Submitted:
14 February 2024
Posted:
15 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Study Design
Anthropometric and Body Composition Variables and Biochemical Parameters
Stool Shape and Consistency
Life Habits
Intestinal Microbiota Analysis
Metagenomic Analysis by DNA Sequencing
Bioinformatics Analysis of Sequencing Data
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohajan, D.; Mohajan, H.K. Obesity and Its Related Diseases: A New Escalating Alarming in Global Health. Paradigm Academic Press: J Innov Med Res. 2023; 2:3. [CrossRef]
- Kodaira, K.; Abe, F.C.; Galvão, T.F.; Silva, M.T. Time-trend in excess weight in Brazilian adults: A systematic review and meta-analysis. PLoS ONE. 2021; 16: e0257755. [CrossRef]
- Abevanoli, L.; Scarpellini, E.; Colica, C.; Boccuto, L.; Salehi, B.; Sharifi–Rad J.; Aiello, V.; Romano, B.; De Lorenzo, A.; Izzo, A.A.; Capasso, R. Gut Microbiota and Obesity: A Role for Probiotics. Nutrients. 2019; 11:2690. [CrossRef]
- Breton, J.; Galmiche, M.; Dechelotte, P. Dysbiotic Gut Bacteria in Obesity: An Overview of the Metabolic Mechanisms and Therapeutic Perspectives of Next-Generation Probiotics. Microorganisms. 2022; 10:452. [CrossRef]
- Brasil, Ministério da Saúde, Vigitel Brasil 2006-2021 : vigilância de fatores de risco e proteção para doenças crônicas por inquérito telefônico : estimativas sobre frequência e distribuição sociodemográfica do estado nutricional e consumo alimentar nas capitais dos 26 estados brasileiros e no Distrito Federal entre 2006 e 2021 : estado nutricional e consumo alimentar [recurso eletrônico] / Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Análise em Saúde e Vigilância de Doenças Não Transmissíveis. – Brasília: Ministério da Saúde, 2022.
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism. 2019; 92:6–10. [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Suganthy, N.; Chaiyasut, C. A Review on Role of Microbiome in Obesity and Antiobesity Properties of Probiotic Supplements. BioMed Res Int. 2019. [CrossRef]
- Masood, A.; Alsheddi, L.; Alfayadh, L.; Bukhari, B.; Elawad, R.; Alfadda, A.A. Dietary and Lifestyle Factors Serve as Predictors of Successful Weight Loss Maintenance Postbariatric Surgery. J Obes. 2019; 7295978. [CrossRef]
- Borgeraas, H.; Johnson, L.K.; Skattebu, J.; Hertel, J.K.; Hjelmesaeth, J. Effects of probiotics on body weight, body mass index, fat mass and fat percentage in subjects with overweight or obesity: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2018; 19:219–232. [CrossRef]
- Duranti, S.; Ferrario, C.; Sinderen, D.V.; Ventura, M.; Turroni, F. Obesity and microbiota: an example of an intricate relationship. Nutr Genes. 2017; 12:18. [CrossRef]
- Vijay, A.; Valdes, A.M. Role of the gut microbiome in chronic diseases: a narrative review. Eur J Clin Nutr. 2022; 76:489-501. [CrossRef]
- Brusaferro, A.; Cozzali, R.; Orabona, C.; Biscarini A, Farinelli E, Cavalli E et al. Is It Time to Use Probiotics to Prevent or Treat Obesity? Nutrients. 2018; 10(11):1613. [CrossRef]
- Parkar, S.G.; Kalsbeek, A.; Cheeseman, J.F. Potential Role for the Gut Microbiota in Modulating Host Circadian Rhythms and Metabolic Health. Microorganisms. 2019; 7:41. [CrossRef]
- Aya, V.; Flórez, A.; Perez, L.; Ramírez, J.D. Association between physical activity and changes in intestinal microbiota composition: A systematic review. PLoS ONE. 2021; 16:e0247039. [CrossRef]
- Hadi, A.; Sepandi, M.; Marx, W.; Moradi, S.; Parastouei, K. Clinical and psychological responses to synbiotic supplementation in obese or overweight adults: A randomized clinical trial. Complement Ther Med. 2019; 47:102216. [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021; 19:55-71. [CrossRef]
- Sankararaman, S.; Noriega, K.; Velayuthan, S.; Sferra, T.; Martindale, R. Gut Microbiome and Its Impact on Obesity and Obesity-Related Disorders. Curr Gastroenterol. 2023; 25:31–44. [CrossRef]
- Kobyliak, N.; Falalyeyeva, T.; Boyko, N.; Tsyryuk, O.; Beregova, T.; Ostapchenko, L. Probiotics and nutraceuticals as a new frontier in obesity prevention and management. Diabetes Res Clin Pract. 2018; 141:190–199. [CrossRef]
- Vallianou, N.; Stratigou, T.; Christodoulatos, G.S.; Tsigalou, C.; Dalamaga, M. Probiotics, Prebiotics, Synbiotics, Postbiotics, and Obesity: Current Evidence, Controversies, and Perspectives. Curr Obes. 2020; 9:179–192. [CrossRef]
- Wang, Z.; Xin, S.; Ding, L.; Ding, W.; Hou, Y.; Liu, C.Q.; Zhang, X.D. The Potential Role of Probiotics in Controlling Overweight/Obesity and Associated Metabolic Parameters in Adults: A Systematic Review and Meta-Analysis. Evid Based Complement Alternat Med. 2019. [CrossRef]
- Bui, T.P.N.; Vos, W.M. Next-generation therapeutic bacteria for treating obesity, diabetes, and other endocrine diseases. Best Pract Res Clin Endocrinol Metab. 2021; 35:101504. [CrossRef]
- Park, S.; Bae, J.H. Probiotics for weight loss: a systematic review and meta-analysis. Nutr Res. 2015; 35:566-75. [CrossRef]
- Chattopadhyay, A.; Mythili, S. The journey of gut microbiome – An introduction and its influence on metabolic disorders. Front Biol. 2018; 13:327–341. [CrossRef]
- Boulangé, C.L.; Neves, A.L.; Chilloux, J.; Nicholson, J.K.; Dumas, M.E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Medicine. 2016; 8:42. [CrossRef]
- Wiciński, M.; Gębalski, J.; Gołębiewski, J.; Malinowski, B. Probiotics for the Treatment of Overweight and Obesity in Humans—A Review of Clinical Trials. Microorganisms. 2020; 8:1148. [CrossRef]
- A healthy lifestyle–WHO recommendations [Internet]. [cited 14 September 2022]. Available sur.https://www.who.int/europe/news-room/fact-sheets/item/a-healthy-lifestyle---who-recommendations. 14 September.
- Martinez, A.P.; Azevedo, G.R. The Bristol Stool Form Scale: its translation to Portuguese, cultural adaptation and validation. Rev Latino Am Enfermagem. 2012; 20:583–9. [CrossRef]
- Molina, M.D.C.B.; Benseñor, I.M.; Cardoso, L.D.O.; Velasquez-Melendez, G.; Drehmer, M.; Pereira, T.S.S,. Faria C.P.; Melere, C.; Manato, L.; Gomes, A.L.C.; Fonseca, M.J.M.; Sichieri, R. Reproducibility and relative validity of the Food Frequency Questionnaire used in the ELSA-Brasil. Cad de Saude Publica. 2013; 379-389. [CrossRef]
- Thompson, E. Hamilton Rating Scale for Anxiety (HAM-A). Occup Med. 2015; 65:601. [CrossRef]
- Mollayeva, T.; Thurairajah, P.; Burton, K.; Mollayeva, S.; Shapiro, C.M.; Colantonio, A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Med Rev. 2016; 25:52-73. [CrossRef]
- Matsudo, S.; Araújo, T.; Matsudo, V.; Andrade, D.; Andrade, E.; Oliveira, L.C.; Braggion, G. International physical activity questionnaire (IPAQ): validity and reproducibility study in Brazil. Rev. Bras. Activation. Phys. Health. 2012; 6:5-18.
- Heinsen, F.A.; Fangmann, D.; Müller, N.; Schulte, D.M.; Rühlemann, M.C.; Türk, K.; Settgast, U.; Lieb, W.; Baines, J.F.; Schreiber, S.; Franke, A.; Laudes, M. Beneficial Effects of a Dietary Weight Loss Intervention on Human Gut Microbiome Diversity and Metabolism Are Not Sustained During Weight Maintenance. Obes Facts. 2016;9:379-391. [CrossRef]
- Szulińska, M.; Łoniewski, I.; van Hemert, S.; Sobieska, M.; Bogdański, P. Dose-Dependent Effects of Multispecies Probiotic Supplementation on the Lipopolysaccharide (LPS) Level and Cardiometabolic Profile in Obese Postmenopausal Women: A 12-Week Randomized Clinical Trial. Nutrients. 2018;10:773. [CrossRef]
- Song, E.J.; Han, K.; Lim, T.J.; Lim, S.; Chung, M.J.; Nam, M.H.; Kim, H.; Nam, Y.D. Effect of probiotics on obesity-related markers per enterotype: a double-blind, placebo-controlled, randomized clinical trial. EPMA J. 2020; 11:31–51. [CrossRef]
- Rouxinol-Dias, A.L.; Pinto, A.R.; Janeiro, C.; Rodrigues, D.; Moreira, M.; Dias, J.; Pereira, P. Probiotics for the control of obesity – Its effect on weight change. Porto Biom J. 2016. [CrossRef]
- Aoun, A.; Darwish, F.; Hamod, N. The Influence of the Gut Microbiome on Obesity in Adults and the Role of Probiotics, Prebiotics, and Synbiotics for Weight Loss. Prev Nutr Food Sci. 2020, 25:113-123. [CrossRef]
- Narmaki, E.; Borazjani, M.; Ataie-Jafari, A.; Hariri, N.; Doost, A.H.; Qorbani, M.; Saidpour, A . The combined effects of probiotics and restricted calorie diet on the anthropometric indices, eating behavior, and hormone levels of obese women with food addiction: a randomized clinical trial. Nutr Neurosci. 2022; 25:963-975. [CrossRef]
- Ataey, A.; Jafarvand, E.; Adham, D.; Moradi-Asl, E. The Relationship Between Obesity, Overweight, and the Human Development Index in World Health Organization Eastern Mediterranean Region Countries. J Prev Med Public Health. 2020; 53:98-105. [CrossRef]
- Mazloom. K.; Siddiqi, I.; Covasa, M. Probiotics: How Effective Are They in the Fight against Obesity? Nutrients. 2019; 11:258. [CrossRef]
- Kobyliak, N.; Conte, C.; Cammarota, G.; Haley, A.P.; Styriak, I.; Gaspar, L.; Fusek, J.; Rodrigo, L.; Kruzliak, P. Probiotics in prevention and treatment of obesity: a critical view. Nutr Metab. 2016; 13:14. [CrossRef]
- Quiroga, R.; Nistal, E.; Estébanez, B.; Damn, D.; Juárez-Fernández, M.; Martínez-Flórez, S.; García-Mediavilla, M.V.; Paz, J.A.; González-Gallego, J.; Sánchez-Campos, S.; Cuevas, M.J. Exercise training modulates the gut microbiota profile and impairs inflammatory signaling pathways in obese children. Exp Mol Med. 2020; 52: 1048–1061. [CrossRef]
- Sohail, M.U.; Yassine, H.M.; Sohail, A.; Thani, A.A.A. Impact of Physical Exercise on Gut Microbiome, Inflammation, and the Pathobiology of Metabolic Disorders. Rev Diabet Stud. 2019; 15:35-48. [CrossRef]
- Kern, T.; Blond, M.B.; Hansen, T.H.; Rosenkilde, M.; Quist, J.S.; Gram, A.S.; Ekstrøm CT, Hansen T, Stallknecht B. Structured exercise alters the gut microbiota in humans with overweight and obesity—A randomized controlled trial. Int J Obes. 2020; 44:125–135. [CrossRef]
- Wang, K.; Mehta, R.S.; Ma, W.; Nguyen, L.H.; Wang, D.D.; Ghazi, A.R.; Yan, Y.; Al-Shaar, L.; Wang, Y.; Hang, D.; Fu, B.C.; Ogino, S.; Rimm, E.B.; Hu, F.B.; Carmody, R.N.; Garrett, W.S.; Sun, Q.; Chan, A.T.; Huttenhower, C.; Song, M. The gut microbiome modifies the associations of short- and long-term physical activity with body weight changes. Microbiome. 2023; 11:121. [CrossRef]
- Hughes, R.L.; Pindus, D.M.; Khan, N.A.; Burd, N.A.; Holscher, H.D. Associations between Accelerometer-Measured Physical Activity and Fecal Microbiota in Adults with Overweight and Obesity. Med Sci Sports Exercise. 2023; 55:680-689. [CrossRef]
- Kang, S.S.; Jeraldo, P.R.; Kurti, A.; Miller, M.E.B.; Cook, M.D.; Whitlock, K.; Goldenfeld, N.; Woods, J.A.; White, B.A.; Chia, N.; Fryer, J.D. Diet and exercise orthogonally alter the gut microbiome and reveal independent associations with anxiety and cognition. Mol Neurodegener. 2014; 9:36. [CrossRef]
- Acharya, K.D.; Graham, M.; Raman, H.; Parakoyi, A.E.R.; Corcoran, A.; Belete, M.; Ramaswamy, B.; Koul, S.; Sachar, I.; Derendorf, K.; Wilmer, J.B.; Gottipati, S.; Tetel, M.J. Estradiol-mediated protection against high-fat diet induced anxiety and obesity is associated with changes in the gut microbiota in female mice. Sci Rep. 2023; 13: 4776. [CrossRef]
- Quicho, M.N.B. Microbiota-Derived Metabolites and Their Effects on Anxiety-Related Behavior. Los Angeles: University of California; 2023.
- Cai, Y.; Liu, P.; Zhou, X.; Yuan, J.; Chen, Q. Probiotics therapy show significant improvement in obesity and neurobehavioral disorders symptoms. Front Cell Infect Microbiol. 2023; 13:1178399. [CrossRef]
- Kaunang, T.M.D.; Setiawan, A.A.; Mayulu, N.; Leonita, I.; Wijaya, A.; Yusuf, V.M.; Mahira, M.F.N.A.; Yudisthira, D.; Gunawan, W.B.; Taslim, N.A.; Purnomo, A.F.; Sabrina, N.; Amalia, N.; Permatasari, H.K.; Nurkolis, F. Are probiotics beneficial for obese patients with major depressive disorder? Opinion for future implications and strategies. Front. Nutr. 2023; 10:1205434. [CrossRef]
- Ko, C.Y.; Liu, Q.Q.; Su, H.Z.; Zhang, H.P.; Fan, J.M.; Yang, J.H.; Hu, A.K.; Liu, Y.Q.; Chou, D.; Zeng, Y.M. Gut microbiota in obstructive sleep apnea–hypopnea syndrome: disease-related dysbiosis and metabolic comorbidities. Clin Sci. 2019; 133:905–917. [CrossRef]
- Kuvat, N.; Tanriverdi, H.; Armutcu, F. The relationship between obstructive sleep apnea syndrome and obesity: A new perspective on the pathogenesis in terms of organ crosstalk. Clin Respir J. 2020; 14:595–604. [CrossRef]
- Wang, Y.; Wouw, M.V.D.; Drogos, L.; Mehrabani, E.V.; Reimer, R.A.; Madsen, L.T.; Giesbrecht, G.F. Sleep and the gut microbiota in preschool-aged children. Sleep. 2022; 45:zsac020. [CrossRef]
- Valentini, F.; Evangelisti, M.; Arpinelli, M.; Nardo, G.D.; Borro, M.; Simmaco, M.; Villa, M.P. Gut microbiota composition in children with obstructive sleep apnoea syndrome: a pilot study. Sleep Med. 2020; 76:140-147. [CrossRef]
- Benedict, C.; Vogel, H.; Jonas, W.; Woting, A.; Blaut, M.; Schürmann, A.; Cedernaes, J. Gut microbiota and glucometabolic alterations in response to recurrent partial sleep deprivation in normal-weight young individuals. Molecular Metabolism. 2016; 5:1175-1186. [CrossRef]
- Anderson, J.R.; Carroll, I.; Azcarate-Peril, M.A.; Rochette, A.D.; Heinberg, L.J.; Peat, C.; Steffen, K.; Manderino, L.M.; Mitchell, J.; Gunstad, J. A preliminary examination of gut microbiota, sleep, and cognitive flexibility in healthy older adults. Sleep Med. 2017; 38:104-107. [CrossRef]
- Sen, P.; Molinero-Perez, A.; O’Riordan, K.J.; McCafferty, C.P.; O’Halloran, K.D.; Cryan, J.F. Microbiota and sleep: awakening the gut feeling. Trends Mol Med. 2021; 27:935-945. [CrossRef]
- Smith, R.P.; Easson, C.; Lyle, S.M.; Kapoor, R.; Donnelly, C.P.; Davidson, E.J.; Parikh, E.; Lopez, J.V.; Tartar, J.L. Gut microbiome diversity is associated with sleep physiology in humans. PLoS ONE. 2019;14:e0222394. [CrossRef]
- Aziz, T.; Hussain, N.; Hameed, Z.; Lin, L. Elucidating the role of diet in maintaining gut health to reduce the risk of obesity, cardiovascular and other age-related inflammatory diseases: recent challenges and future recommendations. Gut Microbes. 2024; 16:2297864. [CrossRef]
- Morales, J.S.; Valenzuela, P.L.; Castillo-García, A.; Butragueño, J.; Jiménez-Pavón, D.; Carrera-Bastos, P.; Lucia, A. The Exposome and Immune Health in Times of the COVID-19 Pandemic. Nutrients. 2022; 14: 24. [CrossRef]
- Rinninella, E.; Tohumcu, E.; Raoul, P.; Fiorani, M.; Cintoni, M.; Mele, M.C.; Cammarota, G.; Gasbarrini, A.; Ianiro, G. The role of diet in shaping human gut microbiota. Best Pract Res Clin Gastroenterol. 2023; 63:101828. [CrossRef]
- Li, J.; Riaz Rajoka, M.S.; Shao, D.; Jiang, C.; Jin, M.; Huang, Q.; Yang, H.; Shi, J. Strategies to increase the efficacy of using gut microbiota for the modulation of obesity. Obes Rev. 2017;18:1260-1271. [CrossRef]
- Gumma, E.Z. Human gut microbiota/microbiome in health and diseases: a review. Antonie van Leeuwenhoek. 2020; 113:2019–2040. [CrossRef]
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell Mol Life Sci. 2019; 76:473-493. [CrossRef]
- Hur, K.Y.; Lee, M.S. Gut Microbiota and Metabolic Disorders. Diabetes Metab J. 2015; 39:198-203. [CrossRef]
- Leo, E.E.M.; Peñafiel, A.M.; Escalante, V.M.H.; Araujo, Z.M.C. Ultra-processed diet, systemic oxidative stress, and breach of immunological tolerance. Nutrition. 2021; 92:111419. [CrossRef]
- Dahiya, D.K.; Renuka, Puniya, M.; Shandilya, U.K.; Dhewa, T.; Kumar, N.; Kumar, S.; Puniya, A.K.; Shukla, P. Gut Microbiota Modulation and Its Relationship with Obesity Using Prebiotic Fibers and Probiotics: A Review. Front Microbiol. 2017; 8:563. [CrossRef]
- Armet, A.M.; Deehan, E.C.; O’Sullivan, A.F.; Mota, J.F.; Field, C.J.; Prado, C.M.; Lucey, A.J.; Walter, J. Rethinking healthy eating in light of the gut microbiome. Cell Host Microbe. 2022; 30:764-785. [CrossRef]
- Yan, S.; Tian, Z.; Li, M.; Li, B.; Cui, W. Effects of probiotic supplementation on the regulation of blood lipid levels in overweight or obese subjects: a meta-analysis. Food Funct. 2019; 10:1747. [CrossRef]
- Shirvani-Rad, S.; Tabatabaei-Malazy, O.; Mohseni, S.; Hasani-Ranjbar, S.; Soroush, A.R.; Hoseini-Tavassol, Z.; Ejtahed, H.S.; Larijani, B. Probiotics as a Complementary Therapy for Management of Obesity: A Systematic Review. Evid Based Complement Alternat Med. 2021; 22:2021:6688450. [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr. 2018; 57:1-24. [CrossRef]
- Asemi, Z.; Zare, Z.; Shakeri, H.; Sabihi, S.S.; Esmaillzadeh, A. Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Ann Nutr Metab. 2013; 63:1-9. [CrossRef]
- Perna, S.; Ilyas, Z.; Giacosa, A.; Gasparri, C.; Peroni, G.; Faliva, M.A.; Rigon, C.; Naso, M.; Riva, A.; Petrangolini, G.; Redha, A.A.; Rondanelli, M. Is Probiotic Supplementation Useful for the Management of Body Weight and Other Anthropometric Measures in Adults Affected by Overweight and Obesity with Metabolic Related Diseases? A Systematic Review and Meta-Analysis. Nutrients. 2021; 13:666. [CrossRef]
- Fontané, L.; Benaiges, D.; Goday, A.; Llauradó, G.; Pedro-Boteta, J. Influence of microbiota and probiotics on obesity. Clin Investig Arterioscler. 2018; 30:271-279. [CrossRef]
- Rehman, A.; Tyree, S.M.; Fehlbaum, S.; DunnGalvin, G.; Panagos, C.G.; Guy, B.; Patel, S.; Dinan, T.G.; Duttaroy, A.K.; Duss, R.; Steinert, R.E. A water-soluble tomato extract rich in secondary plant metabolites lowers trimethylamine-n-oxide and modulates gut microbiota: a randomized, double-blind, placebo-controlled cross-over study in overweight and obese adults. J Nut. 2023; 153:96–105. [CrossRef]
- Vallianou, N.G.; Kounatidis, D.; Tsilingiris, D.; Panagopoulos, F.; Christodoulatos, G.S.; Evangelopoulos, A.; Karampela, I.; Dalamaga, M. The Role of Next-Generation Probiotics in Obesity and Obesity-Associated Disorders: Current Knowledge and Future Perspectives. Int J Mol Sci. 2023; 24:6755. [CrossRef]
- Cerdó, T.; García-Santos, J.A.; Bermúdez, M.G.; Campoy C. The Role of Probiotics and Prebiotics in the Prevention and Treatment of Obesity. Nutrients. 2019;11:635. [CrossRef]
- Aguilera, X.E.L.; Manzano, A.; Pirela, D.; Bermúdez, V. Probiotics and Gut Microbiota in Obesity: Myths and Realities of a New Health Revolution. J Pers Med. 2022; 12:1282. [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients. 2020;12:1474. [CrossRef]
- Włodarczyk, M.; S’lizewska, K. Obesity as the 21st Century’s major disease: The role of probiotics and prebiotics in prevention and treatment. Food Bioscience. 2021. [CrossRef]
- Fitch, A.K.; Bays, H.E. Obesity definition, diagnosis, bias, standard operating procedures (SOPs), and telehealth: An Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022. Obesity Pillars. 2022; 1:100004. [CrossRef]
- Ben Othman, R.; Ben Amor, N.; Mahjoub, F.; Berriche, O.; El Ghali, C.; Gamoudi, A.; Jamoussi, H. A clinical trial about effects of prebiotic and probiotic supplementation on weight loss, psychological profile and metabolic parameters in obese subjects. Endocrinol Diabetes Metab. 2023; 6:e402. [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms. 2020; 8:1715. [CrossRef]
- Arora, T.; Singh, S.; Sharma, R.K. Probiotics: Interaction with gut microbiome and antiobesity potential. Nutrition. 2013; 29:591-6. [CrossRef]
- Perumpail, B.J.; Li, A.A.; John, N.; Sallam, S.; Shah, N.D.; Kwong, W.; Cholankeril, G.; Kim, D.; Ahmed, A. The Therapeutic Implications of the Gut Microbiome and Probiotics in Patients with NAFLD. Diseases. 2019;7:27. [CrossRef]
- Kim, S.K.; Guevarra, R.B.; Kim, Y.T.; Kwon, J.; Kim, H.; Cho, J.H.; Kim, H.B.; Lee, J.H. Role of Probiotics in Human Gut Microbiome-Associated Diseases. J Microbiol Biotechnol. 2019; 29:1335-1340. [CrossRef]
- Cox, A.J.; West, N.P.; Cripps, A.W. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. 2015; 3:207-15. [CrossRef]
- Barengolts, E. Gut microbiota, prebiotics, probiotics, and synbiotics in management of obesity and prediabetes: review of randomized controlled trials. Endocr Pract. 2016; 22:1224-1234. [CrossRef]
- Maioli, T.U.; Borras-Nogues, E.; Torres, L.; Barbosa, S.C.; Martins, V.D.; Langella, P.; Azevedo, V.A.; Chatel, J.M. Possible Benefits of Faecalibacterium prausnitzii for Obesity-Associated Gut Disorders. Front Pharmacol. 2021; 12:740636. [CrossRef]
- Mithieux, G. Gut Microbiota and Host Metabolism: What Relationship. Neuroendocrinology. 2018; 106:352-356. [CrossRef]
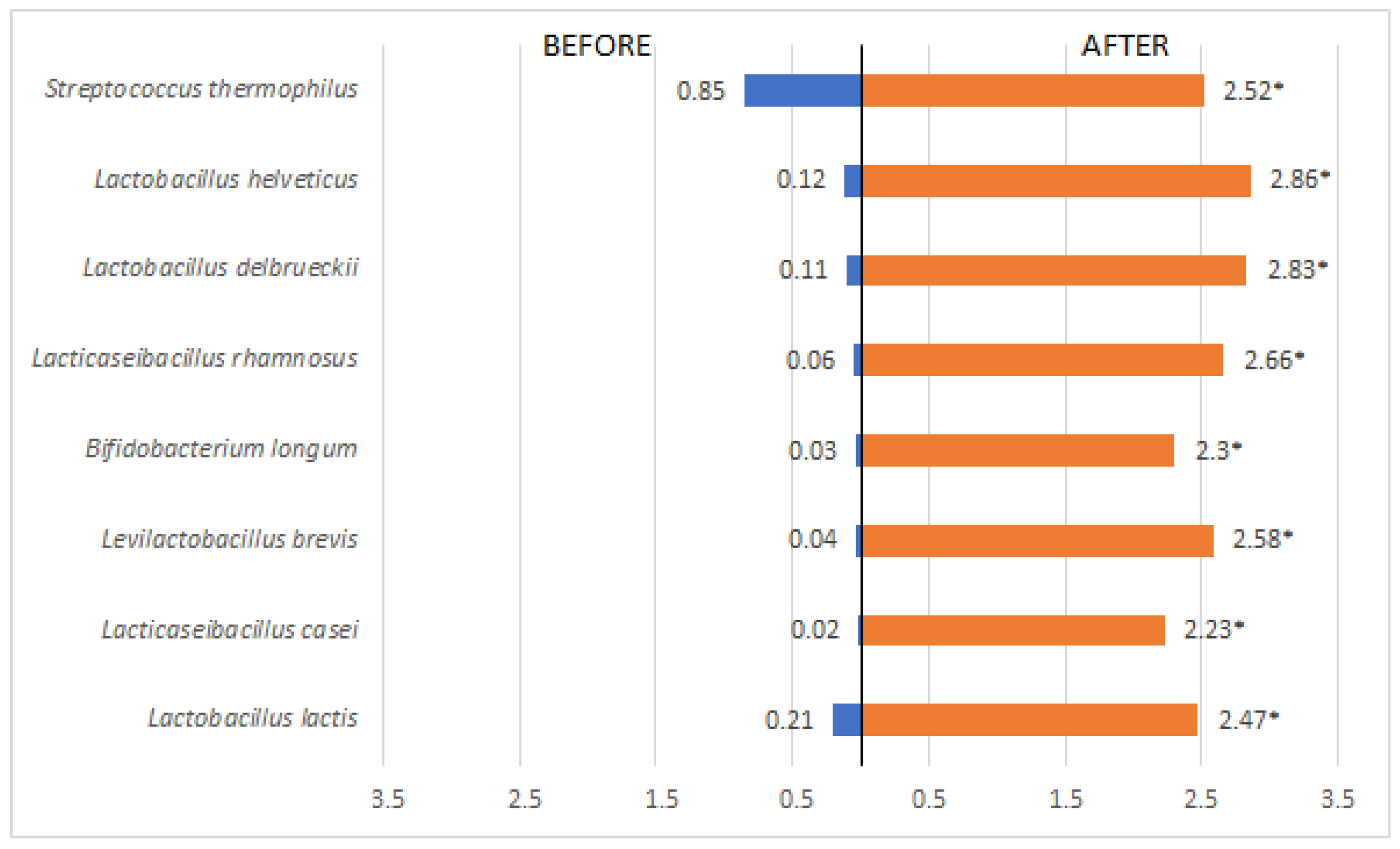
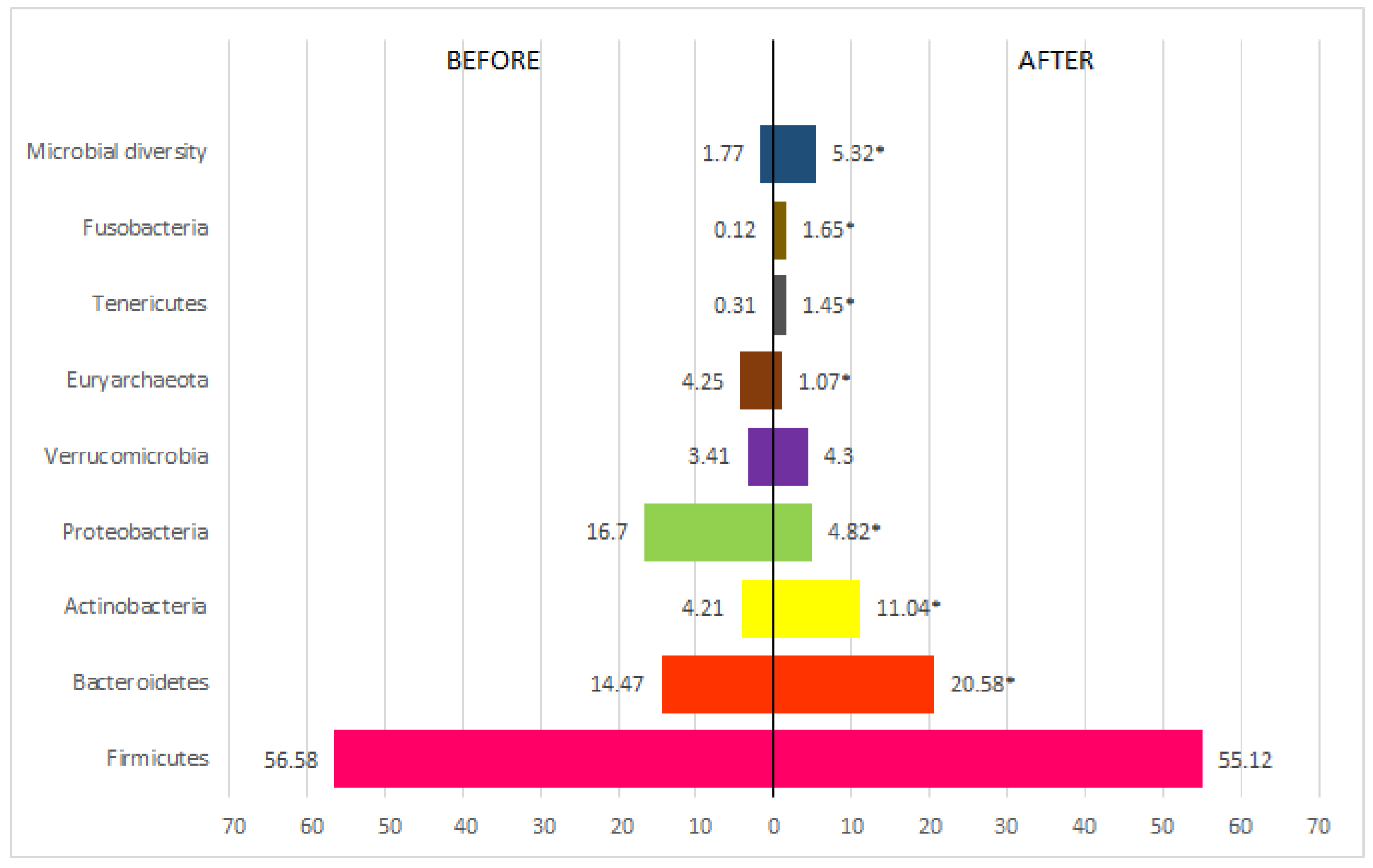
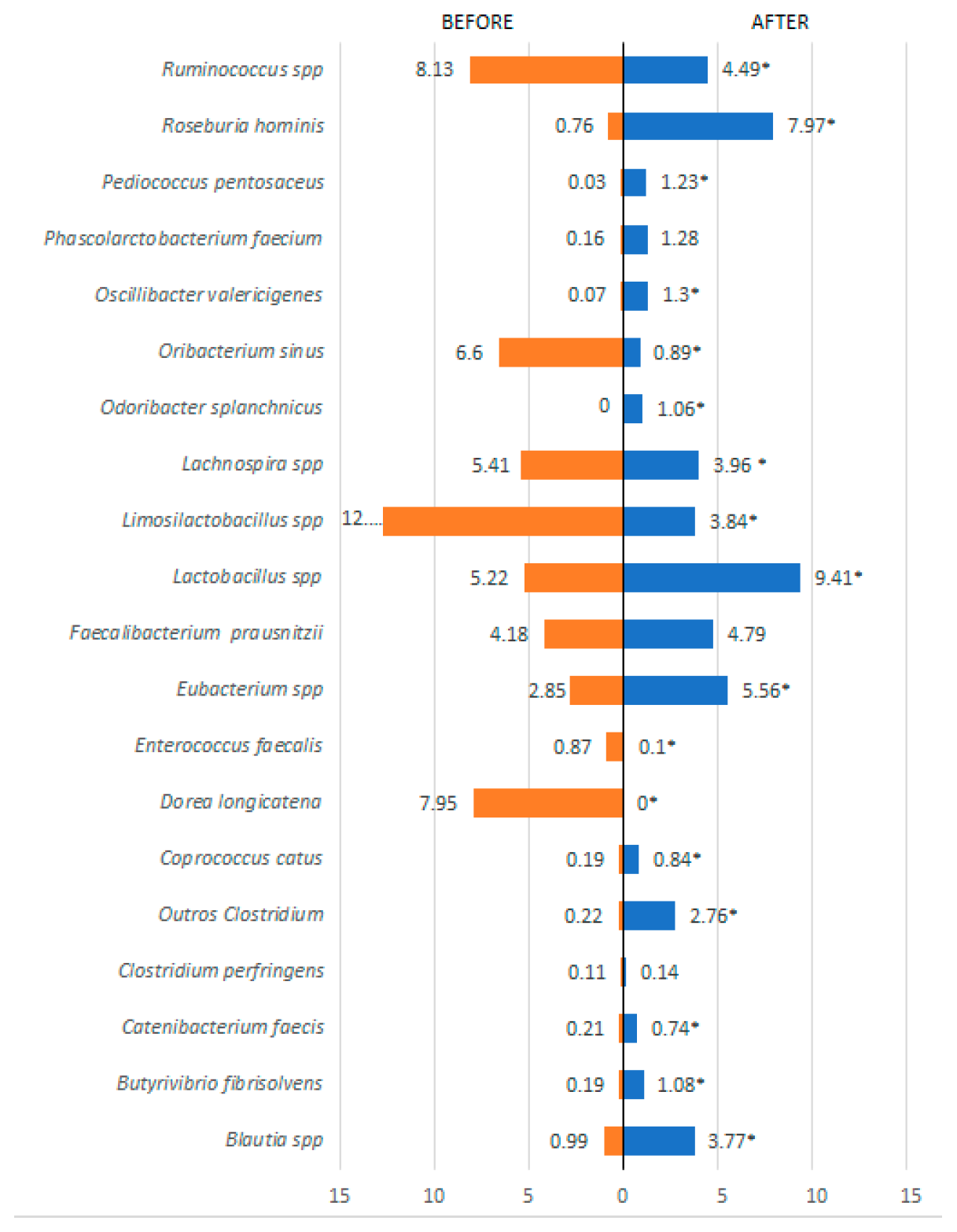
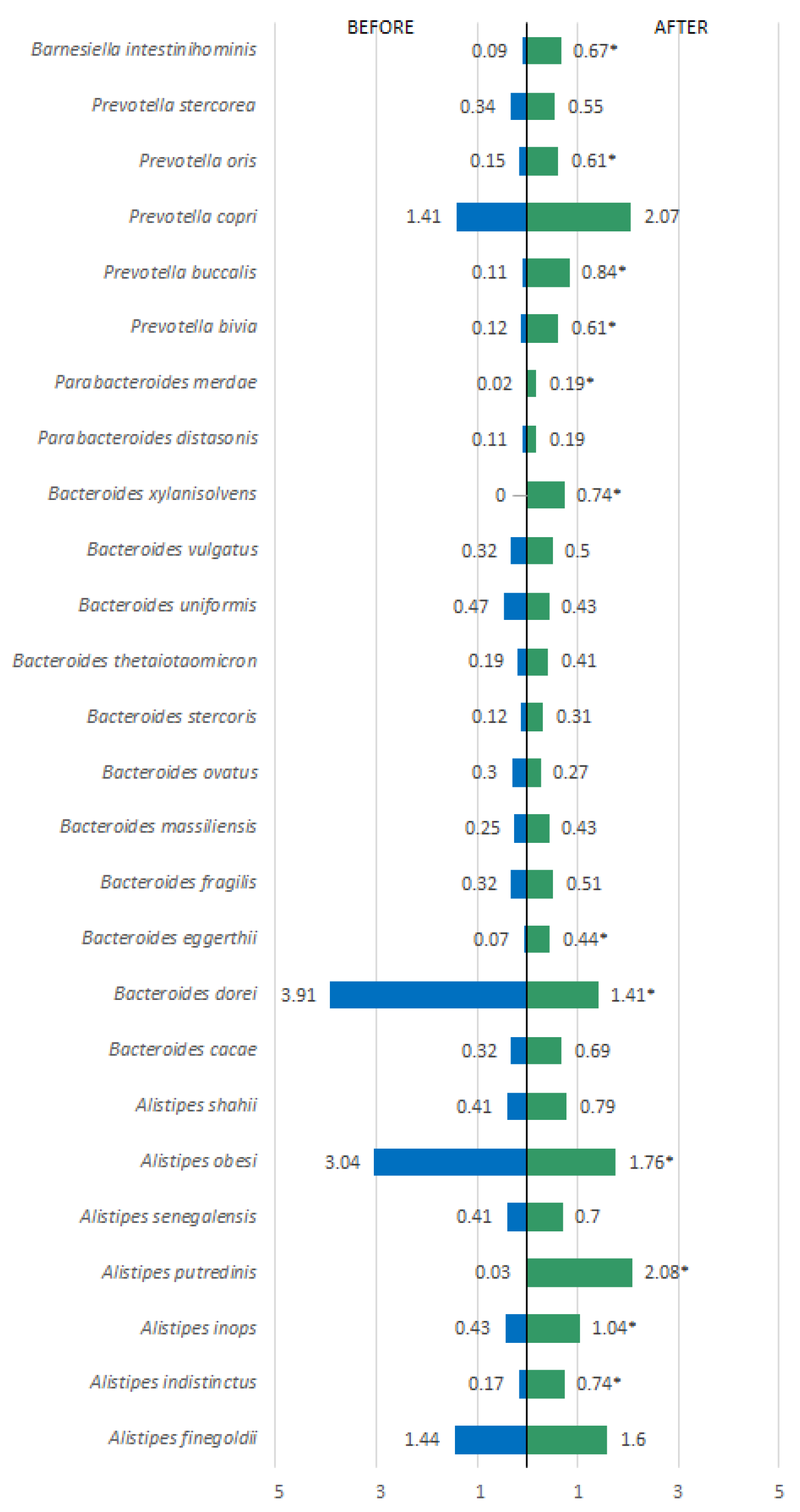
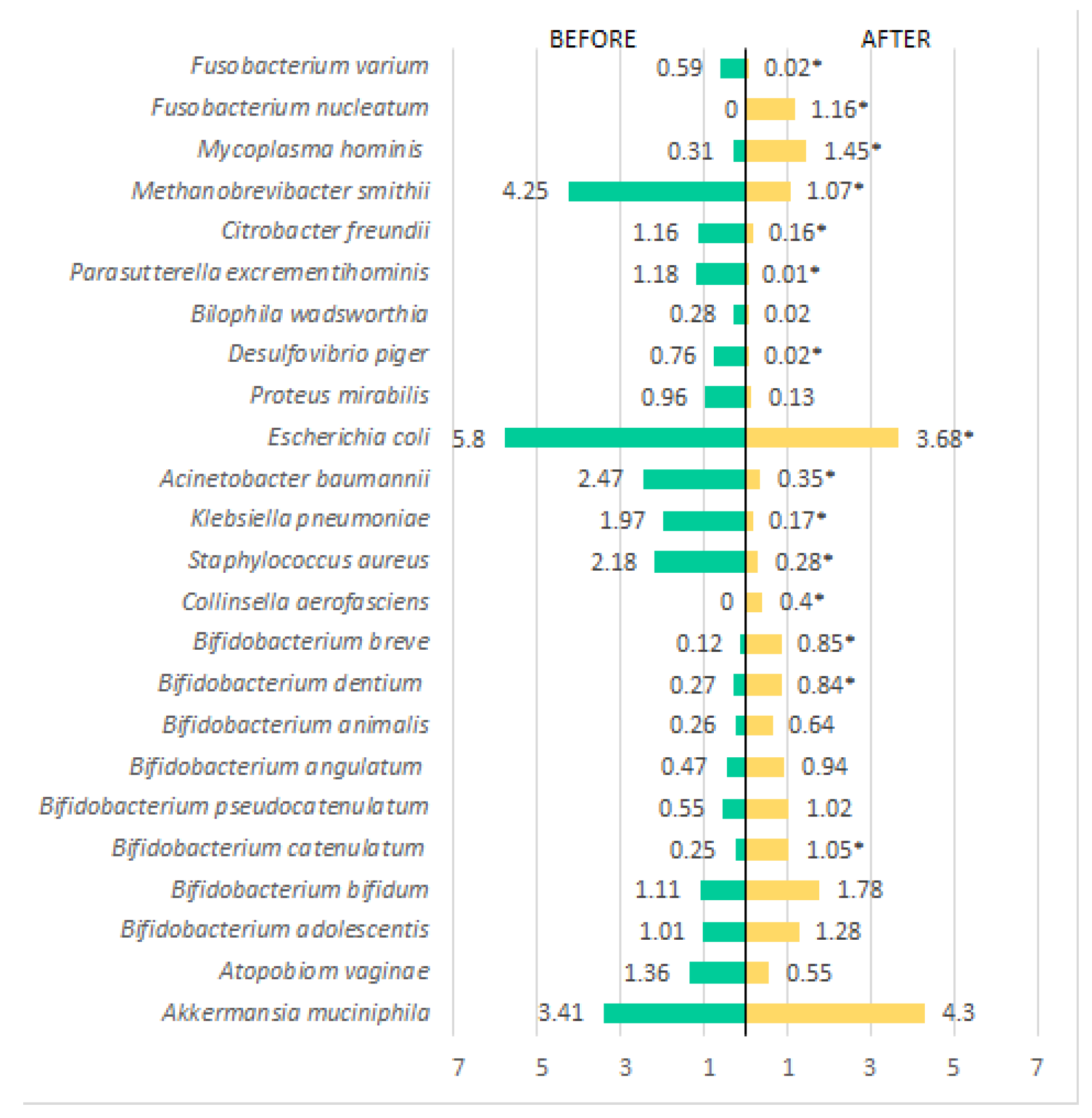
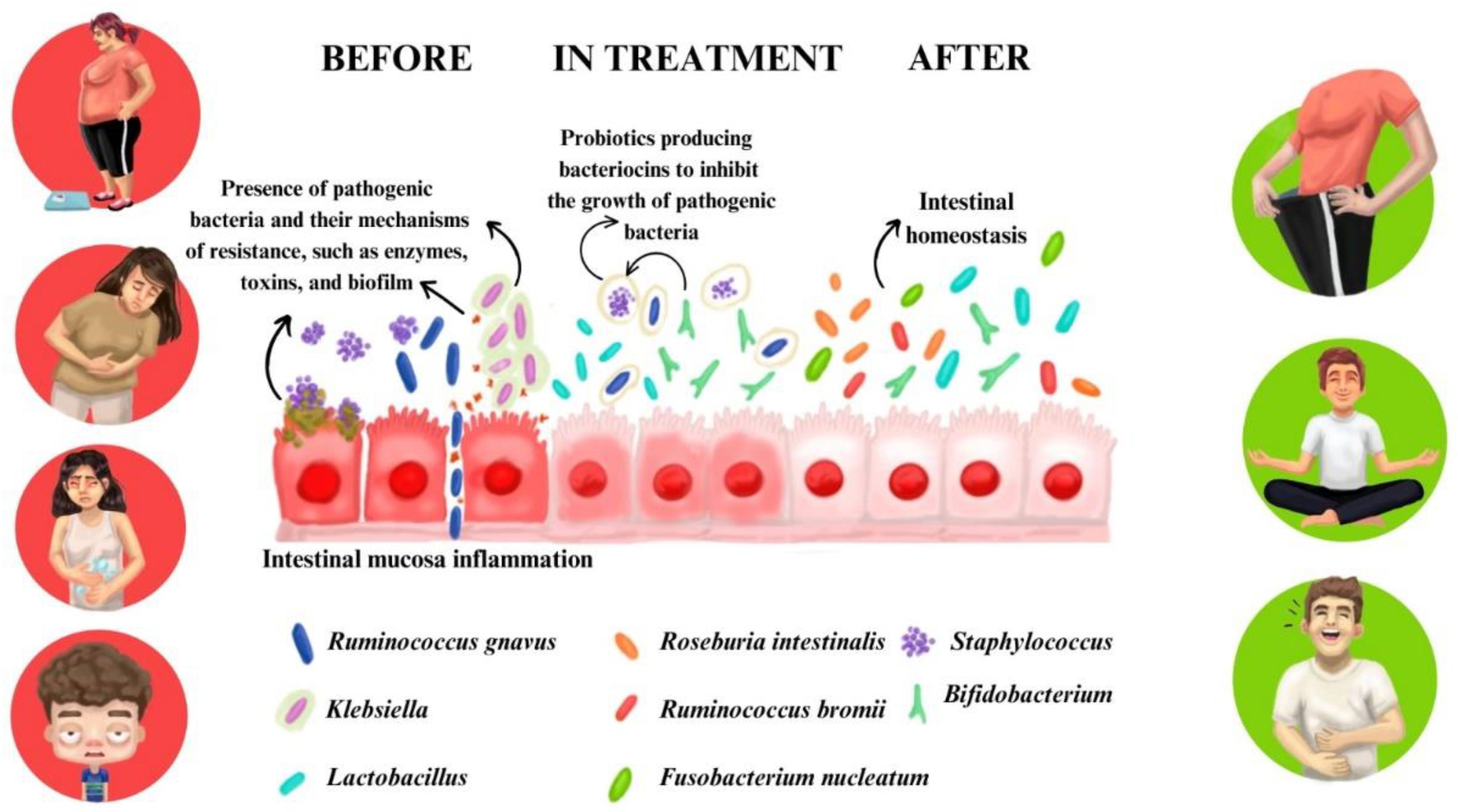
| Variables | Pacients (n=45) | OR (IC)* | Value p** | |
| Before (t0) N(%)1 |
After (t1) N(%) |
|||
| Anxiety - Hamilton | ||||
| No anxiety | 1 (2.2) | 25 (55.6) | 55.00(7.59-307.78) | <0.001 |
| Temporary anxiety | 6 (13.3) | 16 (35.6) | 3.58 (1.14-12.45) | |
| Moderate anxiety | 14(31.1) | 2 (4.4) | 0.10 (0.01-0.51) | |
| Severe anxiety | 24(53.3) | 2 (4.4) | 0.04 (0.00-0.19) | |
| IPAQ | ||||
| Very active | 8 (17.8) | 34 (75.6) | 14.29(4.65-45.58) | <0.001 |
| Active | 9 (20.0) | 8 (17.8) | 0.86 (0.30-2.49) | |
| Irregularly active | 8 (17.8) | 0 (0) | NC2 | |
| Sedentary | 20 (44.4) | 3 (6.7) | 0.09 (0.01-0.35) | |
| Sleep – Pittsburgh | ||||
| Good sleep quality | 14 (31.1) | 39 (86.7) | 14.39 (4.51-49.80) | <0.001 |
| Poor sleep quality | 31 (68.9) | 6 (13.3) | 0.07 (0.02-0.22) | |
| Fiber consumption | ||||
| Yes | 39 (86.7) | 43 (95.6) | 3.30(0.63-17.36) | 0.125 |
| No | 6 (13.3) | 2 (4.4) | 0.30(0.06-1.54) | |
| Water intake (cups/day) | ||||
| 1 | 2 (4.4) | 6 (13.3) | 3.30(0.63-17.36) | <0.001 |
| 2-5 | 31 (68.9) | 5 (11.1) | 0.06(0.02-0.17) | |
| 6-9 | 8 (17.8) | 11 (24.4) | 1.50(0.54-4.16) | |
| ≥ 10 | 4 (8.9) | 23 (51.1) | 10.72(3.29-34.92) | |
| Soda consumption | ||||
| Consumes | 30 (66.7) | 12 (26.7) | 0.18 (0.07-0.45) | <0.001 |
| Does not consume | 15 (33.3) | 33 (73.3) | 5.50(2.23-13.61) | |
| Consumption of processed foods | ||||
| Yes | 41 (91.1) | 9 (20.0) | 0.02(0.01-0.09) | <0.001 |
| No | 4 (8.9) | 36 (80.0) | 41.00(11.63-144.55) | |
| Variables | Patients (n=45) | OR (IC)* | Value p** |
|
| Antes (t0) | Após (t1) | |||
| Weight (kg) (1 | 96.67 ± 14.89 | 90.93 ± 15.01 | - | <0.001 |
| Body fat mass (Kg)( | 39.48 ± 9.58 | 33.19 ± 8.36 | - | <0.001 |
| Lean body mass (kg)( | 28.59 ± 6.17 | 29.21 ±6.42 | - | 0.158 |
| BMI | 34.63 ±4.97 | 33.53 ± 5.35 | - | 0.035 |
| Glucose (mmol/L) | 95.93 ± 9.86 | 87.60 ± 6.49 | - | <0.001 |
| HBa1c (%) | 5.59 ± 0.45 | 5.44 ± 0.36 | - | 0.01 |
| Insulin (µUI/I) | 14.33 ± 7.44 | 8.83 ± 5.14 | - | <0.001 |
| HOMA-IR | 3.36 ± 1.84 | 2.37± 2.65 | - | 0.002 |
| HDL (mmol/L)( | 47.62 ± 12.35 | 56.02 ± 13.70 | - | <0.001 |
| LDL (mmol/L) | 136.36 ± 44.07 | 110.69 ± 39.76 | - | <0.001 |
| Triglycerides (mmol/L) | 171.09 ± 97.66 | 103.64 ± 46.01 | - | <0.001 |
| BMI Classification - N(%)2 | ||||
| Overweight | 0 (0) | 13 (28.9) | NC3 | 0.09 |
| Obesity Grade I | 34 (75.60) | 18 (40) | 0.22(0.09-0.53) | |
| Obesity Grade II | 6 (13.30) | 9 (20) | 1.62(0.53-5.02) | |
| Obesity Grade III | 5 (11.1) | 5 (11.1) | 1.00(0.27-3.72) | |
| Bowel Movement Frequency N(%) | ||||
| Daily | 21 (46.7%) | 40 (89.9%) | 9.14(3.05-27.44) | <0.001 |
| Every Other Day | 10 (22.2%) | 0 (0) | NC | |
| 2 Times/Week | 5 (11.1%) | 3 (6.7%) | 0.54(0.13-2.55) | |
| Every 5 Days or More | 9 (20%) | 1 (2.2%) | 0.09(0.01-0.75) | |
| Bristol Stool Scale - N(%) | ||||
| 3 and 4 | 10 (22.2%) | 34 (75.6) | 10.81(4.07-28.76) | <0.001 |
| Others | 35 (77.8) | 11 (24.4) | 0.09(0.03-0.27) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





