1. Introduction
Portal hypertension (PH) is the main clinical syndrome of liver cirrhosis, which is responsible for most of its complications. PH is defined as a hepatic venous pressure gradient (HVPG) greater than 5 mmHg. Based on portal HVPG, patients with clinically significant portal hypertension (CSPH), showed an HVPG ≥ 10 mmHg [
1]. CSPH is related to an increased risk of developing gastroesophageal varices (GEVs) [
2], overt clinical de-compensation, such as ascites, variceal bleeding, encephalopathy [
3], and hepatocellular carcinoma [
4]. In patients with GEVs, an HVPG > 12 mmHg identifies bleeding risk, while an HVPG < 12 mmHg is associated with protection from bleeding [
5]. Since the risk of GEVs bleeding can be reduced with appropriate medical or endoscopic treatment, in patients with high-risk varices (HRVs) also called “varices needing to treat” (varices with red wale marks or large varices), endoscopic screening of the upper gastrointestinal tract is currently the diagnostic standard. However, a large proportion of cirrhotic patients will not develop HRVs, thus making esophagogastroduodenoscopy (EGD) a non-ideal screening test, that is associated with significant costs and patient discomfort [
6]. Accordingly, in the last decades, increased attention has been dedicated to identifying some accurate non-invasive tests that can rule in and rule out CSPH and HRVs, reducing or avoiding the use of invasive methods such as HVPG measurement and esophagogastroduodenoscopy [
7]. Among laboratory data, platelet count is inversely related to PH, but taken alone, its accuracy for CSPH does not exceed a value of area under curve (AUC) of 0.75 in the literature [
8].
In like manner, although splenomegaly taken alone is a sensitive, but not specific sign of PH, the size of the spleen, combined with platelet count and liver stiffness measurement (LSM) by Transient Elastography (TE) performed with Fibroscan®, provides accurate data on the presence of CSPH/GEVs [
8,
9].
TE, the first and more validated method with the much spreading worldwide, developed to measure liver stiffness (results expressed in kilopascal, [kPa]) and secondly spleen stiffness, exhibited a relatively low specificity in terms of GEVs prediction in one meta-analysis, although the capacity of predicting CSPH was relatively high (sensitivity 90%, specificity 79%) [
10]. However, Fibroscan® exhibits a high measurement failures rate in patients with narrow intercostal spaces, high body mass index, or ascites [
11]. Another limitation is that it is based on M-mode imaging without real-time visualization of the liver parenchyma.
In contrast, new methods integrated in the ultrasound machines can measure the tissues stiffness during a normal exam, enhancing the success rate of measurements [
12] and expressing the results in meter for second (m/sec) or converting in kPa with the Young’s module formula [
13,
14]. The first and more validated among these lasting, is the point shear wave elastography (pSWE) method named also acoustic radiation forced impulse (ARFI) by certain ultrasound companies
The aim of our study was to evaluate the diagnostic value of liver and spleen ARFI, combined with spleen dimension by a conventional abdominal ultrasound and platelet count in new ratio scores, in detecting and predicting the risk of liver-related events in a population of cirrhotic patients of different etiologies.
2. Materials and Methods
We conducted a single-centre prospective and cross-sectional observational study, between February 2017 and February 2021. All consecutive patients with an established diagnosis of liver cirrhosis attending Hepatobiliary Ultrasound Clinic of the Hepatology Unit at Campus Bio-Medico University Hospital for regular surveillance program for hepatocellular carcinoma in the first 6 months of the study (from February to August 2017), were asked to participate to the study. In absence of liver histology, we referred to clinical diagnostic criteria of liver cirrhosis. Specifically, we considered cirrhotic all patients with history of long-lasting liver disease of different etiologies and morphological change of the liver volume and structure evaluated during abdominal ultrasound and/or signs of PH: 1) hypertrophy of the caudate lobe/left lobe of the liver; 2) nodularity of the liver surface in the left lobe evaluated with linear probe; 3) coarse nodular pattern of the liver parenchyma; 4) portosystemic collaterals such as patent umbilical vein; 5) splenomegaly; 6) ascites.
Endoscopic evaluation and grading of oesophageal varices (OEVs) were performed within 3 months of the recruitment, by a single expert endoscopist (>1,000 examinations) who was blinded to the ARFI elastography results. Varices were classified as F1 (straight and small-caliber varices), F2 (tortuous veins forming bead-like appearance), or F3 (tumor-shaped varices) [
15] HRVs were defined as F2 to F3 or F1 with red wale marks, according to the Baveno V criteria [
16].
The exclusion criteria were splenectomy, myeloproliferative syndromes, EGD older than 3 months from the enrollment, bad acoustic window for the liver and the spleen, severe ascites, inability of patient to hold the breath and refusal to informal consent.
pSWE was performed with Acuson S3000 ultrasound system (Siemens®, Munich, Germany) using a 1-4 MHz curved array probe and the software named Virtual touch quantification (VTQ®). All pSWE measures (ARFI elastography) were conducted by two sonographers blinded to the patient’s clinical data. First, a conventional B-mode US im-aging study was conducted, which included evaluation of liver parenchyma and volumes, spleen interpolar diameter and its section area taken in a left longitudinal scan. During the ultrasound exam, the patients were asked to hold their breath in the supine position with the arms extended above the head and ARFI measurements, identified by a region of interest (ROI), were started in the liver and in the spleen. The ROI was characterized by a box with a fixed size of 10x5 mm, far from cysts, biliary ducts, principal blood vessels or liver/spleen lesions. The ROI was placed in the liver parenchyma at the sixth-seventh liver segment two centimeters from to the Glisson’s capsule and perpendicular to its, by an intercostal ultrasound scan between the eighth-ninth right ribs. After, the ROI was placed in the center of the spleen parenchyma, ideally two centimeters from the capsule and far from vascular hilum, by an intercostal ultrasound scan between the eighth-ninth left ribs. The ARFI measurements were expressed in m/sec as median and mean value. ARFI measurement failure was defined as zero valid shots, and unreliable measurements were reported as an interquartile range (IQR) to a median value ratio >30% or success rate <60%. We performed the following ARFI-ratio scores at the enrollment:
ALSDP (ARFI Liver-Spleen Diameter-to-Platelet ratio score): liver stiffness (m/s) x spleen diameter (mm)/platelet count (10³/mm³).
ASSDP (ARFI Spleen-Spleen Diameter-to-Platelet ratio score): spleen stiffness (m/s) x spleen diameter (mm)/platelet count (10³/mm³).
ASSAP (ARFI Spleen-Spleen Area-to-Platelet ratio score): spleen stiffness (m/s) x spleen area (cm2)/platelet count (10³/mm³).
ALSAP (ARFI Liver-Spleen Area-to-Platelet ratio score): liver stiffness (m/s) x spleen area (cm2)/platelet count (10³/mm³).
We also assessed whether or not patients met the following criteria:
Baveno VI criteria: Livers Stiffness ≤ 20 kPa and Platelets count ≥150 x 103 / mm³
Baveno VI EXPANDED criteria: Livers Stiffness ≤ 10 kPa and Platelets count ≥100 x 103 / mm³
Colecchia criteria: Baveno VI criteria (Livers Stiffness ≤ 20 kPa and Platelets count ≥150 x 103 / mm³) plus Spleen Stiffness ≤ 45 kPa
The study protocol was designed to provide 1 year of short-term follow-up (median 14 months, 13-17 months) and 4 years of long-term follow-up (median 46 months, 44-48 months). In the 2 scheduled follow-ups, we registered a laboratory assessment plus clinical patients’ evaluation, registering episodes of ascites, occurrence of new HRVs or oesophageal (OE) bleeding, and liver-related death.
The clinical investigation was conducted according to the principles expressed in the Declaration of Helsinki. The study was approved by the Ethical Committee of the University Hospital, and informed consent was obtained from all the patients due to the prospective nature of the study.
For descriptive statistics, categorical variables were shown as number and proportion, while continuous variables were shown as mean with standard deviation (SD) or median with IQR, as appropriate. The association with incident liver-related events during follow-up, was computed by means of Poisson regressions with robust error variance for binary data and expressed with adjusted risk ratios (aRR) with 95% confidence intervals (CI). Cross-sectional associations of ARFI-ratio scores with actual liver-related events, were estimated by means of logistic regression models and expressed as odds ratios (OR) with 95% CI. Multivariable models were corrected for age, sex, Child-Pugh class, and previous presence of liver decompensation events.
Finally, the predictive capacities were verified through the AUC curve. A p<0.05 was considered statistically significant. All analyses were conducted with R statistics 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
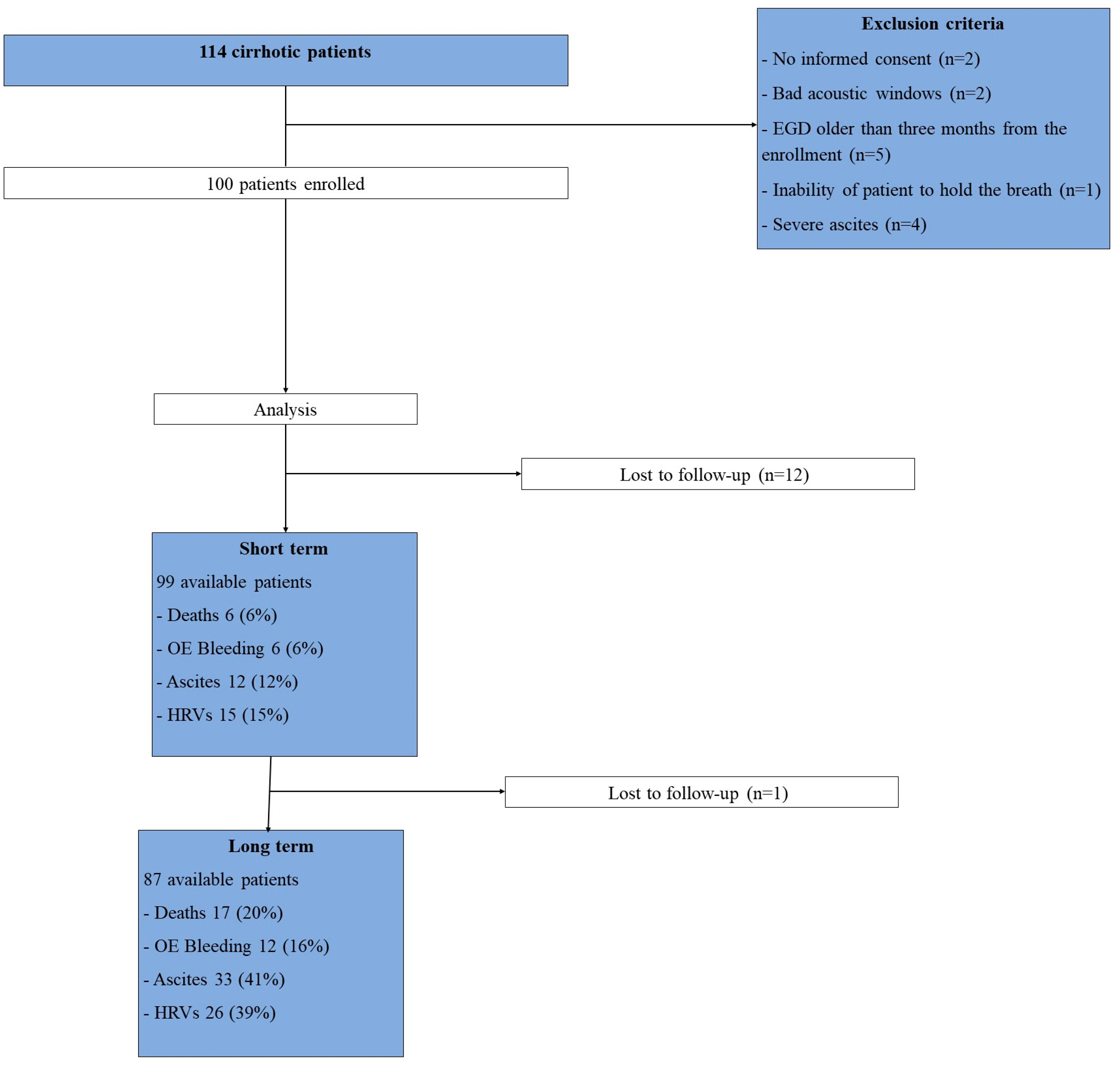
Among 114 patients enrolled, 14 were excluded for these reasons: (1) no informed consent (n = 2), (2) bad acoustic windows (n=2), (3) time between ARFI and endoscopy >3 months (n = 5), (4) inability of patient to hold the breath (n=1), (5) severe ascites (n=4). Finally, 100 subjects with liver cirrhosis (mean age 66.7 years, women 50%, Child-Pugh class A/B/C 86%/12%/2%) were included in the study. 99 and 87 patients were evaluated at the short- and long-term follow-up, respectively (
Figure 1). The most common aetiologies of liver disease were chronic viral hepatitis (33%) and fatty liver (31%), computing for more than half of the population. 10 (10%) subjects had previous OE bleeding at baseline evaluation, while ascites, OEVs and encephalopathy were present in 20 (20%), 20 (20%) and 9 (9%) patients, respectively (
Table 1).
Over a median of 14 months (short-term follow-up), 6 (6%) individuals died while 6 (6%), 12 (12%) and 15 (15%) experienced OE bleeding, new onset of ascites and new evidence of HRVs. One subject was lost at the short-term follow-up. After a median of 46 months (long-term follow-up), 17 (20%) individuals died while 12 (16%), 33 (41%) and 26 (39%) experienced OE bleeding, new onset of ascites and new evidence of HRVs, respectively (
Table 2). 12 subjects were lost at the long-term follow-up.
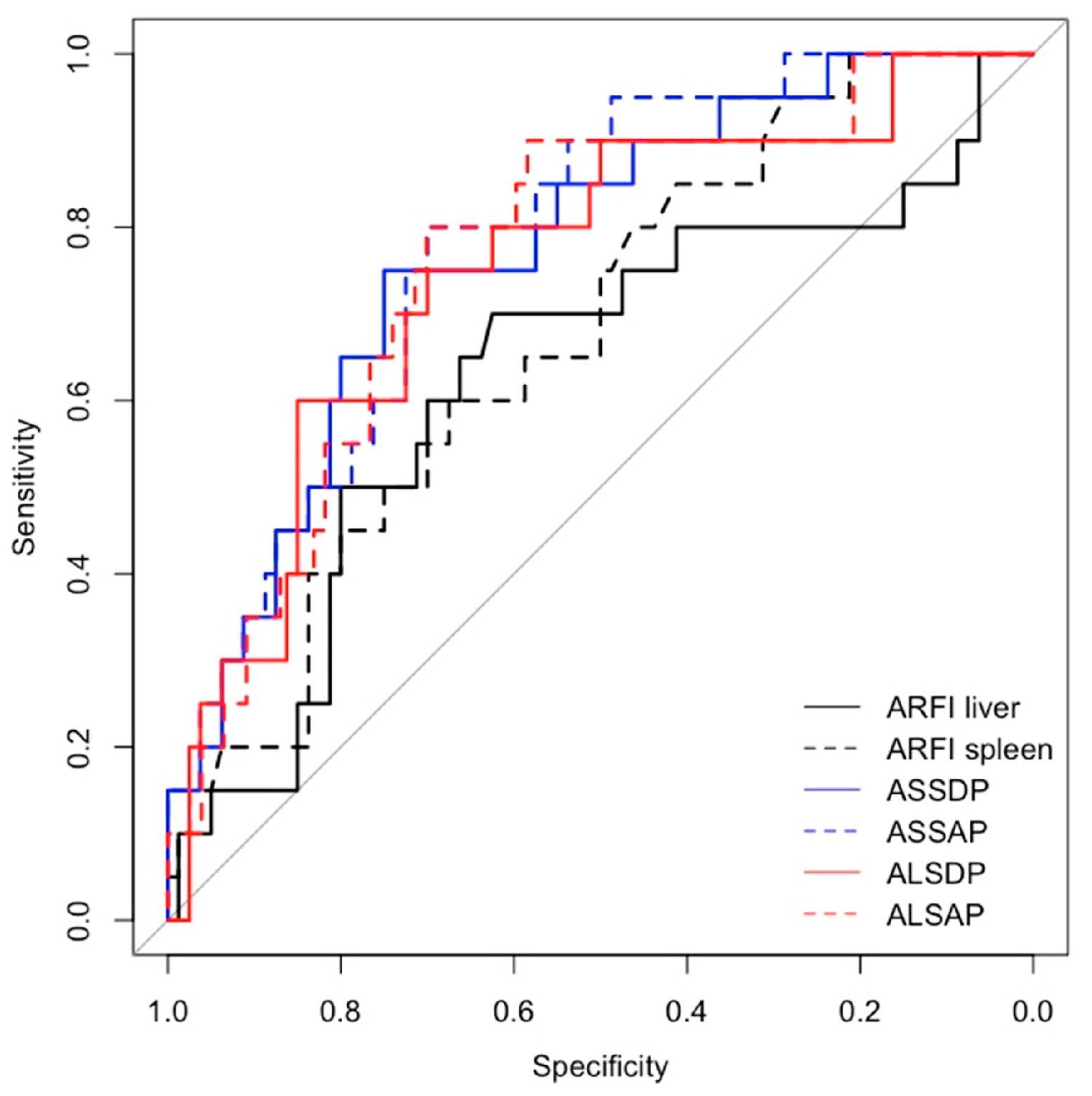
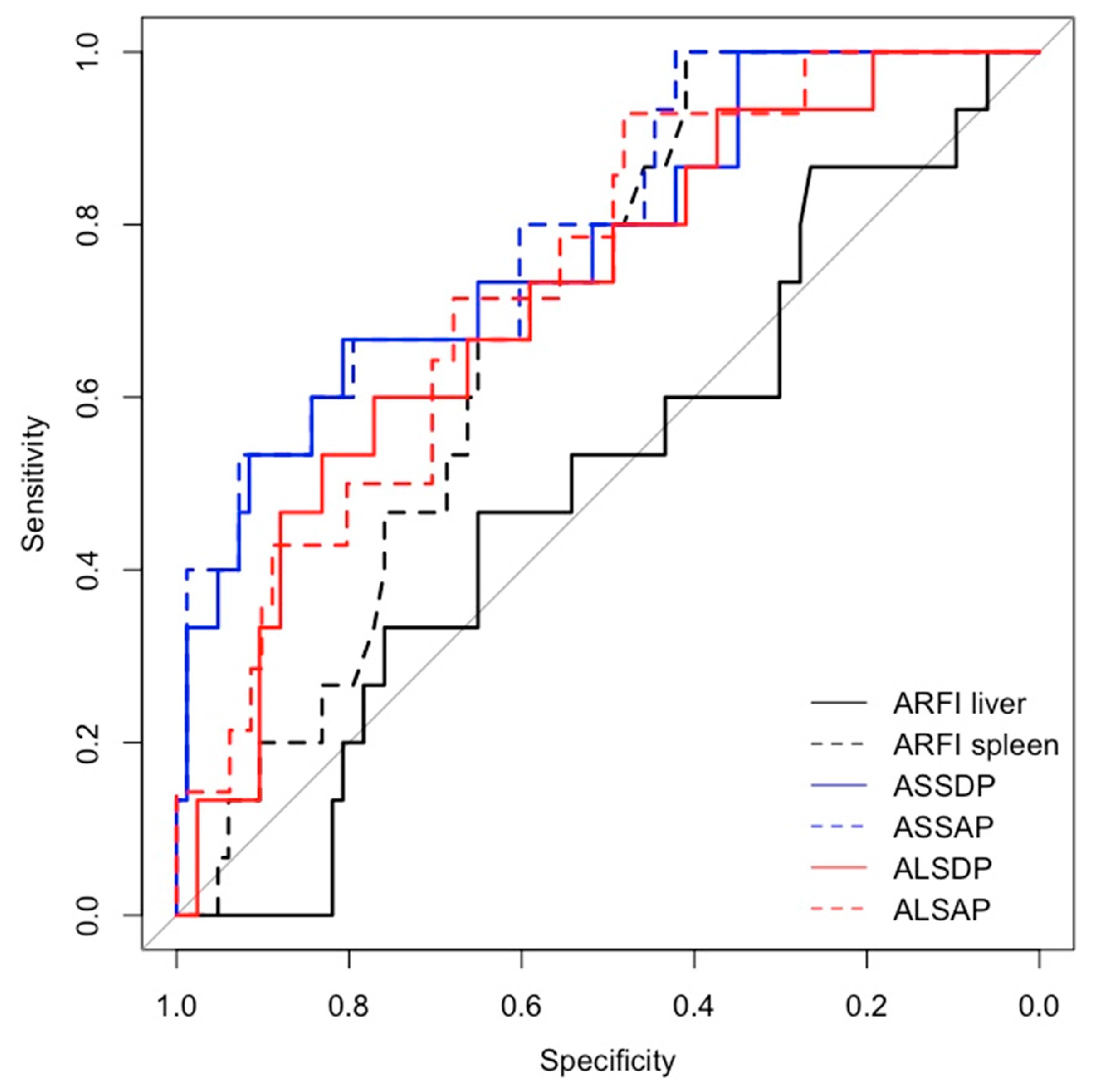
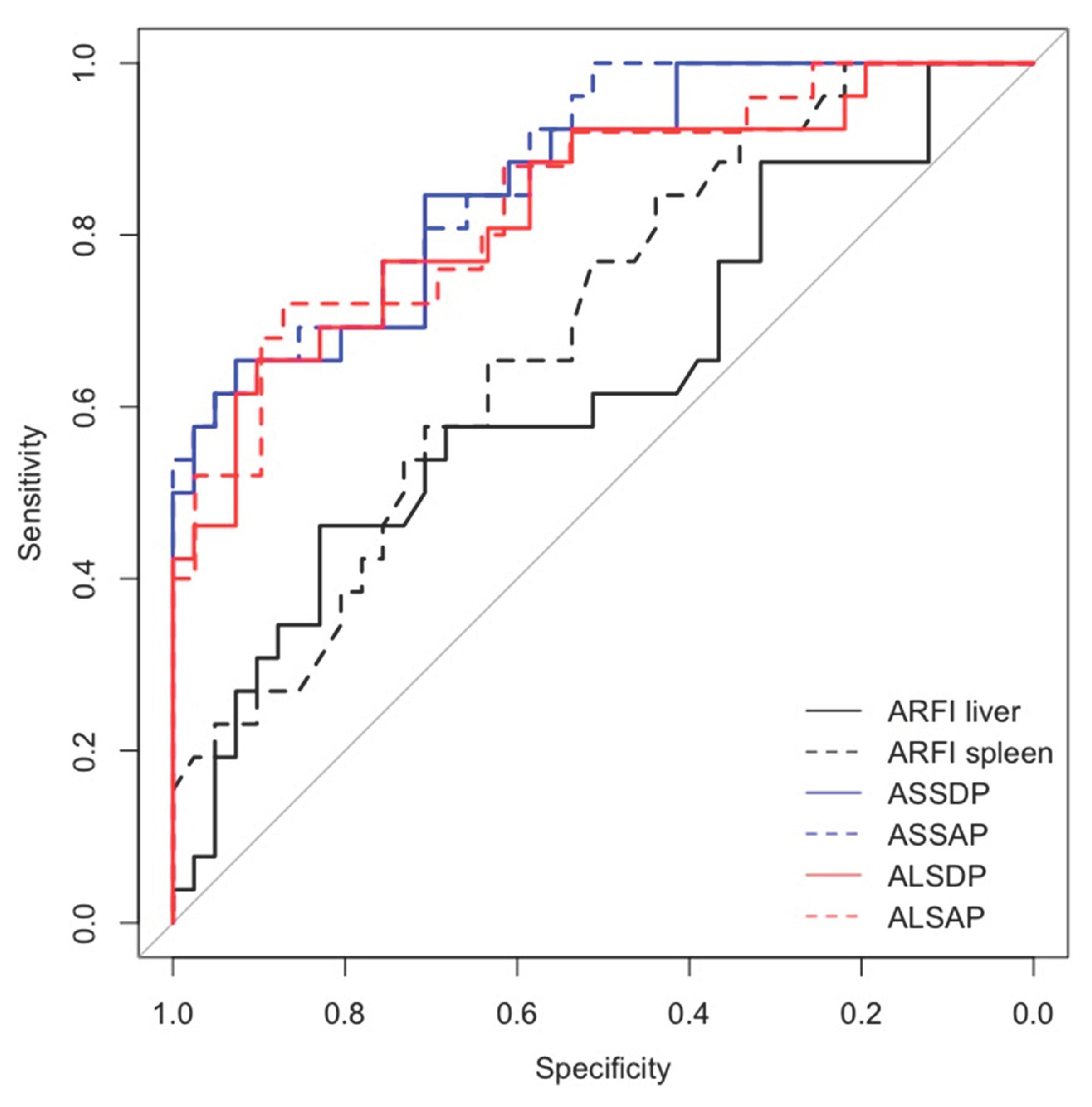
Spleen ARFI, ASSDP, ASSAP, were significantly associated with HRVs in the prospective short- and long-term follow-up and in the cross-sectional study (p<0.05) (
Table 3), while ALSDP and ALSAP were associated with HRVs only in the prospective long-term follow-up and cross-sectional study (p< 0.05). All the ARFI-ratio scores performed better than liver and spleen ARFI alone (AUC > 0.7) in detecting HRVs either at the enrolment and at the follow-up (
Figure 2 and
Figure 3), although ASSAP was the best performing ARFI-ratio score for detecting HRVs at the long-term follow-up (AUC = 0.88) (
Figure 4).
Furthermore, based on AUC values, ARFI ratio scores performed better than other validated combined models or criteria (such as Child-Pugh scores, Baveno VI criteria, Baveno VI expanded criteria and Colecchia criteria [
17]) (AUC values between 0.5-0.6), in predicting the presence or the new occurrence of HRVs (
Table 4). Moreover, only liver ARFI at the long-term follow-up (p=0.039) and spleen ARFI, at the short-term follow-up, identified patients at risk of ascites (p=0.009) in the prospective study. Other ARFI-ratio scores were not associated to any other liver-related event, except for liver ARFI that was slightly associated with OE bleeding (p=0.057) at the short-term follow-up but not in the long-term period (p=0.176), in the prospective study (
Table 4).
4. Discussion
Over the past decades, many efforts have been directed to the research concerning the assessment of the of PH degree with non-invasive tests [
3,
18], to rule out CSPH and specifically HRVs, reducing or avoiding an invasive diagnosis through HVPG and EGD respectively, also stratifying the risk of liver decompensation. Many authors have therefore deepened the role of LSM as a predictor of PH, showing a linear correlation between HVPG and LSM [
7]. The Baveno VI Consensus Conference suggested that in patients with compensated advanced chronic liver disease LSM≤ 20 kPa plus platelets count ≥150x103/mm3, can identify the group of patients whom very unlikely have HRVs (<5%), for whom endoscopy can be avoided [
19]. A possible limitation of the Baveno VI criteria is related to a substantially low number of spared endoscopies (15–25%) [
20]. Therefore, most patients not presenting with HRVs still require endoscopy according to guidelines and it is becoming evident that more accurate non-invasive scores are needed to improve risk stratification. To increase number of spared endoscopies Augustin et al. suggested Baveno VI expanded criteria, a combination of LSM ≤ 25 KPa plus platelet count ≥100X10³/mm [
21].
Spleen stiffness measurement (SSM) by TE or pSWE has showed similar or even better accuracy versus LSM to identify patients at high or low risk of developing HRVs: SSM cut-off of ≤46 kPa by TE has shown better specificity, with same high sensitivity as Baveno VI criteria, in ruling out patients with HRVs. Colecchia et al. proposed Colecchia criteria, a non-invasive prediction model that combined Baveno VI criteria and SSM ≤ 46 kPa; this model can safely avoid more endoscopies than Baveno VI criteria or SSM ≤ 46 kPa taken alone [
17]. Data reported in the literature showed that SSM might be a better marker of PH than LSM in patients with cirrhosis of viral etiology, and a growing body of evidence suggests that this might also be true for cirrhosis related to other causes [
22]. We showed in a past preliminary study published as abstract [
23] that spleen ARFI was not useful to detect HRVs in a cirrhotic population, while in the multivariate analysis LSM (OR 2.4 [1.17-5.34]), platelet count (OR 0.98 [0.97-0.99]) and spleen interpolar diameter (OR 1.16 [0.96-1.36]) were associated with HRVs. In contrast, Jain et al., in a cross-sectional study including 90 patients with liver cirrhosis identified the best cut-off value for liver ARFI of 2.16 m/sec and of 3.04 m/sec for spleen ARFI in detecting OEVs, with a 92 % of accuracy when the stiffness values were taken together [
24]. In a meta-analysis of 16 studies, SSM was able to detect GEVs with higher sensitivity and specificity (0.88 and 0.78, respectively) than LSM (0.83 and 0.66, respectively) [
25]. These data are in line with our results, that showed SSM performing better than LSM in predicting HRVs at the short-term follow-up of the prospective study, with a risk ratio of 2.06 [(CI 95% 1.06-4.03), p= 0.034]. Similarly, SSM showed an OR of 3.04 [(CI 95% 1.23-8.65) p= 0.024] for the presence of HRVs in the cross-sectional study. In a recent meta-analysis of data from 45 studies about the ratio scores combining SSM and LSM with laboratory tests and spleen dimension, SSM and a ratio score between LSM by TE (kPa) X Spleen size (in cm)/Platelet count (number/mm3) [LSPS], were superior to LSM for GEVs detection, with higher sensitivity (0.90 and 0.91 vs 0.85), specificity (0.73 and 0.76 vs 0.64), and AUC (0.899 and 0.851 vs 0.817) [
26].
Interestingly, Park et al. developed a novel ARFI-based prediction model that can accurately identify HRVs in patients with compensated cirrhosis: the ARFI Spleen-Spleen diameter-to platelet ratio score (ASPS), which was calculated as spleen ARFI X spleen diameter/platelet count. To detect HRVs, a negative predictive value of 98.3% was achieved at ASPS <2.83, ruling out the presence of HRVs, whereas a positive predictive value of 100% was achieved ASPS>5.28 [
27].
Nowadays, few studies explored the role of ARFI in predicting HRVs in cirrhotic patients. Huang et al. [
28], in a huge study on 741 consecutive patients, combined liver and spleen ARFI measurements with platelet count, in a strategy named ARP, compared to Baveno VI criteria for oesophageal varices screening. In the training cohort, ARP strategy was defined as LSM < 1.805 m/sec or SSM < 2.445 m/sec and platelet count > 110 x 109/L, sparing 40.6 % of EGDs with a missing rate of HRVs of 3.4 %. In the validation cohort, ARP strategy improved the Baveno VI criteria avoiding unnecessary EGDs [
28]. In another study, Wang et al. prospectively evaluate the performance of liver and spleen ARFI stiffness in a subpopulation of liver cirrhotic patients suffering of chronic hepatitis B under pharmacological viral suppression. The most accurate liver and spleen ARFI cut-off were 1.46 m/sec and 2.28 m/sec respectively. Combining these data with platelet count (> 150 x 109/L), enhanced the capacity of the strategy to spare 38.6 % of EGDs, with a low rate of missed HRVs in the validation cohort (3.4 %) [
29].
Our study demonstrated, in a cohort of patients with prevalent well compensated cirrhosis (only 14 % of the patients showed a liver impaired liver function identified by score of Child-Pugh < B7), a higher diagnostic performance of ARFI-ratio scores in respect of spleen and liver ARFI measurements taken alone, in detecting oesophageal HRVs, both at the time of enrolment. Notably, the highest AUC at the long-term follow-up [0.88 (95% CI 0.79-0.96)] was showed for ASSAP, even better than ASSDP [0.86 (0.78-0.95)], the latter considering inter-polar spleen diameter rather than spleen section area. One possible explanation for the better diagnostic performance of this ARFI-ratio score, could be related to the structural changes of the spleen and volumes secondary to PH [
30], better identified by measurements of the spleen section area than bipolar diameter in cirrhotic patients. Nevertheless, in our population, the use of ARFI-ratio scores does not appear useful in identifying patients at risk of developing clinical events related to cirrhosis and PH, including death and OE bleeding. Only spleen ARFI and liver ARFI were associated in patients at risk of developing ascites in the short- [(aRR (95% CI) 2.58 (1.27-5.24) p=0.009] and long-term follow-up [aRR (95% CI) 1.42 (1.02-1.98) p=0.039)]. This is apparently in disagreement with literature data, in which LSM is a validated predictor of clinical decompensation in patients with chronic liver disease [
31], so that current EASL (European Association for the Study of the Liver) guidelines recommend the use of LSM, in addition to liver function tests, to stratify the risk of clinical decompensation and mortality in compensated chronic liver diseases.
However, there are some limits of the study. Firstly, the study population was prevalent of well compensated liver cirrhosis; secondly since most of the viral patients were affected by hepatitis C virus eradicated by new direct antivirals, antiviral therapy impacted on PH progression reducing the onset of HRVs and clinical events related to liver cirrhosis. Finally, as stated, the spleen measurement is highly sensitive but lowly specific, with a high number of false positive in case of splenomegaly due to non-hepatic causes. Notably, the model needs to be validated in an external population.
5. Conclusions
Our study showed for the first time that combining liver and spleen ARFI measurements with spleen dimension and platelet count, can provide some ratio scores able to identify cirrhotic patients at risk of HRVs in the short- and long-term period. Particularly, the spleen section area seems to perform better than interpolar diameter so that we can suggest of taking this ultrasound parameter during regular surveillance of cirrhotic patients. We need further investigations to include this model in the clinical practice, to select cirrhotic patients needing EGD but, above all, to inform the patients and clinical hepatologists about the risk of HRVs in order to start pharmacological or endoscopic prophylaxis when indicated. Finally, this is a good proof of concept in the field of multiparametric ultrasound of the cirrhotic patients, and it should be spread in the ultrasound room of each liver unit for a faster and more accurate decision making.
Authors’ Contributions: Conceptualization, G.G. and AFM.V.; methodology, G.G. and A.DV.; data curation and statistical analysis, AFM.V. and A.DV.; investigation, P.G., G.G., AFM.V.; writing—original draft preparation, G.G., AFM.V and E.B.; writing—review and editing, G.DP. and V.F.; supervision, G.G and P.B.. All the authors and the cooperators have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of University Campus Bio-Medico of Rome (protocol code 05/17 OSS ComEt CBM; date of approval 27.02.2017).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ALSAP |
ARFI Liver-Spleen Area-to-Platelet ratio score |
| ALSDP |
ARFI Liver-Spleen Diameter-to-Platelet ratio score |
| ARFI |
Acoustic Radiation Forced Impulse |
| ASPS |
ARFI Spleen-Spleen diameter-to platelet ratio score |
| aRR |
adjusted risk ratios |
| ASSAP |
ARFI Spleen-Spleen Area-to-Platelet ratio score |
| ASSDP |
ARFI Spleen-Spleen Diameter-to-platelet ratio score |
| AUC |
area under curve |
| CI |
confidence intervals |
| CSPH |
clinically significant portal hypertension |
| EGD |
esophagogastroduodenoscopy |
| EASL |
European Association for the Study of the Liver |
| GEVs |
gastroesophageal varices |
| HCC |
hepatocellular carcinoma |
| HVPG |
hepatic venous pressure gradient |
| HVRs |
high risk varices |
| IQR |
interquartile range |
| LSM |
liver stiffness measurement |
| TE |
transient tlastography |
| OE |
oesophageal |
| OEVs |
oesophageal varices |
| OR |
odds ratios |
| PH |
portal hypertension |
| pSWE |
point shear wave elastography |
| ROI |
region of interest |
| SSM |
spleen stiffness measurement |
| SD |
standard deviation |
References
- Garcia-Tsao, G.; Abraldes, J. G.; Berzigotti, A.; Bosch, J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatol. 2016, 65, 310–335. [Google Scholar] [CrossRef]
- Groszmann, R. J.; Garcia-Tsao, G.; Bosch, J.; Grace, N.D.; Burroughs, A.K.; Planas, R; Escorsell, A.; Guarcia-Pagan, J.C.; Patch, D.; Matloff, D.; Gao, H.; Makuch, R. Beta-Blockers to Prevent Gastroesophageal Varices in Patients with Cirrhosis. New Eng. J. Med. 2005, 353, 2254–2261. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, C.; Groszmann, R.; Garcia–Tsao, G.; Grace, N.; Burroughs, A.; Planas, R.; Escorsell, A.; Guarcia-Pagan, J.C.; Makuch, R.; Patch, D.S.; Matloff, D.; Bosch, J. Hepatic Venous Pressure Gradient Predicts Clinical Decompensation in Patients with Compensated Cirrhosis. Gastroenterol. 2007, 133, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, C.; Groszmann, R. J.; Garcia-Tsao, G.; Bosch, J.; Grace, N.; Burroughs, A.; Planas, R.; Escorsell, A.; Guarcia-Pagan, J.C.; Makuch, R.; Patch, D.S.; Matloff, D. Hepatic venous pressure gradient predicts development of hepatocellular carcinoma independently of severity of cirrhosis. J. Hepatol. 2009, 50, 923–928. [Google Scholar] [CrossRef]
- Bosch, J.; García-Pagán, J. C. Prevention of variceal rebleeding. Lancet. 2003, 361, 952–954. [Google Scholar] [CrossRef] [PubMed]
- Berzigotti, A.; Bosch, J.; Boyer, T. D. Use of noninvasive markers of portal hypertension and timing of screening endoscopy for gastroesophageal varices in patients with chronic liver disease. Hepatol. 2013, 59, 729–731. [Google Scholar] [CrossRef] [PubMed]
- Berzigotti, A.; Tsochatzis, E.; Boursier, J.; Castera, L.; Cazzagon, N.; Friedrich-Rust, M. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis – 2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef] [PubMed]
- Berzigotti, A.; Seijo, S.; Arena, U.; Abraldes, J. G.; Vizzutti, F.; García–Pagán, J. C.; Pinzani, M.; Bosch, J. Elastography, Spleen Size, and Platelet Count Identify Portal Hypertension in Patients With Compensated Cirrhosis. Gastroenterol. 2013, 144, 102–111. [Google Scholar] [CrossRef]
- Kim, B. K.; Han, K.H.; Park, J. Y.; Ahn, S. H.; Kim, J. K.; Paik, Y. H.; Lee, K.S.; Chon, C.Y.; Kim, D. Y. A Liver Stiffness Measurement-Based, Noninvasive Prediction Model for High-Risk Esophageal Varices in B-Viral Liver Cirrhosis. Am. J. Gastroenterol. 2010, 105, 1382–1390. [Google Scholar] [CrossRef]
- Shi, K.Q.; Fan, Y.C.; Pan, Z.Z.; Lin, X.F.; Liu, W.Y.; Chen, Y.P.; Zheng, M.H. Transient elastography: a meta-analysis of diagnostic accuracy in evaluation of portal hypertension in chronic liver disease. Liver Int. 2012, 33, 62–71. [Google Scholar] [CrossRef]
- Castéra, L.; Foucher, J.; Bernard, P.H.; Carvalho, F.; Allaix, D.; Merrouche, W.; Couzigou, P.; de Lédinghen, V. Pitfalls of liver stiffness measurement: A 5-year prospective study of 13,369 examinations. Hepatol. 2010, 51, 828–35. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.; Calvaruso, V.; Cacopardo, B.; Alessi, N.; Attanasio, M.; Petta, S.; Fatuzzo, F.; Montineri, A.; Mazzola, A.; L’abbate, L.; Nunnari, G.; Bronte, F.; Di Marco, V.; Craxì, A.; Cammà, C. Comparison of Transient Elastography and Acoustic Radiation Force Impulse for Non-Invasive Staging of Liver Fibrosis in Patients With Chronic Hepatitis C. Am. J. Gastroenterol. 2011, 106, 2112–2120. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Palmeri, M. L.; Bouchard, R. R.; Nightingale, R. W.; Nightingale, K. R. An Integrated Indenter-ARFI Imaging System for Tissue Stiffness Quantification. Ultras. Imag. 2008, 30, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Gallotti, A.; D’Onofrio, M.; Pozzi Mucelli, R. Acoustic Radiation Force Impulse (ARFI) technique in ultrasound with Virtual Touch tissue quantification of the upper abdomen. La Rad. Med. 2010, 115, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Idezuki, Y. General rules for recording endoscopic findings of esophagogastric varices (1991). World. J. Surg. 1995, 19, 420–422. [Google Scholar] [CrossRef] [PubMed]
- De Franchis, R. Revising consensus in portal hypertension: Report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J. Hepatol. 2010, 53, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Colecchia, A.; Ravaioli, F.; Marasco, G.; Colli, A.; Dajti, E.; Di Biase, A. R.; Festi, D. A combined model based on spleen stiffness measurement and Baveno VI criteria to rule out high-risk varices in advanced chronic liver disease. J. Hepatol. 2018, 69, 308–317. [Google Scholar] [CrossRef]
- Berzigotti, A.; Rossi, V.; Tiani, C.; Pierpaoli, L.; Zappoli, P.; Riili, A.; Serra, C.; Andreone, P.; Morelli, M.C.; Golfieri, R.; Rossi, C.; Magalotti, D.; Zoli, M. Prognostic value of a single HVPG measurement and Doppler-ultrasound evaluation in patients with cirrhosis and portal hypertension. J. Gastroenterol. 2010, 46, 687–695. [Google Scholar] [CrossRef]
- De Franchis, R. Expanding consensus in portal hypertension. J. Hepatol. 2015, 63, 743–752. [Google Scholar] [CrossRef]
- Jangouk, P.; Turco, L.; De Oliveira, A.; Schepis, F.; Villa, E.; Garcia-Tsao, G. Validating, deconstructing and refining Baveno criteria for ruling out high-risk varices in patients with compensated cirrhosis. Liver Int. 2017, 37, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Augustin, S.; Pons, M.; Maurice, J. B.; Bureau, C.; Stefanescu, H.; Ney, M.; Blasco, H.; Procopet, B.; Tsochatzis, E.; Westbrook, R.H.; Bosch, J.; Berzigotti, A.; Abraldes, J.G.; Genescà, J. Expanding the Baveno VI criteria for the screening of varices in patients with compensated advanced chronic liver disease. Hepatol. 2017, 66, 1980–1988. [Google Scholar] [CrossRef]
- Kim, H. Y.; So, Y. H.; Kim, W.; Ahn, D.W.; Jin Jung, Y.; Woo, H.; Kim, D.; Kim, M.Y.; Baik, S. K. Noninvasive Response Prediction in Prophylactic Carvedilol Therapy for Cirrhotic Patients with Esophageal Varices. J. Hepatol, 2018. [CrossRef]
- Galati, G.; De Vincentis, A.; Guidi, A.; Gallo, P.; Vespasiani-Gentilucci, U.; Picardi, A. Spleen Stiffness Evaluated by Acoustic Radiation Force Impulse (ARFI) Elastography in Cirrhotic Patients is not a Useful Tool to Predict Esophageal Varices Needing Treatment. Ultras. Med. Biol. 2017, 43, S102. [Google Scholar] [CrossRef]
- Jain, A.K.; Bundiwal, A.K.; Jain, S.; Agrawal, P.; Jain, D.; Sircar, S. Evaluation of liver and splenic stiffness by acoustic radiation force impulse for assessment of esophageal varices. Indian J Gastroenterol. 2023. [CrossRef] [PubMed]
- Ma, X.; Wang, L.; Wu, H.; Feng, Y.; Han, X.; Bu, H.; Zhu, Q. Spleen Stiffness Is Superior to Liver Stiffness for Predicting Esophageal Varices in Chronic Liver Disease: A Meta-Analysis. PLoS ONE. 2016, 11, e0165786. [Google Scholar] [CrossRef]
- Manatsathit, W.; Samant, H.; Kapur, S.; Ingviya, T.; Esmadi, M.; Wijarnpreecha, K.; McCashland, T. Accuracy of liver stiffness, spleen stiffness, and LS-spleen diameter to platelet ratio score in detection of esophageal varices: Systemic review and meta-analysis. J. Gastroenterol. and Hepatol. 2018, 33, 1696–1706. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Kim, S. U.; Park, S. Y.; Kim, B. K.; Park, J. Y.; Kim, D. Y.; Ahn, S.H.; Tak, W.Y.; Kweon, Y.O.; Han, K.H. A Novel Model to Predict Esophageal Varices in Patients with Compensated Cirrhosis Using Acoustic Radiation Force Impulse Elastography. PLoS ONE. 2015, 10, e0121009. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, L.; He, R.; Li, S.; Liu, C.; Qi, X.; Li, J. A strategy for varices screening based on acoustic radiation force impulse combined with platelet (CHESS2001): An alternative of Baveno VI criteria. Hepatol Commun. 2022, 6, 3154–3162. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xi, R.; Song, J.; Wen, B.; Zhang, Y.; Zhou, L.; Zhang, X.; Li, Y.; Zhou, F.; Zhu, Y.; Ji, Y.; Lai, Q.; He, Q.; Luo, W.; Qi, T.; Liu, M.; Lan, X.; Dai, L.; Chen, J. Combined model with acoustic radiation force impulse to rule out high-risk varices in HBV-related cirrhosis with viral suppression. Dig. Liver Dis. 2023, 55, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Mejias, M.; Garcia-Pras, E.; Gallego, J.; Mendez, R.; Bosch, J.; Fernandez, M. Relevance of the mTOR signaling pathway in the pathophysiology of splenomegaly in rats with chronic portal hypertension. J. Hepatol. 2010, 52, 529–539. [Google Scholar] [CrossRef]
- Singh, S.; Fujii, L. L.; Murad, M. H.; Wang, Z.; Asrani, S. K.; Ehman, R. L.; Kamath, P. S.; Talwalkar, J. A. Liver Stiffness Is Associated with Risk of Decompensation, Liver Cancer, and Death in Patients With Chronic Liver Diseases: A Systematic Review and Meta-analysis. Clin. Gastroenterol. and Hepatol. 2013, 11, 1573–1584. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).