1. Introduction
With the advent of the COVID-19, it has become even more important to block pathogens at the nasal mucosa entrance. Therefore, to prevent the colonisation of respiratory pathogens, the need for nasal vaccines is prerequisite to overcome the conventional injectable vaccines [
1]. Nasal immunization is considered to be the most effective method of inducing mucosal immunity in the nasopharynx, lungs, and vagina [
2], but whether it can stimulate skin and intestinal mucosal immunity remains unknown.
Most antigens are not sufficient to induce mucosal immunity and require immune-boosting adjuvants such as cholera toxin and heat-labile enterotoxin. However, a clinical study found that an inactivated nasal influenza vaccine containing adjuvants caused facial paralysis (Bell’s palsy) in some people [
3]. Moreover, mucosal adjuvant can impair the olfactory system of mice [
4]. Therefore, nasal immunization should be administered without adjuvants.
Current treatments for allergic dermatitis, rhinitis, and inflammatory bowel disease (IBD) do not offer a cure, but provide temporary relief; While β-2 agonists and inhaled corticosteroids can be used for milder manifestations of asthma, antibodies against type 2-dependent cytokines (IL-4, IL-5, and IL-13) can be used for severe allergies [
5]. However, these treatments often lead to drug resistance or side effects, resulting in a switch to other therapies [
6,
7]. Therefore, safe and highly effective treatments need to be developed.
2. Regulatory T cells for inflammatory or allergic diseases
The implementation of regulatory T (Treg) cells has been known to suppress inflammatory responses [
8,
9]. Treg cells can be utilized to maintain immune homeostasis by relieving excessive inflammation or preventing autoimmunity after a pathogenic event. In murine models, Tregs can regulate both low- and high-level inflammation caused by type 2-hypocytokine and type 2-hypercytokine secretion, respectively, and in human cells, they can alleviate allergic airway inflammation [
5]. Non-toxic Treg cells on the oral and nasal mucosal surfaces are induced similarly to cells in the gut. Tolerance of intestinal Treg is induced only at low antigen delivered, and at high antigen doses, anergy is induced [
10]. Respiratory tolerance is similar to intestinal oral tolerance mechanisms [
11], but it is not known whether nasal and mucosal tolerance can be regulated within the nasal cavity.
Currently, novel microbiome approaches to overcome IBD are either fecal microbiome transplantation (FMT) [
12,
13,
14] or ingestion of microbial strains that induce Treg function that can suppress intestinal inflammation [
15,
16]. However, these methods require antibiotic treatment to eliminate microbial imbalances prior to bacterial treatments. Vancomycin is administered to purge out vegetative
C. difficile, which produces toxins and causes inflammation and diarrhoea, but it does not kill the spore-forms that cause germination when treatment is discontinued. This is because antibiotic treatment causes a lack of beneficial Firmicutes and results in an increase in bile-acid, which in turn allows germination of
C. difficile spores. Therefore, discontinuation of antibiotic therapy and/or incomptlete sterilization may cause recurrence of
C. difficile disease. Recently, oral administration of SER-109 (from the fecal microbiota of healthy donors) has been shown to significantly reduce the recurrence rate of
C. difficile (12%) compared to the placebo group (40%) [
13]. Moreover, SER-109 resulted in a significant improvement in disease-specific quality-of-life scores as early as week 1 compared to patients treated with placebo, with steady and sustained improvement continued through week 8 post-dose [
14]. Moreover, the gut microbiome is subject to dietary modifications even after these treatments [
17,
18]. Therefore, an alternative approach that is not affected by diet would be preferable, and more efficient methods of Treg induction and maintenance are required. To date, it is unknown whether nasal immunization can upregulate Tregs in the skin and gut due to the lack of characterization or vaccination of the nasal mucosa.
3. Lung-gut axis
Interestingly, the alterations in the nasal microbial community including airways also affect the composition of intestinal microbiota. Numerous studies have shown that 2·5 μl of inoculum consisting of fluids, particles, or even microorganisms deposited into the nasal cavity of mice can later be detected in the gastrointestinal tract (GIT) [
19]. This indicates that the mucosal immune system of the GIT may serve as a primary sensor to any foreign antigens that is introduced into the nasal cavity [
20]. For example, manifestations of pneumonia due to
Pseudomonas (P) aeruginosa or multi-drug resistant
Staphylococcus. aureus in lungs are believed to trigger gut injury [
21]. Other way, several gastrointestinal disorders have manifestations in respiratory tract, for example, about half of the IBD patients with known alterations in their intestinal microbiota composition have abnormal lung function. The COPD patients show the intestinal hyper-permeability with a high prevalence of IBD [
22]. Thus, suggesting the “gut–lung axis” as a bi-directional communication network where many respiratory infections are often accompanied by gastrointestinal symptoms [
23]. The communication in the gut-lung axis comprises many direct and indirect pathways.
Accumulating evidence suggests that short chain fatty acids (SCFAs) comprising acetate, lactate, butyrate, and succinate, can be considered as a leading link in the immune axis between the gut and lungs. Indeed, SCFAs are known to modulate immune homeosis and mucosal defence thus contributing to barrier functions. Several
Lactobacillus species are known to secrete lactate producing bacteria, a precursor for SCFA producing bacteria. Additionally, SCFA are known to limit mucosal inflammation by the induction of Tres [
24]. Our findings indicates that microbiota composition in Δpep27-immunizaed colitis mice showed positive correlations with the Treg induction and negative association with the proinflammatory cytokines [
25].
4. Pneumococcal Pep27 induction during invasion and lack of sepsis induction by pep27 mutant
Streptococcus pneumoniae (pneumococcus) is carried asymptomatically in the nasopharynx of healthy individuals and this serves as a major reservoir for pneumococcal infections [
26]. Pneumococcus causes various potentially life-threatening infections such as pneumonia, bacteremia (sepsis), and meningitis [
27]. A prerequisite for invasive pneumonia is that pneumococci must colonize the nasopharynx before they can progress to invasive pneumonia and disseminate to the lung, bloodstream, and central nervous system [
28]. In pneumococci, bacterial lysis releases cell wall components and pneumolysin toxin, and subsequently trigger pro-inflammatory responses. Moreover, mutations in the major autolysin (LytA) reduces pneumococcal virulence [
29].
Pep27 is an effector molecule of the
vncRS operon that mediates vancomycin resistance and autolysis [
30,
31]. However, by microarray analysis, we discovered that a number of pneumococcal genes were induced upon invasion of the human lung cell line A549. We confirmed that these target genes were indeed induced upon invasion of A549 cells by real-time PCR, which demonstrated that not only
pep27, but also
vncR and
vncS were activated by pneumococcal infection. Furthermore, when we constructed 15 mutants of the genes induced during A549 invasion and tested them for attenuation of cytotoxicity in vitro after infection with A549 cells and confirmed reduced toxicity in mice by intranasal infection (pneumonia model) or intraperitoneal injection (sepsis model). Of those genes,
pep27 gene of the
vncRS operon was most prominently induced than normal controls. However, the most significantly induced gene was always
pep27 and the
pep27 mutant was found to have the least toxicity and vaccine efficacy. Lysis-resistant
pep27 mutant (Δpep27) gives rise to reduced cytotoxicity to host cells resulting in decreased inflammation and death [
32,
33]. Thus Δ
pep27 makes the pneumococci incapable of invading into the lungs, blood, and brain [
28], resulting in a virtually non-cytotoxic and highly safe agent that did not cause death after injection into the brains of immunocompromised mice [
34]. Furthermore, intranasal immunization with Δ
pep27, without any adjuvant, demonstrated long-term protective efficacy [
28].
Intranasal immunization with an attenuated erythromycin-resistant Δpep27 and
the inactivated markerless Δ
pep27 could protect a host from lethal pneumococcal challenge serotype independently; it also lowered bacterial colonization in the nasopharynx [
28,
35] suggesting that Δpep27 may be able to provide mucosal immunity against pneumococcal diseases and could represent an efficient mucosal vaccine.
Mechanistically,
vncRS is activated by lactoferrin in serum and is required for the development of pneumonia and sepsis. When the VncS sensor is exposed to lactoferrin, it is phosphorylated and the phosphate group is transferred to the VncR response regulator, allowing the VncRS operon to be induced, which in turn secretes the effector Pep27, which is thought to cause bacterial lysis and release, leading to host lung inflammation. Deletion of the effector Pep27 does not induce lysis and is incompetent to invade into lung and blood, confirming that
pep27 is essential for inflammatory response and sepsis [
34].
5. Intranasal immunization of Δpep27 protects against pathogens and influenza virus infection
During our approach to intranasal immunization using Δpep27 for prevention of pneumococcal diseases, microarray and system biology analyses of human lung cells after a Δpep27 infection unraveled unexpected features predicting preventive effect of influenza virus infection and intestinal abnormality. Thereafter, a series of experiments on this prediction were performed and show that the prediction is true [
25,
36,
37].
Δpep27 provides transient non-specific protection from heterologous bacteria through non-canonical Wnt upregulation. Nasal immunization with Δpep27 can inhibit colonization of
Staphylococcus aureus and
Klebsiella pneumoniae, indicating non-specific resistance to respiratory pathogens [
38].
Injectable pneumococcal vaccines, including the 23-valent polysaccharide vaccine and the 13-valent conjugate vaccine, do not provide mucosal immunity and do not provide complete protection against secondary pneumococcal infection following primary influenza virus infection [
39]. To address these challenges, we determined whether Δpep27 could protect mice against secondary pneumococcal infection following influenza virus infection. Surprisingly, Δpep27 protected mice against secondary pneumococcal infection after influenza virus infection by lowering the influenza virus burden in the lungs. In contrast, the unimmunized group of mice had a nearly 60% higher mortality rate following pneumococcal infection due to higher bacterial loads. Δpep27 vaccination alone can prevent influenza and pneumococcal infections by reducing viral titers in the lungs after infection. Overall, Δpep27 immunization is a novel and safe method to overcome both invasive pneumococcal disease and serious secondary infections following influenza infection during influenza epidemics [
37].
During pneumococcal pneumonia, phagocytes produce H
2O
2 and reactive oxygen species (ROS) for bacterial removal, nonetheless, lung is vulnerable to these oxidative stresses, resulting in extensive cellular and lung damage [
40]. Thus we investigated the therapeutic effect of ∆pep27 immunization on antioxidant Small Proline-Rich Repeat (SPRR) genes in the lungs and its associated consequences on the gut dysbiosis. We observed that Δpep27 significantly increased the levels of the SPRR genes in the lungs suggesting a strengthened alveolar barrier and enhanced resistance to external stressors resulting in a robust regenerative and oxidant stress-relieving mechanism to re-establish immunological tolerance [
36,
41]. Additionally, SPRR genes are involved not only in the establishment of the physical barrier but also in cell migration and wound healing [
41,
42]. Δpep27 on the other hand significantly increased
SPRR genes resulting in a more strengthened alveolar barrier and enhanced resistance to external [
41] including pneumococci suggesting that Δpep27 immunization blocks ROS and oxidative stresses [
36].
Macrophages are classified into M1 and M2 macrophages, which produce inflammatory and anti-inflammatory cytokines, respectively [
43]. Adoptive transfer of M2 macrophages or inducing M2 polarization has been shown to suppress experimental colitis [
44]. M2 macrophages aid in the resolution of inflammation by downregulating inflammatory cytokines and secrete copious amounts of IL-10 and TGF-β thereby protecting against colitis to promote tissue repair and by driving epithelial cell regeneration [
43]. Intranasal Δpep27 immunization upregulates colonic M2 macrophages thereby inhibiting inflammatory milieu [
36].
6. Intranasal immunization of Δpep27 protects allergic diseases
Inactivated serotype 3
S. pneumoniae has been reported to be effective against allergic diseases, including asthma, via Treg upregulation. These inactivated strains in a mouse model significantly suppressed the allergic inflammatory responses that are pivotal in the development and progression of asthma, including Th1 and Th2 cytokine production and eosinophil recruitment to the airways during or after ovalbumin sensitization [
45,
46], but they are toxic and cannot be used as a vaccine.
Intranasal Δpep27 immunization before or after allergen exposure could restore the necessary balance of Th1/Th2 cells by reducing Th2 activity and maintaining Th1 and Treg activity that was disturbed during asthma. Additionally, allergic airway inflammation in the lung was significantly reduced by Δpep27 immunization. Δpep27 immunization may provide long-term protection against asthma without any toxicity [
47].
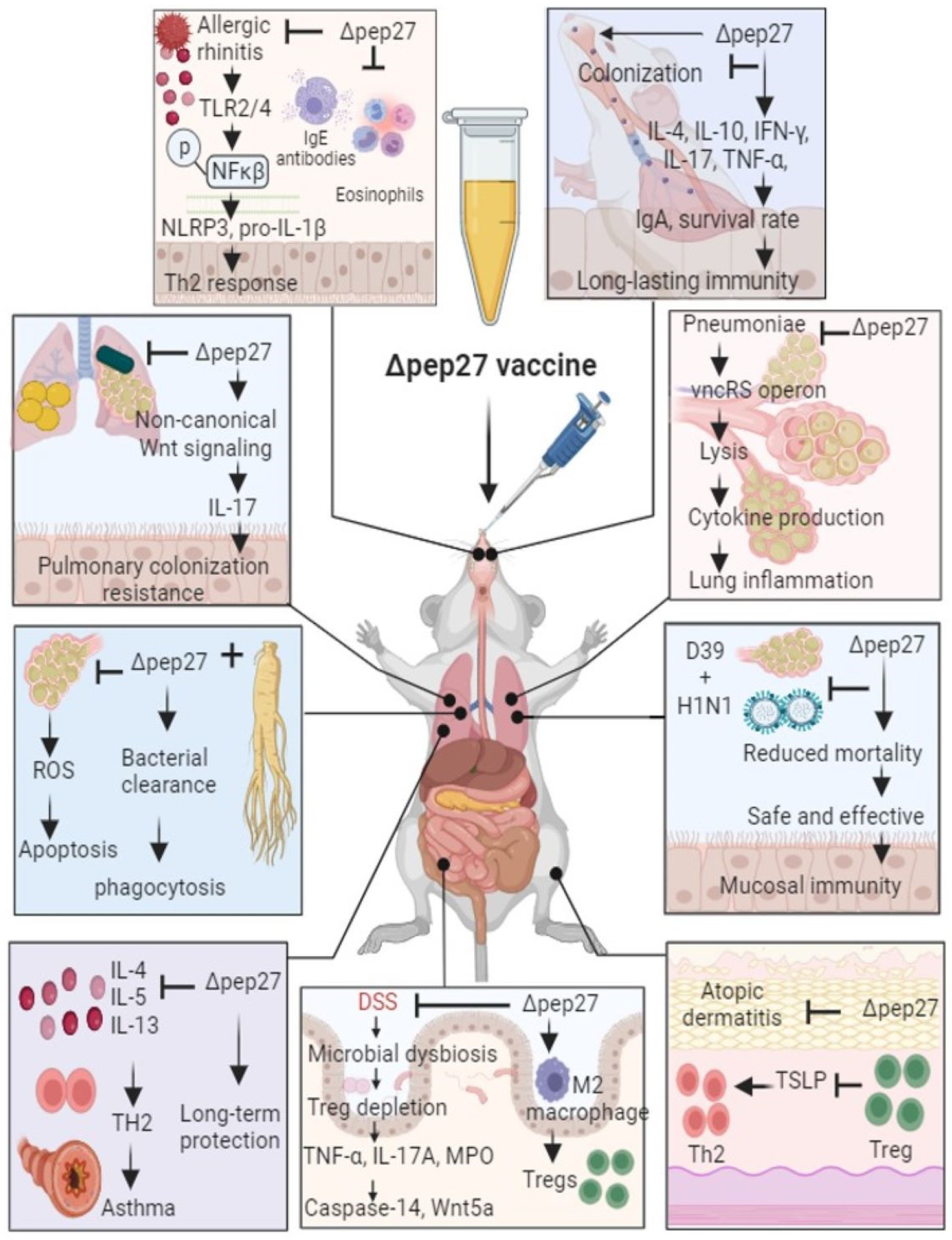
In addition, Δpep27 immunization alleviated allergic symptoms such as sneezing and rubbing frequency and reduced TLR2 and TLR4 expression, Th2 cytokines, and eosinophil infiltration in the nasal mucosa of an ovalbumin (OVA)-induced allergic rhinitis mouse model [
48] (
Figure 1). Mechanistically, Δpep27 reduced the activation of NLRP3 inflammasome in the nasal mucosa by downregulating the TLR signaling pathway and subsequently prevented allergic reactions [
48].
Prophylactic and therapeutic analysis showed that Δ
pep27 could elicit anti-inflammatory Treg-relevant factors and epithelial barrier genes (
Filaggrin,
involucrin, loricrin, and SPRR proteins). Accordingly, pneumococcal Δpep27 immunization upregulated Treg activity, suppressing epidermal collapse, IgE, and TSLP. On the other hand, Treg suppression worsened atopic dermititis through upregulation of TSLP and Th2 and repression of epithelial barrier function compared to the non-suppressed pneumococcal Δpep27 group. In summary, pneumococcal Δ
pep27 immunization alleviated allergic dermatitis symptoms by upregulating Tregs and epithelial barrier functions and suppressing TSLP and Th2 to relieve allergic dermatitis symptoms [
49].
7. Intranasal immunization of Δpep27 protects IBD potentially by anti-oxidative SPRR and antiinflammatory M2 upregulation via Treg induction
Intranasal Δpep27 immunization prevented dextran sulfate sodium (DSS)-induced colitis. ∆pep27 significantly mitigated oxidative stress parameters and down-regulates pro-inflammatory cytokines, and Wnt5a expressions via Treg induction in gut. Moreover, ∆pep27 induces upregulation of the anti-inflammatory genes IL-10 and TGF-β1, as well as M2 macrophages via Treg induction and tight junction genes. Δpep27 also suppresses DSS-induced caspase-14 expression and upregulates Tregs, resulting in healthy microbiota. Inhibition of Treg function confirmed that ∆pep27 has therapeutic effects on gut inflammation and caspase-14 via Treg upregulation. Overall, intranasal immunization with ∆pep27 can attenuate colonic inflammation via Treg induction and could be a highly pragmatic way to re-establish immunological tolerance [
25,
36] (
Figure 1).
8. Conclusion
To date, treating allergic diseases as well as recurrent inflammatory diseases, including IBD, remains a challenging task. Moreover, the causes of these diseases are not fully understood. Although there have been many attempts to use Tregs, which are effective for hypersensitivity reactions such as excessive inflammation or allergies, there were many limitations in terms of functionality. In this study, it was confirmed that Tregs induced by intranasal immunization were stably expressed not only in the nasopharynx and lungs, but also in the skin and intestines, making them effective against inflammation/hypersensitivity reactions. Mechanistically, Δpep27 suppresses oxidative stress levels, which are closely linked to gut dysbiosis, potentially by increasing the SPRR family in the lungs, suggesting that the gut-lung axis is a bi-directional communication network. Furthermore, analysis of key genes in the lungs induced by Δpep27 immunization highlighted mucosal protection, particularly in the lungs and gut, and this mechanism of immune tolerance included normal defense against gut dysbiosis by Tregs. Furthermore, Δpep27 immunization induced M2 macrophages, an antioxidant milieu to mitigate the stress response, and Treg attenuated caspase-14 and Wnt5a expression independent of the inflammatory environment through the lung-gut axis, suggesting a robust anti-inflammatory mucosal tolerance and subsequent restoration of the gut microbiota, ensuring that barrier integrity is maintained to ensure intestinal immune homeostasis. ∆pep27 immunization accelerated appears to promote the development and restoration of functional Treg cells in the skin, internal and respiratory organs as well as in the intestines, perhaps through mucosal Treg infiltration or induction. Therefore, ∆pep27 may be a promising mucosal vaccine candidate treatment in the field of clinical application for allergic and inflammatory diseases. However, inflammatory bowel disease is an intractable multifactorial disorder, and there have been no clinical trials using Δpep27 in allergic and inflammatory bowel disease, so further studies on optimizing the parameters regulated by Δpep27 and the resilience of food-induced changes in Treg expression are needed to confirm its effectiveness in these diseases.
Author Contributions
H.I. and D.-K.R. collected, analyzed, and reviewed the literature, and wrote the main manuscript. H.I. and D.-K.R. prepared the figures.
Conflicts of Interest
The authors declare that they have no conflict of financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Lavelle, E.C.; Ward, R.W. Mucosal vaccines - fortifying the frontiers. Nat. Rev. Immunol. 2022; 22, 236-250. [CrossRef]
- Holmgren, J.; Czerkinsky C. Mucosal immunity and vaccines. Nat. Med. 2005; 11, S45-53. [CrossRef]
- Mutsch, M.; Zhou, W.; Rhodes, P.; Bopp, M.; Chen, R.T.; Linder, T.; Spyr, C.; Steffen, R. Use of the inactivated intranasal influenza vaccine and the risk of Bell’s palsy in Switzerland. N. Engl. J. Med. 2004; 350, 896-903. [CrossRef]
- Fukuyama, Y.; Okada, K.; Yamaguchi, M.; Kiyono, H.; Mori, K.; Yuki, Y. Nasal Administration of Cholera Toxin as a Mucosal Adjuvant Damages the Olfactory System in Mice. PLoS One. 2015; 10, e0139368. [CrossRef]
- Reuter, S.; Raspe, J.; Taube, C. Microbes little helpers and suppliers for therapeutic asthma approaches. Respir. Res. 2024, 25:29. [CrossRef]
- Bonds, R.S.; Midoro-Horiuti, T. Estrogen effects in allergy and asthma. Curr. Opin. Allergy Clin. Immunol. 2013; 13, 92-9. [CrossRef]
- Dubinsky, M.C.; Ferrante, M.; Irving, P.M.; Kamperidis, N.; Kobayashi, T.; Kotze, P.G.; Lambert, J.; Noor, N.M.; Roblin, X.; Roda, G.; Vande Casteele, N.; Yarur, A.J.; Arebi, N.; Danese, S.; Paul, S.; Sandborn, W.J.; Vermeire, S.; Cheifetz, A.S.; Peyrin-Biroulet, L. International consortium for therapeutic drug monitoring of biologics in inflammatory bowel disease: unmet needs and future perspectives. Lancet Gastroenterol Hepatol. 2022; 171-185. [CrossRef]
- Esensten, J.H; Muller, Y.D; Bluestone, J.A; Tang, Q. Regulatory T-celltherapyfor autoimmune and autoinflammatory diseases: The next frontier. J. Allergy Clin. Immunol. 2018; 142, 1710-1718. [CrossRef]
- Baeten, P.; Van Zeebroeck, L.; Kleinewietfeld, M.; Hellings, N, Broux, B. Improving the Efficacy of Regulatory T CellTherapy. Clin. Rev. Allergy Immunol. 2022;62, 363-381. [CrossRef]
- Weiner, H.L.; da Cunha, A.P.; Quintana, F.; Wu, H. Oral tolerance. Immunol. Rev. 2011; 241, 241-59. [CrossRef]
- Galletti, J.G.; de Paiva, C.S. Age-related changes in ocular mucosal tolerance: Lessons learned from gut and respiratory tract immunity. Immunology. 2021, 164:43-56. [CrossRef]
- Britton, G.J.; Contijoch, E.J.; Spindler, M.P.; Aggarwala, V.; Dogan, B.; Bongers, G.; San.; Mateo, L.; Baltus, A.; Das, A.; Gevers, D.; Borody, T.J.; Kaakoush, N.O.; Kamm, M.A.; Mitchell, H.; Paramsothy, S.; Clemente, J.C.; Colombel. J.F.; Simpson, K.W.; Dubinsky, M.C.; Grinspan, A.; Faith, J.J. Defined microbiota transplant restores Th17/RORγt+ regulatory T cell balance in mice colonized with inflammatory bowel disease microbiotas. Proc. Natl. Acad. Sci. USA. 2020; 117, 21536-21545. [CrossRef]
- Feuerstadt, P.; Louie, T.J.; Lashner, B.; Wang, EEL.; Diao, L.; Bryant, J.A.; Sims, M.; Kraft, C.S.; Cohen, S.H.; Berenson, C.S.; Korman, L.Y.; Ford, C.B.; Litcofsky, K.D.; Lombardo, M.J.; Wortman, J.R.; Wu, H.; Auniņs, J.G.; McChalicher, C.W.J.; Winkler, J.A.; McGovern, BH.; Trucksis, M.; Henn, M.R.; von Moltke, L. SER-109, an Oral Microbiome Therapy for Recurrent Clostridioides difficile Infection. N. Engl J. Med. 2022; 386, 220-229. [CrossRef]
- Garey, KW.; Jo, J.; Gonzales-Luna, A.J.; Lapin, B.; Deshpande, A.; Wang, E.; Hasson, B.; Pham, S.V.; Huang, S.P.; Reese, P.R.; Wu, H.; Hohmann, E.; Feuerstadt, P.; Oneto, C.; Berenson, C.S.; Lee, C.; McGovern, B.; vonMoltke, L. Assessment of Quality of Life Among Patients With Recurrent Clostridioides difficile Infection Treated with Investigational Oral Microbiome TherapeuticSER-109: Secondary Analysis of a Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2253570. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, K.; Tanoue, T.; Oshima, K.; Suda, W.; Nagano, Y.; Nishikawa, H.; Fukuda, S.; Saito, T.; Narushima, S.; Hase, K.; Kim, S.; Fritz, J.V.; Wilmes, P.; Ueha, S.; Matsushima, K.; Ohno, H.; Olle, B.; Sakaguchi, S.; Taniguchi, T.; Morita, H.; Hattori, M.; Honda, K. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013, 500, 232–236. [Google Scholar] [CrossRef]
- Van der Lelie, D.; Oka, A.; Taghavi, S.; Umeno, J.; Fan, T.J.; Merrell, K.E.; Watson, S.D.; Ouellette, L.; Liu, B.; Awoniyi, M.; Lai, Y.; Chi, L.; Lu, K.; Henry, C.S.; Sartor, R.B. Rationally designed bacterial consortia to treat chronic immune-mediated colitis and restore intestinal homeostasis. Nat. Commun. 2021, 12, 3105. [Google Scholar] [CrossRef]
- Tan, J.; Taitz, J.; Sun, S.M.; Langford, L.; Ni, D.; Macia, L. Your Regulatory T Cells Are What You Eat: How Diet and Gut Microbiota Affect Regulatory T Cell Development. Front. Nutr. 2022, 9, 878382. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Southam, D.S.; Dolovich, M.; O’Byrne, P.M.; Inman, M.D. Distribution of intranasal instillations in mice: effects of volume, time, body position, and anesthesia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 282, L833–L839. [Google Scholar] [CrossRef]
- Samuelson, D.R.; Welsh, D.A.; Shellito, J.E. Regulation of lung immunity and host defense by the intestinal microbiota. Front. Microbiol. 2015, 6, 1085. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Mande, S.S. Diet, microbiota and gut-lung connection. Front. Microbiol. 2018, 9, 2147. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wen, Q.; Yao, F.; Xu, D.; Huang, Y.; Wang, J. Gut–lung axis: the microbial contributions and clinical implications. Crit. Rev. Microbiol. 2017, 43, 81–95. [Google Scholar] [CrossRef]
- Wypych, T.P.; Wickramasinghe, L.C.; Marsland, B.J. The influence of the microbiome on respiratory health. Nat. Immunol. 2019, 20, 1279–1290. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; Takahashi, M.; Fukuda, N.N.; Murakami, S.; Miyauchi, E.; Hino, S.; Atarashi, K.; Onawa, S.; Fujimura, Y.; Lockett, T.; Clarke, J.M.; Topping, D.L.; Tomita, M.; Hori, S.; Ohara, O.; Morita, T.; Koseki, H.; Kikuchi, J.; Honda, K.; Hase, K.; Ohno, H. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Iqbal, H.; Kim, G.L.; Kim, J.H.; Ghosh, P.; Shah, M.; Lee, W.; Rhee, D.K. Pep27 Mutant Immunization Inhibits Caspase-14 Expression to Alleviate Inflammatory Bowel Disease via Treg Upregulation. Microorganisms 2022, 10, 1871. [Google Scholar] [CrossRef]
- Kwon, H.Y.; Ogunniyi, A.D.; Choi, M.H.; Pyo, S.N.; Rhee, D.K.; Paton, J.C. The ClpP protease of Streptococcus pneumoniae modulates virulence gene expression and protects against fatal pneumococcal challenge. Infect. Immun. 2004, 72, 5646–5653. [Google Scholar] [CrossRef]
- Loughran, A.J.; Orihuela, C.J.; Tuomanen, E.I. Streptococcus pneumoniae: Invasion and Inflammation. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Choi, S.Y.; Kwon, M.K.; Tran, T.D.; Park, S.S.; Lee, K.J.; Bae, S.M.; Briles, D.E.; Rhee, D.K. Streptococcus pneumoniae pep27 mutant as a live vaccine for serotype-independent protection in mice. Vaccine 2012, 30, 2008–2019. [Google Scholar] [CrossRef]
- Kim, G.L.; Luong, T.T.; Park, S.S.; Lee, S.; Ha, J.A.; Nguyen, C.T.; Ahn, J.H.; Park, K.T.; Paik, M.J.; Suhkneung-Pyo; Briles, D.E.; Rhee, D.K. Inhibition of Autolysis by Lipase LipA in Streptococcus pneumoniae Sepsis. Mol. Cells 2017, 40, 935–944. [Google Scholar] [CrossRef]
- Novak, R.; Henriques, B.; Charpentier, E.; Normark, S.; Tuomanen, E. Emergence of vancomycin tolerance in Streptococcus pneumoniae. Nature. 1999, 399, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Lee, S.; Rhee, D.K. Crystal Structure of the Pneumococcal Vancomycin-Resistance Response Regulator DNA-Binding Domain. Mol. Cells 2021, 44, 179–185. [Google Scholar] [CrossRef]
- Kwon, M.K. Mutagenesis of the Pneumococcal Genes Induced during Infection into the Lung Cells and Characterization of the Mutants in Virulence. MS Thesis, School of Pharmacy, Sungkyunkwan University, 2008. [Google Scholar]
- Rhee, D.K. Development of highly effective vaccine for prevention of pneumococcal diseases, Korea Disease Control and Prevention Agency, Research service project, 2009.
- Lee, S.; Ghosh, P.; Kwon, H.; Park, S.S.; Kim, G.L.; Choi, S.Y.; Kim, E.H.; Tran, T.D.; Seon, S.H.; Le, N.T.; Iqbal, H.; Lee, S.; Pyo, S.; Rhee, D.K. Induction of the pneumococcal vncRS operon by lactoferrin is essential for pneumonia. Virulence 2018, 9, 1562–1575. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Tran, T.D.; Briles, D.E.; Rhee, D.K. Inactivated pep27 mutant as an effective mucosal vaccine against a secondary lethal pneumococcal challenge in mice. Clin. Exp. Vaccine Res. 2013, 2, 58–65. [Google Scholar] [CrossRef]
- Iqbal, H.; Kim, G.L.; Kim, J.H.; Ghosh, P.; Shah, M.; Lee, W.; Rhee, D.K. Pneumococcalpep27-mutant inhibits Wnt5a expression via the regulation of T helper cells to attenuate colitis. Int. Immunopharmacol. 2022b, 109:108927. [CrossRef]
- Seon, S.H.; Choi, J.A.; Yang, E.; Pyo, S.; Song, M.K.; Rhee, D.K. Intranasal Immunization with an attenuated pep27 mutant provides protection from Influenza virus and secondary pneumococcal infections. J. Infect. Dis. 2018, 217, 637-640. [CrossRef]
- Kim, G.L.; Lee, S.; Kim, S.J.; Lee, S.O.; Pyo, S.; Rhee, D.K. Pulmonary Colonization Resistance to Pathogens via Noncanonical Wnt and Interleukin-17A by Intranasal pep27Mutant Immunization. J. Infect. Dis. 2018, 217, 1977–1986. [Google Scholar] [CrossRef]
- Metzger, D.W.; Furuya, Y.; Salmon, S.L.; Roberts, S.; Sun, K. Limited efficacy of antibacterial vaccination against secondary serotype 3 pneumococcal pneumonia following influenza infection. J. Infect. Dis. 2015, 212, 445–52. [Google Scholar] [CrossRef] [PubMed]
- Zahlten, J.; Kim, Y.J.; Doehn, J.M.; Pribyl, T.; Hocke, A.C.;, García P, Hammerschmidt, S.; Suttorp, N.; Hippenstiel, S.; Hubner, R.H. Streptococcus pneumoniae-Induced Oxidative Stress in Lung Epithelial Cells Depends on Pneumococcal Autolysis and Is Reversible by Resveratrol. J. Infect. Dis. 2015, 211, 1822-30. [CrossRef]
- Vermeij, W.P.; Backendorf, C. Skin cornification proteins provide global link between ROS detoxification and cell migration during wound healing. PLoS One. 2010, 5, e11957. [Google Scholar] [CrossRef] [PubMed]
- Vermeij, W.P.; Florea, B.I.; Isenia, S.; Alia, A.; Brouwer, J.; Backendorf, C. Proteomic identification of in vivo interactors reveals novel function of skin cornification proteins. J. Proteome Res. 2012, 11, 3068–3076. [Google Scholar] [CrossRef]
- Wang, J.; Chen, W.D.; Wang, Y.D. The Relationship Between Gut Microbiota and Inflammatory Diseases: The Role of Macrophages. Front. Microbiol. 2020, 9, 1065. [Google Scholar] [CrossRef] [PubMed]
- Hunter, M.M.; Wang, A.; Parhar, K.S.; Johnston, M.J.G.; Van Rooijen, N.; Beck, P.L.; McKay, D.M. In vitro-derived alternatively activated macrophages reduce colonic inflammation in mice. Gastroenterology 2010, 138, 1395–1405. [Google Scholar]
- Preston, J.A.; Essilfie, A.T.; Horvat, J.C.; Wade, M.A.; Beagley, K.W.; Gibson, P.G.; Foster, P.S.; Hansbro, P.M. Inhibition of allergic airways disease by immunomodulatory therapy with whole killed Streptococcus pneumoniae. Vaccine 2007, 25, 8154–8162. [Google Scholar] [CrossRef]
- Preston, J.A.; Thorburn, A.N.; Starkey, M.R.; Beckett, E.L.; Horvat, J.C.; Wade, M.A.; O’Sullivan, B.J.; Thomas, R.; Beagley, K.W.; Gibson, P.G.; Foster, P.S.; Hansbro, P.M. Streptococcus pneumoniae infection suppresses allergic airways disease by inducing regulatory T-cells. Eur. Respir J. 2011, 37, 53–64. [Google Scholar] [CrossRef]
- Kim, B.G.; Ghosh, P.; Ahn, S.; Rhee, D.K. Pneumococcal pep27 mutant immunization suppresses allergic asthma in mice. Biochem. Biophys. Res. Commun. 2019, 514, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.I.; Kim, J.H.; Nam, K.E.; Lee, W.; Rhee, D.K. Pneumococcal Δpep27Immunization Attenuates TLRs and NLRP3 Expression and Relieves Murine Ovalbumin-Induced Allergic Rhinitis. J. Microbiol. Biotechnol. 2022, 32, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Ahn, S.; Ghosh, P.; Rhee, D.K. Immunization with a Pneumococcal pep27 Mutant Strain Alleviates Atopic Dermatitis through the Upregulation of Regulatory T-Cell Activity and Epithelial Barrier Function and Suppressing TSLP Expression. J. Invest. Dermatol. 2023, 143, 115–123.e6. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).