Submitted:
12 February 2024
Posted:
14 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
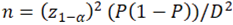 , n = individual sample size, z_(1-α) = 1,96 (when α = 0,05), P – assumed PCOM prevalence for unselected population according to previously published data, D – absolute error. If we take it as prevalence 33% [8,9] (or 0,33) and absolute error as 5%, then the minimum sample size:
, n = individual sample size, z_(1-α) = 1,96 (when α = 0,05), P – assumed PCOM prevalence for unselected population according to previously published data, D – absolute error. If we take it as prevalence 33% [8,9] (or 0,33) and absolute error as 5%, then the minimum sample size: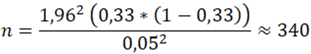
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J.; Network, I.P. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Clin Endocrinol (Oxf) 2018, 89, 251–268. [Google Scholar] [CrossRef]
- Teede, H.J.; Tay, C.T.; Laven, J.; Dokras, A.; Moran, L.J.; Piltonen, T.T.; Costello, M.F.; Boivin, J.; M Redman, L.; A Boyle, J.; et al. Recommendations from the 2023 International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. Fertil Steril 2023, 120, 767–793. [Google Scholar] [CrossRef]
- Huang, Z.; Yong, E.L. Ethnic differences: Is there an Asian phenotype for polycystic ovarian syndrome? Best Pract Res Clin Obstet Gynaecol 2016, 37, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qiao, J. Ethnic differences in the phenotypic expression of polycystic ovary syndrome. Steroids 2013, 78, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Köşüş, N.; Köşüş, A.; Turhan, N.; Kamalak, Z. Do threshold values of ovarian volume and follicle number for diagnosing polycystic ovarian syndrome in Turkish women differ from western countries? Eur J Obstet Gynecol Reprod Biol 2011, 154, 177–181. [Google Scholar] [CrossRef]
- Han, Y.S.; Lee, A.R.; Song, H.K.; Choi, J.I.; Kim, J.H.; Kim, M.R.; Kim, M.J. Ovarian Volume in Korean Women with Polycystic Ovary Syndrome and Its Related Factors. J Menopausal Med 2017, 23, 25–31. [Google Scholar] [CrossRef]
- Lazareva, L.; Suturina, L. International Journal of Biomedicine 2022;12(1):100-103. In: 2022 SLIJoB, 10.21103/Article12(1)_RA6 -D, editors. [CrossRef]
- Azziz, R.; Carmina, E.; Dewailly, D.; Diamanti-Kandarakis, E.; Escobar-Morreale, H.F.; Futterweit, W.; Janssen, O.E.; Legro, R.S.; Norman, R.J.; Taylor, A.E.; et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril 2009, 91, 456–488. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.; Doherty, D.A. The potential implications of a PCOS diagnosis on a woman's long-term health using data linkage. J Clin Endocrinol Metab 2015, 100, 911–919. [Google Scholar] [CrossRef]
- Hsu, M.I. Changes in the PCOS phenotype with age. Steroids 2013, 78, 761–766. [Google Scholar] [CrossRef]
- Belenkaia, L.V.; Lazareva, L.M.; Walker, W.; Lizneva, D.V.; Suturina, L.V. Criteria, phenotypes and prevalence of polycystic ovary syndrome. Minerva Ginecol 2019, 71, 211–223. [Google Scholar] [CrossRef]
- Rao, P.; Bhide, P. Controversies in the diagnosis of polycystic ovary syndrome. Ther Adv Reprod Health 2020, 14, 2633494120913032. [Google Scholar] [CrossRef]
- Dewailly, D.; Lujan, M.E.; Carmina, E.; Cedars, M.I.; Laven, J.; Norman, R.J.; Escobar-Morreale, H.F. Definition and significance of polycystic ovarian morphology: a task force report from the Androgen Excess and Polycystic Ovary Syndrome Society. Hum Reprod Update 2014, 20, 334–352. [Google Scholar] [CrossRef]
- Suturina, L.; Lizneva, D.; Danusevich, I.; Lazareva, L.; Belenkaya, L.; Nadeliaeva, I.a.; Kovalenko, I.; Bazarova, T.; Khomyakova, A.; Natyaganova, L.; et al. The design, methodology, and recruitment rate for the Eastern Siberia PCOS epidemiology&phenotype (ES-PEP) Study. In Proceedings of the 41st Annual Meeting of the Androgen Excess & PCOS Society, Lorne, Victoria, Australia, 10–12 November 2016; p. 76. [Google Scholar]
- Suturina, L.; Lizneva, D.; Atalyan, A.; Lazareva, L.; Belskikh, A.; Bairova, T.; Sholokhov, L.; Rashidova, M.; Danusevich, I.; Nadeliaeva, I.; et al. Establishing Normative Values to Determine the Prevalence of Biochemical Hyperandrogenism in Premenopausal Women of Different Ethnicities from Eastern Siberia. Diagnostics (Basel) 2022, 13. [Google Scholar] [CrossRef]
- Yildiz, B.O.; Bolour, S.; Woods, K.; Moore, A.; Azziz, R. Visually scoring hirsutism. Hum Reprod Update 2010, 16, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Balen, A.H.; Laven, J.S.; Tan, S.L.; Dewailly, D. Ultrasound assessment of the polycystic ovary: international consensus definitions. Hum Reprod Update 2003, 9, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Atalyan, A.V. Atalyan A.V., Kolesnikova L.I., Kolesnikov S. I., Grjibovski A. M., Suturina L. V. Research Electronic Data Capture (REDCap) for Building and Managing Databases for Population-based Biomedical Studies. Ekologiya cheloveka [Human Ecology]. 2019, 2, 52–59. [CrossRef]
- Ahmad, A.K.; Quinn, M.; Kao, C.N.; Greenwood, E.; Cedars, M.I.; Huddleston, H.G. Improved diagnostic performance for the diagnosis of polycystic ovary syndrome using age-stratified criteria. Fertil Steril 2019, 111, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Carmina, E.; Campagna, A.M.; Fruzzetti, F.; Lobo, R.A. AMH measurement versus ovarian ultrasound in the diagnosis of polycystic ovary syndrome in different phenotypes. Endocr Pract 2016, 22, 287–293. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Chen, X.; Zhang, Q.; Wang, W.; Li, Y.; Yang, D. Ovarian volume and follicle number in the diagnosis of polycystic ovary syndrome in Chinese women. Ultrasound Obstet Gynecol 2008, 32, 700–703. [Google Scholar] [CrossRef]
- Dewailly, D.; Alebić, M.; Duhamel, A.; Stojanović, N. Using cluster analysis to identify a homogeneous subpopulation of women with polycystic ovarian morphology in a population of non-hyperandrogenic women with regular menstrual cycles. Hum Reprod 2014, 29, 2536–2543. [Google Scholar] [CrossRef]
- Fulghesu, A.M.; Ciampelli, M.; Belosi, C.; Apa, R.; Pavone, V.; Lanzone, A. A new ultrasound criterion for the diagnosis of polycystic ovary syndrome: the ovarian stroma/total area ratio. Fertil Steril 2001, 76, 326–331. [Google Scholar] [CrossRef]
- Jonard, S.; Robert, Y.; Dewailly, D. Revisiting the ovarian volume as a diagnostic criterion for polycystic ovaries. Hum Reprod 2005, 20, 2893–2898. [Google Scholar] [CrossRef]
- Sujata, K.; Swoyam, S. 2D and 3D Trans-vaginal Sonography to Determine Cut-offs for Ovarian Volume and Follicle Number per Ovary for Diagnosis of Polycystic Ovary Syndrome in Indian Women. J Reprod Infertil 2018, 19, 146–151. [Google Scholar]
- Kim, H.J.; Adams, J.M.; Gudmundsson, J.A.; Arason, G.; Pau, C.T.; Welt, C.K. Polycystic ovary morphology: age-based ultrasound criteria. Fertil Steril 2017, 108, 548–553. [Google Scholar] [CrossRef]
- Le, N.S.V.; Le, M.T.; Nguyen, N.D.; Tran, N.Q.T.; Nguyen, Q.H.V.; Cao, T.N. A Cross-Sectional Study on Potential Ovarian Volume and Related Factors in Women with Polycystic Ovary Syndrome from Infertile Couples. Int J Womens Health 2021, 13, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Lie Fong, S.; Laven, J.S.E.; Duhamel, A.; Dewailly, D. Polycystic ovarian morphology and the diagnosis of polycystic ovary syndrome: redefining threshold levels for follicle count and serum anti-Müllerian hormone using cluster analysis. Hum Reprod 2017, 32, 1723–1731. [Google Scholar] [CrossRef]
- Lujan, M.E.; Jarrett, B.Y.; Brooks, E.D.; Reines, J.K.; Peppin, A.K.; Muhn, N.; Haider, E.; Pierson, R.A.; Chizen, D.R. Updated ultrasound criteria for polycystic ovary syndrome: reliable thresholds for elevated follicle population and ovarian volume. Hum Reprod 2013, 28, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Wongwananuruk, T.; Panichyawat, N.; Indhavivadhana, S.; Rattanachaiyanont, M.; Angsuwathana, S.; Techatraisak, K.; Pratumvinit, B.; Sa-Nga-Areekul, N. Accuracy of anti-Müllerian hormone and total follicles count to diagnose polycystic ovary syndrome in reproductive women. Taiwan J Obstet Gynecol 2018, 57, 499–506. [Google Scholar] [CrossRef]
- Jonard, S.; Dewailly, D. The follicular excess in polycystic ovaries, due to intra-ovarian hyperandrogenism, may be the main culprit for the follicular arrest. Hum Reprod Update 2004, 10, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Hudecova, M.; Holte, J.; Olovsson, M.; Sundström Poromaa, I. Long-term follow-up of patients with polycystic ovary syndrome: reproductive outcome and ovarian reserve. Hum Reprod 2009, 24, 1176–1183. [Google Scholar] [CrossRef]
- Glintborg, D.; Mumm, H.; Ravn, P.; Andersen, M. Age associated differences in prevalence of individual rotterdam criteria and metabolic risk factors during reproductive age in 446 caucasian women with polycystic ovary syndrome. Horm Metab Res 2012, 44, 694–698. [Google Scholar] [CrossRef]
- Wiser, A.; Shalom-Paz, E.; Hyman, J.H.; Sokal-Arnon, T.; Bantan, N.; Holzer, H.; Tulandi, T. Age-related normogram for antral follicle count in women with polycystic ovary syndrome. Reprod Biomed Online 2013, 27, 414–418. [Google Scholar] [CrossRef] [PubMed]
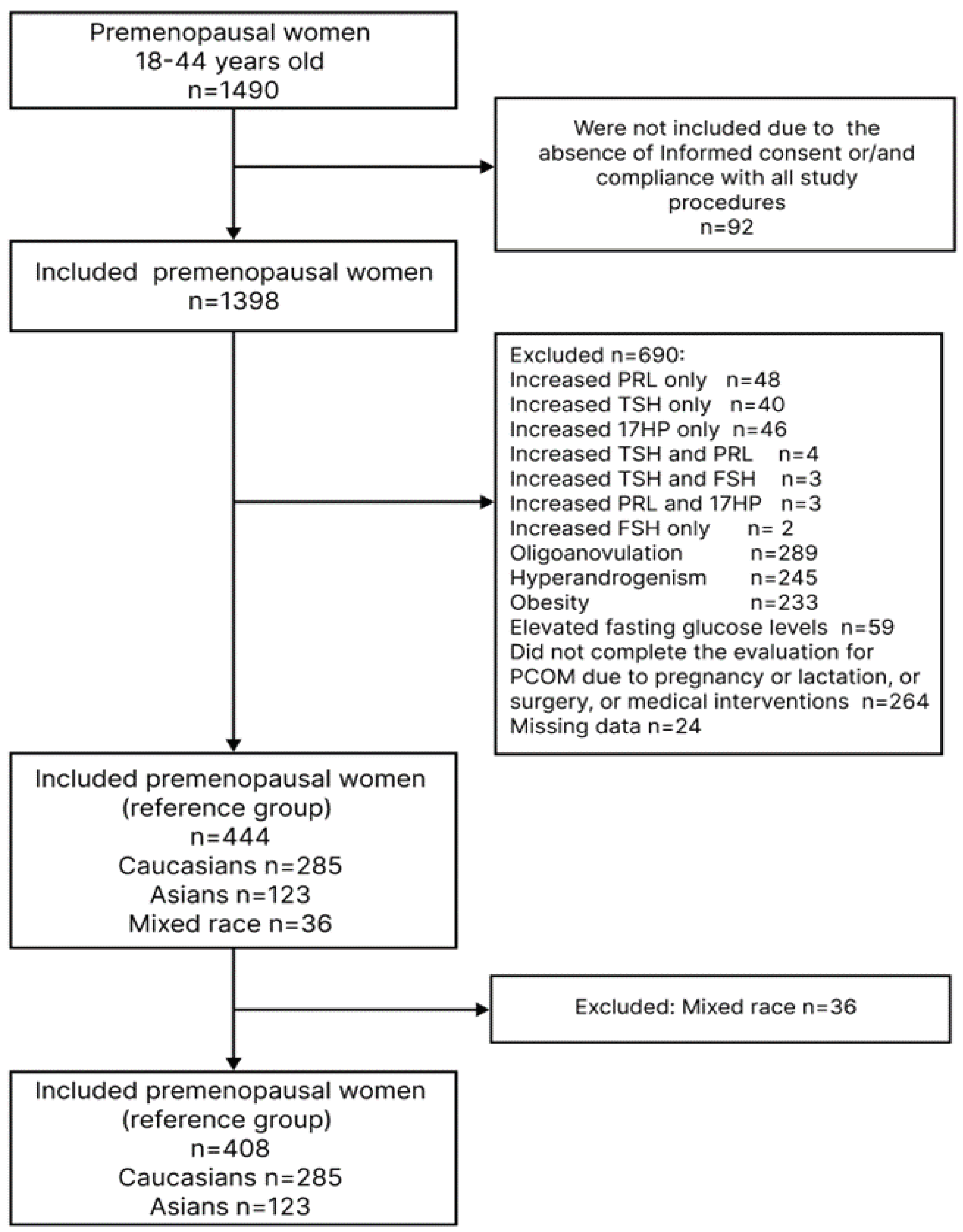
| Parameter | Total n= 408 |
Caucasians(1) n=285 |
Asians(2) n=123 |
p- value |
|---|---|---|---|---|
| Mean ± SD. Median (Lower Q; Upper Q) | ||||
| Age, years |
34.32±5.96 35.00 (30.00;39.00) |
34.04±6.01 34.00 (30.00;39.00) |
34.98±5.81 35.00 (31.00;40.00) |
p1-2=0.680* |
| Anthropometry and vital signs Mean ± SD. Median (Lower Q; Upper Q) | ||||
| WC, cm | 74.14±8.85 73.00 (67.00;80.00) |
73.86±8.95 73.00 (67.00;80.00) |
74.80±8.63 74.00 (68.00;81.00) |
p1-2=0.649* |
| BMI, kg/m2 | 23.79±3.30 23.70 (21.15;26.60) |
23.90±3.29 23.90 (21.20;26.70) |
23.54±3.31 23.70 (20.90;26.30) |
p1-2=0.916* |
| Systolic blood pressure, mm Hg | 119.51±11.52 119.00 (112.00;126.00) |
119.40±11.02 120.00 (112.00;125.00) |
119.75±12.64 117.00 (111.00;126.00) |
p1-2=0.066* |
| Diastolic blood pressure, mm Hg | 76.51±8.61 76.00 (70.00;81.500) |
76.00±8.39 76.00 (70.00;81.00) |
77.71±9.05 77.00 (71.00;83.00) |
p1-2=0.307* |
| Education n/N (%) | p=0.000# | |||
| Doctoral degree | 27/408 (6.62%) | 15/285 (5.26%) | 12/123 (9.76%) | p1-2=0.093** |
| Master’s degree | 286/408 (70.10%) | 185/285 (64.91%) | 101/123 (82.11%) |
p1-2=0.001** |
| Incomplete high school | 16/408 (3.92%) | 13/285 (4.56%) | 3/123 (2.44%) | p1-2=0.311** |
| Bachelor’s degree | 58/408 (14.22%) | 52/285 (18.25%) | 6/123 (4.88%) | p1-2=0.000** |
| Some college | 1/408 (0.25%) | 1/285 (0.35%) | 0/123 (0.00%) | p1-2=0.511** |
| High school or equivalent | 14/408 (3.43%) | 13/285 (4.56%) | 1/123 (0.81%) | p1-2=0.056** |
| Middle school only | 5/408 (1.23%) | 5/285 (1.75%) | 0/123 (0.00%) | p1-2=0.140** |
| Elementary school | 0/408 (0.00%) | 0/285 (0.00%) | 0/123 (0.00%) | NS |
| No degree | 1/408 (0.25%) | 1/285 (0.35%) | 0/123 (0.00%) | p1-2=0.511** |
| Occupation, n/N (%) | p=0.000# | |||
| Legislators. senior officials and managers | 9/408 (2.22%) | 8/285 (2.83%) | 1/123 (0.82%) | p1-2=0.511** |
| Professionals | 179/408 (44.20%) | 108/285 (38.16%) | 71/123 (58.20%) | p1-2=0.206** |
| Technicians and associate professionals | 78/408 (19.26%) | 62/285 (21.91%) | 16/123 (13.11%) | p1-2=0.000** |
| Office clerks | 55/408 (13.58%) | 33/285 (11.66%) | 22/123 (18.03%) | p1-2=0.039** |
| Service workers. and shop and market sales | 21/408 (5.19%) | 13/285 (4.59%) | 8/123 (6.56%) | p1-2=0.084** |
| Skilled agricultural and fishery workers | 1/408 (0.25%) | 1/285 (0.35%) | 0/123 (0.00%) | p1-2=0.511** |
| Craft and related trades workers | 41/408 (10.12%) | 38/285 (13.43%) | 3/123 (2.46%) | p1-2=0.001** |
| Plant and machine operators and assemblers | 8/408 (1.98%) | 8/285 (2.83%) | 0/123 (0.00%) | p1-2=0.059** |
| Elementary occupations | 13/408 (3.21%) | 12/285 (4.24%) | 1/123 (0.82%) | p1-2=0.072** |
| Marital status n/N (%) | р=0.890# | |||
| Single | 97/408 (23.89%) | 68/285 (23.86%) | 29/123 (23.97%) | |
| Married | 220/408 (54.19%) | 152/285 (53.33%) | 68/123 (56.20%) | |
| Living with another | 38/408 (9.36%) | 30/285 (10.53%) | 8/123 (6.61%) | |
| Separated | 5/408 (1.23%) | 4/285 (1.40%) | 1/123 (0.83%) | |
| Divorced | 35/408 (8.62%) | 23/285 (8.07%) | 12/123 (9.92%) | |
| Widowed | 8/408 (1.97%) | 6/285 (2.11%) | 2/123 (1.65%) | |
| Would rather not say | 3/408 (0.74%) | 2/285 (0.70%) | 1/123 (0.83%) | |
| Menstrual and reproductive history Mean ± SD. Median (Lower Q; Upper Q) |
||||
| Age at menarche, years | 13.28±1.35 13.00 (12.00;14.00) |
13.22±1.31 13.00 (12.00;14.0) |
13.41±1.43 13.00 (12.00;14.00) |
p1-2=0.223* |
| Average menstrual cycle length, days | 27.71±2.11 28.00 (27.99;29.00) |
27.61±2.16 28.00 (27.00;28.00) |
27.93±1.97 28.00 (27.00;30.00) |
p1-2=0.251* |
| Parity of pregnancies | 2.36±2.15 2.00 (1.00;3.00) |
2.37±2.19 2.00 (1.00;4.00) |
2.33±2.04 2.00 (1.00;3.00) |
p1-2=0.375* |
| mFG score | 0.54±0.96 0.00 (0.00;1.00) |
0.62±1.02 0.00 (0.00;1.00) |
0.37±0.78 0.00 (0.00;0.00) |
p1-2=0.001* |
| Hormones Mean ± SD. Median (Lower Q; Upper Q) | ||||
| Prolactin, mIU/ml | 333.91±153.57 307.00 (218.00;438.50) |
303.38±134.08 277.00 (202.00;382.00) |
404.64±172.07 399.00 (269.00;509.00) |
p1-2=0.001* |
| TSH, mIU/ml | 1.56±0.73 1.50 (1.00;2.00) |
1.52±0.73 1.40 (1.00;1.90) |
1.66±0.72 1.60 (1.20;2.10) |
p1-2=0.996* |
| LH, mIU/ml | 7.23±10.02 5.30 (3.20;7.50) |
7.52±10.16 5.60 (3.30;7.90) |
6.57±9.67 4.80 (3.20;7.00) |
p1-2=0.533* |
| FSH. mIU/ml | 5.86±3.22 5.40 (3.8;7.0) |
5.96±3.22 5.50 (4.00;7.00) |
5.67±3.22 5.10 (3.60;6.90) |
p1-2=1.00* |
| TT, ng/dl | 25.02±13.74 24.69 (14.93;33.61) |
26.22±14.68 25.70 (16.05;34.83) |
21.78±10.65 22.09 (13.64;29.99) |
p1-2=0.000* |
| SHBG, nmol/l | 86,23±56.21 70.35 (47.00;105.70) |
90.48±59.98 71.00 (51.40;115.00) |
76.38±45.00 65.60 (43.50;96.80) |
p1-2=0.000* |
| FAI | 1.35±1.05 1.15 (0.59;1.83) |
1.42±1.17 1.19 (0.59;1.88) |
1.19±0.70 1.07 (0.57;1.62) |
p1-2=0.000* |
| DHEAS, μg/dl | 160.52±65.36 155.00 (113.00;202.00) |
164.65±68.89 158.00 (118.00;210.00) |
150.87±55.32 145.00 (106.00;187.00) |
p1-2=0.006* |
| 17ОНP, nmol/l | 5.50±3.37 5.15 (2.50;7.00) |
5.63±3.35 5.40 (2.80;7.00) |
5.21±3.41 5.00 (2.10;7.00) |
p1-2=0.810* |
| АМН, ng/ml | 2.85± 2.21 2.20 (1.10;4.40) |
2.91±2.20 2.30 (1.10;4.50) |
2.71±2.22 1.90 (0.57;1.62) |
p1-2=0.900* |
| Total N=408 |
Caucasians N=285 (subgroup 1) |
Asians N=123 (subgroup 2) |
p- value” | |
| n=563* | n=388* | n=175* | ||
| OV | ||||
| Mean±SD (Min-Max) |
6.30±2.31 (0.54;16.98) |
6.58±2.36 (0.54;16.98) |
5.69±2.09 (1.57;14.63) |
p1-2=0.000 |
| Median (Lower Q; Upper Q) |
6.01(4.77;7.37) | 6.305 (5.04;7.78) | 6.00 (4.34;6.63) | |
| 95 Percentile (95%CI) | 10.31(9.86; 11.22) | 10.63(10.01; 11.88) | 9.32(8.57; 10.65) | |
| 97.5 Percentile (95%CI) | 12.3 (10.68; 13.16) | 12.45 (11.09;13.30) | 10.62 (9.34; 13.92) | |
| 98 Percentile (95%CI) | 12.56 (11.28; 13.56) | 12.58 (11.39; 13.76) | 10.66(9.51; 14.29) | |
| FNPO | ||||
| Mean±SD (Min-Max) |
6.85±2.78 (1.00;30.00) |
7.19±3.00 (1.00;30.00) |
6.11±2.03 (1.00;14.00) |
p1-2=0.000 |
| Median (Lower Q; Upper Q) |
6,00 (5.00;8.00) | 7,00 (5.00;8.00) | 6,00 (5.00;6.00) | |
| 95 Percentile (95%CI) | 12 (10,00; 10,72) | 12# (10; 12) | 10# (9; 10) | |
| 97,5 Percentile (95%CI) | 14 (12,00; 14,95) | 14# (12; 14) | 10# (9; 10) | |
| 98 Percentile (95%CI) | 14 (12,00; 14,00) | 15# (13,25; 15,26) | 10.52#(10; 12) | |
|
Total N=408 |
Caucasians N=285 |
Asians N=123 |
p- value (U-test) | ||||
|
<35 yrs, n=269& (1) |
≥35 yrs, n=294& (2) |
<35 yrs., n=194& (1a) |
≥35 yrs, n=194& (2a) |
<35 yrs, n=75& (1b) |
≥35 yrs, n=100& (2b) |
||
| Mean±SD. (Min-Max) |
6.72±2.37 (0.54;16.98) |
5.91±2.2 (0.94;14.63) |
7.09±2.38 (0.54;16.98) |
6.07±2.22 (0.94;13.56) |
5.78±2.07 (1.57;12.72) |
5.62±2.12 (2.2;14.63) |
p1-2=0.000 p1а-1b=0.000 p1а-2a=0.000 p1b-2b=0.596 p1а-1b=0.000 p2а-2b=0.040 |
| Median (Lower Q; Upper Q) |
6.28 (5.08;7.85) | 5.62 (4.39;7.11) | 6.7 (5.5;8.09) | 5.93 (4.6;7.27) | 5.22 (4.44;6.96) | 5.4 (4.17;6.46) | |
| 95 Percentile (95%CI) | 11.32 (10.13. 12.65) |
9.83 (9.32. 10.32) |
12.05## (10.53. 12.89) |
9.85## (9.49. 10.26) |
9.55 (8.43. 11.26) |
9.18 (7.96. 10.88) |
|
| 97,5 Percentile (95%CI) | 12.67 (11.38. 13.81) |
10.47 (9.85. 12.56) |
12.81 (11.6. 14.18) |
10.24 (9.85. 12.56) |
10.2 (9.11. 12.72) |
10.64 (8.59. 14.63) |
|
| 98 Percentile (95%CI) | 12.73 (11.91. 15.15) |
10.6 (9.94. 13.56) |
13.26 (12.33. 15.9) |
10.35 (9.87. 12.56) |
10.39 (9.22. 12.72) |
10.74 (9.02. 14.63) |
|
| Follicle number per ovary (FNPO) in the reference group from unselected population, by age | |||||||
| Mean±SD. (Min-Max) |
7.88±2.9 (3;30) | 5.91±2.29 (1;15) | 8.22±3.14 (3;30) | 6.15±2.47 (1;15) | 7.03±1.94 (3;14) | 5,43±1.83 (1;12) | p1-2=0.000 p1а-1b=0.000 p1а-2a=0.000 p1b-2b=0.000 p1а-1b=0.006 p2а-2b=0.009 |
| Median (Lower Q; Upper Q) |
7 (6;9) | 6 (5;7) | 7 (6;9) | 6 (5;7) | 7 (6;8) | 5 (4;6) | |
| 95 Percentile (95%CI) | 13 (12.0. 14.6)* | 10.35 (9.0, 12.0)* | 14 (13.0, 16.0)## | 12” (11.65, 14.65) | 10 (8.0, 11.0)## | 8.05” (8.0, 10.05) | |
| 97,5 Percentile (95%CI) | 15 (13.0, 16.0) | 12 (11.0, 13.32) | 15 (12.83, 16.0) | 12.17” (12.0, 14.0) |
10.3 (10.0, 14.0) | 9.52” (8.0, 11.52) | |
| 98 Percentile (95%CI) | 15 (13.0, 16.0)* |
12 (10.0, 12.0)* |
15.4**,## (14.0, 18.0) |
13**,” (12.0, 14.0) |
11.04”,## (10.0, 14.0) |
10.02” (8.02, 12.0) |
|
| Author, Year Country, | Setting Study Design # | Total Population, | Ethnicity Controls | Age range | OV, Mean±StD. (Min-Max) For Controls |
OV, UNLs Controls |
FNPO Mean±SD. (Min-Max) For Controls |
FPNO, UNLs Controls |
Transducer Frequency |
|---|---|---|---|---|---|---|---|---|---|
| Ahmad et al. 2019, USA [19]. | Cross-sectional study | Control: 756 (FNPO, OV) PCOS: 245 (FNPO), 297 (OV) |
Caucasians | Overall (20-40) | 6.49±4.98 | 6.75 | 10.01 ± 5.29 | 13 | 4-8 MHz, 4-10 MHz |
| 25 to <30 | 7.31±6.33 | 8.5 | 12.38±5.52 | 15 | |||||
| 30 to <35 | 6.49±4.97 | 7.00 | 10.14±4.8 | 14 | |||||
| 35 to <40 | 5.82±3.39 | 6.25 | 7.96±4.66 | 12 | |||||
| Carmina et al. 2016, Italy [20]. | Retrospective matched controlled study | PCOS: 113 Control: 47 | Caucasians | 19 to 35 years | N/A | 4.4 ± 1.8 | N/A | 10 ± 4 | 8-10 MHz |
| Chen et al. 2008, Сhina [21]. | Age-matched women | PCOS: 432 Control: 153 | Chinese population | N/A | N/A | 6.4 | N/A | 10 | 6 MHz |
| Dewailly et al. 2014; France [22]. | Retrospective study | Control: 521 PCOS: 272 OA+HA (Full-blown): 95 OA+PCOM: 110 HA+PCOM: 67 |
Caucasians | 18 to 40 years | N/A | N/A | N/A | 12.0 | 5-7 MHz |
| Fulghesu et al. 2001, Italy [23]. | Retrospective data analysis. | Control: 30 Multi-Follicular Ovaries (MFO): 27 PCOS: 53 | Caucasians | 18-38 | N/A | 13.21 | N/A | N/A | 6.5 MHz |
| Jonard et al. 2005, France [24]. | Observational cohort study |
Control: 57 PCOS: 98 | Caucasians | Control: 29.0 (24.5– 35.0) PCOS: 27.2 (19.5– 33.0) | 4.75 (3.11–6.86) | 7 | 6.5 (4.5–10.5) | 12.0 | 7 MHz |
| Kar and Swoyam 2018, India [25]]. | PCOS: 86 Control: 45 | Caucasians | 18–45 years | 5.06±2.44 | 6.15 | 7.13±3.51 | 12.0 | 6-12 MHz | |
| Kim et al. 2017, United States / Iceland [26]. | Cross-sectional, case-control design | Control: 666 (Boston) and 32 (Iceland) PCOS: 544 (Boston) and 105 (Iceland) 18 to >44 years. |
Caucasians | ≤24 years | N/A | 12 | N/A | 13 | 4-8 MHz |
| 25–29 years | N/A | 10 | N/A | 14 | |||||
| 30–34 years | N/A | 9 | N/A | 10 | |||||
| 35–39 years | N/A | 8 | N/A | 10 | |||||
| 40–44 years | N/A | 10 | N/A | 9 | |||||
| Köşüş et al. 2011a, Turkey [5]. | Prospective study | Control: 65 PCOS: 251 | Caucasians | N/A | 6.43 | N/A | 8 | 6.5 MHz | |
| Le et al. 2021, Vietnam [27]. |
Cross-sectional study | Control: 273 PCOS: 119 | Asiane | 33.99±4.78 years | 6.08±3.67 | 6.0 | N/A | N/A | 7 MHz |
| Lie Fong et al. 2017, Netherlands / United States [28]. | Retrospective observational cohort study | Control: 297 - Young non-PCOM (Cluster 1): 118 Young PCOM (Cluster 2): 28 Old non-PCOM (Cluster 3): 100 Old PCOM (Cluster 4): 51 PCOS: 700 | Caucasians | Young women | N/A | N/A | 9 (5–24) | 12.25 |
6.5–8 mHz |
| Old women | N/A | N/A | 10.75 | ||||||
| Lujan et al. 2013, United States / Canada [29]. | A diagnostic test study was performed using cross-sectional data | Control: 70 PCOS: 98 | Caucasians | 18–44 years | N/A | 10 | N/A | 26 | 5-9 MHz 6-12 MHz |
| Wongwananuruk et al. 2018, Thailand [30]. | Cross-sectional study | Control: 63 PCOS: 55 | Asiane | 18e45 years of age | 4.66 ± 1.83 | 6.5 | 9.97 ± 3.86 | 15 | 8 MHz |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





