1. Introduction
The proteolytic complex extracted from pineapples (
Ananas comosus), called “bromelain”, is well known to possess anti-inflammatory, antioedema and analgesic properties, which indicates that that it may be prescribed for several conditions characterized by the presence of acute inflammation, with or without oedema [
1]. The proteases that constitute bromelain are cysteine endopeptidases, which catalyze the hydrolysis of the peptide bonds of non-terminal amino acids [
2]. Although this enzymatic complex’s mechanisms of action are not fully understood, several
in-vitro and
in-vivo studies have underlined three targets of action: first, fibrinolytic activity, which proceeds via the activation of factor XI and the modulation of the kallikrein-kinin pathway; second, the regulation of the arachidonic cascade and the production of inflammatory cytokines; and, third, the limitation of neutrophil migration to inflammation sites [
3]. These actions permit bromelain to be potentially effective in several conditions, as has been highlighted in several randomized clinical trials (RCTs), which have demonstrated the anti-inflammatory, analgesic and antioedema activities of bromelain in rheumatoid arthritis, osteoarthritis, perioperative sport injuries, cardiovascular diseases, chronic rhinosinusitis and skin wounds and burns [
4]. In this regard, bromelain may also contribute to reducing the inflammation and oedema caused by oral surgery. A recent meta-analysis of six RCTs has demonstrated that bromelain alleviates postoperative pain 7 days after mandibular third molar surgery (p=0.002) and decreases facial swelling in the early and late postoperative stages (p=0.02 and p=0.0004, respectively) [
5]. Similar results have been obtained in previous meta-analyses performed by Mendes
et al. [
6], de Almeida
et al. [
7], and de Souza
et al. [
8], the last of which also showed improvements in social isolation and sleep quality.
Ibuprofen is the most frequently prescribed analgesic/anti-inflammatory drug in dental surgery, followed by naproxen and acetaminophen [
9]. However, although NSAIDs spontaneously resolve inflammation, generally within a week, this conventional therapy is not free from side effects [
10]. In fact, a percentage of patients have reported excessive NSAIDs dosing, and, whereas doses of ibuprofen under 1200 mg/day only minimally increase the risk of gastrointestinal bleeding, the prescription dose dramatically increases the risk of bleeding (relative risk of 4 vs no medication) [
11]. The risk is higher with prolonged use, but one study has reported that patients starting naproxen are at higher risk than those starting ibuprofen and that the difference is detectable within 14 days [
12]. This suggests that even a few days of use results in increased potential for injury. Some studies have estimated that up to 15,000 people die annually from complications related to NSAIDs treatment in the United States [
13], and their overuse is a potential major health issue.
In recent years, clinical studies have shown that oral supplementation with nutraceuticals may help to reduce inflammation, pain and/or oedema in subjects with chronic inflammatory diseases, reducing the need for NSAIDs [
3]. Brome-Inf® is one of these nutraceutical substances, and is a freeze-dried extract of pineapple, highly concentrated in bioactive peptides and bromelain, marketable as a food supplement or functional food. Although there are several clinical studies on the use of bromelain for the control of the pain and inflammation associated with impacted third molar surgery that have demonstrated reductions in the doses and number of administrations of conventional anti-inflammatory/analgesic drugs [
7], to the best of our knowledge, this is the first study that compares the activity of the pineapple phytocomplex (titrated in bromelain 8%) with purified bromelain. Moreover, bromelain in the form of freeze-dried pineapple is also a functional food with good palatability.
The aim of this study is to investigate the potential role of nutraceutical supplementation in people subjected to mandibular third molar surgery in order to reduce the need for NSAIDs and improve quality of life. The second goal is to evaluate differences in efficacy in the freeze-dried pineapple extract and single component bromelain.
2. Materials and Methods
2.1. Study design and participants
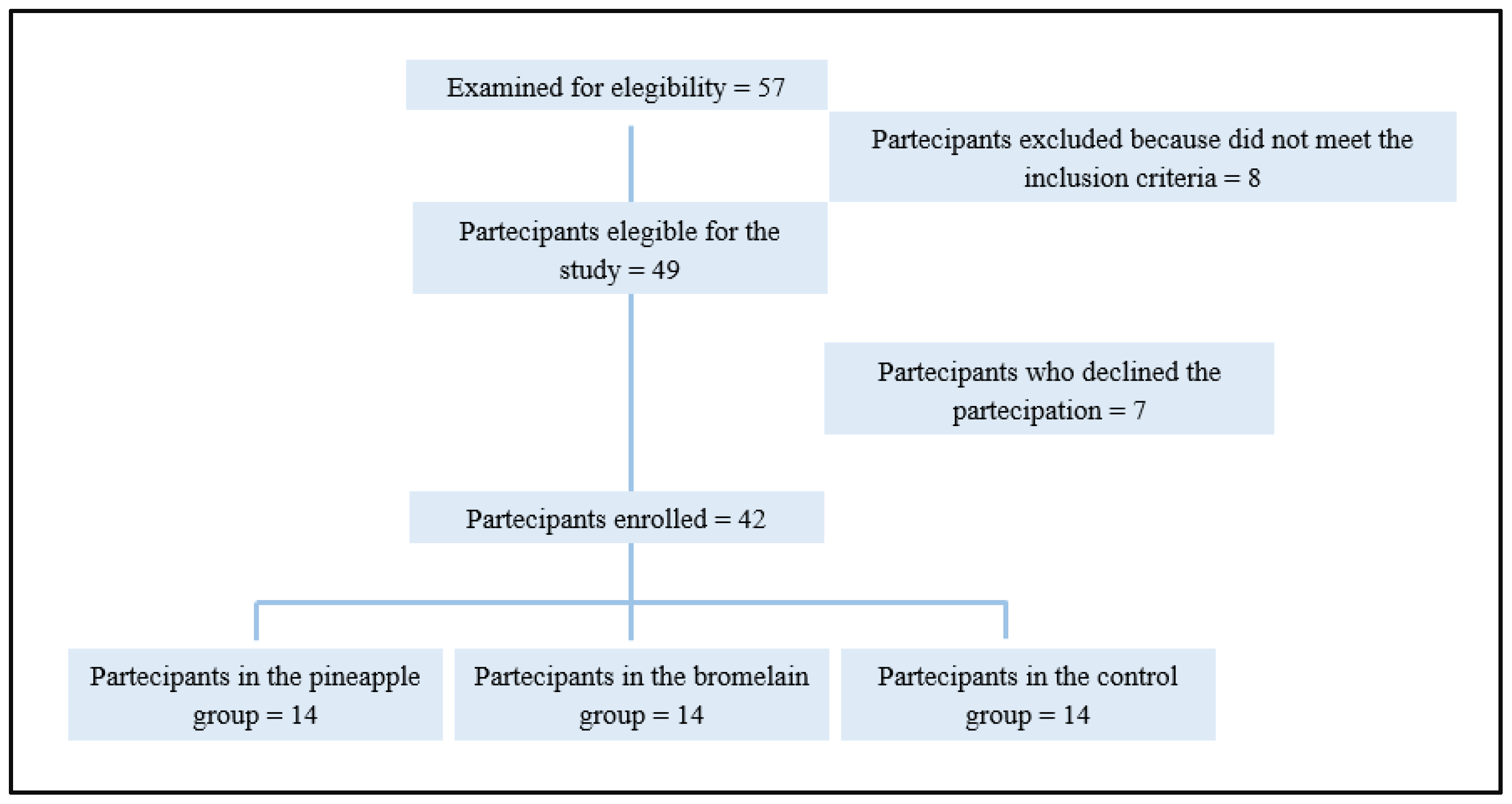
This was a pilot, three-arm, double-blind, randomized study. It involved patients enrolled for third molar surgery who were randomized 1:1:1 (
Figure 1) to receive pineapple extract, purified bromelain or placebo for 7 days after surgery. The study population included 42 healthy individuals belonging to the “Studio Dentistico Pisano Procchio” of Alessandria, who required third molar surgery under local anesthesia. The inclusion criteria were the following; participants aged between 18-35 years that were in good health, who had a partial bony impacted mandibular third molar, were free from pericoronitis and infection at surgery, who had received no medication in the previous 2 weeks, and had no history of allergy to the drugs used in the trial. The exclusion criteria were; the presence of comorbidities or any medical or surgical condition that makes the patient's adherence to the study protocol complex or inconstant, the co-assumption of other supplements, allergies or intolerances to the active ingredient or excipients. Patients were excluded from the study if they had missing data or recall visits, or if they had reported the use of non-trial drugs during the observation period.
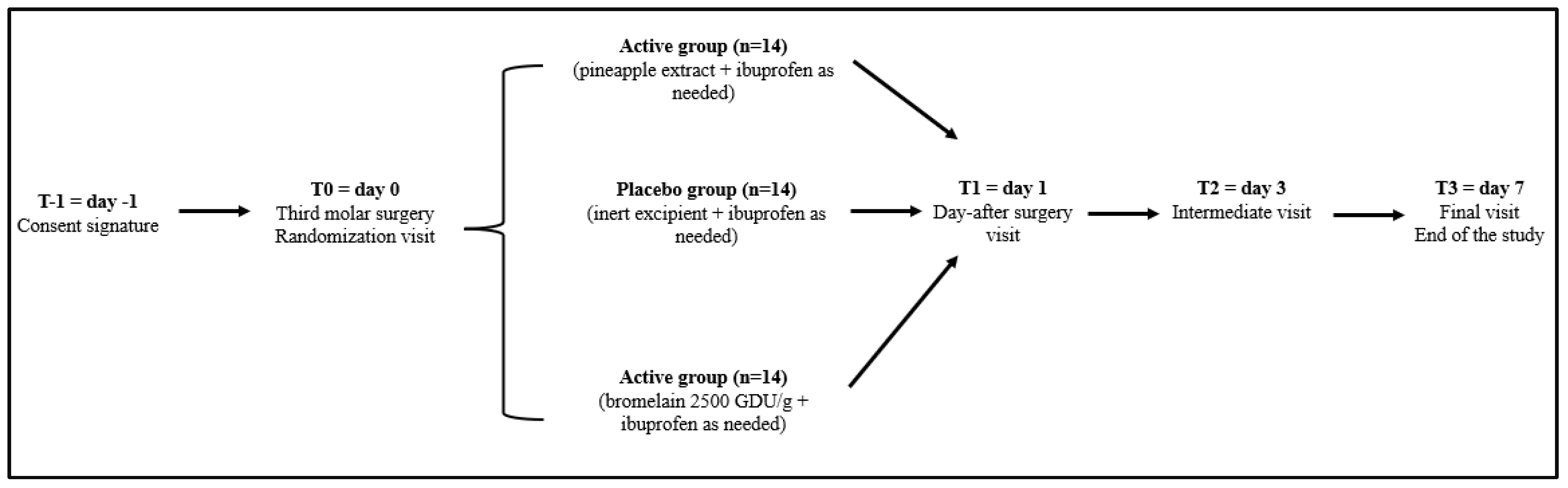
Informed consent was obtained (T=-1) the day before surgery, and participants were then randomized to receive the pineapple extract, purified bromelain or placebo for 7 days. On T=0 (day of surgery), T=1 (day 1), T=2 (day 3), and T=3 (day 7), patients were evaluated for clinical status, in addition to an evaluation of compliance, and the tolerability of the products. The study timeline is described in details in
Figure 2.
2.2. Treatment
After the signature of the consent form (T-1), at the time of randomization (T0), every patient was given either Brome-Inf® (freeze-dried pineapple powder containing 200 mg of bromelain every 2.5 g of powder, with a spoon), bromelain (200 mg of bromelain 2,500 GDU/g every 2.5 g of powder, with a spoon), or placebo (similar in taste and shape) to be taken orally; 2.5 g every 6 hours starting from the morning of surgery and for 3 days after (T2), and 2.5 g every 12 hours for the following 4 days (T3). Subjects were instructed to take ibuprofen 600 mg as needed if pain became significant (for a maximum of t.i.d.). Moreover, postoperatively, all patients in the study received amoxicillin + clavulanic acid (1 g t.i.d.) for 5 days after surgery.
For the entire duration of the study, patients were instructed to take the assigned treatment at approximately the same time each day, preferably on an empty stomach. Patients were examined 1, 3, and 7 days after surgery. Pain, swelling and trismus were measured at each follow-up visit. The patients received the QoL questionnaire to complete on day 4 after surgery and returned at suture removal by day 7. The total number of rescue analgesic tablets taken during this period was also recorded.
The study products were manufactured and packaged by Studio 3 Farma srl (Torre di Mosto, Italy), in accordance with Quality Management System ISO 9001:2015.
Randomization was performed centrally using computer-generated codes. Participants and investigators were blinded to group assignments. The alphanumeric codes (X, Y, Z) for randomization were kept closed inside an envelope that was kept in a locked drawer in the main investigator's desk. It was opened at the end of the study by the principal investigator.
2.3. Product preparation
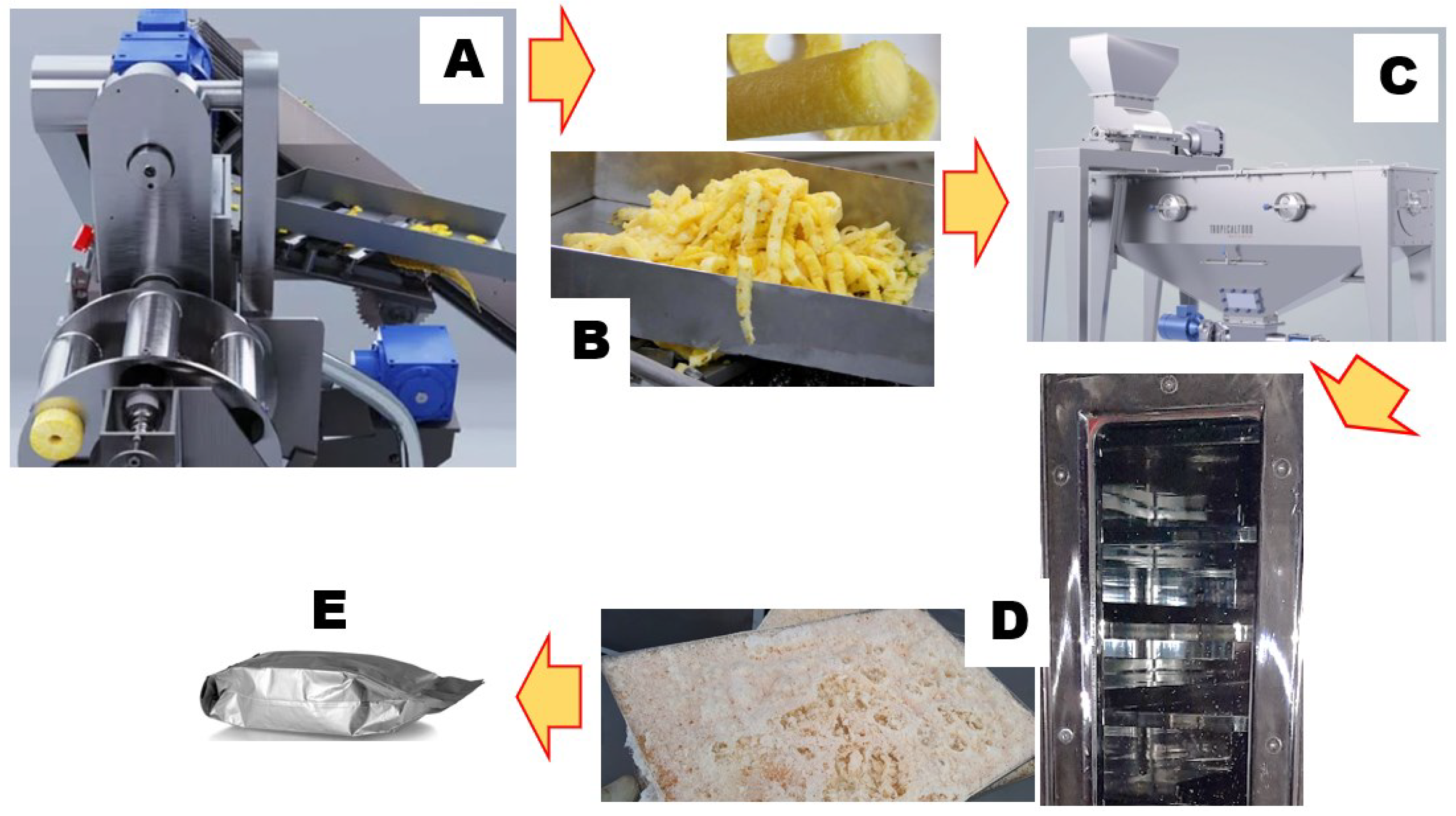
In this work, we scaled up a previously reported lab-scale process [
14]. Thoroughly washed, size-selected pineapples (
Ananas comosus L.) at a uniform ripening stage were processed into fruit slices (rings) using the GINACA – TFGK-5, a processing machine obtained from Tropical Food Machinery srl (Busseto, Italy). This automatic cylinder-forming machine produces cored cylinders from calibrated pineapple fruit. It has an automated and continuous system for loading, transporting and centering the fruit, as well as a processing group that facilitates peeling, cutting and coring. The surplus pineapple core and external pulp were reclaimed for juice production, while the remaining peels were expelled. Subsequently, the cored cylinders and pulp were promptly chilled to 4°C, mechanically blended using a pilot-scale blender (Waring Commercial, Stamford, CT, USA), and subjected to centrifugation at 5,000 g and 4°C (Beckman Instruments, Palo Alto, CA, USA), effectively separating insoluble particles from the juice. Dry matter analysis was conducted on 50 g of juice, dried to a constant weight at 105 °C overnight in a laboratory dry oven, in adherence to the established standards outlined in the AOAC method 922.10. The resulting residue was quantified and reported as a percentage of the initial material. The outcomes are expressed as grams of solid matter per 100 g of fresh pineapple. Subsequently, the juice underwent rapid cooling in liquid nitrogen and was subjected to freeze-drying using a Criofarma C560-12 unit followed by fast packaging under vacuum.
The scheme depicted in
Figure 3 summarizes the Brome-Inf® preparation steps, from byproduct to final product: toward a circular economy strategy.
2.4. Efficacy assessment
The primary endpoint of the study was to compare the need for ibuprofen intake in the three groups. The primary outcome variables were pain, swelling, trismus and QoL scores recorded after surgery. Post-operative pain was evaluated using a VAS, 10 cm in length, ranging from 0 for ‘‘no pain’’ to 10 for ‘‘the worse possible pain’’. Facial swelling in the operation side was evaluated using two facial measurements; tragus-pogonion and gonion-lateral canthus. The preoperative sum of the two values (in millimeters) was taken as the baseline for that side. Trismus was measured as the difference in the interincisal distance at maximal mouth opening before and after surgery.
The effect on QoL was measured using a questionnaire that has been fully described and validated in a previously published report [
15]. The questionnaire includes a number of items addressing social isolation, working isolation, eating ability and variation in diet, speaking ability, sleep impairment and physical appearance. Recovery for each QoL item was defined as a number on a 4-point scale. The scale included the following responses: not at all (coded 0), little (coded 1), quite a lot (coded 2), and very much (coded 3). The total score range was 0-42, with higher scores indicating poorer QoL. The other outcome variables were demographic, including age, gender and body mass index (BMI). The intraoperative variables included the duration of surgery (in minutes from the incision to the last suture). The postoperative variables included the number of rescue analgesic tablets taken by the patients up to day 7.
2.5. Assessment of safety and tolerability
Safety and tolerability were evaluated using continuous monitoring over the study to detect any adverse events and evaluate the clinical safety of treatment. Treatment compliance and the occurrence of adverse effects were monitored using a diary sheet organized on tables with the possibility to indicate the assumption nutraceutical or placebo treatment, eventual ibuprofen intake and number of administrations, and side effects.
2.6. Statistical analysis
Personal data and physiological/pathological anamnesis were only collected at the enrolment visit (T-1), and treatment compliance only in T3.
The sample size was chosen to achieve a power of 80%, with a level of significance equal to 0.05 for a specified difference in pain at a mean of 1 cm recorded on the VAS. A desired sample size of 14 patients per group was found necessary to fit a statistical model for analyzing the differences among the study groups.
Data were incrementally entered, over the study period, into an electronic sheet (Excel, Microsoft, Windows 2003, Redmond, WA), double checked for errors and then processed using the GraphPad Prism 8.0.2 software version for Windows. A descriptive analysis of each of the variables was made. The demographic and clinical characteristics of the patients were analyzed using One way analysis of variance (ANOVA) followed by a Bonferroni’s post-test. A significance level <0.05 was considered statistically significant for all tests conducted.
3. Results
Forty-nine people that needed to undergo the extraction of a single mandibular third molar under local anesthesia and had fulfilled all the inclusion criteria were enrolled. However, seven patients were later excluded because they did not attend follow-up visits or had used non-study drugs. Thus, 42 patients, who had attended the follow-up visits and completed the questionnaire were included in the final analysis. The mean patient age (19 men and 23 women) was 22.8 (range 19 to 27). No statistically significant differences in the demographical characteristics of the subjects, or in parameters related to the surgical procedure, were found in the study groups (
Table 1), except for the BMI of patients assigned to bromelain group which was found to be significantly lower than the BMI of the placebo group (p=0.0002).
Regarding perceived pain, a significant reduction in pain (VAS-10) was observed in the Brome-inf® and bromelain group compared to the placebo group (p<0.0001 for both, at all intervals) (
Table 2). In addition, patients in the Brome-inf® and bromelain groups reported approximately half the average intake of ibuprofen compared to the placebo group (p<0.0001 for both active groups).
The highest swelling measurements were reported 1 day after surgery in all study groups (
Table 2). The difference in the magnitude of swelling between the bromelain group and the placebo group was significant (p=0.037) at day 1. A comparison of the groups also revealed a significant reduction in swelling at days 3 and 7 in both the bromelain and Brome -Inf® groups (p<0.0001), compared with the placebo group.
The mean baseline measurements of interincisal distance were 46, 44 and 45 mm in the placebo, bromelain and Brome-Inf® groups, respectively. In all groups, trismus was at its maximum 1 day after surgery and had subsided at the subsequent follow-up intervals. However, a comparison of the groups failed to reveal significant differences (
Table 2).
Regarding the QoL measurements, both active groups demonstrated a significant reduction of scores in all areas (social, work, eating, sleep, speech and appearance) compared with the placebo group (
Table 3). A significant improvement was also seen in the total QoL score for both bromelain and Brome-Inf® groups compared to the placebo group (Placebo vs Bromelain P=0.0001; Placebo vs Brome-Inf® P=0.0002).
No side effects were reported during treatment. Moreover, both Brome-Inf® and bromelain supplementation showed good palatability and excellent compliance (100%). No cases of alveolar osteitis or wound infection were reported during the study period.
4. Discussion
In the last few decades, a new paradigm in healthcare that focuses on diet and nutrition has emerged. A more health-conscious consumer pool with increased expendable income in the Western world has shifted consumer trends towards the purchase of dietary supplements, functional foods and nutraceuticals with the goal of maintaining optimal health and preventing chronic pathologies that affect quality of life and reduce lifespan [
16]. Epidemiological studies suggest that there exists an association between the assumption of nutraceuticals and the prevention of several diseases [
17].
The nutraceutical market is currently a multi-billion-euro industry and has received an astonishing worldwide response. It was valued at approximately
$383 billion in 2016 and was expected to reach approximately
$561 billion by 2022 prior to the coronavirus 2019 (COVID-19) pandemic [
18]. In addition, the value of the nutraceuticals industry is already more than 25% of the value of the pharmaceutical industry [
18].
One of the most interesting nutraceuticals, bromelain is widely used for the prevention or cogestion of numerous diseases that are characterized by the presence of inflammation, oedema and algesia. Although several clinical trials have demonstrated the efficacy of bromelain supplementation in the reduction of pain [
19], inflammation [
20] and oedematous components [
21], its commercial cost is high while the isolation and purification of bromelain from pineapple (fruit, stem, core and leaves) is still a challenge, making up 70–90% of the total production cost of the final extract [
22]. In addition, despite new feasible methods of protein purification (e.g., membrane filtration, reverse micellar systems, aqueous two-phase extraction and chromatographic techniques) and new biotechnological processes developed to mitigate production costs, several limitations still create problems for the efficiency of product recovery from crude-plant extracts and the effectiveness of the obtained extract. The enzyme complex tends to be irreversibly inactivated at high temperatures (such as during the pasteurization process), whereas the progressive concentration of bromelain in crude pineapple juice during the purification process can induce spontaneous enzymatic deactivation [
3]. In this context, the use of a freeze-dried pineapple juice extract obtained from by-products (core and peel of
Ananas comosus) that, thus, respects the concepts of “zero waste approach” and the “circular economy”, has been shown to preserve a good quantity of total bromelain (up to 8% of dry weight) in its active form.
This study investigates the effect of lyophilized pineapple extract (titrated and standardized in bromelain) and purified bromelain on postoperative sequelae and QoL measures after the surgical removal of the impacted lower third molars. Our study, based on work by Majid
et al. [
23], demonstrates that the oral intake of bromelain in multiple daily-doses, starting on the day of surgery and continuing for 7 days, resulted in a significant effect on the clinical and QoL status of these patients. In particular, the regular assumption of bromelain, both as a functional food and in its purified form (200 mg every 6 hours starting on the morning of surgery and continuing for 3 days after, and 200 mg every 12 hours for the following 4 days), has been observed to significantly reduce ibuprofen intake compared with placebo group, acting as a painkiller and inflammation treatment. In this regard, previous studies have demonstrated that the effects of bromelain are comparable to those of pre-emptive diclofenac sodium or ibuprofen in the third molar surgery setting [
6,
7,
8]. Moreover, both groups (pineapple and bromelain) were observed to have a positive effect on QoL measurements after third molar removal, with this likely being due to their anti-oedema, anti-inflammatory and analgesic effects. Moreover, they display excellent safety profiles (no adverse reaction reported) and good palatability. In this context, the pineapple extract and bromelain study groups showed a marked antiphlogistic effect, which was higher than that of the placebo group (characterized by the statistically higher consumption of ibuprofen). Ibuprofen was chosen as a reference drug in the present study to represent the NSAIDs family, and, as expected, it showed a significant analgesic and antiphlogistic effect during the early postoperative period in the placebo group.
Bromelain has shown therapeutic benefits in doses as small as 160 mg/day. However, it is thought, for most conditions, that the best results occur at doses of 750-1,000 mg/day in four divided doses [
3], which was the regimen used in the present study. Although bromelain’s mechanisms of action have not yet been fully established, it appears to act by removing cell-surface molecules such as CD128a/CXCR1, CD128b/CXCR2, CD16, CD14, CD44 and CD21, which are important for leukocyte trafficking, cellular adhesion, the induction of pro-inflammatory mediators and immunomodulatory effects on T cells. Bromelain also modulates proinflammatory prostaglandins through the inhibition of thromboxane A2 and prostaglandin E. It also modulates P-selectin-mediated neutrophil recruitment [
24] and regulates the plasma fibrinogen levels and blood levels of bradykinin [
25]. These mechanisms make it potentially effective against several conditions associated with inflammation, justifying its use as a potential alternative to NSAIDs.
Several risk factors for oedema, pain and trismus after third molar surgery have been reported by different investigators and have included age, gender, operative time and surgical experience [
26]. The bias of such factors or their dominance in one group or another, which would affect the reading of our results, was minimized by the randomization of the treatment allocation and the strict inclusion criteria. In addition, the surgical phase was performed by the same surgeon in all cases to avoid possible operator variability. Double-blinding also enabled us to overcome any possible personal bias from the patients and the surgeon [
27].
This study also demonstrates that the supplements provide a significant improvement in QoL, highlighting that pineapple extract may be an adjuvant to help improve QoL in individuals subjected to third molar surgery. Moreover, to the best of our knowledge, this is the first study to investigate the effect of the entire phytocomplex of lyophilized pineapple by-product on the QoL status of patients after oral surgery and compare the effects with bromelain as a single component. This may be particularly important especially in the context of circular economy; starting from the waste products from the pineapple food chain, it was possible to obtain a particularly effective titrated and standardized extract, adopting the so-called “zero waste approach”. In this regard, one of the most relevant aspects of the study concerns the overlap in the results obtained from the pineapple extract and the single component bromelain. Although the dosages of bromelain were comparable in the two active groups, the purified bromelain exhibited superior enzymatic activity (2,500 GDU/g vs 400 GDU/g of pineapple extract). Consequently, it is important to consider whether the evaluation of the enzymatic activity through the measurement of GDU is a predictive method for
in-vivo effects, and, above all, whether the impact of the entire phytocomplex is to be preferred over the single protease mixture. In fact, several studies have shown that the proteolytic activity of bromelain is only partly connected to its pharmacological effects, suggesting that evaluating the whole phytocomplex, including non-protein factors, is of great importance [
28]. These aspects require extensive future research.
Our study was limited by the small number of participants involved. However, it highlights the need for further large-scale RCTs to examine the analgesic and anti-oedema efficacy of pineapple extract and purified bromelain. Larger and more extensive studies are also still needed to verify the scalability in the production of pineapple extracts from food industry by-products, to analyze the final cost of the raw material on industrial production and conduct a detailed analysis into the cost/benefit ratio of this nutraceutical, to evaluate, in in-vitro studies, the active ingredients present in the freeze-dried pineapple phytocomplex that may have an additive effect to bromelain, to study the pharmacokinetics of bromelain, which are almost completely unknown, and to evaluate the long-term efficacy and safety profile of bromelain (even at high doses), before considering the prescription of this nutraceutical in clinical practice.