Submitted:
16 February 2024
Posted:
16 February 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Materials and Methods
Results and Discussion
The Difference of Photosynthetic Parameters between F. tikoua Bur. and A. philoxeroides
The Difference of Chlorophyll Fluorescence Parameters between F. tikoua Bur. and A. philoxeroides
Dissipation of Light Energy Absorbed by Leaves of F. tikoua Bur. and A. philoxeroides
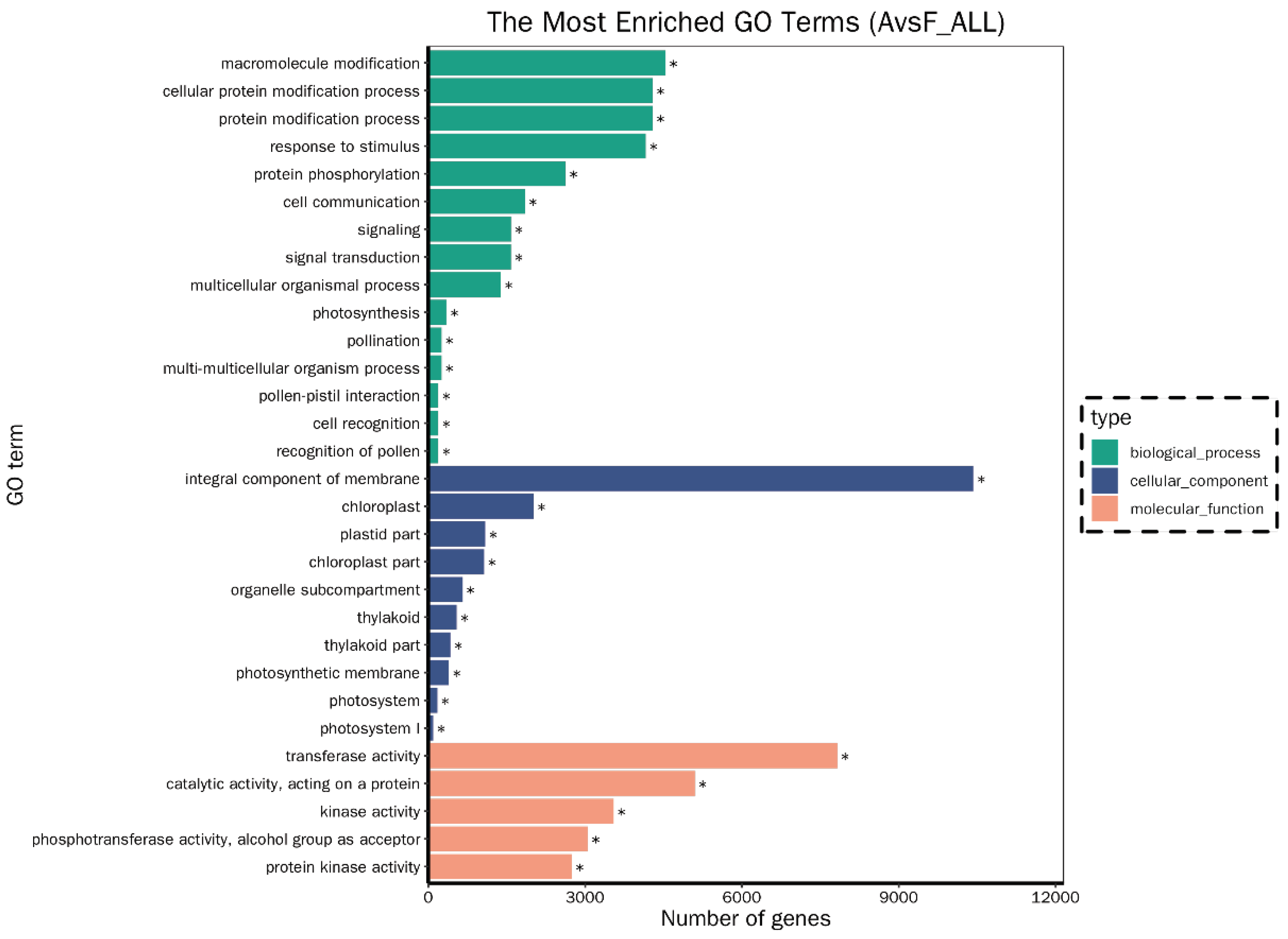
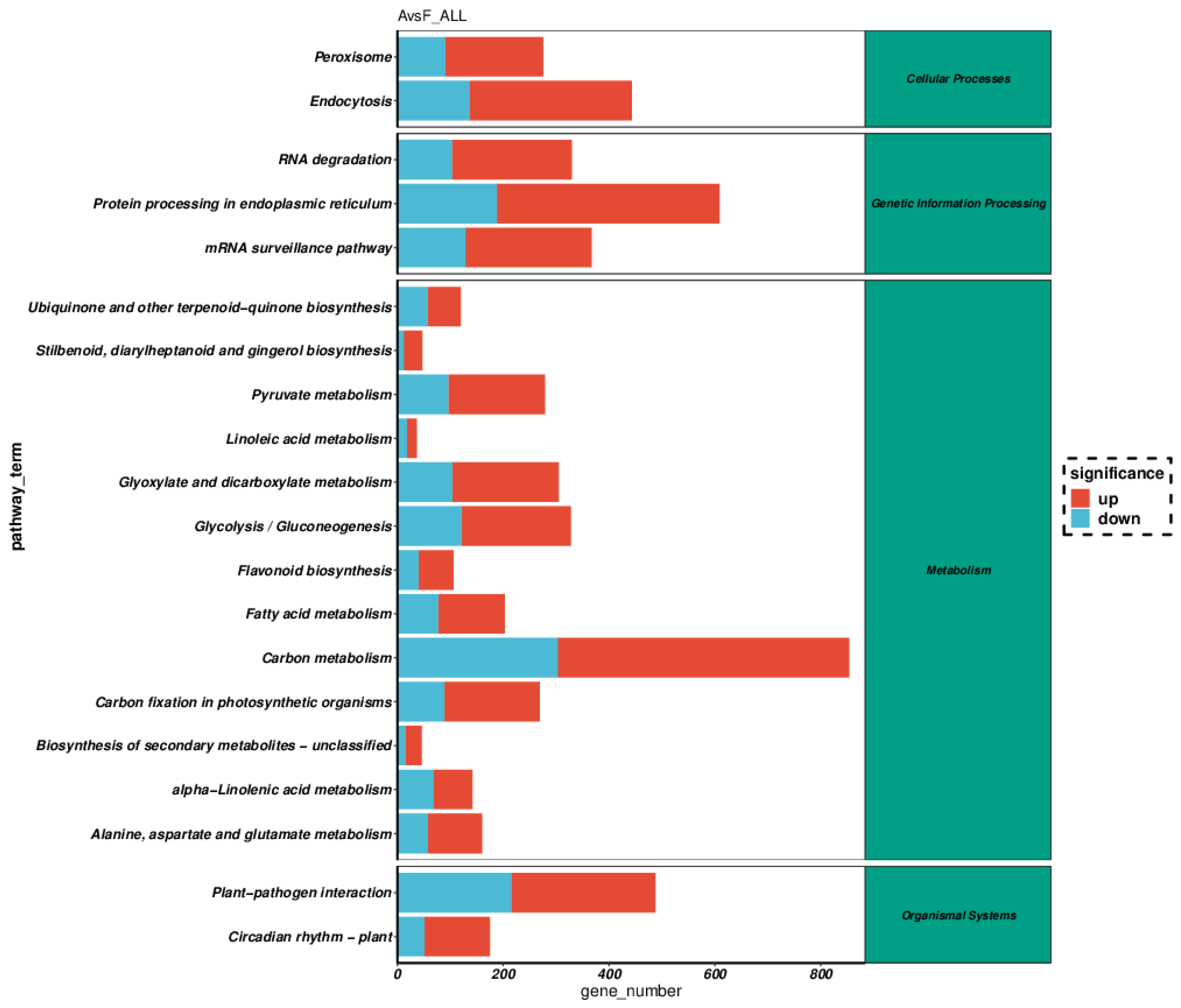
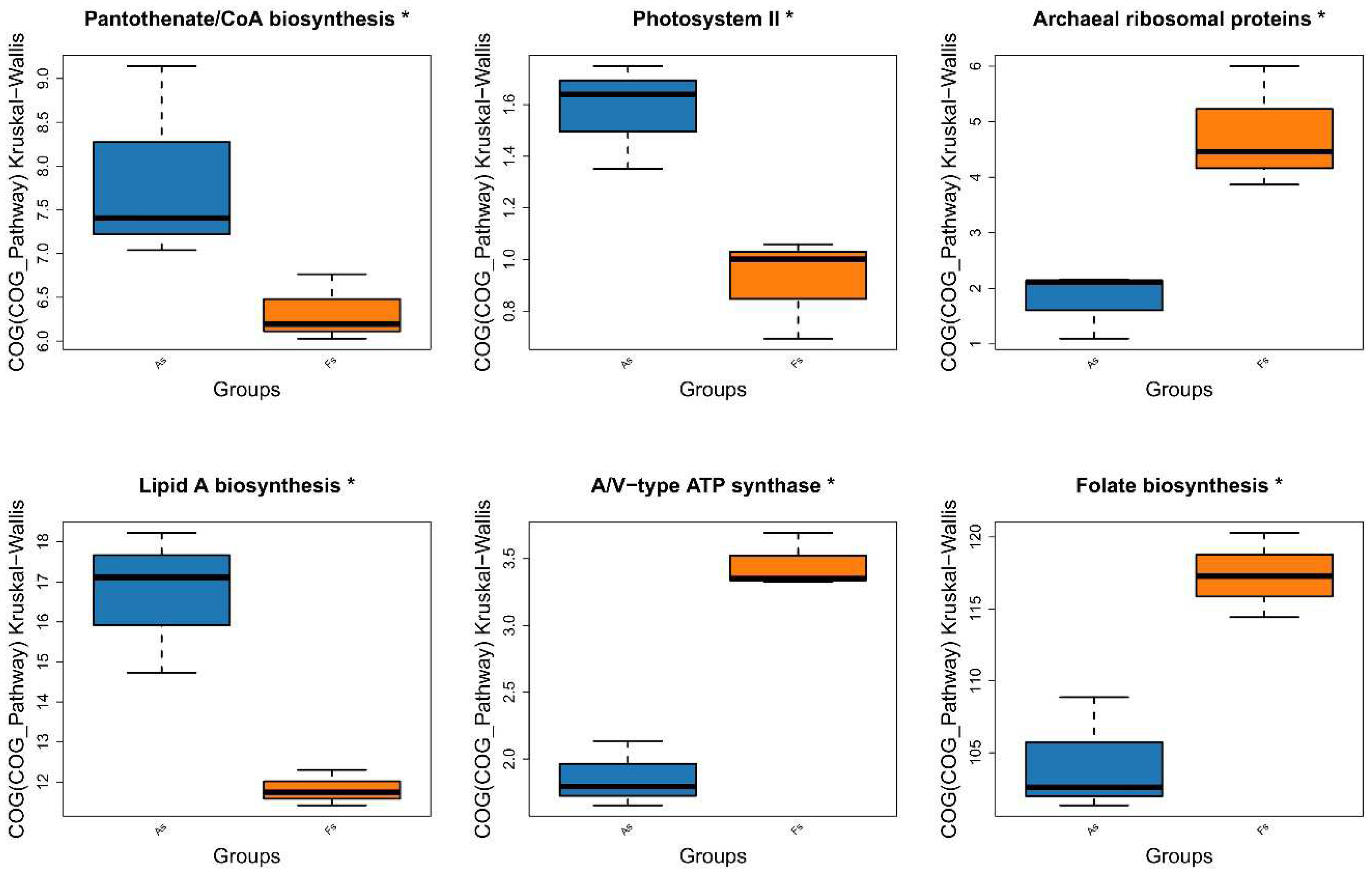
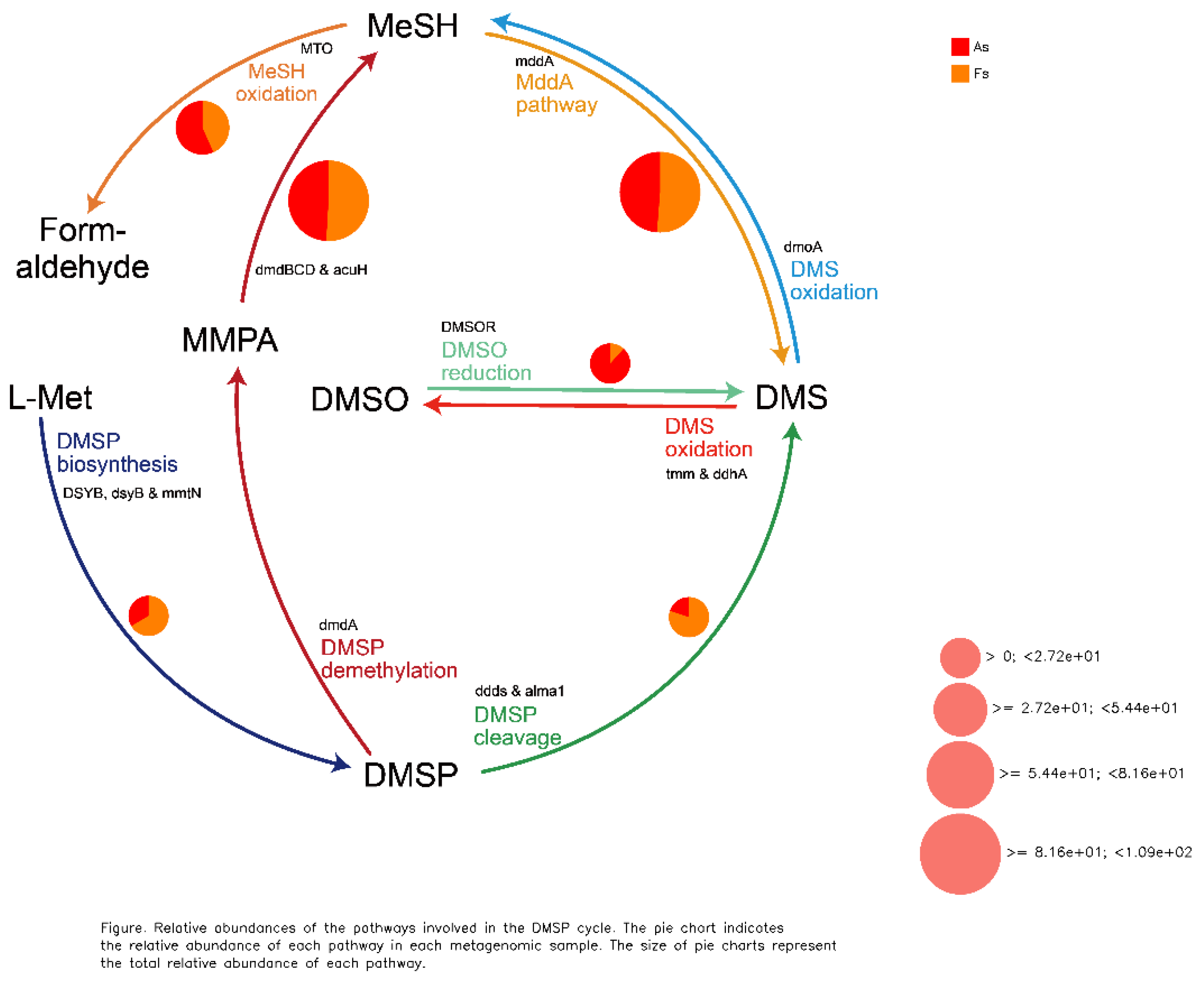
Differences in Gene Expression Profiles between F. tikoua Bur. and A. philoxeroides
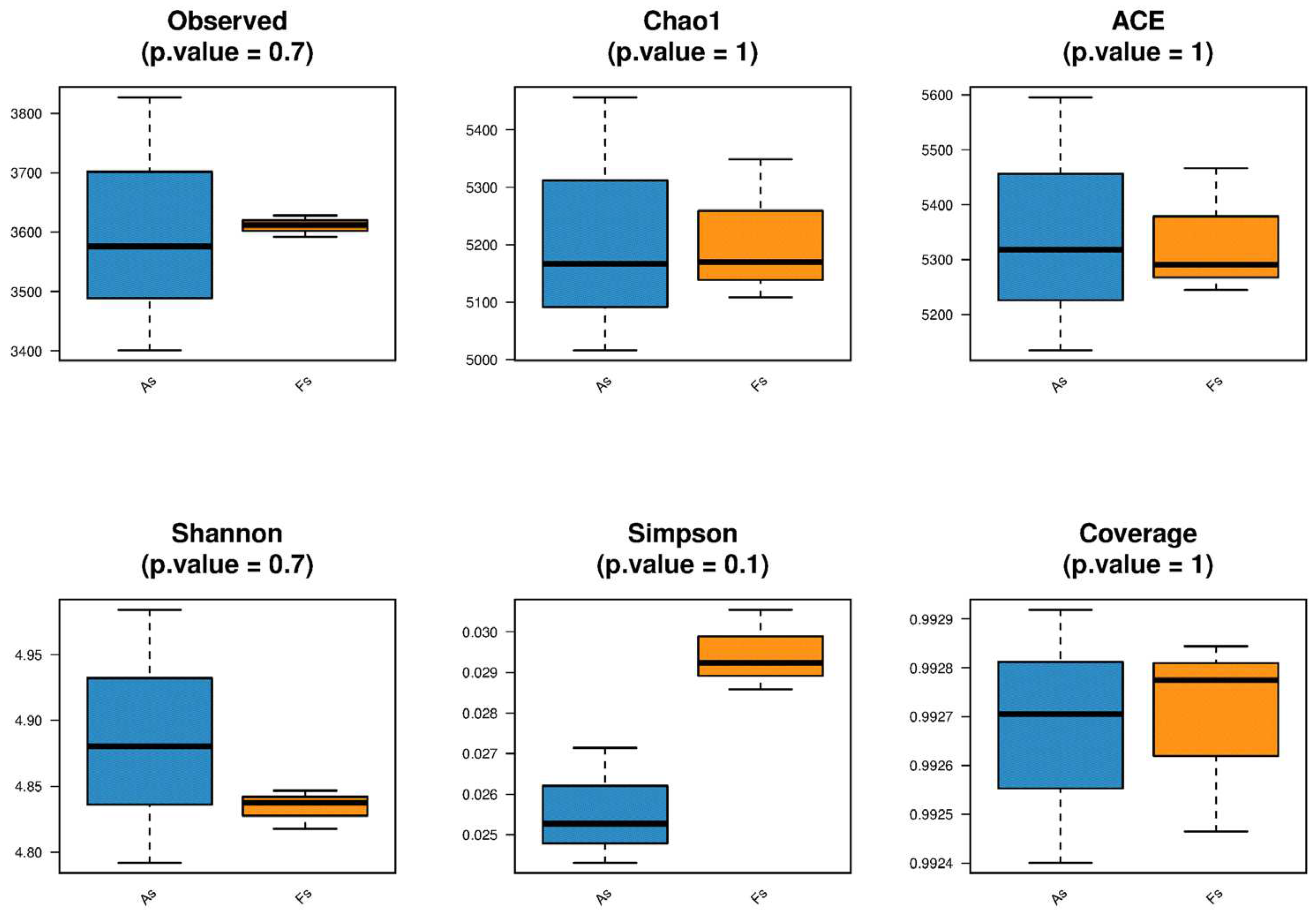
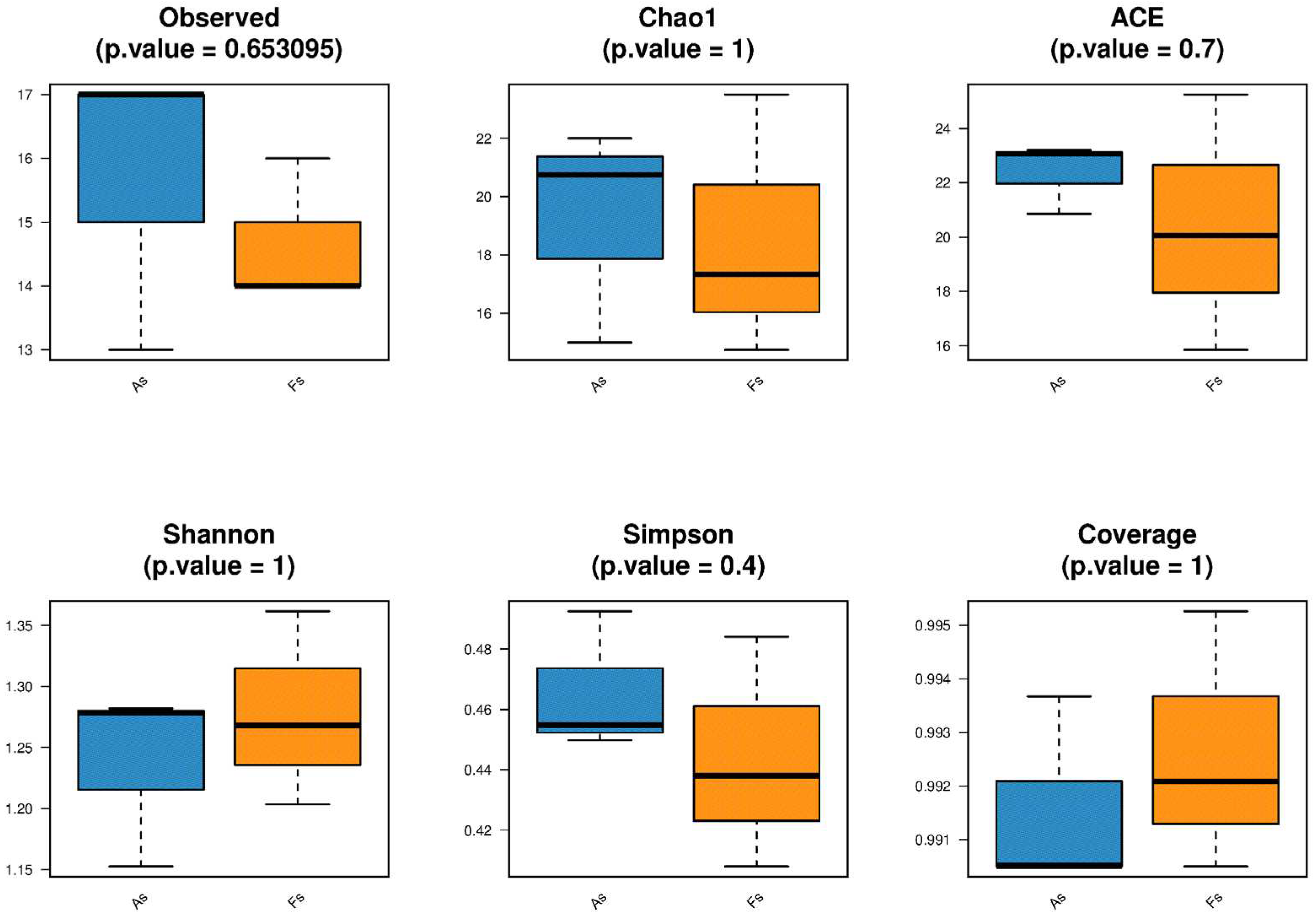
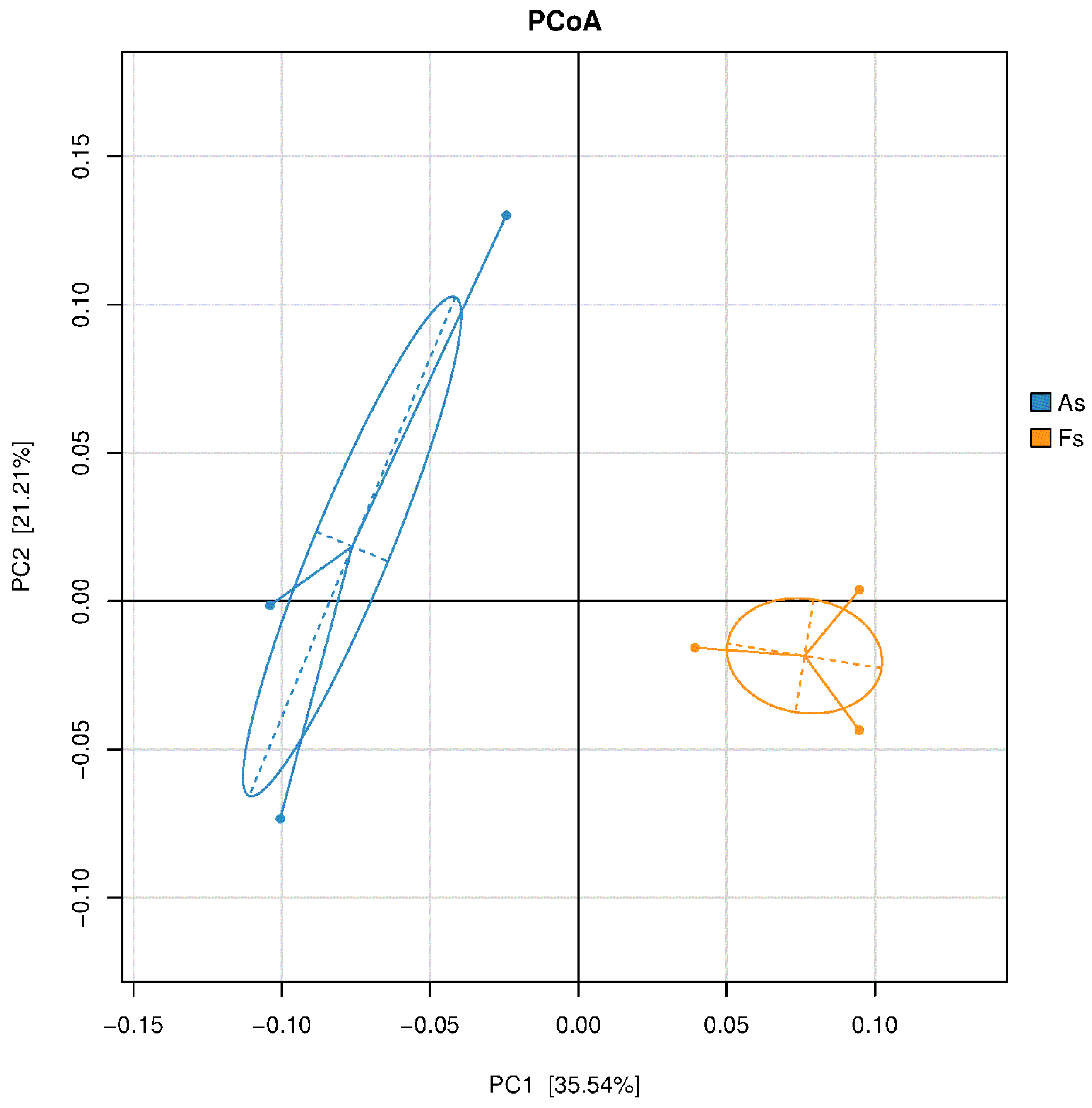
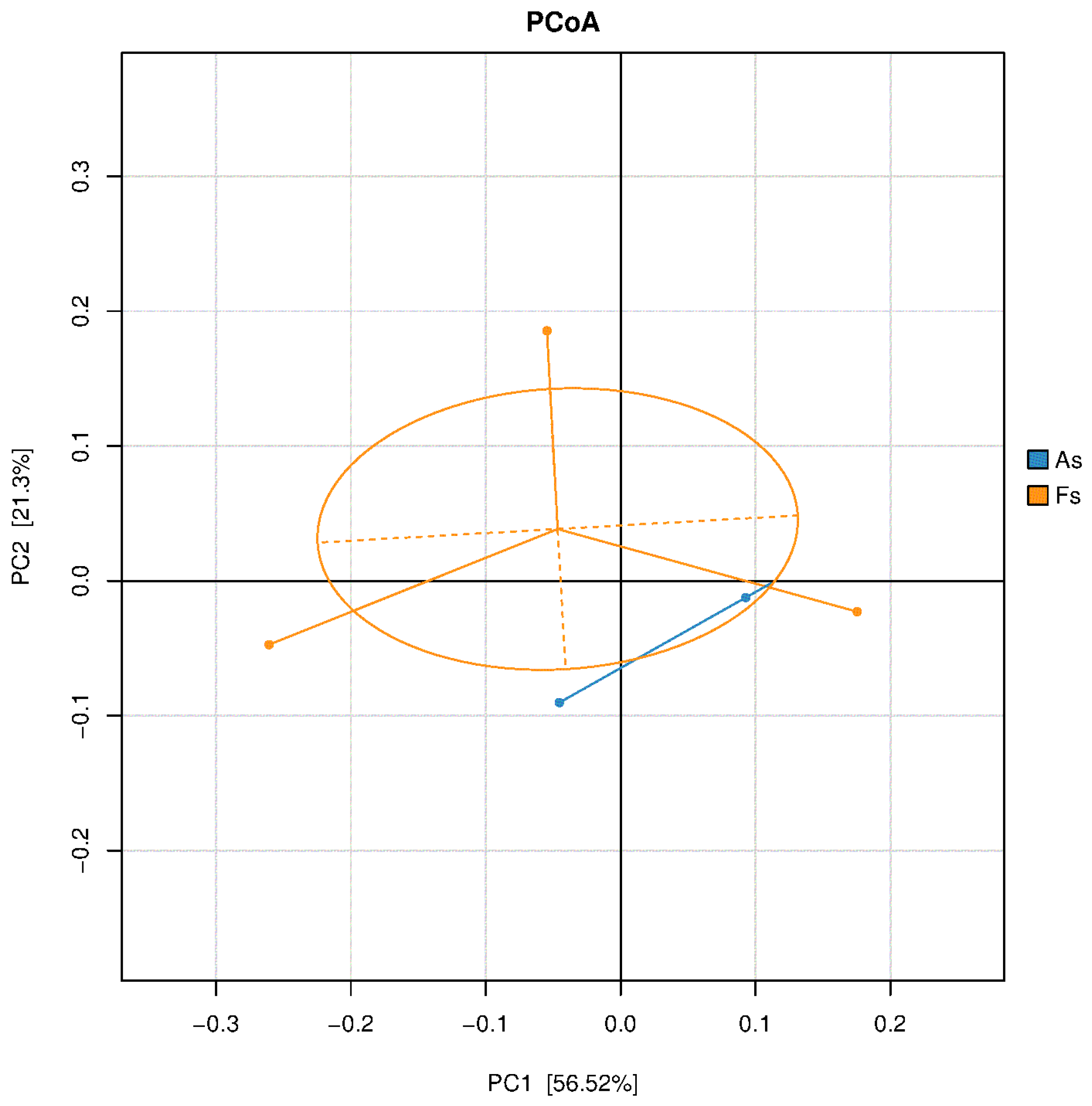
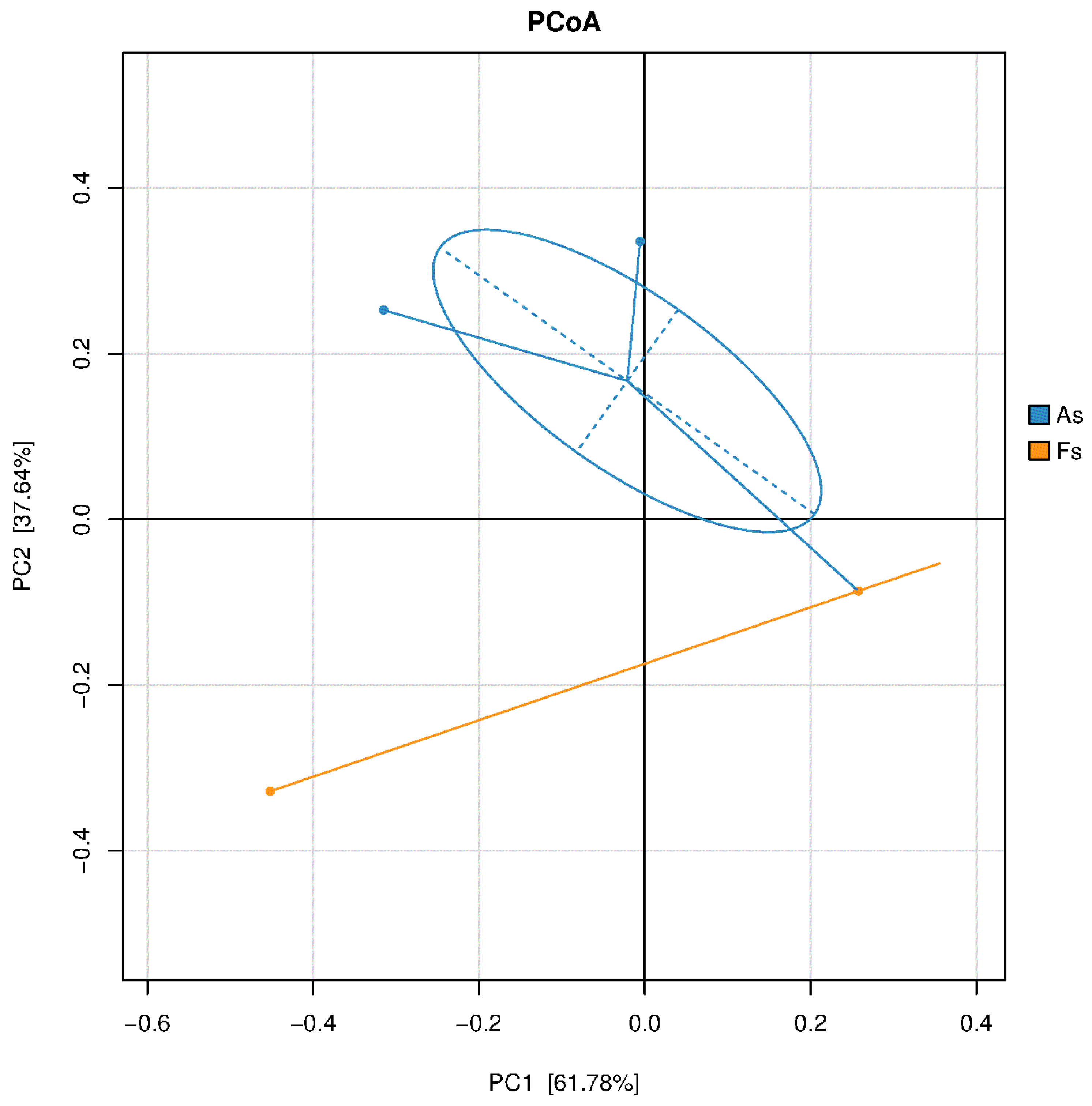
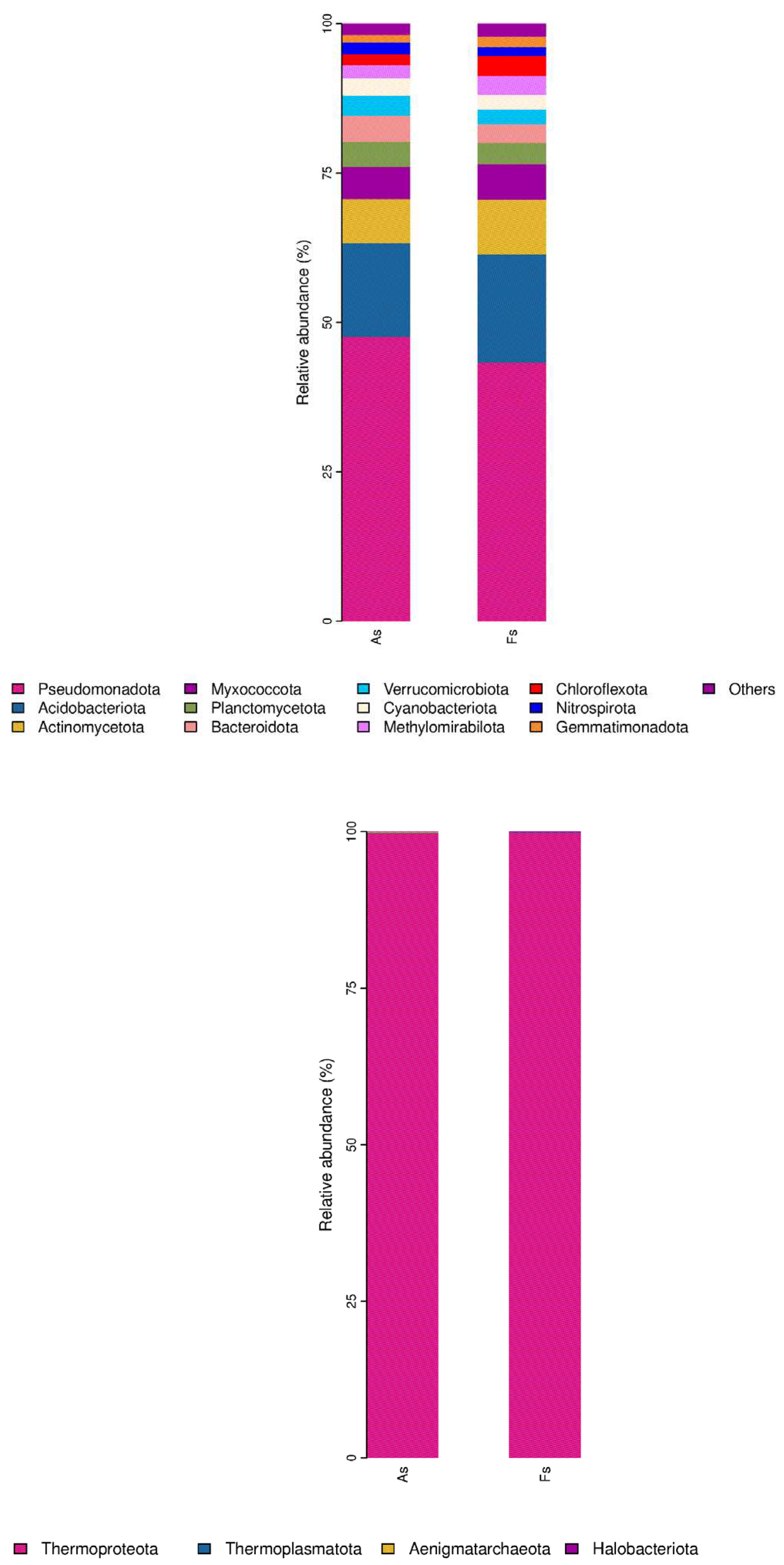
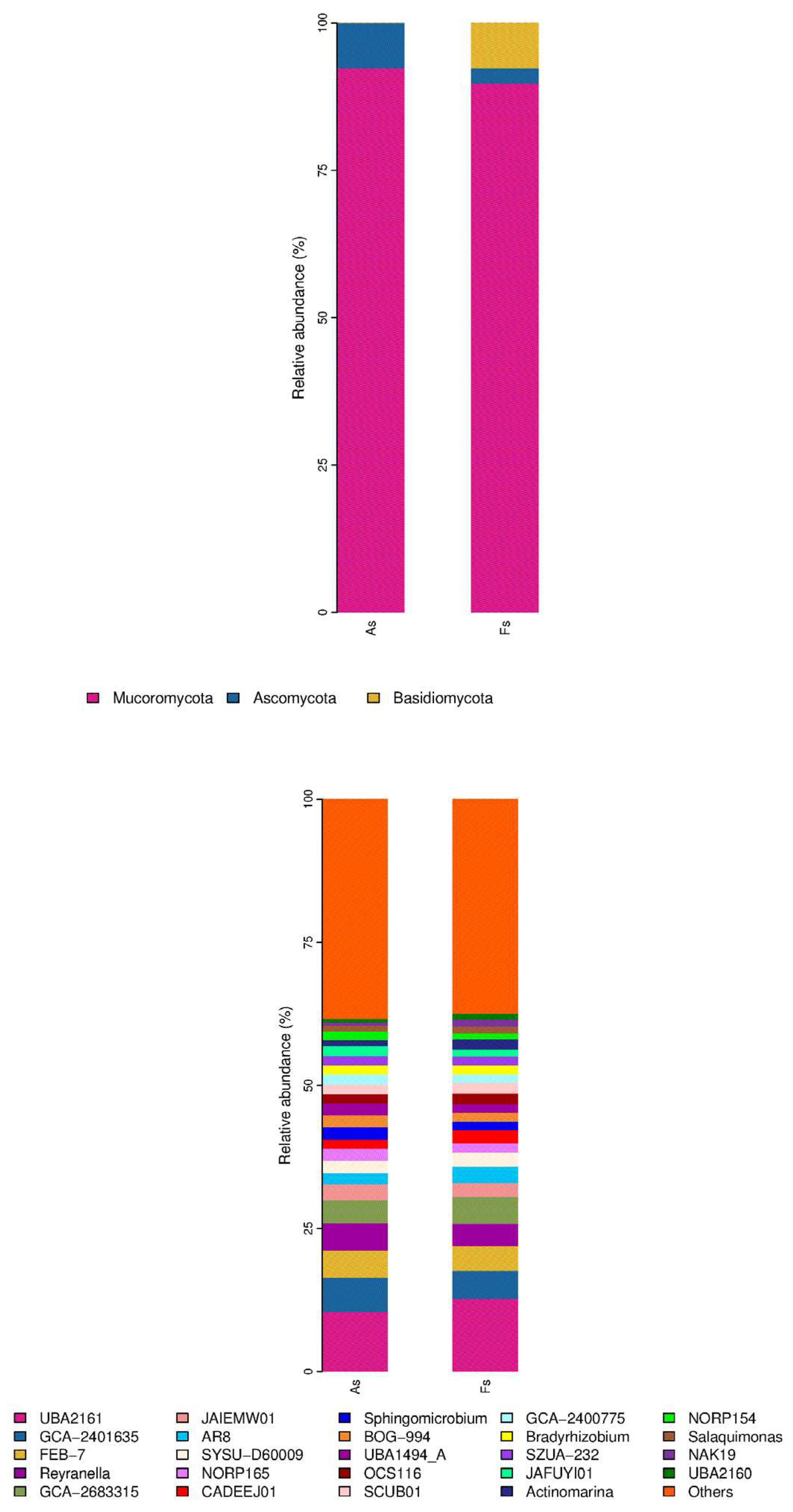
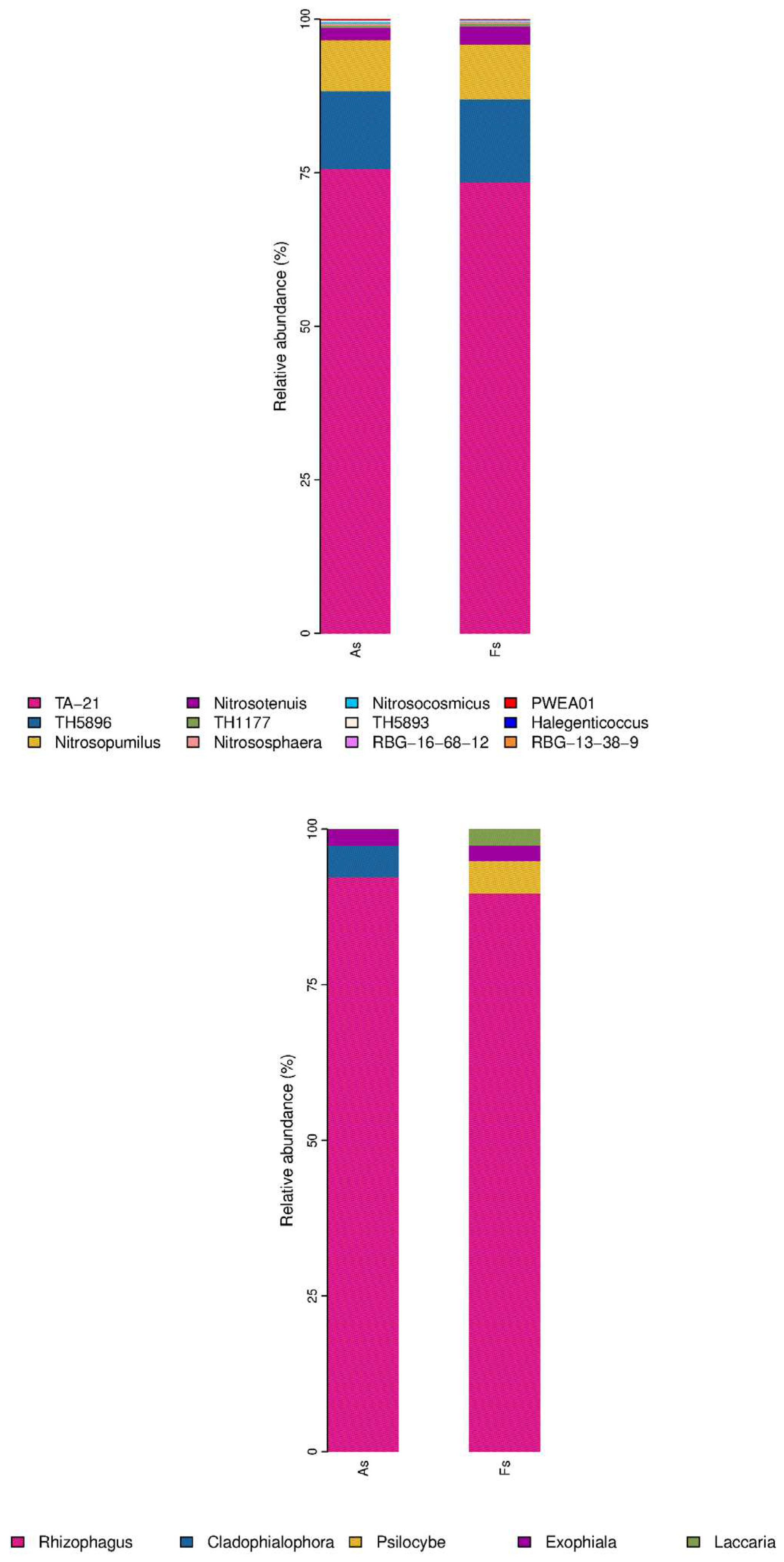
Differences in Rhizosphere Microbial Abundance between F. tikoua Bur. and A. philoxeroides
Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bell, C.W., Asao, S., Calderón, F.J., Wolk, B.H., & Wallenstein, M.D. (2015). Plant nitrogen uptake drives rhizosphere bacterial community assembly during plant growth. Soil Biology & Biochemistry, 85, 170-182. [CrossRef]
- Chen, Y., Xu, T., Fu, W., Hu, Y., Hu, H., You, L., Chen, B. (2021).Soil organic carbon and total nitrogen predict large-scale distribution of soil fungal communities in temperate and alpine shrub ecosystems. Eur. J. Soil Biol., 102, 103270. [CrossRef]
- Chen, Y., Huang, M.,Wu, W., Wang, A., Bao, T., Zheng, C., Chou, L., Tzeng, H., Tu, S.(2016).The floral scent of Ficus pumila var. pumila and its effect on the choosing behavior of pollinating wasps of Wiebesia pumilae.Acta Ecol. Sin. 36: 321-326. [CrossRef]
- Demmig-Adams, B., Adams III, W., Barker, D., Logan, B., Bowling, D., Verhoeven, A. (1996). Using chlorophyll fluorescence to assess the fraction of absorbed light allocated to thermal dissipation of excess excitation.Physiologia Plantarum,98(2):253-264. [CrossRef]
- Gao, L., Wei, C., He, Y., Tang, X., Chen, W., Xu, H., Wu, Y., Wilschut, R. A., & Lu, X. (2023). Aboveground herbivory can promote exotic plant invasion through intra- and interspecific aboveground-belowground interactions. The New phytologist, 237(6), 2347-2359. [CrossRef]
- Gibbons, S. M., Lekberg, Y., Mummey, D. L., Sangwan, N., Ramsey, P. W., & Gilbert, J. A. (2017). Invasive Plants Rapidly Reshape Soil Properties in a Grassland Ecosystem. mSystems, 2(2), e00178-16. 2. [CrossRef]
- Gioria, M., & Osborne, B. A. (2014). Resource competition in plant invasions: Emerging patterns and research needs. Frontiers in Plant Science, 5, 103442. [CrossRef]
- Fan, S., Yu, D., & Liu, C. (2013). The invasive plant Alternanthera philoxeroides was suppressed more intensively than its native congener by a native generalist: implications for the biotic resistance hypothesis. PloS one, 8(12), e83619. [CrossRef]
- Fan, S., Yu, H., Dong, X., Wang, L., Chen, X., Yu, D., & Liu, C. (2016). Invasive plant Alternanthera philoxeroides suffers more severe herbivory pressure than native competitors in recipient communities. Sci Rep, 6, 36542. [CrossRef]
- Farquhar, G., Sharkey, T. (1982). Stomatal conductance and photosynthesis. Annual Review of Plant Physiology, 33(3):317-345. [CrossRef]
- Guidi, L., Lo Piccolo, E., & Landi, M. (2019). Chlorophyll Fluorescence, Photoinhibition and Abiotic Stress: Does it Make Any Difference the Fact to Be a C3 or C4 Species?. Frontiers in plant science, 10, 174. [CrossRef]
- Hu, S., Gao, H., Li, J., Wang, Y., Gao, A., Wen, J., Balah, M.A., & Wu, A. (2023). The latitudinal and longitudinal allelopathic patterns of an invasive alligator weed (Alternanthera philoxeroides) in China. PLOS ONE, 18(1): e0280866. [CrossRef]
- Li, C., Bo, H., Song, B., Chen, X.C., Cao, Q.q., Yang, R.r., Ji,S.p., Wang, L.f., & Liu, J. (2022). Reshaping of the soil microbiome by the expansion of invasive plants: shifts in structure, diversity, co-occurrence, niche breadth, and assembly processes. Plant Soil 477:629-646. [CrossRef]
- Li, X.; Zhang, Y.; Kong,F.L.; Naz, M.; Zhou, J.Y.; Qi, S.S.;Dai, Z.C.; Du, D.L. (2023).Invasive Plant Alternanthera philoxeroides BenefitsMore Competition Advantage fromRhizosphere Bacteria Regardless ofthe Host Source. Plants, 12, 2085. [CrossRef]
- Liu, M., Pan, X., Zhang, Z., Kleunen M., & Li, B. (2020). Testing the shifting defense hypothesis for constitutive and induced resistance and tolerance. J Pest Sci 93, 355-364. [CrossRef]
- Liu, Z., Ge, X., Fu, Z., & Liu, J. (2020). Alternanthera philoxeroides invasion affects the soil seed bank of reed community. Environmental and Experimental Botany, 180, 104196. [CrossRef]
- Liu, Z., Yu, H., Sun,X., & Ding, J. (2020). Effects of elevated temperature on chemistry of an invasive plant, its native congener and their herbivores. J Plant Ecol, 2022,15(3):450-460. [CrossRef]
- Liu, B., Yan, J., Li, W., Yin, L., Li, P., Yu, H., Xing, L., Cai, M., Wang, H., Zhao, M., Zheng, J., Sun, F., Wang, Z., Jiang, Z., Ou, Q., Li, S., Qu, L., Zhang, Q., Zheng, Y., Qiao, X., … Wan, F. (2020). Mikania micrantha genome provides insights into the molecular mechanism of rapid growth. Nature communications, 11(1), 340. 1. [CrossRef]
- Mao R., Lu X., Ding, J. (2011).Effects of a nematode Meloidogyne incognita and its interaction with above-ground herbivory on an invasive wetland plant, alligator weed (Alternanthera philoxeroides) Plant Species Biology, 26(1):73-83. [CrossRef]
- Manoharan, B., Qi, S.-S., Dhandapani, V., Chen, Q., Rutherford, S., Wan, J.S., Jegadeesan, S., Yang, H.-Y., Li, Q., Li, J., Dai, Z.C., and Du, D.L. (2019).Gene Expression Profiling Reveals Enhanced Defense Responses in an Invasive Weed Compared to Its Native Congener During Pathogenesis. Int. J. Mol. Sci., 20, 4916. [CrossRef]
- Oku, H., Inafuku, M., Takamine, T., Nagamine, M., Saitoh, S., & Fukuta, M. (2014). Temperature threshold of isoprene emission from tropical trees, Ficus virgata and Ficus septica. Chemosphere, 95, 268–273. [CrossRef]
- Prentis, P. J., Wilson, J. R., Dormontt, E. E., Richardson, D. M., & Lowe, A. J. (2008). Adaptive evolution in invasive species. Trends in plant science, 13(6), 288-294. [CrossRef]
- Putten, W., Klironomos, J., & Wardle, D. (2007).Microbial ecology of biological invasions. ISME J 1, 28-37. [CrossRef]
- Stefanowicz, A.M., Stanek, M., Majewska, M.L., Nobis, M., & Zubek, S. (2019).Invasive plant species identity affects soil microbial communities in a mesocosm experiment.Applied Soil Ecology,136:168-177. [CrossRef]
- Stirbet, A., Lazár, D., Kromdijk, J., & Govindjee . (2018).Chlorophyll a fluorescence induction: Can just a one-second measurement be used to quantify abiotic stress responses?. Photosynthetica 56:86-104. [CrossRef]
- Sun, J., Javed, Q., Du, Y., Azeem, A., Abbas, A., Iqbal, B., He, Y.H.,Xiang,Y., & Du, D.L.(2022). Invasive Alternanthera philoxeroides has performance advantages over natives under flooding with high amount of nitrogen. Aquat Ecol, 56, 891-903. [CrossRef]
- Tie, D., Hu, H., Yu, X., Shu, Y., & Zhang, J. (2020). Responses of photosynthetic characteristics and chlorophyll fluorescence parameters of Phoebe zhennan saplings to cadmium stress. Acta Ecologica Sinica, 40(11) :3738-3746.
- Wang, T., Hu, J., Gao, Y., Yu, D., & Liu, C. (2017). Disturbance, Trait Similarities, and Trait Advantages Facilitate the Invasion Success of Alternanthera philoxeroides (Mart.) Griseb. Clean-soil Air Water, 45, 1600378. [CrossRef]
- Wang, T., Hu, J., Wang, R., Liu, C., & Yu, D. (2018). Tolerance and resistance facilitate the invasion success of alternanthera philoxeroides in disturbed habitats: a reconsideration of the disturbance hypothesis in the light of phenotypic variation. Environmental & Experimental Botany, 153, 135-142. [CrossRef]
- Wang, Y., Chen, C., Xiong, Y., Wang, Y., & Li, Q. (2021). Combination effects of heavy metal and inter-specific competition on the invasiveness of Alternanthera philoxeroides. Environmental and Experimental Botany, 189, 104532. [CrossRef]
- Wang, Y., Xiong, Y., Wang, Y., & Li, Q. (2021). Long period exposure to serious cadmium pollution benefits an invasive plant (Alternanthera philoxeroides) competing with its native congener (Alternanthera sessilis). The Science of the total environment, 786, 147456. [CrossRef]
- Wang, X., Wang, X., Wang,W., Wang, J., & Yu, F. (2022). Effects of InvasivePlant Diversity on Soil MicrobialCommunities. Diversity, 14, 992. [CrossRef]
- Wang, M., Chen, S., Chen, L., & Wang, D. (2019). Saline stress modifies the effect of cadmium toxicity on soil archaeal communities. Ecotoxicology and environmental safety, 182, 109431. [CrossRef]
- Wei, H., He, M., Lu, X., & Ding, J. (2016).Differences in interactions of aboveground and belowground herbivores on the invasive plant Alternanthera philoxeroides and native host A. sessilis. Biol Invasions, 18, 3437-3447. [CrossRef]
- Weis, E., & Berry, J.A. (1988). Plants and high temperature stress. Symposia of the Society for Experimental Biology,42:329-346.
- Wu, Q. Effect of drought stress and nitrogen on root morphology,physiological characteristics and yield formation of sorghum. Shenyang:Shenyang Agricultural University, 2017.
- , Tang, H., Fu, L., Tan, J., Govindjee, G., & Guo, Y. (2023). Determination of Fv/Fm from Chlorophyll a Fluorescence without Dark Adaptation by an LSSVM Model. Plant Phenomics, 5. [CrossRef]
- Xu, C. Y., Schooler, S. S., & Van Klinken, R. D. (2012). Differential influence of clonal integration on morphological and growth responses to light in two invasive herbs. PloS one, 7(4), e35873. 4. [CrossRef]
- Xu, C., Ge, Y., & Wang, J. (2019). Molecular basis underlying the successful invasion of hexaploid cytotypes of Solidago canadensis L.: Insights from integrated gene and miRNA expression profiling. Ecology and evolution, 9(8), 4820-4852. [CrossRef]
- Yin, L., Liu, B., Wang, H., Zhang, Y., Wang, S., Jiang, F., Ren, Y., Liu, H., Liu, C., Wan, F., Wang, H., Qian, W., & Fan, W. (2020). The Rhizosphere Microbiome of Mikania micrantha Provides Insight Into Adaptation and Invasion. Frontiers in microbiology, 11, 1462. [CrossRef]
- You, W., Yu, D., Liu, C. Xie, D., & Xiong, W. (2013).Clonal integration facilitates invasiveness of the alien aquatic plant Myriophyllum aquaticum L. under heterogeneous water availability. Hydrobiologia, 718, 27-39. [CrossRef]
- Zhang, K. M., Shen, Y., Zhou, X. Q., Fang, Y. M., Liu, Y., & Ma, L. Q. (2016). Photosynthetic electron-transfer reactions in the gametophyte of Pteris multifida reveal the presence of allelopathic interference from the invasive plant species Bidens pilosa. Journal of photochemistry and photobiology. B, Biology, 158, 81–88. [CrossRef]
- Zhang, X., Wang, G., Zhang, S., Chen, S., Wang, Y., Wen, P., Ma, X., Shi, Y., Qi, R., Yang, Y., Liao, Z., Lin, J., Lin, J., Xu, X., Chen, X., Xu, X., Deng, F., Zhao, L., Lee, Y. L., Wang, R., … & Ming, R. (2020). Genomes of the Banyan Tree and Pollinator Wasp Provide Insights into Fig-Wasp Coevolution. Cell, 183(4), 875-889.e17. [CrossRef]
- Zheng, Y. L., Feng, Y. L., Zhang, L. K., Callaway, R. M., Valiente-Banuet, A., Luo, D. Q., Liao, Z. Y., Lei, Y. B., Barclay, G. F., & Silva-Pereyra, C. (2015). Integrating novel chemical weapons and evolutionarily increased competitive ability in success of a tropical invader. The New phytologist, 205(3), 1350–1359. [CrossRef]

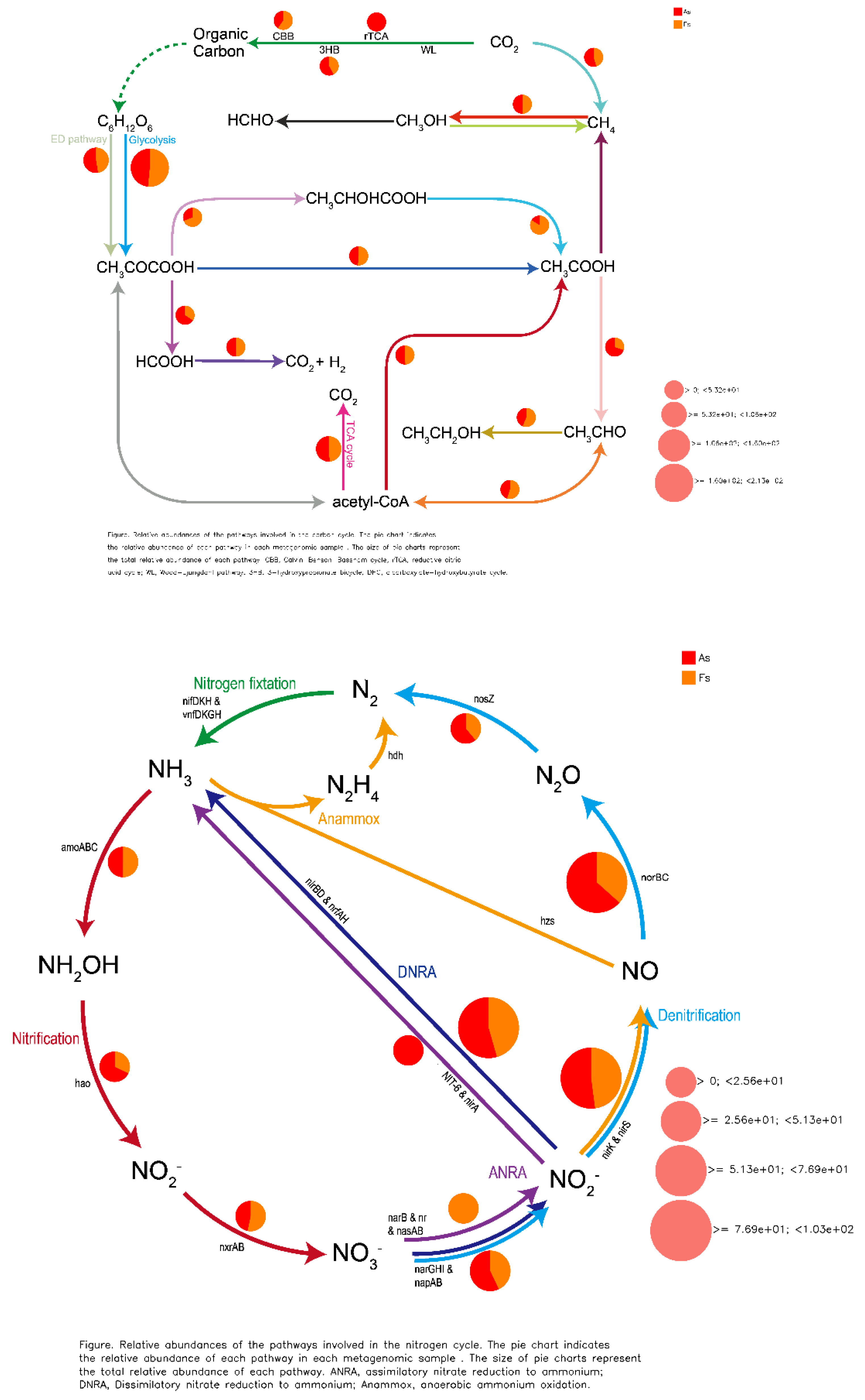
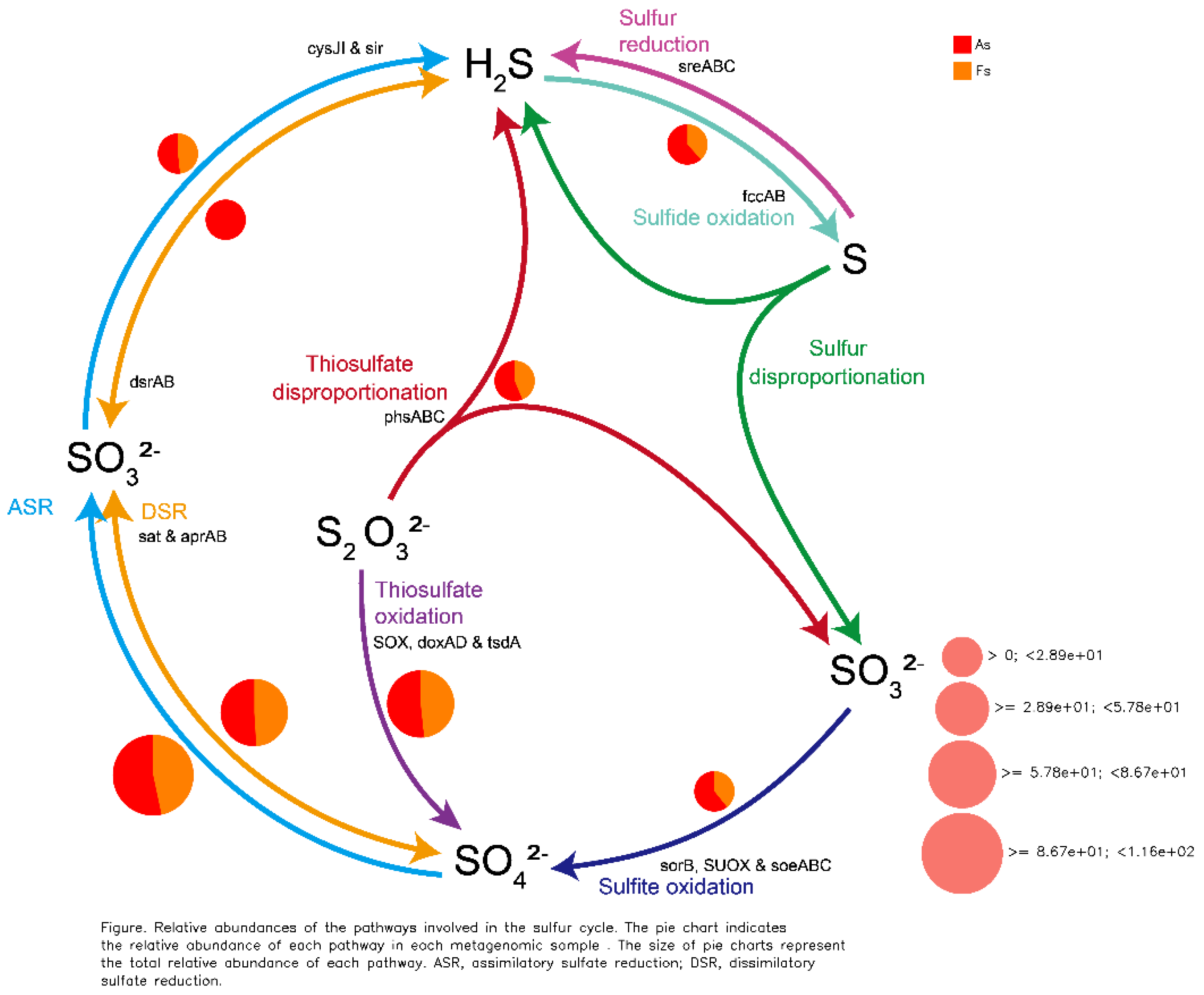
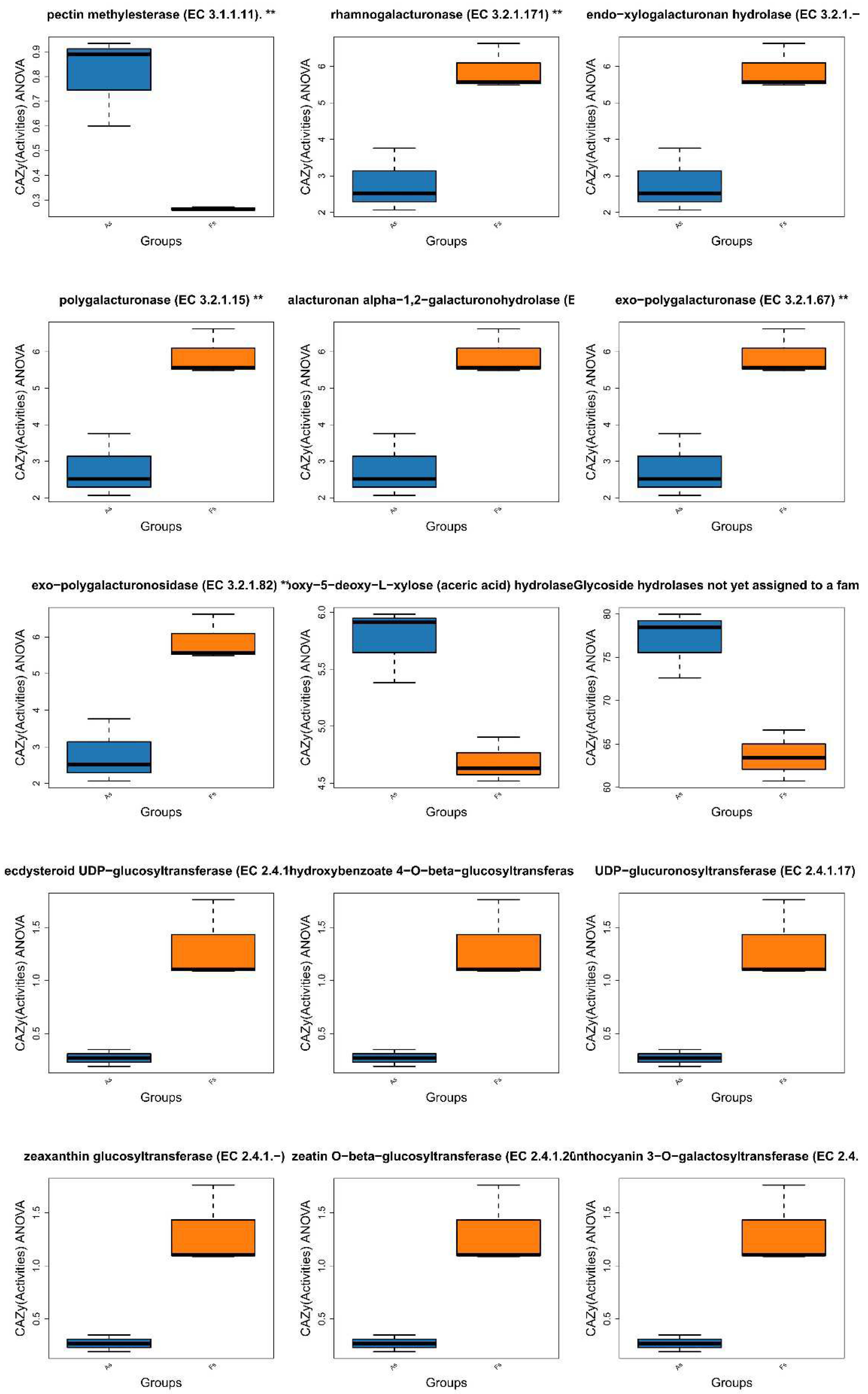
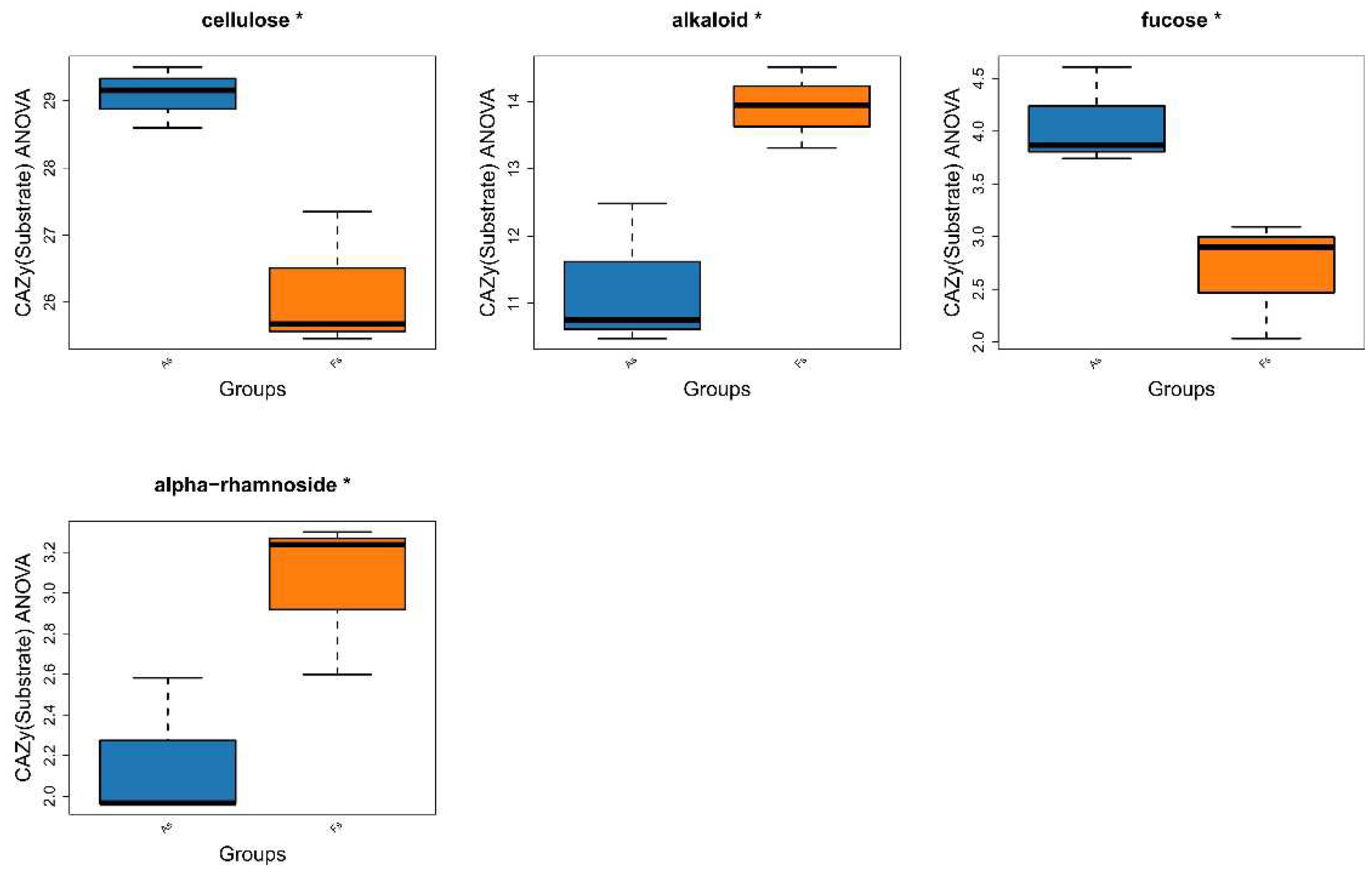
| Time | Parameters | F. tikoua Bur. | A. philoxeroides |
| Oct.16th | E(×10-2molm⁻²s⁻¹) | 0.49±0.00bB | 0.63±0.35aA |
| A(µmolm⁻²s⁻¹) | 11.10±2.49aA | 11.99±0.40aA | |
| Ci(µmolmol⁻¹) | 321.63±2.03aA | 324.38±9.01aA | |
| LS | 20.80±0.32aA | 19.24±2.21aA | |
| WUE(µmolmmol-1) | 2.66±0.01aA | 2.39±0.49aA | |
| gsw(×103molm⁻²s⁻¹) | 289.88±2.44bA | 319.96±23.34aA | |
| gbw(×103molm⁻²s⁻¹) | 2834.21±0.14aA | 2831.93±1.78bA | |
| gtw(×103molm⁻²s⁻¹) | 278.15±7.89aA | 286.29±20.10aA | |
| gtc(×103molm⁻²s⁻¹) | 175.21±5.01aA | 180.38±13.34aA | |
| T (℃) | 28.41±0.19aA | 27.53±0.08bB | |
| VPDKpa) | 1.51±0.02bB | 1.92±0.06aA | |
| RHcham(%) | 59.87±0.06aA | 47.17±0.80bA | |
| Oct.30th | E(×10-2molm⁻²s⁻¹) | 1.10±0.03bA | 1.30±0.20aA |
| A(µmolm⁻²s⁻¹) | 16.32±0.2aA | 16.65±0.95aA | |
| Ci(µmolmol⁻¹) | 313.23±2.41aA | 325.15±10.46aA | |
| LS | 21.52±0.60aA | 17.20±2.14bA | |
| WUE(µmolmmol-1) | 1.31±0.20aA | 1.38±0.13aA | |
| gsw(×103molm⁻²s⁻¹) | 385.53±6.78bA | 453.74±20.80aA | |
| gbw(×103molm⁻²s⁻¹) | 2842.71±0.67aA | 2840.81±0.89bA | |
| gtw(×103molm⁻²s⁻¹) | 364.97±13.50bA | 416.94±17.57aA | |
| gtc(×103molm⁻²s⁻¹) | 230.45±8.62bA | 263.65±11.24aA | |
| T (℃) | 30.42±0.09aA | 30.13±0.07bA | |
| VPDleaf(Kpa) | 2.65±0.02bA | 2.85±0.06aA | |
| RHcham(%) | 35.74±0.11aA | 34.60±0.16bB |
| Time | Parameter | F. tikoua Bur. | A. philoxeroides |
| Oct.16th | F0 | 252.87±2.60 aA | 211.32±1.74 bA |
| Fm | 1318.64±83.09 aA | 1275.75±44.33 aA | |
| Fv/Fm | 0.81±0.01 bA | 0.83±0.01 aA | |
| Fv/F0 | 4.21±1.24 bA | 5.04±1.56 aA | |
| ΦPSII | 0.36±0.03 aA | 0.28±0.02 bA | |
| ETR(µmolm⁻² s⁻¹) | 151.99±10.55 aA | 117.82±7.95 bA | |
| NPQ | 1.56±0.11 aA | 1.38±1.78 bA | |
| Fv'/Fm' | 0.62±0.01 bA | 0.71±0.03 aA | |
| qP | 0.58±0.04 aA | 0.53±0.17 aA | |
| [Y(NO)] | 0.25±0.01bA | 0.31±0.00aA | |
| [Y(NPQ)] | 0.37±0.01aA | 0.40±0.04aA | |
| Oct.30th | F0 | 243.12±11.72 aA | 166.01±48.07 bA |
| Fm | 1251.25±117.48 aA | 1090.54±5.28 aA | |
| Fv/Fm | 0.81±0.01 bA | 0.84±0.01 aA | |
| Fv/F0 | 4.15±1.25 bA | 5.57 ±1.22 aA | |
| ΦPSII | 0.41±0.01 aA | 0.25±0.02 bA | |
| ETR(µmolm⁻² s⁻¹) | 166.50±4.43 aA | 106.10±6.83 bA | |
| NPQ | 1.79±0.25 aA | 1.54±0.26 bA | |
| Fv'/Fm' | 0.63±0.00 bA | 0.68±0.00 aA | |
| qP | 0.62±0.01 aA | 0.60±0.07 aA | |
| [Y(NO)] | 0.21±0.02bA | 0.30±0.00aA | |
| [Y(NPQ)] | 0.38±0.01aA | 0.31±0.03aA |
| Time | Parameter | F. tikoua Bur. | A. philoxeroides |
| Oct.16th | P % | 34.09±1.72aA | 26.63±3.75aA |
| D % | 38.44±0.75aA | 31.85±0.86bA | |
| Ex % | 27.47±2.48bA | 41.51±2.88aA | |
| Oct.30th | P % | 38.80±0.03aA | 23.98±1.75bA |
| D % | 37.03±0.02aA | 31.36±0.36bA | |
| Ex % | 24.17±0.06bA | 44.65±1.39aA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





