Introduction
Prescribed burning, recognized as controlled or planned fire, has been an integral part of woodland ecosystems since ancient times, serving as a fundamental natural process [
1]. The deliberate initiation of fires within designated areas under specific conditions, prescribed fire aims to manipulate fire behavior to achieve specific effects, demanding continuous consideration before, during, and after the fire event [
2]. In the forests of the mid-western United States, a noticeable shift away from oak dominance is observed [
3]. As resettlement has led to a continuous decline in woodland extent, the successful regeneration of oak forests relies on maintaining sufficient oak seedling densities in anticipation of future canopy disturbances [
4]. The prevailing fire regimes in the eastern United States exert significant influence over the composition, function, and structure of ecosystems where oaks predominate [
5].
Fire serves as a crucial factor in sustaining oak-hickory forests by inducing selective mortality or modifying the growth rates of surviving trees [
6]. While the immediate stress imposed by fire is characterized by high temperatures, long-term exposure can lead to cambial injury, subsequently increasing susceptibility to insect attacks and diseases [
7]. The insulating properties of tree bark shield cambial tissues from the intense heat generated by fires, and the thickness of bark, which increases with tree size, correlates with fire resistance [
8]. Factors such as bark heat capacity, sprouting ability as seedlings, and storage capacity further contribute to the overall fire resistance of tree species [
9]. However, the continuous occurrence of fires has altered the species composition within oak woodlands, leading to the emergence of new plant species. Oak seedlings suffer mortality and injury under continuous fire conditions, impacting the overall ecosystem. Fire exhibits both short- and long-term effects on tree mortality, with periodic fires positively influencing oak saplings, regeneration, and leaf production. In contrast, continuous fire negatively affects leaf production and the count of oak saplings [
10]. In the oak woodlands subjected to continuous fire, there is a notable emergence of sassafras, prompting the necessity for further research to understand the changing dynamics of oak woodlands and the influence of sassafras on the ecosystem.
The oak, a sizable broad-leaf tree, thrives abundantly in temperate climate zones across the northern hemisphere. Beyond its lignocellulosic composition, oak leaves boast elevated levels of cutins, suberins, and tannins, contributing to inhibitory effects on fungal growth [
11]. These additional constituents render oak litter less susceptible to microbial degradation. In contrast to low-molecular-weight phenolics, condensed tannins within oak leaves have been observed to impede decomposition and nitrogen mineralization [
12]. Notably, the litter from Quercus petraea, a species of oak, is recognized as one of the most recalcitrant types, displaying a slow decomposition rate [
13]. Comparatively, Quercus leaf litter, when juxtaposed with wood, exhibits higher nitrogen content, evident in their distinct carbon-to-nitrogen (C/N) ratios of 30–90 and 150, respectively. Additionally, the leaf litter contains a considerably greater abundance of p-hydroxyphenyl lignin units [
13].
Sassafras exhibits a marked aversion to shaded environments, displaying limited seed reproduction within densely canopied forests. Nevertheless, it thrives as a pioneer species in abandoned fields and readily colonizes disturbed or early-stage secondary forests. The removal of the overarching forest canopy, achieved through various means like controlled burns, contributes to an increase in sassafras species density. Conversely, in the face of escalating competition and the gradual closure of forest canopies, the significance of sassafras diminishes. Although relatively rare, the species endures in old-growth stands. Sassafras displays adaptability across elevations, ranging from a few meters above sea level to as high as 1,220 meters [
14]. To gain a comprehensive understanding of sassafras dynamics in the continually burned woodland, it becomes imperative to evaluate specific leaf weight, leaf decomposition rates, sapling density, and chlorophyll levels.
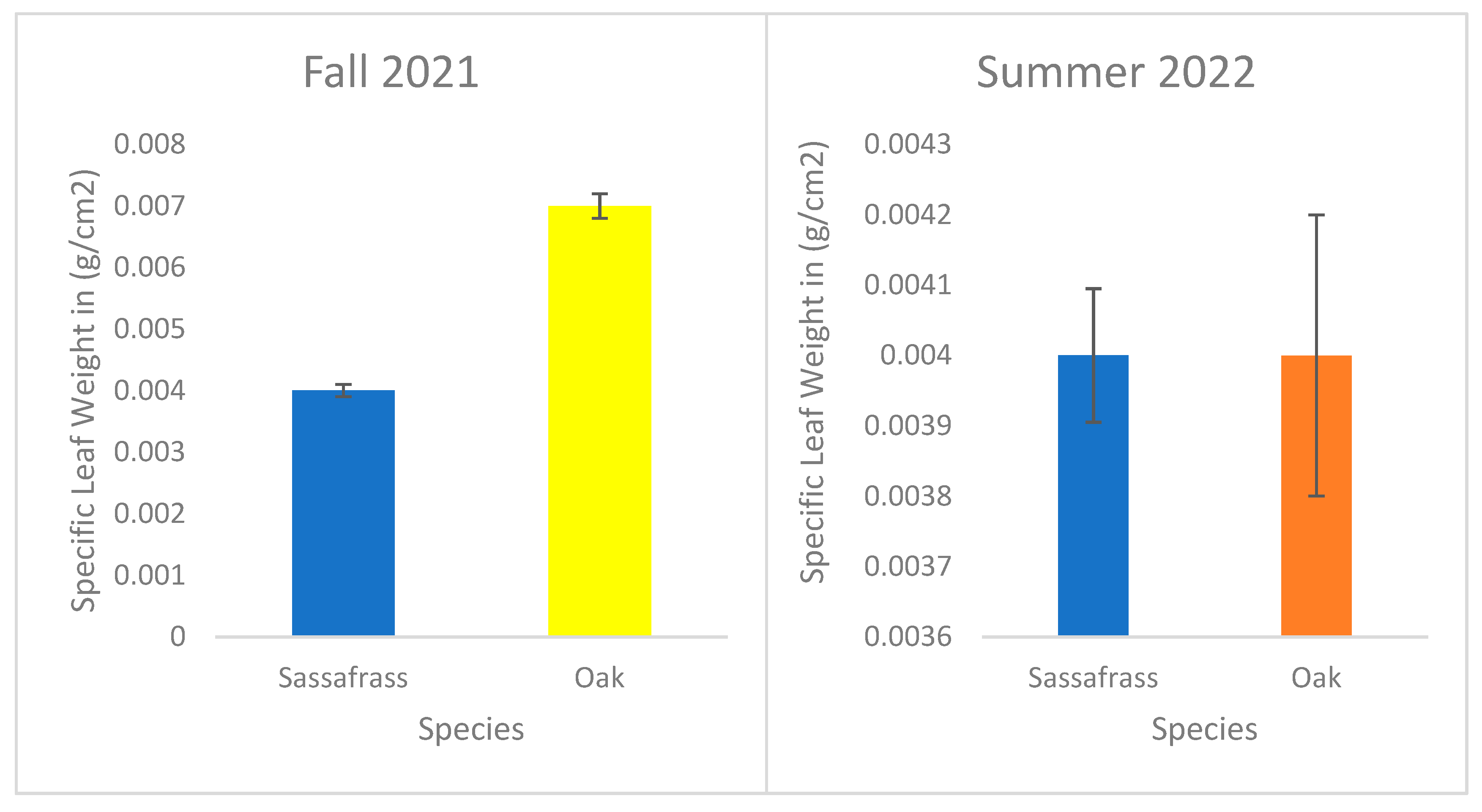
Specific leaf weight (SLW), denoting the leaf mass per unit leaf area (g/cm²), serves as a pivotal metric illuminating a plant’s resource allocation strategy, photosynthetic capacity, and overall vitality. The variability in SLW offers insights into a species’ adaptive responses to diverse environmental conditions, shedding light on its seasonal adjustments. In this study, two prevalent tree species were chosen, anticipating differences in growth patterns and physiological traits. The positive correlation between specific leaf weight (leaf weight/leaf area) and photosynthesis per unit leaf area has been observed across various genotypes [
15]. Photosynthesis on a leaf area basis typically exhibits positive associations with nitrogen content, protein levels, and photosynthetic enzyme concentrations per unit of leaf area [
16]. Consequently, the heightened photosynthesis on a leaf area basis for leaves with high specific leaf weight is likely attributed to a more concentrated presence of the photosynthetic apparatus per unit leaf area. The allocation of dry matter to leaf area plays a critical role in determining plant growth rates during early developmental stages. Previous research [
17] has indicated that species with lower specific leaf weight values tend to exhibit higher relative growth rates, presumably because, for an equivalent amount of leaf dry matter, plants with lower specific leaf weight boast a greater leaf area, enabling them to intercept more light.
Leaf litter decomposition plays a pivotal role in nutrient cycling within terrestrial ecosystems [
18]. This intricate process encompasses leaching, mechanical breakdown, digestion by saprophagous soil organisms, and the enzymatic degradation of chemical compounds by saprotrophic microbiota [
19]. In the natural woodland setting, leaf litter is typically a diverse mixture contributed by various plant species annually. However, many managed forests, particularly monocultures, witness leaf litter predominantly composed of a single species. The initial rate of decomposition is commonly associated with crucial nutrients such as nitrogen (N) and phosphorus (P), both recognized as limiting factors for microbial litter decomposition [
20]. As decomposition progresses to the later stages, the composition of the litter’s carbon source may undergo alterations, particularly as easily degradable carbohydrates become depleted. During this phase, the degradation rate of lignin assumes a prominent role in the decomposition process. Consequently, the limiting factors may vary, with elevated nitrogen concentrations potentially inhibiting the formation of the ligninase system in various white-rot fungi [
21]. Conversely, manganese (Mn) can enhance decomposition rates due to its role as a cofactor for Mn-peroxidase, a crucial enzyme in the ligninase system [
22].
The investigation of decay processes at the soil surface often employs the widely adopted litterbag method. This method involves placing fresh leaf litter into designated bags, which are then inserted into the soil’s litter layer. Periodic retrieval of these bags allows for the measurement of the remaining litter quantity. The selection of mesh size is a critical consideration, aiming to strike a balance between facilitating organism entry to the litter and minimizing excessive particle loss. Researchers frequently experiment with varying mesh sizes in litterbags to manipulate microbial composition and observe its effects on the decay dynamics [
23]. Opting for a minute mesh size not only excludes specific organisms but also serves as an effective deterrent against particle loss reaching the mineral soil. In environments with concentrated sunlight, such as areas with ample light exposure, the utilization of fiberglass mesh emerges as a practical recommendation. This is due to the susceptibility of nylon and other materials to degradation under prolonged exposure to UV light, making fiberglass a more durable alternative in such settings [
24]. There existed a notable gap in the examination of sassafras ecology within the context of the continuously burned site. This research aimed to fill this void by conducting an evaluation of both sapling density, specific leaf weight, physiology and mass loss resulting from decomposition in the oak-hickory woodland subject to continuous fire application. The continuous application of fire occurred at regular 2–3-year intervals, commencing in the year 1980 [
10].
Data Collection
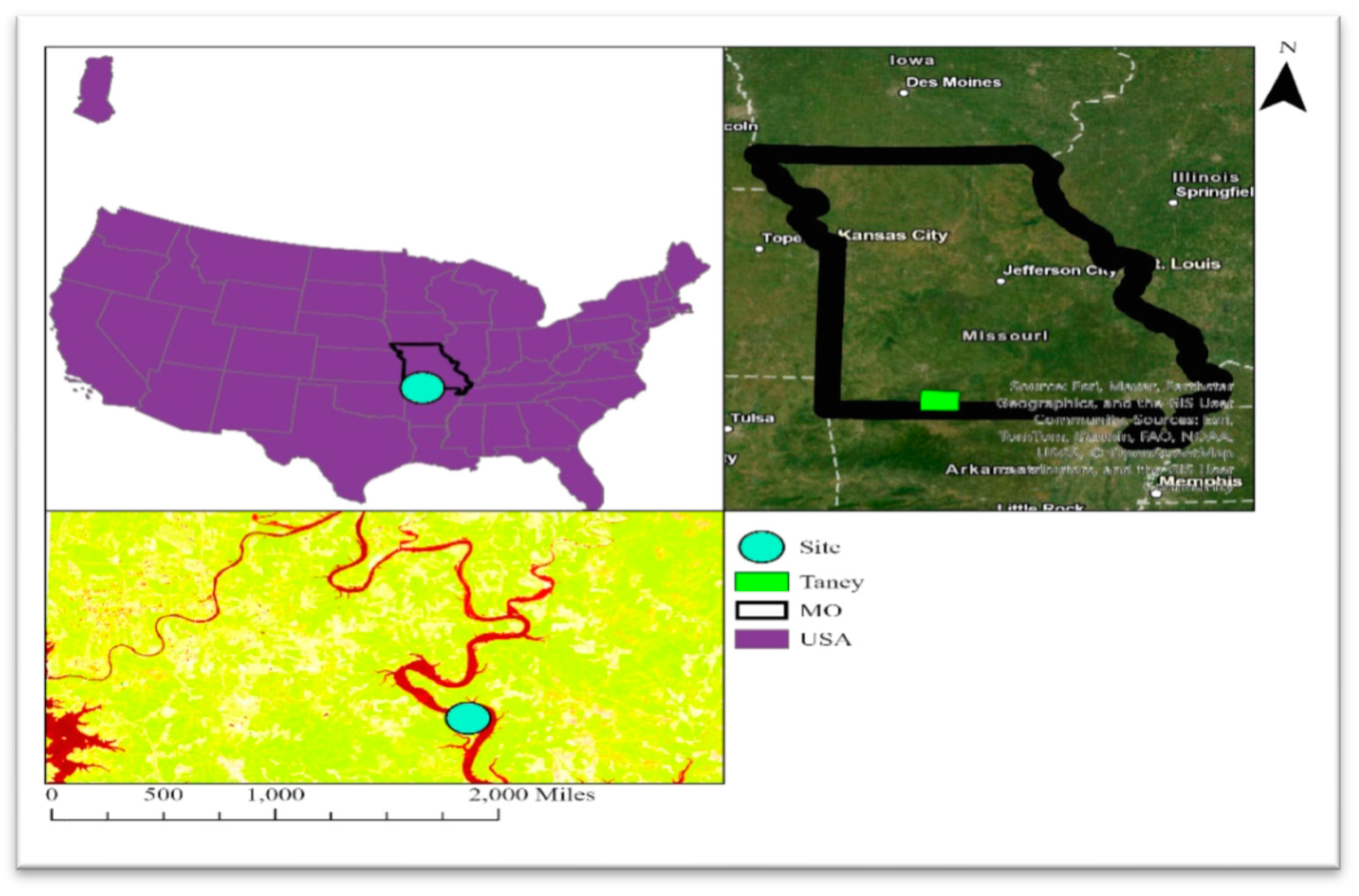
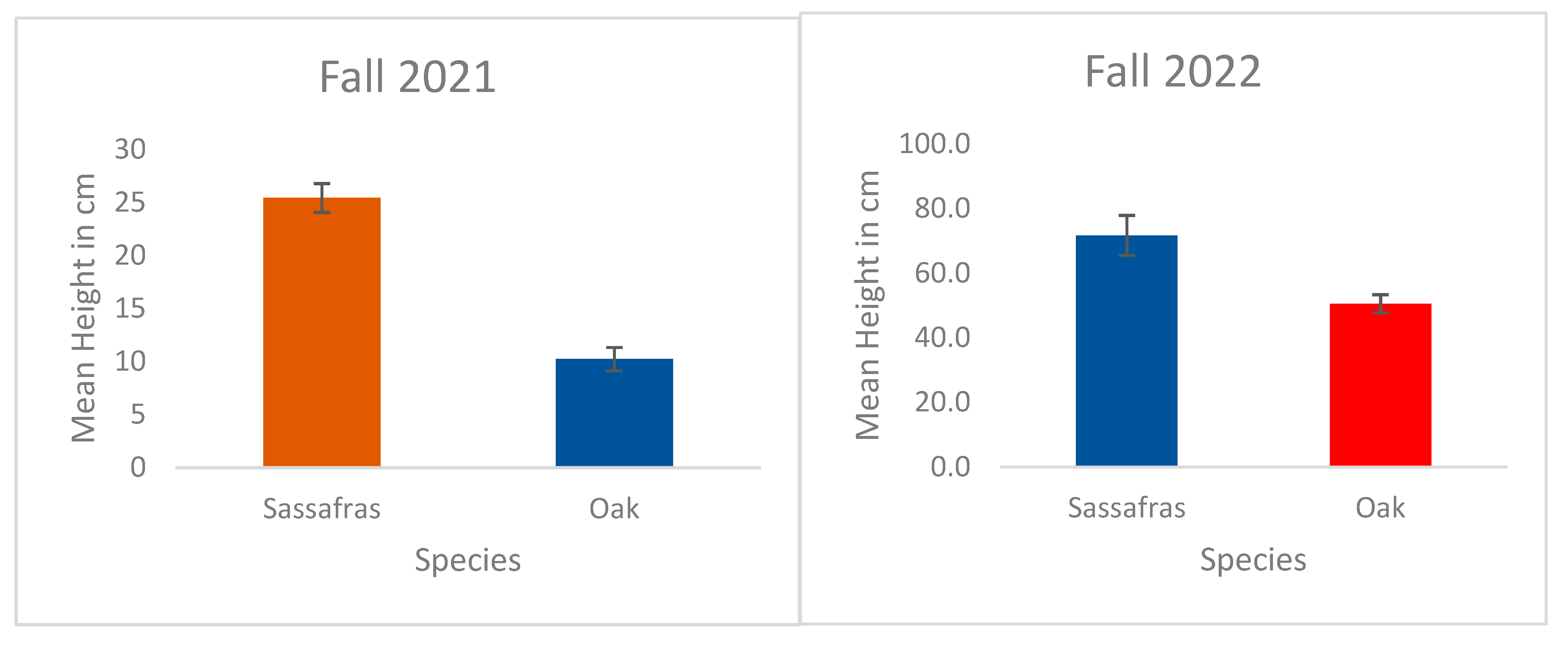
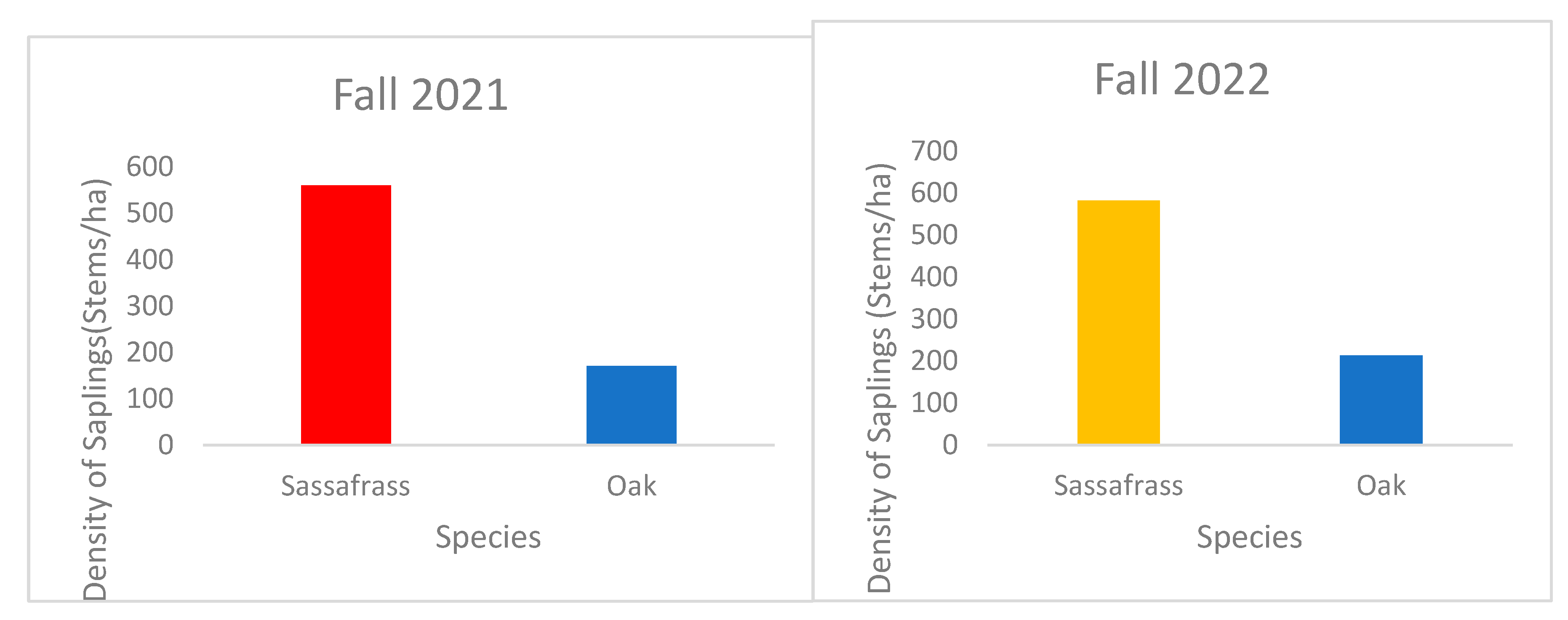
A 0.1ha linear transect was made in the reference plot. Ten blocks were made within the linear transects, and the sapling count was done for the sassafras and oak in fall 2021 and 2022. Saplings density was estimated based on the count. Saplings’ height of oak and sassafras was evaluated in the 0.1 ha transects using a cm-scale attached to the PVC tube. The leaves were collected in October 2022 for the specific leaf weight. A sharp knife was used, and the leaves were collected haphazardly, and were placed in zip-lock bags and placed in cooler, and finally brought to the lab. The leaf area was estimated by leaf area meter, and for each leaf, the area was measured three times, and then the average was calculated. After measuring the leaf area, all leaves were oven-dried at 80°C to a constant mass to remove any excess moisture on the leaves before being weighed again. Specific leaf weight was calculated by dividing the oven-dried weight of the leaf by its corresponding leaf area.
MC-100 Chlorophyll Concentration Meter (Apogee Instruments, Inc., Logan, UT, USA) was used for the chlorophyll concentration measurement. Clipping the sensor onto the leaves was done for the measurement. Measurement was done on the different saplings’ leaves. Selection was done haphazardly. In summer 2022 leaves of oak and sassafras were collected for the water potential measurement. Water potential was measured using a Scholander pressure bomb. Leaves were collected haphazardly, and leaf petiole were used for the estimation of water potential. Leaves without any deformation or visible stress were used.
The leaf collection was again done in December 2021 for the decomposition rate estimation based on time. A comparison of the decomposition rate was done between oak and sassafras. Leaves were kept for air drying for three months, and in March 2022, leaves were placed in a litter bag of 20 cm × 20 cm in size made with 1-2mm mesh were used. This mesh size prevents interference due to macro-fauna but allows litter colonization with microbes. Litter bags filled with 8 g of air-dried white oak and sassafras leaves. The random blocks of 110m length and 5m width were laid in BSFS. Altogether, six blocks were made in two rows. Each block was separated by 25m. In each block, 30 litter bags total of which 15 was for oak and 15 was for sassafras were randomly placed 15-20cm below the soil surface. Leaves were left to decompose for two to eight months (April 1 to November 30) in the year 2022. Decomposed leaves were collected at five collection intervals that included 61 days (June 1), 105 days (July 15), 153 days (August 12), 198 days (October 15), and 244 days (November 30)—the experimental period ended after 244 days (November 30, 2022). After collection, the samples were oven-dried at 800C until constant weight. After drying, the air-dried plant material was carefully extracted from each litter bag. All the non-plant materials were removed. The remaining air-dried plant material was weighed to calculate the leaf litter decomposition rate.
Discussion
Patterns of forest development are dependent on the legacies of the past. Oak is the dominating woodland in the Ozark, Missouri [
26]. The decrease in stem densities observed, indicative of self-thinning, was consistent with expectations. However, the notable increase in mortality among smaller trees in the reference plots provided evidence in favor of the theory that continuous burning expedited stand development by facilitating stem exclusion processes [
26]. The site’s capacity to support trees is inherently tied to the size of those trees, leading to an expected pattern of density-dependent mortality as stands mature and individual trees increase in size, thereby ensuring predictability in ecosystem dynamics [
27]. However, oak woodland management is complex, and fire is the only tool for its management [
26]. Numerous land managers within the Missouri Ozarks are expressing keen interest in employing prescribed burning as a tool to rejuvenate woodland ecosystems on lands with a historical precedent of regular fire occurrences [
28]. While prescribed burning offers a potential means to rapidly achieve desired structural and compositional goals, there remains a significant gap in our understanding regarding the exclusive impacts of fire on forest development. Periodic fire in woodland was more effective for management than the reference and control plots [
10]. The leaf productivity of oak was found to be higher in the periodically burned plots than the other [
10], which is consistent with our analysis as in reference sites, lots of sassafras saplings were observed. This analysis showed that the oak woodland’s reference site fire application system benefits sassafras rather than oak. The sapling’s density, height, leaf decomposition, and specific leaf weight were all observed to be high for sassafras. If it continues, it will alter the soil composition and overall ecosystem. Further research on the soil composition, leaf nutrient analysis of sassafras, and general soil nutrient cycle is needed to understand the reference plots better. The appropriate method for sassafras reduction is required, or the periodic fire is only needed on Ozark for oak management as periodic fire favors the oak saplings and overall leaf production.