Submitted:
13 February 2024
Posted:
13 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
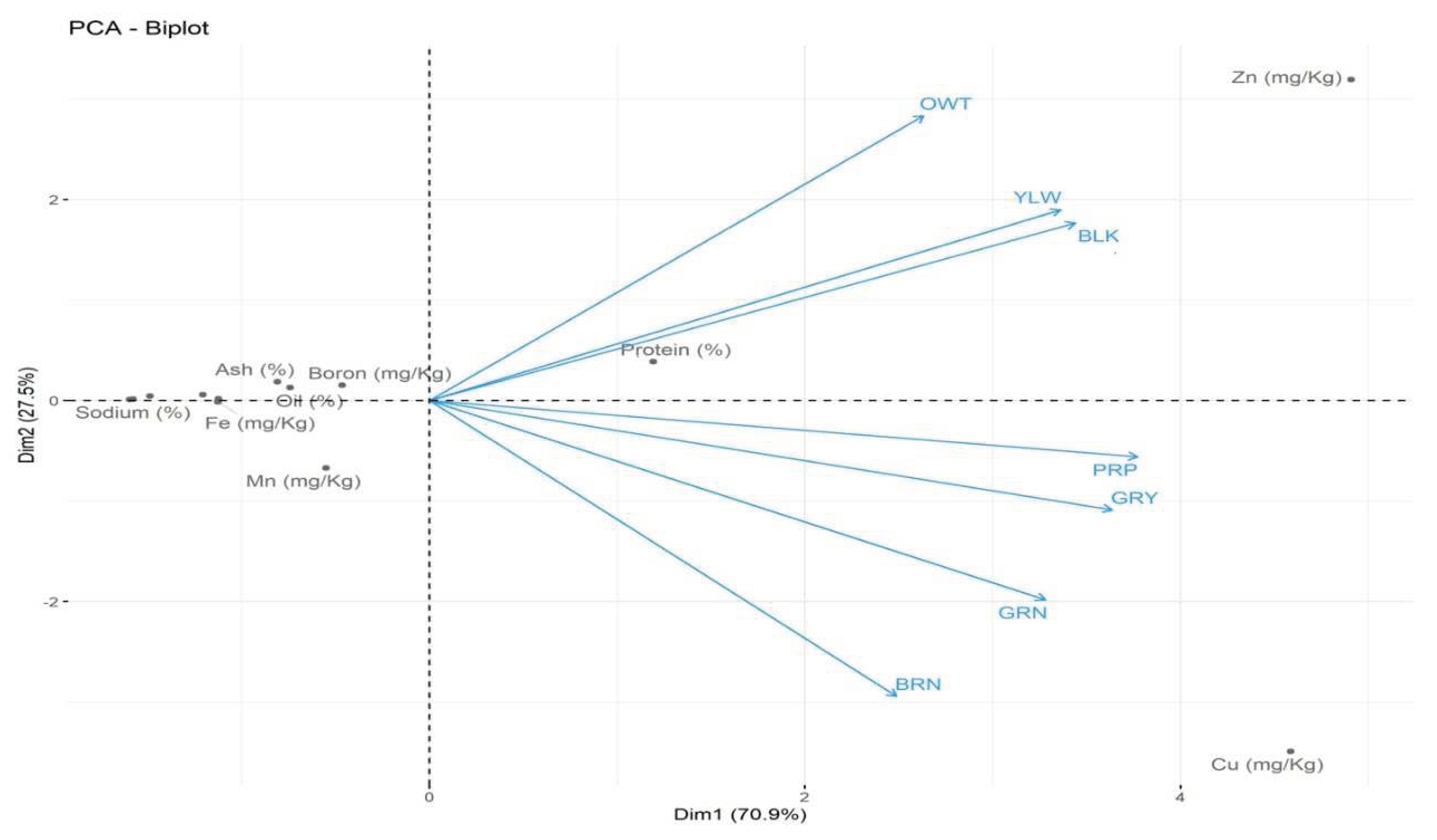
2.1. Physical characteristics of Indigenous Adlay accessions
2.2. Physicochemical, triglycerides and functional groups of indigenous Adlay accessions
2.2.1. Physicochemical characterization
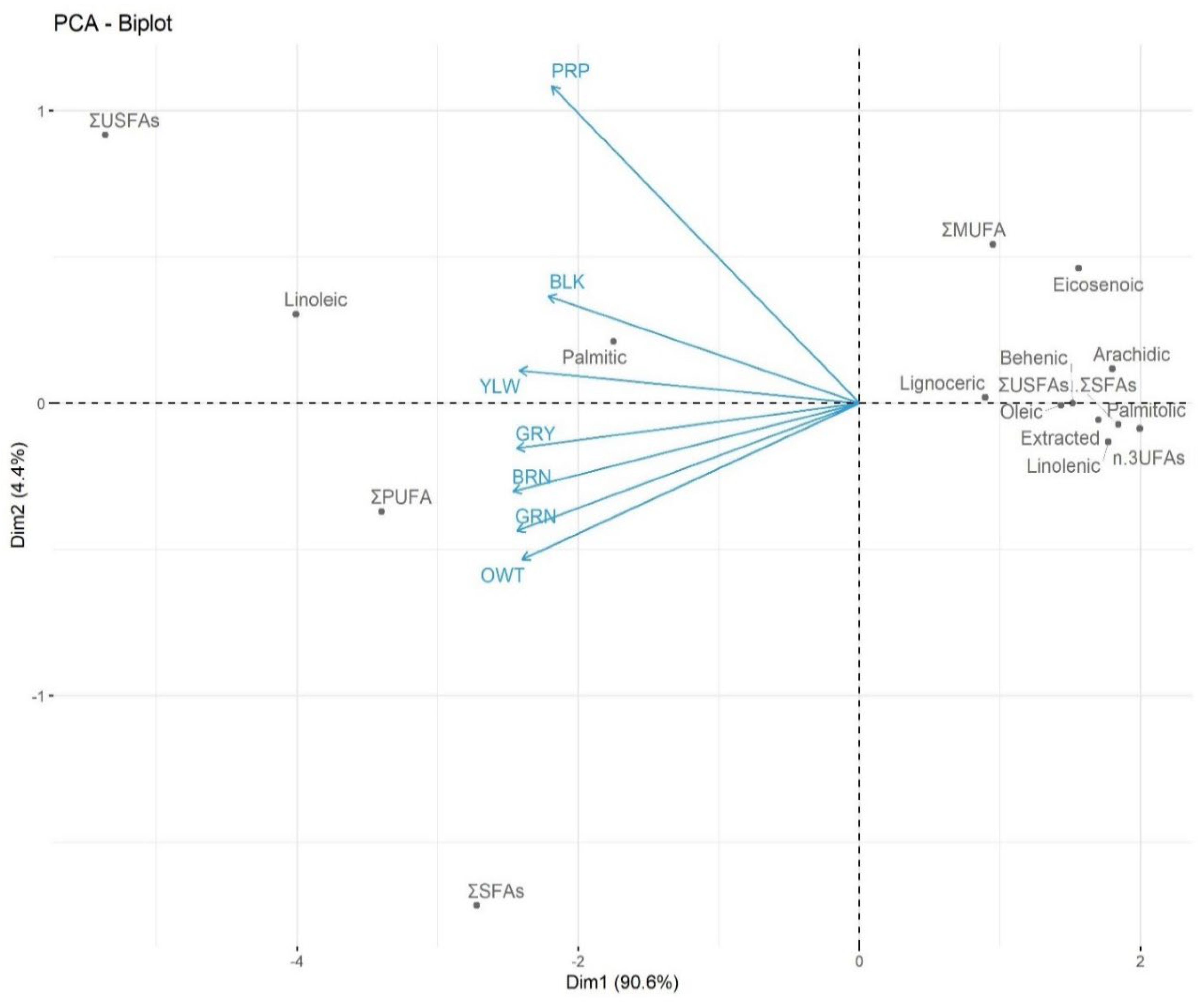
2.2.2. Wild adlay triglyceride composition
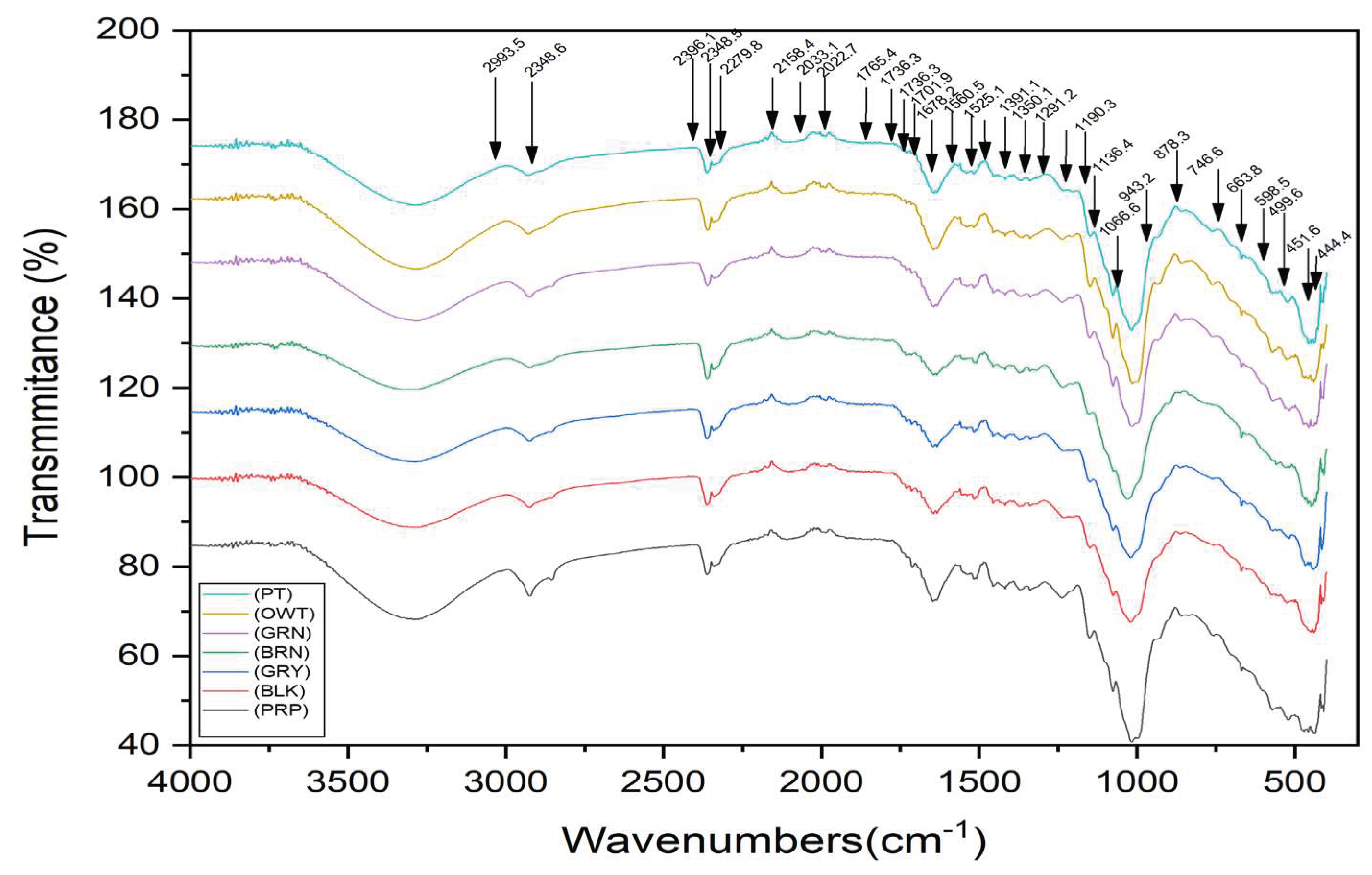
2.2.3. Identification of functional groups or bonds in Adlay flour using FTIR
3. Experimental sections
3.1. Materials
3.2. Physical characteristics determination of Adlay seed
3.3. Physicochemical, triglycerides composition and functional groups determination
3.3.1. Physicochemical parameters measurement
3.3.2. Ash, crude fiber, and mineral determination
3.3.3. Triglyceride composition determination
3.3.4. Functional groups/bonds frequencies determination in Adlay flour using FTIR
4. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, A.; Cottrell, J.J.; Dunshea, F.R. Characterization, Antioxidant Potential, and Pharmacokinetics Properties of Phenolic Compounds from Native Australian Herbs and Fruits. Plants 2023, 12, 993. [Google Scholar] [CrossRef] [PubMed]
- Zahid, H.F.; Ali, A.; Ranadheera, C.S.; Fang, Z.; Ajlouni, S. Identification of phenolics profile in freeze-dried apple peel and their bioactivities during in vitro digestion and colonic fermentation. Int. J. Mol. Sci. 2023, 24, 1514. [Google Scholar] [CrossRef]
- Ali, A.; Cottrell, J.J.; Dunshea, F.R. Identification and characterization of anthocyanins and non-anthocyanin phenolics from Australian native fruits and their antioxidant, antidiabetic, and anti-Alzheimer potential. Food Res. Int. 2022, 162, 111951. [Google Scholar] [CrossRef]
- Weng, W.F.; Peng, Y.; Pan, X.; Yan, J.; Li, X.D.; Liao, Z.Y.; Cheng, J.P.; Gao, A.J.; Yao, X.; Ruan, J.J.; Zhou, M.L. Adlay, an ancient functional plant with nutritional quality, improves human health. Front.Nutr. 2022, 9, 1019375. [Google Scholar] [CrossRef] [PubMed]
- Li, X.K. Research progress on chemical constituents and pharmacological effects of Coicis Semen. Chinese Tradational Herb Drugs. 2020, 24, 5645–5657. [Google Scholar]
- Igbokwe, C.J.; Wei, M.; Feng, Y.; Duan, Y.; Ma, H.; Zhang, H. Coix seed: A review of its physicochemical composition, bioactivity, processing, application, functionality, and safety aspects. Food Rev. Int. 2022, 38(sup1), 921–939. [Google Scholar] [CrossRef]
- Devaraj, R.D.; Jeepipalli, S.P.; Xu, B. Phytochemistry and health promoting effects of Job’s tears (Coix lacryma-jobi)-A critical review. Food Biosi. 2020, 34, 100537. [Google Scholar] [CrossRef]
- Zeng, Y.; Yang, J.; Chen, J.; Pu, X.; Li, X.; Yang, X.; Yang, L.E.; Ding, Y.; Nong, M.; Zhang, S.; He, J. Actional mechanisms of active ingredients in functional food adlay for human health. Molecules 2022, 27, 4808. [Google Scholar] [CrossRef]
- Soni, J.K.; Lalramhlimi, B.; Kumar, A.; Navik, O.; Sailo, L.; Doley, S. Coix: an underutilized functional food crop of Mizoram. Genetic Resources and Crop Evolution. 2023, 1, 1–7. [Google Scholar] [CrossRef]
- Deng, S.F.; Ying, Z.Y.; Yang, Y.Q.; Lin, Z.G.; Chen, M. Research progress of functional ingredients on adlay. Chin Agri Sci Bullet. 2017, 33, 123–128. [Google Scholar]
- Andriansyah, R.C.E.; Hidayat, D.D.; Kuala, S.I.; Luthfiyanti, R.; Surahman, D.N.; Indriati, A.A. Comparative Study between Wild and Cultivated Varieties of Adlay Grains for Some Engineering Properties. In Proceedings of the 16th ASEAN Food Conference (16thAFC2019)-Outlook and Opportunities of Food Technology and Culinary for Tourism Industry; 2019; pp. 104–113, ISBN 978-989-758-467-1. [Google Scholar]
- Xi, X.J. Zhu, Y.G.; Tong, Y.P.; Yang, X.L.; Tang, N.N.; Ma, S.M.; Cheng, Z. Assessment of the genetic diversity of different Job’s tears (Coix lacryma-jobi L.) accessions and the active composition and anticancer effect of its seed oil. PloS one. 2016, 11, e0153269. [Google Scholar] [CrossRef]
- Hou, J.J.; Cao, C.M.; Xu, Y.W.; Yao, S.; Cai, L.Y.; Long, H.L.; Guo, D.A. Exploring lipid markers of the quality of coix seeds with different geographical origins using supercritical fluid chromatography mass spectrometry and chemometrics. Phytomedicine 2018, 45, 1–7. [Google Scholar] [CrossRef]
- Wen, A., Xie, C., Mazhar, M., Wang, C., Zeng, H., Qin, L. and Zhu, Y., 2020. Tetramethyl pyrazine from Adlay (Coix lacryma jobi) biotransformation Bacillus subtilis and its quality characteristics. J Food Sci Technol. 2020, 57, 4092–4102.
- Mendoza, A.J.A.; Sabellano, F.M., Jr. Baco, L.T.; Nabua, W.C.; Pantallano, E.S. Varietal performance of Adlai (Coix lacryma-jobi L.). NMSCST Research Journal 2015, 3, 139–147. [Google Scholar]
- Vujic, D.N.; Acanski, M.M. Performance of GCMS for differentiation of various types of flours by creating dendrogram of Lipid soluble extract. CI&CEQ. 2012, 18, 555–581. [Google Scholar] [CrossRef]
- Liaotrakoon, W.; Liaotrakoon, V.; Wongsaengtham, W.; Rodsiri, S. Influence of dry-and wet-milling processes on physicochemical properties, syneresis, pasting profile and microbial count of job’s tear flour. Int. Food Res. J. 2014, 21, 1745–1749. [Google Scholar]
- Ding, Y.; Zhang, G.; Ni, C.; Yu, G.; Cheng, J.; Zheng, H. Understanding the mechanism of change in morphological structures, visualization features, and physicochemical characteristics of adlay seeds (Coix lacryma-jobi L.): The role of heat soaking. J Cereal Sci. 2020, 91, 102892. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, Y.; Zhu, X. Xu, R.; Shen, H.; Zhang, Q.; Ge, X. The Effect of Different Extraction Methods on Extraction Yield, Physicochemical Properties, and Volatile Compounds from Field Muskmelon Seed Oil. Foods 2022, 11, 721. [Google Scholar] [CrossRef]
- Xu, L.; Chen, L.; Ali, B.; Yang, N.A.; Chen, Y.; Wu, F.; Jin, Z.; Xu, X. Impact of germination on nutritional and physicochemical properties of adlay seed (Coixlachryma-jobi L.). Food chem. 2017, 229, 312–318. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X.; Rong, Y.Z.; Wu, J.H.; Yang, Y.J.; Wang, Z.W. Rapid determination of fat, protein, and amino acid content in coix seed using near-infrared spectroscopy technique. Food Anal Methods. 2015, 8, 334–342. [Google Scholar] [CrossRef]
- He, C.J.; Li, Z..; Liu, H.; Zhang, H.; Wang, L.; Chen, H. Chemical compositions and antioxidant activity of adlay seed (Coixlachryma-jobi L.) oil extracted from four main producing areas in China. J food Sci. 2020, 85, 123–131. [CrossRef]
- Zhao, W.Y.; Gong, Y.; Huang, S.; Yu, H.; Lu, Y. Optimization, and kinetics for the refluxing extractions process of Coix seed oil. Chin J Bioproc Eng E. 2010, 8, 1–5.
- Fatima, A., Ali, S., Ijaz, M., Mahmood, R., Sattar, S., Khan, J., and Ahmad, M. Boron application improves the grain yield and quality of fine grain rice cultivars in Punjab, Pakistan. Pak. J. Agri. Sci. 2018, 55, 761–766.
- Hore, D.K.; Rathi, R.S. Characterization of Job’s tears germplasm in North-East India. Nat. Prod. Radiance. 2008, 6, 50–54. [Google Scholar]
- Coludo, F.E.; Janairo, G.C. Effects of Soil Chemistry on the Physico-Chemical Characteristics of the Grains of Adlay (Coix lacryma-jobi Linn) Grown in Bukidnon, Philippines. Ann. Trop. Res. 2015, 2015 37, 26–43. [Google Scholar] [CrossRef]
- Hu, A.; Zhang, Z.; Zheng, J.; Wang, Y.; Chen, Q.; Liu, R.; Liu, X.; Zhang, S. Optimizations, and comparison of two supercritical extractions of adlay oil. IFST . 2012, 13, 128–133.
- Lawrence, G.D. Dietary fat and health: dietary recommendations in the context of scientific evidence. Adv Nutr. 2013, 4, 294–302. [Google Scholar] [CrossRef]
- Mensink, R.P.; Zock, P.L.; Kester, A.D.; Katan, M.B. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003, 77, 1146–55. [Google Scholar] [CrossRef]
- Ni, C.; Li, B.; Ding, Y.; Wu, Y.; Wang, Q.; Wang, J.; Cheng, J. Anti-cancer properties of coix seed oil against HT-29 colon cells through regulation of the PI3K/AKT signaling pathway. Foods 2021, 10, 2833. [Google Scholar] [CrossRef]
- Peng J, Gao W, Peng C, He C, Zhang Q, Bi W. Characteristics of germplasm resources in Coix from Xishuangbanna. Zhongguo Zhong Yao Za Zhi. 2010, 35, 415–418, In Chinese. [PubMed]
- Yang, Y.; Du, S.Y.; Sun, Y.Q.; Han, T.; Jia, M.; Qin, L.P. Determination of effective contents triolein and coixol in Coix lacryma jobi var. mayuen from different origins. Chin Trad Herb Drugs 2017, 48, 578–558. [Google Scholar]
- Kuhnen, S.; Ogliari, J.B.; Dias, P.F.; Boffo, E.F.; Correia, I.; Ferreira, A.G.; Maraschin, M. ATR-FTIR spectroscopy and chemometric analysis applied to discrimination of landrace maize flours produced in southern Brazilian. Int. J. Food sci. Technol. 2010, 45, 1673–1681. [Google Scholar] [CrossRef]
- Ali, M.; Al-Hattab.T.; Al-Hydary. Extraction of date palm seed oil (Phoenix dactylifera) by soxhlet apparatus. IJAET 2015, 8, 261–271.
- Abdul, R.; Che, M.Y.B. Determination of extra virgin olive oil in quaternary mixture using FTIR spectroscopy and multivariate calibration. Spectroscopy. 2011, 26, 203–211. [Google Scholar]
- Timilsena, Y.P.; Vongsvivut, J.; Adhikari, R.; Adhikari, B. Physicochemical and thermal characteristics of Australian chia seed oil. Food Chem. 2017, 228, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Gurmani, Z.A.; Khan, S.U. Quality characteristics integration and relationship in basmati rice is useful for checking adulteration and admixture. J. Agric. Crop Res. 2019, 7, 148–169. [Google Scholar] [CrossRef]
- Ratnayake, W.M.N.; Hansen, S.L.; Kennedy, M.P. Evaluation of the CP-Sil 88 and SP-2560 GC columns used in the recently approved AOCS Official Method Ce 1h-05: Determination of c/s-, trans-, saturated, monounsaturated, and polyunsaturated fatty acids in vegetable or non-ruminant animal oils and fats. JAOC. 2006, 83, 475–488. [Google Scholar] [CrossRef]
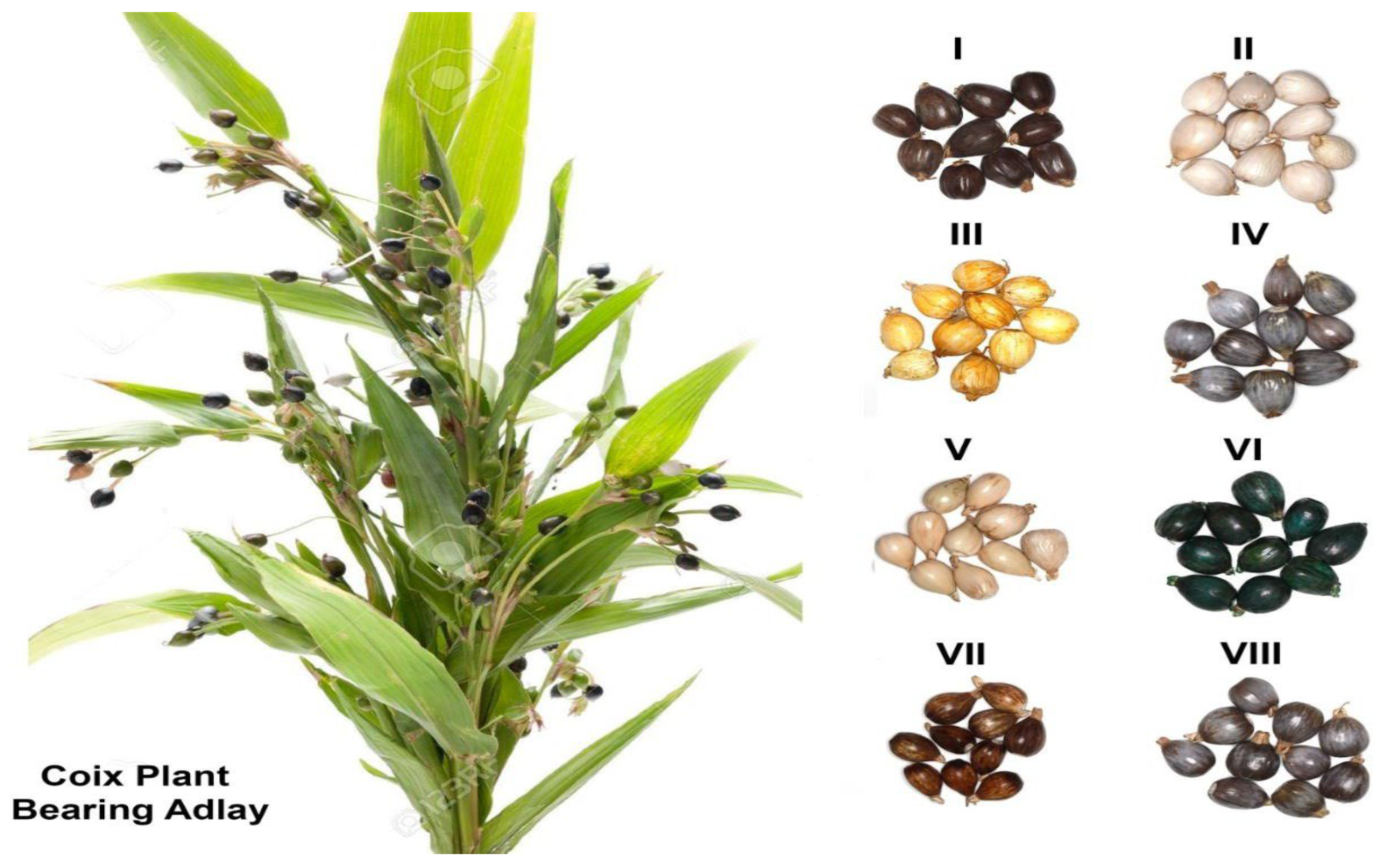
| Parameters Description | Indigenous Adlay Collections (1000 Grains Each) | |||||||
|---|---|---|---|---|---|---|---|---|
| PRP | BLK | BRN | GRN | GRY | YLW | OWT | Av: ±STDev | |
| Shell texture | Stony | Stony | Stony | Hard | Hard | Stony | Hard | NA |
| Appearance* | Shiny pears, | Shiny, rounded | Shiny, irregular | Shiny, irregular | Shiny, oval | Shiny, rounded | Shiny, pears | NA |
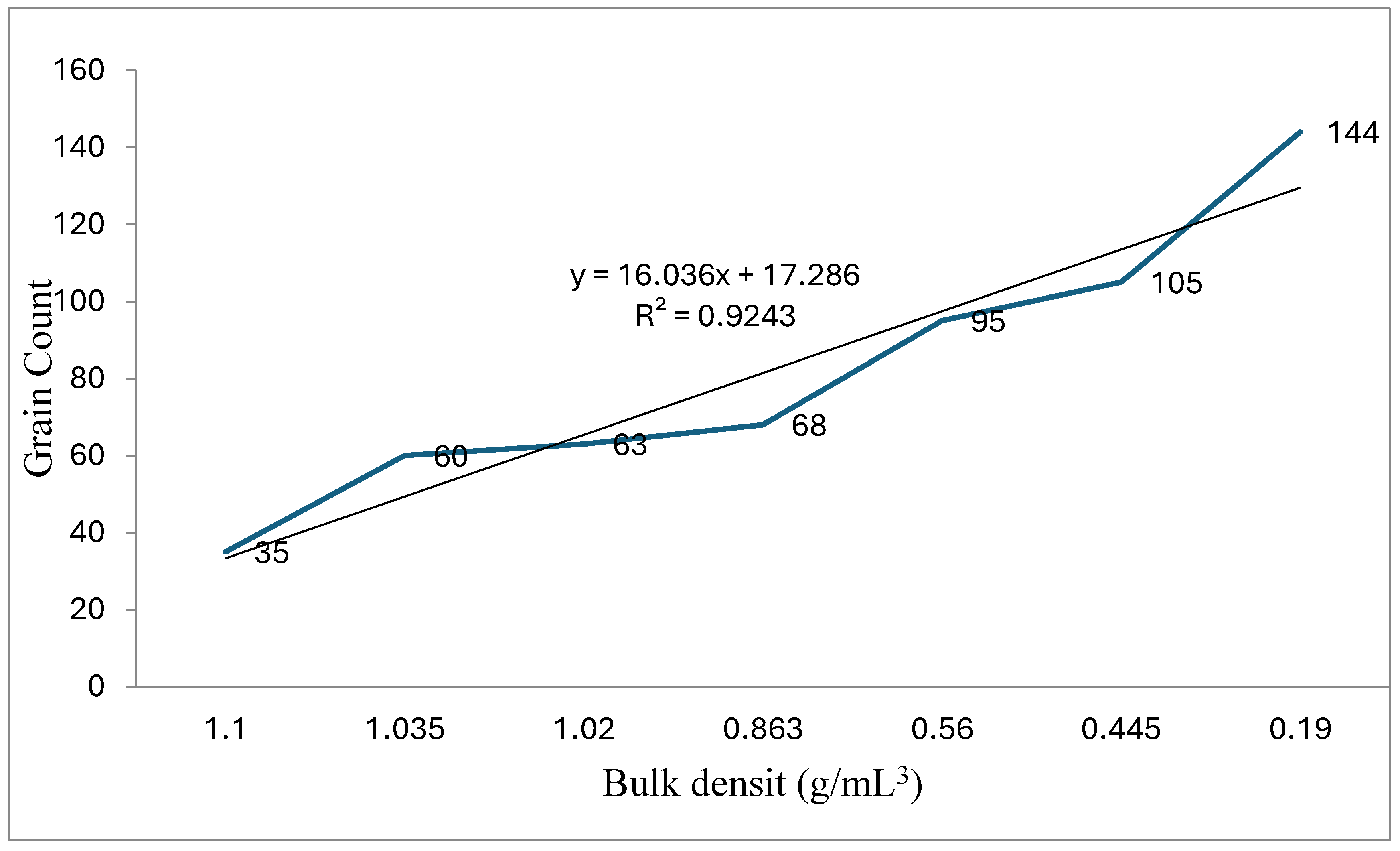
| Grain count | 35a | 63b | 60c | 68d | 105e | 95f | 144g | 82 ± 36.03 |
| Length (cm) | 0.95a | 0.84c | 0.84c | 0.91b | 0.81d | 0.78e | 0.91b | 0.86 ±0.062 |
| Breadth (cm) | 0.7c | 0.63e | 0.65d | 0.8a | 0.75b | 0.65d | 0.75b | 0.71 ±0.064 |
| Shape (lbr) | 1.2b | 1.33a | 1.29a | 1.14c | 1.08c | 1.19b | 1.22b | 1.04 ±0.46 |
| bead dia(mm) | 0.2a | 0.2a | 0.2a | 0.2a | 0.2a | 0.2a | 0.2a | 0.2 |
| bead vol mm3 | 0.295a | 0.262b | 0.264b | 0.286a | 0.255b | 0.245b | 0.287a | 0.27 ±0.02 |
| Weight (g) | 0.286a | 0.159b | 0.17c | 0.15c | 0.1d | 0.11d | 0.07e | 0.15±0.07 |
| BD (g/ml3) | 0.624a | 0.61a | 1.035b | 0.863c | 0.234d | 0.34e | 0.185f | 0.45± 0.19 |
| SD (g/mL3) ϯ | 1.1a | 1.02a | 1.035a | 0.863b | 0.445c | 0.56d | 0.19e | 0.745±0.35 |
| GD (g/ml3) | 0.031a | 0.02b | 0.02b | 0.013c | 0.004d | 0.006d | 0.001d | 0.014 ±0.011 |
| FD (g/mL3) | 0.667b | 0.966a | 0.588b | 0.5c | 0.5c | 0.476c | 0.385d | 0.58 ±0.19 |
| BP (%) | 63.6a | 63.6a | 45.6b | 45.6b | 52.73c | 52.6c | 53.5c | 53.89 ±7.41 |
| Proximate Parameter | Indigenous Adlay Collections | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| PRP | BLK | BRN | GRN | GRY | YLW | OWT | Av: ±STDEV | ||
| moisture (%) | 11.9c | 11.9c | 12.2b | 13.2a | 11.6d | 12.2b | 12b | 12.4 ±0.51 | |
| protein (%) | 15.57b | 15.63b | 15.8a | 12.01e | 15.58b | 14.30c | 13.73d | 14.66 ± 1.4 | |
| oil (%) | NMR | 4.29a | 4.51a | 4.78a | 4.13b | 4.63b | 4.77b | 4.07c | 4.45 ±0.294 |
| Extract | 4.31c | 4.57b | 4.76a | 4.07d | 4.69b | 4.71b | 4.19c | 4.5 ± 0.3 | |
| fiber (%) | 1.79e | 1.98b | 2.37a | 1.94b | 1.97b | 2.21d | 1.88e | 2.02 ±0.2 | |
| ash (%) | 2.6a | 2.0b | 1.88c | 2.63a | 2.0b | 1.95b | 2.63a | 2.24 ±0.36 | |
| P (%) | 0.232c | 0.2d | 0.2d | 0.3a | 0.27b | 0.23c | 0.25c | 0.3 ±0.04 | |
| K (%) | 0.34c | 0.3c | 0.28d | 0.48b | 0.64a | 0.42b | 0.68a | 0.45 ±0.16 | |
| Ca (%) | 1.2c | 0.9d | 1.3c | 2.1a | 1.5b | 2.2a | 0.6e | 1.4 ±0.6 | |
| Na (%) | 0.28a | 0.16b | 0.28a | 0.16b | 0.17b | 0.21c | 0.2c | 0.21 ±0.052 | |
| B (mg/Kg) | 3.0c | 4.0a | 3.9a | 3.41b | 3.0b | 2.0d | 3.25b | 3.2 ±0.7 | |
| Fe (mg/Kg) | 2.9a | 1.011e | 2.3b | 1.67c | 1.045e | 1.44d | 1.0e | 1.6 ± 0.73 | |
| Cu(mg/Kg) | 43.53a | 8.00e | 37.0b | 34.5c | 15.3d | 6.64f | 0.56g | 20.8 ±17.18 | |
| Zn (mg/Kg | 36.623a | 22.157b | 1.27f | 13.24d | 9.2e | 20.37c | 20.37c | 17.61±11.21 | |
| Mn (mg/Kg | 5.8c | 1.56e | 8.3a | 7.06b | 2.04d | 1.78e | BDL | 4.4 ±3.0 | |
| FAs (% av: ±STDEV) | BLK | BRN | GRN | OWT | GRY | YLW | PRP | Av ±SDEV |
|---|---|---|---|---|---|---|---|---|
| Palmitic (C16:0) (26.3 ± 4) | 24.5d | 27.5c | 29.7b | 30.1a | 22e | 20.6f | 29.6b | 26.3 ±1.4 |
| Palmitolic (C16:1) | ---- | --- | --- | ---- | --- | 2.02 | --- | 2.02 ±0.0 |
| Oleic (C18:1n9C (4.2±0.63) | 3.7e | 3.92d | 4.2c | 4.79b | 3.5f | 3.7e | 5.2a | 4.2 ±1.4 |
| Linoleic(C18:2n6C)43.5±1.4 | 52.0a | 43.5c | 37.6d | 34.7e | 51b | 52.5a | 33.0f | 43.5 ±1.4 |
| Linolenic(C18:3n9C) | 1.09c | 0.99c | 1.04c | 1.01c | 4.1b | 5.8a | ----- | 2.4 ±1.4 |
| Arachidic (C20:0) | --- | 2.35c | --- | --- | 2.8b | 1.31d | 4.81a | 2.82 ±1.5 |
| eicosenoic (C20:1) (3.13 ±4) | 3.8b | ---- | 1.96d | 2.62c | ---- | 2.1d | 11.4a | 4.4 ±1.4 |
| Behenic (C22:0) | 3.1d | 4.65b | 3.71c | 4.99a | ---- | 2.6e | 5.3a | 4.06 ±1.1 |
| Lignoceric(C24:0) | 4.7f | 7.52d | 9.89c | 10.9a | 3.5g | 7.2e | 10.62b | 7.8 ±2.9 |
| Solvent Extract Oil (%) | 2.51b | 2.91a | 1.75e | 1.91d | 2.6a | 2.68a | 2.2c | 2.4 ±0.43 |
| ΣSFAs | 32.3 | 39.67 | 43.3 | 45.99 | 38.3 | 31.71 | 5.40 | 33.8 ±13.58 |
| ΣUSFAs | 60.59 | 48.41 | 44.8 | 43.12 | 58.6 | 66.12 | 49.6 | 53.04 ±8.7 |
| ΣMUFA | 6.5 | 3.92 | 6.16 | 7.41 | 3.5 | 7.82 | 16.6 | 7.42 ±4.4 |
| ΣPUFA | 6.29 | 44.04 | 38.64 | 35.71 | 55.1 | 58.3 | 33.0 | 38.73 ± 7.2 |
| n-3UFAs | 1.09 | 0.99 | 1.04 | 1.01 | 4.1 | 5.8 | ---- | 2.38 ±2.1 |
| Ratio of ΣUSFAs/ ΣSFAs | 1.88 | 1.2 | 1.1 | 0.93 | 1.53 | 2.08 | 0.98 | 1.4± 0.45 |
| Peak (s) at Animated cm-1 | Origin Relevant to Peak (s) | Functional Group (s) Reached Out | Indigenous Adlay Grains Flours | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PRP | YLW | OWT | BLK | GRY | GRN | BRN | |||
| Frequencies 2348/49 to 3350/ 3635 Description Originating Peaks | |||||||||
| 3350/3653 | bold/alone/broader or semi rounded peak sym. stretch - H bonded OH | Aliphatic or / Aryl Alcohols/ carboxylic/ carbohydrate known window | Frequency (3350-3653), appearance (broader/ wider) shape (semi global/ rounded) bold and alone found uniformly and equally in all collection evident H-bonded OH group symmetric stretch pertinent to -COOH; and or carbohydrates; and or R-OH/Ar-OH | ||||||
| ~ 3000 | -CH3; >CH2 symmetric stretching | Well known window (defined Basic skeleton) | 2965; 2994 |
same separated
band type |
at 2963 | same bands | |||
| 2863/65 | aldehydic H symmetric stretch shoulder type |
typical defined window
HC=O near R- stretch |
shoulder type | ||||||
| 2348/49 | O=C=O stretch | CO2 indication as background contamination | CO2 presence without any detectable difference in samples indicates background atmospheric CO2 interception, that may be avoided by T at <2%. It is a well-defined window | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





