Introduction
Antimicrobial resistance (AMR) in foodborne pathogens poses a significant hazard to public health worldwide (Alsayeqh et al., 2021). The demand for food-animals is high especially poultry, is projected to increase by 17.8% by 2030 (Oecd/Fao, 2021). Globally, chickens account for 91% of the world’s total poultry population (Erdaw and Beyene, 2022). In Uganda, over 85% of the livestock farmers rear poultry for livelihood and as a means of income generation (UBOS and ICF, 2018). Poultry is one of the main AMR threats and risk of transmitting AMR genes in the food chain to humans (Sevilla-Navarro et al., 2022). AMR directly affects global economic growth due to costs associated with infections by resistant pathogens, with developing countries in Africa bearing the biggest burden of its negative effects (Ayukekbong et al., 2017, Morel et al., 2020).
Unlike other antimicrobials, a continuous emergence and rapid dissemination of resistance to antibiotics among pathogens has been reported (Liu et al., 2016). The increase is mainly attributed to the irrational use of antibiotics in animal and human health (Argudín et al., 2017). Several risk factors contribute to the emergence of AMR including inadequate veterinary healthcare, lack of monitoring and regulatory services, intervention of excessive informal animal health service providers, and farmers’ knowledge gap on AMR, which have resulted in the misuse and overuse of antibiotics in all types of animal farming (Al Amin et al., 2020). Non-therapeutic use of antibiotics for growth promotion, and prevention and control of disease on poultry farms further predisposes to AMR emergence (Schar et al., 2018, Moffo et al., 2021).
Resistant microbes may be disseminated directly or indirectly between animals and humans when exposed (Wang et al., 2021). Cultural norms such as gender roles and management systems underlying animal production and close interactions between humans and animals have been reported as key drivers for AMR spillover and amplification especially in poultry (Muleme et al., 2023). Laxity in policy implementation, unskilled human resources, and weak surveillance systems for AMR are underlying gaps in AMR control in Uganda (Muleme et al., 2023). Other factors associated with human exposure to resistant pathogens include proximity to poultry, shared water source, poultry wastes as fertilizer and gender roles (Roess et al., 2015). Increase in drug resistant animal-derived pathogens that threaten animals and humans health (Li et al., 2021), can result from exchange of resistance genes between commensal and pathogenic microbes (Crofts et al., 2017).
Escherichia coli (E. coli ) is one of the commensal microbes within the gastrointestinal tract of animals (Kabiswa et al., 2018). However, strains known to cause illnesses in animals and humans have emerged in the recent past (Mitchell et al., 2015), likewise resistant ones. E. coli play an important ecological role and can be used as a bioindicator of AMR (Ramos et al., 2020). Transmission of foodborne resistant pathogens from reservoirs, particularly poultry, to the human population does occur (Aworh et al., 2021). A study in Tanzania showed that resistant E. coli are widely distributed among humans, animals and environment (Subbiah et al., 2020). In Uganda, a high prevalence of multidrug resistant (MDR) E. coli (88.4%) in clinically sick poultry has been reported (Kakooza et al., 2021). Monitoring and surveillance of resistant pathogens across the human–animal and environmental interface is one of the best approaches for decision-making and reducing AMR (Mathew et al., 2020). In 2017, the World Health Organization (WHO) published a list of 12 priority antibiotic-resistant pathogens, among which was E. coli (Asokan et al., 2019).
Poultry production accounts for one-third of global meat production (FAO, 2020) and poultry are among food-animals often raised under intensive conditions utilizing large amounts of antimicrobials, a practice that is driving AMR (Imam et al., 2020). Despite the rapid growth of poultry production, extensive use of antibiotics and the rapidly increasing population, limited data exists on the prevalence, resistance patterns and the risk factors associated with MDR E. coli in Uganda. Available data were on antimicrobial use, patterns and drivers of AMR mainly central (Kakooza et al., 2023, Muleme et al., 2022) and western (Samuel et al., 2023) regions of Uganda. West Nile region is one of the refugee hosting regions, with Arua as the only city with high population and rapidly increasing chicken production to address the high food demand, yet the epidemiology and factors associated with MDR E. coli carriage on chicken farms are not well known. The human population dynamics and demand for animal-based protein especially chicken and chicken products could drive the emergency and spread of MDR pathogens within humans and animals in west Nile region. We therefore sought to determine the prevalence, describe the AMR patterns and identify risk factors associated with MDR E. coli carriage on chicken farms, to inform policy and clinical management of E. coli infections.
Materials and methods
Study area and setting
The study was conducted in Arua district located in west Nile sub-region in northern Uganda (3° 01’ 28.80” N, 30° 54’ 21.59” E). Due to the new city status, Arua’s boundaries have increased from 10.5Km2 to 60Km2 and the population has grown seven-fold, from 60,000 to about 400,000 people (Capici, 2021). The district is currently providing shelter to over 200,000 refugees (Capici, 2021). With the high influx of refugees from Southern Sudan and Democratic Republic of Congo, the demand for food and healthcare has increased. The explosive population may modify the food production system into intensified production systems, which are driver of AMR (Imam et al., 2020). Over 85% of the livestock farmers in the district rear poultry for livelihood (UBOS and ICF, 2018). The health workers in the region reported unrestricted open-market sale of veterinary and human drugs especially in the refugee areas.
Study design
A cross-sectional study was conducted among chicken rearing households with apparently healthy chickens from 6th April to 31st May 2023. The study included households keeping chicken of a flock size of at least 50 chickens for commercial and semi-commercial purposes, and had prior use of antibiotics. In a flock size of above 50, chickens are often kept under intensive management system associated with high amount of antibiotic use (Imam et al., 2020) and exposure to humans due the close proximity (Muleme et al., 2022). The chickens should have been intended for human consumption and had reached slaughter weight for broiler or were “spent” layers. Farms with apparently sick chicken and had administered antibiotics within one week prior to the study were excluded. Additional data were obtained on socioeconomic and environmental factors using an observation checklist and a semi-structured questionnaire. While the environmental factors included biosecurity measures, source of water, production system and flock size. Household members aged 18-years and above, and directly involved in the day to day management of the farm were interviewed. All respondents gave informed consent prior to sample collection or the interviews.
Sampling Procedure
A multistage sampling approach was used to reach the primary (farm) and secondary (chicken and human) sampling units of the study. A total of 158 chicken farms were included, derived using the modified Kish Leslie sample size determination formula (Leslie, 1965). Using Steve Bennett’s formula of cluster sample size calculation (Bennett et al., 1991), 55 parishes were determined to be sufficient. All 11 sub-counties were selected with the help of the district veterinary office based on the farming characteristics. A maximum of six parishes were selected randomly from each sub-county and 82 villages from each parish based on the proportion to size probability distribution. Lists of sub-counties, parishes and villages were obtained from the district veterinary office. A minimum distance of 100 meters between farms was considered. At this distance the possibility of cross-contamination resulting from close proximity would be minimised. From each farm, 10 fresh faecal samples were picked and pooled in Amies transport media with charcoal to form a farm sample.
Data collection procedures
Study teams composed of one guide and an animal health officer, who identified, recruited and collected data from farming households that fulfilled the inclusion criteria. The study teams were trained on data collection and laboratory standard operating procedures prior to the study. A pre-tested semi-structured questionnaire was administered to the manager or owner of the farm with knowledge of the key activities around the farm so as to assess the risks associated with MDR E. coli carriage. The independent variables collected were environmental and socioeconomic factors which included veterinary healthcare service provision, management and production systems, biosecurity measures, monitoring and regulatory services, farmers’ knowledge and marital status, antimicrobial use, source of livelihood and food supply system. Fresh chicken faecal samples were collected, from the farm unit with the oldest flock, by the animal health officer. The samples were transported in Ziplocs and under cold chain (4 - <10oC) to the National Microbiology Reference Laboratory at the National Animal Disease Diagnostics and Epidemiology Center (NADDEC) in Entebbe within 72 hours of collection for analysis.
Isolation and Identification of Escherichia coli
A swab with faecal sample was obtained from the Amies with charcoal transport media and inoculated in sterile brain heart infusion (BHI) and incubated aerobically at 37oC for 18– 24 hours. A loopful of homogenised turbid suspension was streaked on MacConkey agar with crystal violet (OxoidTM, UK) and incubated aerobically at 37°C for 18–24 hours. Presumptive colonies obtained were purified by sub-culturing on 5% sheep blood agar and incubated aerobically at 37°C for 18–24 hours. The pure colonies were subjected to Gram staining, oxidase test and then to standard identification biochemical tests that included Sulphide-Indole-Motility (SIM) media, Triple Sugar Iron (TSI), urease and citrate utilization according to standard operating procedures to identify E. coli. Isolates that were lactose fermenting on MacConkey, Gram negative rods, oxidase negative, sulphur, urea and citrate negative, but positive for indole and motility with overall acid reactions on TSI were identified as E. coli. All identified E. coli isolates were re-subcultured onto 5% blood agar to obtain fresh colonies of 18– 24 hours old in preparation for antibiotic susceptibility testing.
Antimicrobial Susceptibility Testing
Antimicrobial susceptibility testing (AST) for
E. coli isolates was conducted following standard operating procedure, using Kirby-Bauer disc diffusion on Mueller-Hinton agar method (Hudzicki, 2009) and interpreted as either resistant, intermediate or susceptible according to clinical breakpoints as defined by the Clinical and Laboratory Standards Institute (CLSI) 2023 guidelines (version 33). Isolates with an intermediate susceptible result were considered resistant. The antimicrobials chosen were commonly used antibiotics and those listed by WHO (WHO, 2017) shown in
Table 2. Standard reference strain of
E. coli (ATCC 25922), was used to quality control antibiotic discs potency and Mueller Hinton Agar (MHA) performance characteristics. Multidrug resistance was defined as resistance to three or more classes of antimicrobials (Tang et al., 2017). A farm was defined as “positive” for a resistant
E. coli if at least one isolate resistant to the antimicrobial drug under study was isolated from the farm.
Table 1.
List of antimicrobials used for susceptibility testing ((all antibiotic discs were from OxoidTM, UK).
Table 1.
List of antimicrobials used for susceptibility testing ((all antibiotic discs were from OxoidTM, UK).
| WHO CIA classification |
Antimicrobial agent |
Class |
WHO-AWaRe |
Disc Charges |
| Critically important |
Ampicillin |
Penicillin |
Access |
10µg |
| Gentamicin |
Aminoglycoside |
10 µg |
| Ceftriaxone |
Cephalosporine |
Watch |
30 µg |
| Ciprofloxacin |
Fluoroquinolone |
5 µg |
| Imipenem |
Carbapenem |
10 µg |
| Cefepime |
Cephalosporine |
30µg |
| Highly important |
Chloramphenicol |
Amphenicol |
Access |
30 µg |
| Cotrimoxazole |
Diaminopyrimidine/sulphonamide |
25 µg |
| Tetracycline |
Tetracycline |
30 µg |
Statistical Analysis
Data were entered into Microsoft Excel®, cleaned and exported to STATA Statistical Package (version 16.0 Stata Corp LP, College Station, TX, USA) for analysis. The study employed univariate, bivariate and multivariable levels of analysis to assess factors associated with carriage of MDR E. coli on chicken farms. Descriptive statistics were populated in one-way tabulations, frequencies and percentages were reported in tables and figures. Prevalence was defined as the total number of farms with MDR E. coli isolates out of the total number of chicken farms under consideration. Resistance of E. coli isolates were categorized as resistant or susceptible using the CLSI (version 33) guidelines. At bivariate analysis, the study assessed for the statistical association between covariates using the Pearson chi-square test. The study employed a modified Poisson regression modelling approach at bivariate and multivariable analysis to generate prevalence ratios (PR) and the respective 95% confidence intervals (95% CI). Variables with p-value less than 0.25 and those deemed biologically important to the study from literature and expert opinion were included in multivariable analysis. At the multivariable level, a forward stepwise regression model approach was used where variables <0.05 level of significance were considered statistically significant and therefore associated with MDR. The variables, which formed the final model, were assessed for interaction by including an interaction term (each of the interaction terms was assessed for its statistical significance).
ETHICAL APPROVAL
Ethical approval was obtained from the Makerere University School of Public Health Ethics Committee in April 2023 (Approval number: MakSPH-REC/04/04/2023/182/04/04/2024). Permission was sought from the district veterinary office prior to commencement of the study. A written consent was obtained from the farm owners to be enrolled in the study.
Results
Characteristics of poultry farming households
This study was conducted among 158 poultry farming households. Majority 60% (95/158) of the chicken farmers were females, 87% (137/158) married, 54% (85/158) aged 35 or more years, and 47% (74/158) had attained tertiary education. In more than half, 59% (93/158), of households, poultry was a source of livelihood, 65% (102/158) had kept chicken for utmost 5 years and 55% (85/158) were not aware of AMR (
Table 2).
Table 2.
Background characteristics of chicken farmers.
Table 2.
Background characteristics of chicken farmers.
| Factor |
Sub category |
Frequency (n) |
Percentage (%) |
| Sex |
Male |
63 |
39.9 |
| |
Female |
95 |
60.1 |
| Marital status |
Married |
137 |
86.7 |
| |
Single |
21 |
13.3 |
| Education status |
Informal |
14 |
8.9 |
| |
Primary |
30 |
19.0 |
| |
Secondary |
40 |
25.3 |
| |
Tertiary |
74 |
46.8 |
| Age (years) |
18 – 24 |
15 |
9.5 |
| |
25 – 34 |
58 |
36.7 |
| |
>35 |
85 |
53.8 |
| Poultry as source of livelihood |
Yes |
93 |
58.9 |
| No |
65 |
41.1 |
| Duration as poultry farmer (years) |
<=5 |
102 |
64.6 |
| |
>5 |
56 |
35.4 |
| Washed hands after farming |
Yes |
46 |
29.1 |
| |
No |
112 |
70.9 |
| Aware of AMR |
Yes |
71 |
45.5 |
| |
No |
85 |
54.5 |
Characteristics of chicken farms
Overall, 50% (83/158) of the farms were rural, 68% (107/158) small scale and had no footbath, 53% (84/158) used borehole as water source and 84% (133/158) had full time staff and sought veterinary services from private veterinarians. Over 70% (111/158) farm used farm manure as fertilizer and 67% (105/158) had other livestock (
Table 3).
Prevalence of multidrug resistant E. coli species on chicken farms
The prevalence of MDR was 62.7% (95% CI: 55.0 – 70.3). Multidrug resistance varied significantly by gender of the farmer (p = 0.004); being higher on female managed farms than male managed farms (68.7% vs 31.3% respectively). Additionally, the prevalence of MDR significantly varied by farmer’s level of education (p = 0.017) and was substantially higher (64%) of MDR on farms without footbath (
Table 4).
The resistance patterns of E. coli species on chicken farms
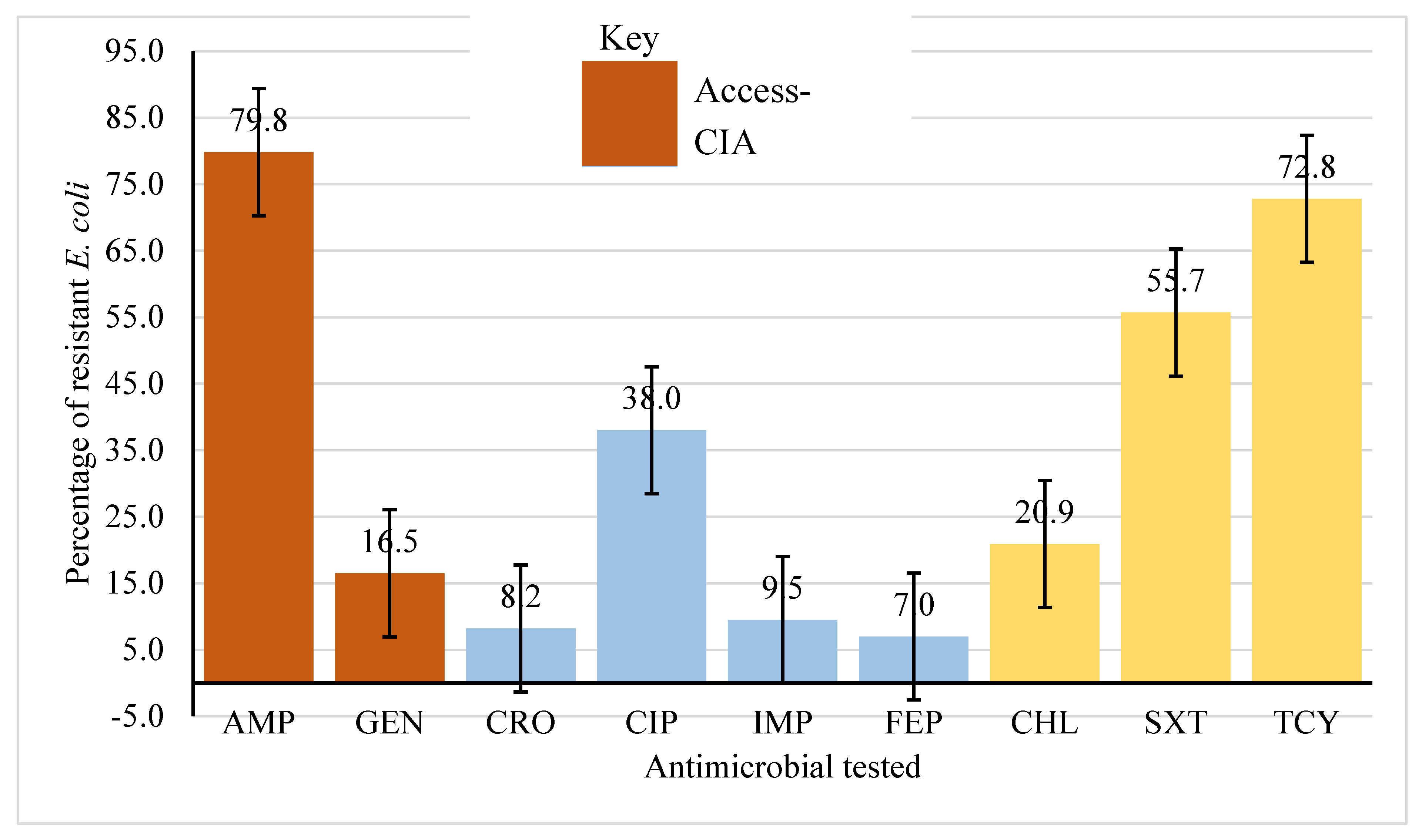
All farms used antibiotic, with tetracycline (83.6%), sulfadimidine (32.9%) and cotrimoxazole (9.2%) as the most used. On average, among the common WHO CIA and WHO AWaRe categories for human medicine, higher resistance (50%) was observed among WHO Access and Highly Important antimicrobials (Access-HIA), followed by Critically Important category (Access-CIA), 48%. Relatively lower resistance (16%) was observed among the Watch and Critically Important Antimicrobials category (Watch-CIA). Among the Access-HIA category, highest resistance was observed against tetracycline, 72.9% (95% CI 65.2 – 79.2) while among the Access-CIA, resistance was highest against ampicillin 79.8% (95% CI 72.7 – 85.4). Among the Watch-CIA category, highest resistance was observed against ciprofloxacin, 38% (95% CI 30.7 – 45.9) and lowest against cefepime, 7% (95% CI 3.9 – 12.3) (
Figure 1).
Public health risk factors associated with carriage and spread of MDR E. coli on chicken farms of farming households
Factors independently associated with carriage and spread of MDR
E. coli included gender, absence of footbath, education level and antimicrobial dose regimen. Chicken farms managed by males had lower prevalence of MDR
E. coli (APR = 0.72, 95% CI: 0.55 – 0.93) compared to those managed by females. The prevalence of MDR
E. coli was significantly (p = 0.002) higher among chicken farms that had no footbath (APR = 1.48, 95% CI: 1.16 – 1.88). Farms managed by farmers with education level higher than primary had a significantly lower likelihood of MDR
E. coli (
Table 5): secondary (APR=0.64, 95% CI: 0.46 – 0.88) and tertiary education level (APR = 0.60, 95% CI: 0.47 – 0.75). Furthermore, farmers who administered the drug doses as prescribed were less likely to have farms with MDR
E. coli (APR = 0.76, p = 0.022) (Table 6).
After assessing for effect modification, the study found a significant association between the prevalence of MDR E. coli, education level of the farmer and presence of footbath on farms. The farmers who attained tertiary education level but had no footbath on farms were two times more likely to have farms with MDR E. coli (APR = 1.74, p = 0.01).
Discussion
The study found a high prevalence (62.7%) of MDR E. coli on chicken farms. High resistance was observed against ampicillin (79.8%), tetracycline (72.8%), cotrimoxazole (55.7%), and ciprofloxacin (38%). Female gender was associated with high prevalence of MDR E. coli (68.7%) while significantly varied with farmers’ level of education. Presence of footbath, males as farm managers, secondary education or higher, and adherence to recommended antibiotic doses were protective against E. coli resistance while education with no footbath posed a heightened risk. There is need for context-specific collaborative strategies, to address knowledge on biosecurity on farms, and prudent use of antimicrobials among chicken farming communities, which considers gender dimensions to safeguard both animal and human health.
Multidrug resistant E. coli on chicken farms posed a significant public health threat because of E. coli’s tendency to disseminate AMR genes to intestinal bacteria in humans (Sevilla-Navarro et al., 2022, Wang et al., 2021). The MDR E. coli prevalence in this study was higher than the prevalence (37%) observed in western Uganda (Samuel et al., 203) and the global average (40%) (Roth et al., 2019), implying a high burden of MDR E. coli on the Arua district farms. However, the results were similar to findings from a Vietnam study (63.3%) (Nhung et al., 2022), but lower than the prevalence (71%) in Nepal (Koju et al., 2022). According to a 2022 systematic review in Sub-Saharan Africa, the prevalence of MDR E. coli ranged from 98.7% in Uganda to 100% in Ghana (Azabo et al., 2022). The differences in MDR E. coli prevalence could be due to differences in husbandry systems, sample types and interpretation of antimicrobial susceptibility. The study in Nepal used cecum samples while the other study in Uganda used layer chickens under a deep litter intensive husbandry system, compared to on-farm fresh faecal samples collected from all chicken husbandry systems in this study. Furthermore, the study in Nepal used the minimum inhibitory concentration (MIC) CLSI guideline while our study used Kirby-Bauer disc diffusion on Mueller-Hinton agar method to interpret the antimicrobial susceptibility. Unlike MIC method which is quantitative, disc diffusion provides a qualitative AST result (Gajic et al., 2022). These high prevalence of MDR E. coli on chicken farms could reveal the burden in animal health. The MDR E. coli on chicken farms could spillover and amplified among human population and environment. Infections by MDR E. coli in humans, certainly drive the cost of treatment high, morbidity and mortality in both animals and humans compromising quality of health outcomes and economic growth (Alsayeqh et al., 2021) thus, posing significant threat to public health.
There were significant differences in prevalence of MDR E. coli by gender and education level of the farmer. Differences in MDR E. coli prevalence by gender and education level have been observed in other studies (Ozturk et al., 2019, Boamah et al., 2016). Knowledge and gender influence farmers’ decision on when and how to use antimicrobials (Ozturk et al., 2019, Boamah et al., 2016), hence education targeting gender dimensions may be essential to promote responsible antimicrobial use practices and mitigating the emergency and spread of MDR E. coli on chicken farms, to prevent potential spillover into human population. The study found low prevalence of MDR E. coli on farms managed by males compared to those managed by females, consistent with other similar studies (Mudenda et al., 2023, Ozturk et al., 2019). The low prevalence of MDR E. coli on male managed farms could be attributed to the gender-based socioeconomic differences (Erdaw and Beyene, 2022, Galiè et al., 2015). Males compared to females counters have higher access to information and financial resources to seek healthcare in a household including veterinary services (Erdaw and Beyene, 2022). Access to information and financial resources influences decision on seeking for veterinary services and prudent antimicrobial use, thus promoting irrational antimicrobial use which drives emergency and spread of resistant pathogens including MDR E. coli (Ozturk et al., 2019). In contrast, a study conducted in Zambia reported females being more aware on antimicrobials and AMR, and they had good health seeking behaviour compared to their male counterparts (Mwansa et al., 2023). Good health seeking behaviour is associated with low prevalence of AMR and MDR (Mwansa et al., 2023, Pham-Duc and Sriparamananthan, 2021). The observed differences in these studies could be attributed to the variation in the socioeconomic practices of the study populations (Pham-Duc and Sriparamananthan, 2021). The high prevalence of MDR E. coli reported in our study could also be attributed to the observed differences in education status of the farmers. The study found significant difference in prevalence of MDR E. coli by education level of the farmer, where almost all the chicken farms managed by farmers who did not attained any formal education status had MDR E. coli. The high prevalence of MDR E. coli among female gender reported in this study indicates that gender differences on AMR are greatly contextual and intersect with other sociodemographic factors, particularly education status (Pham-Duc and Sriparamananthan, 2021). Socioeconomic status and gender plays a critical role in health promotion of the family and community (Goodwin et al., 2005, Al-Mustapha et al., 2020). Chicken contributes to nutrition security directly and indirectly through the sale and home consumption of chickens and eggs at both the household and community levels (Alders et al., 2018), thus the observed high prevalence of MDR E. coli especially on farms managed by females could impact negatively on maternal and child nutrition. Therefore, these can have a significant negative public health impact.
There was high resistance against ampicillin (Access-CIA), and tetracycline and cotrimoxazole (Access-HIA). Higher ampicillin resistance has been observed in Cameroon (77%) but a lower resistance was observed in Tanzania [52.3%] (Kimera et al., 2021). Similar results were reported in Nepal [66%] (Koju et al., 2022), Vietnam [21.0% – 73.3%] (Nhung et al., 2022), Cameroon [58.4%] (Moffo et al., 2021) and Tanzania [54%] (Kiiti et al., 2021). This situation may result from inadequate veterinary healthcare systems, which influences irrational use of antimicrobials and drives AMR emergence and spread (Al Amin et al., 2020, WHO, 2014, Kalam et al., 2022). Gentamycin (Access-CIA) resistance was higher in Cameroon (38%) (Aworh et al., 2019) compared to our study findings. Lower resistance was observed against the Watch-CIA with highest resistance being against ciprofloxacin. The lower E. coli resistance to Watch-CIA: cefepime and ceftriaxone (7%) and imipenem (11.6%) was similar to retrospective study done in the Central Diagnostic Laboratory (CDL) in Makerere University in Uganda (Kakooza et al., 2021). These drugs are uncommon for use in livestock in Uganda therefore limiting bacterial exposures. However, the observed resistance is an indication of a raising resistance against drugs considered as last-resort in human medicine which is worrisome for public health. The high resistance to ciprofloxacin could be attributed to the high level of use of enrofloxacin in poultry (Roth et al., 2017). The high E. coli resistance to Access-HIA in this study was similar to that in Tanzania: chloramphenicol (34.2%), cotrimoxazole (56.7%) and tetracycline (68.4%) (Kimera et al., 2021). However, tetracycline resistance in this study was lower than that reported in Bangladesh [100%] (Sarker et al., 2019), Malaysia [91.2%] (Elmi et al., 2021) and Nepal [86%] (Koju et al., 2022). In contrast, a study in Canada reported lower resistance against chloramphenicol (17%) (Varga et al., 2019). This could be attributed to differences in sample sources and variation in implementation of drug related and AMR mitigation policies (Muleme et al., 2022, Myers et al., 2022). The observed resistance patterns to these critically important antimicrobial agents in human Medicine in this present study is worrisome for public health since it may limit treatment options compromising health outcomes (Kaye et al., 2021).
Sex and education of a farmer, absence of footbath on the farm and following dose regimen were associated with MDR E. coli consistent with other studies (Hassan et al., 2021, Al Amin et al., 2020, WHO, 2014, Imam et al., 2020). Chicken farm being managed by male sex was protective against MDR E. coli consistent to those reported in Zambia (Mudenda et al., 2023) and Pakistan (Saeed et al., 2023). The less likelihood of male managed chicken farm carring MDR E. coli could be attributed to good husbandry practices and knowledge on AMR by the male gender compared to their female counterparts (Mudenda et al., 2022, Pham-Duc and Sriparamananthan, 2021). The observed association could also be due to high likelihood of males in accessing other sources of income to facilitate good husbandry needs on the farms compared to their female counterparts (Al-Mustapha et al., 2020). The observed high likelihood of female managed farms having high prevalence of carriage of MDR E. coli could pose a significant public health threat because of the critical roles female gender (women and girls) play at household and community levels (Gautron et al., 2023). Gender determines the division of labour in many contexts especially in Low- and Middle-Income Countries (LMICs). In most of the farming communities in Sub Saharan Africa (SSA), female gender particularly, women take care of sick animals not destined for slaughter (Barasa, 2019). These roles increases their exposure to resistant zoonotic pathogen including MDR E. coli (Barasa, 2019). Women and girls are also main players in household activities and taking care of children including household water, sanitation and hygiene (WASH) activities (Gautron et al., 2023). These roles played by female gender coupled with the observed heightened risk of MDR E. coli on chicken farms managed by females could lead to spillover and amplification into the human population especially chicken farming communities due to these interaction (Muleme et al., 2023). The negative public health impact could potentially affect these chicken farming communities especially, mothers and children due to lack of access to clean WASH which leads to increased likelihood of exposure to resistant zoonotic pathogens (Gautron et al., 2023, Roess et al., 2015, Barasa and Virhia, 2022).
In this study, farms without a footbath were more likely to have a high prevalence of MDR E. coli similar to study in Sub-Saharan Africa (Muleme et al., 2022, Afakye et al., 2020). Presence of a footbath on farms is one of the basic biosecurity measures. The poor biocontainment practices, and close interactions between humans and animals as observed among the farming households in this study, may pose a potential pathway for spread of resistant pathogens between human-animal interface. The observed high likelihood of farms without footbath having high risk of carriage of MDR E .coli shows the potential for environmental contamination, thus limiting access to clean WASH. Lack of access to clean water for both humans and animals have a significant public health threat as it provides pathway for transmission of resistant zoonotic pathogens between human-animal and environment interface (Medlicott et al., 2020). Inadequate sanitation exposes household members especially children to chicken faecal matter which increases the exposure to antibiotic resistant pathogens (Gautron et al., 2023). Infections by these zoonotic pathogens could lead to high morbidity, mortality among humans and animals, and food insecurity among the chicken farming communities. A farmer attaining at least a secondary education was associated with a lower risk of MDR E. coli on the chicken farms similar to finding of a study in Bangladesh (Al Amin et al., 2020). This could be attributed to enhanced knowledge on AMR and antimicrobial use. However, this study found a significant interaction between education and absence of footbath on chicken farms. Importantly, farmers who attained a tertiary education level but had no footbath on their farms were more likely to have farms with MDR E. coli. This means that absence of footbath on chicken farms could modify the effect of education on occurrence of MDR E. coli (Muleme et al., 2023, Muleme et al., 2022). This study indicates that public health capacity building interventions should be highly contextual since education alone could not be enough to contain emergence and spread of MDR E. coli on chicken farms.
There were a number of methodological limitations recognized in this study, including using responses from farmers to test for association with MDR E. coli which could have introduced a recall bias. However, this limitation was mitigated by using a validated semi-structured questionnaire. Secondly, the study did not perform molecular characterization of all the MDR isolates. However, the phenotypic analysis conducted was based on the internationally recognized and standardized CLSI protocol and guidelines, thus reflecting the real magnitude and pattern of AMR in the study setting. This study provides data on the prevalence and risk factors associated with carriage of MDR E. coli on chicken farms which can be used to guide evidence-based veterinary and public health policies for the containment of emergence and spread of AMR between human, animal and environment interfaces.
Conclusions
There was high prevalence of MDR E. coli on chicken farms which varied by sex and education level of the farmers. Resistance to ampicillin, tetracycline and cotrimoxazole among the Access antibiotic class and ciprofloxacin among the Watch antibiotic class was common. Footbath, adherence to recommended antibiotic doses and secondary education or higher protected against E. coli resistance, while education with no footbath posed a heightened risk. Antimicrobial stewardship and infection prevention strategies are urgently required on chicken farms. Further studies should consider environmental and human samples from the chicken farmers to compare the resistance patterns in these communities. Genotypic studies should be performed on isolates in order to understand the flow of resistomes across the human, animal, and environment interface.
List of abbreviations
| AMR: |
Antimicrobial resistance |
|
E. coli: |
Escherihia coli |
| CLSI: |
Clinical and Laboratory Standards Institute guidelines |
| MDR |
Multidrug resistance |
| WHO AWaRe: |
World Health Organization’s Access, Watch, Reserve classification of Antibiotics for Human Medicine |
| WHO CIA: |
World Health Organization’s List of Critically Important Antimicrobials for Human Medicine |
| HIA: |
Highly Important Antimicrobial agents in human |
Author Contributions
CAN, CGO, DM, JM, RW conceptualized and designed the study. CAN, FK, DMB, JM, SAA, EI supported and provided administrative support. CAN, SAA, EI, DK conducted investigation. CAN, EO carried out formal data analysis. CAN, CGO, DM supervised and written the original draft manuscript. All authors read and approved the final manuscript as submitted.
Funding
This research was supported by Fleming Fund Country Grant (Grant Number No: RFP/CG2/Uganda).
Data Availability Statement
Data will be availed upon request.
Acknowledgments
We are grateful to the respondents who participated in this study. We also appreciated the management of the National Animal Disease Diagnostic and Epidemiology Center (NADDEC) of the Ministry of Agriculture, Animal Industry and Fisheries (MAAIF) for allowing the study to be conducted without any inconveniences encountered. We wish to appreciate the support provided by the Arua District Veterinary Office during mobilization of farmers, farm identification and sample collection.
Conflicts of Interest
The authors have declared that no competing interests exist.
References
- FAO 2020. Meat Market Review, Overview of global meat market developments in 2019, April 2020. Rome.
- Afakye, K.; Kiambi, S.; Koka, E.; Kabali, E.; Dorado-Garcia, A.; Amoah, A.; Kimani, T.; Adjei, B.; A Caudell, M. The Impacts of Animal Health Service Providers on Antimicrobial Use Attitudes and Practices: An Examination of Poultry Layer Farmers in Ghana and Kenya. Antibiotics 2020, 9, 554. [Google Scholar] [CrossRef] [PubMed]
- Al-Mustapha, A.I.; Adetunji, V.O.; Heikinheimo, A. Risk Perceptions of Antibiotic Usage and Resistance: A Cross-Sectional Survey of Poultry Farmers in Kwara State, Nigeria. Antibiotics 2020, 9, 378. [Google Scholar] [CrossRef]
- Al Amin, A.; Hoque, M.N.; Siddiki, A.Z.; Saha, S.; Kamal, M. Antimicrobial resistance situation in animal health of Bangladesh. Vet.-World 2020, 13, 2713–2727. [Google Scholar] [CrossRef]
- Alders, R.G.; Dumas, S.E.; Rukambile, E.; Magoke, G.; Maulaga, W.; Jong, J.; Costa, R. Family poultry: Multiple roles, systems, challenges, and options for sustainable contributions to household nutrition security through a planetary health lens. Matern. Child Nutr. 2018, 14, e12668. [Google Scholar] [CrossRef] [PubMed]
- Alsayeqh, A.F.; Baz, A.H.A.; Darwish, W.S. Antimicrobial-resistant foodborne pathogens in the Middle East: a systematic review. Environ. Sci. Pollut. Res. 2021, 28, 68111–68133. [Google Scholar] [CrossRef] [PubMed]
- Argudín, M.A.; Deplano, A.; Meghraoui, A.; Dodémont, M.; Heinrichs, A.; Denis, O.; Nonhoff, C.; Roisin, S. Bacteria from Animals as a Pool of Antimicrobial Resistance Genes. Antibiotics 2017, 6, 12. [Google Scholar] [CrossRef]
- Asokan, G.V.; Ramadhan, T.; Ahmed, E.; Sanad, H. WHO Global Priority Pathogens List: A Bibliometric Analysis of Medline-PubMed for Knowledge Mobilization to Infection Prevention and Control Practices in Bahrain. Oman Med. J. 2019, 34, 184–193. [Google Scholar] [CrossRef]
- Aworh, M.K.; Kwaga, J.; Okolocha, E.; Mba, N.; Thakur, S. Prevalence and risk factors for multi-drug resistant Escherichia coli among poultry workers in the Federal Capital Territory, Abuja, Nigeria. PLoS ONE 2019, 14, e0225379. [Google Scholar] [CrossRef]
- Aworh, M.K.; Kwaga, J.K.P.; Hendriksen, R.S.; Okolocha, E.C.; Thakur, S. Genetic relatedness of multidrug resistant Escherichia coli isolated from humans, chickens and poultry environments. Antimicrob. Resist. Infect. Control. 2021, 10, 1–13. [Google Scholar] [CrossRef]
- Ayukekbong, J.A.; Ntemgwa, M.; Atabe, A.N. The threat of antimicrobial resistance in developing countries: causes and control strategies. Antimicrob. Resist. Infect. Control. 2017, 6, 1–8. [Google Scholar] [CrossRef]
- Azabo, R.; Dulle, F.; Mshana, S.E.; Matee, M.; Kimera, S. Antimicrobial use in cattle and poultry production on occurrence of multidrug resistant Escherichia coli. A systematic review with focus on sub-Saharan Africa. Front. Veter- Sci. 2022, 9, 1000457. [Google Scholar] [CrossRef] [PubMed]
- BARASA, V. 2019. WaArusha Agro-pastoralist Experiences with Risk of Febrile Illness: An Ethnographic Study of Social Drivers of Zoonoses and Rural Health-seeking Behaviours in Monduli District, Northern Tanzania. University of Sussex.
- Barasa, V.; Virhia, J. Using Intersectionality to Identify Gendered Barriers to Health-Seeking for Febrile Illness in Agro-Pastoralist Settings in Tanzania. Front. Glob. Women’s Heal. 2022, 2, 746402. [Google Scholar] [CrossRef] [PubMed]
- Bennett, S.; Woods, T.; Liyanage, W.M.; Smith, D.L. A simplified general method for cluster-sample surveys of health in developing countries. World Health Statistics Quarterly 1991, 44, 98–106. [Google Scholar]
- BOAMAH, V. E. , AGYARE, C., ODOI, H. & DALSGAARD, A. 2016. Practices and factors influencing the use of antibiotics in selected poultry farms in Ghana.
- CAPICI, V. 2021. Refugeehood in Uganda’s rapidly urbanizing cities: An investigation of the South Sudanese refugees’ use of assets and community self-reliance to overcome humanitarian protection challenges in Arua city, Northern Uganda.
- Crofts, T.S.; Gasparrini, A.J.; Dantas, G. Next-generation approaches to understand and combat the antibiotic resistome. Nat. Rev. Microbiol. 2017, 15, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Elmi, S.A.; Simons, D.; Elton, L.; Haider, N.; Hamid, M.M.A.; Shuaib, Y.A.; Khan, M.A.; Othman, I.; Kock, R.; Osman, A.Y. Identification of Risk Factors Associated with Resistant Escherichia coli Isolates from Poultry Farms in the East Coast of Peninsular Malaysia: A Cross Sectional Study. Antibiotics 2021, 10, 117. [Google Scholar] [CrossRef] [PubMed]
- Erdaw, M.M.; Beyene, W.T. Trends, prospects and the socio-economic contribution of poultry production in sub-Saharan Africa: a review. World’s Poult. Sci. J. 2022, 78, 835–852. [Google Scholar] [CrossRef]
- Gajic, I.; Kabic, J.; Kekic, D.; Jovicevic, M.; Milenkovic, M.; Culafic, D.M.; Trudic, A.; Ranin, L.; Opavski, N. Antimicrobial Susceptibility Testing: A Comprehensive Review of Currently Used Methods. Antibiotics 2022, 11, 427. [Google Scholar] [CrossRef]
- Galiè, A.; Mulema, A.; Benard, M.A.M.; Onzere, S.N.; E Colverson, K. Exploring gender perceptions of resource ownership and their implications for food security among rural livestock owners in Tanzania, Ethiopia, and Nicaragua. Agric. Food Secur. 2015, 4, 2. [Google Scholar] [CrossRef]
- GAUTRON, J. M. , TU THANH, G., BARASA, V., VOLTOLINA, G. Using intersectionality to study gender and antimicrobial resistance in low-and middle-income countries. Health Policy and Planning 2023, 38, 1017–1032. [Google Scholar] [CrossRef]
- GOODWIN, P. Y. , GARRETT, D. A., GALAL, O. Women and family health: The role of mothers in promoting family and child health. International Journal of Global Health and Health Disparities 2005, 4, 30–42. [Google Scholar]
- HASSAN, M. M. , KALAM, M. A., ALIM, M. A., SHANO, S., NAYEM, M. R. K., BADSHA, M. R., AL MAMUN, M. A., HOQUE, A., TANZIN, A. Z., NATH, C., KHANOM, H., KHAN, S. A., ISLAM, M. M., UDDIN, M. B. & ISLAM, A. Knowledge, Attitude, and Practices on Antimicrobial Use and Antimicrobial Resistance among Commercial Poultry Farmers in Bangladesh. Antibiotics 2021, 10, 784. [Google Scholar]
- HUDZICKI, J. Kirby-Bauer disk diffusion susceptibility test protocol. American society for microbiology 2009, 15, 55–63. [Google Scholar]
- Imam, T.; Gibson, J.S.; Foysal, M.; Das, S.B.; Das Gupta, S.; Fournié, G.; Hoque, A.; Henning, J. A Cross-Sectional Study of Antimicrobial Usage on Commercial Broiler and Layer Chicken Farms in Bangladesh. Front. Veter- Sci. 2020, 7, 576113. [Google Scholar] [CrossRef] [PubMed]
- Kabiswa, W.; Nanteza, A.; Tumwine, G.; Majalija, S. Phylogenetic Groups and Antimicrobial Susceptibility Patterns of Escherichia coli from Healthy Chicken in Eastern and Central Uganda. J. Veter- Med. 2018, 2018, 1–6. [Google Scholar] [CrossRef] [PubMed]
- KAKOOZA, S. , MUWONGE, A., NABATTA, E., ENEKU, W., NDOBOLI, D., WAMPANDE, E., MUNYIIRWA, D., KAYAGA, E., TUMWEBAZE, M. A., AFAYOA, M., SSAJJAKAMBWE, P., TAYEBWA, D. S., TSUCHIDA, S., OKUBO, T., USHIDA, K., SAKURAI, K. & MUTEBI, F. A retrospective analysis of antimicrobial resistance in pathogenic Escherichia coli and Salmonella spp. isolates from poultry in Uganda. Int J Vet Sci Med 2021, 9, 11–21. [Google Scholar] [PubMed]
- Kakooza, S.; Tayebwa, D.S.; Njalira, K.R.; Kayaga, E.B.; Asiimwe, I.; Komugisha, M.; Wanyana, M.; Kisekka, R.; Kyabarongo, A.; Kiryabwire, D.H.; et al. Reflections on Drivers for the Emergence and Spread of Antimicrobial Resistant Bacteria Detected from Chickens reared on Commercial Layer Farms in Mukono District, Uganda. Veter- Med. Res. Rep. 2023, 14, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Kalam, A.; Rahman, S.; Alim, A.; Shano, S.; Afrose, S.; Jalal, F.A.; Akter, S.; Khan, S.A.; Islam, M.; Uddin, B.; et al. Knowledge, Attitudes, and Common Practices of Livestock and Poultry Veterinary Practitioners Regarding the AMU and AMR in Bangladesh. Antibiotics 2022, 11, 80. [Google Scholar] [CrossRef]
- Kaye, K.S.; Gupta, V.; Mulgirigama, A.; Joshi, A.V.; E Scangarella-Oman, N.; Yu, K.; Ye, G.; Mitrani-Gold, F.S. Antimicrobial Resistance Trends in Urine Escherichia coli Isolates From Adult and Adolescent Females in the United States From 2011 to 2019: Rising ESBL Strains and Impact on Patient Management. Clin. Infect. Dis. 2021, 73, 1992–1999. [Google Scholar] [CrossRef]
- Kiiti, R.W.; Komba, E.V.; Msoffe, P.L.; Mshana, S.E.; Rweyemamu, M.; Matee, M.I.N. Antimicrobial Resistance Profiles of Escherichia coli Isolated from Broiler and Layer Chickens in Arusha and Mwanza, Tanzania. Int. J. Microbiol. 2021, 2021, 1–9. [Google Scholar] [CrossRef]
- Kimera, Z.I.; Mgaya, F.X.; Misinzo, G.; Mshana, S.E.; Moremi, N.; Matee, M.I.N. Multidrug-Resistant, Including Extended-Spectrum Beta Lactamase-Producing and Quinolone-Resistant, Escherichia coli Isolated from Poultry and Domestic Pigs in Dar es Salaam, Tanzania. Antibiotics 2021, 10, 406. [Google Scholar] [CrossRef]
- KOJU, P. , SHRESTHA, R., SHRESTHA, A., TAMRAKAR, S., RAI, A., SHRESTHA, P., MADHUP, S. K., KATUWAL, N., SHRESTHA, A., SHRESTHA, A. Antimicrobial Resistance in E. coli Isolated from Chicken Cecum Samples and Factors Contributing to Antimicrobial Resistance in Nepal. Tropical Medicine and Infectious Disease 2022, 7, 249. [Google Scholar] [CrossRef] [PubMed]
- LESLIE, K. 1965. “Survey sampling’‘ Systematic Biology. 643.
- Li, X.; Zhang, Z.; Xu, D.; Wu, C.; Li, J.; Zheng, Y. A Prediction Method for Animal-Derived Drug Resistance Trend Using a Grey-BP Neural Network Combination Model. Antibiotics 2021, 10, 692. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Y.; Wang, Y.; Walsh, T.R.; Yi, L.-X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Mathew, P.; Jaguga, C.; Mpundu, M.; Chandy, S.J. Building knowledge and evidence base on antimicrobial resistance in Africa, through ‘One Health’ based surveillance. Clin. Epidemiology Glob. Heal. 2020, 8, 313–317. [Google Scholar] [CrossRef]
- MEDLICOTT, K. , WESTER, A., GORDON, B., MONTGOMERY, M., TAYLER, E., SUTHERLAND, D., SCHMOLL, O., DE-SOUZA, M., KOO-OSHIMA, S. & DA-BALOGH, K. J. W. F. O. R. R. 2020. Technical brief on water, sanitation, hygiene and wastewater management to prevent infections and reduce the spread of antimicrobial resistance.
- MITCHELL, N. M. , JOHNSON, J. R., JOHNSTON, B., CURTISS, R., 3RD & MELLATA, M. Zoonotic potential of Escherichia coli isolates from retail chicken meat products and eggs. Appl Environ Microbiol 2015, 81, 1177–1187. [Google Scholar] [PubMed]
- Moffo, F.; Mouiche, M.M.M.; Djomgang, H.K.; Tombe, P.; Wade, A.; Kochivi, F.L.; Dongmo, J.B.; Mbah, C.K.; Mapiefou, N.P.; Ngogang, M.P.; et al. Poultry Litter Contamination by Escherichia coli Resistant to Critically Important Antimicrobials for Human and Animal Use and Risk for Public Health in Cameroon. Antibiotics 2021, 10, 402. [Google Scholar] [CrossRef] [PubMed]
- Morel, C.M.; Alm, R.A.; Ardal, C.; Bandera, A.; Bruno, G.M.; Carrara, E.; Colombo, G.L.; de Kraker, M.E.A.; Essack, S.; Frost, I.; et al. A one health framework to estimate the cost of antimicrobial resistance. Antimicrob. Resist. Infect. Control. 2020, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mudenda, S.; Bumbangi, F.N.; Yamba, K.; Munyeme, M.; Malama, S.; Mukosha, M.; Hadunka, M.A.; Daka, V.; Matafwali, S.K.; Siluchali, G.; et al. Drivers of antimicrobial resistance in layer poultry farming: Evidence from high prevalence of multidrug-resistant Escherichia coli and enterococci in Zambia. Veter- World 2023, 16, 1803–1814. [Google Scholar] [CrossRef] [PubMed]
- Mudenda, S.; Mukosha, M.; Godman, B.; Fadare, J.; Malama, S.; Munyeme, M.; Hikaambo, C.N.; Kalungia, A.C.; Hamachila, A.; Kainga, H.; et al. Knowledge, Attitudes, and Practices of Community Pharmacy Professionals on Poultry Antibiotic Dispensing, Use, and Bacterial Antimicrobial Resistance in Zambia: Implications on Antibiotic Stewardship and WHO AWaRe Classification of Antibiotics. Antibiotics 2022, 11, 1210. [Google Scholar] [CrossRef]
- MULEME, J. , KIBIRA, S. P., SSEMPEBWA, J. C., MUGAMBE, R. K., KANKYA, C., MUNYEME, M., KISAKA, S., ISUNJU, J. B., NINSIIMA, L. R. & MUSOKE, D. 2023. Health workers’ perspectives on the occurrence and management of antimicrobial resistance at the human-animal-environment interface in Uganda.
- MULEME, J. , MUSOKE, D., BALUGABA, B. E., KISAKA, S., MAKUMBI, F. E., BUREGYEYA, E., ISUNJU, J. B., WAMBI, R., MUGAMBE, R. K. & KANKYA, C. J. M. 2022. Epidemiology of Extended-spectrum beta-lactamase-producing Escherichia coli at the human-animal-environment interface in Wakiso district, Uganda. 2022.11. 12.22282228.
- Mwansa, M.; Mukuma, M.; Mulilo, E.; Kwenda, G.; Mainda, G.; Yamba, K.; Bumbangi, F.N.; Muligisa-Muonga, E.; Phiri, N.; Silwamba, I.; et al. Determination of antimicrobial resistance patterns of Escherichia coli isolates from farm workers in broiler poultry production and assessment of antibiotic resistance awareness levels among poultry farmers in Lusaka, Zambia. Front. Public Health 2023, 10, 998860. [Google Scholar] [CrossRef]
- Myers, J.; Hennessey, M.; Arnold, J.-C.; McCubbin, K.D.; Lembo, T.; Mateus, A.; Kitutu, F.E.; Samanta, I.; Hutchinson, E.; Davis, A.; et al. Crossover-Use of Human Antibiotics in Livestock in Agricultural Communities: A Qualitative Cross-Country Comparison between Uganda, Tanzania and India. Antibiotics 2022, 11, 1342. [Google Scholar] [CrossRef] [PubMed]
- Nhung, N.T.; Yen, N.T.P.; Dung, N.T.T.; Nhan, N.T.M.; Phu, D.H.; Kiet, B.T.; Thwaites, G.; Geskus, R.B.; Baker, S.; Carrique-Mas, J.; et al. Antimicrobial resistance in commensal Escherichia coli from humans and chickens in the Mekong Delta of Vietnam is driven by antimicrobial usage and potential cross-species transmission. JAC-Antimicrobial Resist. 2022, 4, dlac054. [Google Scholar] [CrossRef] [PubMed]
- OECD/FAO 2021. OECD-FAO agricultural outlook 2021–2030. OECD Publishing Paris, France.
- Ozturk, Y.; Celik, S.; Sahin, E.; Acik, M.N.; Cetinkaya, B. Assessment of Farmers’ Knowledge, Attitudes and Practices on Antibiotics and Antimicrobial Resistance. Animals 2019, 9, 653. [Google Scholar] [CrossRef] [PubMed]
- Pham-Duc, P.; Sriparamananthan, K. Exploring gender differences in knowledge and practices related to antibiotic use in Southeast Asia: A scoping review. PLoS ONE 2021, 16, e0259069. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.; Silva, V.; Dapkevicius, M.d.L.E.; Caniça, M.; Tejedor-Junco, M.T.; Igrejas, G.; Poeta, P. Escherichia coli as Commensal and Pathogenic Bacteria among Food-Producing Animals: Health Implications of Extended Spectrum β-Lactamase (ESBL) Production. Animals 2020, 10, 2239. [Google Scholar] [CrossRef] [PubMed]
- Roess, A.A.; Winch, P.J.; Akhter, A.; Afroz, D.; Ali, N.A.; Shah, R.; Begum, N.; Seraji, H.R.; El Arifeen, S.; Darmstadt, G.L.; et al. Household Animal and Human Medicine Use and Animal Husbandry Practices in Rural Bangladesh: Risk Factors for Emerging Zoonotic Disease and Antibiotic Resistance. Zoonoses Public Health 2015, 62, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Roth, N.; Käsbohrer, A.; Mayrhofer, S.; Zitz, U.; Hofacre, C.; Domig, K.J. The application of antibiotics in broiler production and the resulting antibiotic resistance in Escherichia coli: A global overview. Poult. Sci. 2019, 98, 1791–1804. [Google Scholar] [CrossRef] [PubMed]
- Roth, N.; Mayrhofer, S.; Gierus, M.; Weingut, C.; Schwarz, C.; Doupovec, B.; Berrios, R.; Domig, K.J. Effect of an organic acids based feed additive and enrofloxacin on the prevalence of antibiotic-resistant E. coli in cecum of broilers. Poult. Sci. 2017, 96, 4053–4060. [Google Scholar] [CrossRef]
- Saeed, M.A.; Saqlain, M.; Waheed, U.; Ehtisham-Ul-Haque, S.; Khan, A.U.; Rehman, A.U.; Sajid, M.; Atif, F.A.; Neubauer, H.; El-Adawy, H. Cross-Sectional Study for Detection and Risk Factor Analysis of ESBL-Producing Avian Pathogenic Escherichia coli Associated with Backyard Chickens in Pakistan. Antibiotics 2023, 12, 934. [Google Scholar] [CrossRef]
- SAMUEL, M. , FREDRICK WABWIRE, T., TUMWINE, G. & WAISWA, P. J. V. M. I. Antimicrobial Usage by Small-Scale Commercial Poultry Farmers in Mid-Western District of Masindi Uganda: Patterns, Public Health Implications, and Antimicrobial Resistance of E. coli. 2023.
- SARKER, M. S. , MANNAN, M. S., ALI, M. Y., BAYZID, M., AHAD, A. & BUPASHA, Z. B. Antibiotic resistance of Escherichia coli isolated from broilers sold at live bird markets in Chattogram, Bangladesh. J Adv Vet Anim Res 2019, 6, 272–277. [Google Scholar]
- Schar, D.; Sommanustweechai, A.; Laxminarayan, R.; Tangcharoensathien, V. Surveillance of antimicrobial consumption in animal production sectors of low- and middle-income countries: Optimizing use and addressing antimicrobial resistance. PLoS Med. 2018, 15, e1002521. [Google Scholar] [CrossRef]
- Sevilla-Navarro, S.; Catalá-Gregori, P.; Torres-Boncompte, J.; Orenga, M.T.; Garcia-Llorens, J.; Cortés, V. Antimicrobial Resistance Trends of Escherichia coli Isolates: A Three-Year Prospective Study of Poultry Production in Spain. Antibiotics 2022, 11, 1064. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, M.; Caudell, M.A.; Mair, C.; Davis, M.A.; Matthews, L.; Quinlan, R.J.; Quinlan, M.B.; Lyimo, B.; Buza, J.; Keyyu, J.; et al. Antimicrobial resistant enteric bacteria are widely distributed amongst people, animals and the environment in Tanzania. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Tang, K.L.; Caffrey, N.P.; Nóbrega, D.B.; Cork, S.C.; E Ronksley, P.; Barkema, H.W.; Polachek, A.J.; Ganshorn, H.; Sharma, N.; Kellner, J.D.; et al. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: a systematic review and meta-analysis. Lancet Planet. Health 2017, 1, e316–e327. [Google Scholar] [CrossRef] [PubMed]
- UBOS, I. J. K. U. & ICF 2018. Uganda demographic and health survey 2016.
- Varga, C.; Guerin, M.T.; Brash, M.L.; Slavic, D.; Boerlin, P.; Susta, L. Antimicrobial resistance in fecal Escherichia coli and Salmonella enterica isolates: a two-year prospective study of small poultry flocks in Ontario, Canada. BMC Veter- Res. 2019, 15, 1–10. [Google Scholar] [CrossRef]
- Wang, Y.; Lyu, N.; Liu, F.; Liu, W.J.; Bi, Y.; Zhang, Z.; Ma, S.; Cao, J.; Song, X.; Wang, A.; et al. More diversified antibiotic resistance genes in chickens and workers of the live poultry markets. Environ. Int. 2021, 153, 106534. [Google Scholar] [CrossRef]
- WHO 2014. Antimicrobial resistance global report on surveillance: 2014 summary. WHO.
- WHO 2017. Integrated surveillance of antimicrobial resistance in foodborne bacteria: application of a one health approach: guidance from the WHO Advisory Group on Integrated Surveillanec of Antimicrobial Resistance (AGISAR).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).