Submitted:
16 February 2024
Posted:
19 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Collection and Extraction of Campsis radicans L. Plant Samples
2.2. Determination of Campsis radicans L. Plant’s Mineral Content
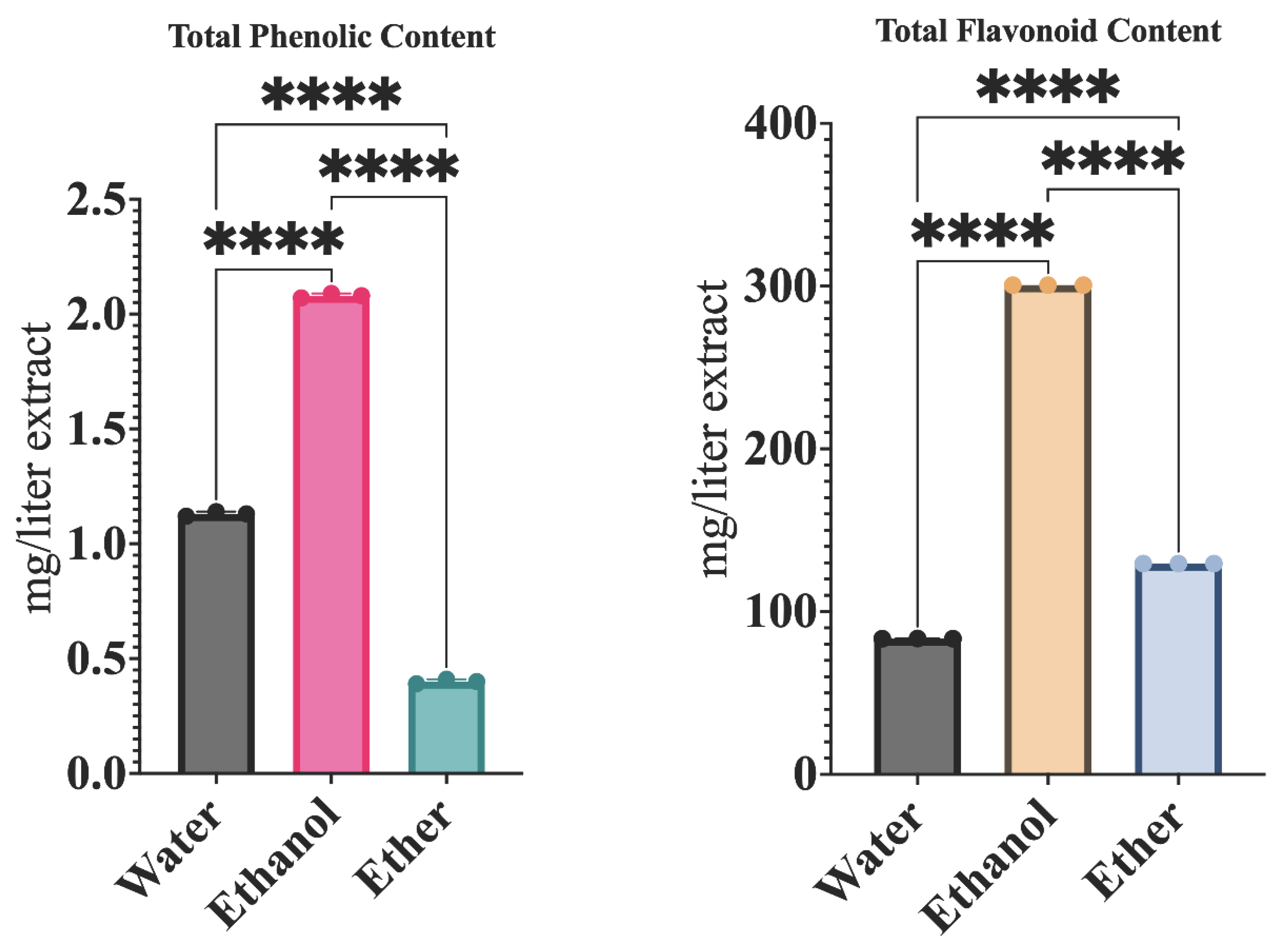
2.3. Determination of Total Phenolic Content
2.4. Determination of Total Flavonoid Content
2.5. Determination of antioxidant capacity of Campsis radicans L. plant extracts
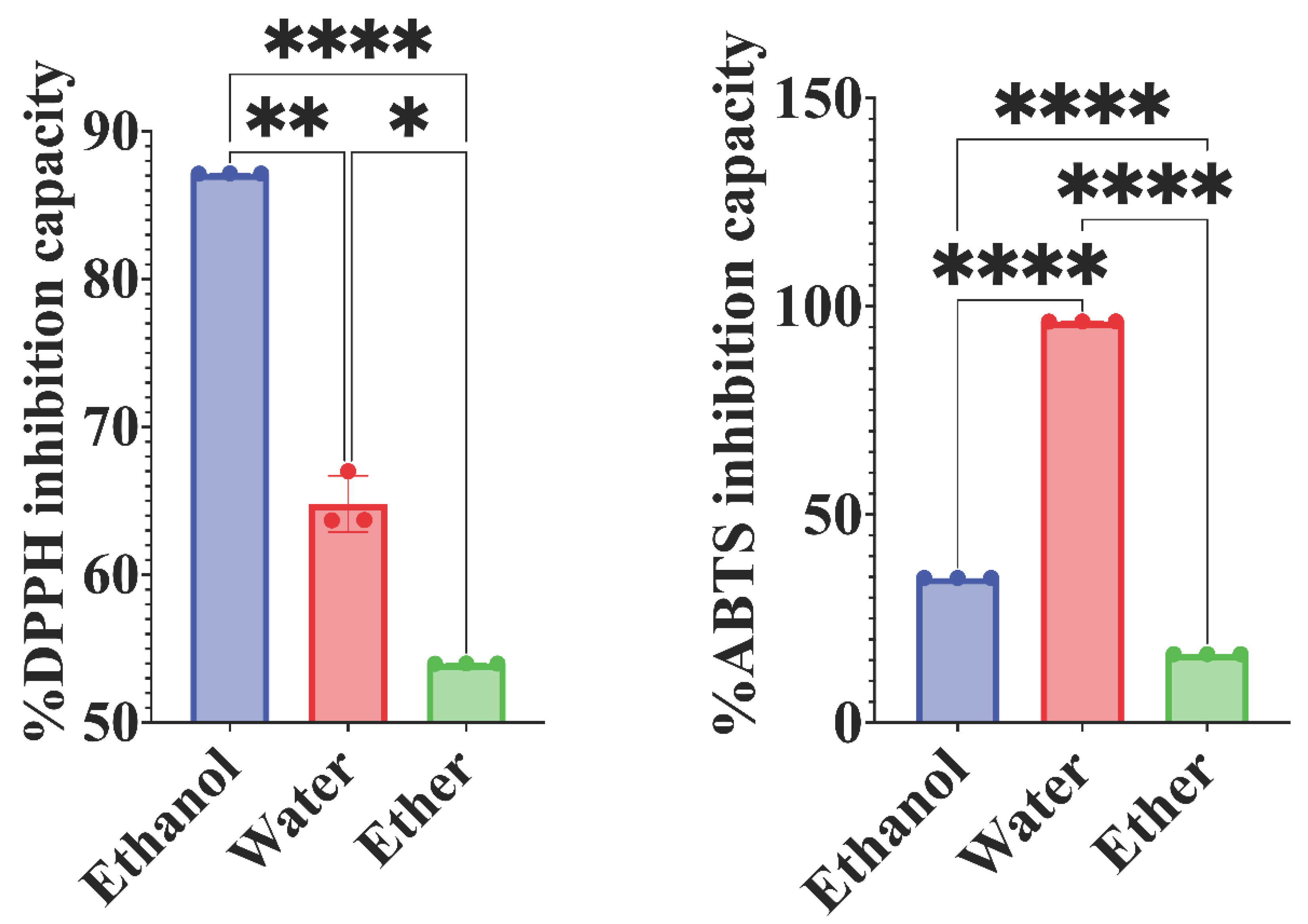
2.5.1. DPPH method
2.5.2. ABTS method
2.6. Cell culture
2.7. Cell Proliferation and Viability
2.8. MTT test
2.9. ADMET and drug similarity estimation for ligands
2.10. Finding and Preparing the Target Protein
2.11. Molecular Docking
2.12. Statistical analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singh, M.; Kumar, D.; Sharma, D.; Singh, G. TYPHONIUM FLAGELLIFORME: A MULTIPURPOSE PLANT. Int. Res. J. Pharm. 2013, 4, 45–48. [Google Scholar] [CrossRef]
- Nobili, S.; Lippi, D.; Witort, E.; Donnini, M.; Bausi, L.; Mini, E.; Capaccioli, S. Natural Compounds for Cancer Treatment and Prevention. Pharmacol. Res. 2009, 59, 365–378. [Google Scholar] [CrossRef]
- Jing, L.; Mohamed, M.; … A.R.-J. of M.; 2010, undefined Phytochemicals, Antioxidant Properties and Anticancer Investigations of the Different Parts of Several Gingers Species (Boesenbergia Rotunda, Boesenbergia. academia.eduLJ Jing, M Mohamed, A Rahmat, MFA BakarJournal of Medicinal Plants Research, 2010•academia.edu.
- Research, H.M.-J. of M.P.; 2010, undefined Studies of the Anticancer Effect of Sesquiterpene Lactone from Carpesium Rosulatum. academicjournals.org.
- Ghavami, G.; Sardari, S.; Res, M.S.-J.M.P.; 2010, undefined Anticancerous Potentials of Achillea Species against Selected Cell Lines. academicjournals.orgG Ghavami, S Sardari, MA ShokrgozarJ Med Plants Res, 2010•academicjournals.org 2010, 4, 2411–2417.
- Cho, W. Supportive Cancer Care with Chinese Medicine; 2010. [Google Scholar]
- Borrelli, F.; Capasso, R.; … A.R.-A. pharmacology; 2004, undefined Green Tea and Gastrointestinal Cancer Risk. Wiley Online LibraryF Borrelli, R Capasso, A Russo, E ErnstAlimentary pharmacology & therapeutics, 2004•Wiley Online Library 2004, 19, 497–510. [CrossRef]
- Sakarkar, D.; Deshmukh, V. Ethnopharmacological Review of Traditional Medicinal Plants for Anticancer Activity. 2011.
- Azaizeh, H.; Saad, B.; Khalil, K.; … O.S.--B.C. and; 2006, undefined The State of the Art of Traditional Arab Herbal Medicine in the Eastern Region of the Mediterranean: A Review. hindawi.comH Azaizeh, B Saad, K Khalil, O SaidEvidence-Based Complementary and Alternative Medicine, 2006•hindawi.com 2006, 3, 229–235. [CrossRef]
- Wen, J.; Jansen, R.K. Morphological and Molecular Comparisons of Campsis Grandiflora and C. Radicans (Bignoniaceae), an Eastern Asian and Eastern North American Vicariad Species Pair. Plant Syst. Evol. 1995, 196, 173–183. [Google Scholar] [CrossRef]
- Uddin, M.; of, M.H.-J. of the A.S.; 2016, undefined Plant Diversity of Dhaka University Campus, Bangladesh. researchgate.netMZ Uddin, MA HassanJournal of the Asiatic Society of Bangladesh, Science, 2016•researchgate.net 2016, 42, 49–68. [CrossRef]
- Moore, M. Medicinal Plants of the Desert and Canyon West; UNM Press, 1989; ISBN 0890135916. [Google Scholar]
- Islam, M.; Jannat, T.; Kuddus, Md.R.; Rashid, M.A.; Haque, M.R. In Vitro and in Vivo Evaluation of Pharmacological Potentials of Campsis Radicans L. Clin. Phytoscience 2019, 5. [Google Scholar] [CrossRef]
- Capanoglu, E.; De Vos, R.C.H.; Hall, R.D.; Boyacioglu, D.; Beekwilder, J. Changes in Polyphenol Content during Production of Grape Juice Concentrate. Food Chem. 2013, 139, 521–526. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT - Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Karacaer, N.T.; Kerimoğlu, B.; Baran, T.; Tarhan, M.; Menteş, A.; Öztürk, K. Antitumor and Apoptotic Effects of New-Generation Platinum Compounds on Human Leukemia Cell Lines HL-60 and K562. Biol. (Bratisl) 2022, 77, 249–260. [Google Scholar] [CrossRef]
- Bestwick, C.S.; Milne, L. Influence of Galangin on HL-60 Cell Proliferation and Survival. Cancer Lett. 2006, 243, 80–89. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Ntie-Kang, F.; Lifongo, L.L.; Mbah, J.A.; Owono Owono, L.C.; Megnassan, E.; Mbaze, L.M.; Judson, P.N.; Sippl, W.; Efange, S.M.N. In Silico Drug Metabolism and Pharmacokinetic Profiles of Natural Products from Medicinal Plants in the Congo Basin. Silico Pharmacol. 2013, 1. [Google Scholar] [CrossRef] [PubMed]
- Zoete, V.; Daina, A.; Bovigny, C.; Michielin, O. SwissSimilarity: A Web Tool for Low to Ultra High Throughput Ligand-Based Virtual Screening. J. Chem. Inf. Model. 2016, 56, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Youle, R.J.; Tjandra, N. Structure of Bax: Coregulation of Dimer Formation and Intracellular Localization. Cell 2000, 103, 645–654. [Google Scholar] [CrossRef]
- Souers, A.J.; Leverson, J.D.; Boghaert, E.R.; Ackler, S.L.; Catron, N.D.; Chen, J.; Dayton, B.D.; Ding, H.; Enschede, S.H.; Fairbrother, W.J.; et al. ABT-199, a Potent and Selective BCL-2 Inhibitor, Achieves Antitumor Activity While Sparing Platelets. Nat. Med. 2013, 19, 202–208. [Google Scholar] [CrossRef]
- Fang, B.; Fu, G.; Agniswamy, J.; Harrison, R.W.; Weber, I.T. Caspase-3 Binds Diverse P4 Residues in Peptides as Revealed by Crystallography and Structural Modeling. Apoptosis 2009, 14, 741–752. [Google Scholar] [CrossRef]
- Trott, O.; chemistry, A.O.-J. of computational; 2010, undefined AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. Wiley Online LibraryO Trott, AJ OlsonJournal of computational chemistry, 2010•Wiley Online Library 2010, 31, 455–461. [CrossRef]
- Bil Der, T.; StAnojkovIC-SebIC, A.; PIvIC, R.; joSIC, D.; DInIC, Z.; StAnojkovIC, A.; Author, C.; Formülasyonlarda Yaygın Olarak Kullanılan Seçilmiş Tıbbi Bitkilerin Ağır Metal İçeriği ESER BİLGİSİ Araştırma Makalesi Sorumlu Yazar, B. Heavy Metals Content in Selected Medicinal Plants Commonly Used As. dergipark.org.trA Stanojkovic-Sebic, R Pivic, D Josic, Z Dinic, A StanojkovicJournal of Agricultural Sciences, 2015•dergipark.org.tr 2015, 21, 317–325. [CrossRef]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant Activity and Phenolic Compounds of 112 Traditional Chinese Medicinal Plants Associated with Anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. ACS Publ. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Ghose, A.; … V.V.-J. of combinatorial; 1999, undefined A Knowledge-Based Approach in Designing Combinatorial or Medicinal Chemistry Libraries for Drug Discovery. 1. A Qualitative and Quantitative Characterization Of. ACS Publ. 1999, 1, 55–68. [CrossRef]
| Element | Campsis radicans L. Heavy Metal Concentrations (mg/kg) | Normal Concentrations in Plants (mg/kg) | Toxic Concentrations in Plants (mg/kg) |
|---|---|---|---|
| As | Not Detected | 10-60 | <2 |
| Cd | Not Detected | <0.1-1 | 10 |
| Co | Not Detected | 0.05-0.5 | 30-40 |
| Cr | 119.66 ± 0,06 | 0.1-1 | 2 |
| Cu | 1372.9 ± 4.59 | 3-15 | 20 |
| Fe | 5742.15 ± 3.24 | 50-200 | >500 |
| Mn | 31.92 ± 0.16 | 15-100 | 400 |
| Mo | 1.92 ± 0.17 | 0.1-0.2 | >0.2 |
| Ni | 649.3 ± 1.8 | 0.1-5 | 30 |
| Pb | 3.51 ± 0.18 | 1.0-5.0 | 20 |
| Ti | Not Detected | 5-50 | >50 |
| V | 11.66 ± 0.03 | 0.5-1 | >1 |
| Zn | 25.62 ± 0.61 | 15-100 | 200 |
| Systematic name | Carbon number | PubChem CID |
|---|---|---|
| Pentadecanoic Acid | C 15:0 | 13849 |
| Palmitic Acid | C 16:0 | 985 |
| Stearic acid | C 18:0 | 5281 |
| Linoleic acid | C 18:2 ω6 | 5280450 |
| Protein | Ligands | Binding Energy (kj/mol) | Number of Hydrogen Bonds | Bonded Amino Acids |
|---|---|---|---|---|
| Bax | Levoglucosan | -3.7 | 3 | ASP 71 - LYS 119 |
| Propanoic acid, 2-hydroxy-, 1-methylethyl ester, (2S)- | -3.3 | 0 | PRO 88 - LEU 120 - ALA 124 - LEU 132 | |
| 4-Methyl-3,6,9-trioxadecan-1-ol | -3.4 | 3 | ARG 34 - LYS 119 | |
| 4-Vinylbenzoic acid | -5.2 | 1 | THR 85 - PRO 88 - LEU 120 - LYS 123 - ALA 124 - LEU 132 - ILE 136 | |
| Dimethyl malate | -3.3 | 3 | ARG 34 - LYS 119 | |
| Levoglucosenone | -3.8 | 2 | LYS 123 - THR 127 | |
| L-Proline, 1-(1-methylethyl)-5-oxo-, methyl ester | -3.7 | 4 | LYS 119 - LUE 122 - LYS 123 | |
| 4-Methoxyphenethyl alcohol | -4.3 | 0 | PRO 88 - LYS 123 - ALA 124 | |
| Trimethyl citrate | -3.8 | 5 | ILE 80 - LYS 119 - LYS 123 | |
| Bcl-2 | Levoglucosan | -4.3 | 2 | ALA 97 - GLY 142 |
| Propanoic acid, 2-hydroxy-, 1-methylethyl ester, (2S)- | -3.6 | 2 | ALA 97 - GLY 142 | |
| 4-Methyl-3,6,9-trioxadecan-1-ol | -3.2 | 1 | GLY 142 | |
| 4-Vinylbenzoic acid | -4.5 | 1 | ALA 97 - TRP 141 - VAL 145 - LEU 198 | |
| Dimethyl malate | -3.9 | 2 | ALA 97 - ARG 104 | |
| Levoglucosenone | -4.4 | 1 | GLY 142 | |
| L-Proline, 1-(1-methylethyl)-5-oxo-, methyl ester | -4.3 | 0 | PHE 101 - ARG 104 | |
| 4-Methoxyphenethyl alcohol | -4.7 | 0 | ALA 97 - ASP 100 - PHE 101 - ARG 104 - VAL 145 | |
| Trimethyl citrate | -4.2 | 5 | PHE 101 - ARG 104 - GLY 142 | |
| Caspase-3 | Levoglucosan | -4.9 | 4 | ARG 207 - PHE 250 |
| Propanoic acid, 2-hydroxy-, 1-methylethyl ester, (2S)- | -3.7 | 2 | HIS 121 - TYR 204 - SER 205 - TRP 206 - ARG 207 - PHE 256 | |
| 4-Methyl-3,6,9-trioxadecan-1-ol | -3.8 | 2 | TYR 204 - SER 205 - TRP 206 - ARG 207 - PHE 256 | |
| 4-Vinylbenzoic acid | -4.5 | 2 | CYS 163 - SER 205 - TRP 206 - PHE 256 | |
| Dimethyl malate | -3.9 | 2 | ARG 207 | |
| Levoglucosenone | -3.9 | 2 | TRP 214 - PHE 250 | |
| L-Proline, 1-(1-methylethyl)-5-oxo-, methyl ester | -4.3 | 2 | SER 205 - TRP 206 - ARG 207 | |
| 4-Methoxyphenethyl alcohol | -4.0 | 1 | TRP206 - PHE 250 - PHE 256 | |
| Trimethyl citrate | -4.3 | 10 | THR 62 - SER 63 - SER 65 - SER. 205 - ARG 207 - SER 209 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





