Submitted:
13 February 2024
Posted:
14 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Background
2.1. Battery Basics
2.1.1. Energy
2.1.2. Power
2.1.3. Voltage
2.1.4. State of the Charge (SOC)
2.1.5. Capacity
2.1.6. Voltage Drops
2.1.7. Self-Discharge
2.1.8. Electrical Double Layer
2.1.9. Lithiation/ Delithiation
2.1.10. Particle Pulverization
2.1.11. Lithium Plating
2.1.12. Solid Electrolyte Interface
2.1.13. Charge/Discharge Test
2.1.14. Cyclic Voltammetry Test
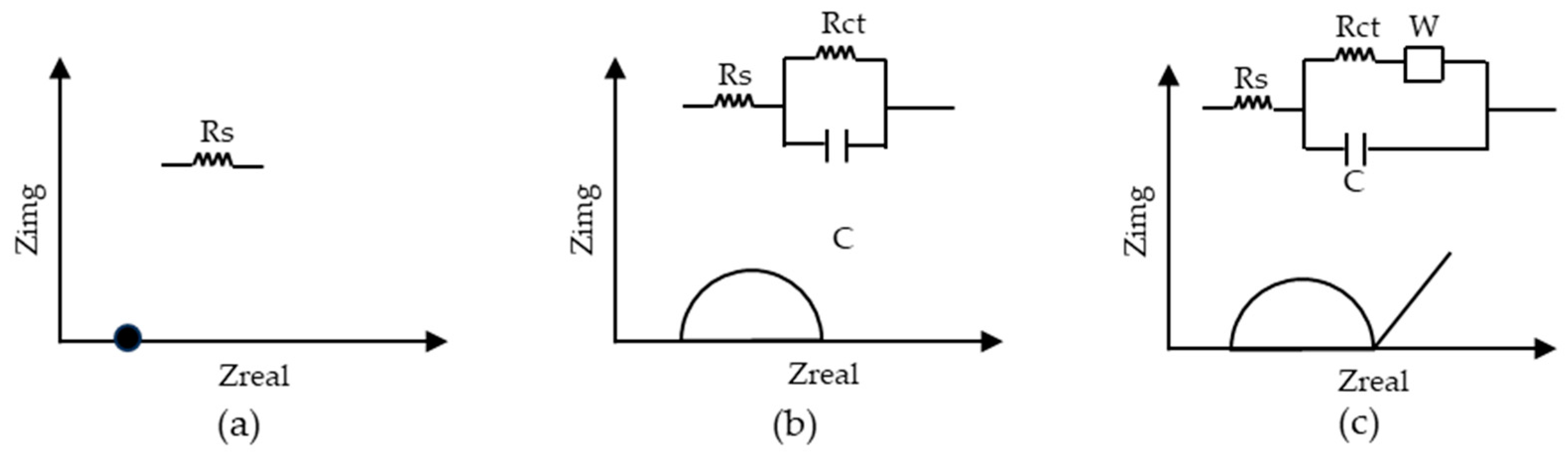
2.1.15. Electrochemical Impedance Spectroscopy (EIS)
- Ohmic resistance (Rs) is related to the ionic and electronic conductivity of various components in the battery, including the electrolyte, electrodes, and current collector. This is measured at low frequencies, and on the Nyquist plot, it is represented by the real part of the impedance (Figure 1a).
- Charge transfer resistance (Rct) is the resistance related to the electrochemical reactions occurring at the interface layer of the electrode-electrolyte. Information about the kinetics of the charge transfer process, such as lithium intercalation at the electrode surface, is provided by this resistance. On the Nyquist plot, this resistance is observed as a semicircle, with the radius of the semicircle representing the charge transfer resistance. This region corresponds to the high-frequency range. An improvement in battery performance is indicated by a reduction in the radius/diameter of this semicircle, suggesting that the charge transfer processes at the electrode-electrolyte interface are more efficient and faster. This layer also serves as a capacitance that stores charges transferring slowly to the electrode. In the equivalent circuit, it is represented as a capacitance in parallel to the charge transfer resistance (Figure 1b).
- Warburg impedance (W) is related to the diffusion (mass transport) of lithium ions into the solid electrode and electrolyte. On the Nyquist plot, it is represented by a sloped line. This region corresponds to the medium frequency range on the plot. The slope of the line reflects the diffusion coefficient of the species. A steeper slope indicates more difficult ion diffusion, while a shallower slope suggests easier mass transport and diffusion. The tail of this impedance is also significant. Tail extensions or deviations from the line indicate additional electrochemical processes occurring in the battery (Figure 1c).
2.2. Basic Types of Batteries
2.2.1. Lead-Acid
2.2.2. NiCd
2.2.3. Ni-MH
2.2.4. Li-Ion
2.3. Basic Geometries
2.3.1. Thin Film
2.3.2. 3D Porous Structure
2.4. Impact of 3D Printing on Battery Performance
- High Resolution and Mechanical Stability: The advent of 3D printing technology have revolutionized the precision and resolution of the battery designs which directly affects the energy and power density and the overall battery performance [90,91,92,93,94,95,96,97]. Furthermore, the ability to fabricate high-resolution geometries through 3D printing results in enhanced mechanical stability [94]. Engineering designs at the microscopic scale make it possible to control the battery structure precisely, ensuring enhanced mechanical performance. Battery properties, particularly during electrochemical reactions when components undergo changes that can impact structural integrity, benefit from mechanical stability [98]. With 3D printing advantages in high resolution, the risk of electrode breakage and battery failure due to structural instability is eliminated which increases the overall reliability of the battery [99].
- Energy density and power density: 3D printing with the ability to control the design makes it possible to increase the active material loading inside the structure in the less volume which results in higher energy density [100,101,102,103]. On the other hand, 3D printing ability to finely control the geometry of battery components plays a critical role in elevating this energy transfer rate within the structure, ultimately resulting in higher power density [104,105,106].
- Customizability and size: One of the advantages of 3D printing is the design control which leads to the customizability of the structure. Furthermore, depending on the method and the device resolution, the size can be controlled, and the part can be fabricated in a wide range of scales for the production of miniaturized batteries [107,108].
- Efficient Production Process: In contrast to the conventional method, which consist of multiple steps including slurry preparation, tape casting, material drying, calendaring, material cutting, assembly, electrolyte filling, and final packaging, 3D printing offers notable efficiency. In the 3D printing process, the steps include material preparation, part geometry design, 3D printing, assembly, and optional electrolyte filling, depending on the chosen 3D printing method [109,110,111,112]. One of the advantages of 3D printing in battery production is the potential reduction in fabrication time which is attributed to the straightforward process with fewer steps. Nevertheless, it is crucial to note that the overall fabrication time depends on the specific method employed and any post-treatment requirements [31].
- Ability to fabricate all-solid-state batteries: Solid-state batteries, utilizing solid electrolytes instead of liquid counterparts, offer high dimensional integrity, excellent mechanical properties, and enhanced safety [115]. 3D printing, with its precision and design control, facilitates the engineering and fabrication of solid-state electrolytes compatible with electrode configurations which results in the all-solid-state batteries through which all the components can be printed on top of each other. This approach eliminates the need for glove boxes, making production more cost-efficient and environmentally friendly [116,117,118,119].
2.5. Goals of Geometric Design for Batteries
- Energy density and power density: The design helps the user to fabricate the battery component based on the mechanical configuration of the device which makes it possible to customize the shape and size of the battery. With the design freedom, batteries can be fabricated with complicated integration and controlled distance between the components to receive the best properties of the battery. The 3D printed electrodes facilitate the ion transfer which results in high energy density and high power density [30,120,121].
- Cycle life and safety: The arrangement of electrodes and the distribution of active materials impact uniform charge and discharge cycles, thus affecting cycle life [122]. Additionally, the geometry can improve thermal management, preventing overheating and enhance safety [123]. Moreover, proper separator and electrolyte design, as well as internal pressure management mechanisms, contribute to safety and longevity [102,124].
3. Review
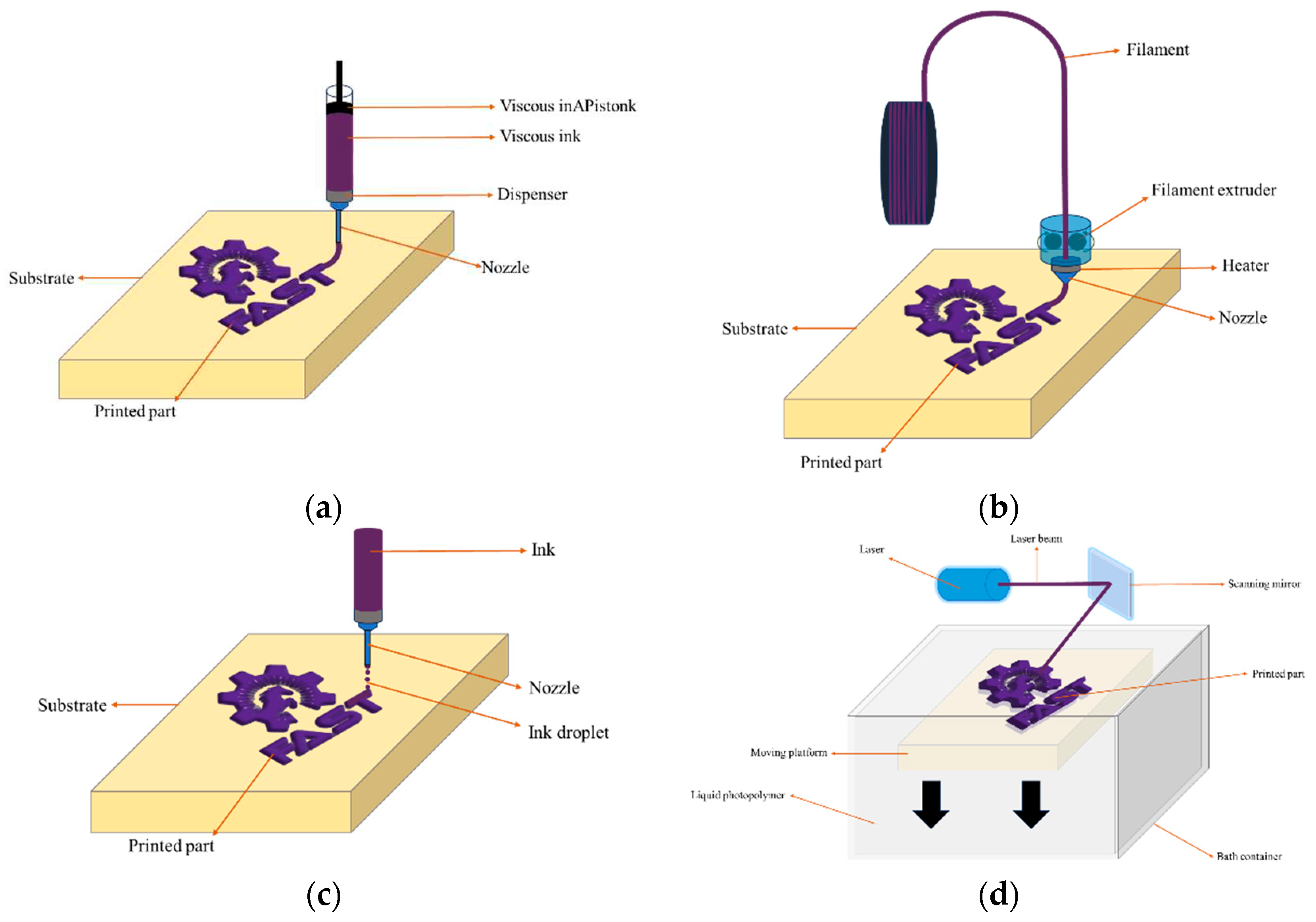
3.1. DIW
3.2. FFF
3.3. IJP
3.4. SLA
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Electricity in the, U.S. - U.S. Energy Information Administration (EIA). Available online: https://www.eia.gov/energyexplained/electricity/electricity-in-the-us.php (accessed on 19 January 2024).
- Pearce, J.M.; Parncutt, R. Quantifying Global Greenhouse Gas Emissions in Human Deaths to Guide Energy Policy. Energies 2023, 16, 6074. [Google Scholar] [CrossRef]
- D’Amato, G.; Cecchi, L. Effects of Climate Change on Environmental Factors in Respiratory Allergic Diseases. Clinical & Experimental Allergy 2008, 38, 1264–1274. [Google Scholar] [CrossRef]
- Haines, A.; Kovats, R.; Campbell-Lendrum, D.; Corvalan, C. Climate Change and Human Health: Impacts, Vulnerability, and Mitigation. The Lancet 2006, 367, 2101–2109. [Google Scholar] [CrossRef] [PubMed]
- Denkenberger, D.C.; Pearce, J.M. Feeding Everyone: Solving the Food Crisis in Event of Global Catastrophes That Kill Crops or Obscure the Sun. Futures 2015, 72, 57–68. [Google Scholar] [CrossRef]
- Barnes, D.F.; Floor, W.M. RURAL ENERGY IN DEVELOPING COUNTRIES: A Challenge for Economic Development. Annu. Rev. Energy. Environ. 1996, 21, 497–530. [Google Scholar] [CrossRef]
- Stern, N.H.; Treasury, G.B. The Economics of Climate Change: The Stern Review; Cambridge University Press, 2007; ISBN 978-0-521-70080-1. [Google Scholar]
- Peters, G.P.; Hertwich, E.G. CO 2 Embodied in International Trade with Implications for Global Climate Policy. Environ. Sci. Technol. 2008, 42, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Heidari, N.; Pearce, J.M. A Review of Greenhouse Gas Emission Liabilities as the Value of Renewable Energy for Mitigating Lawsuits for Climate Change Related Damages. Renewable and Sustainable Energy Reviews 2016, 55, 899–908. [Google Scholar] [CrossRef]
- Pryor, S.C.; Barthelmie, R.J. Climate Change Impacts on Wind Energy: A Review. Renewable and Sustainable Energy Reviews 2010, 14, 430–437. [Google Scholar] [CrossRef]
- Global Wind and Solar Energy Share in Electricity Mix 2022 Available online:. Available online: https://www.statista.com/statistics/1302047/global-wind-and-solar-energy-share-electricity-mix/ (accessed on 26 September 2023).
- Hub, I.S.K. Wind and Solar Will Provide 50% of Electricity in 2050, BNEF Report Finds | News | SDG Knowledge Hub | IISD.
- Yu, H.; Helland, H.; Yu, X.; Gundersen, T.; Sin, G. Optimal Design and Operation of an Organic Rankine Cycle (ORC) System Driven by Solar Energy with Sensible Thermal Energy Storage. Energy Conversion and Management 2021, 244, 114494. [Google Scholar] [CrossRef]
- Ali, U. Bloomberg New Energy Outlook 2019: The Future of the Energy Sector. Power Technology 2019.
- Adeh, E.H.; Good, S.P.; Calaf, M.; Higgins, C.W. Solar PV Power Potential Is Greatest Over Croplands. Sci Rep 2019, 9, 11442. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Orosz, M.S.; Kumar, P. Thermo-Economic Evaluation of ORCs for Various Working Fluids. Applied Thermal Engineering 2016, 109, 841–853. [Google Scholar] [CrossRef]
- Zhu, Z.; Jiang, T.; Ali, M.; Meng, Y.; Jin, Y.; Cui, Y.; Chen, W. Rechargeable Batteries for Grid Scale Energy Storage. Chem. Rev. 2022, 122, 16610–16751. [Google Scholar] [CrossRef] [PubMed]
- Kebede, A.A.; Kalogiannis, T.; Van Mierlo, J.; Berecibar, M. A Comprehensive Review of Stationary Energy Storage Devices for Large Scale Renewable Energy Sources Grid Integration. Renewable and Sustainable Energy Reviews 2022, 159, 112213. [Google Scholar] [CrossRef]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling Lithium-Ion Batteries from Electric Vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Nair, N.-K.C.; Garimella, N. Battery Energy Storage Systems: Assessment for Small-Scale Renewable Energy Integration. Energy and Buildings 2010, 42, 2124–2130. [Google Scholar] [CrossRef]
- Joseph, P.K.; Devaraj, E. Design of Hybrid Forward Boost Converter for Renewable Energy Powered Electric Vehicle Charging Applications. IET Power Electronics 2019, 12, 2015–2021. [Google Scholar] [CrossRef]
- Ransome, T. Lithium Ion Battery. Available online: https://www.renewables4u.com.au/lithium-ion-battery/ (accessed on 19 January 2024).
- Mottaghi, M.; Rahman, M.; Kulkarni, A.; Pearce, J.M. AC/off-Grid Photovoltaic Powered Open-Source Ball Mill. HardwareX 2023, 14, e00423. [Google Scholar] [CrossRef] [PubMed]
- Mottaghi, M.; Bai, Y.; Kulkarni, A.; Pearce, J.M. Open Source Scientific Bottle Roller. HardwareX 2023, 15, e00445. [Google Scholar] [CrossRef]
- Dhankani, K.C.; Pearce, J.M. Open Source Laboratory Sample Rotator Mixer and Shaker. HardwareX 2017, 1, 1–12. [Google Scholar] [CrossRef]
- Vadivel, D.; Branciforti, D.S.; Kerroumi, O.; Dondi, M.; Dondi, D. Mostly 3D Printed Chemical Synthesis Robot. HardwareX 2022, 11, e00310. [Google Scholar] [CrossRef] [PubMed]
- Wittbrodt, B.T.; Glover, A.G.; Laureto, J.; Anzalone, G.C.; Oppliger, D.; Irwin, J.L.; Pearce, J.M. Life-Cycle Economic Analysis of Distributed Manufacturing with Open-Source 3-D Printers. Mechatronics 2013, 23, 713–726. [Google Scholar] [CrossRef]
- Pearce, J.M. Open-Source Lab: How to Build Your Own Hardware and Reduce Research Costs. Elsevier, 2013; ISBN 978-0-12-410486-0. [Google Scholar]
- Laplume, A.O.; Petersen, B.; Pearce, J.M. Global Value Chains from a 3D Printing Perspective. J Int Bus Stud 2016, 47, 595–609. [Google Scholar] [CrossRef]
- Lyu, Z.; Lim, G.J.H.; Koh, J.J.; Li, Y.; Ma, Y.; Ding, J.; Wang, J.; Hu, Z.; Wang, J.; Chen, W.; et al. Design and Manufacture of 3D-Printed Batteries. Joule 2021, 5, 89–114. [Google Scholar] [CrossRef]
- Pang, Y.; Cao, Y.; Chu, Y.; Liu, M.; Snyder, K.; MacKenzie, D.; Cao, C. Additive Manufacturing of Batteries. Adv. Funct. Mater. 2020, 30, 1906244. [Google Scholar] [CrossRef]
- Battery Technology Handbook.
- HISTORY | Primary Batteries. 2009; 555–564. [CrossRef]
- PRIMARY BATTERIES | Overview. 2009; 22–27. [CrossRef]
- When to Use Rechargeable Batteries. Available online: https://www.consumerreports.org/electronics-computers/batteries/when-to-use-rechargeable-batteries-a1076298884/ (accessed on 14 January 2024).
- Lopes, P.P.; Stamenkovic, V.R. Past, Present, and Future of Lead–Acid Batteries. Science 2020, 369, 923–924. [Google Scholar] [CrossRef] [PubMed]
- The Role of Energy Storage in Low-Carbon Energy Systems. In Storing Energy; Elsevier, 2016; pp. 3–22.
- Viswanathan, B. Chapter 12 - Batteries. In Energy Sources; Viswanathan, B., Ed.; Elsevier: Amsterdam, 2017; pp. 263–313. ISBN 978-0-444-56353-8. [Google Scholar]
- Burheim, O.S. Secondary Batteries. In Engineering Energy Storage; Elsevier, 2017; pp. 111–145. ISBN 978-0-12-814100-7. [Google Scholar]
- May, G.J.; Davidson, A.; Monahov, B. Lead Batteries for Utility Energy Storage: A Review. Journal of Energy Storage 2018, 15, 145–157. [Google Scholar] [CrossRef]
- A General Discussion of Li Ion Battery Safety. Electrochemical Society Interface 2012. [CrossRef]
- Flora, G.; Gupta, D.; Tiwari, A. Toxicity of Lead: A Review with Recent Updates. Interdisciplinary Toxicology 2012, 5, 47–58. [Google Scholar] [CrossRef]
- Posada, J.O.G.; Rennie, A.J.R.; Villar, S.P.; Martins, V.L.; Marinaccio, J.; Barnes, A.; Glover, C.F.; Worsley, D.A.; Hall, P.J. Aqueous Batteries as Grid Scale Energy Storage Solutions. Renewable and Sustainable Energy Reviews 2017, 68, 1174–1182. [Google Scholar] [CrossRef]
- Quansah, D.A. Comparative Study of Electricity Storage Batteries for Solar Photovoltaic Home Systems. Doctoral dissertation, 2008. [Google Scholar]
- Marin-Garcia, G.; Vazquez-Guzman, G.; Sosa, J.M.; Lopez, A.R.; Martinez-Rodriguez, P.R.; Langarica, D. Battery Types and Electrical Models: A Review. In Proceedings of the 2020 IEEE International Autumn Meeting on Power, Electronics and Computing (ROPEC); IEEE: Ixtapa, Mexico, 4 November 2020; pp. 1–6. [Google Scholar]
- Poullikkas, A. A Comparative Overview of Large-Scale Battery Systems for Electricity Storage. Renewable and Sustainable Energy Reviews 2013, 27, 778–788. [Google Scholar] [CrossRef]
- Ramachandra Rao, S. Resource Recovery from Process Wastes. In Waste Management Series; Elsevier, 2006; Volume 7, pp. 375–457. ISBN 978-0-08-045131-2. [Google Scholar]
- Beaudin, M.; Zareipour, H.; Schellenberg, A.; Rosehart, W. Energy Storage for Mitigating the Variability of Renewable Electricity Sources. In Energy Storage for Smart Grids; Elsevier; pp. 1–33. ISBN 978-0-12-410491-4.
- Avril, S.; Arnaud, G.; Florentin, A.; Vinard, M. Multi-Objective Optimization of Batteries and Hydrogen Storage Technologies for Remote Photovoltaic Systems. Energy 2010, 35, 5300–5308. [Google Scholar] [CrossRef]
- Divya, K.C.; Østergaard, J. Battery Energy Storage Technology for Power Systems—An Overview. Electric Power Systems Research 2009, 79, 511–520. [Google Scholar] [CrossRef]
- Parker, C.D. APPLICATIONS – STATIONARY | Energy Storage Systems: Batteries. In Encyclopedia of Electrochemical Power Sources; Elsevier, 2009; pp. 53–64. ISBN 978-0-444-52745-5. [Google Scholar]
- Lemaire-Potteau, E.; Perrin, M.; Genies, S. BATTERIES | Charging Methods. In Encyclopedia of Electrochemical Power Sources; Elsevier, 2009; pp. 413–423. ISBN 978-0-444-52745-5. [Google Scholar]
- Zhu, W.H.; Zhu, Y.; Davis, Z.; Tatarchuk, B.J. Energy Efficiency and Capacity Retention of Ni–MH Batteries for Storage Applications. Applied Energy 2013, 106, 307–313. [Google Scholar] [CrossRef]
- Abdin, Z.; Khalilpour, K.R. Single and Polystorage Technologies for Renewable-Based Hybrid Energy Systems. In Polygeneration with Polystorage for Chemical and Energy Hubs; Elsevier, 2019; pp. 77–131. ISBN 978-0-12-813306-4. [Google Scholar]
- Iclodean, C.; Varga, B.; Burnete, N.; Cimerdean, D.; Jurchiş, B. Comparison of Different Battery Types for Electric Vehicles. IOP Conf. Ser.: Mater. Sci. Eng. 2017, 252, 012058. [Google Scholar] [CrossRef]
- Bernard, P.; Lippert, M. 2015- Nickel–Cadmium and Nickel–Metal Hydride Battery Energy Storage. In Electrochemical Energy Storage for Renewable Sources and Grid Balancing; Elsevier, 2015; pp. 223–251. ISBN 978-0-444-62616-5. [Google Scholar]
- German, J.M. Hybrid Electric Vehicles. In Encyclopedia of Energy; Elsevier, 2004; pp. 197–213 ISBN 978-0-12-176480-7.
- Tsais, P.-J.; Chan, L.I. 2013- Nickel-Based Batteries: Materials and Chemistry. In Electricity Transmission, Distribution and Storage Systems; Elsevier, 2013; pp. 309–397 ISBN 978-1-84569-784-6.
- Aktaş, A.; Kirçiçek, Y. Solar Hybrid Systems and Energy Storage Systems. In Solar Hybrid Systems; Elsevier, 2021; pp. 87–125 ISBN 978-0-323-88499-0.
- Cao, C.; Steinrück, H.-G. 2023- Molecular-Scale Synchrotron X-Ray Investigations of Solid-Liquid Interfaces in Lithium-Ion Batteries. In Encyclopedia of Solid-Liquid Interfaces; Elsevier, 2024; pp. 391–416 ISBN 978-0-323-85670-6.
- Soto, A.; Berrueta, A.; Mateos, M.; Sanchis, P.; Ursúa, A. 2022- Impact of Micro-Cycles on the Lifetime of Lithium-Ion Batteries: An Experimental Study. Journal of Energy Storage 2022, 55, 105343. [Google Scholar] [CrossRef]
- Keçili, R.; Arli, G.; Hussain, C.M. 2020- Future of Analytical Chemistry with Graphene. In Comprehensive Analytical Chemistry; Elsevier, 2020; Vol. 91, pp. 355–389 ISBN 978-0-323-85371-2.
- Lei, H.; Han, Y.Y. 2019- The Measurement and Analysis for Open Circuit Voltage of Lithium-Ion Battery. J. Phys.: Conf. Ser. 2019, 1325, 012173. [Google Scholar] [CrossRef]
- Gao, X.; Liu, K.; Su, C.; Zhang, W.; Dai, Y.; Parkin, I.P.; Carmalt, C.J.; He, G. From Bibliometric Analysis: 3D Printing Design Strategies and Battery Applications with a Focus on Zinc-Ion Batteries. SmartMat 2024, 5, e1197. [Google Scholar] [CrossRef]
- Torabi, F.; Ahmadi, P. Battery Technologies. In Simulation of Battery Systems; Elsevier, 2020; pp. 1–54 ISBN 978-0-12-816212-5.
- Delannoy, P.-E.; Riou, B.; Brousse, T.; Le Bideau, J.; Guyomard, D.; Lestriez, B. Ink-Jet Printed Porous Composite LiFePO4 Electrode from Aqueous Suspension for Microbatteries. Journal of Power Sources 2015, 287, 261–268. [Google Scholar] [CrossRef]
- Zhou, L.; Ning, W.; Wu, C.; Zhang, D.; Wei, W.; Ma, J.; Li, C.; Chen, L. 3D-Printed Microelectrodes with a Developed Conductive Network and Hierarchical Pores toward High Areal Capacity for Microbatteries. Advanced Materials Technologies 2019, 4. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, C.; Xie, H.; Gao, T.; Yao, Y.; Pastel, G.; Han, X.; Li, Y.; Zhao, J.; Fu, K. (Kelvin); et al. 3D-Printed All-Fiber Li-Ion Battery toward Wearable Energy Storage. Advanced Functional Materials 2017, 27, 1703140. [Google Scholar] [CrossRef]
- Huang, J.; Yang, J.; Li, W.; Cai, W.; Jiang, Z. Electrochemical Properties of LiCoO2 Thin Film Electrode Prepared by Ink-Jet Printing Technique. Thin Solid Films 2008, 516, 3314–3319. [Google Scholar] [CrossRef]
- Solid State Thin-Film Lithium Battery Systems. Current Opinion in Solid State and Materials Science 1999, 4, 479–482. [CrossRef]
- Schwenzel, J.; Thangadurai, V.; Weppner, W. Developments of High-Voltage All-Solid-State Thin-Film Lithium Ion Batteries. Journal of Power Sources 2006, 154, 232–238. [Google Scholar] [CrossRef]
- Clement, B.; Lyu, M.; Sandeep Kulkarni, E.; Lin, T.; Hu, Y.; Lockett, V.; Greig, C.; Wang, L. Recent Advances in Printed Thin-Film Batteries. Engineering 2022, 13, 238–261. [Google Scholar] [CrossRef]
- Ding, J.; Shen, K.; Du, Z.; Li, B.; Yang, S. 3D-Printed Hierarchical Porous Frameworks for Sodium Storage. ACS Appl. Mater. Interfaces 2017, 9, 41871–41877. [Google Scholar] [CrossRef] [PubMed]
- Additive Manufacturing Enabled, Microarchitected, Hierarchically Porous Polylactic-Acid/Lithium Iron Phosphate/Carbon Nanotube Nanocomposite Electrodes for High Performance Li-Ion Batteries. Journal of Power Sources 2021, 494, 229625. [CrossRef]
- Saleh, M.S.; Li, J.; Park, J.; Panat, R. 3D Printed Hierarchically-Porous Microlattice Electrode Materials for Exceptionally High Specific Capacity and Areal Capacity Lithium Ion Batteries. Additive Manufacturing 2018, 23, 70–78. [Google Scholar] [CrossRef]
- Liu, C.; Qiu, Y.; Liu, Y.; Xu, K.; Zhao, N.; Lao, C.; Shen, J.; Chen, Z. Novel 3D Grid Porous Li4Ti5O12 Thick Electrodes Fabricated by 3D Printing for High Performance Lithium-Ion Batteries. J Adv Ceram 2022, 11, 295–307. [Google Scholar] [CrossRef]
- Liu, C.; Cheng, X.; Li, B.; Chen, Z.; Mi, S.; Lao, C. Fabrication and Characterization of 3D-Printed Highly-Porous 3D LiFePO4 Electrodes by Low Temperature Direct Writing Process. Materials 2017, 10, 934. [Google Scholar] [CrossRef]
- Martinez, A.C.; Maurel, A.; Aranzola, A.P.; Grugeon, S.; Panier, S.; Dupont, L.; Hernandez-Viezcas, J.A.; Mummareddy, B.; Armstrong, B.L.; Cortes, P.; et al. Additive Manufacturing of LiNi1/3Mn1/3Co1/3O2 Battery Electrode Material via Vat Photopolymerization Precursor Approach. Sci Rep 2022, 12, 1–13. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, Q.; Ling, W.; Dai, H.; Chen, H.; Liu, J.; Qiu, Y.; Zhong, L. Porous Electrode Materials for Zn-Ion Batteries: From Fabrication and Electrochemical Application. Batteries 2022, 8, 223. [Google Scholar] [CrossRef]
- Yang, Y.; Ai, L.; Yu, S.; He, J.; Xu, T.; Chen, D.; Shen, L. 3D-Printed Porous GO Framework Enabling Dendrite-Free Lithium-Metal Anodes. ACS Applied Energy Materials 2022. [Google Scholar] [CrossRef]
- Chen, C.; Li, S.; Notten, P.H.L.; Zhang, Y.; Hao, Q.; Zhang, X.; Lei, W. 3D Printed Lithium-Metal Full Batteries Based on a High-Performance Three-Dimensional Anode Current Collector. ACS Appl. Mater. Interfaces 2021, 13, 24785–24794. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, L.; Lin, Q.; Tang, M.; Wu, Y.; Ke, C. Hierarchical-Coassembly-Enabled 3D-Printing of Homogeneous and Heterogeneous Covalent Organic Frameworks. Journal of the American Chemical Society 2019. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Chu, Y.; Pan, L.; Wu, B.; Zou, L.; He, J.; Han, M.; Zhao, T.; Zeng, L. 3D Printing Critical Materials for Rechargeable Batteries: From Materials, Design and Optimization Strategies to Applications. Int. J. Extrem. Manuf. 2023, 5, 042008. [Google Scholar] [CrossRef]
- Menon, A.; Khan, A.; Balakrishnan, N.T.M.; Raghavan, P.; Leon Y Leon, C.A.; Khan, H.A.; Fatima, M.J.J.; Owuor, P.S. Advances in 3D Printing for Electrochemical Energy Storage Systems. J. Mater. Sci. Technol. Res. 2021, 8, 50–69. [Google Scholar] [CrossRef]
- Thakur, A.R.; Dong, X. Experimental and Numerical Studies of Slurry-Based Coextrusion Deposition of Continuous Carbon Fiber Micro-Batteries to Additively Manufacture 3D Structural Battery Composites. Composites Part B: Engineering 2023, 255, 110632. [Google Scholar] [CrossRef]
- Cheng, M. Direct Ink Writing of Polymer Batteries. Ph.D., University of Illinois at Chicago: United States -- Illinois, 2020.
- Ragones, H.; Menkin, S.; Kamir, Y.; Gladkikh, A.; Mukra, T.; Kosa, G.; Golodnitsky, D. Towards Smart Free Form-Factor 3D Printable Batteries. Sustainable Energy & Fuels 2018, 2, 1542–1549. [Google Scholar] [CrossRef]
- Ponnada, S.; Babu Gorle, D.; Chandra Bose, R.S.; Sadat Kiai, M.; Devi, M.; Venkateswara Raju, C.; Baydogan, N.; Kar Nanda, K.; Marken, F.; Sharma, R.K. Current Insight into 3D Printing in Solid-State Lithium-Ion Batteries: A Perspective. Batteries & Supercaps 2022, 5, e202200223. [Google Scholar] [CrossRef]
- Nyika, J.; Mwema, F.M.; Mahamood, R.M.; Akinlabi, E.T.; Jen, T. Advances in 3D Printing Materials Processing-Environmental Impacts and Alleviation Measures. Advances in Materials and Processing Technologies 2022, 8, 1275–1285. [Google Scholar] [CrossRef]
- Mao, M.; He, J.; Li, X.; Zhang, B.; Lei, Q.; Liu, Y.; Li, D. The Emerging Frontiers and Applications of High-Resolution 3D Printing. Micromachines 2017, 8, 113. [Google Scholar] [CrossRef]
- Park, Y.-G.; Yun, I.; Chung, W.G.; Park, W.; Lee, D.H.; Park, J.-U. High-Resolution 3D Printing for Electronics. Advanced Science 2022, 9, 2104623. [Google Scholar] [CrossRef]
- Ahn, D.; Stevens, L.M.; Zhou, K.; Page, Z.A. Rapid High-Resolution Visible Light 3D Printing. ACS Central Science 2020. [Google Scholar] [CrossRef]
- High-Resolution PLA-Based Composite Scaffolds via 3-D Printing Technology. Acta Biomaterialia 2013, 9, 5521–5530. [CrossRef]
- Toward High Resolution 3D Printing of Shape-Conformable Batteries via Vat Photopolymerization: Review and Perspective. Available online: https://ieeexplore.ieee.org/abstract/document/9568946/ (accessed on 27 October 2023).
- Fonseca, N.; Thummalapalli, S.V.; Jambhulkar, S.; Ravichandran, D.; Zhu, Y.; Patil, D.; Thippanna, V.; Ramanathan, A.; Xu, W.; Guo, S.; et al. 3D Printing-Enabled Design and Manufacturing Strategies for Batteries: A Review. Small 2023, 2302718. [Google Scholar] [CrossRef]
- Emerging Application of 3D-Printing Techniques in Lithium Batteries: From Liquid to Solid. Materials Today 2022, 59, 161–181. [CrossRef]
- 3D Printing for Rechargeable Lithium Metal Batteries. Energy Storage Materials 2021, 38, 141–156. [CrossRef]
- Mu, T.; Xiang, L.; Wan, X.; Lou, S.; Du, C.; Zuo, P.; Yin, G. Ultrahigh Areal Capacity Silicon Anodes Realized via Manipulating Electrode Structure. Energy Storage Materials 2022, 53, 958–968. [Google Scholar] [CrossRef]
- Zhang, M.; Mei, H.; Chang, P.; Cheng, L. 3D Printing of Structured Electrodes for Rechargeable Batteries. J. Mater. Chem. A 2020, 8, 10670–10694. [Google Scholar] [CrossRef]
- Lyu, Z.; Lim, G.J.H.; Guo, R.; Kou, Z.; Wang, T.; Guan, C.; Ding, J.; Chen, W.; Wang, J. 3D-Printed MOF-Derived Hierarchically Porous Frameworks for Practical High-Energy Density Li–O2 Batteries. Advanced Functional Materials 2019, 29, 1806658. [Google Scholar] [CrossRef]
- Gao, X.; Yang, X.; Wang, S.; Sun, Q.; Zhao, C.; Li, X.; Liang, J.; Zheng, M.; Zhao, Y.; Wang, J.; et al. A 3D-Printed Ultra-High Se Loading Cathode for High Energy Density Quasi-Solid-State Li–Se Batteries. Journal of Materials Chemistry A 2020, 8, 278–286. [Google Scholar] [CrossRef]
- All 3D Printing Lithium Metal Batteries with Hierarchically and Conductively Porous Skeleton for Ultrahigh Areal Energy Density. Energy Storage Materials 2023, 54, 304–312. [CrossRef]
- 3D Printing of Fast Kinetics Reconciled Ultra-Thick Cathodes for High Areal Energy Density Aqueous Li–Zn Hybrid Battery. Science Bulletin 2022, 67, 1253–1263. [CrossRef]
- Wang, J.; Sun, Q.; Gao, X.; Wang, C.; Li, W.; Holness, F.B.; Zheng, M.; Li, R.; Price, A.D.; Sun, X.; et al. Toward High Areal Energy and Power Density Electrode for Li-Ion Batteries via Optimized 3D Printing Approach. ACS Appl. Mater. Interfaces 2018, 10, 39794–39801. [Google Scholar] [CrossRef] [PubMed]
- Marschewski, J.; Brenner, L.; Ebejer, N.; Ruch, P.; Michel, B.; Poulikakos, D. 3D-Printed Fluidic Networks for High-Power-Density Heat-Managing Miniaturized Redox Flow Batteries. Energy & Environmental Science 2017, 10, 780–787. [Google Scholar] [CrossRef]
- Li, J.; Du, Z.; Ruther, R.E.; An, S.J.; David, L.A.; Hays, K.; Wood, M.; Phillip, N.D.; Sheng, Y.; Mao, C.; et al. Toward Low-Cost, High-Energy Density, and High-Power Density Lithium-Ion Batteries. JOM 2017, 69, 1484–1496. [Google Scholar] [CrossRef]
- Wei, T.-S.; Ahn, B.Y.; Grotto, J.; Lewis, J.A. 3D Printing of Customized Li-Ion Batteries with Thick Electrodes. Advanced Materials 2018, 30, 1703027. [Google Scholar] [CrossRef]
- Shi, H.; Cao, J.; Sun, Z.; Ghazi, Z.A.; Zhu, X.; Han, S.; Ren, D.; Lu, G.; Lan, H.; Li, F. 3D Printing Enables Customizable Batteries. Batteries & Supercaps 2023, 6, e202300161. [Google Scholar] [CrossRef]
- Design and Fabrication of Multifunctional Structural Batteries. Journal of Power Sources 2009, 189, 646–650. [CrossRef]
- Robust Free-Standing Electrodes for Flexible Lithium-Ion Batteries Prepared by a Conventional Electrode Fabrication Process. Electrochimica Acta 2017, 247, 371–380. [CrossRef]
- Roberts, M.; Johns, P.; Owen, J.; Brandell, D.; Edstrom, K.; Enany, G.E.; Guery, C.; Golodnitsky, D.; Lacey, M.; Lecoeur, C.; et al. 3D Lithium Ion Batteries—from Fundamentals to Fabrication. Journal of Materials Chemistry 2011, 21, 9876–9890. [Google Scholar] [CrossRef]
- Hao, F.; Han, F.; Liang, Y.; Wang, C.; Yao, Y. Architectural Design and Fabrication Approaches for Solid-State Batteries. MRS Bulletin 2018, 43, 775–781. [Google Scholar] [CrossRef]
- Bhosale, V.S.; Gaikwad, P.M.; Maladkar, N.P.; Desai, K.V. A Review on Use of 3D Printing for Battery Manufacturing. 2022.
- Comprehensive Review on Various Additive Manufacturing Techniques and Its Implementation in Electronic Devices. Journal of Manufacturing Systems 2022, 62, 477–502. [CrossRef]
- Bates, A.M.; Preger, Y.; Torres-Castro, L.; Harrison, K.L.; Harris, S.J.; Hewson, J. Are Solid-State Batteries Safer than Lithium-Ion Batteries? Joule 2022, 6, 742–755. [Google Scholar] [CrossRef]
- He, Y.; Chen, S.; Nie, L.; Sun, Z.; Wu, X.; Liu, W. Stereolithography Three-Dimensional Printing Solid Polymer Electrolytes for All-Solid-State Lithium Metal Batteries. Nano Lett. 2020, 20, 7136–7143. [Google Scholar] [CrossRef] [PubMed]
- Zekoll, S.; Marriner-Edwards, C.; Hekselman, A.K.O.; Kasemchainan, J.; Kuss, C.; Armstrong, D.E.J.; Cai, D.; Wallace, R.J.; Richter, F.H.; Thijssen, J.H.J.; et al. Hybrid Electrolytes with 3D Bicontinuous Ordered Ceramic and Polymer Microchannels for All-Solid-State Batteries. Energy Environ. Sci. 2018, 11, 185–201. [Google Scholar] [CrossRef]
- Processing and Manufacturing of next Generation Lithium-Based All Solid-State Batteries. Current Opinion in Solid State and Materials Science 2022, 26, 101003. [CrossRef]
- Schnell, J.; Tietz, F.; Singer, C.; Hofer, A.; Billot, N.; Reinhart, G. Prospects of Production Technologies and Manufacturing Costs of Oxide-Based All-Solid-State Lithium Batteries. Energy & Environmental Science 2019, 12, 1818–1833. [Google Scholar] [CrossRef]
- Li, H.; Liang, J. Recent Development of Printed Micro-Supercapacitors: Printable Materials, Printing Technologies, and Perspectives. Adv. Mater. 2020, 32, 1805864. [Google Scholar] [CrossRef]
- Lin, Y.; Gao, Y.; Fang, F.; Fan, Z. Recent Progress on Printable Power Supply Devices and Systems with Nanomaterials. Nano Res. 2018, 11, 3065–3087. [Google Scholar] [CrossRef]
- Ma, J.; Zheng, S.; Chi, L.; Liu, Y.; Zhang, Y.; Wang, K.; Wu, Z.-S. 3D Printing Flexible Sodium-Ion Microbatteries with Ultrahigh Areal Capacity and Robust Rate Capability. Advanced Materials 2022, 34, 2205569. [Google Scholar] [CrossRef]
- Thermal Management of Lithium-Ion Battery Cells Using 3D Printed Phase Change Composites. Applied Thermal Engineering 2020, 171, 115126. [CrossRef]
- 3D Printed Separator for the Thermal Management of High-Performance Li Metal Anodes. Energy Storage Materials 2018, 12, 197–203. [CrossRef]
- Lewis, J.A. Direct Ink Writing of 3D Functional Materials. Adv. Funct. Mater. 2006, 16, 2193–2204. [Google Scholar] [CrossRef]
- Tagliaferri, S.; Panagiotopoulos, A.; Mattevi, C. Direct Ink Writing of Energy Materials. Materials Advances 2021, 2, 540–563. [Google Scholar] [CrossRef]
- Saadi, M. a. S.R.; Maguire, A.; Pottackal, N.T.; Thakur, M.S.H.; Ikram, M.M.; Hart, A.J.; Ajayan, P.M.; Rahman, M.M. Direct Ink Writing: A 3D Printing Technology for Diverse Materials. Advanced Materials 2022, 34, 2108855. [Google Scholar] [CrossRef] [PubMed]
- High-Precision Resistance Strain Sensors of Multilayer Composite Structure via Direct Ink Writing: Optimized Layer Flatness and Interfacial Strength. Composites Science and Technology 2021, 201, 108530. [CrossRef]
- Chen, B.; Willenbacher, N. High-Precision Direct Ink Writing of Li6.4La3Zr1.4Ta0.6O12. Journal of the European Ceramic Society 2022, 42, 7491–7500. [Google Scholar] [CrossRef]
- Loaldi, D.; Piccolo, L.; Brown, E.; Tosello, G.; Shemelya, C.; Masato, D. Hybrid Process Chain for the Integration of Direct Ink Writing and Polymer Injection Molding. Micromachines 2020, 11. [Google Scholar] [CrossRef]
- Yuk, H.; Zhao, X. A New 3D Printing Strategy by Harnessing Deformation, Instability, and Fracture of Viscoelastic Inks. Advanced Materials 2017, 30. [Google Scholar] [CrossRef] [PubMed]
- Rocha, V.G.; Saiz, E.; Tirichenko, I.S.; García-Tuñón, E. Direct Ink Writing Advances in Multi-Material Structures for a Sustainable Future. J. Mater. Chem. A 2020, 8, 15646–15657. [Google Scholar] [CrossRef]
- Yirmibesoglu, O.D.; Simonsen, L.E.; Manson, R.; Davidson, J.; Healy, K.; Menguc, Y.; Wallin, T. Multi-Material Direct Ink Writing of Photocurable Elastomeric Foams. Commun Mater 2021, 2, 1–14. [Google Scholar] [CrossRef]
- Xu, C.; Quinn, B.; Lebel, L.L.; Therriault, D.; L’Espérance, G. Multi-Material Direct Ink Writing (DIW) for Complex 3D Metallic Structures with Removable Supports. ACS Appl. Mater. Interfaces 2019, 11, 8499–8506. [Google Scholar] [CrossRef] [PubMed]
- Renteria, A.; Balcorta, V.H.; Marquez, C.; Rodriguez, A.A.; Renteria-Marquez, I.; Regis, J.; Wilburn, B.; Patterson, S.; Espalin, D.; Tseng, T.-L. (Bill); et al. Direct Ink Write Multi-Material Printing of PDMS-BTO Composites with MWCNT Electrodes for Flexible Force Sensors. Flex. Print. Electron. 2022, 7, 015001. [Google Scholar] [CrossRef]
- Cadiou, T.; Demoly, F.; Gomes, S. A Hybrid Additive Manufacturing Platform Based on Fused Filament Fabrication and Direct Ink Writing Techniques for Multi-Material 3D Printing. Int J Adv Manuf Technol 2021, 114, 3551–3562. [Google Scholar] [CrossRef]
- Mantelli, A.; Romani, A.; Suriano, R.; Levi, M.; Turri, S. Direct Ink Writing of Recycled Composites with Complex Shapes: Process Parameters and Ink Optimization. Advanced Engineering Materials 2021, 23. [Google Scholar] [CrossRef]
- Wei, M.; Zhang, F.; Wang, W.; Alexandridis, P.; Zhou, C.; Wu, G. 3D Direct Writing Fabrication of Electrodes for Electrochemical Storage Devices. Journal of Power Sources 2017, 354. [Google Scholar] [CrossRef]
- Li, Q.; Dong, Q.; Wang, J.; Xue, Z.; Li, J.; Yu, M.; Zhang, T.; Wan, Y.; Sun, H. Direct Ink Writing (DIW) of Graphene Aerogel Composite Electrode for Vanadium Redox Flow Battery. Journal of Power Sources 2022, 542, 231810. [Google Scholar] [CrossRef]
- Zhu, C.; Schorr, N.B.; Qi, Z.; Wygant, B.R.; Turney, D.E.; Yadav, G.G.; Worsley, M.A.; Duoss, E.B.; Banerjee, S.; Spoerke, E.D.; et al. Direct Ink Writing of 3D Zn Structures as High-Capacity Anodes for Rechargeable Alkaline Batteries. Small Structures 2023, 4, 2200323. [Google Scholar] [CrossRef]
- Liu, Z.; Tian, X.; Liu, M.; Duan, S.; Ren, Y.; Ma, H.; Tang, K.; Shi, J.; Hou, S.; Jin, H.; et al. Direct Ink Writing of Li 1.3 Al 0.3 Ti 1.7 (PO 4 ) 3 -Based Solid-State Electrolytes with Customized Shapes and Remarkable Electrochemical Behaviors. Small 2021, 17, 2002866. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Gu, Y.; Sharma, J.; Hong, K.; Li, J. A Conformal Heat-Drying Direct Ink Writing 3D Printing for High-Performance Lithium-Ion Batteries. Materials Today Chemistry 2023, 32, 101672. [Google Scholar] [CrossRef]
- Li, L.; Tan, H.; Yuan, X.; Ma, H.; Ma, Z.; Zhao, Y.; Zhao, J.; Wang, X.; Chen, D.; Dong, Y. Direct Ink Writing Preparation of LiFePO4/MWCNTs Electrodes with High-Areal Li-Ion Capacity. Ceramics International 2021, 47, 21161–21166. [Google Scholar] [CrossRef]
- Rasul, M.G.; Cheng, M.; Jiang, Y.; Pan, Y.; Shahbazian-Yassar, R. Direct Ink Printing of PVdF Composite Polymer Electrolytes with Aligned BN Nanosheets for Lithium-Metal Batteries. ACS Nanosci. Au 2022, acsnanoscienceau.1c00056. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, N.; Xu, K.; Li, Y.; Mwizerwa, J.P.; Shen, J.; Chen, Z. High-Performance LiFePO4 and SiO@C/Graphite Interdigitated Full Lithium-Ion Battery Fabricated via Low Temperature Direct Write 3D Printing. Materials Today Energy 2022, 29, 101098. [Google Scholar] [CrossRef]
- FDM, vs. FFF: Differences and Comparison. Available online: https://www.xometry.com/resources/3d-printing/fdm-vs-fff-3d-printing/ (accessed on 24 January 2024).
- Jones, R.; Haufe, P.; Sells, E.; Iravani, P.; Olliver, V.; Palmer, C.; Bowyer, A. RepRap – the Replicating Rapid Prototyper. Robotica 2011, 29, 177–191. [Google Scholar] [CrossRef]
- Sells, E.; Bailard, S.; Smith, Z.; Bowyer, A.; Olliver, V. RepRap: The Replicating Rapid Prototyper: Maximizing Customizability by Breeding the Means of Production. In Handbook of Research in Mass Customization and Personalization; World Scientific Publishing Company, 2009; pp. 568–580 ISBN 978-981-4280-25-9.
- Bowyer, A. 3D Printing and Humanity’s First Imperfect Replicator. 3D Printing and Additive Manufacturing 2014, 1, 4–5. [Google Scholar] [CrossRef]
- Reyes, C.; Somogyi, R.; Niu, S.; Cruz, M.A.; Yang, F.; Catenacci, M.J.; Rhodes, C.P.; Wiley, B.J. Three-Dimensional Printing of a Complete Lithium Ion Battery with Fused Filament Fabrication. ACS Appl. Energy Mater. 2018, acsaem.8b00885. [Google Scholar] [CrossRef]
- 3D Printing a Complete Lithium Ion Battery with Fused Filament Fabrication | Request PDF Available online:. Available online: https://www.researchgate.net/publication/327735139_3D_Printing_a_Complete_Lithium_Ion_Battery_with_Fused_Filament_Fabrication (accessed on 2 November 2023).
- Mecheter, A.; Tarlochan, F. Fused Filament Fabrication Three-Dimensional Printing: Assessing the Influence of Geometric Complexity and Process Parameters on Energy and the Environment. Sustainability 2023, 15, 12319. [Google Scholar] [CrossRef]
- Sola, A. Materials Requirements in Fused Filament Fabrication: A Framework for the Design of Next-Generation 3D Printable Thermoplastics and Composites. Macromolecular Materials and Engineering 2022, 307, 2200197. [Google Scholar] [CrossRef]
- Maurel, A.; Courty, M.; Fleutot, B.; Tortajada, H.; Prashantha, K.; Armand, M.; Grugeon, S.; Panier, S.; Dupont, L. Highly Loaded Graphite–Polylactic Acid Composite-Based Filaments for Lithium-Ion Battery Three-Dimensional Printing. Chem. Mater. 2018, 30, 7484–7493. [Google Scholar] [CrossRef]
- Baechler, C.; DeVuono, M.; Pearce, J.M. Distributed Recycling of Waste Polymer into RepRap Feedstock. Rapid Prototyping Journal 2013, 19, 118–125. [Google Scholar] [CrossRef]
- Cruz Sanchez, F.A.; Boudaoud, H.; Hoppe, S.; Camargo, M. Polymer Recycling in an Open-Source Additive Manufacturing Context: Mechanical Issues. Additive Manufacturing 2017, 17, 87–105. [Google Scholar] [CrossRef]
- Cruz Sanchez, F.A.; Boudaoud, H.; Camargo, M.; Pearce, J.M. Plastic Recycling in Additive Manufacturing: A Systematic Literature Review and Opportunities for the Circular Economy. Journal of Cleaner Production 2020, 264, 121602. [Google Scholar] [CrossRef]
- Dertinger, S.C.; Gallup, N.; Tanikella, N.G.; Grasso, M.; Vahid, S.; Foot, P.J.S.; Pearce, J.M. Technical Pathways for Distributed Recycling of Polymer Composites for Distributed Manufacturing: Windshield Wiper Blades. Resources, Conservation and Recycling 2020, 157, 104810. [Google Scholar] [CrossRef]
- Fully Metallic Copper 3D-Printed Electrodes via Sintering for Electrocatalytic Biosensing. Applied Materials Today 2021, 25, 101253. [CrossRef]
- Mo, F.; Guo, B.; Liu, Q.; Ling, W.; Liang, G.; Chen, L.; Yu, S.; Wei, J. Additive Manufacturing for Advanced Rechargeable Lithium Batteries: A Mini Review. Front. Energy Res. 2022, 10, 986985. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Chen, J.; Wang, Z.; Tang, B. Hybrid Energy Storage System Design for Mobile Multi-Material Fused Deposition Modeling. AIP Advances 2020, 10, 075322. [Google Scholar] [CrossRef]
- Anzalone, G.C.; Wijnen, B.; Pearce, J.M. Multi-Material Additive and Subtractive Prosumer Digital Fabrication with a Free and Open-Source Convertible Delta RepRap 3-D Printer. Rapid Prototyping Journal 2015, 21, 506–519. [Google Scholar] [CrossRef]
- Nanomaterials-Based Additive Manufacturing for Mass Production of Energy Storage Systems: 3D Printed Batteries and Supercapacitors.
- Maurel, A. Thermoplastic Composite Filaments Formulation and 3D-Printing of a Lithium-Ion Battery via Fused Deposition Modeling. phdthesis, Université de Picardie Jules Verne, 2020.
- Challenges of 3D Printing in LIB Electrodes: Emphasis on Material-Design Properties, and Performance of 3D Printed Si-Based LIB Electrodes. Journal of Power Sources 2022, 543, 231840. [CrossRef]
- Laureto, J.J.; Pearce, J.M. Anisotropic Mechanical Property Variance between ASTM D638-14 Type i and Type Iv Fused Filament Fabricated Specimens. Polymer Testing 2018, 68, 294–301. [Google Scholar] [CrossRef]
- Fused Filament Fabrication of Polymer Materials: A Review of Interlayer Bond. Additive Manufacturing 2021, 37, 101658. [CrossRef]
- Beydaghi, H.; Abouali, S.; Thorat, S.B.; Del Rio Castillo, A.E.; Bellani, S.; Lauciello, S.; Gentiluomo, S.; Pellegrini, V.; Bonaccorso, F. 3D Printed Silicon-Few Layer Graphene Anode for Advanced Li-Ion Batteries. RSC Adv. 2021, 11, 35051–35060. [Google Scholar] [CrossRef]
- Maurel, A.; Grugeon, S.; Fleutot, B.; Courty, M.; Prashantha, K.; Tortajada, H.; Armand, M.; Panier, S.; Dupont, L. Three-Dimensional Printing of a LiFePO4/Graphite Battery Cell via Fused Deposition Modeling. Sci Rep 2019, 9, 18031. [Google Scholar] [CrossRef]
- Gao, W.; Michalička, J.; Pumera, M. Hierarchical Atomic Layer Deposited V 2 O 5 on 3D Printed Nanocarbon Electrodes for High-Performance Aqueous Zinc-Ion Batteries. Small 2022, 18, 2105572. [Google Scholar] [CrossRef]
- Foster, C.W.; Zou, G.; Jiang, Y.; Down, M.P.; Liauw, C.M.; Garcia-Miranda Ferrari, A.; Ji, X.; Smith, G.C.; Kelly, P.J.; Banks, C.E. Next-Generation Additive Manufacturing: Tailorable Graphene/Polylactic(Acid) Filaments Allow the Fabrication of 3D Printable Porous Anodes for Utilisation within Lithium-Ion Batteries. Batteries & Supercaps 2019, 2, 448–453. [Google Scholar] [CrossRef]
- Hu, X.; Chen, Y.; Xu, W.; Zhu, Y.; Kim, D.; Fan, Y.; Yu, B.; Chen, Y. 3D-Printed Thermoplastic Polyurethane Electrodes for Customizable, Flexible Lithium-Ion Batteries with an Ultra-Long Lifetime. Small 2023, 19, 2301604. [Google Scholar] [CrossRef] [PubMed]
- Maurel, A.; Armand, M.; Grugeon, S.; Fleutot, B.; Davoisne, C.; Tortajada, H.; Courty, M.; Panier, S.; Dupont, L. Poly(Ethylene Oxide)−LiTFSI Solid Polymer Electrolyte Filaments for Fused Deposition Modeling Three-Dimensional Printing. J. Electrochem. Soc. 2020, 167, 070536. [Google Scholar] [CrossRef]
- Yang, P.; Fan, H.J. Inkjet and Extrusion Printing for Electrochemical Energy Storage: A Minireview. Advanced Materials Technologies 2020, 5, 2000217. [Google Scholar] [CrossRef]
- Sousa, R.E.; Costa, C.M.; Lanceros-Méndez, S. Advances and Future Challenges in Printed Batteries. ChemSusChem 2015, 8, 3539–3555. [Google Scholar] [CrossRef]
- Fabrication of Modern Lithium Ion Batteries by 3D Inkjet Printing: Opportunities and Challenges. Heliyon 2022, 8, e12623. [CrossRef] [PubMed]
- Sowade, E.; Polomoshnov, M.; Willert, A.; Baumann, R.R. Toward 3D-Printed Electronics: Inkjet-Printed Vertical Metal Wire Interconnects and Screen-Printed Batteries. Advanced Engineering Materials 2019, 21, 1900568. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, Q.; Liu, L.; Xu, J.; Yan, M.; Jiang, Z. A Novel and Facile Route of Ink-Jet Printing to Thin Film SnO2 Anode for Rechargeable Lithium Ion Batteries. Electrochimica Acta 2006, 51, 2639–2645. [Google Scholar] [CrossRef]
- Lawes, S.; Sun, Q.; Lushington, A.; Xiao, B.; Liu, Y.; Sun, X. Inkjet-Printed Silicon as High Performance Anodes for Li-Ion Batteries. Nano Energy 2017, 36, 313–321. [Google Scholar] [CrossRef]
- Chen, T.; Wang, Y.; Yang, Y.; Huang, F.; Zhu, M.; Ang, B.T.W.; Xue, J.M. Heterometallic Seed-Mediated Zinc Deposition on Inkjet Printed Silver Nanoparticles Toward Foldable and Heat-Resistant Zinc Batteries. Advanced Functional Materials 2021, 31, 2101607. [Google Scholar] [CrossRef]
- Kushwaha, A.; Jangid, M.K.; Bhatt, B.B.; Mukhopadhyay, A.; Gupta, D. Inkjet-Printed Environmentally Friendly Graphene Film for Application as a High-Performance Anode in Li-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 7911–7921. [Google Scholar] [CrossRef]
- Kushwaha, A.; Sharma, A.; Bhatt, B.B.; Mukhopadhyay, A.; Gupta, D. Inkjet-Printed Graphene-Modified Aluminum Current Collector for High-Voltage Lithium-Ion Battery. ACS Appl. Energy Mater. 2023, 6, 4168–4178. [Google Scholar] [CrossRef]
- Viviani, P.; Gibertini, E.; Iervolino, F.; Levi, M.; Magagnin, L. Carbon Additive Effect on the Electrochemical Performances of Inkjet Printed Thin-Film Li4Ti5O12 Electrodes. Journal of Manufacturing Processes 2021, 72, 411–418. [Google Scholar] [CrossRef]
- Kolchanov, D.S.; Mitrofanov, I.; Kim, A.; Koshtyal, Y.; Rumyantsev, A.; Sergeeva, E.; Vinogradov, A.; Popovich, A.; Maximov, M.Yu. Inkjet Printing of Li-Rich Cathode Material for Thin-Film Lithium-Ion Microbatteries. Energy Technology 2020, 8, 1901086. [Google Scholar] [CrossRef]
- Pei, M.; Shi, H.; Yao, F.; Liang, S.; Xu, Z.; Pei, X.; Wang, S.; Hu, Y. 3D Printing of Advanced Lithium Batteries: A Designing Strategy of Electrode/Electrolyte Architectures. J. Mater. Chem. A 2021, 9, 25237–25257. [Google Scholar] [CrossRef]
- Overview on the Applications of Three-Dimensional Printing for Rechargeable Lithium-Ion Batteries. Applied Energy 2020, 257, 114002. [CrossRef]
- Tian, X.; Zhou, K. 3D Printing of Cellular Materials for Advanced Electrochemical Energy Storage and Conversion. Nanoscale 2020, 12, 7416–7432. [Google Scholar] [CrossRef]
- Narita, K.; Saccone, M.A.; Sun, Y.; Greer, J.R. Additive Manufacturing of 3D Batteries: A Perspective. Journal of Materials Research 2022, 37, 1535–1546. [Google Scholar] [CrossRef]
- Recent Advances in 3D Printed Electrode Materials for Electrochemical Energy Storage Devices. Journal of Energy Chemistry 2023, 81, 272–312. [CrossRef]
- Cheng, M.; Deivanayagam, R.; Shahbazian-Yassar, R. 3D Printing of Electrochemical Energy Storage Devices: A Review of Printing Techniques and Electrode/Electrolyte Architectures. Batteries & Supercaps 2020, 3, 130–146. [Google Scholar] [CrossRef]
- A Comprehensive Review of the Photopolymerization of Ceramic Resins Used in Stereolithography. Additive Manufacturing 2020, 35, 101177. [CrossRef]
- Brinckmann, S.A.; Patra, N.; Yao, J.; Ware, T.H.; Frick, C.P.; Fertig, R.S. Stereolithography of SiOC Polymer-Derived Ceramics Filled with SiC Micronwhiskers. Advanced Engineering Materials 2018, 20, 1800593. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, R.; He, Z.; Zhao, K.; Pan, L. Printing 3D Gel Polymer Electrolyte in Lithium-Ion Microbattery Using Stereolithography. J. Electrochem. Soc. 2017, 164, A1852–A1857. [Google Scholar] [CrossRef]
- Norjeli, M.F.; Tamchek, N.; Osman, Z.; Mohd Noor, I.S.; Kufian, M.Z.; Ghazali, M.I.B.M. Additive Manufacturing Polyurethane Acrylate via Stereolithography for 3D Structure Polymer Electrolyte Application. Gels 2022, 8, 589. [Google Scholar] [CrossRef]
- Lee, K.; Shang, Y.; Bobrin, V.A.; Kuchel, R.; Kundu, D.; Corrigan, N.; Boyer, C. 3D Printing Nanostructured Solid Polymer Electrolytes with High Modulus and Conductivity. Advanced Materials 2022, 34, 2204816. [Google Scholar] [CrossRef]
- Katsuyama, Y.; Kudo, A.; Kobayashi, H.; Han, J.; Chen, M.; Honma, I.; Kaner, R.B. A 3D-Printed, Freestanding Carbon Lattice for Sodium Ion Batteries. Small 2022, 18, 2202277. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Wang, C.; Wang, L.; Lu, B.; Gao, F.; Shao, D. DLP Printing of a Flexible Micropattern Si/PEDOT:PSS/PEG Electrode for Lithium-Ion Batteries. Chem. Commun. 2022, 58, 7642–7645. [Google Scholar] [CrossRef] [PubMed]
- Yoko, A.; Oshima, Y. Recovery of Silicon from Silicon Sludge Using Supercritical Water. The Journal of Supercritical Fluids 2013, 75, 1–5. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Y.; Wu, J.; Shao, R.; Jiang, R.; Tie, Z.; Jin, Z. A Review on Recent Advances for Boosting Initial Coulombic Efficiency of Silicon Anodic Lithium Ion Batteries. Small 2022, 18, 2102894. [Google Scholar] [CrossRef] [PubMed]
- Pearce, J.M. Economic Savings for Scientific Free and Open Source Technology: A Review. HardwareX 2020, 8, e00139. [Google Scholar] [CrossRef] [PubMed]
- Dobbelaere, T.; Vereecken, P.M.; Detavernier, C. A USB-Controlled Potentiostat/Galvanostat for Thin-Film Battery Characterization. HardwareX 2017, 2, 34–49. [Google Scholar] [CrossRef]
- Sylvestrin, G.R.; Scherer, H.F.; Hideo Ando Junior, O. Hardware and Software Development of an Open Source Battery Management System. IEEE Latin America Transactions 2021, 19, 1153–1163. [Google Scholar] [CrossRef]
- Fleming, J.; Amietszajew, T.; McTurk, E.; Towers, D.P.; Greenwood, D.; Bhagat, R. Development and Evaluation of In-Situ Instrumentation for Cylindrical Li-Ion Cells Using Fibre Optic Sensors. HardwareX 2018, 3, 100–109. [Google Scholar] [CrossRef]
- Carloni, A.; Baronti, F.; Di Rienzo, R.; Roncella, R.; Saletti, R. An Open-Hardware and Low-Cost Maintenance Tool for Light-Electric-Vehicle Batteries. Energies 2021, 14, 4962. [Google Scholar] [CrossRef]
- Yensen, N.; Allen, P.B. Open Source All-Iron Battery for Renewable Energy Storage. HardwareX 2019, 6, e00072. [Google Scholar] [CrossRef]
- Koirala, D.; Yensen, N.; Allen, P.B. Open Source All-Iron Battery 2.0. HardwareX 2021, 9, e00171. [Google Scholar] [CrossRef] [PubMed]
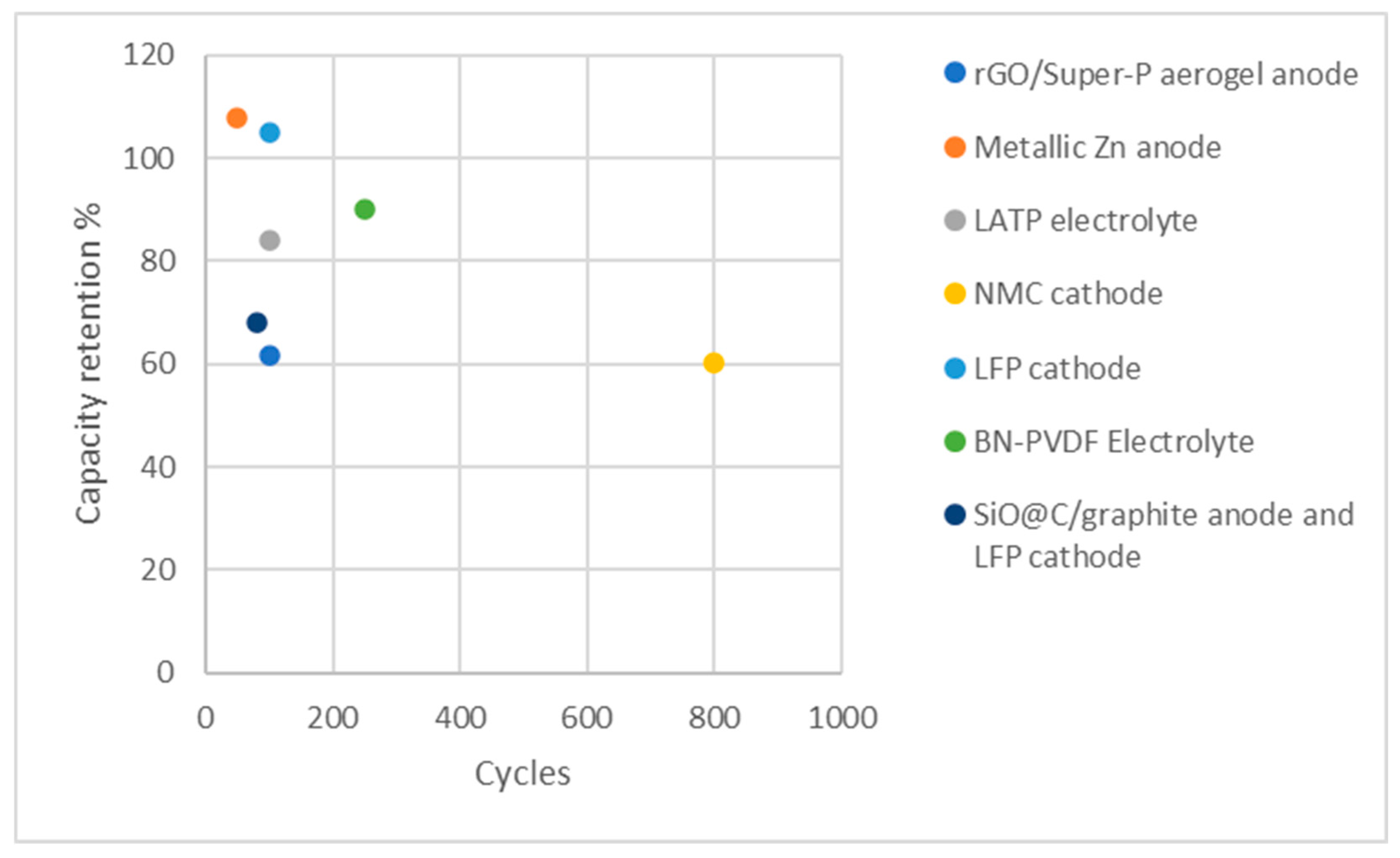
| Printed component | Discharge Capacity | Coulombic efficiency | Cycle numbers | Reference |
| Anode: rGO/Super-P aerogel | 848.4 mA h at 80 mA cm−2 | More than 95% | 100 | [139] |
| Anode: metallic Zn | 214.85 mAh g−1 at 25 mA cm−2 | 87% | 650 | [140] |
| Electrolyte: LATP | 150 mAh g-1 at 0.5 C | 100% | 100 | [141] |
| Cathode: NMC | 107.5 mAh g−1 at a current density of 1 C | 99.9% | 800 | [142] |
| Cathode: LFP | 150 mA h g−1 at 0.5 C | 99.9% | 100 - 500 | [143] |
| Electrolyte: BN-PVDF | 132 mAh g–1 at 1C rate | N/A | 130 | [144] |
| Anode: SiO@C/graphite Cathode: LFP |
75 mAh g-1 at 0.3 C | 100% | 40 | [145] |
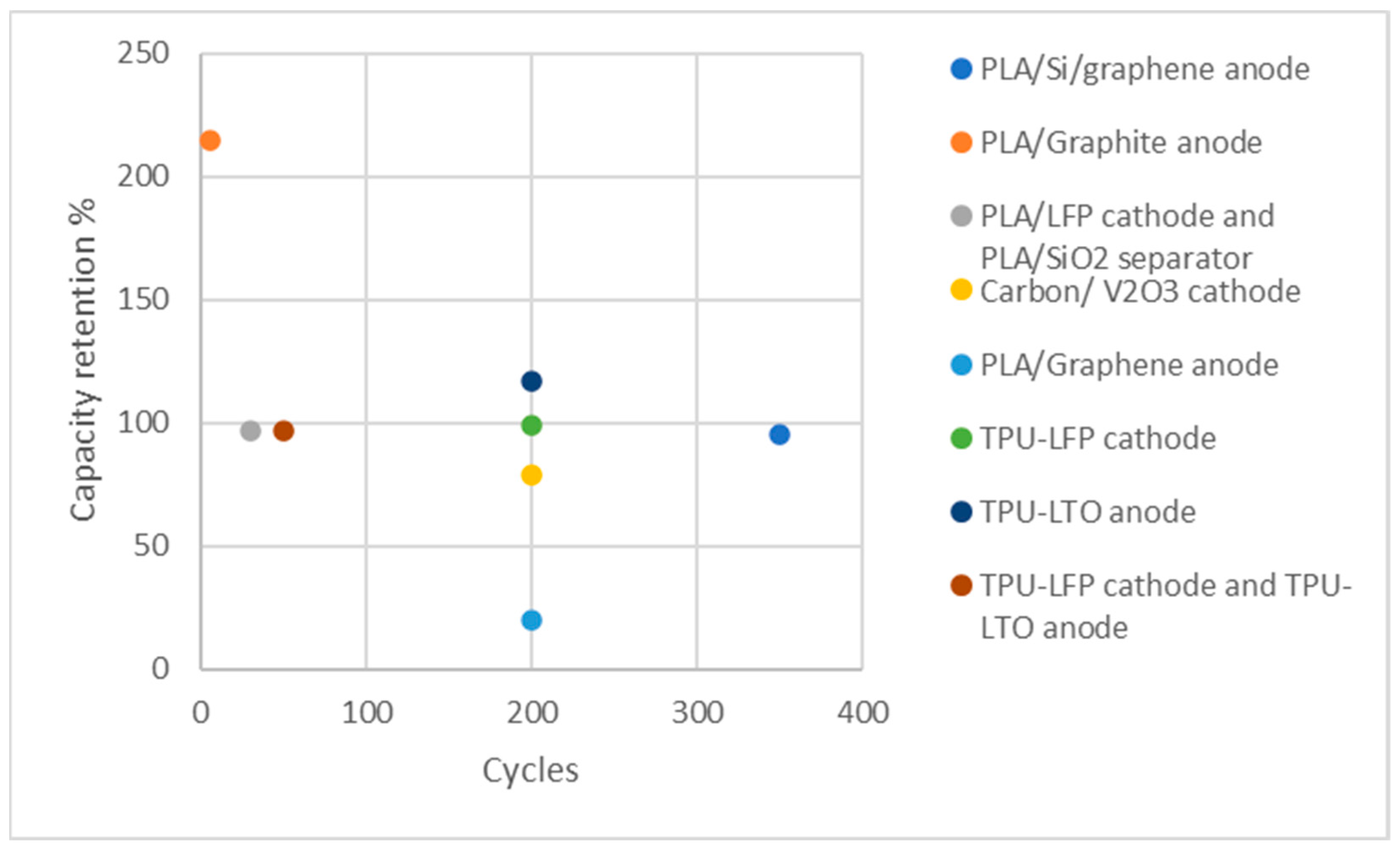
| Printed component | Discharge Capacity | Coulombic efficiency | Cycle numbers | Reference |
| Anode: PLA/Si/graphene | 327 mA h g−1 at the current density of 20 mA g−1 | 96% | 350 | [168] |
| Anode: PLA/Graphite | 200 mAh g-1 at the current density of 18.6 mA g-1 (C/20) | N/A | 5 | [154] |
| Cathode: PLA/LFP Separator: PLA/SiO2 |
165 mAh g-1 at C/20 | N/A | ~30 | [169] |
| Cathode: Carbon/ V2O3 | 183 mAh g-1 at 3 A g−1 current density | 99.99% | 200 | [170] |
| Anode: PLA/Graphene | 100 mAh g-1 at 40 mA g−1 | 99.9% | 200 | [171] |
| Cathode: TPU-LFP | 113.1 mAh g-1 at the rate of 0.3 C | 99.75% | 200 | [172] |
| Anode: TPU-LTO | 120.0 mAh g-1 at the rate of 0.3 C | 100.39% | 200 | [172] |
| Printed component | Discharge Capacity | Coulombic efficiency | Cycle numbers | Reference |
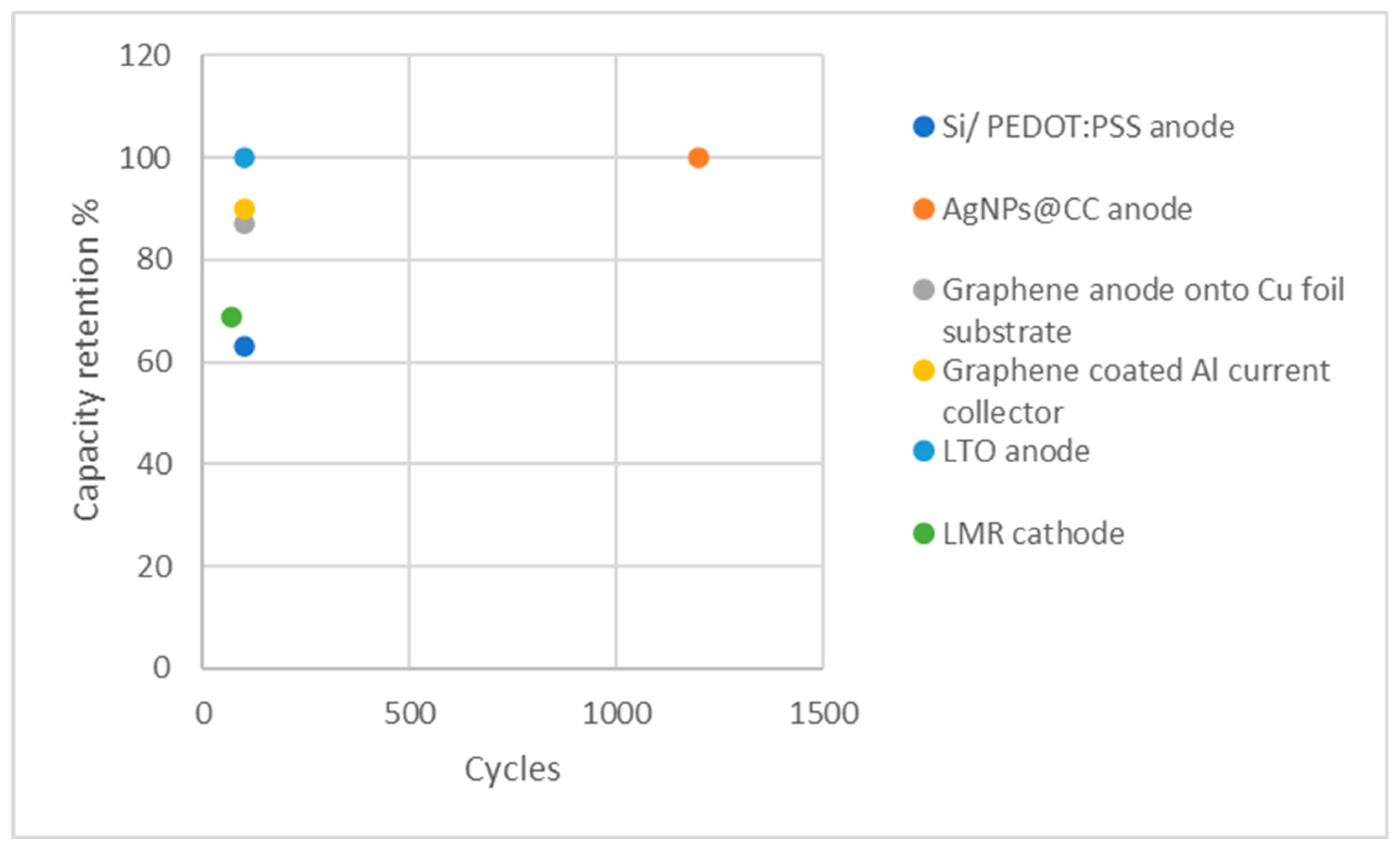
| Anode: Si/ PEDOT:PSS | 1700 mA h g−1 at 0.1 C | 98.6% | 100 | [179] |
| Anode: AgNPs@CC | 184 mAh g-1 at 5 A g-1 | 99.5% | 1200 & 800 | [180] |
| Anode: graphene onto Cu foil substrate | 520 mAh g-1 at 2C | 99% | 100 | [181] |
| Current collector: graphene coated Al | 180 mAh g-1 at C/5 | N/A | 100 | [182] |
| Anode: LTO | 128 mAh g−1 at 0.5 C | 100% | 100 | [183] |
| Cathode: LMR | 240 mAh g⁻¹ at 0.01 C | N/A | 70 | [184] |
| Printed component | Discharge Capacity | Coulombic efficiency | Cycle numbers | Other properties | Reference |
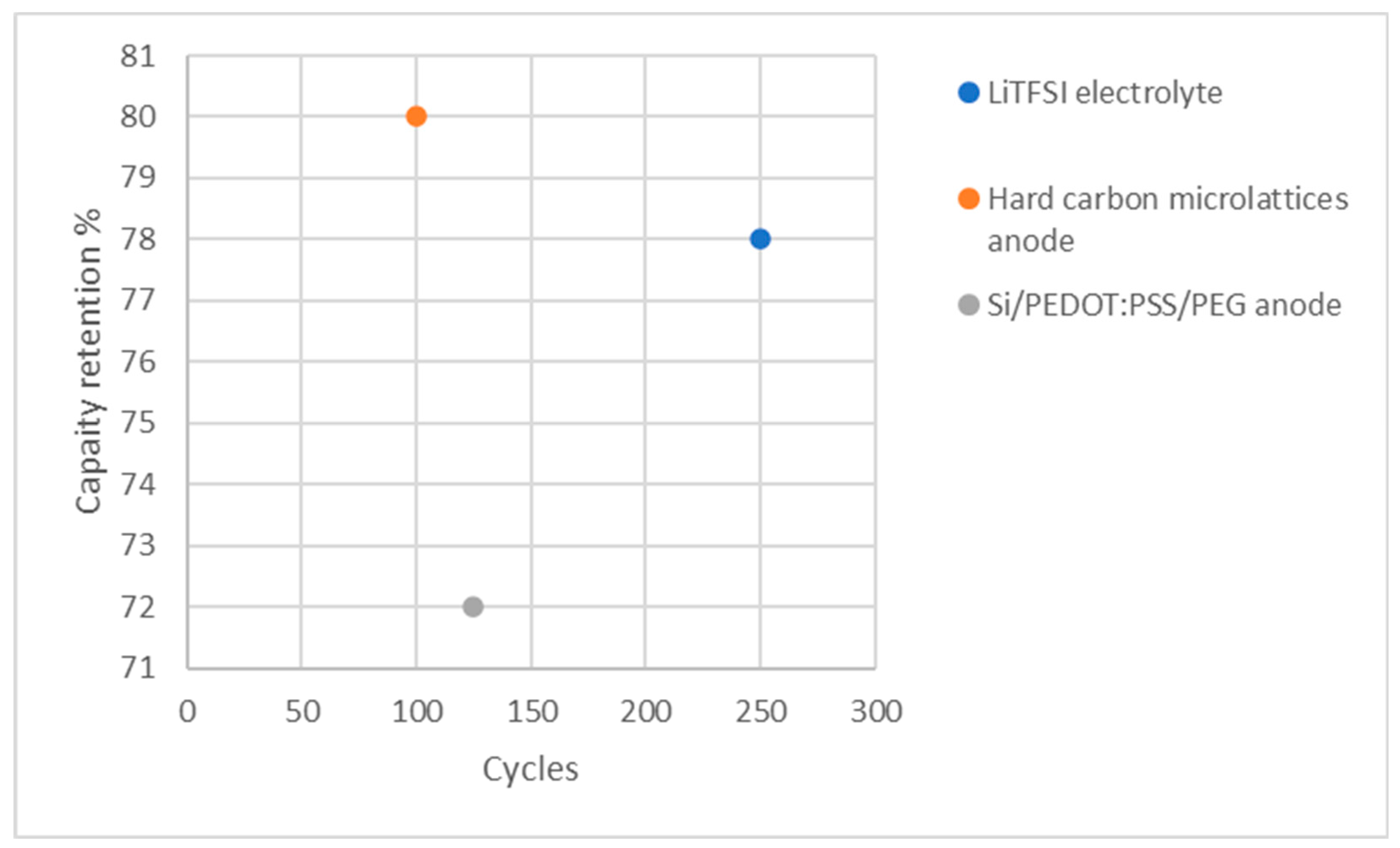
| Electrolyte: LiTFSI | 166 mAh g–1 at 0.1 C | 100% | 250 | Ionic conductivity: 3.7 × 10–4 S cm–1 | [116] [116] |
| Electrolyte: PEG-base gel polymer | 1.4 µAh cm-2 at 5 µA | N/A | 2 | Ionic conductivity: 4.8 × 10−3 S cm–1 | [193] [193] |
| Electrolyte: PUA-base gel polymer | N/A | N/A | N/A | Ionic conductivity: 1.24 × 10−3 S cm–1 | [194] [194] |
| Electrolyte: LAGP solid electrolyte | N/A | N/A | N/A | Ionic conductivity: 1.6 × 10−4 S cm−1 | [117] [117] |
| Electrolyte: PEO solid electrolyte | N/A | N/A | N/A | Ionic conductivity: 3 × 10−4 S cm−1 | [195] [195] |
| Anode: hard carbon microlattices | 225 mAh g-1 at 5 mA g−1 | 99.4% | 2 | N/A | [196] [196] |
| Anode: Si/PEDOT:PSS/PEG | 1105 mAh g-1 at 800 mA g−1 | 86.3% | 125 | N/A | [197] [197] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





