Submitted:
16 February 2024
Posted:
16 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Marine Indole Alkaloids
2.1. Simple indole alkaloids, (SIAs)
2.1.1. C-3- acyclic subtituted simple indole alkaloids
2.1.2. C3-(Iminoimidazolidin- and Pirazin-)-subtituted simple indole alkaloids
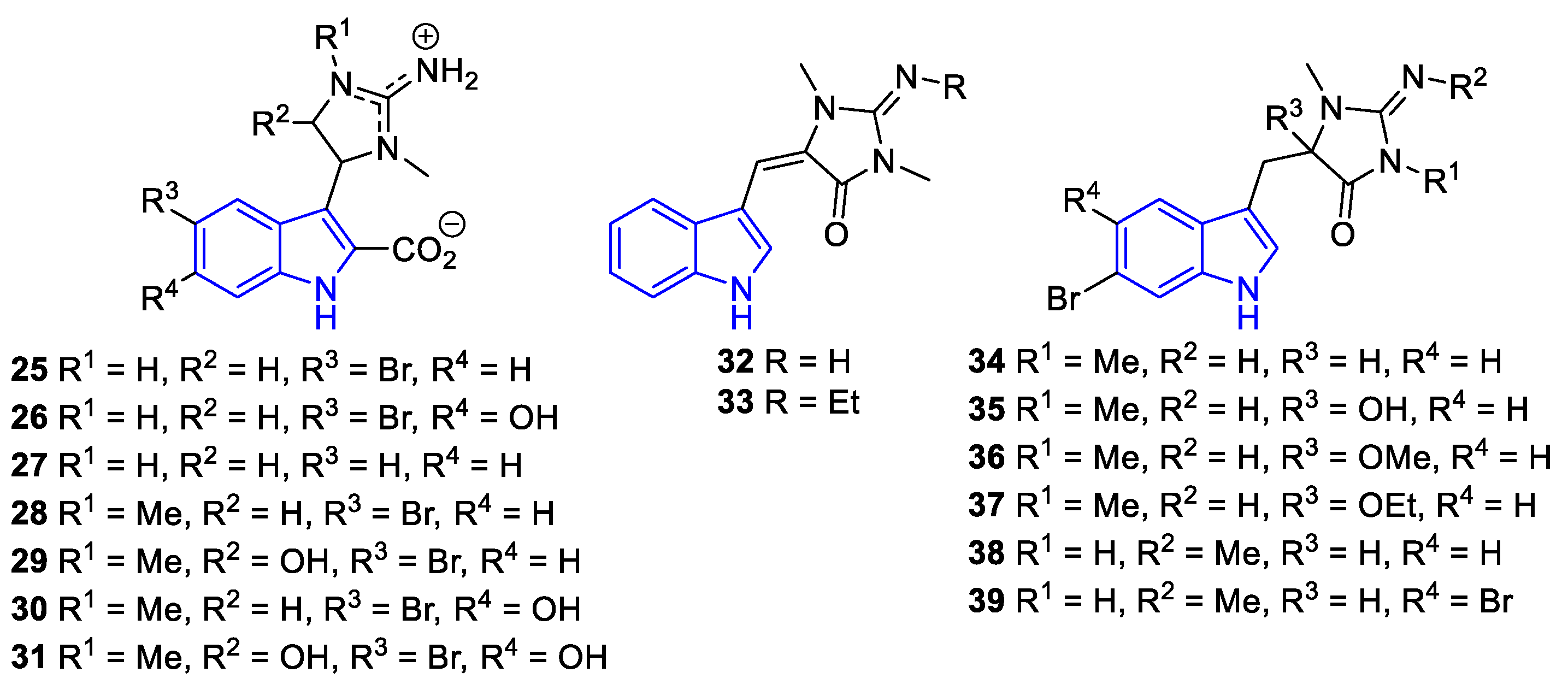
2.1.3. Bis-/tri-indole alkaloids.
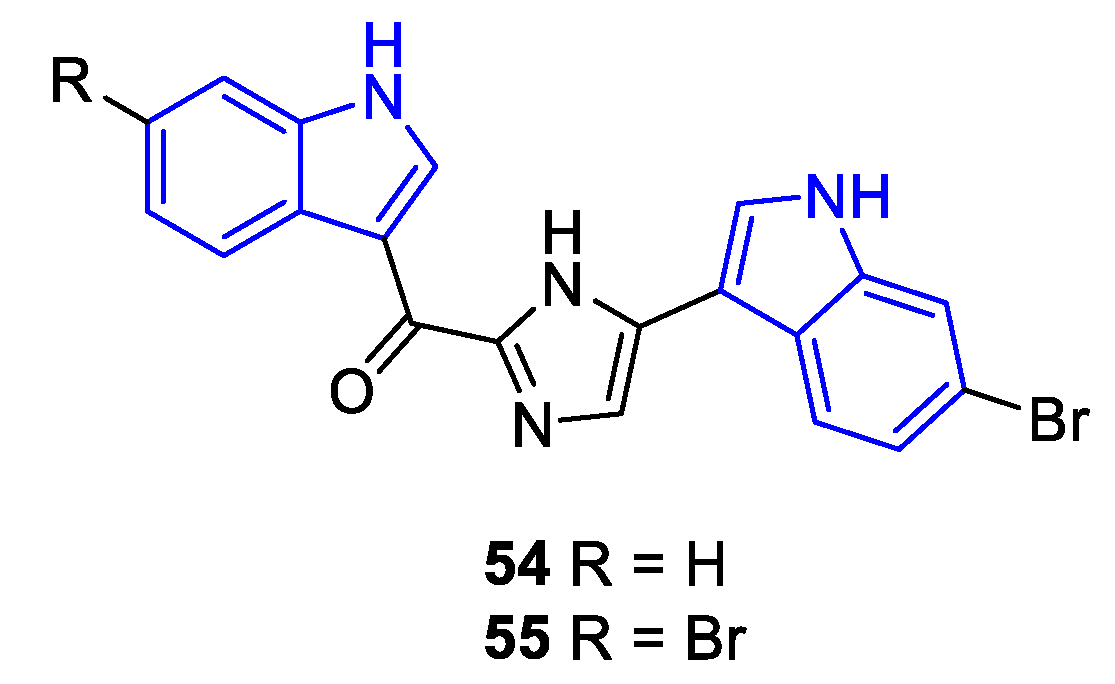
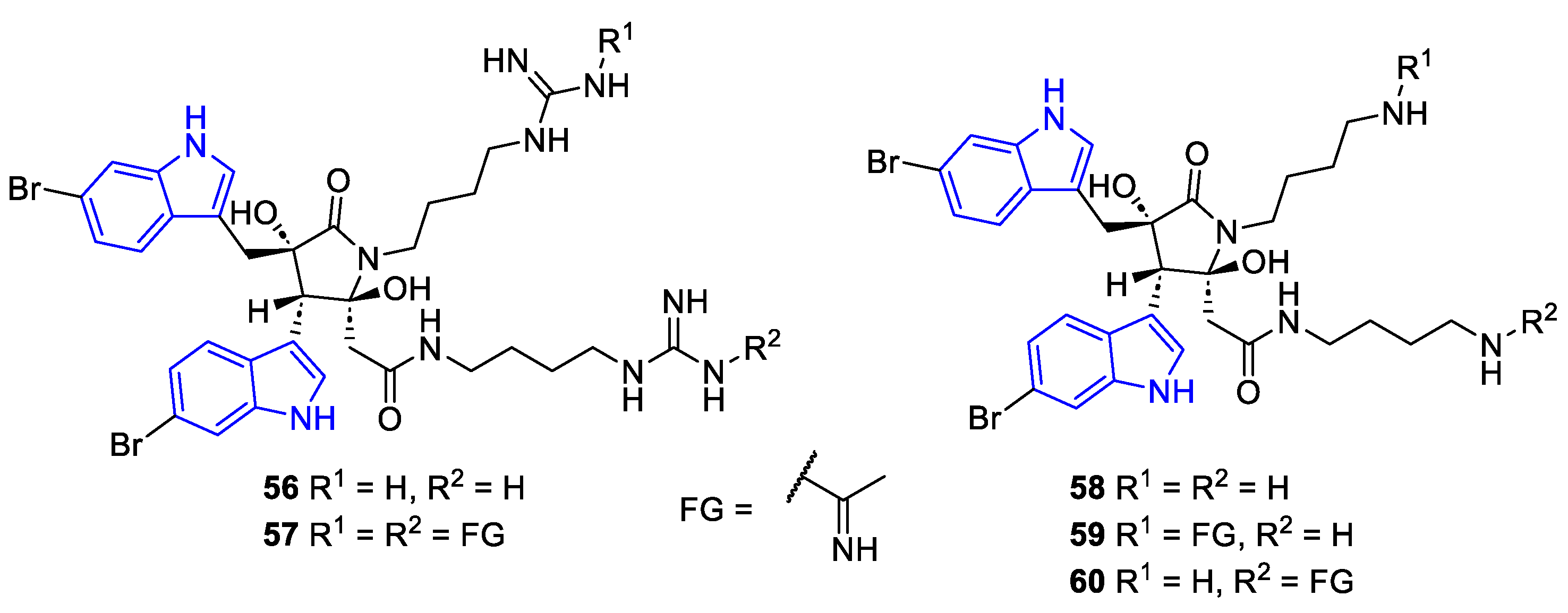
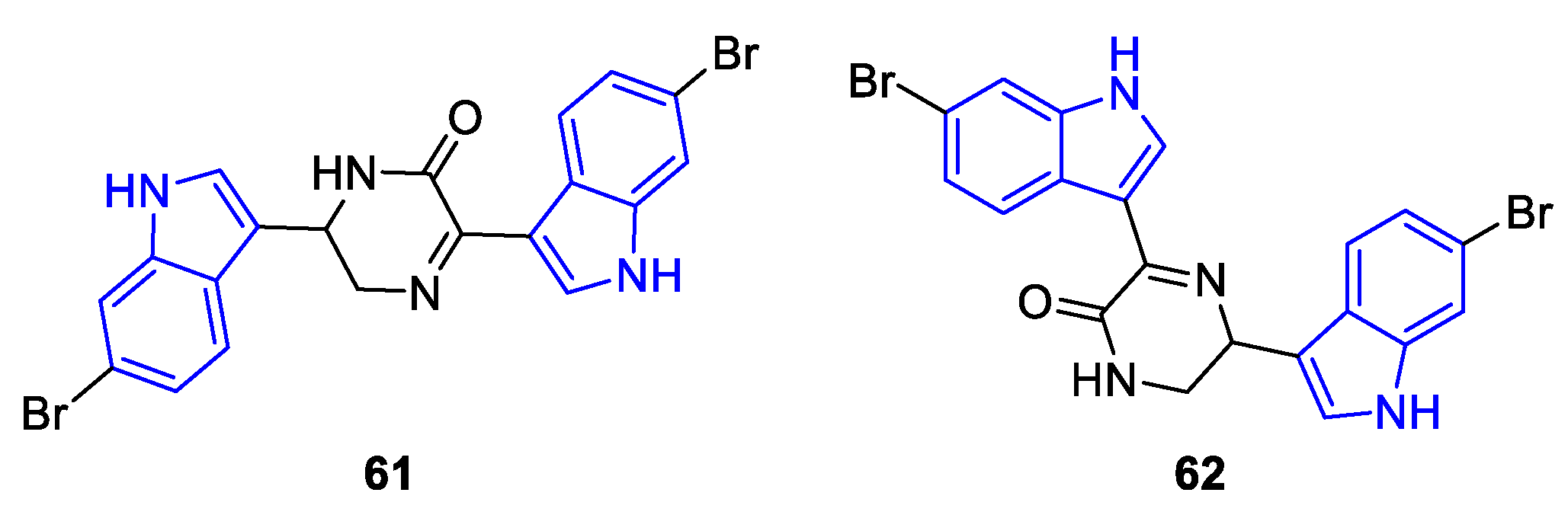
2.2. Prenylated Indole Alcaloids (PIA)
2.2.1. Diketopiperazinas (DKPs) indole alkaloids
2.2.1.1. Simple diketopiperazines
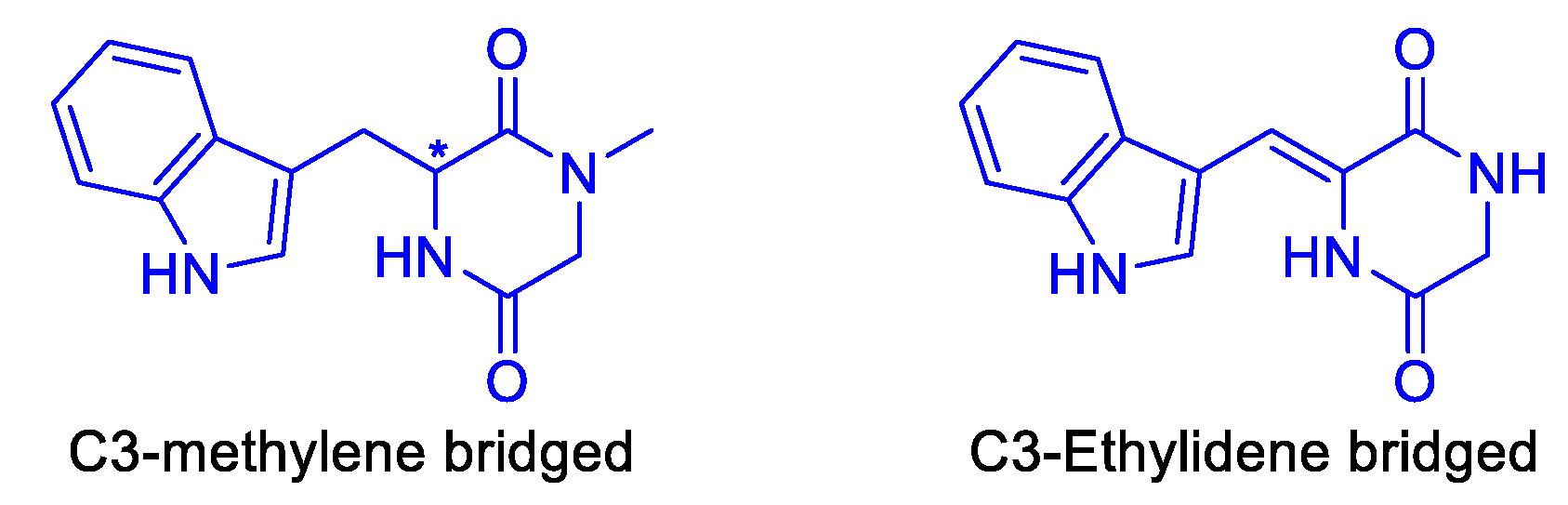
Attached at C-3 with a methylene bridge
Attached at C3 with an ethylidene bridge
Bis-indole diketopiperazine
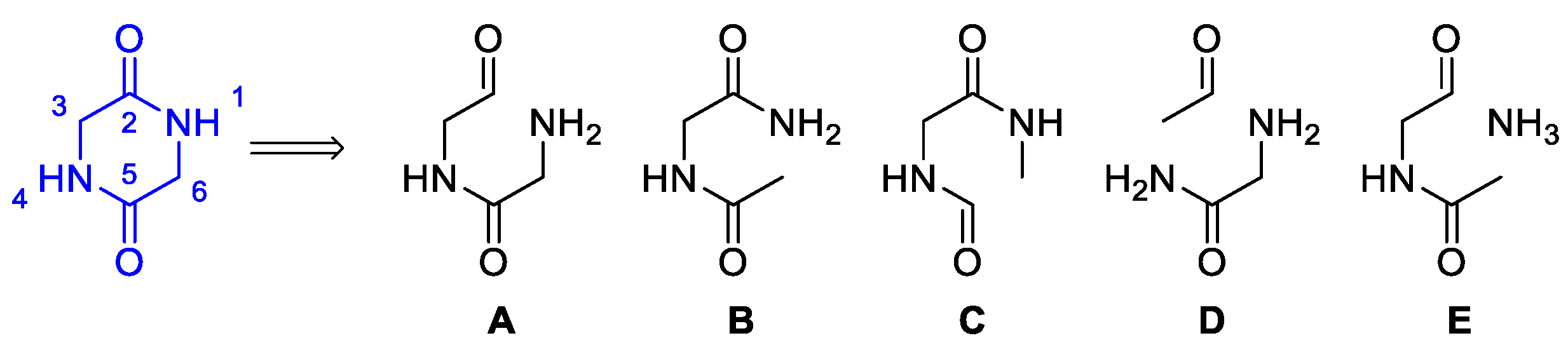
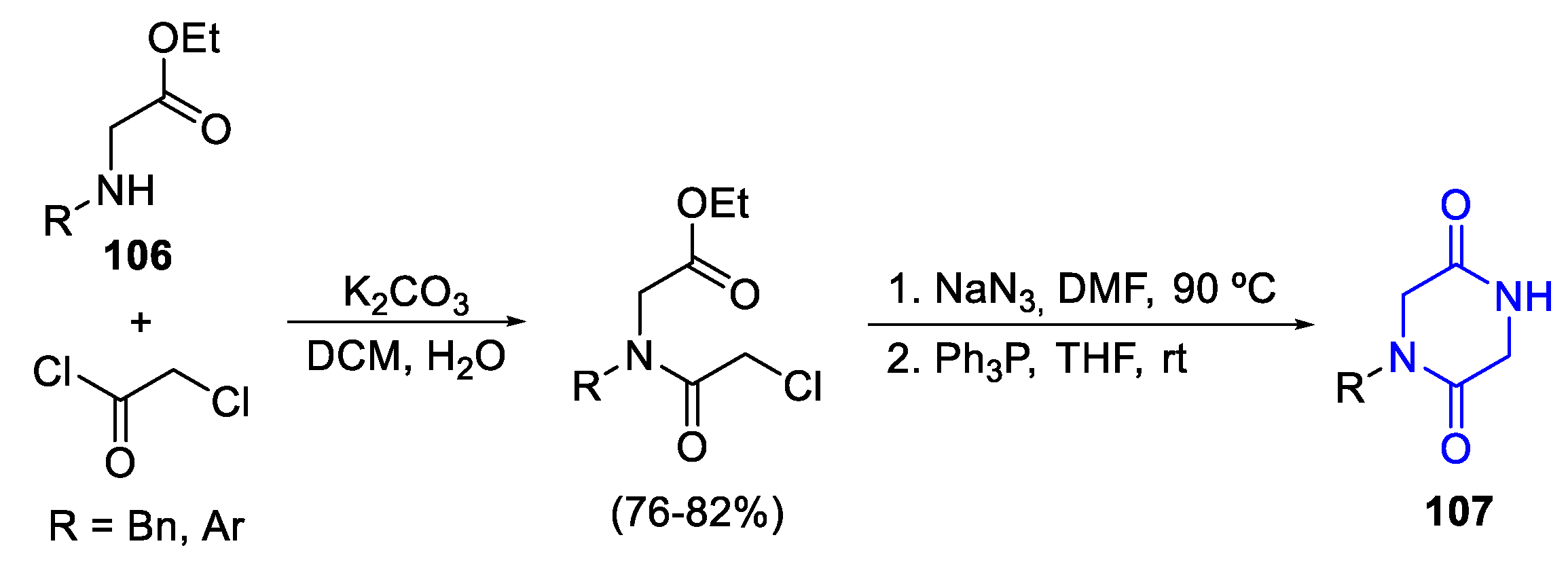
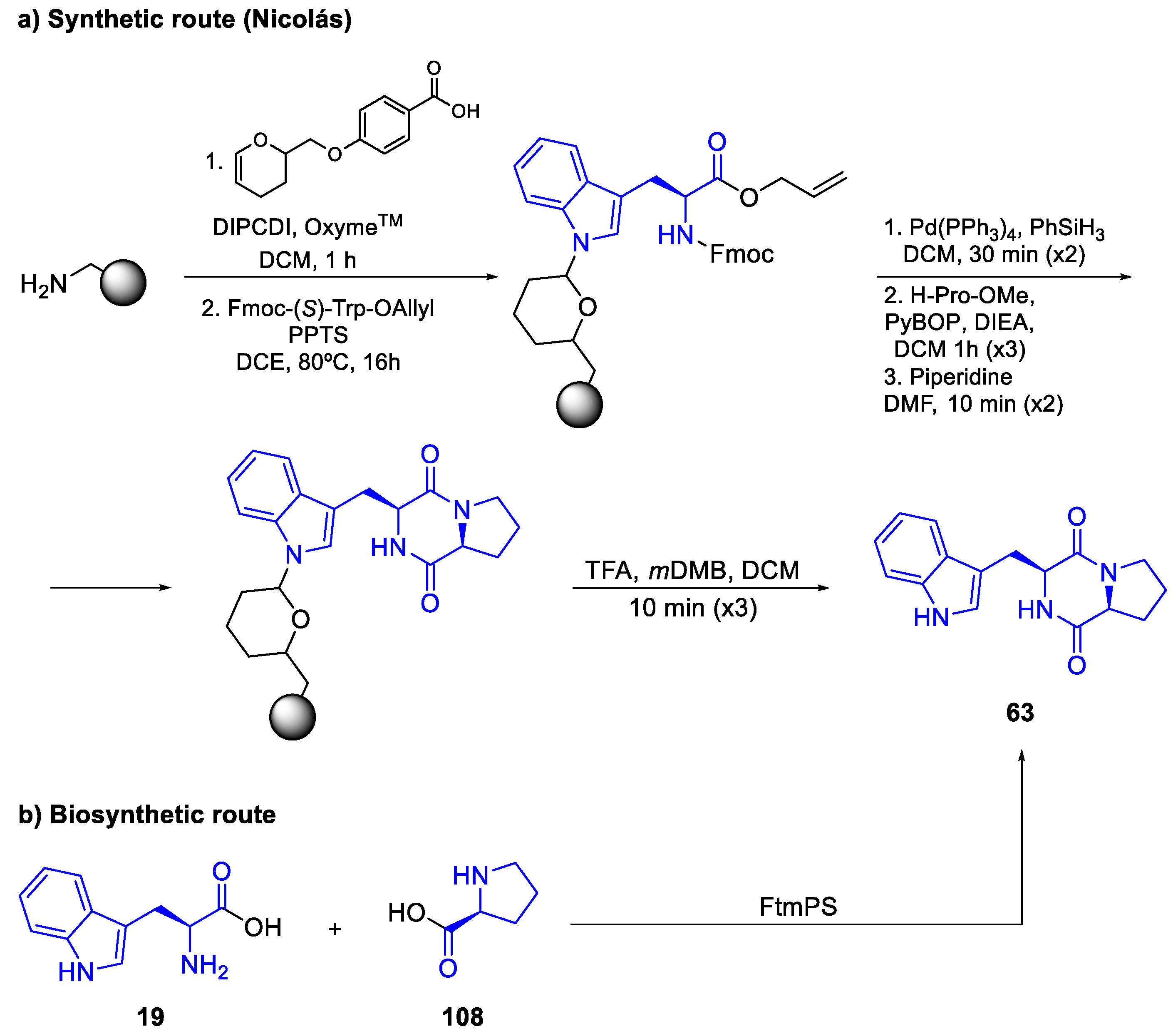
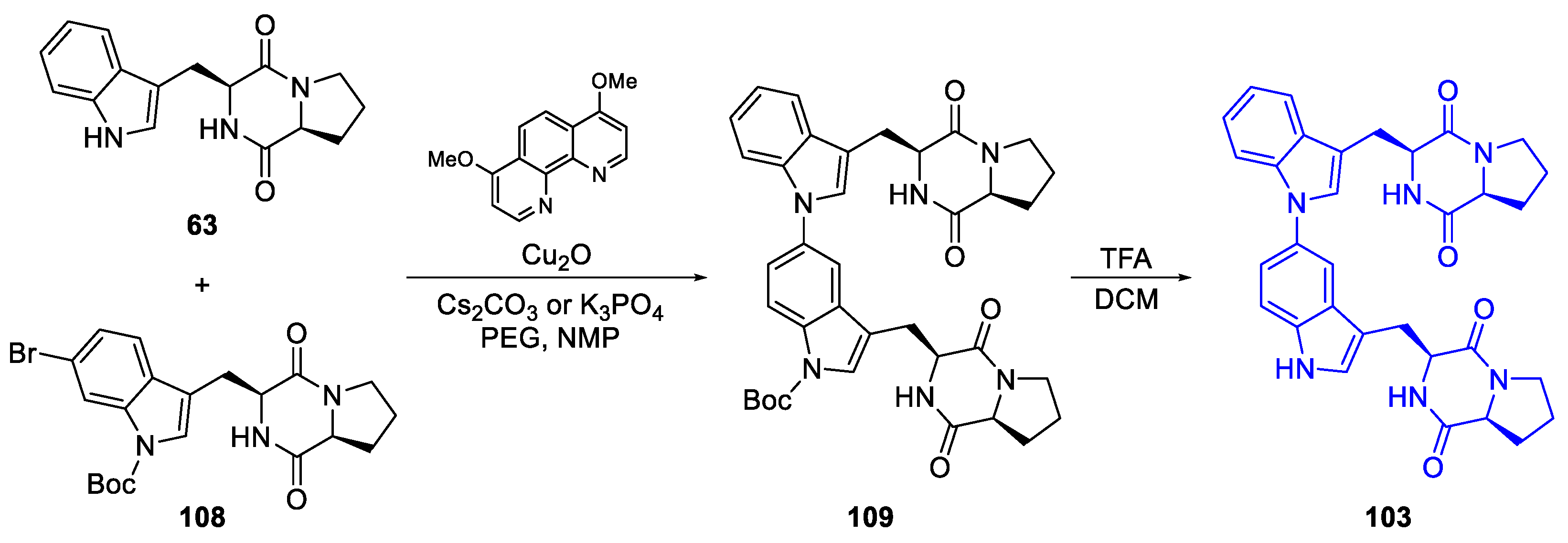
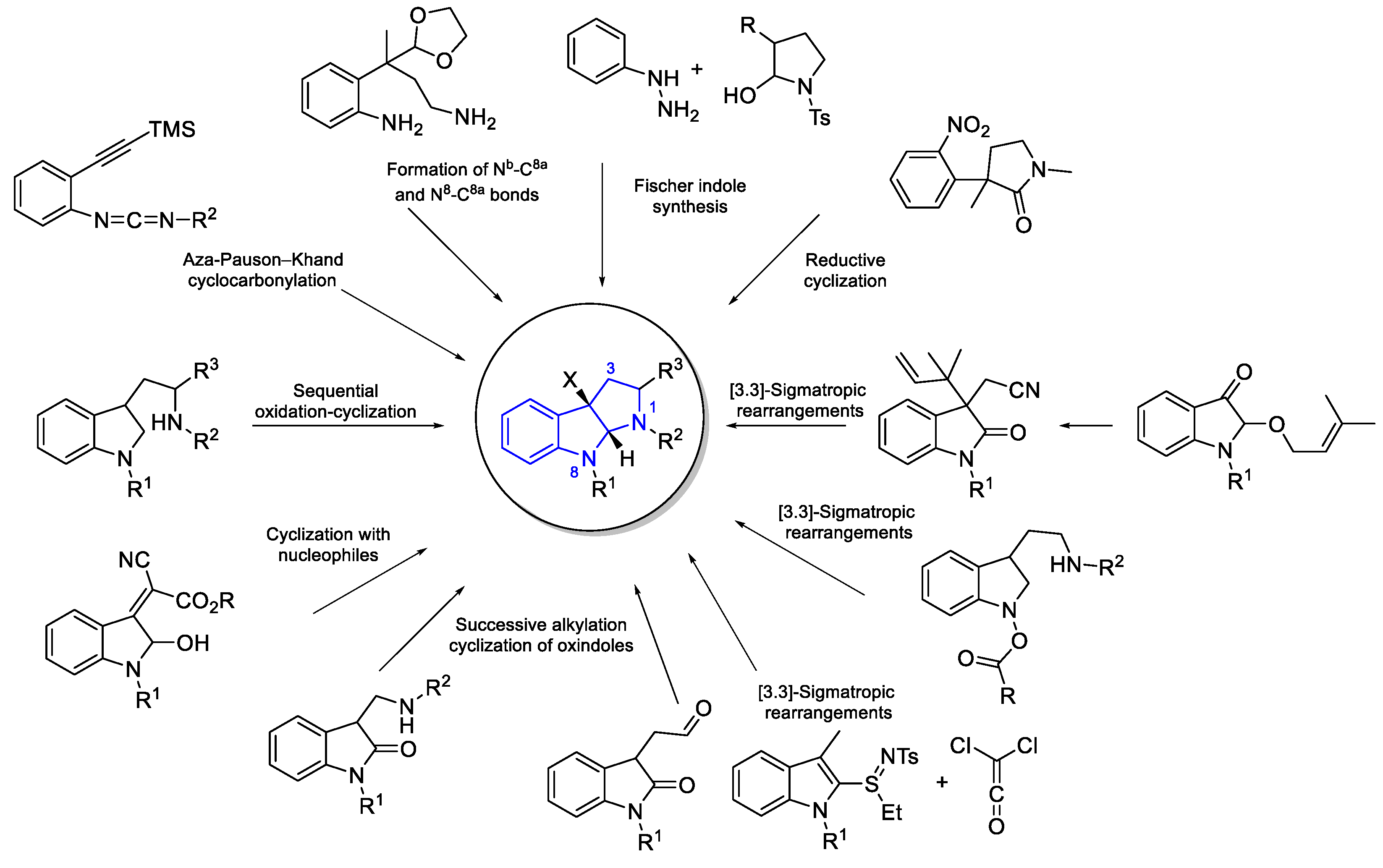
- Synthetic routes
2.2.1.2. DKPs featuring dimethylpyranoindole
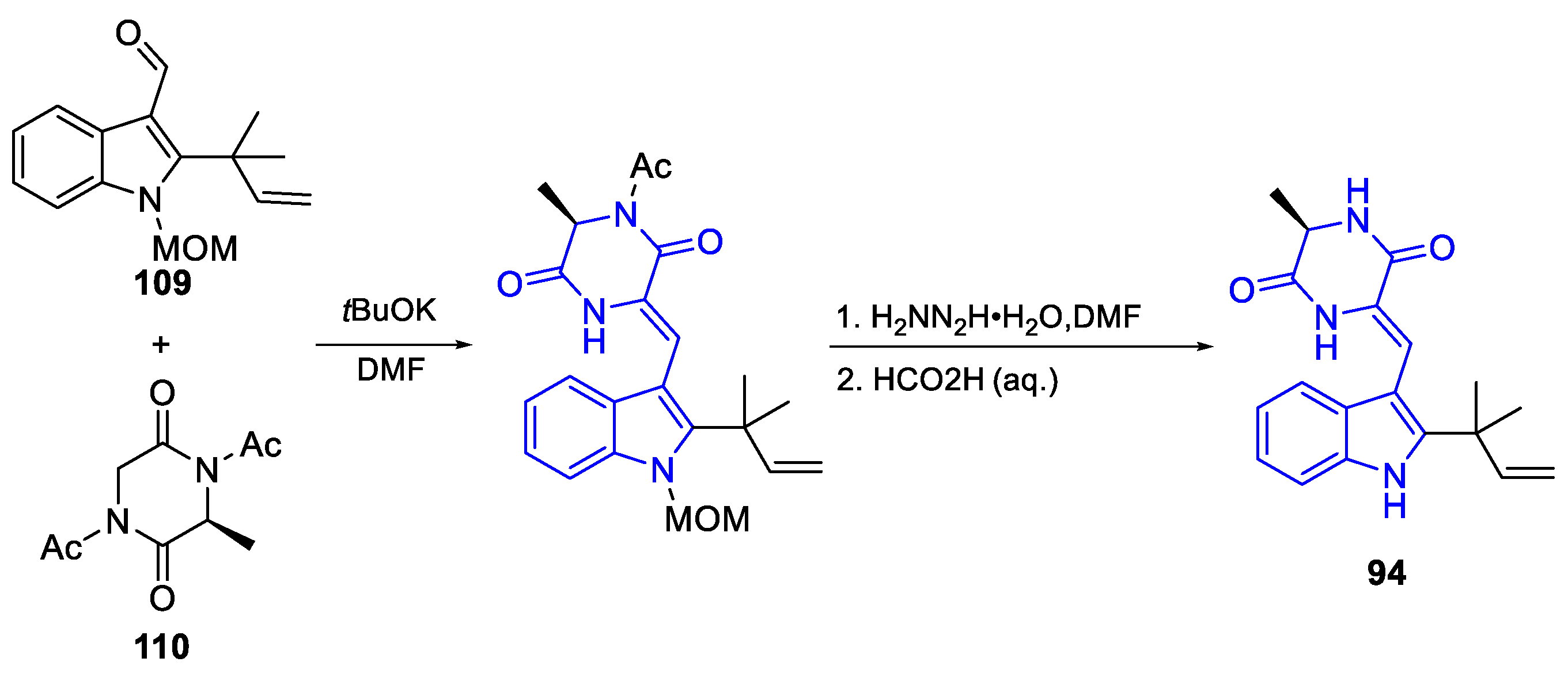
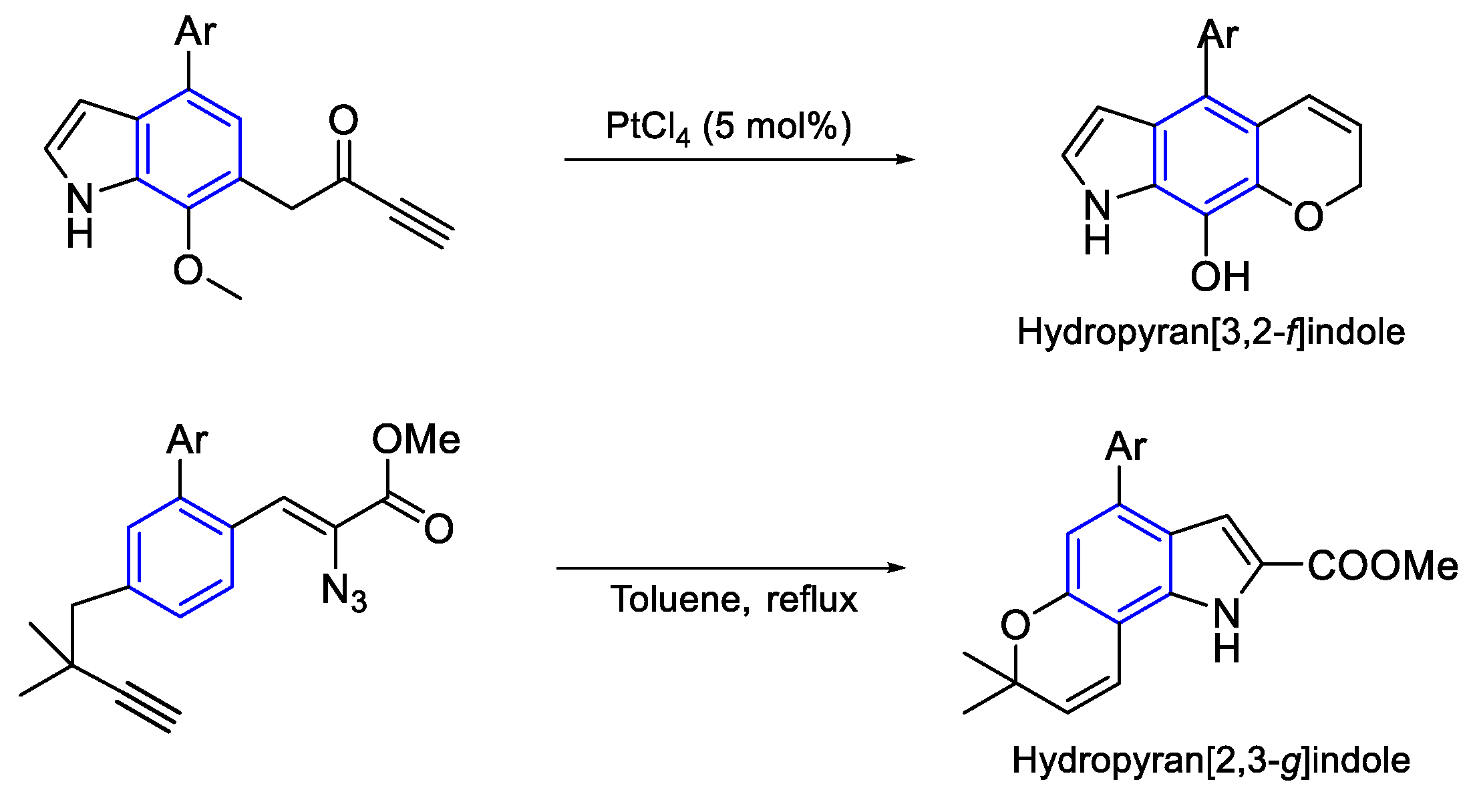
- Synthesis of Brevianamides Byciclo[2.2.2]diazaoctano alkaloids
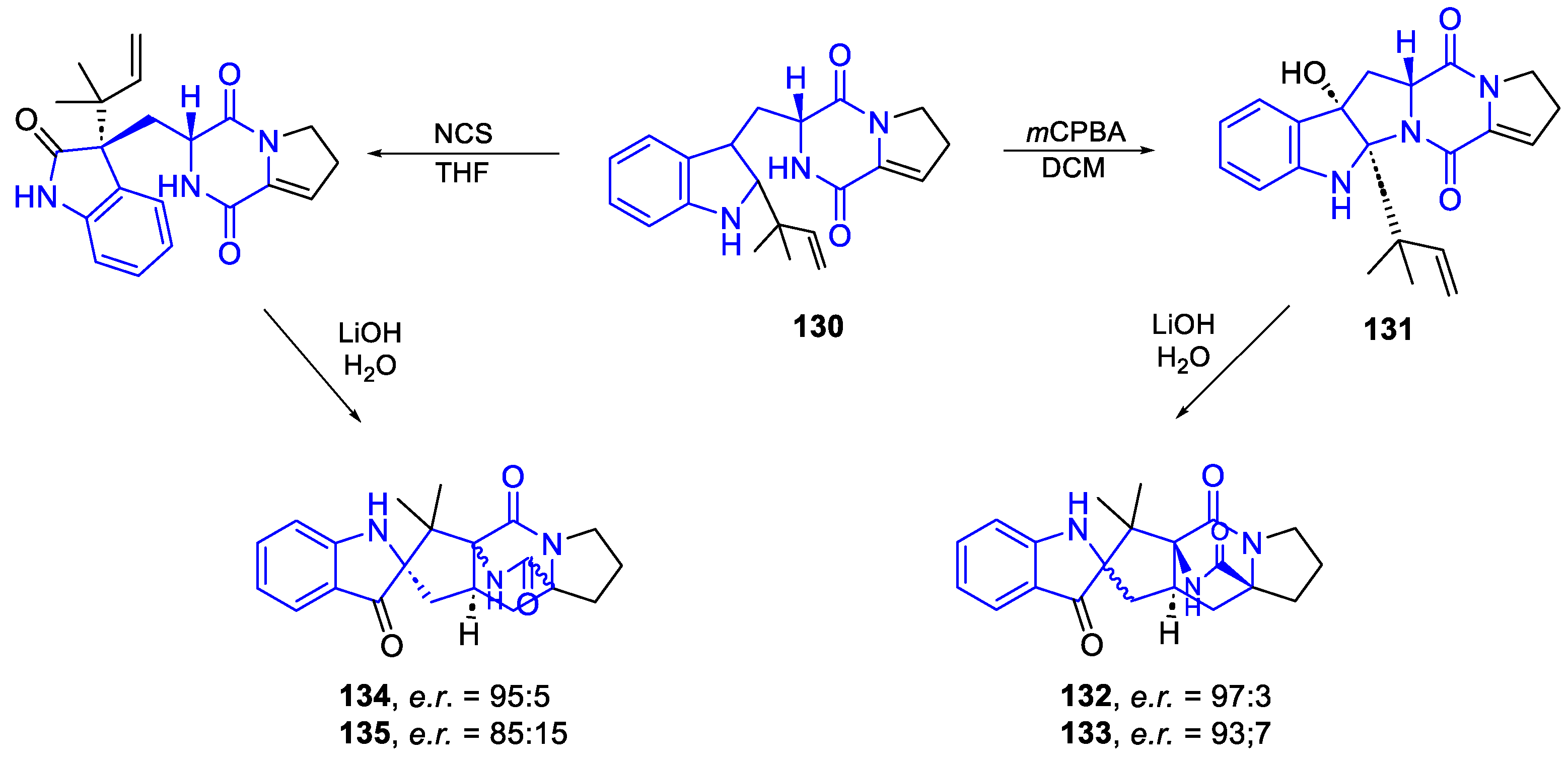
- Synthesis general of hydropyranoindole alkaloids
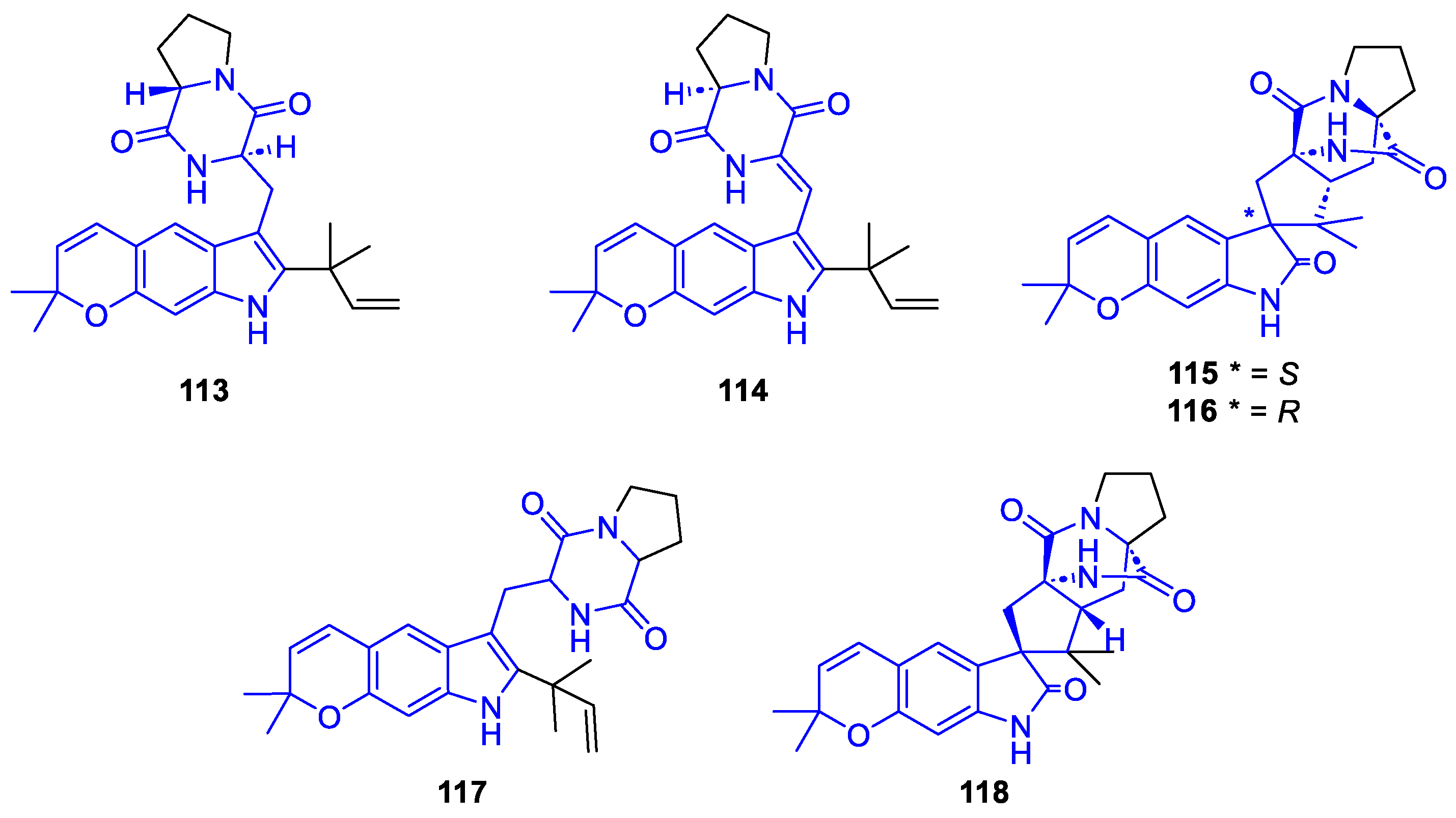
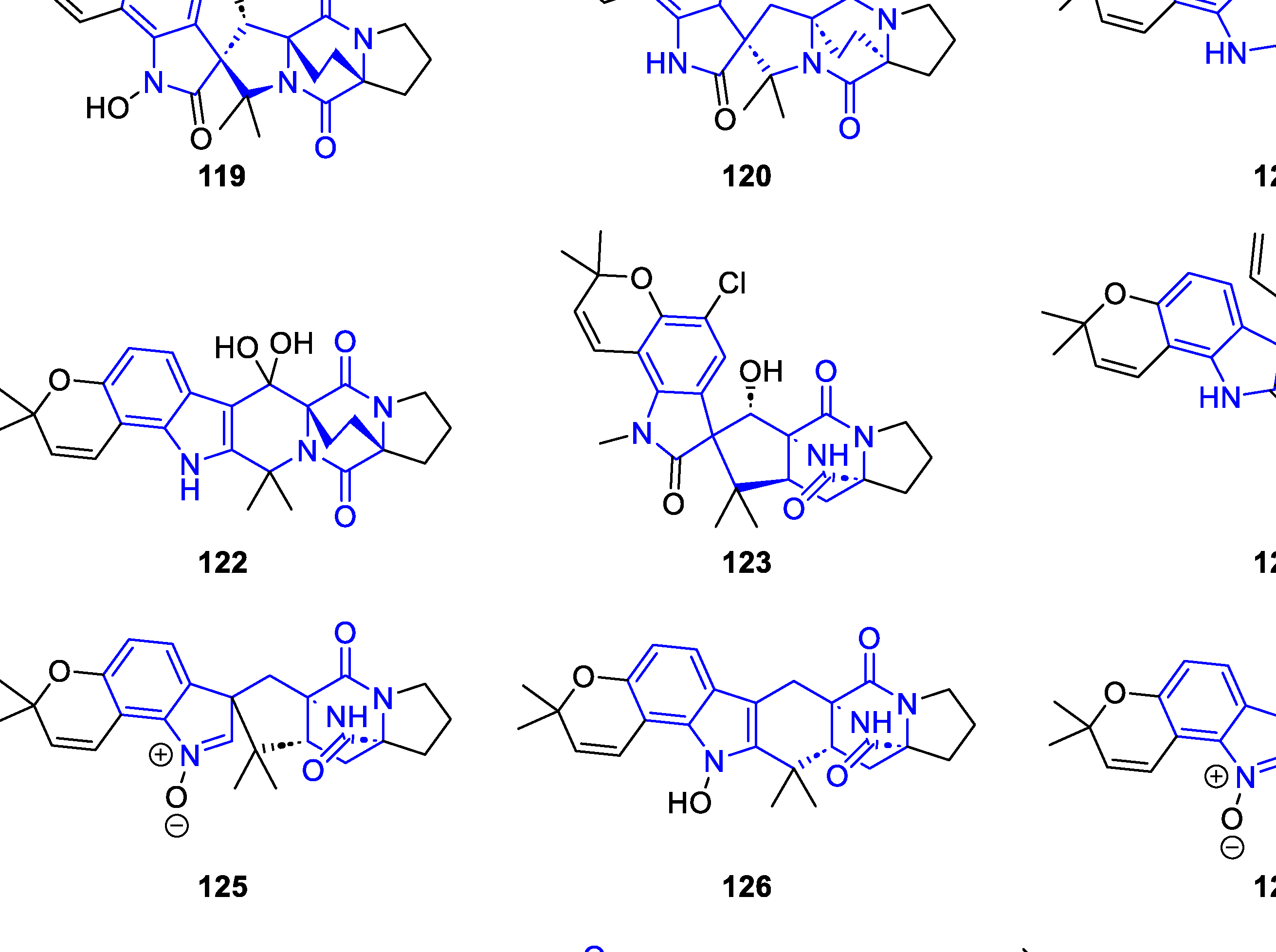
2.2.1.3. Spirocyclic DKP alkaloids
2.2.1.4. Other polycyclic DKP alkaloids
- General Synthesis of indole DKP alkaloids
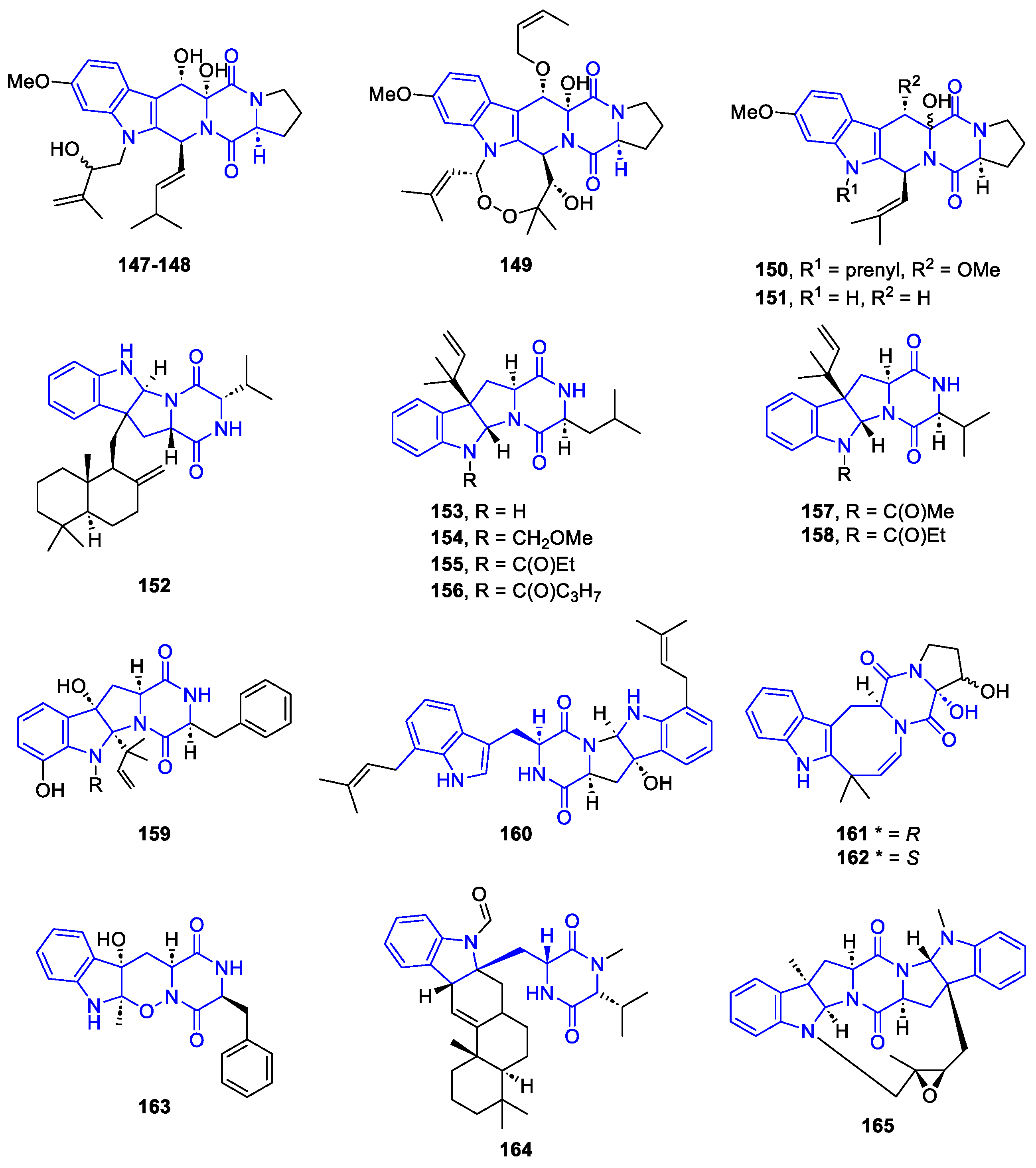
2.2.2. HexahydroPyrrolo[2,3-b]indol (HPI) derivatives
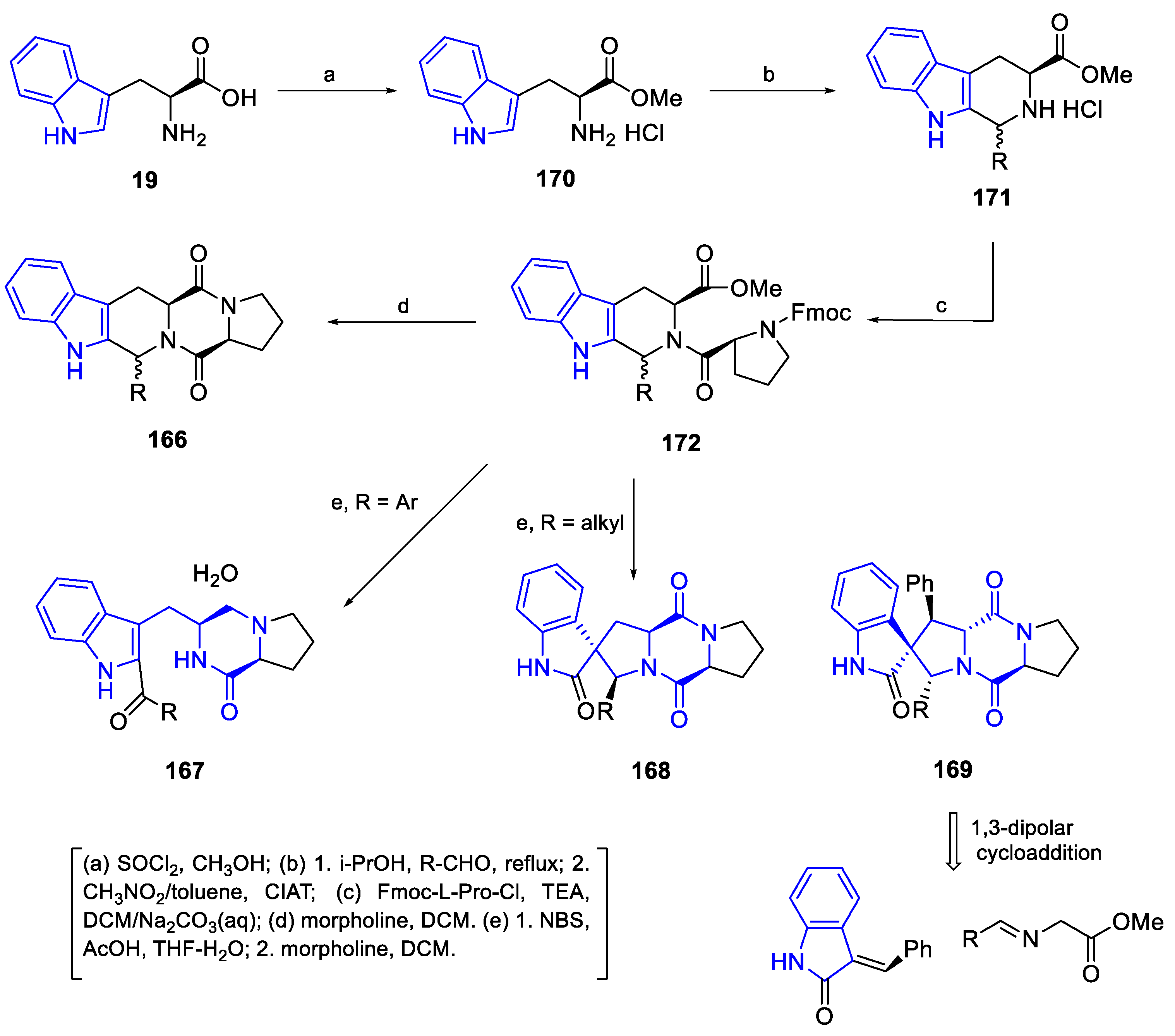
- Synthesis of HexahydroPyrrolo[2,3-b]indol (HPI) derivatives
Synthesis of Flustramines
Synthesis of HPI tricyclic skeleton
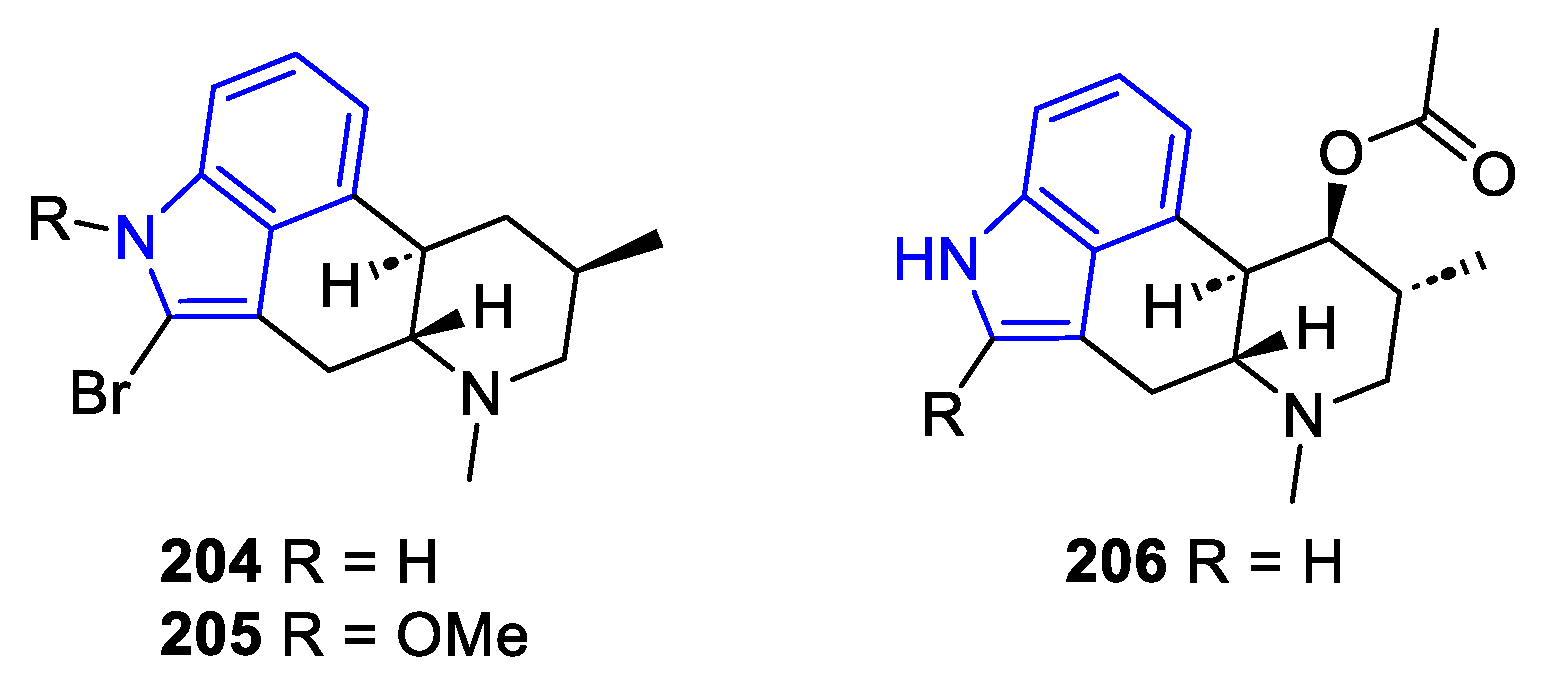
2.2.3. Indolactam alkaloids
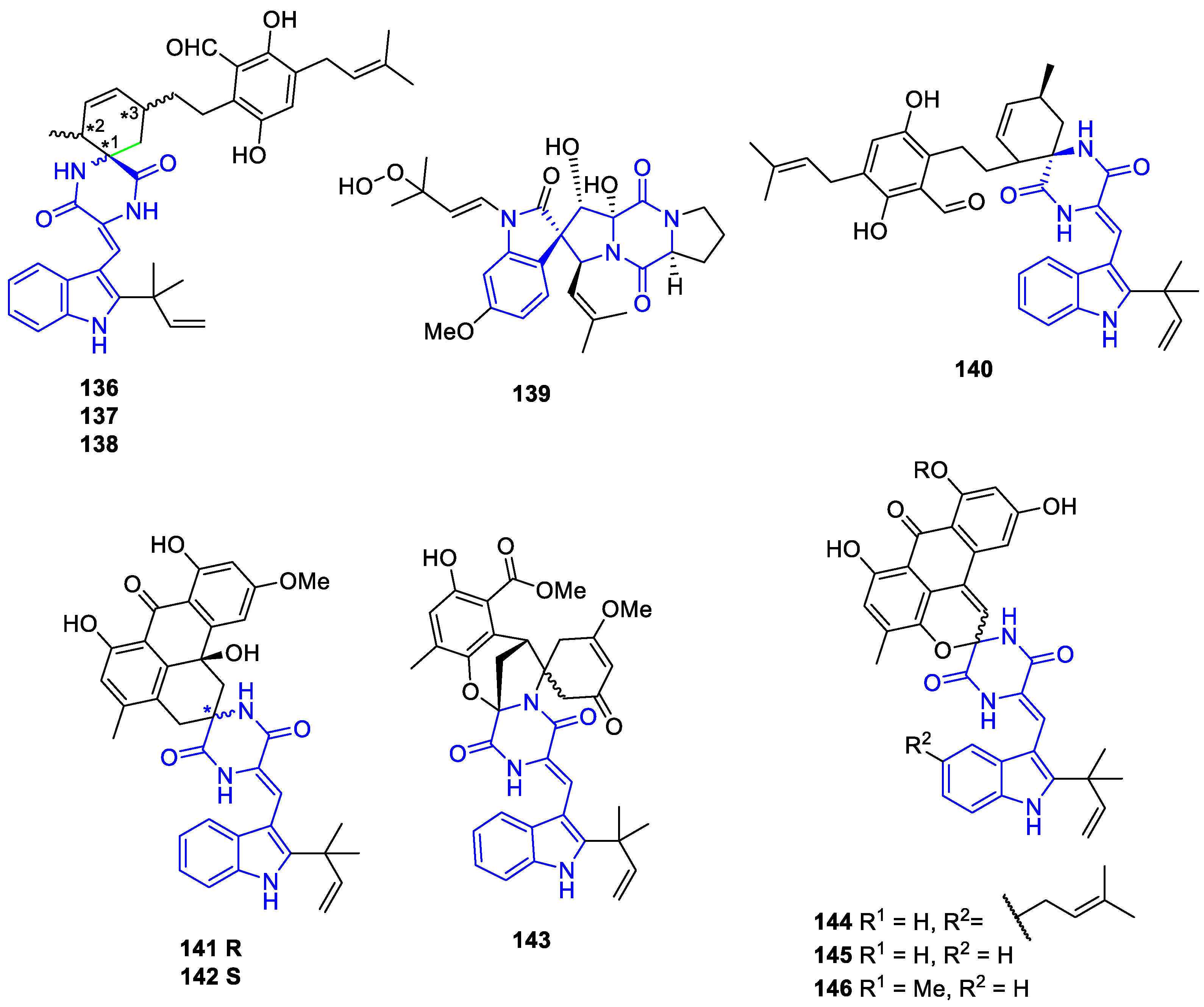
2.2.4. Other Polycyclic Indole alkaloids
2.2.6. Ergot alkaloids
2.3. Annelated indole alkaloids
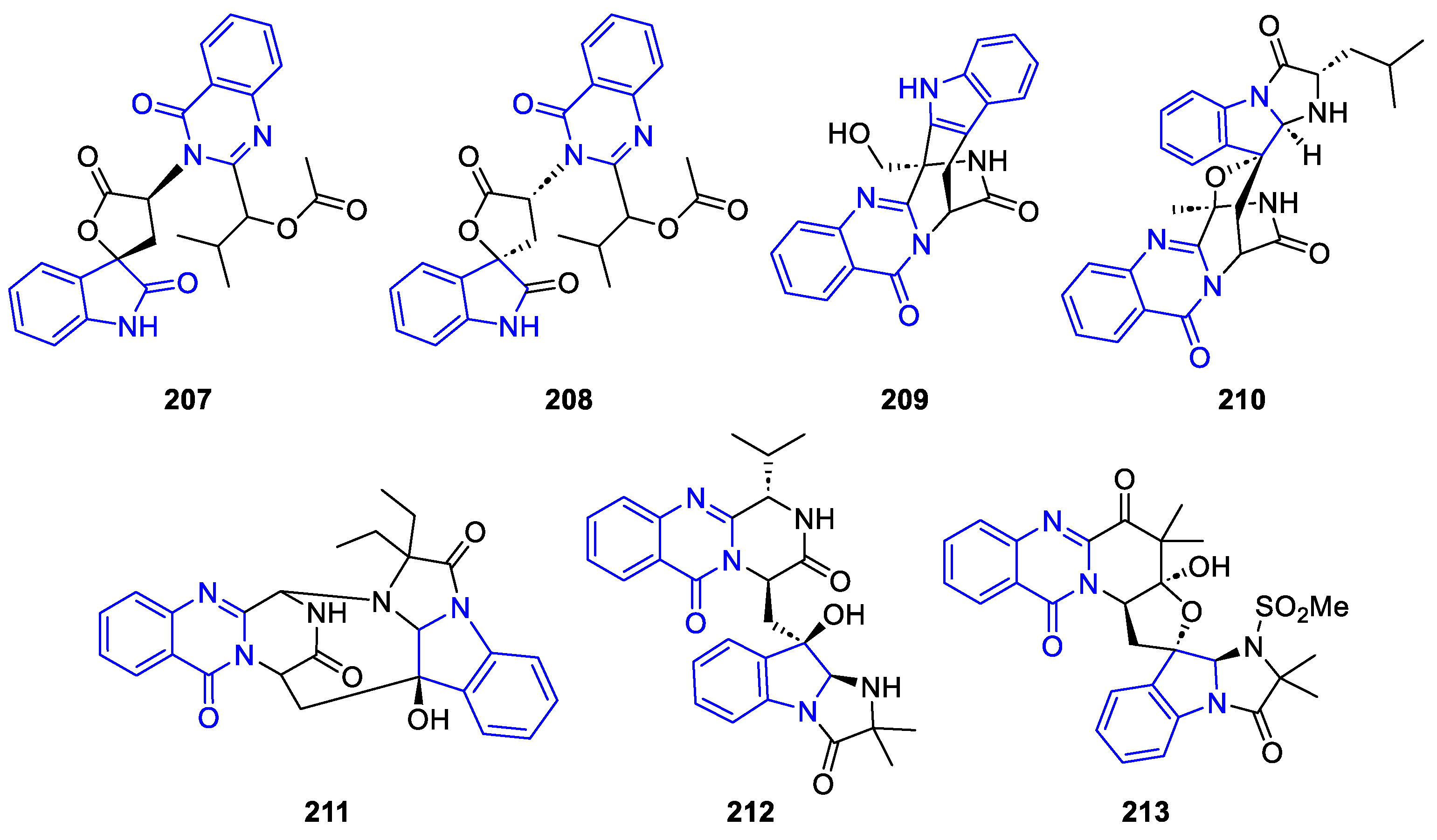
2.3.1. Quinazoline(inone)-containing annelated indole
2.3.2. Imidazolone-containing pyrrolidinone
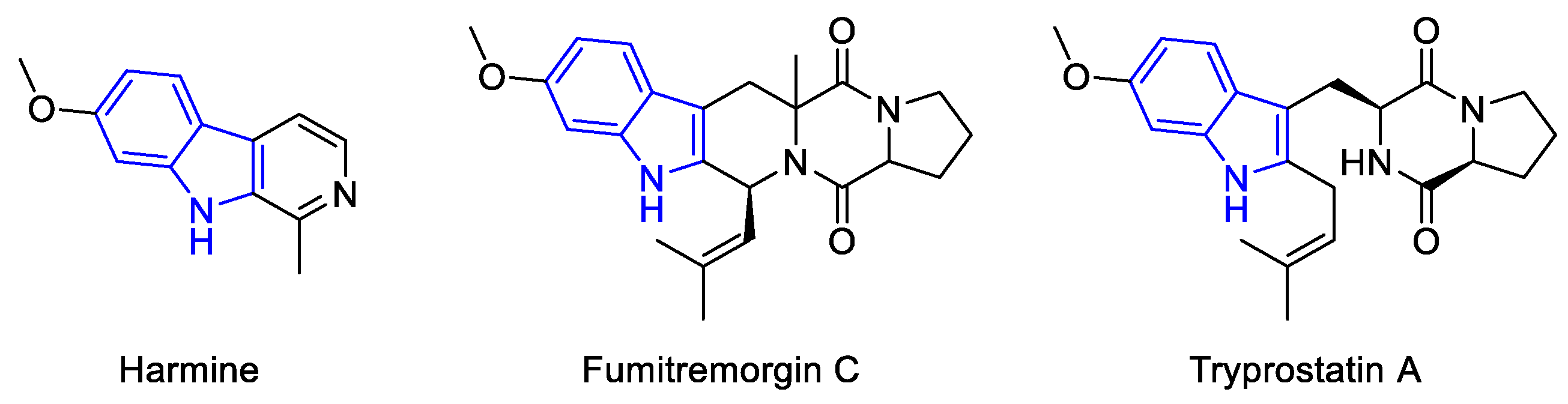
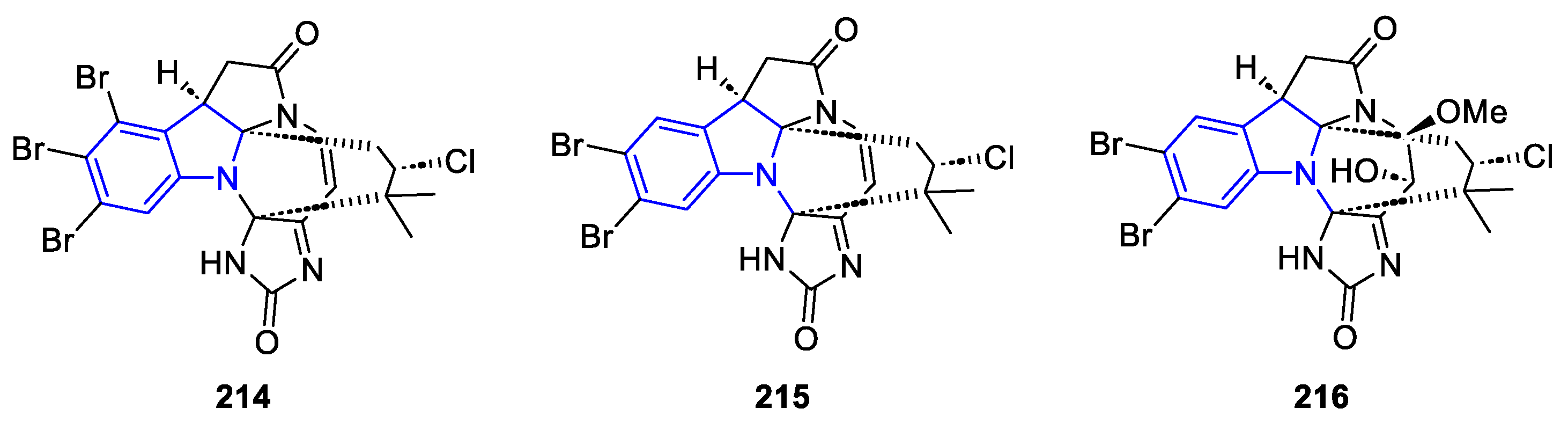
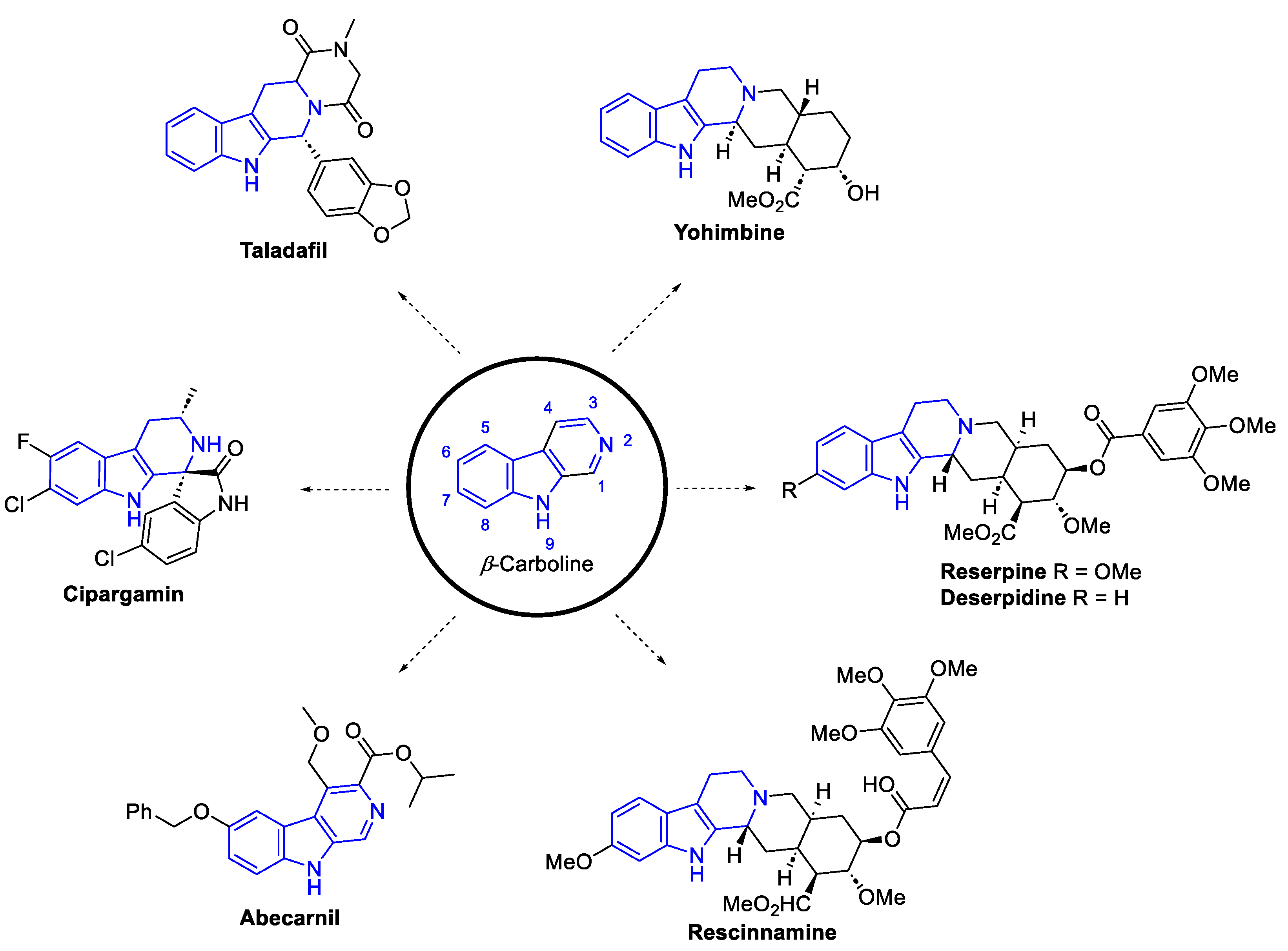
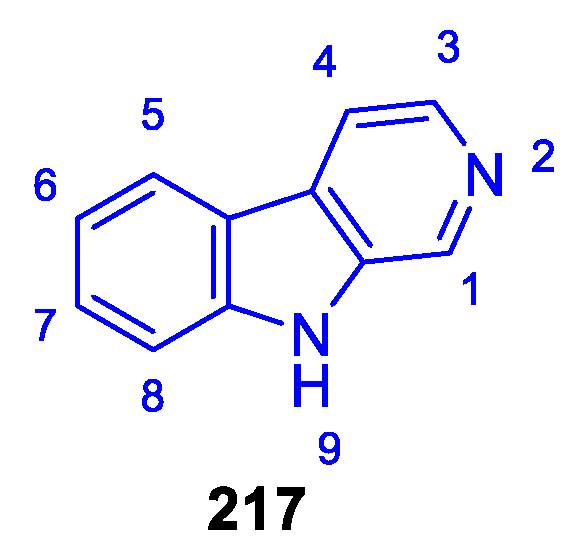
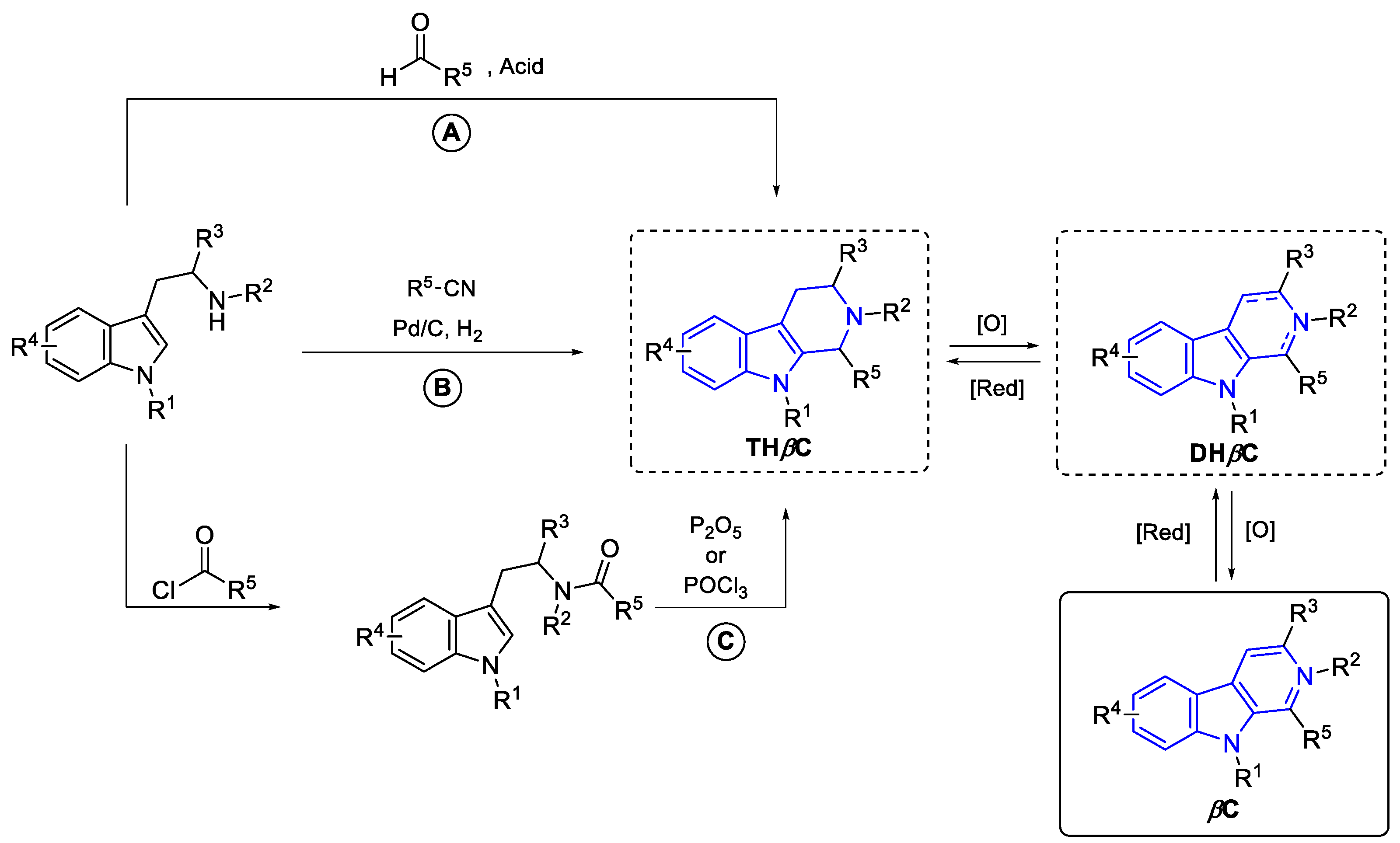
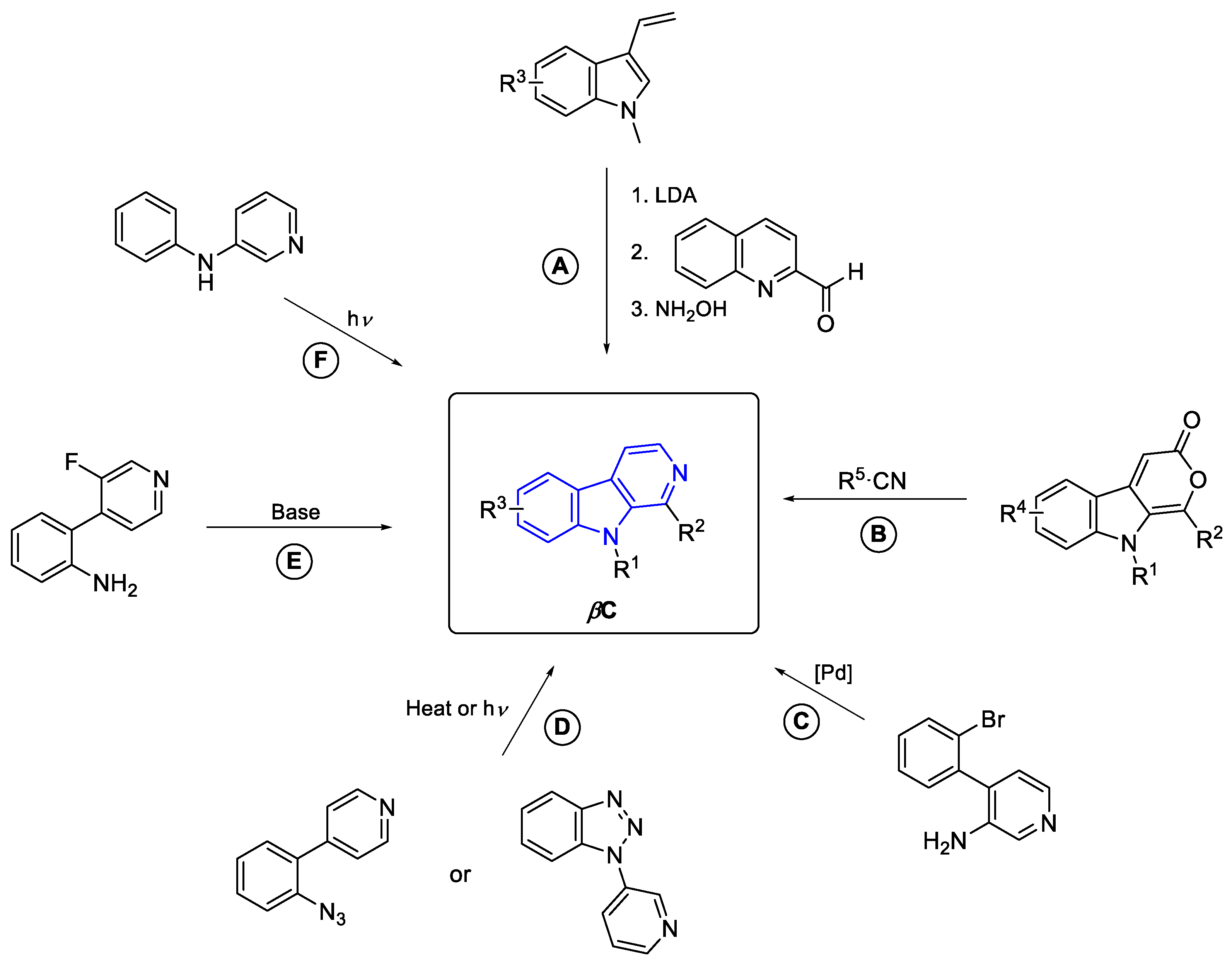
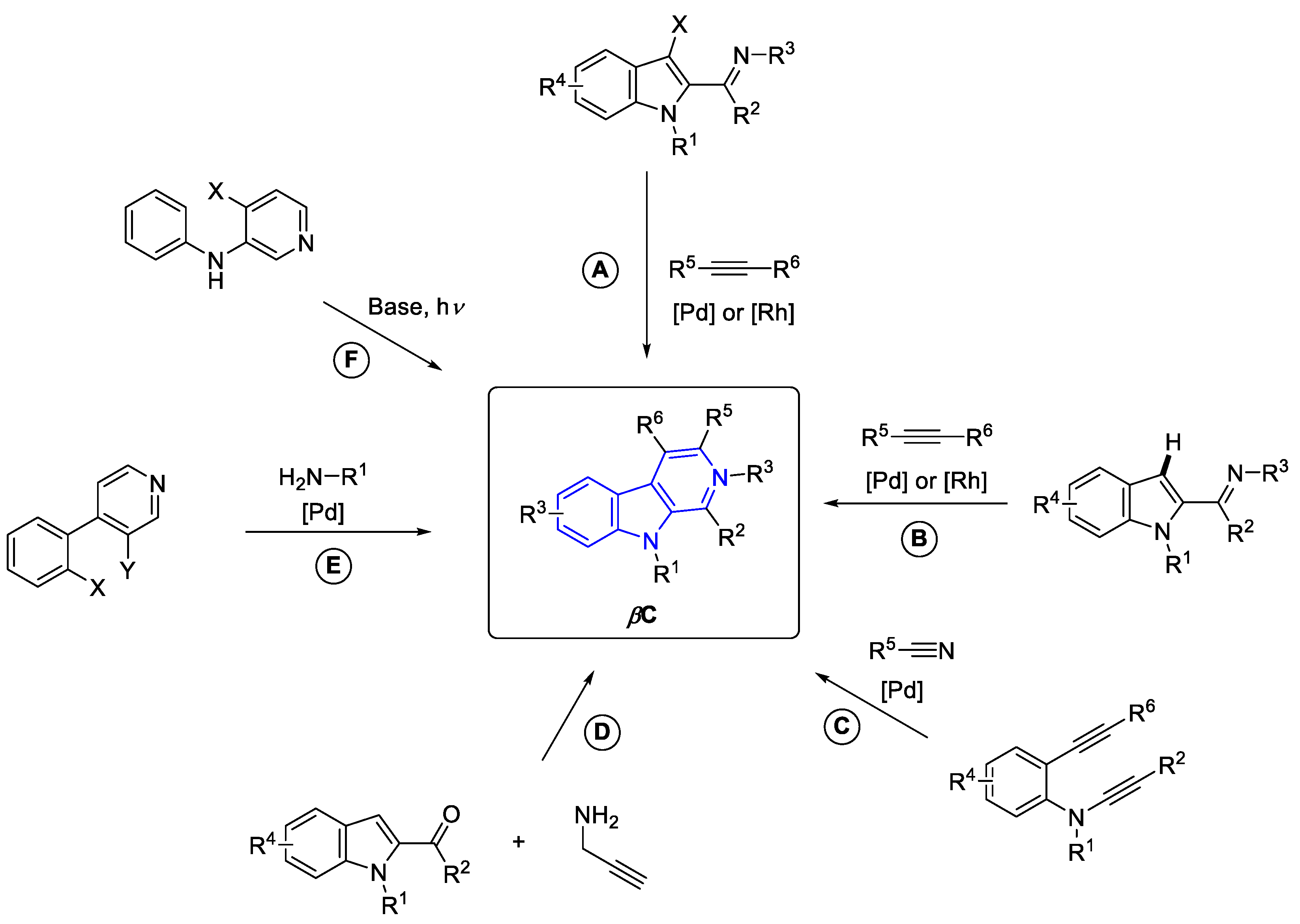
2.3.3. β-carbolines
2.2.1.1. β-Carboline monomers
- ‘Simple’β-Carbolines
- C1-substituted (DH/TH)-Carbolines:
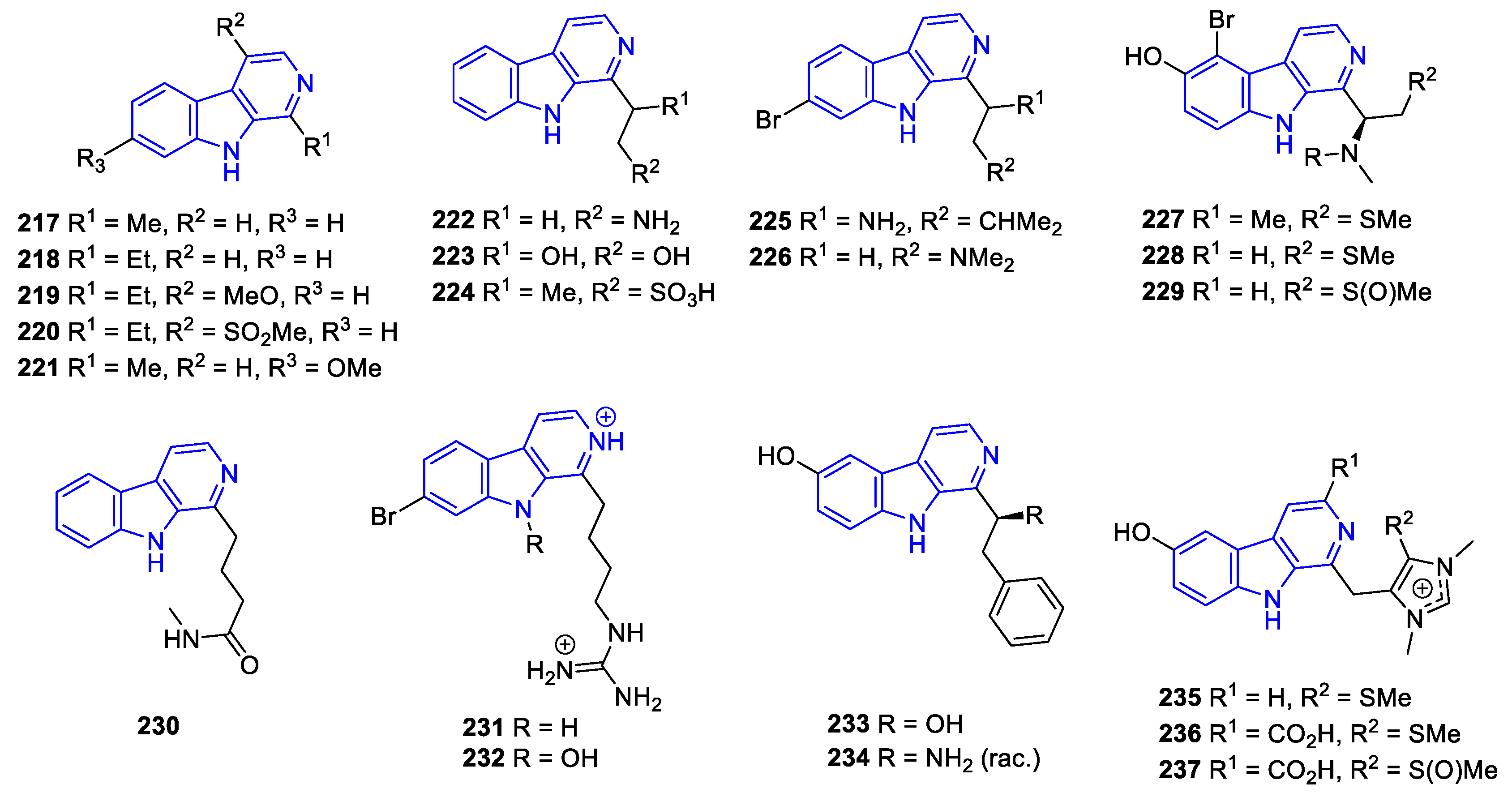
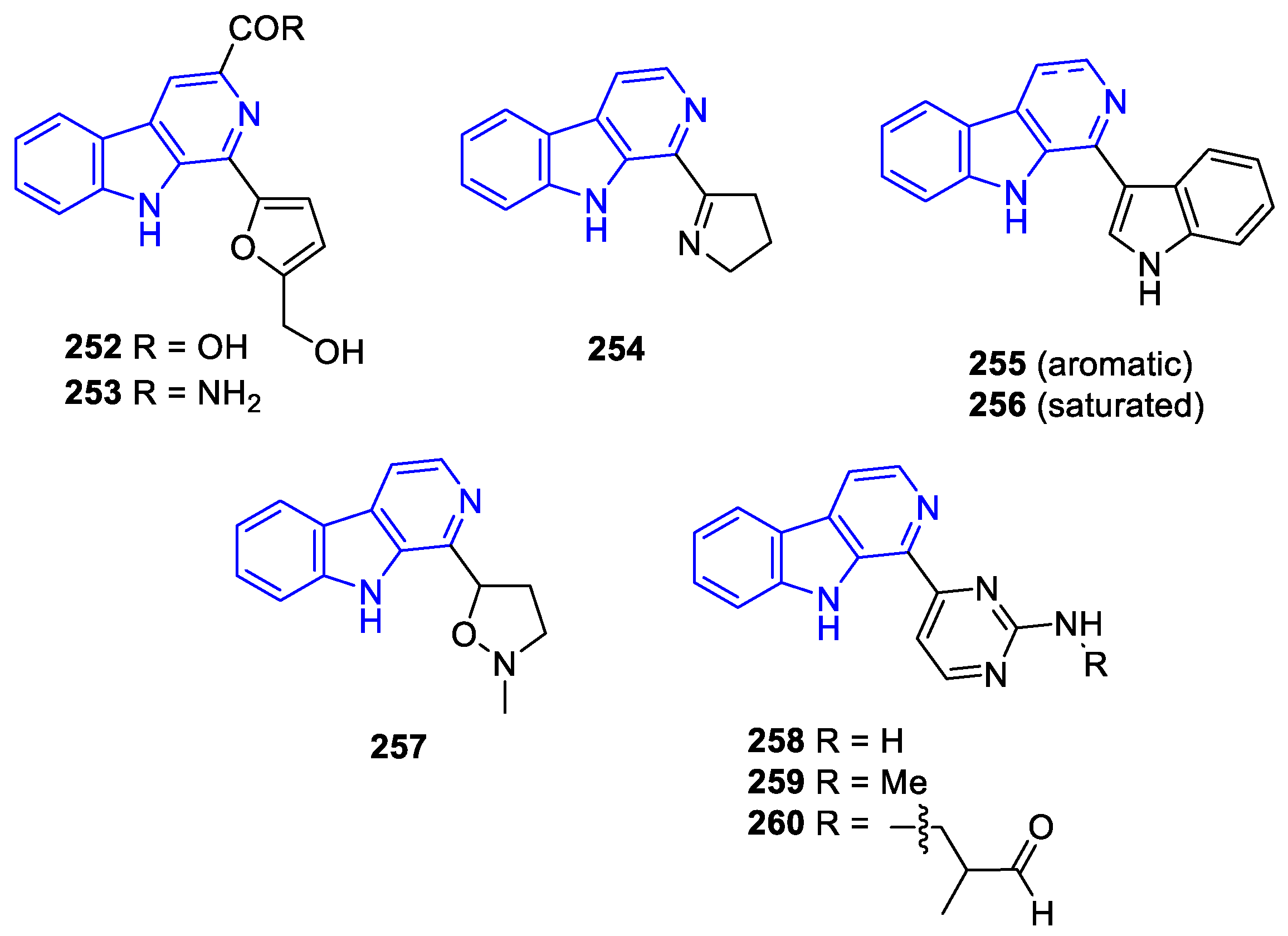
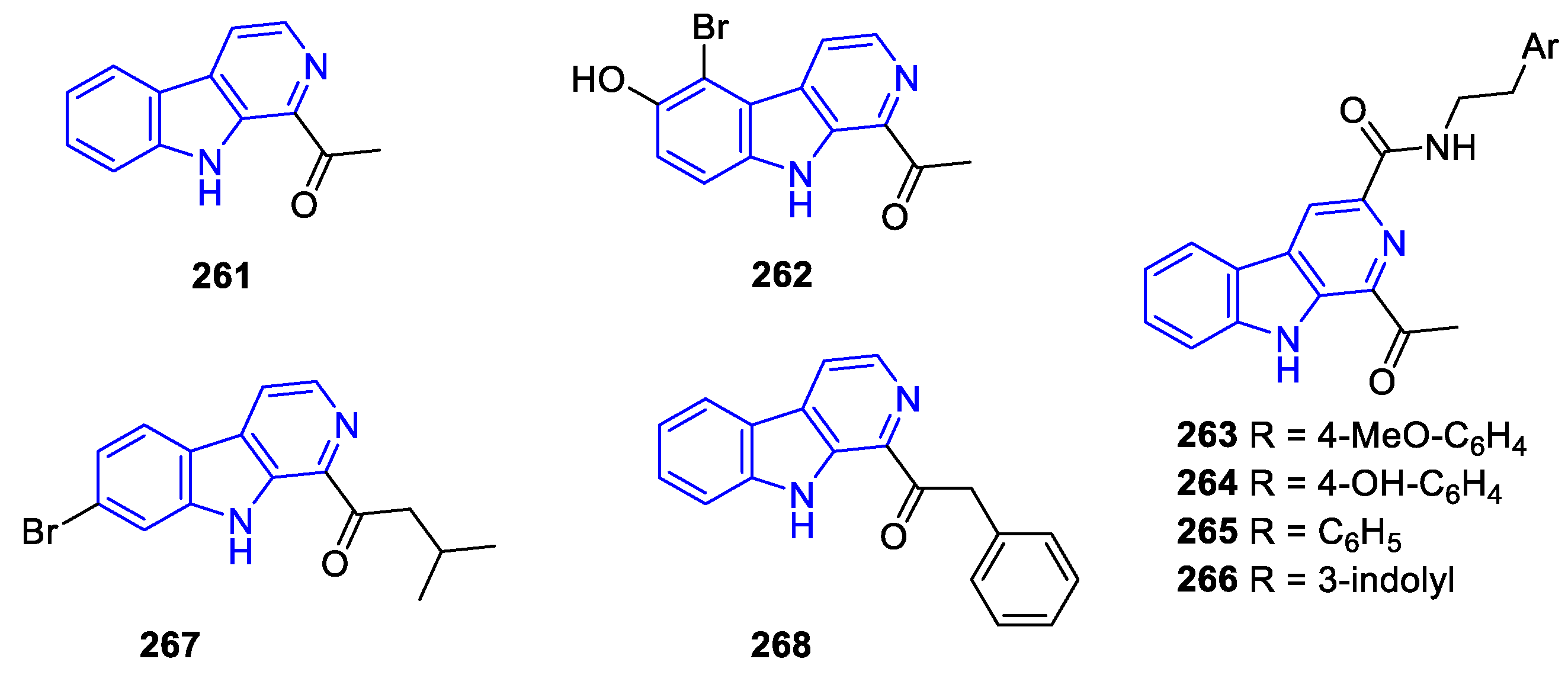
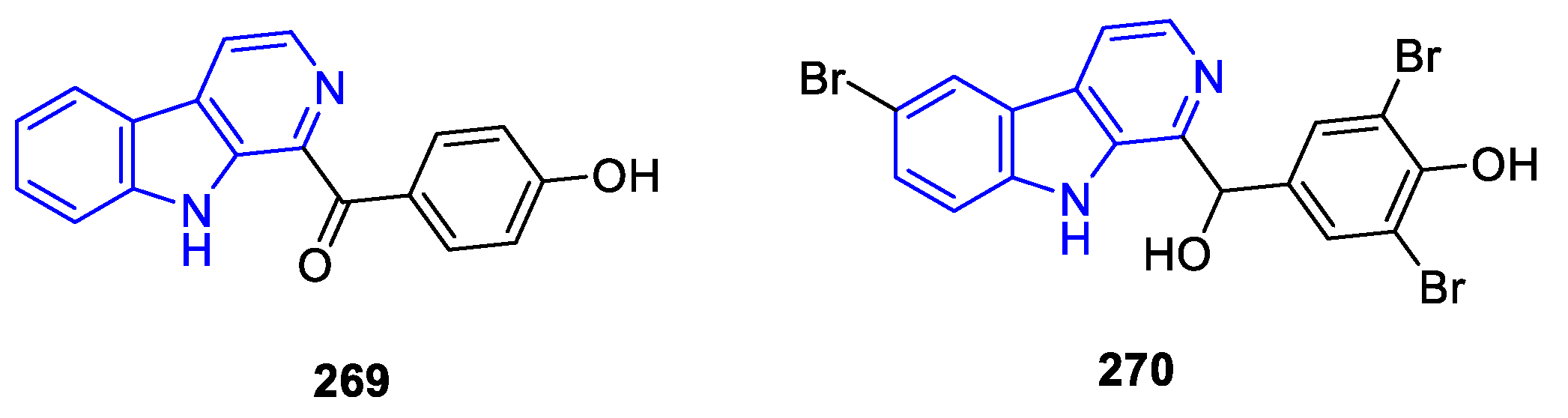
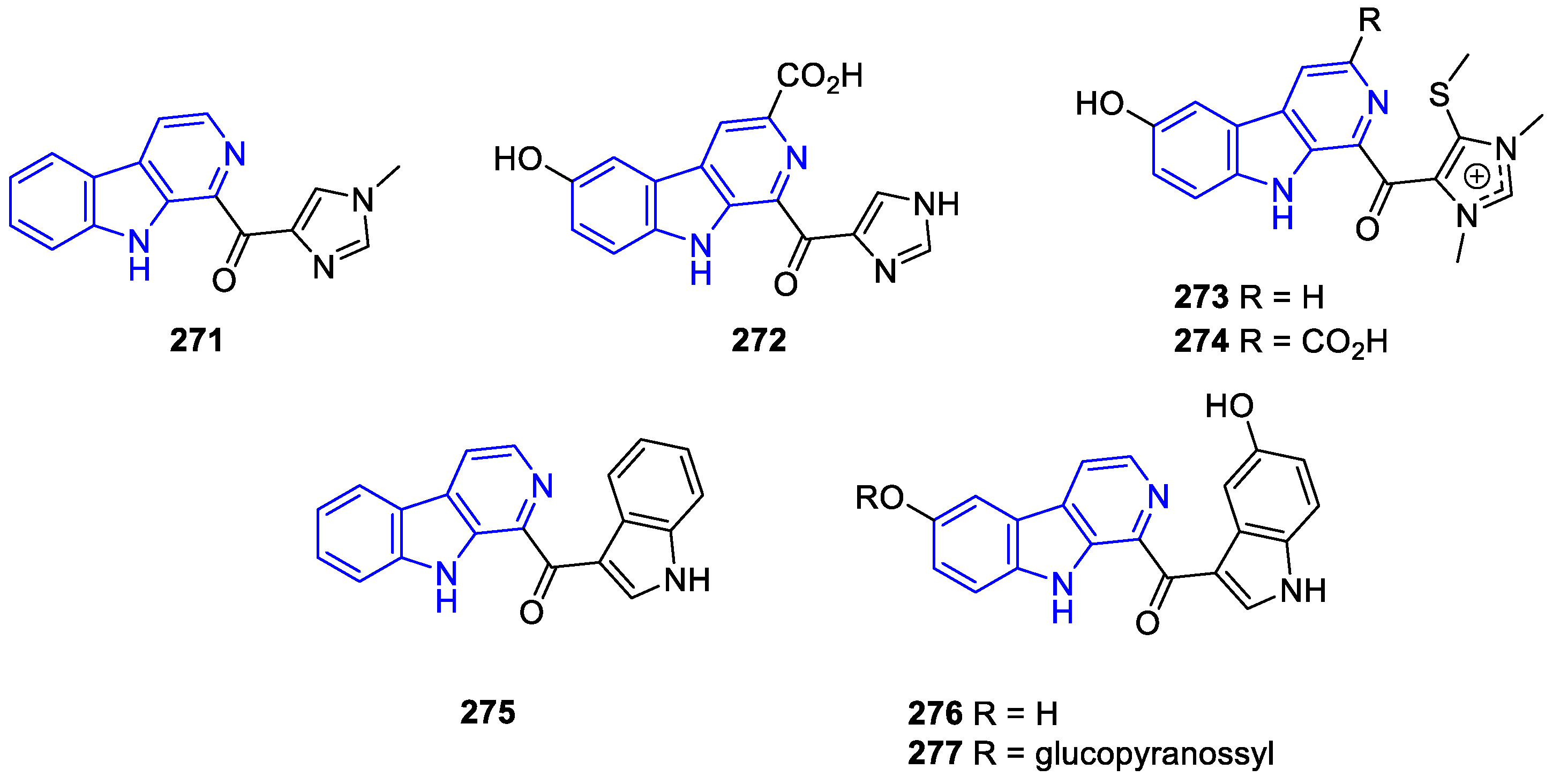
- Manzamines:
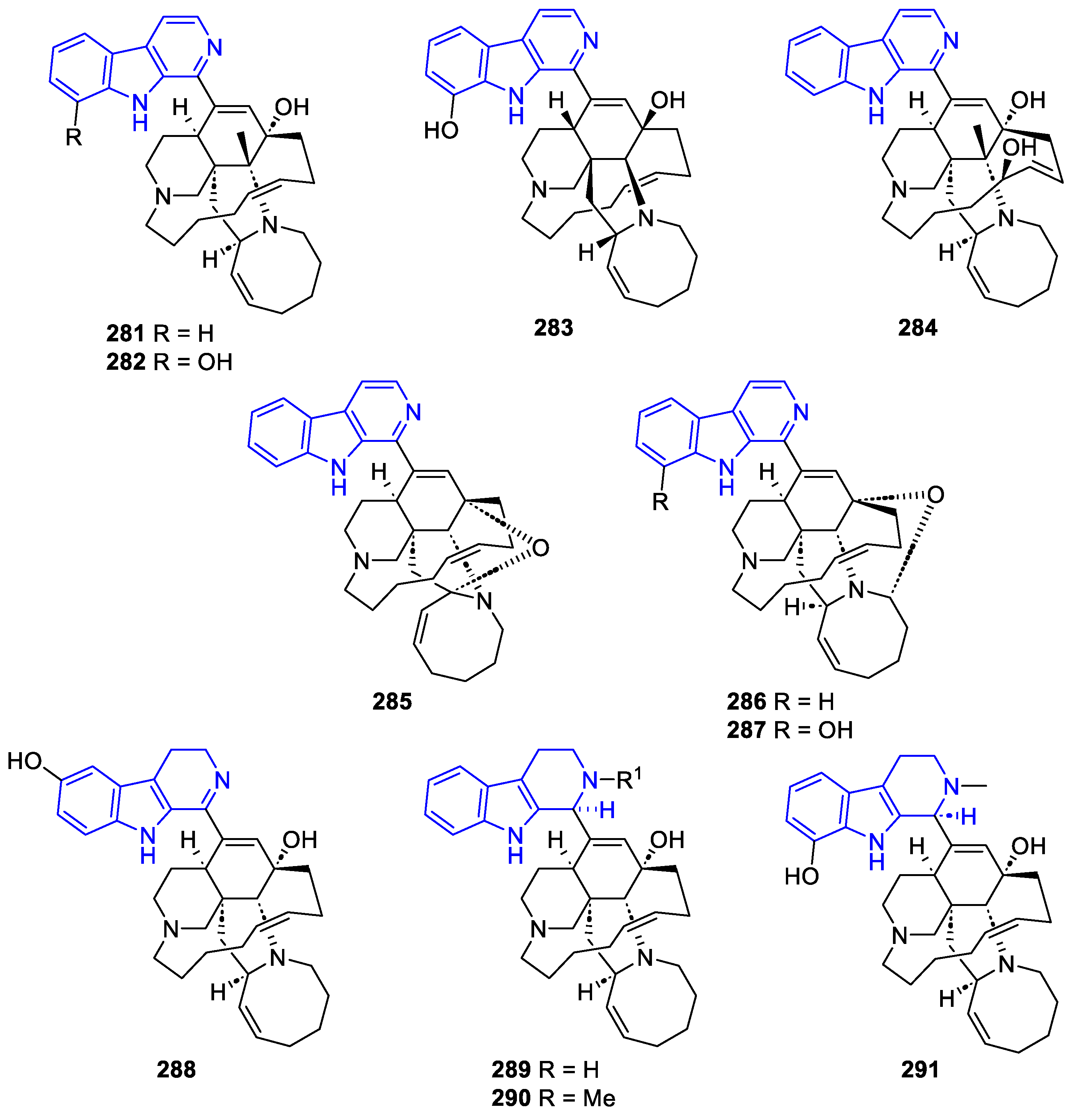
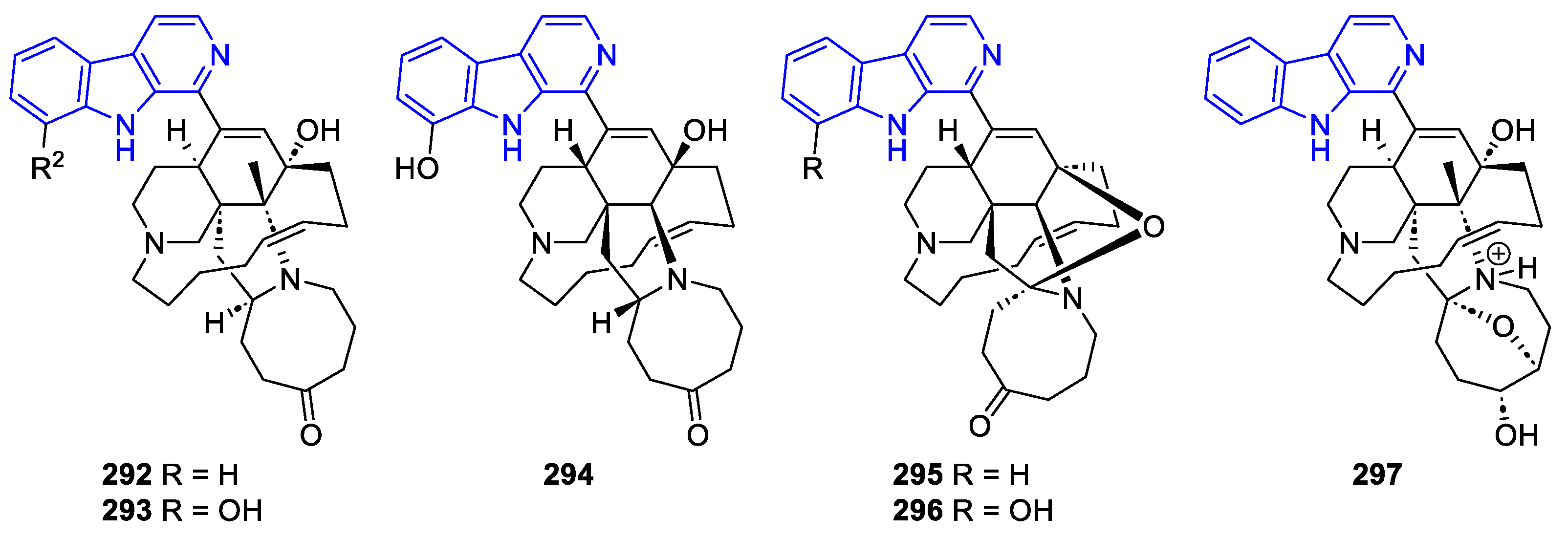
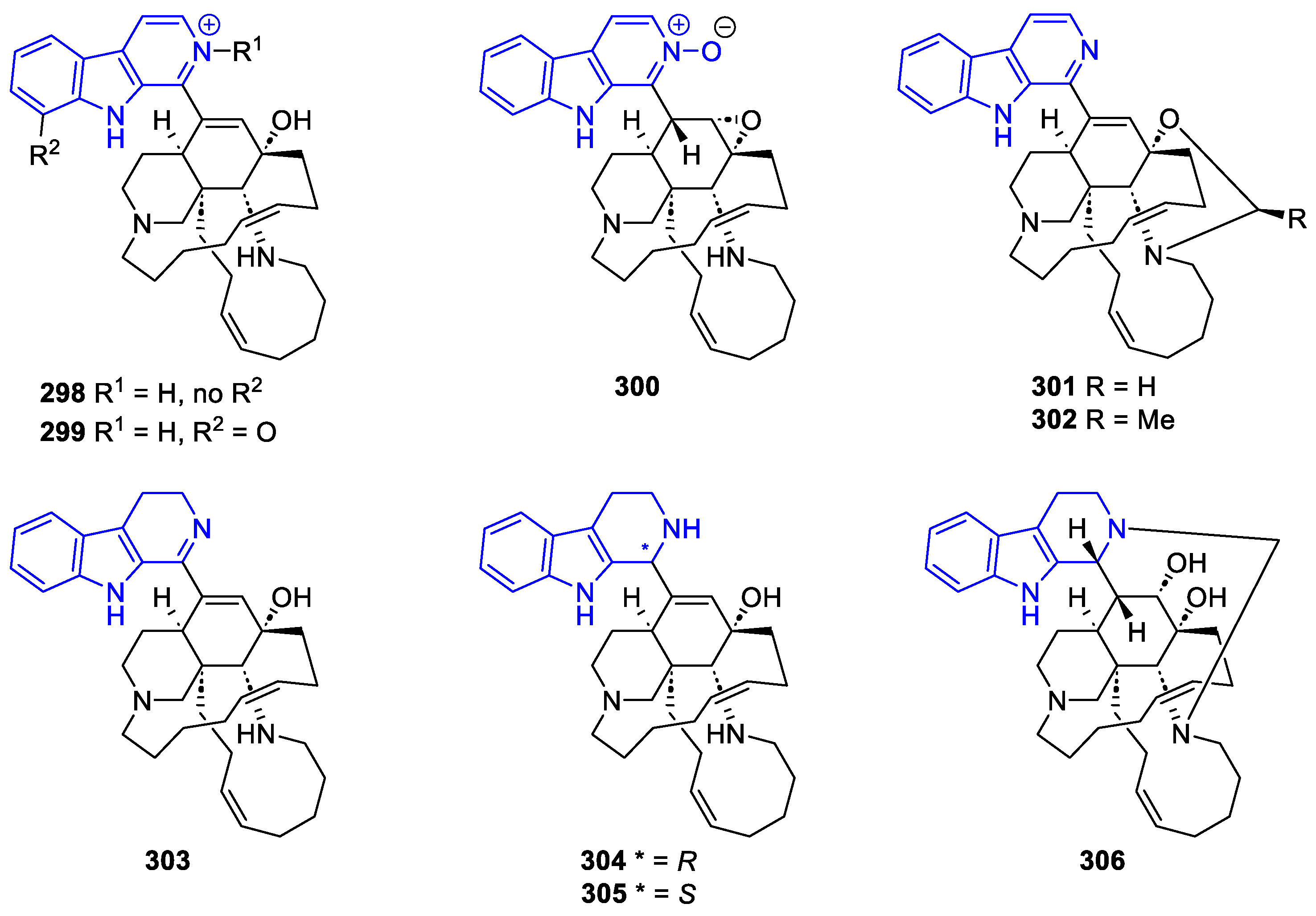
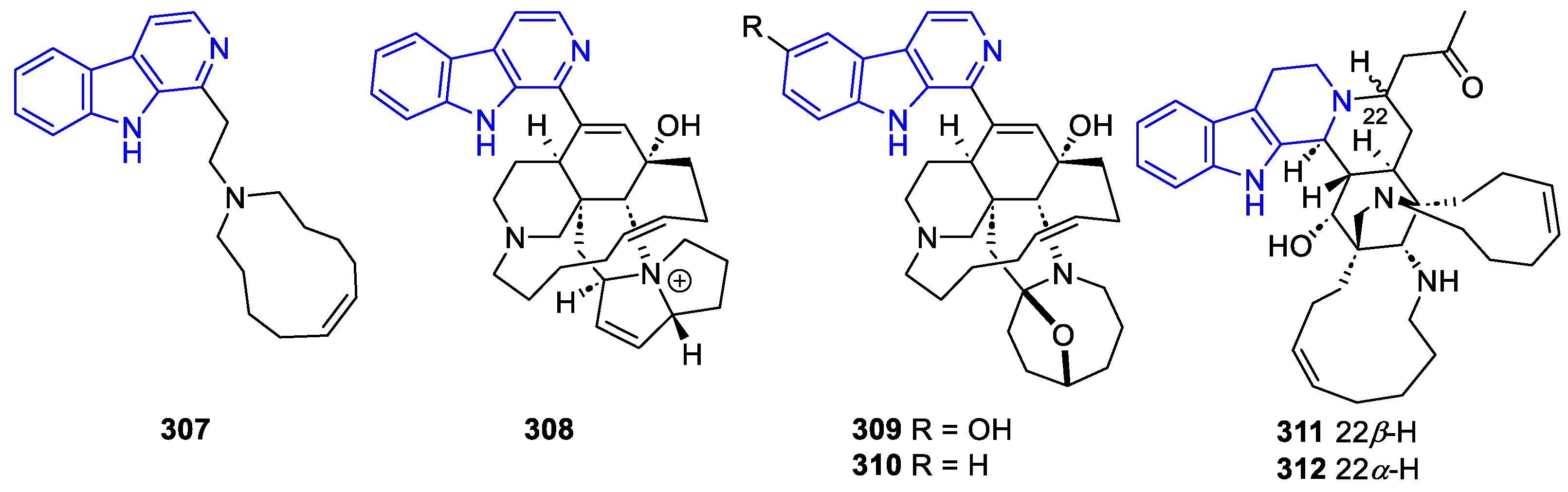
- N2-substituted (DH/TH)β-Carbolines:
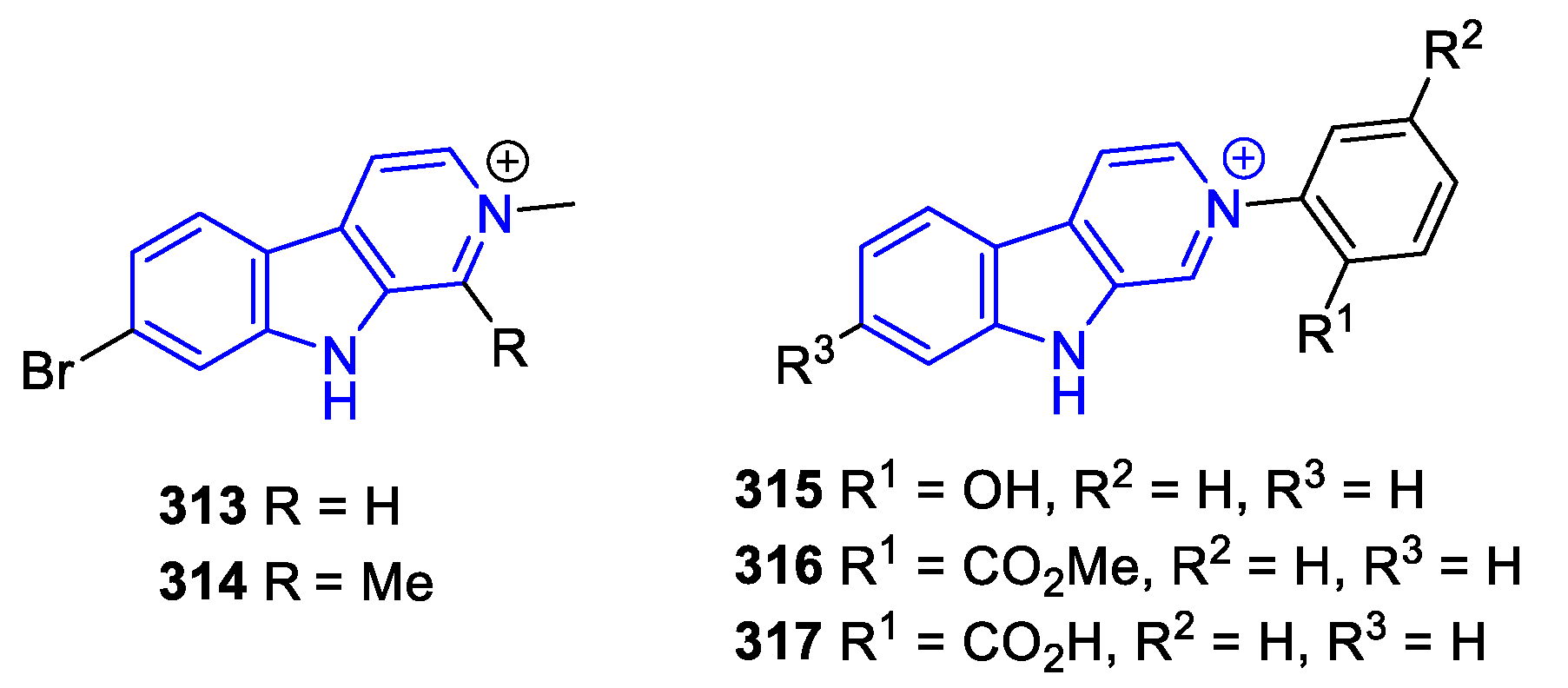
- C3-substituted (DH/TH)β-Carbolines:
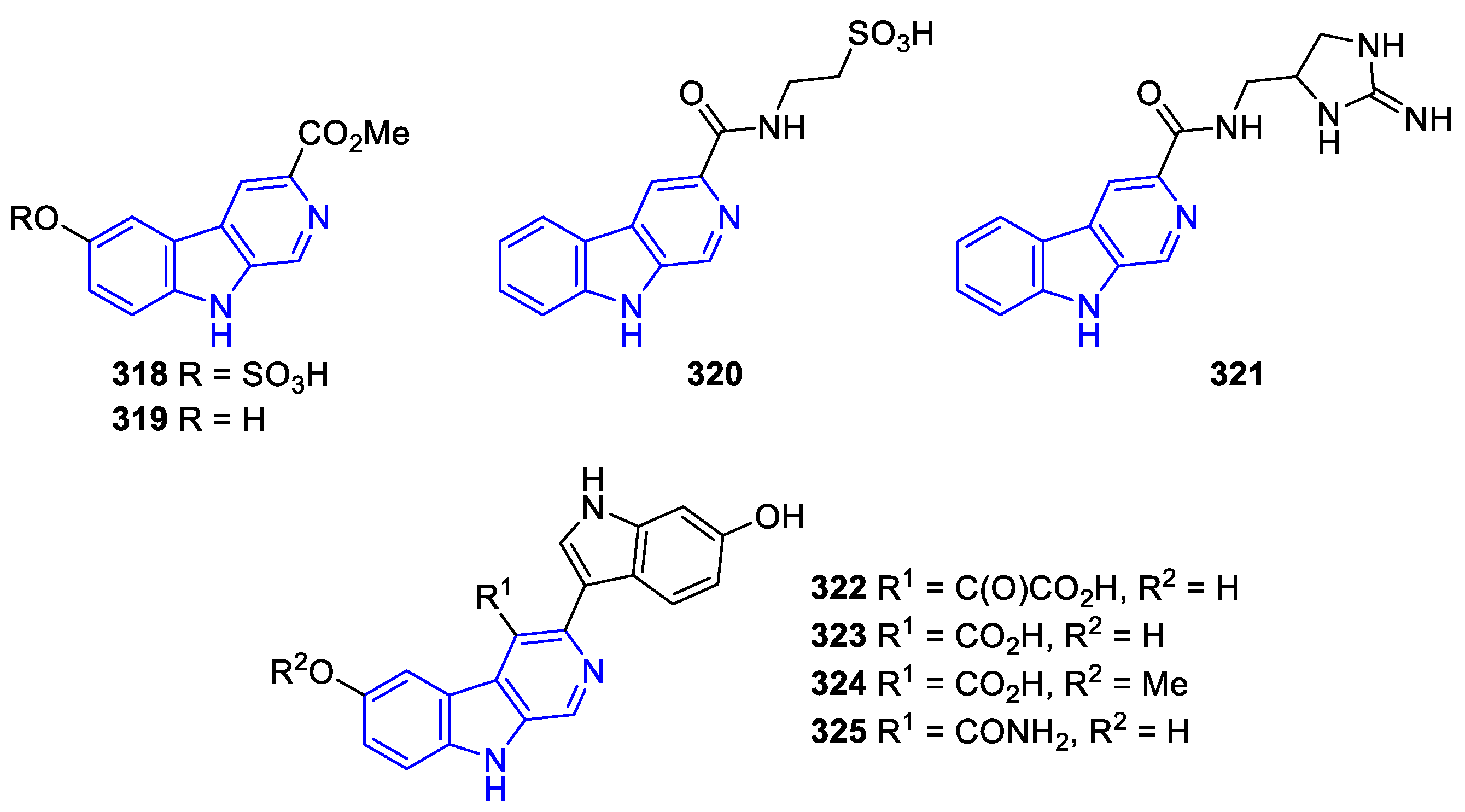
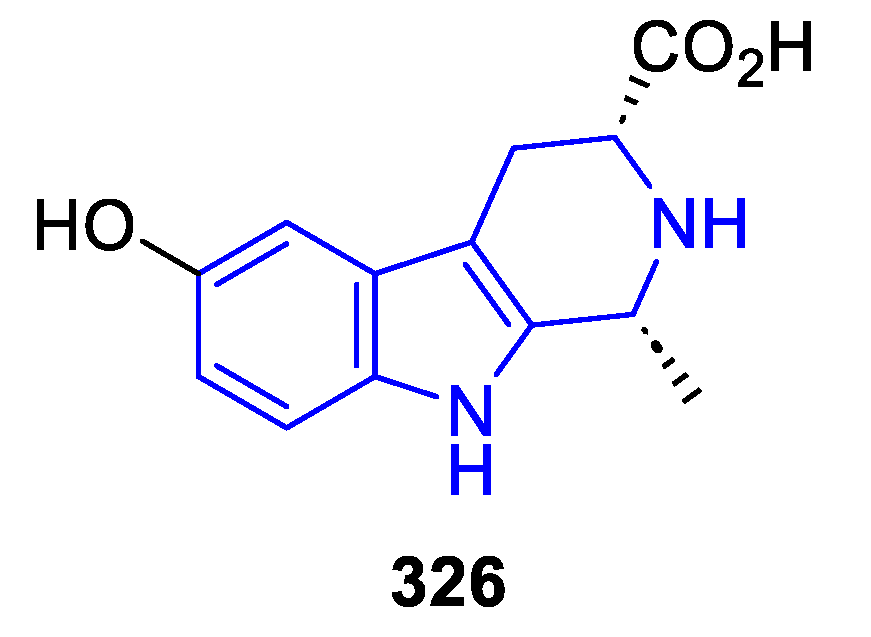
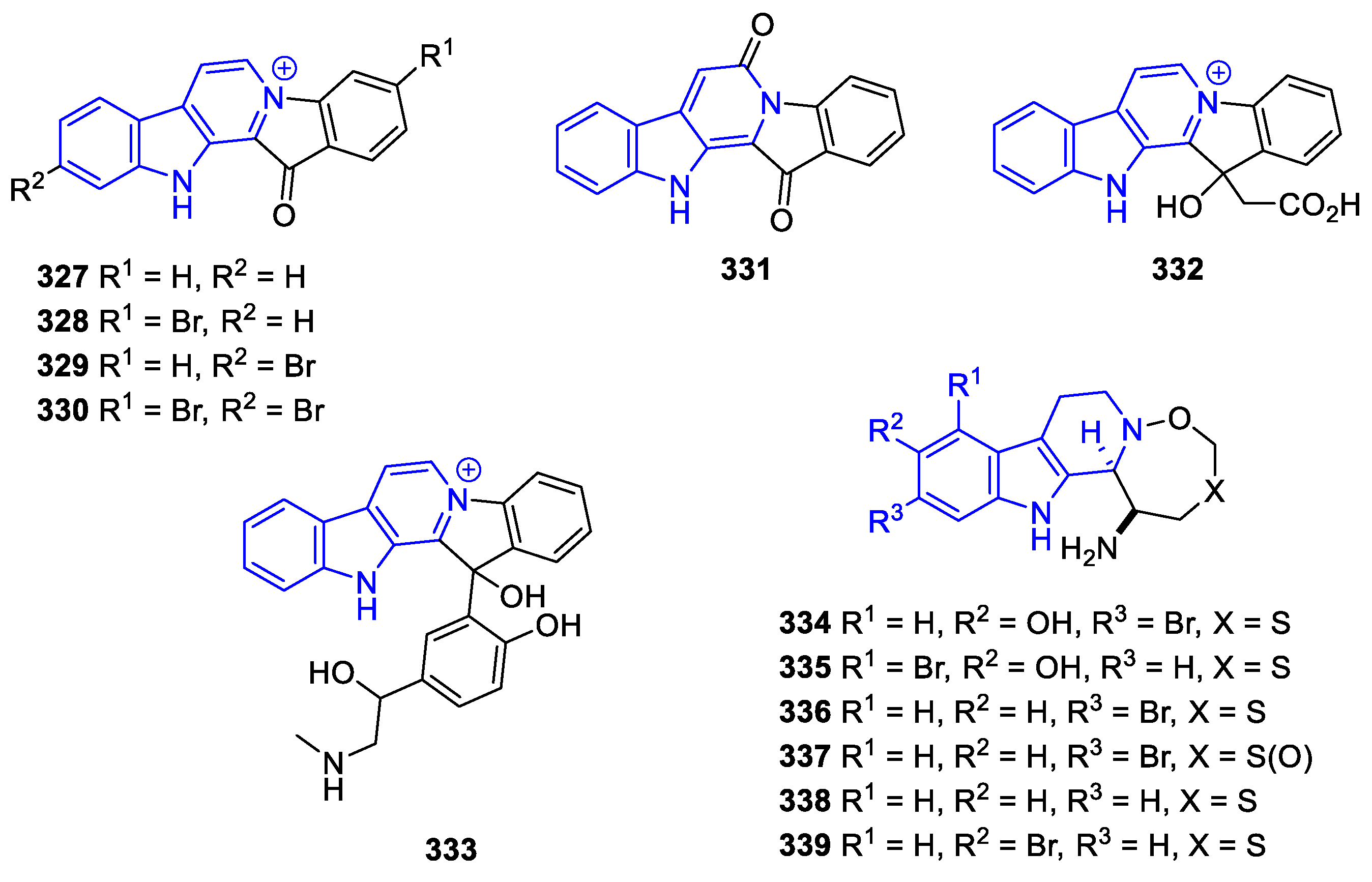
Annelated β-carbolines
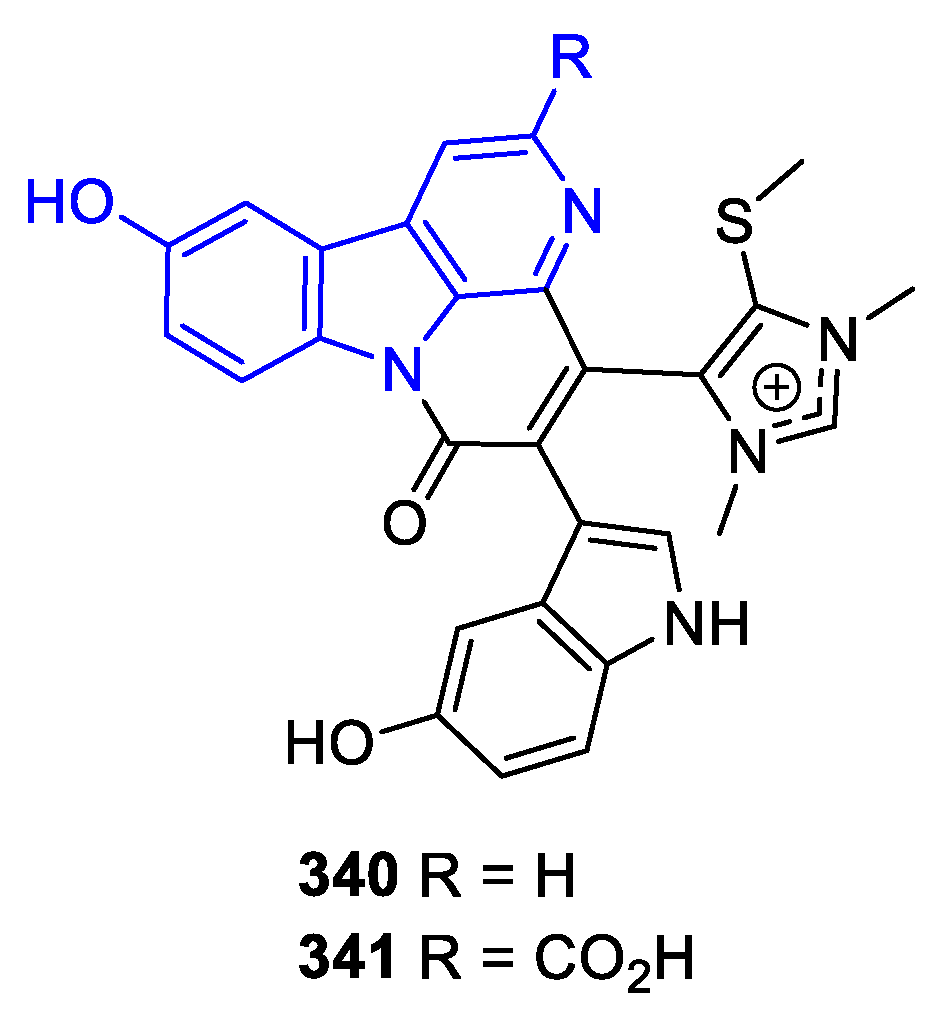
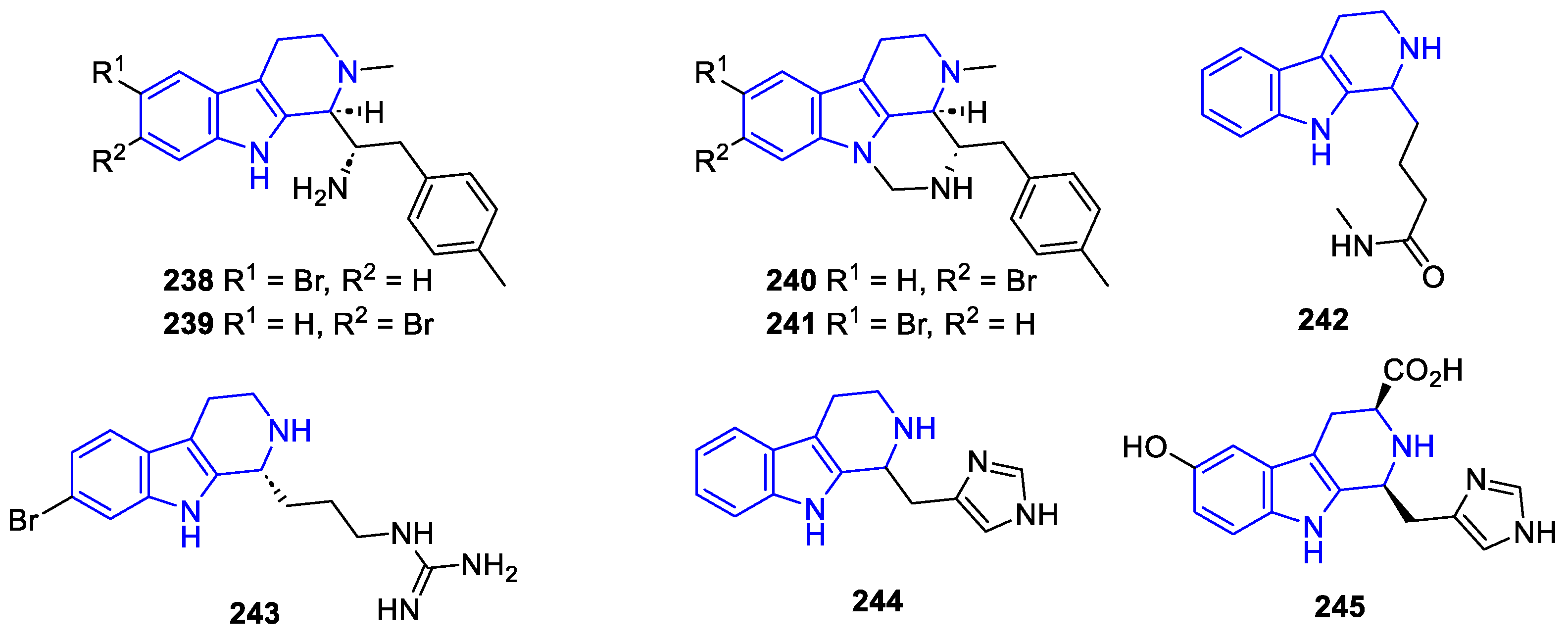
2.2.2.2. β-Carboline dimers
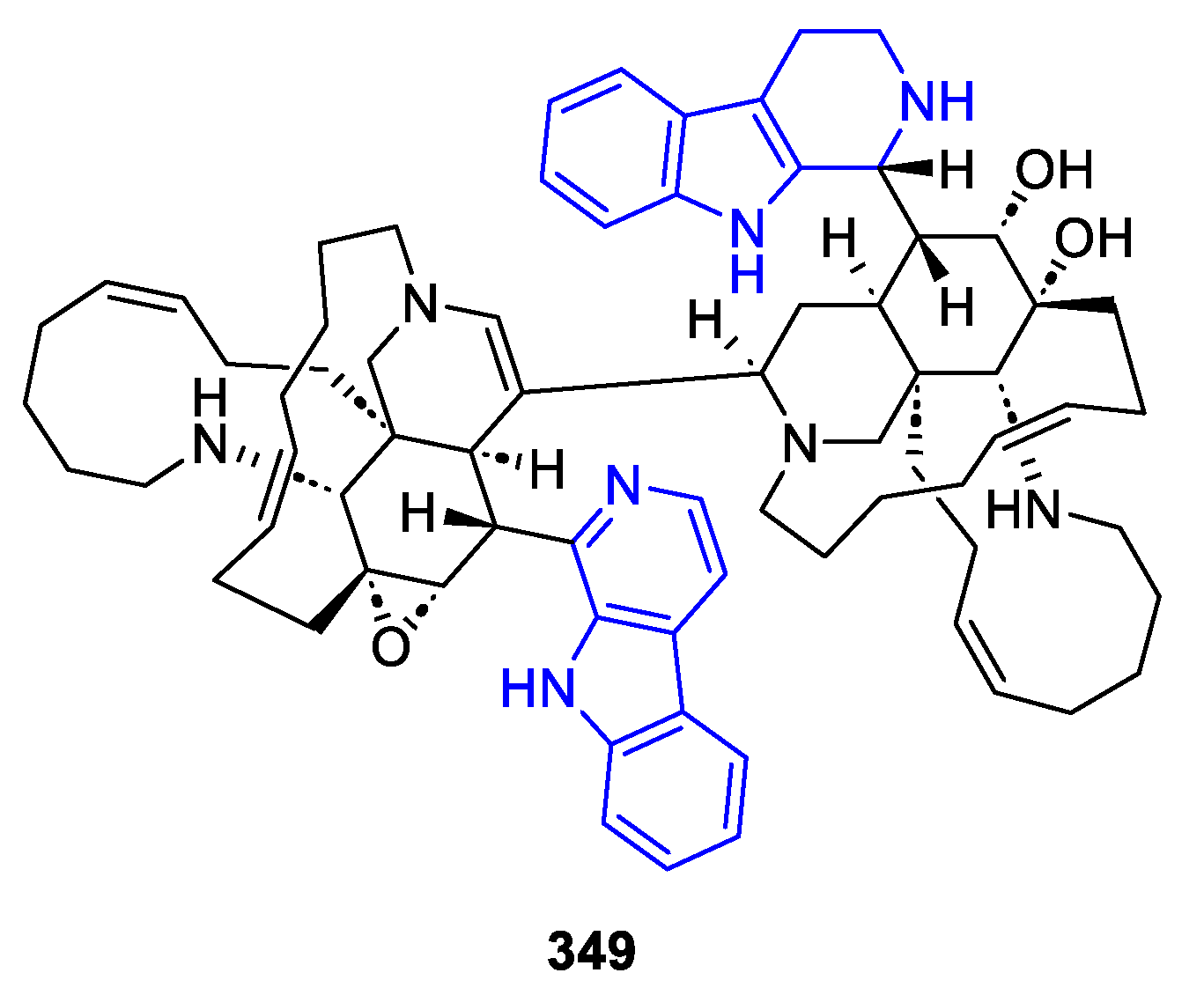
- 1,1-Linked dimers
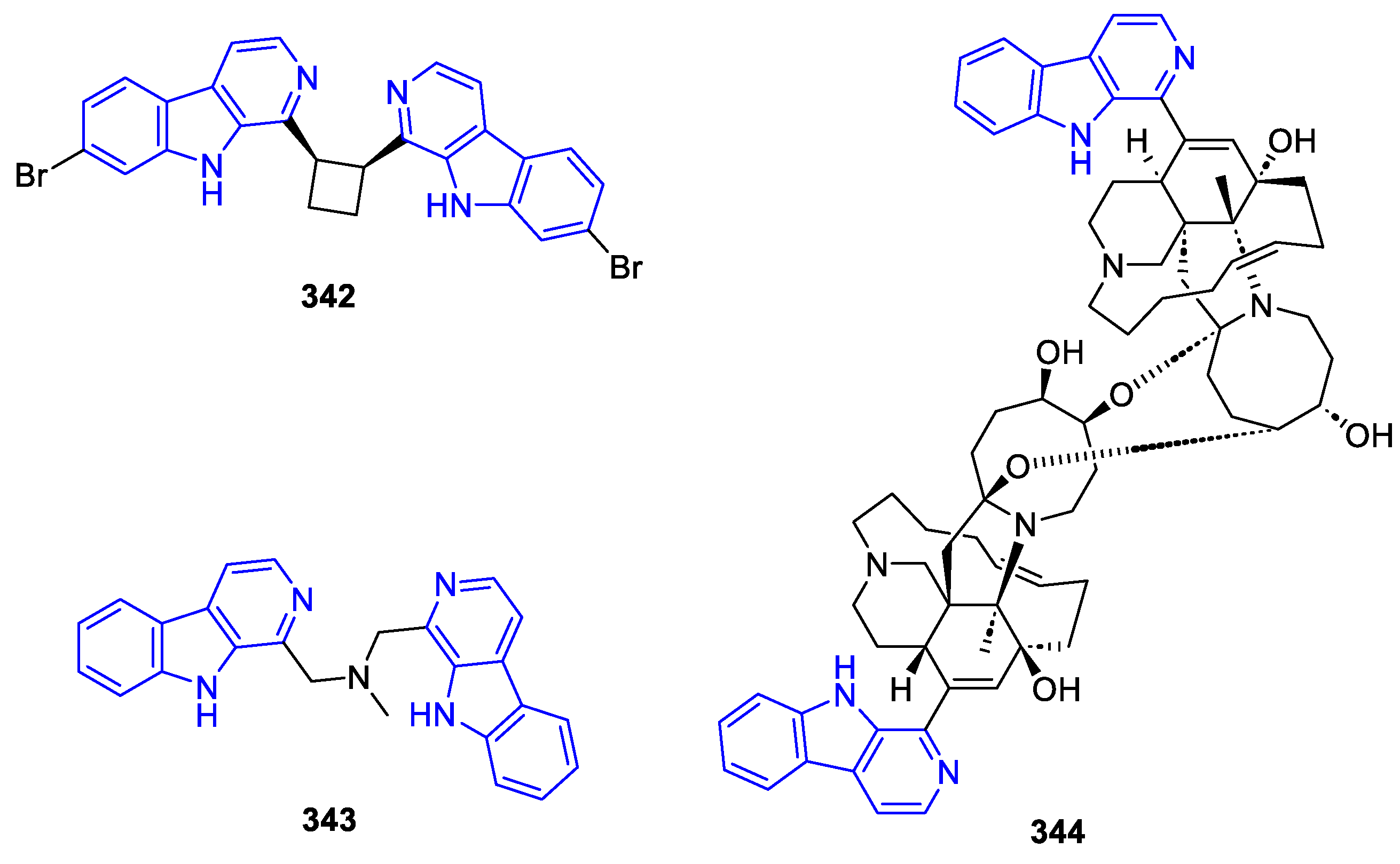
- 9,9-Linked dimers
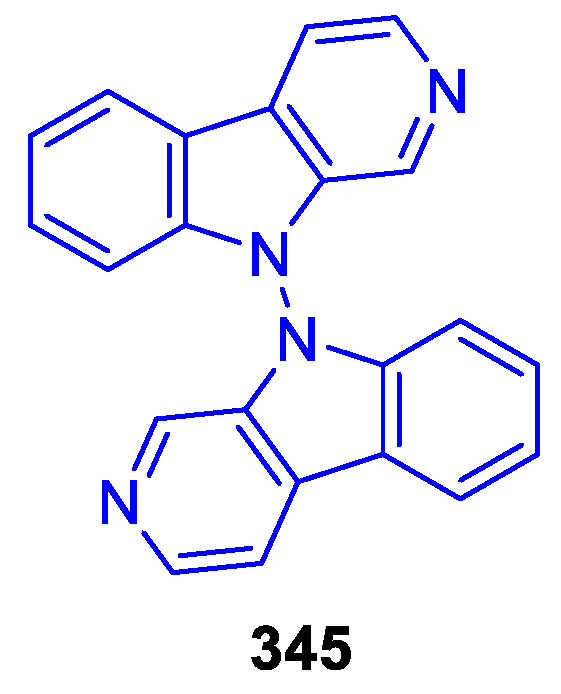
- ‘Hybrid’ dimers
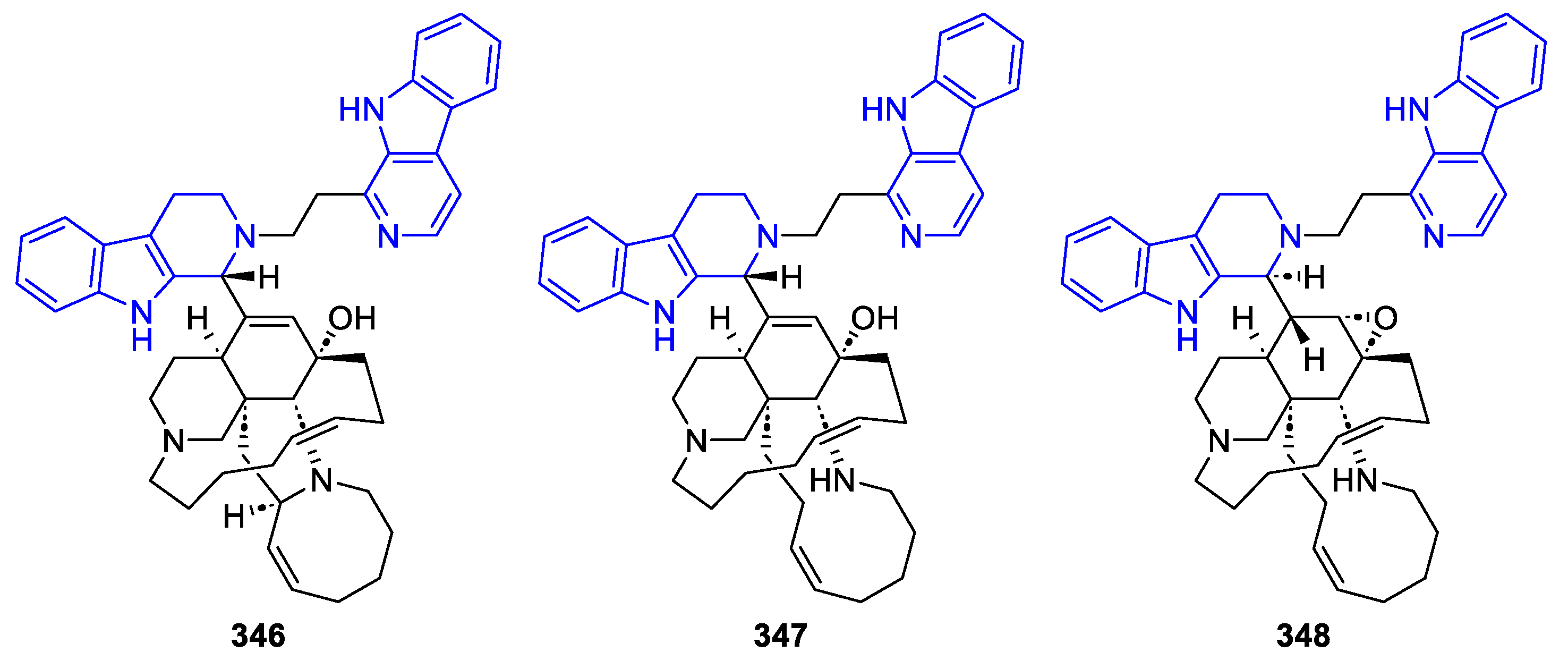
General syntheses of β-Carboline alkaloids
2.3.4. Other annelated indole alkaloids
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Meaning | Abbreviation | Meaning |
| 5-HT | 5-hydroxytryptamine receptors | HIV | Human Immunodeficiency Virus |
| 5-HT2A | Hydroxytryptamine 2A | HL-60 | Promyelocytic leukemia cell line |
| 5-HT2C | 5-Hydroxytryptamine 2C | HSV | Herpes Simplex Virus |
| A/WSN/33 (H1N1) |
Influenza A Virus Subtype H1N1 | HT-29 | Human colon cancer cell line |
| A549 | Adenocarcinomic human alveolar basal epithelial cell line | IC50 | Half-Maximum Inhibitory Concentration |
| AChE | Acetylcholinesterase | K562 | Human myelogenous leukemia cell line |
| B16-F10 | Melanoma cell line | KB | Human epithelial carcinoma cell line |
| βC | β-Carboline | L1210 | Mouse lymphocytic leukemia cell line |
| BCG | Bacille Calmette-Guérin | L5178Y | Mouse lymphoma cell line |
| BChE | Butyrylcholinesterase | LMM3 | Human Melanoma Cells |
| CK1 | Casein Kinase 1 Delta | LoVo | Human colorectal cancer cell lines |
| CLK1 | CDC-like Kinase 1 | MCF-7 | Human breast cancer cell line |
| DCE | 1,2-Dicloroetane | MDA-MB-231 | Human Metastatic Breast Carcinoma Cells |
| DCM | Dichloromethane | MDA-MB-435 | Human Breast Carcinoma Cell line |
| DHβC | Dihydro-b-Carboline | MDCK | Madin-Darby canine kidney |
| DKP | Diketopiperazine | MIC | Minimum Inhibitory Concentration |
| DMA | N,N-Dimethylacetamide | MRC-9 | Human lung cancer cell line |
| DMAPP | Dimethylallyl pyrophosphate | MRSA | Methicillin-Resistant Staphylococcus aureus |
| DMF | N,N-Dimethylformamide | NCI-H460 | Human non-small cell lung carcinoma cell line |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl | NFB | Nuclear Factor κB |
| Dyrk1A | Dual-Specificity Tyrosine-Phosphorylation Regulated Kinase 1A | OVCAR-3 | Human high-grade serous ovarian adenocarcinoma cell line |
| ED50 | dose of a medication that produces the intended pharmacological effect in 50% of the patient population studied | P388 | Leukemia cell line |
| FDA | Food and Drug Administration | PC3 | Human prostatic adenocarcinoma cell line |
| GSK-3β | Glycogen Synthase Kinase 3 Beta | PD | Parkinson’s Disease |
| H12999 | Human non-small cell lung carcinoma cell line | PIA | Prenylated Indole Alkaloid |
| H37Rv | Mycobacterium tuberculosis strain | PPi | Pyrophosphate |
| H522-T1 | human non-small cell lung cancer cell line | PTP | Protein Tyrosine Phosphatase |
| HCT-116 | Human colon cancer cell line | RD | Human Rhabdomyosarcoma Cells |
| HCT-8 | human colon carcinoma cell line | SIA | Simple Indole Alkaloid |
| HEK293 | Human Embryonic Kidney cell line | THβC | Tetrahydro-b-Carboline |
| HEK293 T9 | Non-malignant human kidney cell line | THF | Tetrahydrofuran |
| HeLa | Human Cervical Epidermoid Carcinoma Cells | U937 | Human histiocytic lymphoma cell line |
| Hep2 | Human Epithelial Carcinoma Cells | USF-HO25 | University of South Florida - Human Osteosarcoma 25 |
| Hep-G2 | Human hepatocellular carcinoma cell line | UV | Ultraviolet |
References
- Karthikeyan, A.; Joseph, A.; Nair, B.G. Promising Bioactive Compounds from the Marine Environment and Their Potential Effects on Various Diseases. J. Genet. Eng. Biotechnol. 2022, 20, 14–52. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2023, 40, 275–325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; An, R.; Wang, J.; Sun, N.; Zhang, S.; Hu, J.; Kuai, J. Exploring Novel Bioactive Compounds from Marine Microbes. Curr. Opin. Microbiol. 2005, 8, 276–281. [Google Scholar] [CrossRef]
- Malve, H. Exploring the Ocean for New Drug Developments: Marine Pharmacology. J. Pharm. Bioall. Sci. 2016, 8, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Eichler, B.; Klausner, E.A.; Duffy-Matzner, J.; Zheng, W. Lead/Drug Discovery from Natural Resources. Molecules 2022, 27, 8280–8310. [Google Scholar] [CrossRef] [PubMed]
- Taori, K.; Paul, V.J.; Luesch, H. Structure and Activity of Largazole, a Potent Antiproliferative Agent from the Floridian Marine Cyanobacterium Symploca Sp. J. Am. Chem. Soc. 2008, 130, 1806–1807. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.; Taori, K.; Kim, H.; Hong, J.; Luesch, H. Total Synthesis and Molecular Target of Largazole, a Histone Deacetylase Inhibitor. J. Am. Chem. Soc. 2008, 130, 8455–8459. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Sun, K.; Sun, J. Recent Advances of Marine Natural Indole Products in Chemical and Biological Aspects. Molecules 2023, 28, 2204–2227. [Google Scholar] [CrossRef]
- Walter, T.; Veldmann, K.H.; Götker, S.; Busche, T.; Rückert, C.; Kashkooli, A.B.; Paulus, J.; Cankar, K.; Wendisch, V.F. Physiological Response of Corynebacterium Glutamicum to Indole. Microorganisms 2020, 8, 1945–1968. [Google Scholar] [CrossRef]
- Huang, Z.; Yin, L.; Guan, L.; Li, Z.; Tan, C. Novel Piperazine-2,5-Dione Analogs Bearing 1H-Indole: Synthesis and Biological Effects. Bioorg. Med. Chem. Lett. 2020, 30, 127654–127658. [Google Scholar] [CrossRef]
- Wei, C.; Zhang, J.; Shi, J.; Gan, X.; Hu, D.; Song, B. Synthesis, Antiviral Activity, and Induction of Plant Resistance of Indole Analogues Bearing Dithioacetal Moiety. J. Agric. Food Chem. 2019, 67, 13882–13891. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yao, J.; Guo, K.; He, F.; Chen, K.; Lin, Z.; Liu, S.; Huang, J.; Wu, Q.; Fang, M.; et al. Design, Synthesis, and Biological Evaluation of 5-((8-Methoxy-2-Methylquinolin-4-Yl)Amino)-1H-Indole-2-Carbohydrazide Derivatives as Novel Nur77 Modulators. Eur. J. Med. Chem. 2020, 204, 112608. [Google Scholar] [CrossRef]
- Sreenivasulu, R.; Reddy, K.T.; Sujitha, P.; Kumar, C.G.; Raju, R.R. Synthesis, Antiproliferative and Apoptosis Induction Potential Activities of Novel Bis(Indolyl)Hydrazide-Hydrazone Derivatives. Bioorg. Med. Chem. 2019, 27, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Diao, P.-C.; Jian, X.-E.; Chen, P.; Huang, C.; Yin, J.; Huang, J.C.; Li, J.-S.; Zhao, P.-L. Design, Synthesis and Biological Evaluation of Novel Indole-Based Oxalamide and Aminoacetamide Derivatives as Tubulin Polymerization Inhibitors. Bioorg. Med. Chem. Lett. 2020, 30, 126816–126822. [Google Scholar] [CrossRef]
- Vijayakumar, B.G.; Ramesh, D.; Joji, A.; Jayachandra Prakasan, J.; Kannan, T. In Silico Pharmacokinetic and Molecular Docking Studies of Natural Flavonoids and Synthetic Indole Chalcones against Essential Proteins of SARS-CoV-2. E. J. Pharmacol. 2020, 886, 173448–173459. [Google Scholar] [CrossRef]
- Hoffman, R.L.; Kania, R.S.; Brothers, M.A.; Davies, J.F.; Ferre, R.A.; Gajiwala, K.S.; He, M.; Hogan, R.J.; Kozminski, K.; Li, L.Y.; et al. Discovery of Ketone-Based Covalent Inhibitors of Coronavirus 3CL Proteases for the Potential Therapeutic Treatment of COVID-19. J. Med. Chem. 2020, 63, 12725–12747. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Wang, T.; Lu, J.; Ding, C.Z.; Hu, L.; Hu, G.; He, H.; Zeng, X.; Li, X.; Sun, D.; et al. Design, Syntheses and Evaluations of Novel Indole Derivatives as Orally Selective Estrogen Receptor Degraders (SERD). Bioorg. Med. Chem. Let.t 2020, 30, 127601–127606. [Google Scholar] [CrossRef]
- Miao, G.; Wang, Y.; Yan, Z.; Zhang, L. Synthesis, in Vitro ADME Profiling and in Vivo Pharmacological Evaluation of Novel Glycogen Phosphorylase Inhibitors. Bioorg. Med. Chem. Lett. 2020, 30, 127117–127123. [Google Scholar] [CrossRef]
- Solangi, M.; Kanwal; Mohammed Khan, K. ; Saleem, F.; Hameed, S.; Iqbal, J.; Shafique, Z.; Qureshi, U.; Ul-Haq, Z.; Taha, M.; et al. Indole Acrylonitriles as Potential Anti-Hyperglycemic Agents: Synthesis, α-Glucosidase Inhibitory Activity and Molecular Docking Studies. Bioorg. Med. Chem. 2020, 28, 115605–115616. [Google Scholar] [CrossRef]
- Méndez, M.; Matter, H.; Defossa, E.; Kurz, M.; Lebreton, S.; Li, Z.; Lohmann, M.; Löhn, M.; Mors, H.; Podeschwa, M.; et al. Design, Synthesis, and Pharmacological Evaluation of Potent Positive Allosteric Modulators of the Glucagon-like Peptide-1 Receptor (GLP-1R). J. Med. Chem. 2020, 63, 2292–2307. [Google Scholar] [CrossRef]
- Purgatorio, R.; De Candia, M.; Catto, M.; Carrieri, A.; Pisani, L.; De Palma, A.; Toma, M.; Ivanova, O.A.; Voskressensky, L.G.; Altomare, C.D. Investigating 1,2,3,4,5,6-Hexahydroazepino[4,3-b]Indole as Scaffold of Butyrylcholinesterase-Selective Inhibitors with Additional Neuroprotective Activities for Alzheimer’s Disease. E. J. Med. Chem. 2019, 177, 414–424. [Google Scholar] [CrossRef]
- Ju, Z.; Su, M.; Hong, J.; La Kim, E.; Moon, H.R.; Chung, H.Y.; Kim, S.; Jung, J.H. Design of Balanced COX Inhibitors Based on Anti-Inflammatory and/or COX-2 Inhibitory Ascidian Metabolites. E. J. Med. Chem. 2019, 180, 86–98. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, B.; Li, J.; Liu, H.; Zhang, Y.; Yang, Z.; Liu, W. Design, Synthesis, Biological Evaluation and Docking Study of Novel Indole-2-Amide as Anti-Inflammatory Agents with Dual Inhibition of COX and 5-LOX. Eur. J. Med. Chem. 2019, 180, 41–50. [Google Scholar] [CrossRef]
- Bai, H.; Cui, P.; Zang, C.; Li, S. Enantioselective Total Synthesis, Divergent Optimization and Preliminary Biological Evaluation of (Indole-N-Alkyl)-Diketopiperazines. Bioorg. Med. Chem. Lett. 2019, 29, 126718–126723. [Google Scholar] [CrossRef]
- Mishra, S.; Kaur, M.; Chander, S.; Murugesan, S.; Nim, L.; Arora, D.S.; Singh, P. Rational Modification of a Lead Molecule: Improving the Antifungal Activity of Indole – Triazole – Amino Acid Conjugates. E. J. Med. Chem. 2018, 155, 658–669. [Google Scholar] [CrossRef]
- Siebenbuerger, L.; Hernandez-Olmos, V.; Abdelsamie, A.S.; Frotscher, M.; Van Koppen, C.J.; Marchais-Oberwinkler, S.; Scheuer, C.; Laschke, M.W.; Menger, M.D.; Boerger, C.; et al. Highly Potent 17β-HSD2 Inhibitors with a Promising Pharmacokinetic Profile for Targeted Osteoporosis Therapy. J. Med. Chem. 2018, 61, 10724–10738. [Google Scholar] [CrossRef] [PubMed]
- Cherigo, L.; Lopez, D.; Martinez-Luis, S. Marine Natural Products as Breast Cancer Resistance Protein Inhibitors. Mar. Drugs 2015, 13, 2010–2029. [Google Scholar] [CrossRef] [PubMed]
- Netz, N.; Opatz, T. Marine Indole Alkaloids. Mar. Drugs 2015, 13, 4814–4914. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Xu, H. Recent Progress of Bioactivities, Mechanisms of Action, Total Synthesis,Structural Modifications and Structure-Activity Relationships of IndoleDerivatives: A Review. MRMC 2022, 22, 2702–2725. [Google Scholar] [CrossRef]
- Kaushik, N.; Kaushik, N.; Attri, P.; Kumar, N.; Kim, C.; Verma, A.; Choi, E. Biomedical Importance of Indoles. Molecules 2013, 18, 6620–6662. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, Y.; Ito, Y.; Suzuki, M.; Hirota, A. Indole Derivatives from a Marine Sponge-Derived Yeast as DPPH Radical Scavengers. J. Nat. Prod. 2009, 72, 2069–2071. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Lee, Y.; Hong, J.; Lee, C.-O.; Jung, J.H. Monoindole Alkaloids from a Marine Sponge Spongosorites Sp. Mar. Drugs 2007, 24(22), 16158–16172. [Google Scholar] [CrossRef]
- He, W.-F.; Xue, D.-Q.; Yao, L.-G.; Li, J.-Y.; Li, J.; Guo, Y.-W. Hainanerectamines A–C, Alkaloids from the Hainan Sponge Hyrtios Erecta. Mar. Drugs 2014, 12, 3982–3993. [Google Scholar] [CrossRef] [PubMed]
- Campos, P.-E.; Pichon, E.; Moriou, C.; Clerc, P.; Trépos, R.; Frederich, M.; De Voogd, N.; Hellio, C.; Gauvin-Bialecki, A.; Al-Mourabit, A. New Antimalarial and Antimicrobial Tryptamine Derivatives from the Marine Sponge Fascaplysinopsis Reticulata. Mar. Drugs 2019, 17, 167. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.C.; Resende, D.I.S.P.; Da Costa, P.M.; Pinto, M.M.M.; Sousa, E. Tryptophan Derived Natural Marine Alkaloids and Synthetic Derivatives as Promising Antimicrobial Agents. Eur. J. Med. Chem. 2021, 209, 112945–112978. [Google Scholar] [CrossRef]
- Li, J.L.; Xiao, B.; Park, M.; Yoo, E.S.; Shin, S.; Hong, J.; Chung, H.Y.; Kim, H.S.; Jung, J.H. PPAR-γ Agonistic Metabolites from the Ascidian Herdmania Momus. J. Nat. Prod. 2012, 75, 2082–2087. [Google Scholar] [CrossRef] [PubMed]
- May Zin, W.W.; Buttachon, S.; Dethoup, T.; Pereira, J.A.; Gales, L.; Inácio, Â.; Costa, P.M.; Lee, M.; Sekeroglu, N.; Silva, A.M.S.; et al. Antibacterial and Antibiofilm Activities of the Metabolites Isolated from the Culture of the Mangrove-Derived Endophytic Fungus Eurotium Chevalieri KUFA 0006. Phytochemistry 2017, 141, 86–97. [Google Scholar] [CrossRef]
- Shady, N.H.; Fouad, M.A.; Ahmed, S.; Pimentel-Elardo, S.M.; Nodwell, J.R.; Kamel, M.S.; Abdelmohsen, U.R. A New Antitrypanosomal Alkaloid from the Red Sea Marine Sponge Hyrtios Sp. J. Antibiot. 2018, 71, 1036–1039. [Google Scholar] [CrossRef]
- Socha, A.M.; Long, R.A.; Rowley, D.C. Bacillamides from a Hypersaline Microbial Mat Bacterium. J. Nat. Prod. 2007, 70, 1793–1795. [Google Scholar] [CrossRef]
- Che, Q.; Qiao, L.; Han, X.; Liu, Y.; Wang, W.; Gu, Q.; Zhu, T.; Li, D. Anthranosides A–C, Anthranilate Derivatives from a Sponge-Derived Streptomyces Sp. CMN-62. Org. Lett. 2018, 20, 5466–5469. [Google Scholar] [CrossRef]
- Zhong, W.-M.; Wang, J.-F.; Shi, X.-F.; Wei, X.-Y.; Chen, Y.-C.; Zeng, Q.; Xiang, Y.; Chen, X.-Y.; Tian, X.-P.; Xiao, Z.-H.; et al. Eurotiumins A–E, Five New Alkaloids from the Marine-Derived Fungus Eurotium Sp. SCSIO F452. Mar. Drugs 2018, 16, 136–148. [Google Scholar] [CrossRef]
- Youssef, F.S.; Simal-Gandara, J. Comprehensive Overview on the Chemistry and Biological Activities of Selected Alkaloid Producing Marine-Derived Fungi as a Valuable Reservoir of Drug Entities. Biomedicines 2021, 9, 485–516. [Google Scholar] [CrossRef]
- Meng, Z.-H.; Sun, T.-T.; Zhao, G.-Z.; Yue, Y.-F.; Chang, Q.-H.; Zhu, H.-J.; Cao, F. Marine-Derived Fungi as a Source of Bioactive Indole Alkaloids with Diversified Structures. Mar. Life Sci. Technol. 2021, 3, 44–61. [Google Scholar] [CrossRef]
- Izumikawa, M.; Hashimoto, J.; Takagi, M.; Shin-ya, K. Isolation of Two New Terpeptin Analogs—JBIR-81 and JBIR-82—from a Seaweed-Derived Fungus, Aspergillus Sp. SpD081030G1f1. J. Antibiot. 2010, 63, 389–391. [Google Scholar] [CrossRef]
- Li, J.J. Bartoli Indole Synthesis. In Name Reactions: A Collection of Detailed Mechanisms and Synthetic Applications Fifth Edition; Li, J.J., Ed.; Springer International Publishing: Cham, 2014; ISBN 978-3-319-03979-4. [Google Scholar]
- Karimi, S.; Ma, S.; Liu, Y.; Ramig, K.; Greer, E.M.; Kwon, K.; Berkowitz, W.F.; Subramaniam, G. Substituted Pyrrole Synthesis from Nitrodienes. Tetrahedron Lett. 2017, 58, 2223–2227. [Google Scholar] [CrossRef]
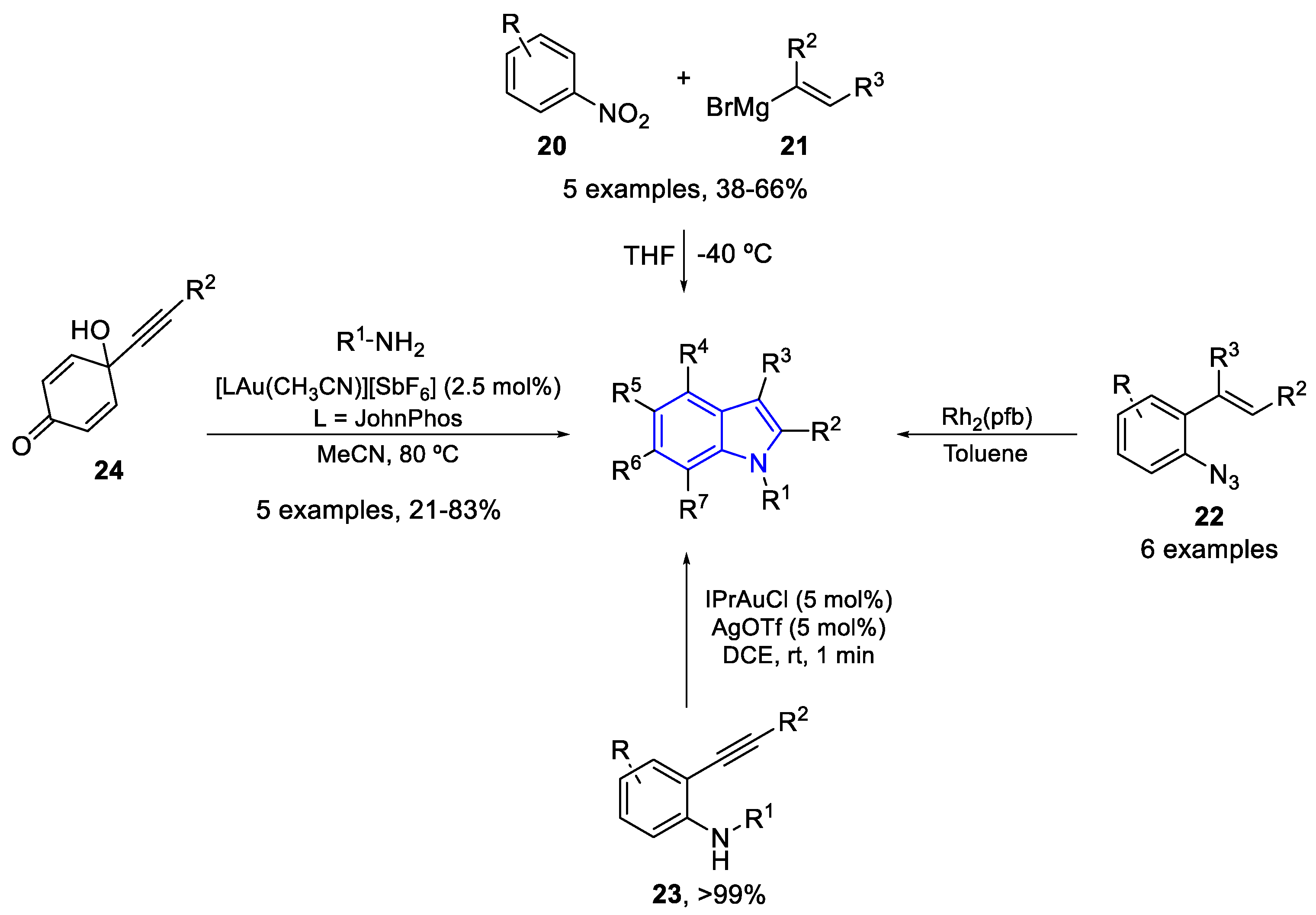
- Kadiyala, V.; Bharath Kumar, P.; Balasubramanian, S.; Karunakar, G.V. Gold-Catalyzed Synthesis of 6-Hydroxyindoles from Alkynylcyclohexadienones and Substituted Amines. J. Org. Chem. 2019, 84, 12228–12236. [Google Scholar] [CrossRef]
- Dorel, R.; Echavarren, A.M. Gold(I)-Catalyzed Activation of Alkynes for the Construction of Molecular Complexity. Chem. Rev. 2015, 115, 9028–9072. [Google Scholar] [CrossRef]
- Capon, R.J.; Peng, C.; Dooms, C. Trachycladindoles A–G: Cytotoxic Heterocycles from an Australian Marine Sponge, Trachycladus Laevispirulifer. Org. Biomol. Chem. 2008, 6, 2765–2772. [Google Scholar] [CrossRef] [PubMed]
- Segraves, N.L.; Crews, P. Investigation of Brominated Tryptophan Alkaloids from Two Thorectidae Sponges: Thorectandra and Smenospongia. J. Nat. Prod. 2005, 68, 1484–1488. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.-F.; Schetz, J.A.; Kelly, M.; Peng, J.-N.; Ang, K.K.H.; Flotow, H.; Leong, C.Y.; Ng, S.B.; Buss, A.D.; Wilkins, S.P.; et al. New Antiinfective and Human 5-HT2 Receptor Binding Natural and Semisynthetic Compounds from the Jamaican Sponge Smenospongia a Urea. J. Nat. Prod. 2002, 65, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Hentz, A. Vers la synthèse totale du Trachycladindole E, développement de nouvelles réactivités des ynamides.
- Stanovnik, B.; Svete, J. The Synthesis of Aplysinopsins, Meridianines, and Related Compounds. ChemInform 2006, 37, chin–200623261. [Google Scholar] [CrossRef]
- Franco, L.H.; Joffé, E.B.D.K.; Puricelli, L.; Tatian, M.; Seldes, A.M.; Palermo, J.A. Indole Alkaloids from the Tunicate Aplidium m Eridianum. J. Nat. Prod. 1998, 61, 1130–1132. [Google Scholar] [CrossRef]
- Xiao, L. A Review: Meridianins and Meridianins Derivatives. Molecules 2022, 27, 8714–8726. [Google Scholar] [CrossRef]
- Han, S.; Zhuang, C.; Zhou, W.; Chen, F. Structural-Based Optimizations of the Marine-Originated Meridianin C as Glucose Uptake Agents by Inhibiting GSK-3β. Mar. Drugs 2021, 19, 149–173. [Google Scholar] [CrossRef] [PubMed]
- Kruppa, M.; Müller, T.J.J. A Survey on the Synthesis of Variolins, Meridianins, and Meriolins—Naturally Occurring Marine (Aza)Indole Alkaloids and Their Semisynthetic Derivatives. Molecules 2023, 28, 947–970. [Google Scholar] [CrossRef]
- Fresneda, P.M.; Molina, P.; Bleda, J.A. Synthesis of the Indole Alkaloids Meridianins from the Tunicate Aplidium Meridianum. Tetrahedron 2001, 57, 2355–2363. [Google Scholar] [CrossRef]
- Karpov, A.S.; Merkul, E.; Rominger, F.; Müller, T.J.J. Concise Syntheses of Meridianins by Carbonylative Alkynylation and a Four-Component Pyrimidine Synthesis. Angew. Chem. Int. Ed. 2005, 44, 6951–6956. [Google Scholar] [CrossRef]
- Kruppa, M.; Sommer, G.A.; Müller, T.J.J. Concise Syntheses of Marine (Bis)Indole Alkaloids Meridianin C, D, F, and G and Scalaridine A via One-Pot Masuda Borylation-Suzuki Coupling Sequence. Molecules 2022, 27, 2233–2243. [Google Scholar] [CrossRef]
- Tibiletti, F.; Simonetti, M.; Nicholas, K.M.; Palmisano, G.; Parravicini, M.; Imbesi, F.; Tollari, S.; Penoni, A. One-Pot Synthesis of Meridianins and Meridianin Analogues via Indolization of Nitrosoarenes. Tetrahedron 2010, 66, 1280–1288. [Google Scholar] [CrossRef]
- Liu, H.-B.; Lauro, G.; O’Connor, R.D.; Lohith, K.; Kelly, M.; Colin, P.; Bifulco, G.; Bewley, C.A. Tulongicin, an Antibacterial Tri-Indole Alkaloid from a Deep-Water Topsentia Sp. Sponge. J. Nat. Prod. 2017, 80, 2556–2560. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Tsuda, M.; Watanabe, K.; Kobayashi, J. Rhopaladins A ∼ D, New Indole Alkaloids from Marine Tunicate Rhopalaea Sp. Tetrahedron 1998, 54, 8687–8690. [Google Scholar] [CrossRef]
- Zoraghi, R.; Worrall, L.; See, R.H.; Strangman, W.; Popplewell, W.L.; Gong, H.; Samaai, T.; Swayze, R.D.; Kaur, S.; Vuckovic, M.; et al. Methicillin-Resistant Staphylococcus Aureus (MRSA) Pyruvate Kinase as a Target for Bis-Indole Alkaloids with Antibacterial Activities. J. Biol. Chem. 2011, 286, 44716–44725. [Google Scholar] [CrossRef]
- Tapiolas, D.M.; Bowden, B.F.; Abou-Mansour, E.; Willis, R.H.; Doyle, J.R.; Muirhead, A.N.; Liptrot, C.; Llewellyn, L.E.; Wolff, C.W.W.; Wright, A.D.; et al. Eusynstyelamides A, B, and C, nNOS Inhibitors, from the Ascidian Eusynstyela Latericius. J. Nat. Prod. 2009, 72, 1115–1120. [Google Scholar] [CrossRef]
- Tadesse, M.; Tabudravu, J.N.; Jaspars, M.; Strøm, M.B.; Hansen, E.; Andersen, J.H.; Kristiansen, P.E.; Haug, T. The Antibacterial Ent-Eusynstyelamide B and Eusynstyelamides D, E, and F from the Arctic Bryozoan Tegella Cf. Spitzbergensis. J. Nat. Prod. 2011, 74, 837–841. [Google Scholar] [CrossRef]
- Gunasekera, S.P.; McCarthy, P.J.; Kelly-Borges, M. Hamacanthins A and B, New Antifungal Bis Indole Alkaloids from the Deep-Water Marine Sponge, Hamacantha Sp. J. Nat. Prod. 1994, 57, 1437–1441. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-M.; Liang, X.-A.; Kong, Y.; Jia, B. Structural Diversity and Biological Activities of Indole Diketopiperazine Alkaloids from Fungi. J. Agric. Food Chem. 2016, 64, 6659–6671. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Zhang, X.; Lai, D.; Zhou, L. Structural Diversity and Biological Activities of the Cyclodipeptides from Fungi. Molecules 2017, 22, 2026–2112. [Google Scholar] [CrossRef] [PubMed]
- Borthwick, A.D. 2,5-Diketopiperazines: Synthesis, Reactions, Medicinal Chemistry, and Bioactive Natural Products. Chem. Rev. 2012, 112, 3641–3716. [Google Scholar] [CrossRef]
- Li, G.-Y.; Yang, T.; Luo, Y.-G.; Chen, X.-Z.; Fang, D.-M.; Zhang, G.-L. Brevianamide J, A New Indole Alkaloid Dimer from Fungus Aspergillus Versicolor. Org. Lett. 2009, 11, 3714–3717. [Google Scholar] [CrossRef]
- Huang, P.; Xie, F.; Ren, B.; Wang, Q.; Wang, J.; Wang, Q.; Abdel-Mageed, W.M.; Liu, M.; Han, J.; Oyeleye, A.; et al. Anti-MRSA and Anti-TB Metabolites from Marine-Derived Verrucosispora Sp. MS100047. Appl. Microbiol. Biotechnol. 2016, 100, 7437–7447. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Liu, X.; Guo, H.; Ren, B.; Chen, C.; Piggott, A.M.; Yu, K.; Gao, H.; Wang, Q.; Liu, M.; et al. Brevianamides with Antitubercular Potential from a Marine-Derived Isolate of Aspergillus Versicolor. Org. Lett. 2012, 14, 4770–4773. [Google Scholar] [CrossRef]
- Asiri, I.A.M.; Badr, J.M.; Youssef, D.T.A. Penicillivinacine, Antimigratory Diketopiperazine Alkaloid from the Marine-Derived Fungus Penicillium Vinaceum. Phytochem. Lett. 2015, 13, 53–58. [Google Scholar] [CrossRef]
- El-Gendy, B.E.-D.M.; Rateb, M.E. Antibacterial Activity of Diketopiperazines Isolated from a Marine Fungus Using T-Butoxycarbonyl Group as a Simple Tool for Purification. Bioorg. Med. Chem. Lett. 2015, 25, 3125–3128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, W.-L.; Fang, Y.-C.; Zhu, T.-J.; Gu, Q.-Q.; Zhu, W.-M. Cytotoxic Alkaloids and Antibiotic Nordammarane Triterpenoids from the Marine-Derived Fungus Aspergillus Sydowi. J. Nat. Prod. 2008, 71, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Di, X.; Rouger, C.; Hardardottir, I.; Freysdottir, J.; Molinski, T.F.; Tasdemir, D.; Omarsdottir, S. 6-Bromoindole Derivatives from the Icelandic Marine Sponge Geodia Barretti: Isolation and Anti-Inflammatory Activity. Mar. Drugs 2018, 16, 437–454. [Google Scholar] [CrossRef] [PubMed]
- Olsen, E.K.; Hansen, E.; Moodie, L.W.K.; Isaksson, J.; Sepčić, K.; Cergolj, M.; Svenson, J.; Andersen, J.H. Marine AChE Inhibitors Isolated from Geodia Barretti: Natural Compounds and Their Synthetic Analogs. Org. Biomol. Chem. 2016, 14, 1629–1640. [Google Scholar] [CrossRef] [PubMed]
- Hedner, E.; Sjögren, M.; Frändberg, P.-A.; Johansson, T.; Göransson, U.; Dahlström, M.; Jonsson, P.; Nyberg, F.; Bohlin, L. Brominated Cyclodipeptides from the Marine Sponge Geodia Barretti as Selective 5-HT Ligands. J. Nat. Prod. 2006, 69, 1421–1424. [Google Scholar] [CrossRef]
- Chen, X.; Si, L.; Liu, D.; Proksch, P.; Zhang, L.; Zhou, D.; Lin, W. Neoechinulin B and Its Analogues as Potential Entry Inhibitors of Influenza Viruses, Targeting Viral Hemagglutinin. E. J. Med. Chem. 2015, 93, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Kamauchi, H.; Kinoshita, K.; Sugita, T.; Koyama, K. Conditional Changes Enhanced Production of Bioactive Metabolites of Marine Derived Fungus Eurotium Rubrum. Bioorg. Med. Chem. Lett. 2016, 26, 4911–4914. [Google Scholar] [CrossRef]
- Du, F.-Y.; Li, X.; Li, X.-M.; Zhu, L.-W.; Wang, B.-G. Indolediketopiperazine Alkaloids from Eurotium Cristatum EN-220, an Endophytic Fungus Isolated from the Marine Alga Sargassum Thunbergii. Mar. Drugs 2017, 15, 24–34. [Google Scholar] [CrossRef]
- Yan, H.; Li, X.; Li, C.; Wang, B. Alkaloid and Anthraquinone Derivatives Produced by the Marine-Derived Endophytic Fungus Eurotium Rubrum. Helv. Chim. Acta 2012, 95, 163–168. [Google Scholar] [CrossRef]
- Du, F.-Y.; Li, X.-M.; Li, C.-S.; Shang, Z.; Wang, B.-G. Cristatumins A–D, New Indole Alkaloids from the Marine-Derived Endophytic Fungus Eurotium Cristatum EN-220. Bioorg. Med. Chem. Lett. 2012, 22, 4650–4653. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, Y.; Li, S.; Hu, C.; Liu, H.; Zhang, W. Indole Diketopiperazine Alkaloids from the Deep-Sea-Derived Fungus Aspergillus Sp. FS445. Nat. Prod. Res. 2022, 36, 5213–5221. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Kim, S.-K.; Kang, J.S.; Choi, H.D.; Rho, J.R.; Son, B.W. Golmaenone, a New Diketopiperazine Alkaloid from the Marine-Derived Fungus Aspergillus Sp. Chem. Pharm. Bull. 2004, 52, 375–376. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.C.; Cullum, R.; Machado, H.; Smith, A.J.; Yang, I.; Rodvold, J.J.; Fenical, W. Photopiperazines A–D, Photosensitive Interconverting Diketopiperazines with Significant and Selective Activity against U87 Glioblastoma Cells, from a Rare, Marine-Derived Actinomycete of the Family Streptomycetaceae. J. Nat. Prod. 2019, 82, 2262–2267. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.-D.; Li, S.; Guo, R.; Nie, J.; Ma, J.-A. Chiral Phosphoric Acid Catalyzed Enantioselective Decarboxylative Alkylation of β-Keto Acids with 3-Hydroxy-3-Indolyloxindoles. Org. Lett. 2015, 17, 1389–1392. [Google Scholar] [CrossRef]
- Cai, S.; Kong, X.; Wang, W.; Zhou, H.; Zhu, T.; Li, D.; Gu, Q. Aspergilazine A, a Diketopiperazine Dimer with a Rare N-1 to C-6 Linkage, from a Marine-Derived Fungus Aspergillus Taichungensis. Tetrahedron Lett. 2012, 53, 2615–2617. [Google Scholar] [CrossRef]
- Boyd, E.M.; Sperry, J. Total Synthesis of (−)-Aspergilazine A. Org. Lett. 2014, 16, 5056–5059. [Google Scholar] [CrossRef]
- Li, P.; Zhang, M.; Li, H.; Wang, R.; Hou, H.; Li, X.; Liu, K.; Chen, H. New Prenylated Indole Homodimeric and Pteridine Alkaloids from the Marine-Derived Fungus Aspergillus Austroafricanus Y32-2. Mar. Drugs 2021, 19, 98–110. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Ray, K.; Ganai, S. Intramolecular Aza-Wittig Reaction: A New Efficient Tool for the Construction of Piperazine 2,5-Dione Derivatives. Synlett 2010, 2010, 2122–2124. [Google Scholar] [CrossRef]
- Ashnagar, A.; Bailey, P.D.; Cochrane, P.J.; Mills, T.J.; Price, R.A. Unusual Rearrangements and Cyclizations Involving Polycyclic Indolic Systems. Arkivoc 2007, 2007, 161–171. [Google Scholar] [CrossRef]
- Torres-García, C.; Díaz, M.; Blasi, D.; Farràs, I.; Fernández, I.; Ariza, X.; Farràs, J.; Lloyd-Williams, P.; Royo, M.; Nicolás, E. Side Chain Anchoring of Tryptophan to Solid Supports Using a Dihydropyranyl Handle: Synthesis of Brevianamide F. Int. J. Pept. Res. Ther. 2012, 18, 7–19. [Google Scholar] [CrossRef]
- Kuramochi, Sk.; Aoki, T.; Nakazaki, A.; Kamisuki, S.; Takeno, M.; Ohnishi, K.; Kimoto, K.; Watanabe, N.; Kamakura, T.; Arai, T.; et al. Synthesis of Neoechinulin A and Derivatives. Synthesis 2008, 23, 3810–3818. [Google Scholar]
- Li, H.; Sun, W.; Deng, M.; Zhou, Q.; Wang, J.; Liu, J.; Chen, C.; Qi, C.; Luo, Z.; Xue, Y.; et al. Asperversiamides, Linearly Fused Prenylated Indole Alkaloids from the Marine-Derived Fungus Aspergillus Versicolor. J. Org. Chem. 2018, 83, 8483–8492. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, X.-M.; Wang, J.-N.; Li, X.; Wang, B.-G. Prenylated Indole Alkaloids from the Marine-Derived Fungus Paecilomyces Variotii. Chin. Chem. Lett. 2015, 26, 313–316. [Google Scholar] [CrossRef]
- Williams, R.M.; Cox, R.J. Paraherquamides, Brevianamides, and Asperparalines: Laboratory Synthesis and Biosynthesis. An Interim Report. Acc. Chem. Res. 2003, 36, 127–139. [Google Scholar] [CrossRef]
- Ding, Y.; Wet, J.R. de; Cavalcoli, J.; Li, S.; Greshock, T.J.; Miller, K.A.; Finefield, J.M.; Sunderhaus, J.D.; McAfoos, T.J.; Tsukamoto, S.; et al. Genome-Based Characterization of Two Prenylation Steps in the Assembly of the Stephacidin and Notoamide Anticancer Agents in a Marine-Derived Aspergillus Sp. J. Am. Chem. Soc. 2010, 132, 12733–12740. [Google Scholar] [CrossRef]
- Klas, K.R.; Kato, H.; Frisvad, J.C.; Yu, F.; Newmister, S.A.; Fraley, A.E.; Sherman, D.H.; Tsukamoto, S.; Williams, R.M. Structural and Stereochemical Diversity in Prenylated Indole Alkaloids Containing the Bicyclo[2.2.2]Diazaoctane Ring System from Marine and Terrestrial Fungi. Nat. Prod. Rep. 2018, 35, 532–558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Y.; Xu, X.-Y.; Peng, J.; Ma, C.-F.; Nong, X.-H.; Bao, J.; Zhang, G.-Z.; Qi, S.-H. Antifouling Potentials of Eight Deep-Sea-Derived Fungi from the South China Sea. J. Ind. Microbiol. Biotechnol. 2014, 41, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Afiyatullov, S.S.; Zhuravleva, O.I.; Antonov, A.S.; Berdyshev, D.V.; Pivkin, M.V.; Denisenko, V.A.; Popov, R.S.; Gerasimenko, A.V.; von Amsberg, G.; Dyshlovoy, S.A.; et al. Prenylated Indole Alkaloids from Co-Culture of Marine-Derived Fungi Aspergillus Sulphureus and Isaria Felina. J. Antibiot. 2018, 71, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Luan, Y.; Kong, X.; Zhu, T.; Gu, Q.; Li, D. Isolation and Photoinduced Conversion of 6-Epi-Stephacidins from Aspergillus Taichungensis. Org. Lett. 2013, 15, 2168–2171. [Google Scholar] [CrossRef]
- Yang, J.; Gong, L.; Guo, M.; Jiang, Y.; Ding, Y.; Wang, Z.; Xin, X.; An, F. Bioactive Indole Diketopiperazine Alkaloids from the Marine Endophytic Fungus Aspergillus Sp. YJ191021. Mar. Drugs 2021, 19, 157–168. [Google Scholar] [CrossRef]
- Peng, J.; Gao, H.; Li, J.; Ai, J.; Geng, M.; Zhang, G.; Zhu, T.; Gu, Q.; Li, D. Prenylated Indole Diketopiperazines from the Marine-Derived Fungus Aspergillus Versicolor. J. Org. Chem. 2014, 79, 7895–7904. [Google Scholar] [CrossRef]
- Godfrey, R.C.; Jones, H.E.; Green, N.J.; Lawrence, A.L. Unified Total Synthesis of the Brevianamide Alkaloids Enabled by Chemical Investigations into Their Biosynthesis. Chem. Sci. 2022, 13, 1313–1322. [Google Scholar] [CrossRef]
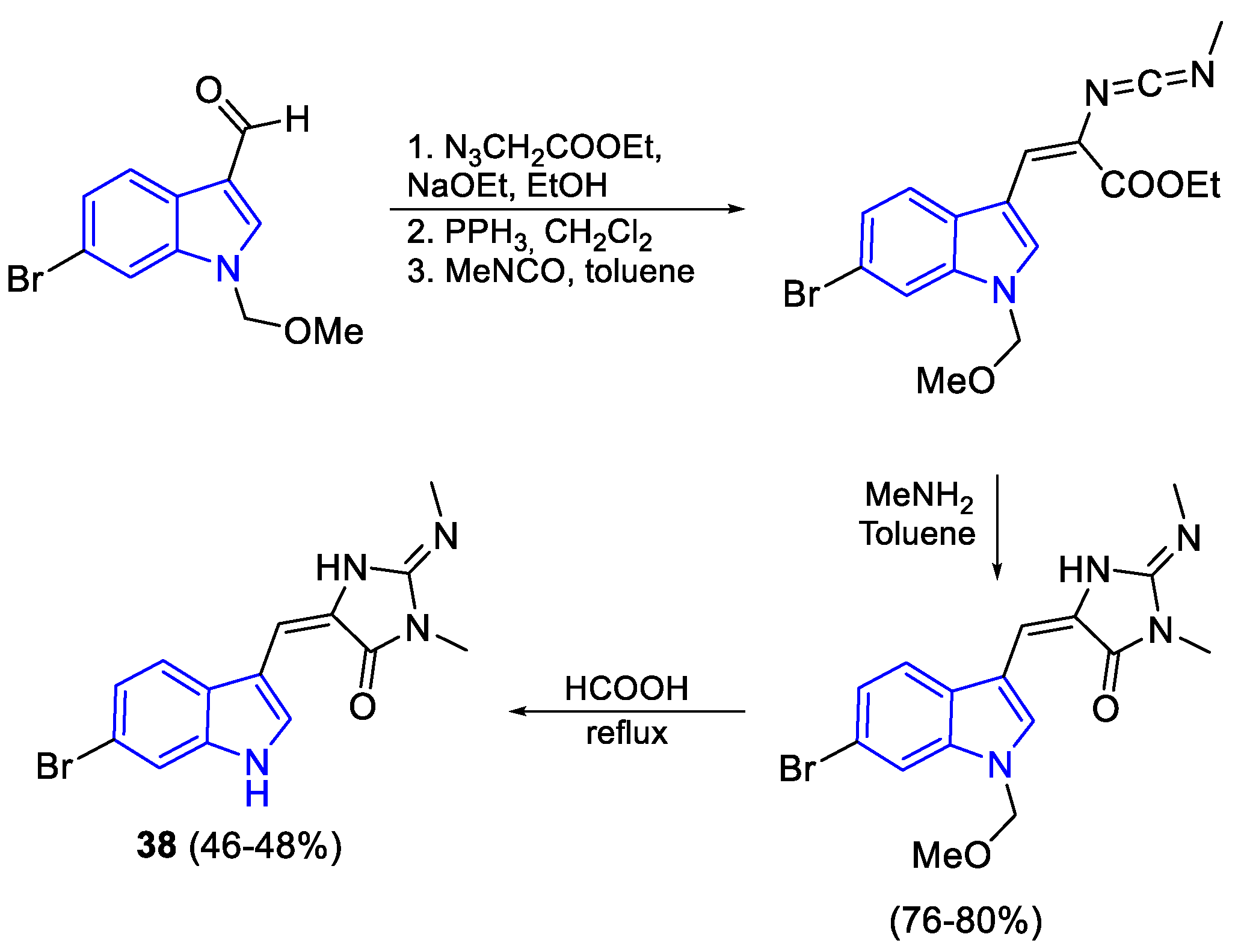
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Saturnino, C.; Sinicropi, M.S. A Comprehensive Review on Pyranoindole-Containing Agents. Curr. Med. Chem. 2022, 29, 3667–3683. [Google Scholar] [CrossRef]
- Wang, C.; Wang, T.; Huang, L.; Hou, Y.; Lu, W.; He, H. Facile Synthetic Approach for 5-Aryl-9-Hydroxypyrano [3,2-f] Indole-2(8H)-One. Arab. J. Chem. 2016, 9, 882–890. [Google Scholar] [CrossRef]
- Grubbs, A.W.; Artman, G.D.; Williams, R.M. Concise Syntheses of the 1,7-Dihydropyrano[2,3-g]Indole Ring System of the Stephacidins, Aspergamides and Norgeamides. Tetrahedron Lett. 2005, 46, 9013–9016. [Google Scholar] [CrossRef]
- Zhong, W.; Wang, J.; Wei, X.; Fu, T.; Chen, Y.; Zeng, Q.; Huang, Z.; Huang, X.; Zhang, W.; Zhang, S.; et al. Three Pairs of New Spirocyclic Alkaloid Enantiomers From the Marine-Derived Fungus Eurotium Sp. SCSIO F452. Front. Chem. 2019, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Fang, Y.; Zhu, T.; Zhang, M.; Lin, A.; Gu, Q.; Zhu, W. Seven New Prenylated Indole Diketopiperazine Alkaloids from Holothurian-Derived Fungus Aspergillus Fumigatus. Tetrahedron 2008, 64, 7986–7991. [Google Scholar] [CrossRef]
- Gao, H.; Liu, W.; Zhu, T.; Mo, X.; Mándi, A.; Kurtán, T.; Li, J.; Ai, J.; Gu, Q.; Li, D. Diketopiperazine Alkaloids from a Mangrove Rhizosphere Soil Derived Fungus Aspergillus Effuses H1-1. Org. Biomol. Chem. 2012, 10, 9501–9506. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Wang, J.; Wei, X.; Chen, Y.; Fu, T.; Xiang, Y.; Huang, X.; Tian, X.; Xiao, Z.; Zhang, W.; et al. Variecolortins A–C, Three Pairs of Spirocyclic Diketopiperazine Enantiomers from the Marine-Derived Fungus Eurotium Sp. SCSIO F452. Org. Lett. 2018, 20, 4593–4596. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-D.; Bao, Y.-R.; Huang, Y.-F.; Hu, D.; Li, X.-X.; Guo, L.-D.; Li, J.; Yao, X.-S.; Gao, H. Three Pairs of Variecolortide Enantiomers from Eurotium Sp. with Caspase-3 Inhibitory Activity. Fitoterapia 2014, 92, 252–259. [Google Scholar] [CrossRef] [PubMed]
- An, C.-Y.; Li, X.-M.; Li, C.-S.; Xu, G.-M.; Wang, B.-G. Prenylated Indolediketopiperazine Peroxides and Related Homologues from the Marine Sediment-Derived Fungus Penicillium Brefeldianum SD-273. Mar. Drugs 2014, 12, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Bunders, C.; Cavanagh, J.; Melander, C. Flustramine Inspired Synthesis and Biological Evaluation of Pyrroloindoline Triazole Amides as Novel Inhibitors of Bacterial Biofilms. Org. Biomol. Chem. 2011, 9, 5476–5481. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Z.-L.; Bai, J.; Zhang, L.-M.; Wu, X.; Zhang, L.; Pei, Y.-H.; Jing, Y.-K.; Hua, H.-M. 2,5-Diketopiperazines from the Marine-Derived Fungus Aspergillus Fumigatus YK-7. Chem. Biodivers. 2012, 9, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Yang, X.; Zhu, T.; Wang, F.; Xiao, X.; Park, H.; Gu, Q. Diketopiperazine Alkaloids from a Deep Ocean Sediment Derived Fungus Penicillium Sp. Chem. Pharm. Bull. (Tokyo) 2009, 57, 873–876. [Google Scholar] [CrossRef] [PubMed]
- Khalil, Z.G.; Huang, X.; Raju, R.; Piggott, A.M.; Capon, R.J. Shornephine A: Structure, Chemical Stability, and P-Glycoprotein Inhibitory Properties of a Rare Diketomorpholine from an Australian Marine-Derived Aspergillus Sp. J. Org. Chem. 2014, 79, 8700–8705. [Google Scholar] [CrossRef]
- Cai, S.; Sun, S.; Peng, J.; Kong, X.; Zhou, H.; Zhu, T.; Gu, Q.; Li, D. Okaramines S–U, Three New Indole Diketopiperazine Alkaloids from Aspergillus Taichungensis ZHN-7-07. Tetrahedron 2015, 71, 3715–3719. [Google Scholar] [CrossRef]
- Ding, L.; Maier, A.; Fiebig, H.-H.; Lin, W.-H.; Hertweck, C. A Family of Multicyclic Indolosesquiterpenes from a Bacterial Endophyte. Org. Biomol. Chem. 2011, 9, 4029–4031. [Google Scholar] [CrossRef]
- Che, Q.; Zhu, T.; Keyzers, R.A.; Liu, X.; Li, J.; Gu, Q.; Li, D. Polycyclic Hybrid Isoprenoids from a Reed Rhizosphere Soil Derived Streptomyces Sp. CHQ-64. J. Nat. Prod. 2013, 76, 759–763. [Google Scholar] [CrossRef]
- Che, Q.; Zhu, T.; Qi, X.; Mándi, A.; Kurtán, T.; Mo, X.; Li, J.; Gu, Q.; Li, D. Hybrid Isoprenoids from a Reeds Rhizosphere Soil Derived Actinomycete Streptomyces Sp. CHQ-64. Org. Lett. 2012, 14, 3438–3441. [Google Scholar] [CrossRef]
- Raju, R.; Piggott, A.M.; Huang, X.-C.; Capon, R.J. Nocardioazines: A Novel Bridged Diketopiperazine Scaffold from a Marine-Derived Bacterium Inhibits P-Glycoprotein. Org. Lett. 2011, 13, 2770–2773. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Ma, Y.; Liu, B.; Chen, P.; Hu, Y.; Zhang, R. Synthesis, Antimicrobial Activity, Structure-Activity Relationship, and Molecular Docking Studies of Indole Diketopiperazine Alkaloids. Front. Chem. 2019, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Adla, S.K.; Sasse, F.; Kelter, G.; Fiebig, H.-H.; Lindel, T. Doubly Prenylated Tryptamines: Cytotoxicity, Antimicrobial Activity and Cyclisation to the Marine Natural Product Flustramine A. Org. Biomol. Chem. 2013, 11, 6119–6130. [Google Scholar] [CrossRef] [PubMed]
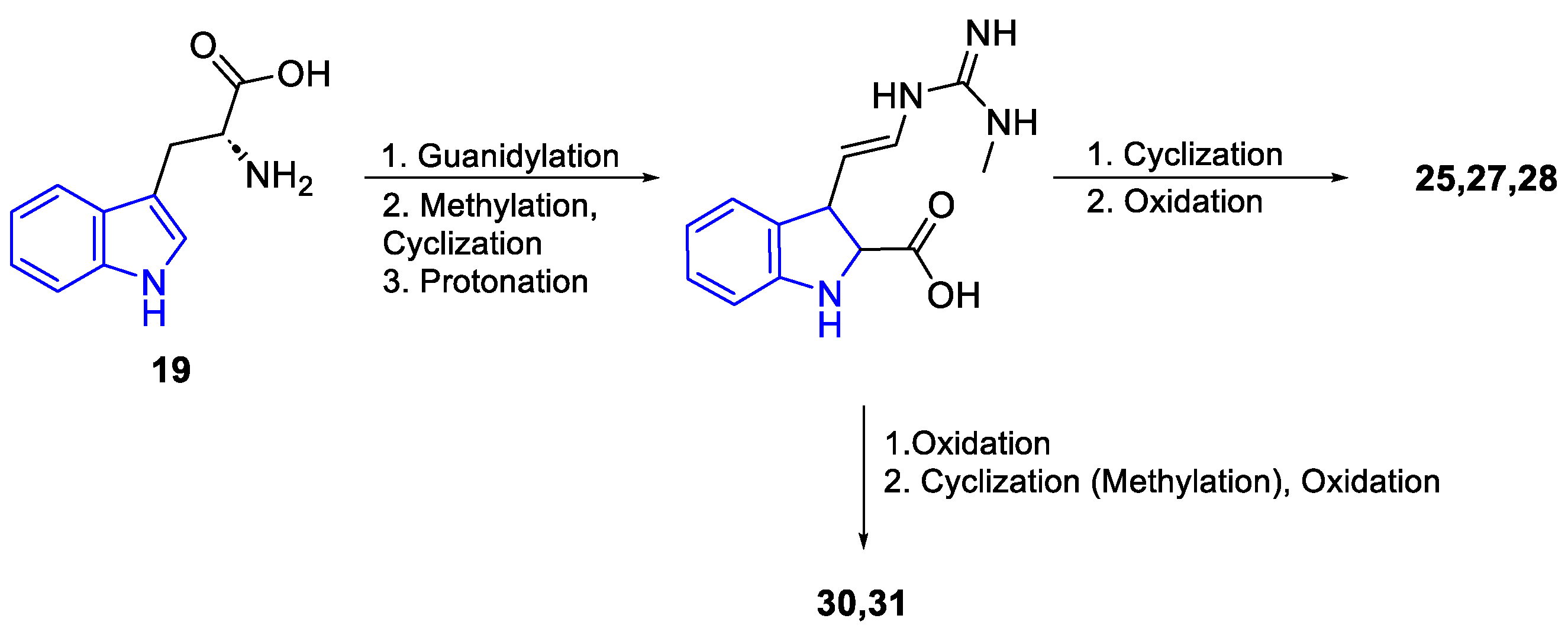
- Ruiz-Sanchis, P.; Savina, S.A.; Albericio, F.; Álvarez, M. Structure, Bioactivity and Synthesis of Natural Products with Hexahydropyrrolo[2,3-b]Indole. Chem. Eur. J. 2011, 17, 1388–1408. [Google Scholar] [CrossRef]
- Huang, H.; Yao, Y.; He, Z.; Yang, T.; Ma, J.; Tian, X.; Li, Y.; Huang, C.; Chen, X.; Li, W.; et al. Antimalarial β-Carboline and Indolactam Alkaloids from Marinactinospora Thermotolerans, a Deep Sea Isolate. J. Nat. Prod. 2011, 74, 2122–2127. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-W.; Liu, X.-J.; Yuan, J.; Li, H.-J.; Mahmud, T.; Hong, M.-J.; Yu, J.-C.; Lan, W.-J. L-Tryptophan Induces a Marine-Derived Fusarium Sp. to Produce Indole Alkaloids with Activity against the Zika Virus. J. Nat. Prod. 2020, 83, 3372–3380. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.-D.; Fan, P.; Zhou, L.-M.; Ma, Q.-Y.; Xie, Q.-Y.; Zheng, H.-Z.; Zheng, Z.-H.; Zhang, R.-S.; Yuan, J.-Z.; Dai, H.-F.; et al. Penerpenes A–D, Four Indole Terpenoids with Potent Protein Tyrosine Phosphatase Inhibitory Activity from the Marine-Derived Fungus Penicillium Sp. KFD28. Org. Lett. 2019, 21, 4864–4867. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.-M.; Kong, F.-D.; Fan, P.; Ma, Q.-Y.; Xie, Q.-Y.; Li, J.-H.; Zheng, H.-Z.; Zheng, Z.-H.; Yuan, J.-Z.; Dai, H.-F.; et al. Indole-Diterpenoids with Protein Tyrosine Phosphatase Inhibitory Activities from the Marine-Derived Fungus Penicillium Sp. KFD28. J. Nat. Prod. 2019, 82, 2638–2644. [Google Scholar] [CrossRef] [PubMed]
- Mugishima, T.; Tsuda, M.; Kasai, Y.; Ishiyama, H.; Fukushi, E.; Kawabata, J.; Watanabe, M.; Akao, K.; Kobayashi, J. Absolute Stereochemistry of Citrinadins A and B from Marine-Derived Fungus. J. Org. Chem. 2005, 70, 9430–9435. [Google Scholar] [CrossRef]
- Du, L.; Li, D.; Zhu, T.; Cai, S.; Wang, F.; Xiao, X.; Gu, Q. New Alkaloids and Diterpenes from a Deep Ocean Sediment Derived Fungus Penicillium Sp. Tetrahedron 2009, 65, 1033–1039. [Google Scholar] [CrossRef]
- Du, L.; Feng, T.; Zhao, B.; Li, D.; Cai, S.; Zhu, T.; Wang, F.; Xiao, X.; Gu, Q. Alkaloids from a Deep Ocean Sediment-Derived Fungus Penicillium Sp. and Their Antitumor Activities. J. Antibiot. 2010, 63, 165–170. [Google Scholar] [CrossRef]
- Sallam, A.A.; Houssen, W.E.; Gissendanner, C.R.; Orabi, K.Y.; Foudah, A.I.; Sayed, K.A.E. Bioguided Discovery and Pharmacophore Modeling of the Mycotoxic Indole Diterpene Alkaloids Penitrems as Breast Cancer Proliferation, Migration, and Invasion Inhibitors. Med. Chem. Commun. 2013, 4, 1360–1369. [Google Scholar] [CrossRef]
- Zhang, P.; Li, X.-M.; Li, X.; Wang, B.-G. New Indole-Diterpenoids from the Algal-Associated Fungus Aspergillus Nidulans. Phytochem. Lett. 2015, 12, 182–185. [Google Scholar] [CrossRef]
- Ivanets, E.V.; Yurchenko, A.N.; Smetanina, O.F.; Rasin, A.B.; Zhuravleva, O.I.; Pivkin, M.V.; Popov, R.S.; Von Amsberg, G.; Afiyatullov, S.S.; Dyshlovoy, S.A. Asperindoles A–D and a p-Terphenyl Derivative from the Ascidian-Derived Fungus Aspergillus Sp. KMM 4676. Mar. Drugs 2018, 16, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Sun, C.; Hou, X.; Che, Q.; Zhang, G.; Gu, Q.; Liu, C.; Zhu, T.; Li, D. Ascandinines A–D, Indole Diterpenoids, from the Sponge-Derived Fungus Aspergillus Candidus HDN15-152. J. Org. Chem. 2021, 86, 2431–2436. [Google Scholar] [CrossRef] [PubMed]
- Makarieva, T.N.; Ilyin, S.G.; Stonik, V.A.; Lyssenko, K.A.; Denisenko, V.A. Pibocin, the First Ergoline Marine Alkaloid from the Far-Eastern Ascidian Eudistoma Sp. Tetrahedron Lett. 1999, 40, 1591–1594. [Google Scholar] [CrossRef]
- Makarieva, T.N.; Dmitrenok, A.S.; Dmitrenok, P.S.; Grebnev, B.B.; Stonik, V.A. Pibocin B, the First N-O-Methylindole Marine Alkaloid, a Metabolite from the Far-Eastern Ascidian Eudistoma Species. J. Nat. Prod. 2001, 64, 1559–1561. [Google Scholar] [CrossRef]
- Kong, F.-D.; Zhang, S.-L.; Zhou, S.-Q.; Ma, Q.-Y.; Xie, Q.-Y.; Chen, J.-P.; Li, J.-H.; Zhou, L.-M.; Yuan, J.-Z.; Hu, Z.; et al. Quinazoline-Containing Indole Alkaloids from the Marine-Derived Fungus Aspergillus Sp. HNMF114. J. Nat. Prod. 2019, 82, 3456–3463. [Google Scholar] [CrossRef]
- Li, Y.-X.; Himaya, S.W.A.; Dewapriya, P.; Zhang, C.; Kim, S.-K. Fumigaclavine C from a Marine-Derived Fungus Aspergillus Fumigatus Induces Apoptosis in MCF-7 Breast Cancer Cells. Mar. Drugs 2013, 11, 5063–5086. [Google Scholar] [CrossRef]
- Limbadri, S.; Luo, X.; Lin, X.; Liao, S.; Wang, J.; Zhou, X.; Yang, B.; Liu, Y. Bioactive Novel Indole Alkaloids and Steroids from Deep Sea-Derived Fungus Aspergillus Fumigatus SCSIO 41012. Molecules 2018, 23, 2379–2388. [Google Scholar] [CrossRef]
- Fremlin, L.J.; Piggott, A.M.; Lacey, E.; Capon, Robert. J. Cottoquinazoline A and Cotteslosins A and B, Metabolites from an Australian Marine-Derived Strain of Aspergillus Versicolor. J. Nat. Prod. 2009, 72, 666–670. [Google Scholar] [CrossRef]
- Zhuang, Y.; Teng, X.; Wang, Y.; Liu, P.; Li, G.; Zhu, W. New Quinazolinone Alkaloids within Rare Amino Acid Residue from Coral-Associated Fungus, Aspergillus Versicolor LCJ-5-4. Org. Lett. 2011, 13, 1130–1133. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-H.; Xu, M.-Y.; Li, H.-J.; Li, J.-Q.; Chen, Y.-X.; Ma, W.-Z.; Li, Y.-P.; Xu, J.; Yang, D.-P.; Lan, W.-J. Amino Acid-Directed Strategy for Inducing the Marine-Derived Fungus Scedosporium Apiospermum F41–1 to Maximize Alkaloid Diversity. Org. Lett. 2017, 19, 4888–4891. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.Ø.; Isaksson, J.; Bayer, A.; Johansen, J.A.; Andersen, J.H.; Hansen, E. Securamine Derivatives from the Arctic Bryozoan Securiflustra Securifrons. J. Nat. Prod. 2017, 80, 3276–3283. [Google Scholar] [CrossRef] [PubMed]
- Adesanya, S.A.; Chbani, M.; Païs, M.; Debitus, C. Brominated β-Carbolines from the Marine Tunicate Eudistoma Album. J. Nat. Prod. 1992, 55, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Sandler, J.S.; Colin, P.L.; Hooper, J.N.A.; Faulkner, D.J. Cytotoxic β-Carbolines and Cyclic Peroxides from the Palauan Sponge Plakortis Nigra. J. Nat. Prod. 2002, 65, 1258–1261. [Google Scholar] [CrossRef]
- Kleks, G.; Duffy, S.; Lucantoni, L.; Avery, V.M.; Carroll, A.R. Orthoscuticellines A–E, β-Carboline Alkaloids from the Bryozoan Orthoscuticella Ventricosa Collected in Australia. J. Nat. Prod. 2020, 83, 422–428. [Google Scholar] [CrossRef]
- Carson, C. c.; Rajfer, J.; Eardley, I.; Carrier, S.; Denne, J. s.; Walker, D. j.; Shen, W.; Cordell, W. h. The Efficacy and Safety of Tadalafil: An Update. BJU International 2004, 93, 1276–1281. [Google Scholar] [CrossRef]
- Wibowo, D.N.S.A.; Soebadi, D.M.; Soebadi, M.A. Yohimbine as a Treatment for Erectile Dysfunction: A Systematic Review and Meta-Analysis. Turk. J. Urol. 2021, 47, 482–488. [Google Scholar] [CrossRef]
- Shamon, S.D.; Perez, M.I. Blood Pressure-lowering Efficacy of Reserpine for Primary Hypertension. Cochrane Database Syst. Rev. 2016, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Moyer, J.H.; Kinard, S.A.; Herschberger, R.; Dennis, E.W. Deserpidine (Canescine) for the Treatment of Hypertension. South. Med. J 1957, 50, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Fife, R.; Maclaurin, J.C.; Wright, J.H. Rescinnamine in Treatment of Hypertension in Hospital Clinic and in General Practice. Br. Med. J. 1960, 2, 1848–1850. [Google Scholar] [CrossRef] [PubMed]
- Stephens, D.N.; Schneider, H.H.; Kehr, W.; Andrews, J.S.; Rettig, K.J.; Turski, L.; Schmiechen, R.; Turner, J.D.; Jensen, L.H.; Petersen, E.N. Abecarnil, a Metabolically Stable, Anxioselective Beta-Carboline Acting at Benzodiazepine Receptors. J. Pharmacol. Exp. Ther. 1990, 253, 334–343. [Google Scholar] [PubMed]
- Bouwman, S.AM.; Zoleko-Manego, R.; Renner, K.C.; Schmitt, E.K.; Mombo-Ngoma, G.; Grobusch, M.P. The Early Preclinical and Clinical Development of Cipargamin (KAE609), a Novel Antimalarial Compound. Travel Med. Infect. Dis. 2020, 36, 101765–101773. [Google Scholar] [CrossRef]
- Dai, J.; Dan, W.; Schneider, U.; Wang, J. β-Carboline Alkaloid Monomers and Dimers: Occurrence, Structural Diversity, and Biological Activities. Eur. J. Med. Chem. 2018, 157, 622–656. [Google Scholar] [CrossRef]
- Herraiz, T. Identification and Occurrence of β-Carboline Alkaloids in Raisins and Inhibition of Monoamine Oxidase (MAO). J. Agric. Food Chem. 2007, 55, 8534–8540. [Google Scholar] [CrossRef]
- Kumla, D.; Shine Aung, T.; Buttachon, S.; Dethoup, T.; Gales, L.; Pereira, J.A.; Inácio, Â.; Costa, P.M.; Lee, M.; Sekeroglu, N.; et al. A New Dihydrochromone Dimer and Other Secondary Metabolites from Cultures of the Marine Sponge-Associated Fungi Neosartorya Fennelliae KUFA 0811 and Neosartorya Tsunodae KUFC 9213. Mar. Drugs 2017, 15, 375–392. [Google Scholar] [CrossRef]
- Di Giorgio, C.; Delmas, F.; Ollivier, E.; Elias, R.; Balansard, G.; Timon-David, P. In Vitro Activity of the β-Carboline Alkaloids Harmane, Harmine, and Harmaline toward Parasites of the Species Leishmania Infantum. Exp. Parasitol. 2004, 106, 67–74. [Google Scholar] [CrossRef]
- Junwised, J.; Saiin, C.; Takasu, K. Synthesis, Cytotoxicity and in Vitro Antimalarial Activity of 1-Ethyl-Beta-Carboline; an Indole Alkaloid of Picrasma Javanica Bl. Proceedings of 57th Kasetsart University Annual Conference: Science and Genetic Engineering, Architecture and Engineering, Agro-Industry, Natural Resources and Environment. Bangkok (Thailand). 2019. [Google Scholar]
- Junwised, J.; Saiin, C.; Takasu, K. Synthesis, Cytotoxicity and in Vitro Antimalarial Activity of 1-Ethyl-Beta-Carboline; an Indole Alkaloid of Picrasma Javanica Bl. Proceedings of 57th Kasetsart University Annual Conference: Science and Genetic Engineering, Architecture and Engineering, Agro-Industry, Natural Resources and Environment. Bangkok (Thailand). 2019. [Google Scholar]
- Patel, K.; Gadewar, M.; Tripathi, R.; Prasad, S.; Patel, D.K. A Review on Medicinal Importance, Pharmacological Activity and Bioanalytical Aspects of Beta-Carboline Alkaloid “Harmine. ” Asian Pac. J. Trop. Biomed. 2012, 2, 660–664. [Google Scholar] [CrossRef]
- Kobayashi, J.; Cheng, J.F.; Ohta, T.; Nozoe, S.; Ohizumi, Y.; Sasaki, T. Eudistomidins B, C, and D: Novel Antileukemic Alkaloids from the Okinawan Marine Tunicate Eudistoma Glaucus. J. Org. Chem. 1990, 55, 3666–3670. [Google Scholar] [CrossRef]
- Suzuki, T.; Kubota, T.; Kobayashi, J. Eudistomidins H–K, New β-Carboline Alkaloids from the Okinawan Marine Tunicate Eudistoma Glaucus. Bioorg. Med. Chem. Lett. 2011, 21, 4220–4223. [Google Scholar] [CrossRef]
- Rashid, M.A.; Gustafson, K.R.; Boyd, M.R. New Cytotoxic N-Methylated Beta-Carboline Alkaloids from the Marine Ascidian Eudistoma Gilboverde. J. Nat. Prod. 2001, 64, 1454–1456. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.M.; Mohamed, G.A. Ingenine E, a New Cytotoxic β-Carboline Alkaloid from the Indonesian Sponge Acanthostrongylophora Ingens. J. Asian Nat. Prod. Res. 2017, 19, 504–509. [Google Scholar] [CrossRef]
- Chan, S.T.S.; Pearce, A.N.; Page, M.J.; Kaiser, M.; Copp, B.R. Antimalarial β-Carbolines from the New Zealand Ascidian Pseudodistoma Opacum. J. Nat. Prod. 2011, 74, 1972–1979. [Google Scholar] [CrossRef]
- Schupp, P.; Poehner, T.; Edrada, R.; Ebel, R.; Berg, A.; Wray, V.; Proksch, P. Eudistomins W and X, Two New β-Carbolines from the Micronesian Tunicate Eudistoma Sp. J. Nat. Prod. 2003, 66, 272–275. [Google Scholar] [CrossRef]
- Iinuma, Y.; Kozawa, S.; Ishiyama, H.; Tsuda, M.; Fukushi, E.; Kawabata, J.; Fromont, J.; Kobayashi, J. Gesashidine A, a β-Carboline Alkaloid with an Imidazole Ring from a Thorectidae Sponge. J. Nat. Prod. 2005, 68, 1109–1110. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Mohamed, G.; Al Haidari, R.; El-Kholy, A.; Zayed, M. Ingenine F: A New Cytotoxic Tetrahydro Carboline Alkaloid from the Indonesian Marine Sponge Acanthostrongylophora Ingens. Phcog. Mag. 2018, 14, 231–234. [Google Scholar] [CrossRef]
- Davis, R.A.; Duffy, S.; Avery, V.M.; Camp, D.; Hooper, J.N.A.; Quinn, R.J. (+)-7-Bromotrypargine: An Antimalarial β-Carboline from the Australian Marine Sponge Ancorina Sp. Tetrahedron Lett. 2010, 51, 583–585. [Google Scholar] [CrossRef]
- Patil, A.D.; Freyer, A.J.; Carte, B.; Taylor, P.B.; Johnson, R.K.; Faulkner, D.J. Haploscleridamine, a Novel Tryptamine-Derived Alkaloid from a Sponge of the Order Haplosclerida: An Inhibitor of Cathepsin K. J. Nat. Prod. 2002, 65, 628–629. [Google Scholar] [CrossRef]
- Tanaka, N.; Momose, R.; Takahashi-Nakaguchi, A.; Gonoi, T.; Fromont, J.; Kobayashi, J. Hyrtimomines, Indole Alkaloids from Okinawan Marine Sponges Hyrtios Spp. Tetrahedron 2014, 70, 832–837. [Google Scholar] [CrossRef]
- Prinsep, M.R.; Blunt, J.W.; Munro, M.H. New Cytotoxic Beta-Carboline Alkaloids from the Marine Bryozoan, Cribricellina Cribraria. J. Nat. Prod. 1991, 54, 1068–1076. [Google Scholar] [CrossRef]
- Yan, W.; Zhao, S.S.; Ye, Y.H.; Zhang, Y.Y.; Zhang, Y.; Xu, J.Y.; Yin, S.M.; Tan, R.X. Generation of Indoles with Agrochemical Significance through Biotransformation by Chaetomium Globosum. J. Nat. Prod. 2019, 82, 2132–2137. [Google Scholar] [CrossRef]
- Shi, Y.; Xu, Z.; Tan, R.; Lei, X. Divergent Total Synthesis of Chaetoglines C to F. J. Org. Chem. 2019, 84, 8766–8770. [Google Scholar] [CrossRef]
- Yan, W.; Ge, H.M.; Wang, G.; Jiang, N.; Mei, Y.N.; Jiang, R.; Li, S.J.; Chen, C.J.; Jiao, R.H.; Xu, Q.; et al. Pictet–Spengler Reaction-Based Biosynthetic Machinery in Fungi. Proc. Natl. Acad. Sci. 2014, 111, 18138–18143. [Google Scholar] [CrossRef] [PubMed]
- Gohil, V.M.; Brahmbhatt, K.G.; Loiseau, P.M.; Bhutani, K.K. Synthesis and Anti-Leishmanial Activity of 1-Aryl-β-Carboline Derivatives against Leishmania Donovani. Bioorg. Med. Chem. Lett. 2012, 22, 3905–3907. [Google Scholar] [CrossRef]
- Kong, Y.; Wang, L.-H.; Liu, L.; Zheng, L.-H.; Bao, Y.-L.; Liu, X.-X.; Wang, S.-Y.; Song, Z.-B. Immunomodulatory Effects of Flazin from Crassostrea Sikamea on Splenic Lymphocytes of Sprague-Dawley Rats. Chin. J. Nat. Med. 2021, 19, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.-G.; Wang, Y.-H.; Wang, R.-R.; Dong, Z.-J.; Yang, L.-M.; Zheng, Y.-T.; Liu, J.-K. Synthesis of Analogues of Flazin, in Particular, Flazinamide, as Promising Anti-HIV Agents. Chem. Biodivers. 2008, 5, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, J.; Harbour, G.C.; Gilmore, J.; Rinehart, K.L.Jr. Eudistomins A, D, G, H, I, J, M, N, O, P, and Q, Bromo, Hydroxy, Pyrrolyl and Iminoazepino.Beta.-Carbolines from the Antiviral Caribbean Tunicate Eudistoma Olivaceum. J. Am. Chem. Soc. 1984, 106, 1526–1528. [Google Scholar] [CrossRef]
- VanWagenen, B.C.; Cardellina, J.H. Short, Efficient Syntheses of the Antibiotic Eudistomins I and T. Tetrahedron Lett. 1989, 30, 3605–3608. [Google Scholar] [CrossRef]
- Rajesh, R.P.; Murugan, A. Spectroscopic Identification of Brominated, Non-Brominated Alkaloids and Evaluation of Antimicrobial Activity of Eudistomin-I, Eudistomin H, from Green Ascidian Eudistoma Viride. J. App. Pharm. Sci. 2019, 9, 116–123. [Google Scholar] [CrossRef]
- V. Serdyuk, O.; A. Kolodina, A. Eudistomin U, Isoeudistomin U, and Related Indole Compounds: Synthesis and Biological Activity. Heterocycles 2018, 96, 1171. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, G.A. Ingenines C and D, New Cytotoxic Pyrimidine-β-Carboline Alkaloids from the Indonesian Sponge Acanthostrongylophora Ingens. Phytochem. Lett. 2016, 18, 168–171. [Google Scholar] [CrossRef]
- Dhaneesha, M.; Umar, Md.; Merlin, T.S.; Krishnan, K.P.; Sukumaran, V.; Sinha, R.K.; Anas, A.; Fu, P.; MacMillan, J.B.; Sajeevan, T.P. Pseudonocardia Cytotoxica Sp. Nov., a Novel Actinomycete Isolated from an Arctic Fjord with Potential to Produce Cytotoxic Compound. Antonie van Leeuwenhoek 2021, 114, 23–35. [Google Scholar] [CrossRef]
- Shin, H.J.; Lee, H.-S.; Lee, D.-S. The Synergistic Antibacterial Activity of 1-Acetyl-Beta-Carboline and Beta-Lactams against Methicillin-Resistant Staphylococcus Aureus (MRSA). J. Microbiol. Biotechnol. 2010, 20, 501–505. [Google Scholar]
- Li, J.; Tang, Y.; Jin, H.-J.; Cui, Y.-D.; Zhang, L.-J.; Jiang, T. An Efficient Synthesis Method Targeted to Marine Alkaloids Marinacarbolines A–D and Their Antitumor Activities. J. Asian Nat. Prod. Res. 2015, 17, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Byun, W.S.; Lim, H.; Hong, J.; Bae, E.S.; Lee, S.B.; Kim, Y.; Lee, J.; Lee, S.K.; Hong, S. Design, Synthesis, and Biological Activity of Marinacarboline Analogues as STAT3 Pathway Inhibitors for Docetaxel-Resistant Triple-Negative Breast Cancer. J. Med. Chem. 2023, 66, 3106–3133. [Google Scholar] [CrossRef] [PubMed]
- Won, T.H.; Jeon, J.; Lee, S.-H.; Rho, B.J.; Oh, K.-B.; Shin, J. Beta-Carboline Alkaloids Derived from the Ascidian Synoicum Sp. Bioorg. Med. Chem. 2012, 20, 4082–4087. [Google Scholar] [CrossRef]
- Wang, W.; Nam, S.-J.; Lee, B.-C.; Kang, H. β-Carboline Alkaloids from a Korean Tunicate Eudistoma Sp. J. Nat. Prod. 2008, 71, 163–166. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, P.; Bijian, K.; Ren, S.; Wan, S.; Alaoui-Jamali, M.A.; Jiang, T. Total Synthesis and Biological Activity of Marine Alkaloid Eudistomins Y1–Y7 and Their Analogues. Mar. Drugs 2013, 11, 1427–1439. [Google Scholar] [CrossRef]
- Yang, G.; Xie, H.; Wang, C.; Zhang, C.; Yu, L.; Zhang, L.; Liu, X.; Xu, R.; Song, Z.; Liu, R.; et al. Design, Synthesis, and Discovery of Eudistomin Y Derivatives as Lysosome-Targeted Antiproliferation Agents. Eur. J. Med. Chem. 2023, 250, 115193–115204. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Hu, J.-F.; Kazi, A.B.; Li, Z.; Avery, M.; Peraud, O.; Hill, R.T.; Franzblau, S.G.; Zhang, F.; Schinazi, R.F.; et al. Manadomanzamines A and B: A Novel Alkaloid Ring System with Potent Activity against Mycobacteria and HIV-1. J. Am. Chem. Soc. 2003, 125, 13382–13386. [Google Scholar] [CrossRef] [PubMed]
- Inman, W.D.; Bray, W.M.; Gassner, N.C.; Lokey, R.S.; Tenney, K.; Shen, Y.Y.; TenDyke, K.; Suh, T.; Crews, P. A β-Carboline Alkaloid from the Papua New Guinea Marine Sponge Hyrtios Reticulatus. J. Nat. Prod. 2010, 73, 255–257. [Google Scholar] [CrossRef]
- Bourguet-Kondracki, M.L.; Martin, M.T.; Guyot, M. A New β-Carboline Alkaloid Isolated from the Marine Sponge Hyrtios Erecta. Tetrahedron Lett. 1996, 37, 3457–3460. [Google Scholar] [CrossRef]
- Szabó, T.; Dancsó, A.; Volk, B.; Milen, M. First Total Synthesis of β-Carboline Alkaloid Trigonostemine G and Its Derivatives. Nat. Prod. Res. 2021, 35, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sun, X.; Xu, B.; Bijian, K.; Wan, S.; Li, G.; Alaoui-Jamali, M.; Jiang, T. Total Synthesis and Bioactivity of the Marine Alkaloid Pityriacitrin and Some of Its Derivatives. Eur. J. Med. Chem. 2011, 46, 6089–6097. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.-J.; Xu, D.-P.; Zhou, T.; Zhou, Y.; Li, S.; Li, H.-B. Bioactivities and Health Benefits of Wild Fruits. Int. J. Mol. Sci. 2016, 17, 1258–1285. [Google Scholar] [CrossRef]
- Sauleau, P.; Martin, M.-T.; Dau, M.-E.T.H.; Youssef, D.T.A.; Bourguet-Kondracki, M.-L. Hyrtiazepine, an Azepino-Indole-Type Alkaloid from the Red Sea Marine Sponge Hyrtios Erectus. J. Nat. Prod. 2006, 69, 1676–1679. [Google Scholar] [CrossRef]
- Kashtoh, H.; Baek, K.-H. Recent Updates on Phytoconstituent Alpha-Glucosidase Inhibitors: An Approach towards the Treatment of Type Two Diabetes. Plants 2022, 11, 2722–2747. [Google Scholar] [CrossRef]
- Ha, N.T.T.; Quan, P.M.; Van Tuyen, N.; Tra, N.T.; Anh, L.T.T.; Son, N.T. Chemical Constituents of Alocasia Odora Rhizomes and Their Biological Activities: Experimental and Molecular Docking Approaches. Rev. Bras. Farmacogn. 2022, 32, 819–826. [Google Scholar] [CrossRef]
- Liew, L.P.P.; Fleming, J.M.; Longeon, A.; Mouray, E.; Florent, I.; Bourguet-Kondracki, M.-L.; Copp, B.R. Synthesis of 1-Indolyl Substituted β-Carboline Natural Products and Discovery of Antimalarial and Cytotoxic Activities. Tetrahedron 2014, 70, 4910–4920. [Google Scholar] [CrossRef]
- Pereira, M.D.P.; Silva, T. da; Aguiar, A.C.C.; Oliva, G.; Guido, R.V.C.; Yokoyama-Yasunaka, J.K.U.; Uliana, S.R.B.; Lopes, L.M.X. Chemical Composition, Antiprotozoal and Cytotoxic Activities of Indole Alkaloids and Benzofuran Neolignan of Aristolochia Cordigera. Planta Med. 2017, 83, 912–920. [Google Scholar] [CrossRef]
- Oku, N.; Matsunaga, S.; Fusetani, N. Shishijimicins A−C, Novel Enediyne Antitumor Antibiotics from the Ascidian Didemnum Proliferum. J. Am. Chem. Soc. 2003, 125, 2044–2045. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Lu, Z.; Li, R.; Woods, J.R.; Sohn, T. Total Synthesis of Shishijimicin A. J. Am. Chem. Soc. 2015, 137, 8716–8719. [Google Scholar] [CrossRef]
- Nakamura, H.; Deng, S.; Kobayashi, J.; Ohizumi, Y.; Tomotake, Y.; Matsuzaki, T.; Hirata, Y. Keramamine-A and -B, Novel Antimicrobial Alkaloids from the Okinawan Marine Sponge Pellina Sp. Tetrahedron Lett. 1987, 28, 621–624. [Google Scholar] [CrossRef]
- Sakai, Ryuichi. ; Higa, Tatsuo.; Jefford, C.W.; Bernardinelli, Gerald. Manzamine A, a Novel Antitumor Alkaloid from a Sponge. J. Am. Chem. Soc. 1986, 108, 6404–6405. [Google Scholar] [CrossRef]
- Rao, K.V.; Kasanah, N.; Wahyuono, S.; Tekwani, B.L.; Schinazi, R.F.; Hamann, M.T. Three New Manzamine Alkaloids from a Common Indonesian Sponge and Their Activity against Infectious and Tropical Parasitic Diseases. J. Nat. Prod. 2004, 67, 1314–1318. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-C.; Kuo, T.-T.; Chang, H.-Y.; Liu, W.-S.; Hsia, S.-M.; Huang, T.-C. Manzamine A Exerts Anticancer Activity against Human Colorectal Cancer Cells. Mar. Drugs 2018, 16, 252–267. [Google Scholar] [CrossRef] [PubMed]
- Kallifatidis, G.; Hoepfner, D.; Jaeg, T.; Guzmán, E.A.; Wright, A.E. The Marine Natural Product Manzamine A Targets Vacuolar ATPases and Inhibits Autophagy in Pancreatic Cancer Cells. Mar. Drugs 2013, 11, 3500–3516. [Google Scholar] [CrossRef] [PubMed]
- Hamann, M.; Alonso, D.; Martín-Aparicio, E.; Fuertes, A.; Pérez-Puerto, M.J.; Castro, A.; Morales, S.; Navarro, M.L.; del Monte-Millán, M.; Medina, M.; et al. Glycogen Synthase Kinase-3 (GSK-3) Inhibitory Activity and Structure–Activity Relationship (SAR) Studies of the Manzamine Alkaloids. Potential for Alzheimer’s Disease. J. Nat. Prod. 2007, 70, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Palem, J.R.; Mudit, M.; Hsia, S.V.; Sayed, K.A.E. Discovery and Preliminary Structure-Activity Relationship of the Marine Natural Product Manzamines as Herpes Simplex Virus Type-1 Inhibitors. Z. Naturforsch. C 2017, 72, 49–54. [Google Scholar] [CrossRef]
- Ichiba, T.; Corgiat, J.M.; Scheuer, P.J.; Kelly-Borges, M. 8-Hydroxymanzamine A, a Beta-Carboline Alkaloid from a Sponge, Pachypellina Sp. J. Nat. Prod. 1994, 57, 168–170. [Google Scholar] [CrossRef]
- Ashok, P.; Ganguly, S.; Murugesan, S. Manzamine Alkaloids: Isolation, Cytotoxicity, Antimalarial Activity and SAR Studies. Drug Discov. Today 2014, 19, 1781–1791. [Google Scholar] [CrossRef]
- El Sayed, K.A.; Kelly, M.; Kara, U.A.K.; Ang, K.K.H.; Katsuyama, I.; Dunbar, D.C.; Khan, A.A.; Hamann, M.T. New Manzamine Alkaloids with Potent Activity against Infectious Diseases. J. Am. Chem. Soc. 2001, 123, 1804–1808. [Google Scholar] [CrossRef]
- Watanabe, D.; Tsuda, M.; Kobayashi, J. Three New Manzamine Congeners from Amphimedon Sponge. J. Nat. Prod. 1998, 61, 689–692. [Google Scholar] [CrossRef]
- Yousaf, M.; El Sayed, K.A.; Rao, K.V.; Lim, C.W.; Hu, J.-F.; Kelly, M.; Franzblau, S.G.; Zhang, F.; Peraud, O.; Hill, R.T.; et al. 12,34-Oxamanzamines, Novel Biocatalytic and Natural Products from Manzamine Producing Indo-Pacific Sponges. Tetrahedron 2002, 58, 7397–7402. [Google Scholar] [CrossRef]
- Yousaf, M.; Hammond, N.L.; Peng, J.; Wahyuono, S.; McIntosh, K.A.; Charman, W.N.; Mayer, A.M.S.; Hamann, M.T. New Manzamine Alkaloids from an Indo-Pacific Sponge. Pharmacokinetics, Oral Availability, and the Significant Activity of Several Manzamines against HIV-I, AIDS Opportunistic Infections, and Inflammatory Diseases. J. Med. Chem. 2004, 47, 3512–3517. [Google Scholar] [CrossRef]
- Crews, P.; Cheng, X.-C.; Adamczeski, M.; Rodríguez, J.; Jaspars, M.; Schmitz, F.J.; Traeger, S.C.; Pordesimo, E.O. 1,2,3,4-Tetrahydro-8-Hydroxymanzamines, Alkaloids from Two Different Haplosclerid Sponges. Tetrahedron 1994, 50, 13567–13574. [Google Scholar] [CrossRef]
- Ichiba, T.; Sakai, R.; Kohmoto, S.; Saucy, G.; Higa, T. New Manzamine Alkaloids from a Sponge of the Genus Xestospongia. Tetrahedron Lett. 1988, 29, 3083–3086. [Google Scholar] [CrossRef]
- El-Desoky, A.H.; Kato, H.; Eguchi, K.; Kawabata, T.; Fujiwara, Y.; Losung, F.; Mangindaan, R.E.P.; de Voogd, N.J.; Takeya, M.; Yokosawa, H.; et al. Acantholactam and Pre-Neo-Kauluamine, Manzamine-Related Alkaloids from the Indonesian Marine Sponge Acanthostrongylophora Ingens. J. Nat. Prod. 2014, 77, 1536–1540. [Google Scholar] [CrossRef]
- Yamada, M.; Takahashi, Y.; Kubota, T.; Fromont, J.; Ishiyama, A.; Otoguro, K.; Yamada, H.; Ōmura, S.; Kobayashi, J. Zamamidine C, 3,4-Dihydro-6-Hydroxy-10,11-Epoxymanzamine A, and 3,4-Dihydromanzamine J N-Oxide, New Manzamine Alkaloids from Sponge Amphimedon Sp. Tetrahedron 2009, 65, 2313–2317. [Google Scholar] [CrossRef]
- Kim, C.-K.; Riswanto, R.; Won, T.H.; Kim, H.; Elya, B.; Sim, C.J.; Oh, D.-C.; Oh, K.-B.; Shin, J. Manzamine Alkaloids from an Acanthostrongylophora Sp. Sponge. J. Nat. Prod. 2017, 80, 1575–1583. [Google Scholar] [CrossRef]
- Furusato, A.; Kato, H.; Nehira, T.; Eguchi, K.; Kawabata, T.; Fujiwara, Y.; Losung, F.; Mangindaan, R.E.P.; de Voogd, N.J.; Takeya, M.; et al. Acanthomanzamines A–E with New Manzamine Frameworks from the Marine Sponge Acanthostrongylophora Ingens. Org. Lett. 2014, 16, 3888–3891. [Google Scholar] [CrossRef]
- Tsuda, M.; Watanabe, D.; Kobayashi, J. Ma’eganedin A, a New Manzamine Alkaloid from Amphimedon Sponge. Tetrahedron Lett. 1998, 39, 1207–1210. [Google Scholar] [CrossRef]
- Longley, R.E.; McConnell, O.J.; Essich, E.; Harmody, D. Evaluation of Marine Sponge Metabolites for Cytotoxicity and Signal Transduction Activity. J. Nat. Prod. 1993, 56, 915–920. [Google Scholar] [CrossRef]
- Kobayashi, M.; Chen, Y.-J.; Aoki, S.; In, Y.; Ishida, T.; Kitagawa, I. Four New β-Carboline Alkaloids Isolated from Two Okinawan Marine Sponges of Xestospongia Sp. and Haliclona Sp. Tetrahedron 1995, 51, 3727–3736. [Google Scholar] [CrossRef]
- Edrada, R.A.; Proksch, P.; Wray, V.; Witte, L.; Müller, W.E.G.; Van Soest, R.W.M. Four New Bioactive Manzamine-Type Alkaloids from the Philippine Marine Sponge Xestospongia Ashmorica. J. Nat. Prod. 1996, 59, 1056–1060. [Google Scholar] [CrossRef]
- Tadokoro, Y.; Nishikawa, T.; Ichimori, T.; Matsunaga, S.; Fujita, M.J.; Sakai, R. N-Methyl-β-Carbolinium Salts and an N-Methylated 8-Oxoisoguanine as Acetylcholinesterase Inhibitors from a Solitary Ascidian, Cnemidocarpa Irene. ACS Omega 2017, 2, 1074–1080. [Google Scholar] [CrossRef]
- Segraves, N.L.; Lopez, S.; Johnson, T.A.; Said, S.A.; Fu, X.; Schmitz, F.J.; Pietraszkiewicz, H.; Valeriote, F.A.; Crews, P. Structures and Cytotoxicities of Fascaplysin and Related Alkaloids from Two Marine Phyla—Fascaplysinopsis Sponges and Didemnum Tunicates. Tetrahedron Lett. 2003, 44, 3471–3475. [Google Scholar] [CrossRef]
- Izzati, F.; Warsito, M.F.; Bayu, A.; Prasetyoputri, A.; Atikana, A.; Sukmarini, L.; Rahmawati, S.I.; Putra, M.Y. Chemical Diversity and Biological Activity of Secondary Metabolites Isolated from Indonesian Marine Invertebrates. Molecules 2021, 26, 1898–1920. [Google Scholar] [CrossRef]
- Tran, T.D.; Pham, N.B.; Ekins, M.; Hooper, J.N.A.; Quinn, R.J. Isolation and Total Synthesis of Stolonines A–C, Unique Taurine Amides from the Australian Marine Tunicate Cnemidocarpa Stolonifera. Mar. Drugs 2015, 13, 4556–4575. [Google Scholar] [CrossRef]
- Ravinder, K.; Vijender Reddy, A.; Krishnaiah, P.; Ramesh, P.; Ramakrishna, S.; Laatsch, H.; Venkateswarlu, Y. Isolation and Synthesis of a Novel β-Carboline Guanidine Derivative Tiruchanduramine from the Indian Ascidian Synoicum Macroglossum. Tetrahedron Lett. 2005, 46, 5475–5478. [Google Scholar] [CrossRef]
- Youssef, D. T. A. Hyrtioerectines A−C, Cytotoxic Alkaloids from the Red Sea Sponge Hyrtios Erectus. J. Nat. Prod. 2005, 68, 9–1416. [Google Scholar] [CrossRef]
- Wang, C.; Wang, S.; Li, H.; Hou, Y.; Cao, H.; Hua, H.; Li, D. Marine-Derived Lead Fascaplysin: Pharmacological Activity, Total Synthesis, and Structural Modification. Mar. Drugs 2023, 21, 226–242. [Google Scholar] [CrossRef]
- Charan, R.D.; McKee, T.C.; Gustafson, K.R.; Pannell, L.K.; Boyd, M.R. Thorectandramine, a Novel β-Carboline Alkaloid from the Marine Sponge Thorectandra Sp. Tetrahedron Lett. 2002, 43, 5201–5204. [Google Scholar] [CrossRef]
- Lake, R.J.; Blunt, J.W.; Munro, M.H.G. Eudistomins From the New Zealand Ascidian Ritterella Sigillinoides. Aust. J. Chem. 1989, 42, 1201–1206. [Google Scholar] [CrossRef]
- Chatwichien, J.; Basu, S.; Murphy, M.E.; Hamann, M.T.; Winkler, J.D. Design, Synthesis, and Biological Evaluation of β-Carboline Dimers Based on the Structure of Neokauluamine. Tetrahedron Lett. 2015, 56, 3515–3517. [Google Scholar] [CrossRef]
- Suzuki, K.; Nomura, I.; Ninomiya, M.; Tanaka, K.; Koketsu, M. Synthesis and Antimicrobial Activity of β-Carboline Derivatives with N2-Alkyl Modifications. Bioorg. Med. Chem. Lett. 2018, 28, 2976–2978. [Google Scholar] [CrossRef]
- Ohtani, I.I.; Ichiba, T.; Isobe, M.; Kelly-Borges, M.; Scheuer, P.J. Kauluamine, an Unprecedented Manzamine Dimer from an Indonesian Marine Sponge, Prianos Sp. J. Am. Chem. Soc. 1995, 117, 10743–10744. [Google Scholar] [CrossRef]
- Zulkifli, S.Z.; Pungot, N.H.; Saaidin, A.S.; Jani, N.A.; Mohammat, M.F. Synthesis and Diverse Biological Activities of Substituted Indole β-Carbolines: A Review. Nat. Prod. Res. 2023, 0, 1–14. [Google Scholar] [CrossRef]
- Dalpozzo, R. The Chiral Pool in the Pictet–Spengler Reaction for the Synthesis of β-Carbolines. Molecules 2016, 21, 699–717. [Google Scholar] [CrossRef] [PubMed]
- Pakhare, D.S.; Kusurkar, R.S. Synthesis of Tetrahydro-β-Carbolines, β-Carbolines, and Natural Products, (±)-Harmicine, Eudistomin U and Canthine by Reductive Pictet Spengler Cyclization. Tetrahedron Lett. 2015, 56, 6012–6015. [Google Scholar] [CrossRef]
- Whaley, W.M.; Govindachari, T.R. The Preparation of 3,4-Dihydroisoquinolines and Related Compounds by the Bischler-Napieralski Reaction. In Organic Reactions; John Wiley & Sons, Ltd., 2011; pp. 74–150. ISBN 978-0-471-26418-7. [Google Scholar]
- Hibino, S.; Sugino, E.; Yamochi, T.; Kuwata, M.; Hashimoto, H.; Sato, K.; Amanuma, F.; Karasaw, Y. Syntheses and Sleeping-Time-Prolonging Effect of Nitramarine and Related Compounds. Chem. Pharm. Bull. (Tokyo), 1987, 35, 2261–2265. [Google Scholar] [CrossRef] [PubMed]
- Broeck, P.V.; Doren, P.V.; Hoornaert, G. Reaction of 3H-Pyrano[3,4-b]Indol-3-Ones and 3H-2-Benzopyran-3-Ones with Heterodienophiles: A Two Step Synthesis for Some 9H-Pyrido[3,4-b]Indoles and Isoquinolines. Synthesis 1992, 1992, 473–476. [Google Scholar] [CrossRef]
- Iwaki, T.; Yasuhara, A.; Sakamoto, T. Novel Synthetic Strategy of Carbolines via Palladium-Catalyzed Amination and Arylation Reaction. J. Chem. Soc., Perkin Trans. 1, 1510. [Google Scholar] [CrossRef]
- Smith, P.A.S.; Boyer, J.H. The Synthesis of Heterocyclic Compounds from Aryl Azides. II. Carbolines and Thienoindole1. J. Am. Chem. Soc. 1951, 73, 2626–2629. [Google Scholar] [CrossRef]
- Rocca, P.; Marsais, F.; Godard, A.; Queguiner, G. Connection between Metalation and Cross-Coupling Strategies. A New Convergent Route to Azacarbazoles. Tetrahedron 1993, 49, 49–64. [Google Scholar] [CrossRef]
- Clark, V.M.; Cox, A.; Herbert, E.J. The Photocyclisation of Anilino-Pyridines to Carbolines. J. Chem. Soc. C 1968, 831–833. [Google Scholar] [CrossRef]
- Arshad, A.S.M.; Mordi, M.N. Carboline Regioisomers Based on Unified Synthetic Approaches. Adv. Synt Catal. 2023, 365, 2126–2146. [Google Scholar] [CrossRef]
- Devi, N.; Kumar, S.; Pandey, S.K.; Singh, V. 1(3)-Formyl-β-Carbolines: Potential Aldo-X Precursors for the Synthesis of β-Carboline-Based Molecular Architectures. Asian J. Org. Chem. 2018, 7, 6–36. [Google Scholar] [CrossRef]
- Qi, S.-H.; Su, G.-C.; Wang, Y.-F.; Liu, Q.-Y.; Gao, C.-H. Alkaloids from the South China Sea Black Coral Antipathes Dichotoma. Chem. Pharm. Bull. 2009, 57, 87–88. [Google Scholar] [CrossRef] [PubMed]
- Lyakhova, E.G.; Kolesnikova, S.A.; Kalinovsky, A.I.; Afiyatullov, S.Sh.; Dyshlovoy, S.A.; Krasokhin, V.B.; Minh, C.V.; Stonik, V.A. Bromine-Containing Alkaloids from the Marine Sponge Penares Sp. Tetrahedron Lett. 2012, 53, 6119–6122. [Google Scholar] [CrossRef]
- Choi, E.J.; Park, J. -S.; Kim, Y. -J.; Jung, J. -H.; Lee, J.K.; Kwon, H.C.; Yang, H.O. Apoptosis-inducing Effect of Diketopiperazine Disulfides Produced by Aspergillus Sp. KMD 901 Isolated from Marine Sediment on HCT116 Colon Cancer Cell Lines. J. Appl. Microbiol. 2011, 110, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Wang, Y.; Liu, P.; Dong, T.; Zhu, W. Thiodiketopiperazines from the Marine-Derived Fungus Phoma Sp. OUCMDZ-1847. J. Nat. Prod. 2014, 77, 132–137. [Google Scholar] [CrossRef] [PubMed]

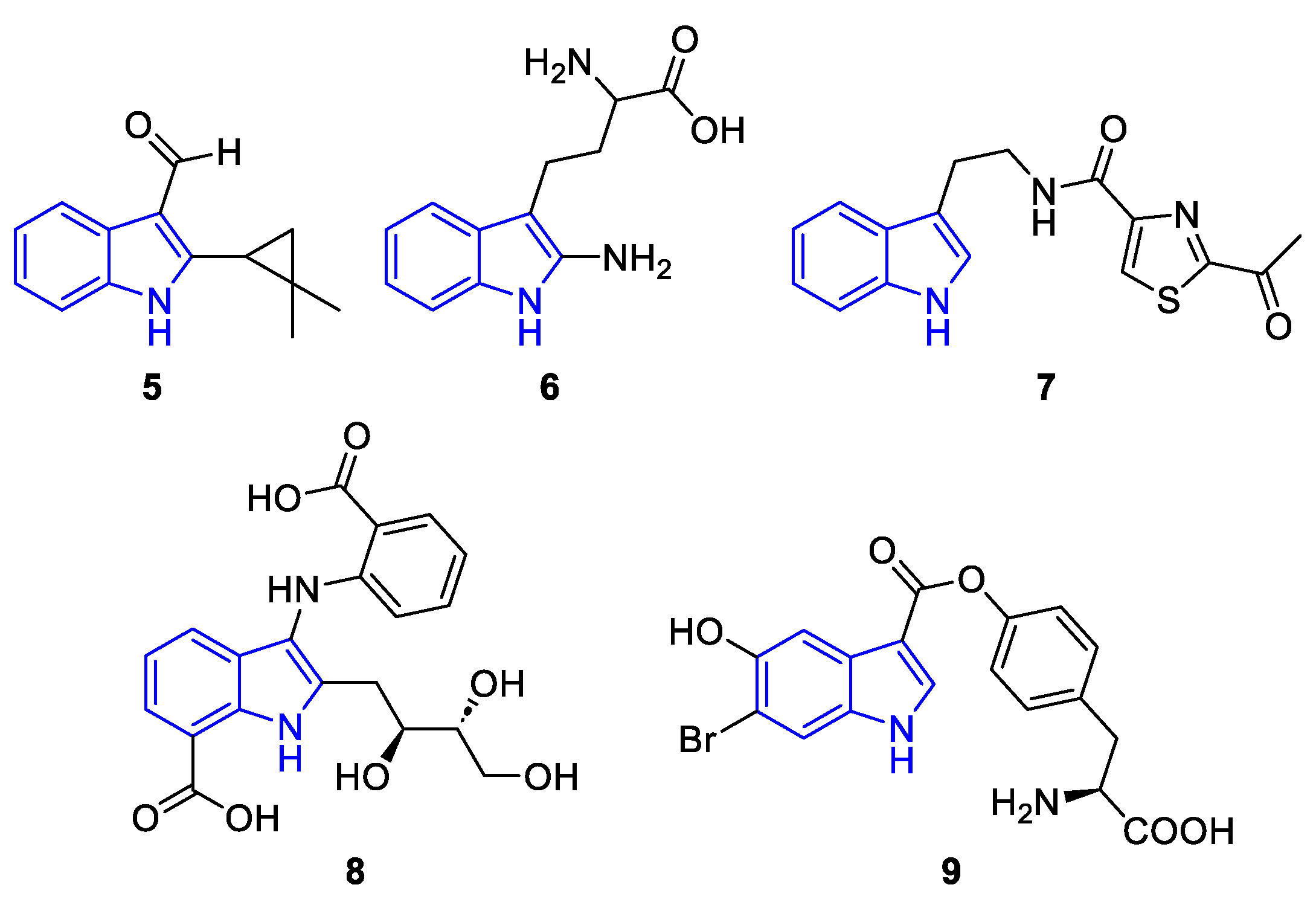
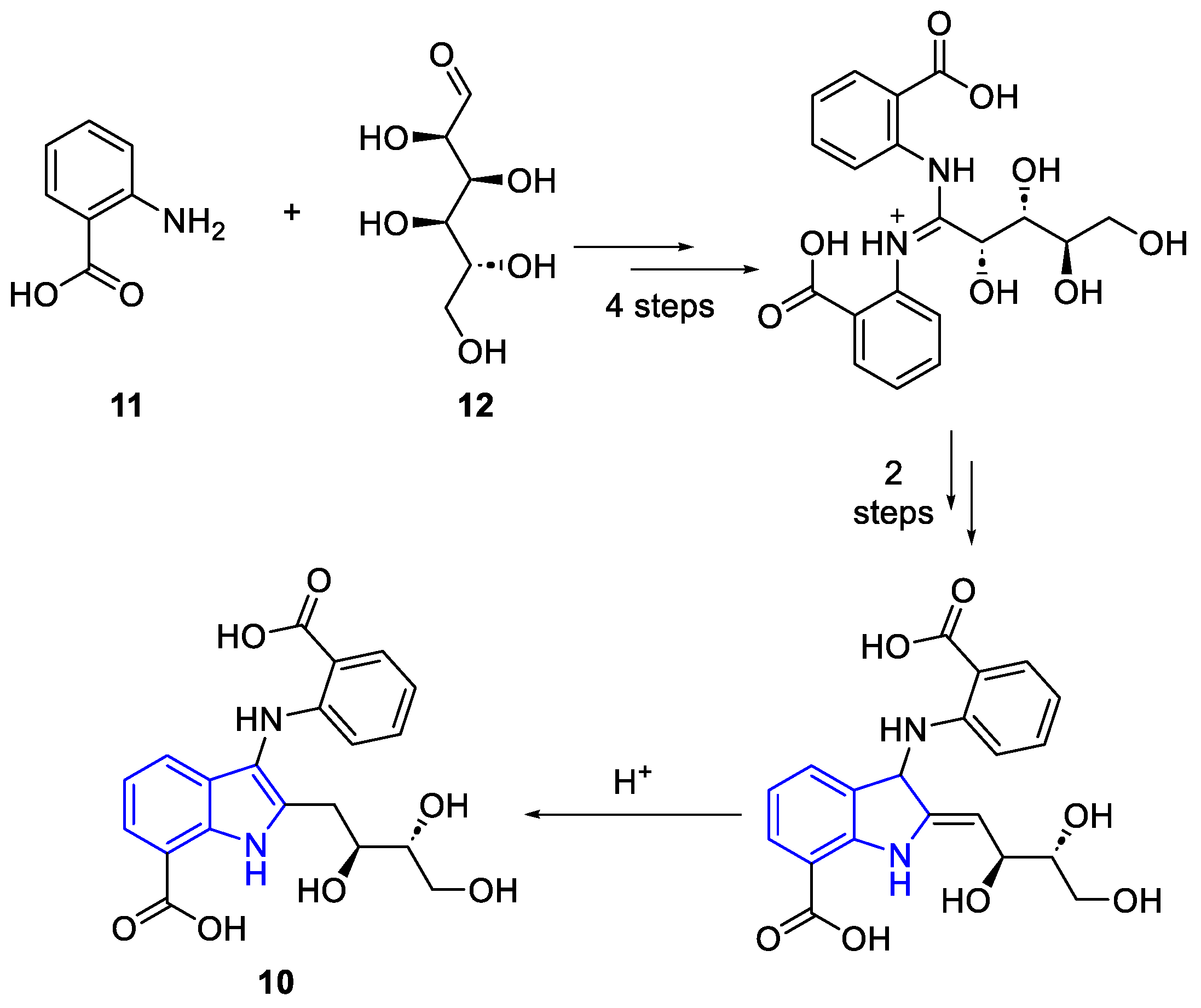
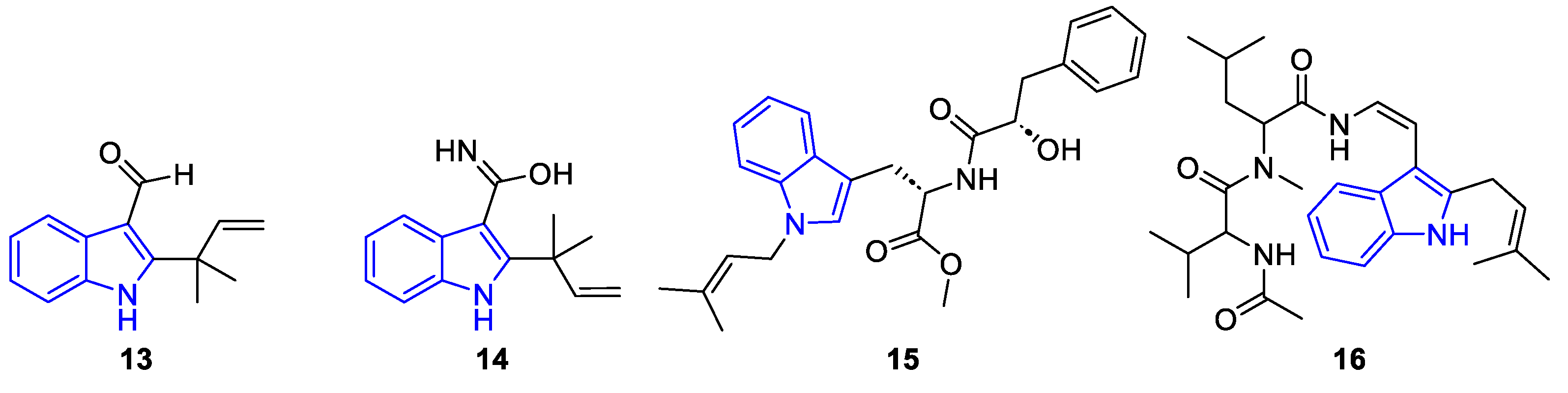
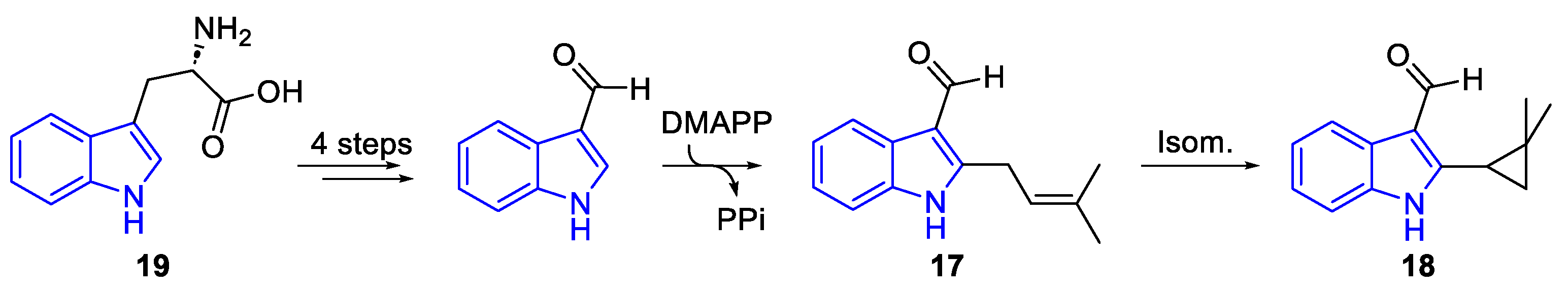
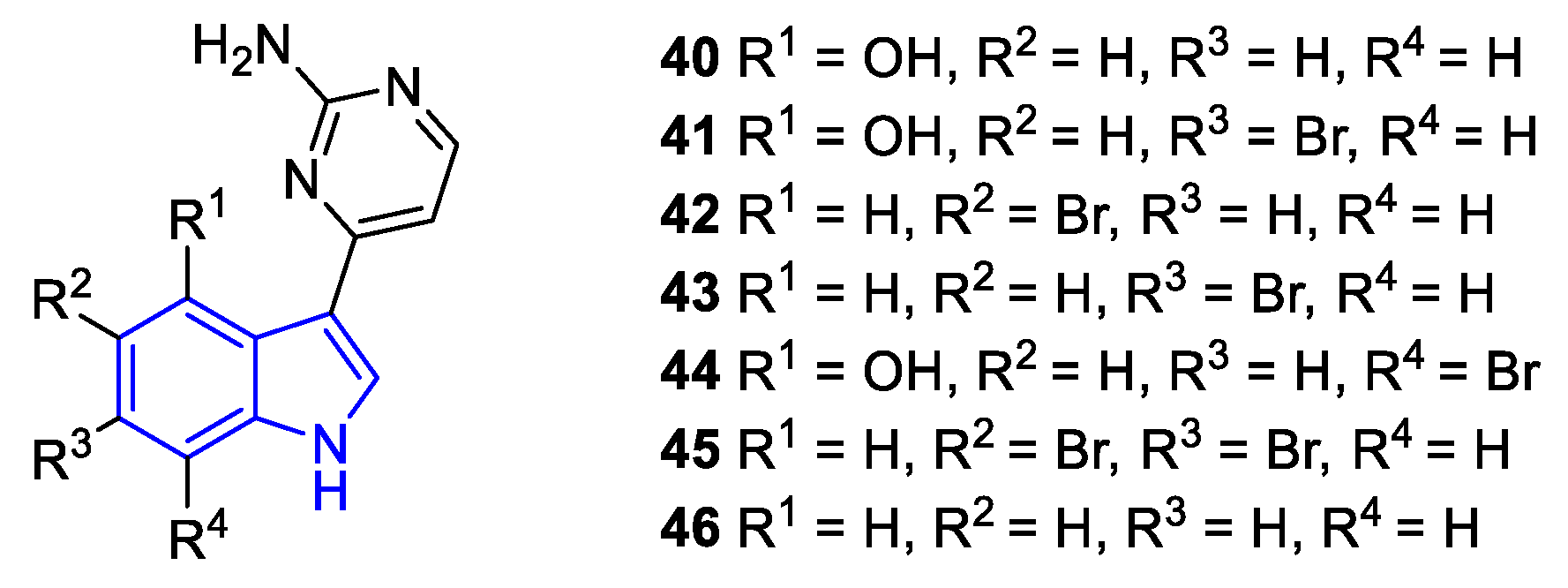
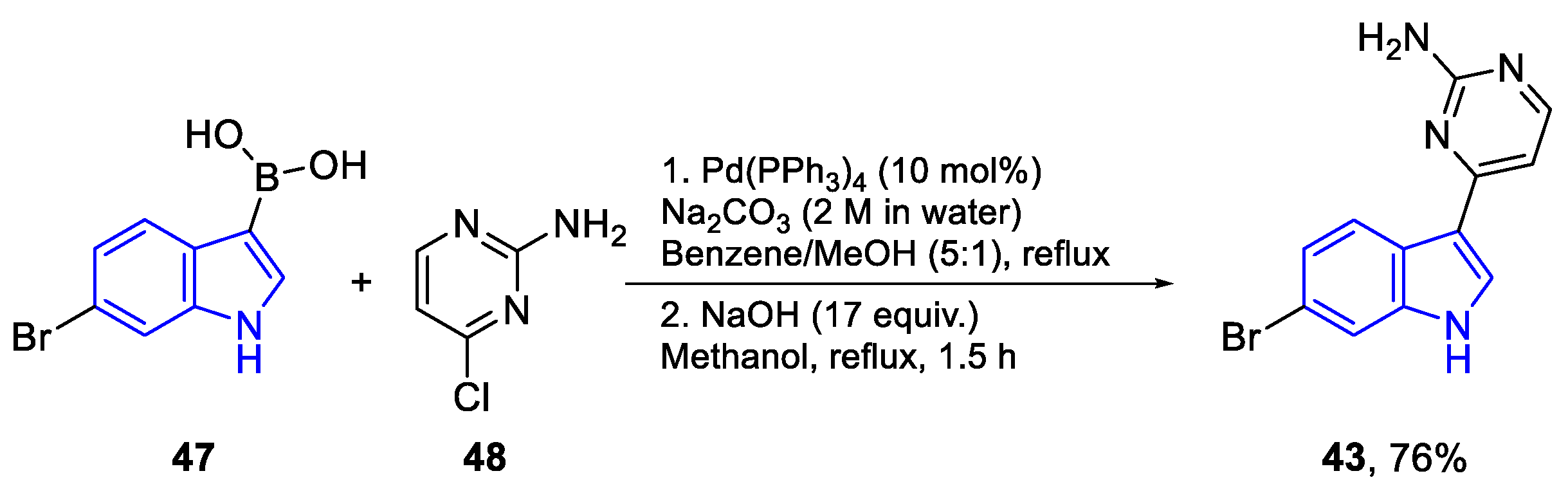
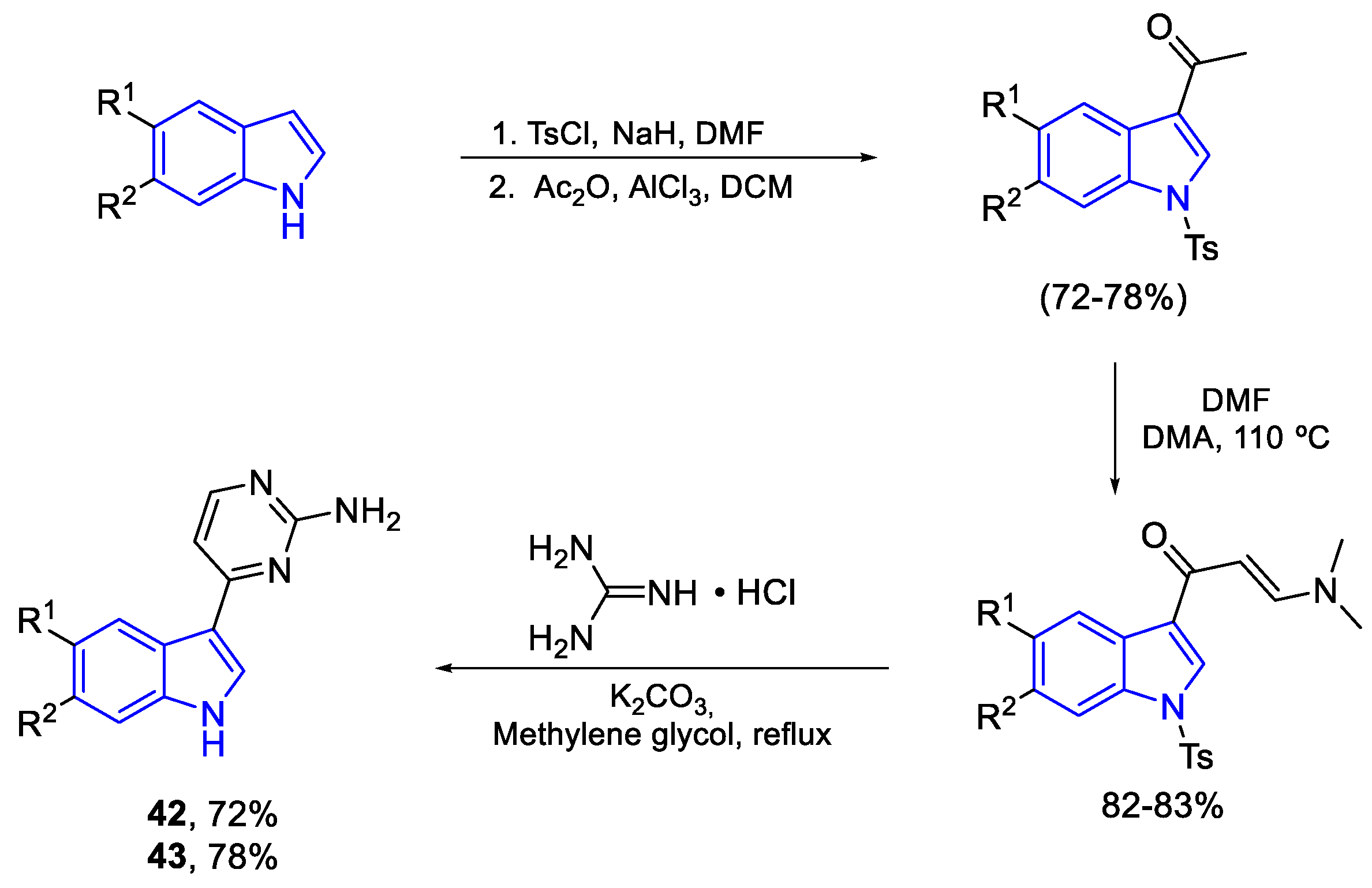
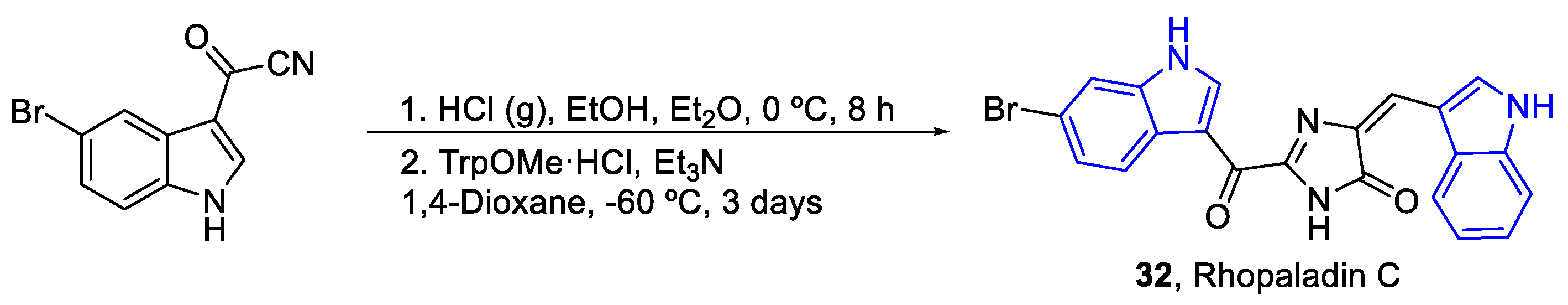
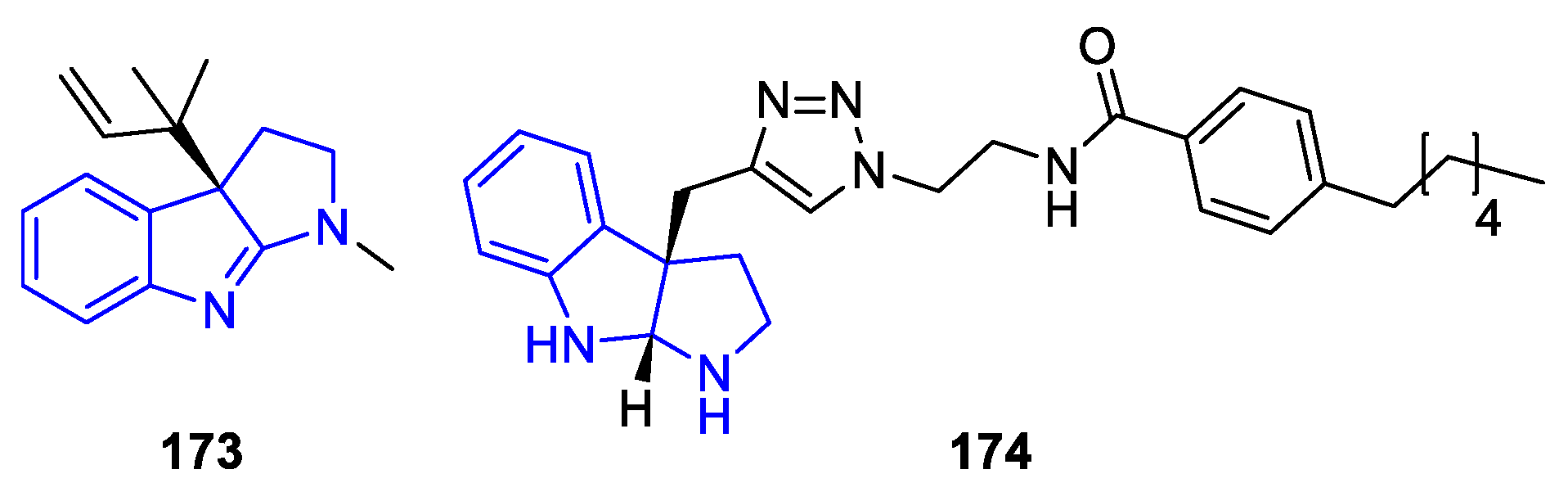
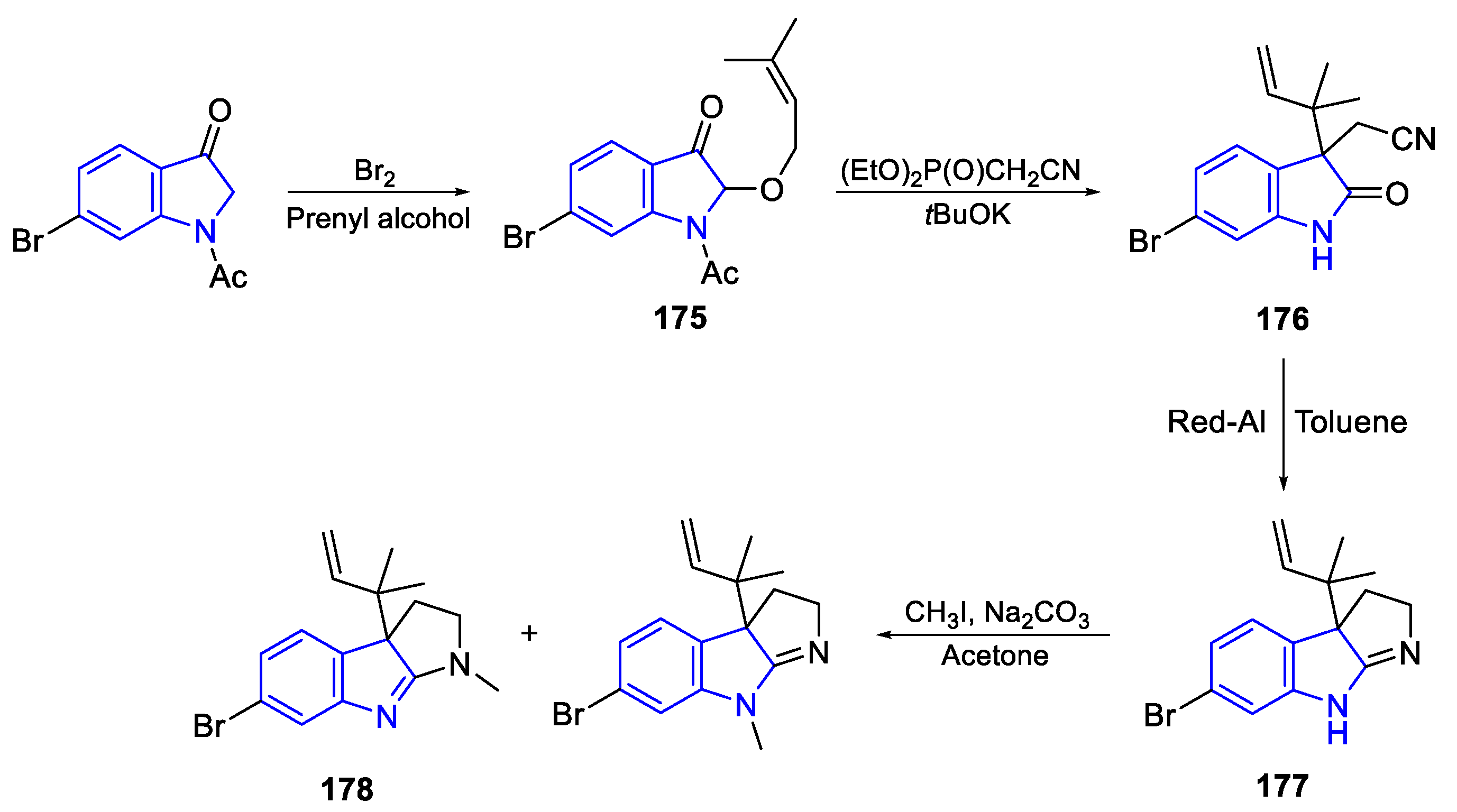
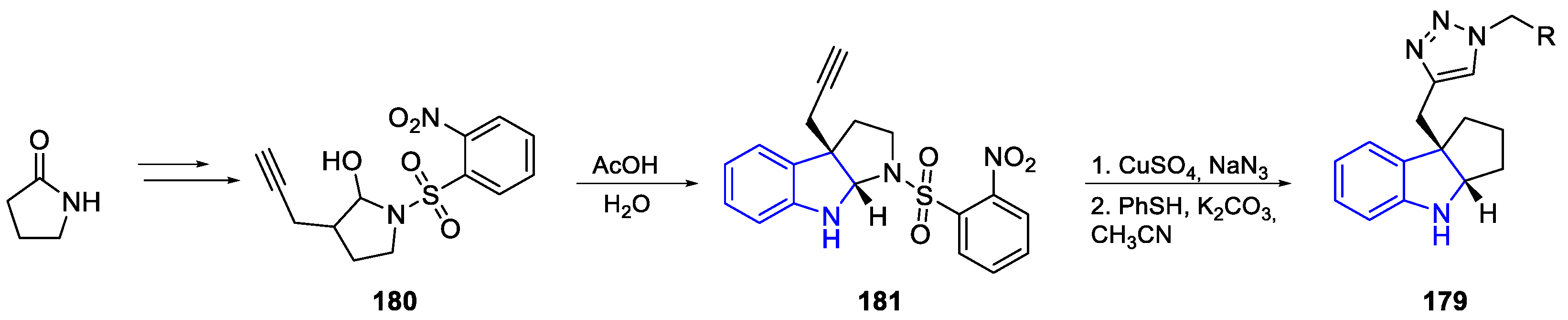
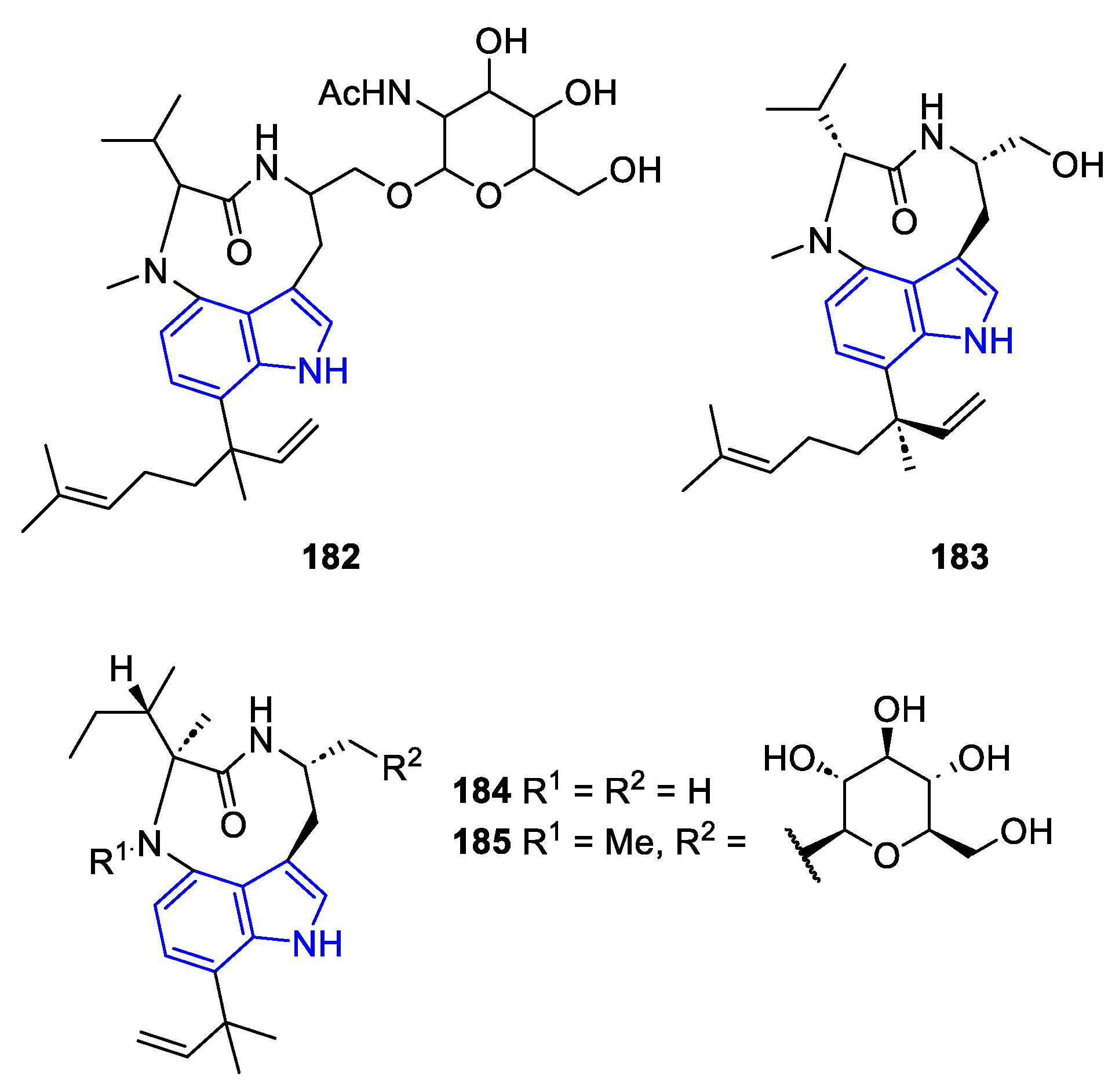

| Meridianin | Anticancer effects |
Prevention of Alzheimer’s Disease |
Antimalarian effects |
Antitubercular effects |
|---|---|---|---|---|
| A (40) | Hela | GSK-3β, CK1δ, Dyrk1A and CLK1 2 | P. falciparum | nd 1 |
| B (41) | PTP, Hep2, U937, LMM3 | nd | nd | |
| C (42) | PTP, Hep2, HT29, RD, U937, LMM3, Hela, MDA-MB-231, A549 | P. falciparum | M. tuberculosis | |
| D (43) | PTP, Hep2, HT29, RD, U937, LMM3, Hela, A549 | nd | M. smegatis 3 | |
| E (44) | PTP, Hep2, U937, LMM3 | nd | nd | nd |
| F (45) | Hep2, U937, LMM3 | nd | nd | nd |
| G (46) | Hela | Dyrk1A | P. falciparum | M. tuberculosis |
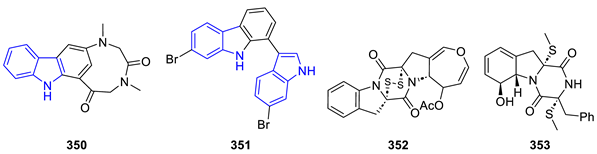 | ||
| Name annelated Indol | Structure | Cytotoxicity [Reference] |
| Antipathine A | 350 | SGC-791, Hep-G2 [256] |
| Indolyl-carbazole | 351 | HL-60, HeLa [257] |
| Deoxyapoaranotin | 352 | HCT-116 1 [258] |
| Phomazine B | 353 | HL-60, HCT-116, K562, MGC-803, A549 [259] 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





