | Strong membrane rim heterogeneous texture |
various membrane rim, homogeneous or patterned |
| Cell diameter (major axis) |
10.5-18µm | 9.5-14µm | 8-18µm | >18µm | >8-18µm | >10 µm | 6-18µm |
| EpCam staining intensity | Dim - Low | Low | Dim - High | Dim - Low | Dim - Low | Dim - Low | Dim |
| EpCam staining distribution | Partly strong, all cell or outer membrane | Clear membrane | Intracellular or partial/ exceeding Nucleus location |
Intracellular or Partial | Intracellular or Partial | Intracellular or Partial | Intracellular, often overlaid with nucleus |
| Nucleus Staining | Low - High | Dim-Low | Dim-Low | Low-High | Dim-Low | Dim-High | Dim -Low |
| Nucleus texture | Homogeneous, no clear centre, | Homogeneous or rimed | Heterogeneous, polarized, nucleoli | Heterogeneous | Heterogeneous | Heterogeneous | homogeneous |
| Nucleus shape | cell shape aligned | oval to round | Variable, not aligned | Variable, not aligned | Variable, not aligned | oval to round | Oval, round |
| N/C-Ratio | >0.8 | ~0.5 | ~0.5 – 0.8 | ~0.5 – 0.8 | ~0.5 – 0.8 | ~0.5 – 0.8 | ~0.5 <1 |
| Cancer Association | Tumor | Systemic | Systemic | Tumor | Systemic | Tumor | Systemic/Tumor |
3.3. Diagnostic Performance
| Cancer present N = 14 |
Cancer absent & cancer naïve N = 43 |
Predictive values | |
|---|---|---|---|
| tCTC cut off at 1 cell/5 mL | |||
| tCTC ≥ 1 and (tCEC ≥ 1 or CEE ≥ 225)* : Positive test | 10 | 3 | PPV: 76.9% |
| (tCTC = 0 or tCEC = 0) and (tCTC = 0 or CEE < 225)*: Negative test | 4 | 40 | NPV: 90.9% |
| Sensitivity & specificity | Sensitivity: 71.4% | Specificity: 93.0% | |
| tCTC cut off at 2 cells/5 mL | |||
| tCTC ≥ 2 and (tCEC ≥ 1 or CEE ≥ 225)*: Positive test | 7 | 0 | PPV: 100% |
| (tCTC < 2 or tCEC = 0) and (tCTC < 2 or CEE < 225)*: Negative test | 7 | 43 | NPV: 86.0% |
| Sensitivity & specificity | Sensitivity: 50.0% | Specificity: 100% | |
| Cancer, before & after surgery N = 28 |
Cancer naïve N = 29 |
Predictive values | |
|---|---|---|---|
| sCTC only; cut off at 3 or 4 cells/5 mL (see text) | |||
| sCTC ≥ 3 or 4*: Positive test | 21 | 1 | PPV: 95.5% |
| sCTC < 3 or 4*: Negative test | 7 | 28 | NPV: 80.0% |
| Sensitivity & specificity | Sensitivity: 75.0% | Specificity: 96.6% | |
| sCTC cut off at 3 or 4 cells/5 mL combined with aCEC, aCIC or aCEB | |||
| sCTC ≥ 3 or 4 and (aCEC ≥ 1 or aCIC ≥ 1 or aCEB ≥ 1)*: Positive test | 16 | 0 | PPV: 100% |
| (sCTC < 3 or 4 or aCEC = 0) and (sCTC < 3 or 4 or aCIC = 0) and (sCTC < 3 or 4 or aCEB = 0)*: Negative test | 12 | 29 | NPV: 70.1% |
| Sensitivity & specificity | Sensitivity: 57.1% | Specificity: 100% | |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Burstein, H.J.; Curigliano, G.; Loibl, S.; et al. Estimating the benefits of therapy for early-stage breast cancer: the St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Annals of Oncology 2019, 30, 1541–1557. [Google Scholar] [CrossRef]
- Pedersen, R.N.; Esen, B.Ö.; Mellemkjær, L.; Christiansen, P.; Ejlertsen, B.; Lash, T.L.; Nørgaard, M.; Cronin-Fenton, D. The incidence of breast cancer recurrence 10-32 years after primary diagnosis. JNCI: Journal of the National Cancer Institute 2022, 114, 391–399. [Google Scholar] [CrossRef]
- Elder, E.E.; Kennedy, C.W.; Gluch, L.; Carmalt, H.L.; Janu, N.C.; Joseph, M.G.; Donellan, M.J.; Molland, J.G.; Gillett, D.J. Patterns of breast cancer relapse. European Journal of Surgical Oncology 2006, 32, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Ellington, T.D.; Miller, J.W.; Henley, S.J.; Wilson, R.J.; Wu, M.; Richardson, L.C. Trends in breast cancer incidence, by race, ethnicity, and age among women aged≥ 20 years—United States, 1999–2018. Morbidity and Mortality Weekly Report 2022, 71, 43. [Google Scholar] [CrossRef] [PubMed]
- Retsky, M.; Demicheli, R.; Forget, P.; De Kock, M.; Gukas, I.; ARogers, R.; Baum, M.; Sukhatme, V.; SVaidya, J. Reduction of breast cancer relapses with perioperative non-steroidal anti-inflammatory drugs: new findings and a review. Current medicinal chemistry 2013, 20, 4163–4176. [Google Scholar] [CrossRef] [PubMed]
- Elkholi, I.E.; Lalonde, A.; Park, M.; Côté, J.F. Breast cancer metastatic dormancy and relapse: An enigma of microenvironment (s). Cancer Research 2022, 16. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, Z.; Chen, Z.; Zhang, T.; Lu, Y. Multiple tumor types may originate from bone marrow-derived cells. Neoplasia 2006, 8, 716–IN3. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Cole, S.W. Chronic inflammation and breast cancer recurrence. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 2009, 27, 3418. [Google Scholar] [CrossRef]
- Pierce, B.L.; Ballard-Barbash, R.; Bernstein, L.; et al. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. Journal of Clinical Oncology 2009, 27, 3437. [Google Scholar] [CrossRef]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer immunoediting: from immunosurveillance to tumor escape. Nature immunology 2002, 3, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Schreier, S.; Triampo, W. The blood circulating rare cell population. What is it and what is it good for? Cells 2020, 9, 790. [Google Scholar] [CrossRef] [PubMed]
- Hida, K.; Hida, Y.; Shindoh, M. Understanding tumor endothelial cell abnormalities to develop ideal anti-angiogenic therapies. Cancer science 2008, 99, 459–466. [Google Scholar] [CrossRef]
- Allard, W.J.; Matera, J.; Miller, M.C.; et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clinical cancer research 2004, 10, 6897–6904. [Google Scholar] [CrossRef]
- Lin, A.Y.; Wang, D.D.; Li, L.; Lin, P.P. Identification and comprehensive co-detection of necrotic and viable aneuploid cancer cells in peripheral blood. Cancers 2021, 13, 5108. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Gong, Y.; Wang, Y.; Xie, J.; Cheng, J.; Huang, Q. Comprehensive atlas of circulating rare cells detected by se-ifish and image scanning platform in patients with various diseases. Frontiers in Oncology 2022, 12. [Google Scholar] [CrossRef]
- Pantel, K.; Alix-Panabières, C. Liquid biopsy and minimal residual disease—latest advances and implications for cure. Nature Reviews Clinical Oncology 2019, 16, 409–424. [Google Scholar] [CrossRef]
- Schreier, S.; Triampo, W. Systemic cytology. A novel diagnostic approach for assessment of early systemic disease. Medical Hypotheses 2021, 156, 110682. [Google Scholar] [CrossRef]
- Pachmann, K. Current and potential use of MAINTRAC method for cancer diagnosis and prediction of metastasis. Expert Review of Molecular Diagnostics 2015, 15, 597–605. [Google Scholar] [CrossRef]
- Bhakdi, S.C.; Suriyaphol, P.; Thaicharoen, P.; et al. Accuracy of tumour-associated circulating endothelial cells as a screening biomarker for clinically significant prostate cancer. Cancers. 2019, 11, 1064. [Google Scholar] [CrossRef] [PubMed]
- Schreier, S.; Budchart, P.; Borwornpinyo, S.; Arpornwirat, W.; Triampo, W. Circulating erythroblast abnormality associated with systemic pathologies may indicate bone marrow damage. Journal of Circulating Biomarkers 2021, 10, 14. [Google Scholar] [CrossRef]
- Schreier, S.; Budchart, P.; Borwornpinyo, S.; et al. New inflammatory indicators for cell-based liquid biopsy: association of the circulating CD44+/CD24− non-hematopoietic rare cell phenotype with breast cancer residual disease. Journal of Cancer Research and Clinical Oncology 2022, 1–2. [Google Scholar] [CrossRef]
- Li, W.; Ma, G.; Deng, Y.; Chen, W.; Liu, Z.; Chen, F.; Wu, Q. Systemic immune-inflammation index is a prognostic factor for breast cancer patients after curative resection. Frontiers in Oncology 2021, 4516. [Google Scholar] [CrossRef]
- Schreier, S.; Sawaisorn, P.; Udomsangpetch, R.; Triampo, W. Advances in rare cell isolation: an optimization and evaluation study. Journal of Translational Medicine 2017, 15, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Schreier, S.; Borwornpinyo, S.; Udomsangpetch, R.; Triampo, W. An update of circulating rare cell types in healthy adult peripheral blood: findings of immature erythroid precursors. Annals of Translational Medicine 2018, 6. [Google Scholar] [CrossRef]
- Wizenty, J.; Ashraf, M.I.; Rohwer, N.; et al. Autofluorescence: A potential pitfall in immunofluorescence-based inflammation grading. Journal of immunological methods 2018, 456, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Heintzelman, D.L.; Lotan, R.; Richards-Kortum, R.R. Characterization of the autofluorescence of polymorphonuclear leukocytes, mononuclear leukocytes and cervical epithelial cancer cells for improved spectroscopic discrimination of inflammation from dysplasia. Photochemistry and photobiology 2000, 71, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Li, K.; Xin, Y.; Tang, K.; Yang, M.; Wang, G.; Tan, Y. Fluid shear stress regulates the survival of circulating tumor cells via nuclear expansion. Journal of Cell Science 2022, 135, jcs259586. [Google Scholar] [CrossRef] [PubMed]
- El-Heliebi, A.; Kroneis, T.; Zöhrer, E.; Haybaeck, J.; Fischereder, K.; Kampel-Kettner, K.; Zigeuner, R.; Pock, H.; Riedl, R.; Stauber, R.; Geigl, J.B. Are morphological criteria sufficient for the identification of circulating tumor cells in renal cancer? Journal of translational medicine 2013, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Erdbruegger, U.; Haubitz, M.; Woywodt, A. Circulating endothelial cells: a novel marker of endothelial damage. Clinica chimica acta 2006, 373, 17–26. [Google Scholar] [CrossRef]
- Bull, T.M.; Golpon, H.; Hebbel, R.P.; Solovey, A.; Cool, C.D.; Tuder, R.M.; Geraci, M.W.; Voelkel, N.F. Circulating endothelial cells in pulmonary hypertension. Thrombosis and haemostasis 2003, 90, 698–703. [Google Scholar]
- Boos, C.J.; Lip, G.Y.; Blann, A.D. Circulating endothelial cells in cardiovascular disease. Journal of the American College of Cardiology 2006, 48, 1538–1547. [Google Scholar] [CrossRef]
- Tokunaga, O.; Satoh, T.; Yamasaki, F.; Wu, L. Multinucleated variant endothelial cells (MVECs) in human aorta: chromosomal aneuploidy and elevated uptake of LDL. InSeminars in thrombosis and hemostasis 1998 (Vol. 24, No. 03, pp. 279-284). Copyright© 1998 by Thieme Medical Publishers, Inc.
- Kolenčík, D.; Narayan, S.; Thiele, J.A.; et al. Circulating tumor cell kinetics and morphology from the liquid biopsy predict disease progression in patients with metastatic colorectal cancer following resection. Cancers 2022, 14, 642. [Google Scholar] [CrossRef]
- Rossi, G.; Ignatiadis, M. Promises and pitfalls of using liquid biopsy for precision medicine. Cancer research 2019, 79, 2798–2804. [Google Scholar] [CrossRef] [PubMed]
- Goeminne, J.C.; Guillaume, T.; Symann, M. Pitfalls in the detection of disseminated non-hematological tumor cells. Annals of oncology 2000, 11, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Marrinucci, D.; Bethel, K.; Bruce, R.H.; et al. Case study of the morphologic variation of circulating tumor cells. Human pathology. 2007, 38, 514–519. [Google Scholar] [CrossRef]
- Marrinucci, D.; Bethel, K.; Luttgen, M.; Bruce, R.H.; Nieva, J.; Kuhn, P. Circulating tumor cells from well-differentiated lung adenocarcinoma retain cytomorphologic features of primary tumor type. Archives of pathology & laboratory medicine 2009, 133, 1468–1471. [Google Scholar]
- Marrinucci, D.; Bethel, K.; Lazar, D.; Fisher, J.; Huynh, E.; Clark, P.; Bruce, R.; Nieva, J.; Kuhn, P. Cytomorphology of circulating colorectal tumor cells: a small case series. Journal of oncology 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- Hüsemann, Y.; Geigl, J.B.; Schubert, F.; et al. Systemic spread is an early step in breast cancer. Cancer cell 2008, 13, 58–68. [Google Scholar] [CrossRef]
- Christiansen, P.; Mele, M.; Bodilsen, A.; Rocco, N.; Zachariae, R. Breast-conserving surgery or mastectomy?: Impact on survival. Annals of Surgery Open 2022, 3, e205. [Google Scholar] [CrossRef]
- Hellman, S.; Lecture, K.M. Natural history of small breast cancers. Journal of clinical oncology. 1994, 12, 2229–2234. [Google Scholar] [CrossRef] [PubMed]
- Van Dalum, G.; Van der Stam, G.J.; Tibbe, A.G.; et al. Circulating tumor cells before and during follow-up after breast cancer surgery. International journal of oncology 2015, 46, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Trzpis, M.; McLaughlin, P.M.; de Leij, L.M.; Harmsen, M.C. Epithelial cell adhesion molecule: more than a carcinoma marker and adhesion molecule. The American journal of pathology 2007, 171, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Bethel, K.; Luttgen, M.S.; Damani, S.; Kolatkar, A.; Lamy, R.; Sabouri-Ghomi, M.; Topol, S.; Topol, E.J.; Kuhn, P. Fluid phase biopsy for detection and characterization of circulating endothelial cells in myocardial infarction. Physical biology 2014, 11, 016002. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Ang, R.R.; Duffy, S.P.; Bazov, J.; Chi, K.N.; Black, P.C.; Ma, H. Morphological differences between circulating tumor cells from prostate cancer patients and cultured prostate cancer cells. PloS one 2014, 9, e85264. [Google Scholar] [CrossRef] [PubMed]
- Slade, M.J.; Coombes, R.C. The clinical significance of disseminated tumor cells in breast cancer. Nature clinical practice Oncology 2007, 4, 30–41. [Google Scholar] [CrossRef]
- Hartkopf, A.D.; Taran, F.A.; Wallwiener, M.; et al. Prognostic relevance of disseminated tumour cells from the bone marrow of early stage breast cancer patients–results from a large single-centre analysis. European journal of cancer 2014, 50, 2550–2559. [Google Scholar] [CrossRef]
- Neeman, E.; Ben-Eliyahu, S. Surgery and stress promote cancer metastasis: new outlooks on perioperative mediating mechanisms and immune involvement. Brain, behavior, and immunity 2013, 30, S32–40. [Google Scholar] [CrossRef]
- Cima, I.; et al. "Tumor-derived circulating endothelial cell clusters in colorectal cancer.". Science translational medicine 2016, 8, 345ra89–345ra89. [Google Scholar] [CrossRef]
- Mehran, R.; et al. "Tumor endothelial markers define novel subsets of cancer-specific circulating endothelial cells associated with antitumor efficacy. " Cancer research 2014, 74, 2731–2741. [Google Scholar] [CrossRef]
- Jiang, X.; et al. "Microfluidic isolation of platelet-covered circulating tumor cells.". Lab on a Chip 2017, 17, 3498–3503. [Google Scholar] [CrossRef] [PubMed]
- Rack, B.; et al. "Circulating tumor cells predict survival in early average-to-high risk breast cancer patients.". Journal of the National Cancer Institute 2014, 106, dju066. [Google Scholar] [CrossRef] [PubMed]
| Patient code | Age* (years) | Comorbidities/ Vaccination | Histological characteristic | Lymphatic/Node involvement | IHC markers | Type of surgery | Systemic immune-inflammation index (SII)** |
|---|---|---|---|---|---|---|---|
| BR001 | 51 | No comorbidity/ Covid-19 vaccination 1 month before enrollment | Invasive lobular carcinoma, 26 mm, Grade 2, Free margin (Closest margin 1 mm) |
Lymphovascular invasion | ER 95% PR 95% Her-2 0% Ki67 30% |
Breast-conserving surgery (BCS) + Sentinel lymph node biopsy (SLNB) |
High (3,279) |
| BR002 | 74 | Diabetes type II, Peripheral vascular disease | Invasive ductal carcinoma, 36 mm, Grade 2, Free margin (Closest margin 12 mm) | Lymphovascular invasion Axillary node 3 of 11 |
ER < 1% PR < 1% Her-2 1+ Ki67 15% |
Mastectomy + Axillary lymph node dissection (ALND) |
High (581) |
| BR003 |
80 | No comorbidity/Covid-19 Vaccination 1 month before enrollment |
Left: Invasive ductal carcinoma, 45 mm, Free margin (Closest margin 3 mm) |
Left: Lymphovascular invasion |
Left: ER 99% PR 20% Her-2 0% Ki67 40% |
Left: Mastectomy with SLNB |
High (913) |
|
Right: Invasive ductal carcinoma, 24 mm, Free margin (Closest margin 8 mm) |
Right: Lymphovascular invasion Axillary node 3 of 5 |
Right: ER 99% PR 20% Her-2 0 Ki67 40% |
Right: Mastectomy with ALND |
||||
| BR005 | 34 | No comorbidity | Invasive ductal carcinoma, 20 mm, Grade III, Free margin (Closest margin 2 mm) | Lymphovascular invasion | ER 0% PR 0% Her-2 2+ Ki67 90% |
BCS + SLNB |
High (1,488) |
| BR006 | 55 | No comorbidity | Invasive ductal carcinoma, 28 mm, Grade III, Free margin (Closest margin 2 mm) | Lymphovascular invasion Axilliary node 4 of 48 |
ER 0% PR 0% Her-2 3+ Ki67 50% |
Mastectomy + ALND |
Low (469) |
| BR007 | 53 | No comorbidity | Invasive ductal carcinoma, 25 mm, Grade III, Free margin (Closest margin 5 mm) | Axilliary node 11 of 27 | Triple negative Ki67-60% |
Mastectomy + ALND |
Low (209) |
| BR008 | 58 | No comorbidity / Covid-19 vaccination 1 month before enrollment | Invasive ductal carcinoma, 30 mm, Grade III, Free margin (Closest margin 5 mm) | No involvement | Triple negative Ki67 50% |
BCS + SLNB |
High (515) |
| BR009 | 59 | No comorbidity/ Covid-19 vaccination 3 months before enrollment | Invasive breast carcinoma, 2 mm, Grade II, Free margin (Closest margin 8 mm) | No involvement | ER 100% PR 80% Her-2 0 Ki67 10% |
Mastectomy + SLNB |
Low (246) |
| BR012 | 62 | Peptic ulcer | Invasive papillary carcinoma, 19 mm, Grade III, Free margin (Closest margin 33 mm) | Axillary node 1 of 3 |
ER < 1% PR < 1% Her-2 3+ Ki67 30% |
Mastectomy + SLNB |
Low (456) |
| BR013 | 69 | Diabetes, Peptic ulcer/ Influenza vaccination 1 month before enrollment | Invasive ductal carcinoma, 13 mm, Grade II, Free margin (Closest margin 5 mm) | No involvement | ER 95% PR 20% Her-2 0 Ki67 25% |
Mastectomy + SLNB | Low (259) |
| BR014 | 61 | No comorbidity | Invasive ductal carcinoma, 19 mm, Grade II, Free margin | No involvement | ER < 1% PR < 1% Her-2 3+ Ki67 30% |
Mastectomy + SLNB | Low (224) |
| BR016 | 40 | Peptic ulcer/ Covid-19 infection 3 months before enrollment | DCIS (left) and Invasive ductal carcinoma (right) Grade II, Free margin (Closest margin 7 mm) | No involvement | ER 80% PR < 1% Her-2 0 Ki67 10% |
Mastectomy + ALND with reconstruction | Low (263) |
| BR017 | 66 | No comorbidity | Invasive ductal carcinoma, 33 mm, Grade III, Free margin (Closest margin 6 mm) | No involvement | ER 100% PR < 0% Her-2 2+ Ki67 25% |
Mastectomy + SLNB | Low (448) |
| BR018 | 51 | No comorbidity | Invasive ductal carcinoma, 12 mm, Grade III, Free margin (Closest margin 1 mm) | No involvement | Triple negative Ki67 60% |
BCS + SLNB | Low (454) |
| Cell type | Phenotype | Description | Diagnostic Implication | References |
|---|---|---|---|---|
| Circulating Epithelial Cell Events (CEE and CTC) |
EpCam+/CD45-/Hoechst+ | EpCam+ heterogeneous cell type composition including CEE and CTC | Multi-pathology association; Cancer association |
[14] |
| Circulating Erythroblasts (CEB) |
EpCam-/CD71+/CD45-/Hoechst+ | CD71+ bone marrow-derived rare cells indicating bone marrow damage (BMD) represented by dyserythropoiesis | Multi-pathology association; Cancer association |
[21] |
| Circulating Endothelial-like cells (CEC) | (CD31+)/CD45-/Hoechst+ | Vascular- and bone marrow-derived rare cells representative of vascular dysfunction, repair and neo-angiogenesis | Multi-pathology association; Cancer association |
[13,15] |
| Circulating Inflammatory cells (CIC) | EpCam-/CD71-CD44+/CD24-/CD45-/Hoechst+ | Leukocyte-like cells with unknown origin associated with systemic inflammation | Multi-pathology association; Cancer association |
[22] |
| Cancer before surgery N = 14 |
Cancer naïve N = 29 |
Predictive values | |
|---|---|---|---|
| SCTC ≥ 3 or 4 (class3 only) and tCTC ≥ 2* or cancer-presence CASA positive or cancer-absence CASA positive: Positive test | 12 | 1 | PPV: 92.3% |
| sCTC < 3 or 4 and tCTC < 2*: Negative test | 2 | 28 | NPV: 93.3% |
| Sensitivity & specificity | Sensitivity: 85.7% | Specificity: 96.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




