Submitted:
16 February 2024
Posted:
19 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Isolation of Δ8-THC and its derivatives
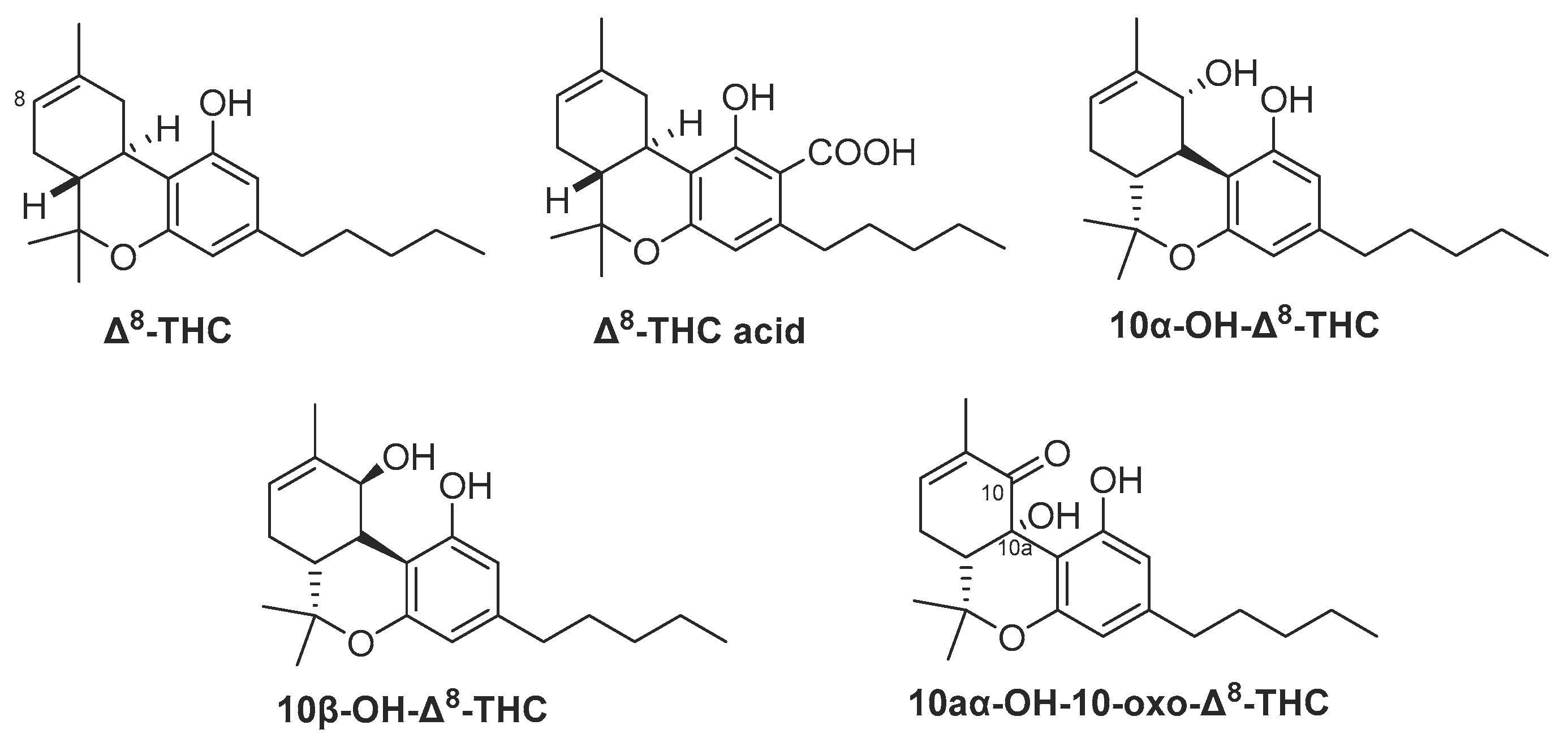
3. Is Δ8-THC a Natural Secondary Metabolite or an Artifact?
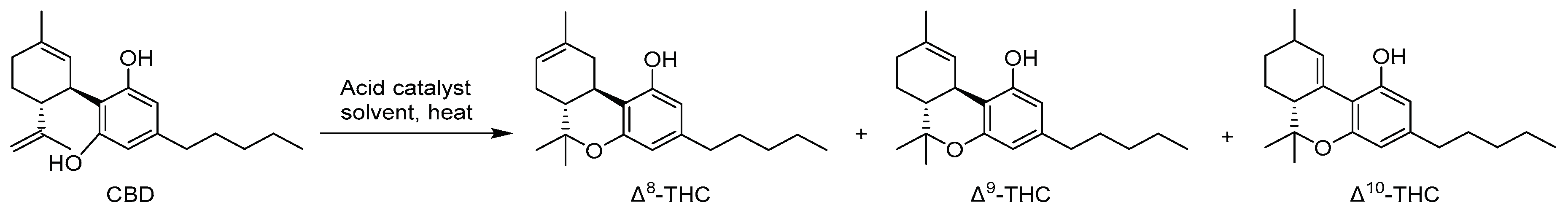
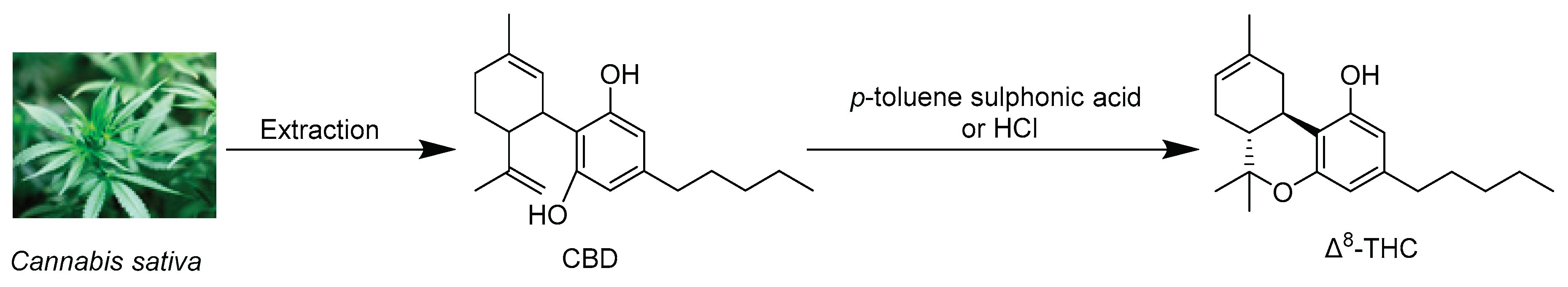
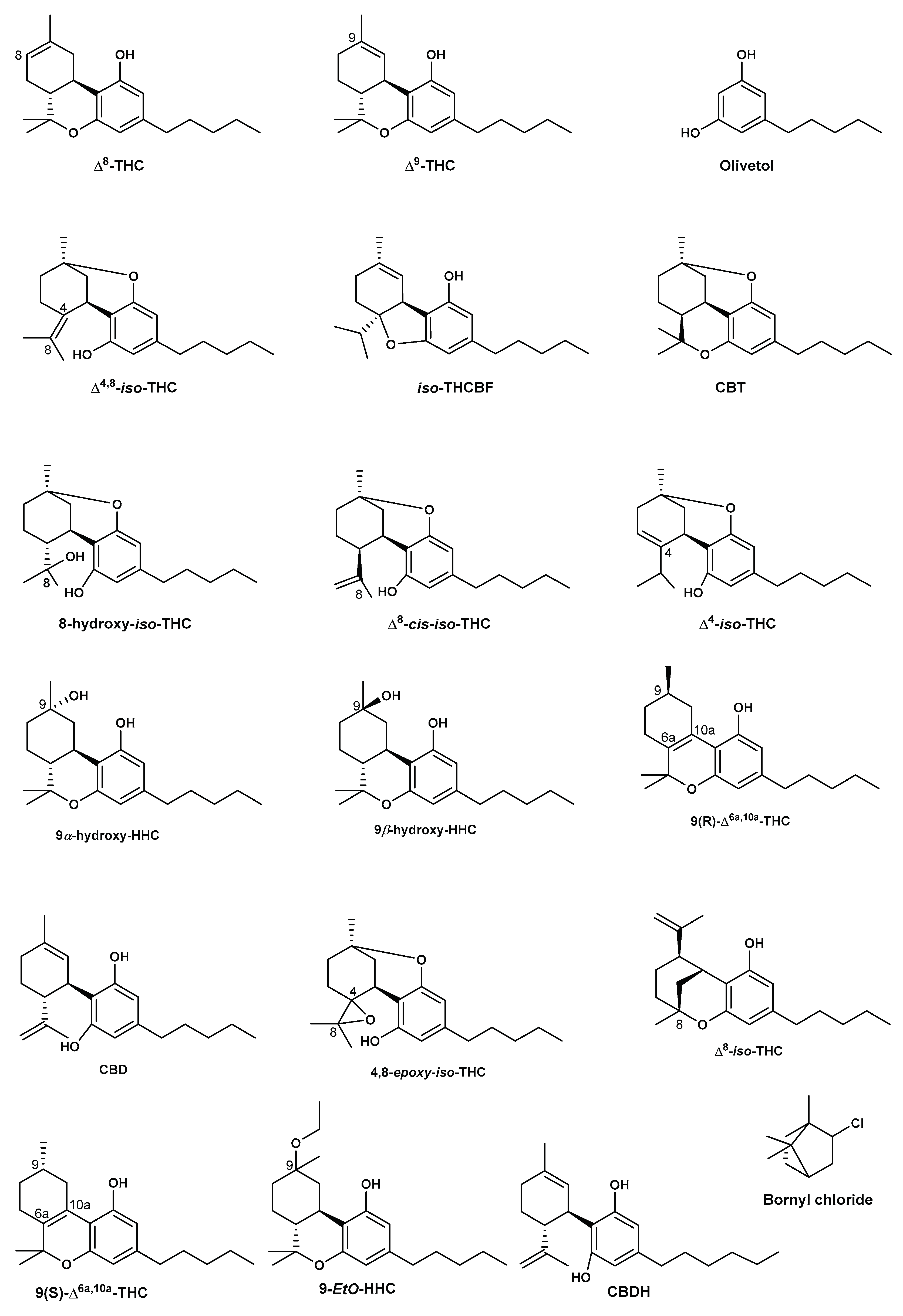
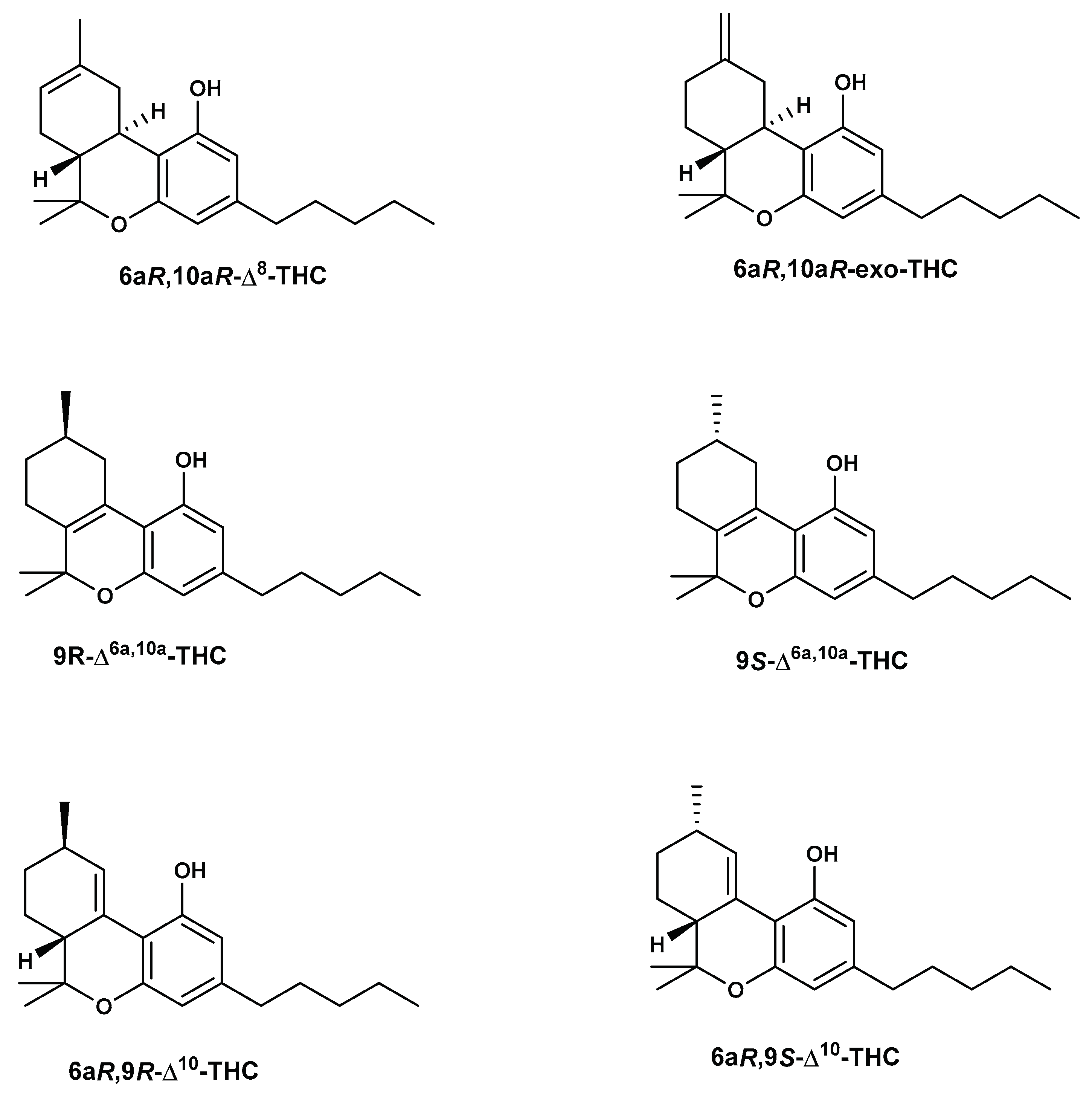
4. Synthesis of Δ8-THC
5. Analysis of Δ8-THC
5.1. Analysis of Δ8-THC in cannabis biomass and cannabis-derived products
5.2. Analysis of Δ8-THC, impurities, and possible contaminants in commercial consumer products
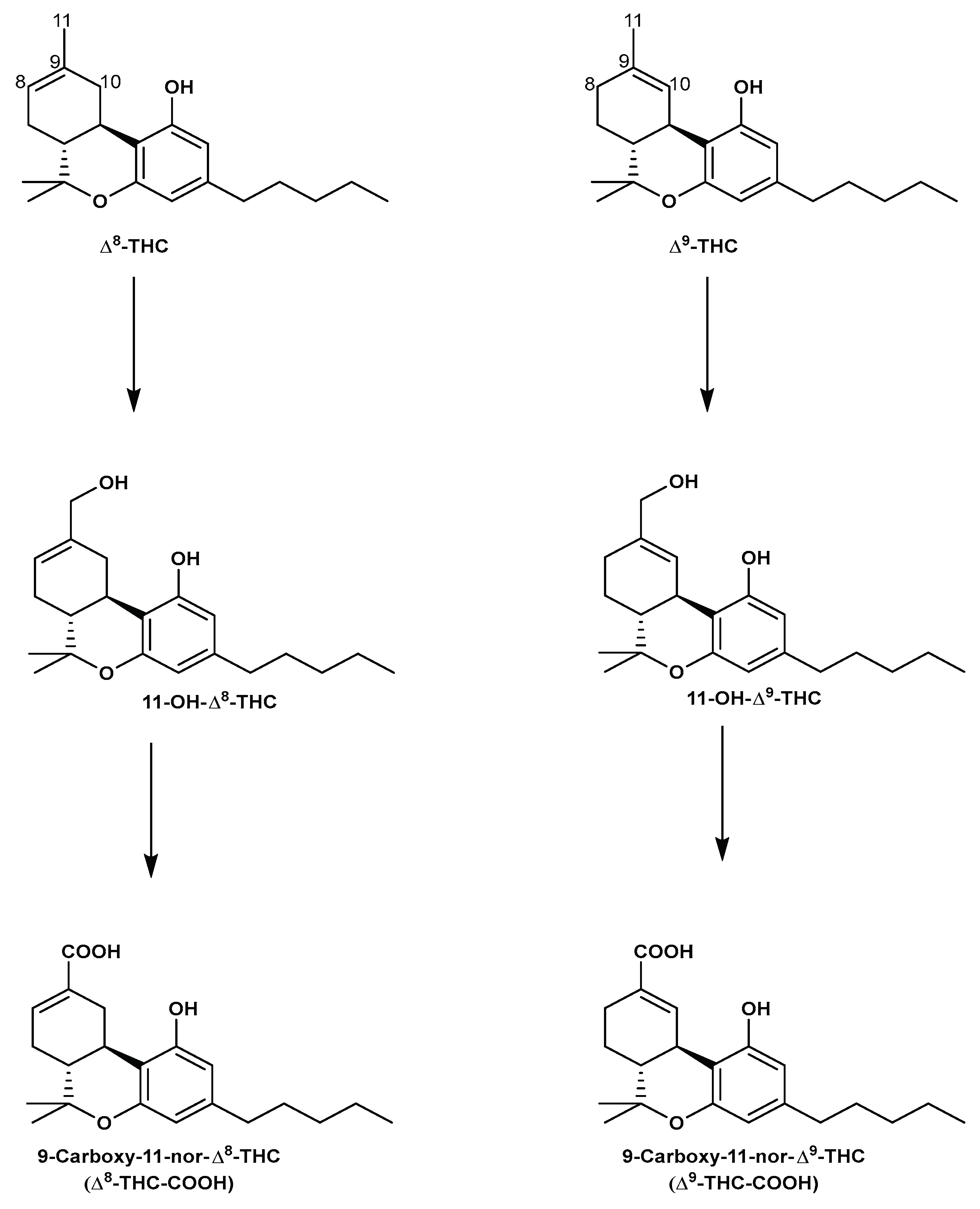
5.3. Analysis of Δ8-THC and Δ9-THC metabolites in different biological matrices.
5.4. Overestimation of Δ9-THC -COOH Levels: A Special Concern
| Matrix | Analytical technique (Analyte) |
Sample preparation | MRMs | Internal Standard (IS) | Reference |
|---|---|---|---|---|---|
| Oral fluid | HPLC-MS/MS (Δ8-THC) |
SPE: I 0 µL of IS+ 400 µL of 2% phosphoric acid+ 400 µL of oral fluid samples, washed with 400 µL of water/methanol (95:5, v/v) and eluted by 400 µL of acetonitrile/methanol (90: 10, v/v) | Quantifier 315.1→123.0 (m/z) a qualifier 315.1→135.1 (m/z) |
d9-Δ8-THC, d3-Δ9-THC, d3-CBD and d3-CBN. d9-Δ8-THC: 324.21→123.1 (m/z) |
[80] |
| Whole blood and serum |
LC-ESI-MS/MS (Δ8-THC-COOH) |
200 µL blood samples+ 20 µL of IS+ 600 µL of ACN, mixing, and centrifugation, evaporated to dryness+ reconstituted in 200 µL of ACN/ H2O/ Formic acid, 60/40/0.1: v/v/v | (m/z, 345→327 (quantifier), 345→299 (qualifier) |
THC-d3 and 11-OH-THC-d3, 10ng, THC-COOH-d3 |
[83] |
| Human urine |
GC/MS (Δ8-THC-COOH) |
after derivatization and Cannabinoid immunoassay |
m/z 488→473, 371 +ve (SIM). |
Δ9-THC-COOH | [84] |
| Postmortem urine |
GC/MS (Δ8-THC-COOH) |
SPE after alkaline hydrolysis and derivatization using BSTFA with I% trimethylchlorosilane |
(Δ9-THC-COOH: m/z, 371,473 and 488; Δ8-THC-COOH: m/z 488, 473, and 432) |
d9-Δ9-THC-COOH: (m/z 374, 476, and 491) |
[85] |
5.5. Stability of Δ8-THC and its metabolites
6. Pharmacology of Δ8-THC
6.1. CB1 and CB2 activation
6.2. Neurotransmitters’ levels and activities
6.3. Cognitive functions
6.4. Analgesic and hypothermic activities
6.5. Antiepileptic activities
6.6. Cardiovascular activities
6.7. Gastro-intestinal tract activities
6.8. Anticancer activities
| Examined cells, organs or system | Type of Study | Results | Reference |
|---|---|---|---|
| Mice, Lewis lung cells, L1210 leukemia cells, and bone marrow cells |
In vitro and In vivo | Δ8-THC showed a dosage-dependent reduction in DNA synthesis. | [135] |
| L1210 murine leukemia.. | In vivo | Among the compounds examined, Δ8-THC exhibited the highest potency, with a remarkable 99% inhibition of DNA synthesis. |
[136] |
| Mice and L1210 murine leukemia |
In vivo and in vitro | Δ8--THC didn’t Inhibit cancer cells’ respiration. | [137] |
| Human cells which metabolize polycyclic hydrocarbon carcinogens |
In vitro | Δ8-THC caused a dose-dependent inhibition of cancer cell growth in addition to a dose-dependent inhibition of [3H]thymidine, [3H]uridine and [3H]leucine incorporation. | [138] |
| Neuroblastoma cell membranes |
In vitro | Δ8-THC inhibited adenylate cyclase in plasma membranes | [139] |
| Human mdr1-gene transfected mouse lymphoma cells | In vitro | Δ8-THC exhibited membrane-associated antitumor effects and reversal of multidrug resistance. |
[140] |
| Human oral Tu183 cancer cells | In vitro | Δ8-THC exhibited dose-dependent potent inhibition against cancer cellular respiration |
[141] |
| Human oral cancer cell | In vitro | Δ8-THC promoted apoptosis and autophagy. Furthermore, it hindered cell migration and invasion. It decreased the production of reactive oxygen and increased levels of glutathione and its expression. it downregulated the expressions of cyclin D1, p53, NOXA, PUMAα, and DRAM, but upregulated the expressions of p21 and H2AX. |
[142] |
| Mice carrying Lewis lung carcinoma |
In vivo | Δ8-THC led to a dose-dependent retardation of tumor growth. Δ8-THC increased the life span of the treated mice and decreased primary tumor size. | [143,144] |
6.9. Immunomodulatory activities
| Examined cells, organs, or system |
Study Type | Results | Reference |
|---|---|---|---|
| Lymphocytes | In vitro | Δ8-THC led to a dose-dependent inability of lymphocytes to integrate the [3H] thymidine. |
[145] |
| T and B lymphocyte | In vitro | Δ8-THC inhibited the mitogen-induced T and B lymphocyte proliferation. |
[146] |
| Mouse macrophage J774-1 cells |
In vitro | Δ8-THC induced cell death of J774-1 cells in a concentration- and/or exposure time-dependent manner. Associated with vacuole formation, chromatin condensation, cell swelling, and nuclear fragmentation. |
[147] |
| BALB/c mice, Plasma and Spleen |
In vivo | Δ8-THC demonstrated a notable inhibition of direct hemolytic plaque-forming cells in the spleen on day 4. |
[148] |
| Guinea pig, Skin | In vivo | moderate (Grade III) sensitizers, causing allergic contact dermatitis. |
[149] |
| Rats, Brain | In vivo | Suppression of autoimmune encephalomyelitis by Δ8-THC was noticed and attributed to its influence on the secretion of corticosterone. |
[150] |
| Rats, Brain | In vivo | Δ8-THC reduced lever-pressing behaviour by activating the arachidonic acid cascade, leading to an elevated production of prostaglandin E2 in the brain through the CB1 receptor. |
[151] |
| Rats, Brain | In vivo | Δ8-THC suppressed lever-pressing behaviour through the activation of the prostanoid EP3 receptor by elevation of prostaglandin E2. |
[152] |
| Human B-lymphoblastoid and mouse fibroblast cell | In vitro | Δ8-THC showed antioxidative effect by preventing serum-deprived cell death that induced by anhydroretinol. | [153] |
6.10. Ocular activities
6.11. Locomotor activities
6.12. Fertility affecting activities
6.13. Antidepressant activities
6.14. Toxicity of Δ8-THC
6.15. Tolerance
6.16. Withdrawal activities
7. Conclusion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kriese, U.; Schumann, E.; Weber, W.; Beyer, M.; Brühl, L.; Matthäus. Oil content, tocopherol composition and fatty acid patterns of the seeds of 51 Cannabis sativa L. genotypes. Euphytica 2004, 137, 339–351. [Google Scholar] [CrossRef]
- Doyle, E.; Spence, A. Cannabis as a medicine? Br. J. Anaesth. 1995, 74, 359–361. [Google Scholar] [PubMed]
- Zuardi, A.W. History of cannabis as a medicine: a review. Braz. J. Psychiatry. 2006, 28, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Crocq, M.-A. History of cannabis and the endocannabinoid system. Dialogues Clin. Neurosci. 2020, 22, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Gaoni, Y.; Mechoulam, R. Isolation, structure, and partial synthesis of an active constituent of hashish. J. Am. Chem. Soc. 1964, 86, 1646–1647. [Google Scholar] [CrossRef]
- ElSohly, M.A.; Radwan, M.M.; Gul, W.; Chandra, S.; Galal, A. Phytochemistry of Cannabis sativa L. In Phytocannabinoids: Unraveling the Complex Chemistry and Pharmacology of Cannabis sativa; Kinghorn, A.D., Falk, H., Gibbons, S., Kobayashi, J.i., Eds.; Springer International Publishing: Cham, 2017; pp. 1–36. [Google Scholar]
- Turner, C.E.; Elsohly, M.A.; Boeren, E.G. Constituents of Cannabis sativa L. XVII. A review of the natural constituents. J. Nat. Prod. 1980, 43, 169–234. [Google Scholar] [CrossRef] [PubMed]
- Radwan, M.M.; Chandra, S.; Gul, S.; ElSohly, M.A. Cannabinoids, phenolics, terpenes and alkaloids of cannabis. Molecules 2021, 26, 2774. [Google Scholar] [CrossRef]
- Pertwee, R.G. Pharmacological Actions of Cannabinoids. In Cannabinoids, Pertwee, R.G., Ed.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2005; pp. 1–51. [Google Scholar]
- Maccarrone, M. Missing pieces to the endocannabinoid puzzle. Trends Mol. Med. 2020, 26, 263–272. [Google Scholar] [CrossRef]
- Alves, P.; Amaral, C.; Teixeira, N.; Correia-da-Silva, G. Cannabis sativa: Much more beyond Δ9-tetrahydrocannabinol. Pharmacol. Res. 2020, 157, 104822. [Google Scholar] [CrossRef]
- Preet, A.; Ganju, R.; Groopman, J. Δ9-Tetrahydrocannabinol inhibits epithelial growth factor-induced lung cancer cell migration in vitro as well as its growth and metastasis in vivo. Oncogene 2008, 27, 339–346. [Google Scholar] [CrossRef]
- Caffarel, M.M.; Andradas, C.; Mira, E.; Pérez-Gómez, E.; Cerutti, C.; Moreno-Bueno, G.; Flores, J.M.; García-Real, I.; Palacios, J.; Mañes, S. Cannabinoids reduce ErbB2-driven breast cancer progression through Akt inhibition. Mol. Cancer 2010, 9, 1–11. [Google Scholar] [CrossRef]
- Laezza, C.; Pagano, C.; Navarra, G.; Pastorino, O.; Proto, M.C.; Fiore, D.; Piscopo, C.; Gazzerro, P.; Bifulco, M. The endocannabinoid system: A target for cancer treatment. Int. J. Mol. Sci. 2020, 21, 747. [Google Scholar] [CrossRef]
- Pagano, C.; Navarra, G.; Coppola, L.; Bifulco, M.; Laezza, C. Molecular mechanism of cannabinoids in cancer progression. Int. J. Mol. Sci. 2021, 22, 3680. [Google Scholar] [CrossRef]
- Institute of Medicine (US). Development of Cannabinoid Drugs. In Marijuana and medicine: Assessing the science base, Joy JE, W.S.J., Benson JA Jr.,, Eds.; National Academies Press Washington (DC), USA, 1999; Volume 1999; pp. 146-168.
- Zeiger, J.S.; Haberstick, B.C.; Corley, R.P.; Ehringer, M.A.; Crowley, T.J.; Hewitt, J.K.; Hopfer, C.J.; Stallings, M.C.; Young, S.E.; Rhee, S.H. Subjective effects to marijuana associated with marijuana use in community and clinical subjects. Drug Alcohol Depend. 2010, 109, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Crean, R.D.; Crane, N.A.; Mason, B.J. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J. Addict. Med. 2011, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Curran, V.H.; Brignell, C.; Fletcher, S.; Middleton, P.; Henry, J. Cognitive and subjective dose-response effects of acute oral Δ 9-tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology 2002, 164, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Serrano, M.J.; Pérez-García, M.; Verdejo-García, A. What are the specific vs. generalized effects of drugs of abuse on neuropsychological performance? Neurosci. Biobehav. Rev. 2011, 35, 377–406. [Google Scholar] [CrossRef] [PubMed]
- D'Souza, D.C.; Perry, E.; MacDougall, L.; Ammerman, Y.; Cooper, T.; Wu, Y.-t.; Braley, G.; Gueorguieva, R.; Krystal, J.H. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology 2004, 29, 1558–1572. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.M.; Di Forti, M. Cannabis and psychosis: what degree of proof do we require? Biol. Psychiatry 2016, 79, 514–515. [Google Scholar] [CrossRef]
- Grotenhermen, F. Review of unwanted actions of cannabis and THC. In Cannabis and cannabinoids. Pharmacology, toxicology, and therapeutic potential; Russo, E.B., Grotenhermen, Franjo, Eds.; Haworth Press: New York, 2002; pp. 233–247. [Google Scholar]
- Leweke, F.M. Acute effects of cannabis and the cannabinoids. In Cannabis and Cannabinoids. Pharmacology, Toxicology and Therapeutic Potential; Russo, E.B., Grotenhermen, Franjo, Eds.; The Haworth Integrative Healing Press: New York, 2002; pp. 249–256. [Google Scholar]
- Hively, R.L.; Mosher, W.A.; Hoffmann, F.W. Isolation of trans-Δ6-tetrahydrocannabinol from marijuana. J. Am. Chem. Soc. 1966, 88, 1832–1833. [Google Scholar] [CrossRef] [PubMed]
- Hanuš, L.; Krejcí, Z. Isolation of two new cannabinoid acids from Cannabis sativa L. of Czechoslovak origin. Acta Univ Olomuc, Fac Med 1975, 74, 161–166. [Google Scholar]
- Radwan, M.M.; ElSohly, M.A.; El-Alfy, A.T.; Ahmed, S.A.; Slade, D.; Husni, A.S.; Manly, S.P.; Wilson, L.; Seale, S.; Cutler, S.J. Isolation and pharmacological evaluation of minor cannabinoids from high-potency Cannabis sativa. J. Nat. Prod. 2015, 78, 1271–1276. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Ross, S.A.; Slade, D.; Radwan, M.M.; Khan, I.A.; ElSohly, M.A. Minor oxygenated cannabinoids from high potency Cannabis sativa L. Phytochemistry 2015, 117, 194–199. [Google Scholar] [CrossRef]
- Hanuš, L.O.; Meyer, S.M.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: a unified critical inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. [Google Scholar] [CrossRef]
- Mechoulam, R.; Braun, P.; Gaoni, Y. Stereospecific synthesis of (-)-. DELTA. 1-and (-)-. DELTA. 1 (6)-tetrahydrocannabinols. J. Am. Chem. Soc. 1967, 89, 4552–4554. [Google Scholar] [CrossRef]
- Zlas, J.; Stark, H.; Seligman, J.; Levy, R.; Werker, E.; Breuer, A.; Mechoulam, R. Early medical use of cannabis. Nature 1993, 363, 215–215. [Google Scholar] [CrossRef]
- Husni, A.S.; McCurdy, C.R.; Radwan, M.M.; Ahmed, S.A.; Slade, D.; Ross, S.A.; ElSohly, M.A.; Cutler, S.J. Evaluation of phytocannabinoids from high-potency Cannabis sativa using in vitro bioassays to determine structure–activity relationships for cannabinoid receptor 1 and cannabinoid receptor 2. Med. Chem. Res. 2014, 23, 4295–4300. [Google Scholar] [CrossRef]
- Hollister, L.E.; Gillespie, H. Delta-8-and delta-9-tetrahydrocannabinol; Comparison in man by oral and intravenous administration. Clin. Pharmacol. Ther. 1973, 14, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Stern, E.; Lambert, D.M. Medicinal chemistry endeavors around the phytocannabinoids. Chem. Biodivers. 2007, 4, 1707–1728. [Google Scholar] [CrossRef] [PubMed]
- Leas, E.C.; Nobles, A.L.; Shi, Y.; Hendrickson, E. Public interest in∆ 8-Tetrahydrocannabinol (delta-8-THC) increased in US states that restricted∆ 9-Tetrahydrocannabinol (delta-9-THC) use. Int. J. Drug Policy 2022, 101, 103557. [Google Scholar] [CrossRef] [PubMed]
- Babalonis, S.; Raup-Konsavage, W.M.; Akpunonu, P.D.; Balla, A.; Vrana, K.E. Δ8-THC: legal status, widespread availability, and safety concerns. Cannabis Cannabinoid Res. 2021, 6, 362–365. [Google Scholar]
- Radwan, M.M.; Wanas, A.S.; Gul, W.; Ibrahim, E.A.; ElSohly, M.A. Isolation and Characterization of Impurities in Commercially Marketed Δ8-THC Products. J. Nat. Prod. 2023, 86, 822–829. [Google Scholar] [CrossRef]
- The U.S. Food and Drug Administration. 5 Things to know about Delta-8-Tetrahydrocannabinol-Delta-8-THC. Available online: https://www.fda.gov/consumers/consumer-updates/5-things-know-about-delta-8-tetrahydrocannabinol-delta-8-thc (accessed on November 19).
- Adams, R. Marihuana: harvey lecture, February 19, 1942. Bull N Y Acad Med 1942, 18, 705. [Google Scholar]
- Krejcí, Z.; Šantavý, F. Isolation of two new cannabinoid acids from Cannabis sativa L. of Czechoslovak origin. Acta Univ Olomuc, Fac Med 1975, 74, 161–166. [Google Scholar]
- Pars, H.G.; Razdan, R.K. Tetrahydrocannabinols and synthetic analogs. Ann. N. Y. Acad. Sci. 1971, 191, 15–22. [Google Scholar] [CrossRef]
- Turner, C.E.; Hadley, K.W.; Fetterman, P.S.; Doorenbos, N.J.; Quimby, M.W.; Waller, C. Constituents of Cannabis sativa L. IV: Stability of cannabinoids in stored plant material. J. Pharm. Sci. 1973, 62, 1601–1605. [Google Scholar] [CrossRef] [PubMed]
- Turner, C. , Hadley KW, Davis KH,. Constituents of Cannabis sativa L. V: Stability of an analytical sample extracted with chloroform. Acta Pharm. Jucoslavica 1973, 23, 89–94. [Google Scholar]
- Rzeppa, S.; Große, J.; Rautenberg, C.; Thieme, D.; Lepp, T.; Vallimäe, H.; Keiler, A.M. Emergence of the less common cannabinoid Δ8-Tetrahydrocannabinol in a doping sample. Drug Test Anal. 2021, 13, 1936–1943. [Google Scholar] [CrossRef] [PubMed]
- Chan-Hosokawa, A.; Nguyen, L.; Lattanzio, N.; Adams, W.R. Emergence of delta-8 tetrahydrocannabinol in DUID investigation casework: method development, validation and application. J. Anal. Toxicol. 2022, 46, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Leas, E.C. The hemp loophole: a need to clarify the legality of delta-8-THC and other hemp-derived tetrahydrocannabinol compounds. Am. J. Public Health 2021, 111, 1927–1931. [Google Scholar] [CrossRef] [PubMed]
- Marzullo, P.; Foschi, F.; Coppini, D.A.; Fanchini, F.; Magnani, L.; Rusconi, S.; Luzzani, M.; Passarella, D. Cannabidiol as the substrate in acid-catalyzed intramolecular cyclization. J. Nat.Prod. 2020, 83, 2894–2901. [Google Scholar] [CrossRef]
- Gaoni, Y.; Mechoulam, R. Hashish—VII: The isomerization of cannabidiol to tetrahydrocannabinols. Tetrahedron 1966, 22, 1481–1488. [Google Scholar] [CrossRef]
- Razdan, R.K. Chemistry and structure-activity relationships of cannabinoids: An overview. In The cannabinoids: Chemical, pharmacologic, and therapeutic aspects; Agurell, S., Dewey, W.L., Willette, R.E., Eds.; Academic Press: New York, U.S.A., 1984; pp. 63–78. [Google Scholar]
- Pertwee, R.G.; Cascio, M.G. Known pharmacological actions of delta-9-tetrahydrocannabinol and of four other chemical constituents of cannabis that activate cannabinoid receptors; Oxford University Press: Oxford, UK, 2014; Volume 115, pp. 137–156. [Google Scholar]
- Masoud, A.; Wingard, D. High performance liquid chromatography with electrochemical detection. 1: Separation of Cannabis constituents and quantitation of Δ9 tetrahydrocannabinol. J. High Resol. Chromatogr. 1979, 2, 118–122. [Google Scholar] [CrossRef]
- Correia, B.; Ahmad, S.M.; Quintas, A. Determination of phytocannabinoids in cannabis samples by ultrasound-assisted solid-liquid extraction and high-performance liquid chromatography with diode array detector analysis. J. Chromatogr. A. 2023, 1705, 464191. [Google Scholar] [CrossRef] [PubMed]
- Elhendawy, M.A.; Radwan, M.M.; Ibrahim, E.A.; Wanas, A.S.; Chandra, S.; Godfrey, M.; ElSohly, M.A. Validation and Quantitation of Fifteen Cannabinoids in Cannabis and Marketed Products Using High-Performance Liquid Chromatography-Ultraviolet/Photodiode Array Method. Cannabis Cannabinoid Res. 2023. [Google Scholar] [CrossRef]
- Duffy, B.C.; Li, L.; Lu, S.; Dittmar, M.A.; Delaney-Baldwin, E.; Durocher, L.A.; Spink, D.C. Chemotyping of∆ 8-THC-Containing e-Liquids Analyzed during the 2019–2020 New York State EVALI Investigation. J. Anal. Toxicol. 2022, 46, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-H.; Avula, B.; ElSohly, M.A.; Radwan, M.M.; Wang, M.; Wanas, A.S.; Mehmedic, Z.; Khan, I.A. Quantitative determination of Δ9-THC, CBG, CBD, their acid precursors and five other neutral cannabinoids by UHPLC-UV-MS. Planta Med. 2018, 84, 260–266. [Google Scholar] [CrossRef]
- Križman, M. A simplified approach for isocratic HPLC analysis of cannabinoids by fine tuning chromatographic selectivity. Eur. Food Res. Technol. 2020, 246, 315–322. [Google Scholar] [CrossRef]
- Song, L.; Carlson, S.; Valenzuela, G.; Chao, M.; Pathipaka, S.B. Development of a validated method for rapid quantification of up to sixteen cannabinoids using ultra-high-performance liquid chromatography diode-array detector with optional electrospray ionization time-of-flight mass spectrometry detection. J. Chromatogr. A. 2022, 1670, 462953. [Google Scholar] [CrossRef]
- Madden, O.; Walshe, J.; Kishore Patnala, P.; Barron, J.; Meaney, C.; Murray, P. Phytocannabinoids-An Overview of the Analytical Methodologies for Detection and Quantification of Therapeutically and Recreationally Relevant Cannabis Compounds. Critical Reviews in Analytical Chemistry 2023, 53, 211–231. [Google Scholar] [CrossRef]
- Christinat, N.; Savoy, M.-C.; Mottier, P. Development, validation and application of a LC-MS/MS method for quantification of 15 cannabinoids in food. Food Chem. 2020, 318, 126469. [Google Scholar] [CrossRef] [PubMed]
- Berman, P.; Futoran, K.; Lewitus, G.M.; Mukha, D.; Benami, M.; Shlomi, T.; Meiri, D. A new ESI-LC/MS approach for comprehensive metabolic profiling of phytocannabinoids in Cannabis. Sci. Rep. 2018, 8, 14280. [Google Scholar] [CrossRef] [PubMed]
- Trofin, I.G.; Vlad, C.C.; Noja, V.V.; Dabija, G. Identification and characterization of special types of herbal cannabis. UPB Sci. Bull., Ser. B 2012, 74, 119–130. [Google Scholar]
- United States H.R.2. Agriculture Improvement Act of 2018. In: 115th United States Congress. Available online: https://www.congress.gov/bill/115th-congress/house-bill/2 (accessed on December 6).
- Drug Enforcement Administration (DEA), D.o.J. Implementation of the Agriculture Improvement Act of 2018. Available online: https://www.federalregister.gov/documents/2020/08/21/2020-17356/implementation-of-the-agriculture-improvement-act-of-2018 (accessed on November 20).
- Razdan, R.K.; Dalzell, H.C.; Handrick, G.R. Hashish. X. Simple one-step synthesis of (-)-. DELTA. 1-tetrahydrocannabinol (THC) from p-mentha-2, 8-dien-1-ol and olivetol. J. Am. Chem. Soc. 1974, 96, 5860–5865. [Google Scholar] [CrossRef]
- Malkov, A.V.; Kočovský, P. Tetrahydrocannabinol revisited: Synthetic approaches utilizing molybdenum catalysts. Collect. Czechoslov. Chem. Commun. 2001, 66, 1257–1268. [Google Scholar] [CrossRef]
- Meehan-Atrash, J.; Rahman, I. Novel Δ8-tetrahydrocannabinol vaporizers contain unlabeled adulterants, unintended byproducts of chemical synthesis, and heavy metals. Chem. Res. Toxicol. 2021, 35, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Ray, C.L.; Bylo, M.P.; Pescaglia, J.; Gawenis, J.A.; Greenlief, C.M. Delta-8 tetrahydrocannabinol product impurities. Molecules 2022, 27, 6924. [Google Scholar] [CrossRef]
- Ciolino, L.A.; Ranieri, T.L.; Brueggemeyer, J.L.; Taylor, A.M.; Mohrhaus, A.S. EVALI vaping liquids part 1: GC-MS cannabinoids profiles and identification of unnatural THC isomers. Front. Chem. 2021, 9, 746479. [Google Scholar] [CrossRef]
- Burstein, S.H.; Menezes, F.; Williamson, E.; MECHOULAM, R. Metabolism of Δ1 (6)-Tetrahydro-cannabinol, an active marihuana constituent. Nature 1970, 225, 87–88. [Google Scholar] [CrossRef]
- Watanabe, K.; Yamaori, S.; Funahashi, T.; Kimura, T.; Yamamoto, I. Cytochrome P450 enzymes involved in the metabolism of tetrahydrocannabinols and cannabinol by human hepatic microsomes. Life Sci 2007, 80, 1415–1419. [Google Scholar] [CrossRef]
- Huestis, M.A. Human cannabinoid pharmacokinetics. Chem. Biodivers. 2007, 4, 1770. [Google Scholar] [CrossRef] [PubMed]
- Tagen, M.; Klumpers, L.E. Review of delta-8-tetrahydrocannabinol (Δ8-THC): Comparative pharmacology with Δ9-THC. Br. J. Pharmacol. 2022, 179, 3915–3933. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Yamamoto, I.; Oguri, K.; Yoshimura, H. Metabolic disposition of delta 8-tetrahydrocannabinol and its active metabolites, 11-hydroxy-delta 8-tetrahydrocannabinol and 11-oxo-delta 8-tetrahydrocannabinol, in mice. Drug Metab. Dispos. 1981, 9, 261–264. [Google Scholar] [PubMed]
- Rosenthal, J.; Howell, M.; Earl, V.; Malik, M. Cannabinoid hyperemesis syndrome secondary to delta-8 THC use. Am. J. Med. 2021, 134, e582–e583. [Google Scholar] [CrossRef] [PubMed]
- Helander, A.; Johansson, M.; Andersson, A.; Villén, T. Analytical and medico-legal problems linked to the presence of delta-8-tetrahydrocannabinol (delta-8-THC): Results from urine drug testing in Sweden. Drug Test Anal. 2022, 14, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Valiveti, S.; Hammell, D.C.; Earles, D.C.; Stinchcomb, A.L. LC–MS method for the estimation of Δ8-THC and 11-nor-Δ8-THC-9-COOH in plasma. J. Pharm. Biomed. Anal. 2005, 38, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Akpunonu, P.; Baum, R.A.; Reckers, A.; Davidson, B.; Ellison, R.; Riley, M.; Trecki, J.; Gerona, R. Sedation and acute encephalopathy in a pediatric patient following ingestion of delta-8-tetrahydrocannabinol gummies. Am. J. Case Rep. 2021, 22, e933488–933481. [Google Scholar] [CrossRef]
- Vikingsson, S.; Hart, E.D.; Winecker, R.E.; Cone, E.J.; Kuntz, D.J.; Clark, M.; Jacques, M.; Hayes, E.D.; Flegel, R.R. Prevalence of∆ 8-tetrahydrocannabinol carboxylic acid in workplace drug testing. J. Anal. Toxicol. 2023, 47, 719–725. [Google Scholar] [CrossRef]
- Sim, Y.E.; Kim, J.W.; Ko, B.J.; Kim, J.Y.; Cheong, J.C.; Pyo, J. Determination of urinary metabolites of cannabidiol, Δ8-tetrahydrocannabinol, and Δ9-tetrahydrocannabinol by automated online μSPE–LC–MS/MS method. J. Chromatogr. B 2023, 1214, 123568. [Google Scholar] [CrossRef]
- Lin, L.; Amaratunga, P.; Reed, J.; Huang, P.; Lemberg, B.L.; Lemberg, D. Quantitation of Δ8-THC, Δ9-THC, cannabidiol and 10 other cannabinoids and metabolites in oral fluid by HPLC–MS-MS. J. Anal. Toxicol. 2022, 46, 76–88. [Google Scholar] [CrossRef]
- Coulter, C.; Wagner, J.R. Cannabinoids in oral fluid: limiting potential sources of cannabidiol conversion to Δ9-and Δ8-tetrahydrocannabinol. J. Anal. Toxicol. 2021, 45, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Milman, G.; Barnes, A.J.; Schwope, D.M.; Schwilke, E.W.; Darwin, W.D.; Goodwin, R.S.; Kelly, D.L.; Gorelick, D.A.; Huestis, M.A. Disposition of cannabinoids in oral fluid after controlled around-the-clock oral THC administration. Clin. Chem. 2010, 56, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Hädener, M.; König, S.; Fabritius, M.M.; Weinmann, W. Using THC-COOH cut-off concentrations for assessing cannabis consumption frequency: A recently detected THC-COOH isomer poses an important analytical problem. Toxichem Krimtech 2017, 84, 168. [Google Scholar]
- Baird, S.N.; Frazee III, C.C.; Garg, U. Evaluation of a Delta-9-Tetrahydrocannabinol Carboxylic Acid (Δ9-THC-COOH) Immunoassay and a Gas Chromatography–Mass Spectrometry (GC-MS) Method for the Detection of Delta-8-Tetrahydrocannabinol Carboxylic Acid (Δ8-THC-COOH). J. Lab. Med 2023, 8, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Crosby, M.M.; Truver, M.T.; Chronister, C.W.; Hoyer, J.L.; Kinsey, A.M.; Brogan, S.C.; Goldberger, B.A. Identification of 11-nor-∆ 8-Tetrahydrocannabinol-9-Carboxylic Acid in Postmortem Urine. J. Anal. Toxicol. 2023, 47, 481–487. [Google Scholar] [CrossRef]
- Pokhai, A.A.; Poklis, J.L.; Williams, G.R.; Wolf, C.E. The preanalytical stability of emerging cannabinoid analogs (delta-8 THC and its metabolites, delta-10 THC and carboxy-HHC) in urine. J. Anal. Toxicol. 2023, 47, 726–731. [Google Scholar] [CrossRef]
- Morin, M.M.; Baum, M. Effects of delta8 tetrahydrocannabinol (THC) on avoidance extinction in rats. Psychon. Bull. Rev. 1986, 24, 385–387. [Google Scholar] [CrossRef]
- Christensen, H.; Freudenthal, R.; Gidley, J.; Rosenfeld, R.; Boegli, G.; Testino, L.; Brine, D.; Pitt, C.; Wall, M. Activity of Δ8-and Δ9-tetrahydrocannabinol and related compounds in the mouse. Science 1971, 172, 165–167. [Google Scholar] [CrossRef]
- Pertwee, R.G.; Ross, R. Cannabinoid receptors and their ligands. Prostaglandins Leukot. Essent. Fatty Acids 2002, 66, 101–121. [Google Scholar] [CrossRef]
- Mackie, K. Cannabinoid receptors: where they are and what they do. J. Neuroendocrinol. 2008, 20, 10–14. [Google Scholar] [CrossRef]
- Mechoulam, R.; Parker, L.A. The endocannabinoid system and the brain. Annu. Rev. Psychol. 2013, 64, 21–47. [Google Scholar] [CrossRef]
- Govaerts, S.J.; Hermans, E.; Lambert, D.M. Comparison of cannabinoid ligands affinities and efficacies in murine tissues and in transfected cells expressing human recombinant cannabinoid receptors. Eur. J. Pharm. Sci. 2004, 23, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Nadipuram, A.K.; Krishnamurthy, M.; Ferreira, A.M.; Li, W.; Moore II, B.M. Synthesis and testing of novel classical cannabinoids: exploring the side chain ligand binding pocket of the CB1 and CB2 receptors. Bioorg. Med. Chem. 2003, 11, 3121–3132. [Google Scholar] [CrossRef]
- Huffman, J.W.; Liddle, J.; Yu, S.; Aung, M.M.; Abood, M.E.; Wiley, J.L.; Martin, B.R. 3-(1′, 1′-Dimethylbutyl)-1-deoxy-Δ8-THC and related compounds: synthesis of selective ligands for the CB2 receptor. Bioorg. Med. Chem. 1999, 7, 2905–2914. [Google Scholar] [CrossRef] [PubMed]
- Hirst, R.A.; Almond, S.L.; Lambert, D.G. Characterisation of the rat cerebella CB1 receptor using SR141716A, a central cannabinoid receptor antagonist. Neurosci. Lett. 1996, 220, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.R.; Compton, D.R.; Semus, S.F.; Lin, S.; Marciniak, G.; Grzybowska, J.; Charalambous, A.; Makriyannis, A. Pharmacological evaluation of iodo and nitro analogs of Δ8-THC and Δ9-THC. Pharmacol. Biochem. Behav.r 1993, 46, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Huffman, J.W.; Miller, J.R.; Liddle, J.; Yu, S.; Thomas, B.F.; Wiley, J.L.; Martin, B.R. Structure–activity relationships for 1′, 1′-dimethylalkyl-Δ8-tetrahydrocannabinols. Bioorg. Med. Chem. 2003, 11, 1397–1410. [Google Scholar] [CrossRef]
- Busch-Petersen, J.; Hill, W.A.; Fan, P.; Khanolkar, A.; Xie, X.-Q.; Tius, M.A.; Makriyannis, A. Unsaturated side chain β-11-hydroxyhexahydrocannabinol analogs. J. Med. Chem 1996, 39, 3790–3796. [Google Scholar] [CrossRef]
- Charalambous, A.; Lin, S.; Marciniak, G.; Banijamali, A.; Friend, F.; Compton, D.; Martin, B.; Makriyannis, A. Pharmacological evaluation of halogenated Δ8-THC analogs. Pharmacol. Biochem. Behav. 1991, 40, 509–512. [Google Scholar] [CrossRef]
- Papahatjis, D.P.; Nahmias, V.R.; Andreou, T.; Fan, P.; Makriyannis, A. Structural modifications of the cannabinoid side chain towards C3-aryl and 1′, 1′-cycloalkyl-1′-cyano cannabinoids. Bioorganic & medicinal chemistry letters 2006, 16, 1616–1620. [Google Scholar]
- Darmani, N.A.; Janoyan, J.J.; Crim, J.; Ramirez, J. Receptor mechanism and antiemetic activity of structurally-diverse cannabinoids against radiation-induced emesis in the least shrew. Eur. J. Pharmacol. 2007, 563, 187–196. [Google Scholar] [CrossRef]
- Domino, E.F. Cannabinoids and the cholinergic system. J Clin Pharmacol. 1981, 21, 249S–255S. [Google Scholar] [CrossRef]
- Tripathi, H.; Vocci, F.; Brase, D.; Dewey, W. Effects of cannabinoids on levels of acetylcholine and choline and on turnover rate of acetylcholine in various regions of the mouse brain. J. Drug Alcohol Res. 1987, 7, 525–532. [Google Scholar]
- Clarke, D.; Jandhyala, B. Acute and chronic effects of tetrahydrocannabinols on monoamide oxidase activity: possible vehicle/tetrahydrocannabinol interactions. Res. commun. chem. pathol. pharmacol. 1977, 17, 471–480. [Google Scholar] [PubMed]
- Avraham, Y.; Ben-Shushan, D.; Breuer, A.; Zolotarev, O.; Okon, A.; Fink, N.; Katz, V.; Berry, E.M. Very low doses of Δ8-THC increase food consumption and alter neurotransmitter levels following weight loss. Pharmacol. Biochem. Behav. 2004, 77, 675–684. [Google Scholar] [CrossRef]
- Darmani, N.A. Cannabinoids of diverse structure inhibit two DOI-induced 5-HT2A receptor-mediated behaviors in mice. Pharmacol. Biochem. Behav.r 2001, 68, 311–317. [Google Scholar] [CrossRef]
- MacLean, K.I.; Littleton, J.M. Environmental stress as a factor in the response of rat brain catecholamine metabolism to Δ8-tetrahydrocannabinol. Eur. J. Pharmacol. 1977, 41, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Poddar, M.; Dewey, W. Effects of cannabinoids on catecholamine uptake and release in hypothalamic and striatal synaptosomes. J. Pharmacol. Exp. Ther. 1980, 214, 63–67. [Google Scholar]
- Sagratella, S.; de Carolis, A.S.; Longo, V. EEG interaction between delta 8-tetrahydrocannabinol and some sedative-anxiolytic drugs. Is the anxiogenic effect of cannabis related to an action on the GABAergic system? Adv. biochem. psychopharmacol. 1986, 41, 203–209. [Google Scholar]
- Little, P.J.; Martin, B.R. The effects of Δ9-tetrahydrocannabinol and other cannabinoids on cAMP accumulation in synaptosomes. Life Sci. 1991, 48, 1133–1141. [Google Scholar] [CrossRef]
- JÄrbe, T.U.; Henriksson, B.G. Effects of Δ 8-THC, and Δ 9-THC on the acquisition of a discriminative positional habit in rats: The transitions between normal and tetrahydrocannabinol-induced states on reversal learning. Psychopharmacologia 1973, 31, 321–332. [Google Scholar] [CrossRef]
- Järbe, T.; Johansson, J.; Henriksson, B. Characteristics of tetrahydrocannabinol (THC)-produced discrimination in rats. Psychopharmacologia 1976, 48, 181–187. [Google Scholar] [CrossRef]
- JÄrbe, T.U.; Henriksson, B.G. Discriminative response control produced with hashish, tetrahydrocannabinols (Δ 8-THC and Δ 9-THC), and other drugs. Psychopharmacologia 1974, 40, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bueno, O.; Carlini, E.; Finkelfarb, E.; Suzuki, J.S. Δ 9-Tetrahydrocannabinol, ethanol, and amphetamine as discriminative stimuli-generalization tests with other drugs. Psychopharmacologia 1976, 46, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Doty, P.; Dykstra, L.A.; Picker, M.J. Discriminative stimulus effects of phencyclidine: pharmacologically specific interactions with Δ9-and Δ8-tetrahydrocannabinol. Drug Alcohol Depend. 1994, 35, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Järbe, T.U.; Henriksson, B.G.; Ohlin, G.C. Delta9-THC as a discriminative cue in pigeons: effects of delta8-THC, CBD, and CBN. Arch. Int. Pharmacodyn. Ther. 1977, 228, 68–72. [Google Scholar] [PubMed]
- Hine, B.; Torrelio, M.; Gershon, S. Analgesic, heart rate, and temperature effects of Δ8-THC during acute and chronic administration to conscious rats. Pharmacology 1977, 15, 65–72. [Google Scholar] [CrossRef]
- Durbin, D.J.; King, J.M.; Stairs, D.J. Behavioral Effects of Vaporized Delta-8 Tetrahydrocannabinol, Cannabidiol, and Mixtures in Male Rats. Cannabis Cannabinoid Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Chesher, G.; Dahl, C.; Everingham, M.; Jackson, D.; Marchant-Williams, H.; Starmer, G. The effect of cannabinoids on intestinal motility and their antinociceptive effect in mice. Br. J. Pharmacol. 1973, 49, 588–594. [Google Scholar] [CrossRef]
- Welch, S.P.; Stevens, D.L. Antinociceptive activity of intrathecally administered cannabinoids alone, and in combination with morphine, in mice. J. Pharmacol. Exp. Ther. 1992, 262, 10–18. [Google Scholar]
- Welch, S.P.; Thomas, C.; Patrick, G.S. Modulation of cannabinoid-induced antinociception after intracerebroventricular versus intrathecal administration to mice: possible mechanisms for interaction with morphine. J. Pharmacol. Exp. Ther. 1995, 272, 310–321. [Google Scholar]
- El-Alfy, A.T.; Ivey, K.; Robinson, K.; Ahmed, S.; Radwan, M.; Slade, D.; Khan, I.; ElSohly, M.; Ross, S. Antidepressant-like effect of Δ9-tetrahydrocannabinol and other cannabinoids isolated from Cannabis sativa L. Pharmacol. Biochem. Behav. 2010, 95, 434–442. [Google Scholar] [CrossRef]
- Welch, S.P.; Huffman, J.W.; Lowe, J. Differential blockade of the antinociceptive effects of centrally administered cannabinoids by SR141716A. J. Pharmacol. Exp. Ther. 1998, 286, 1301–1308. [Google Scholar]
- Consroe, P.F.; Man, D.P.; Chin, L.; Picchioni, A.L. Reduction of audiogenic seizure by Δ8-and Δ9-tetrahydrocannabinols. J. Pharm. Pharmacol. 1973, 25, 764–765. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, M.E.; McCaughran Jr, J.A.; Wada, J.A. Antiepileptic and prophylactic effects of tetrahydrocannabinols in amygdaloid kindled rats. Epilepsia 1978, 19, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Wada, J.A.; Osawa, T.; Corcoran, M.E. Effects of tetrahydrocannabinols on kindled amygdaloid seizures and photogenic seizures in Senegalese baboons, Papio papio. Epilepsia 1975, 16, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Wada, J.A.; Wake, A.; Sato, M.; Corcoran, M.E. Antiepileptic and prophylactic effects of tetrahydrocannabinols in amygdaloid kindled cats. Epilepsia 1975, 16, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Brenda, K.C.; Charles, L.; Charles, R.C. Effects of marihuana cannabinoids on seizure activity in cobalt-epileptic rats. Pharmacol. Biochem. Behav. 1982, 16, 573–578. [Google Scholar]
- Chandradhar, D.; Raymond, D.H. Anticonvulsant activities of Δ-8 and Δ-9 tetrahydrocanabinol and uridine. Toxicol. Appl. Pharmacol. 1975, 31, 452–458. [Google Scholar]
- Johansson, J.; Jarbe, T.; Henriksson, B. Acute and subchronic influences of tetrahydrocannabinols on water and food intake, body weight, and temperature in rats. T.-I.-T. J. Life Sci. 1975, 5, 17–27. [Google Scholar]
- Smiley, K.; Karler, R.; Turkanis, S. Effects of cannabinoids on the perfused rat heart. Res. commun. chem. pathol. pharmacol. 1976, 14, 659–675. [Google Scholar]
- Coffey, R.G.; Yamamoto, Y.; Snella, E.; Pross, S. Tetrahydrocannabinol inhibition of macrophage nitric oxide production. Biochem. Pharmacol. 1996, 52, 743–751. [Google Scholar] [CrossRef]
- Adams, M.; Earnhardt, J.; Dewey, W.; Harris, L. Vasoconstrictor actions of delta8-and delta9-tetrahydrocannabinol in the rat. J. Pharmacol. Exp. Ther. 1976, 196, 649–656. [Google Scholar]
- Abrahamov, A.; Abrahamov, A.; Mechoulam, R. An efficient new cannabinoid antiemetic in pediatric oncology. Life Sci. 1995, 56, 2097–2102. [Google Scholar] [CrossRef]
- Carchman, R.A.; Harris, L.S.; Munson, A.E. The inhibition of DNA synthesis by cannabinoids. Cancer Res. 1976, 36, 95–100. [Google Scholar]
- Tucker, A.N.; Friedman, M.A. Effects of cannabinoids on L1210 murine leukemia. 1. Inhibition of DNA synthesis. Res. commun. chem. pathol. pharmacol. 1977, 17, 703–714. [Google Scholar] [PubMed]
- Tucker, A.N.; Friedman, M.A. Effects of cannabinoids on L1210 murine leukemia. III. Inhibition of respiration. Res. commun. chem. pathol. pharmacol. 1979, 23, 327–332. [Google Scholar] [PubMed]
- Green, L.G.; Stein, J.L.; Stein, G.S. A decreased influence of cannabinoids on macromolecular biosynthesis and cell proliferation in human cells which metabolize polycyclic hydrocarbon carcinogens. Anticancer Res. 1983, 3, 211–217. [Google Scholar] [PubMed]
- Howlett, A.C.; Fleming, R.M. Cannabinoid inhibition of adenylate cyclase. Pharmacology of the response in neuroblastoma cell membranes. Mol. Pharmacol. 1984, 26, 532–538. [Google Scholar] [PubMed]
- Molnár, J.; Szabó, D.; Pusztai, R.; Mucsi, I.; Berek, L.; Ocsovszki, I.; Kawata, E.; Shoyama, Y. Membrane associated antitumor effects of crocine-, ginsenoside- and cannabinoid derivates. Anticancer Res. 2000, 20, 861–867. [Google Scholar] [PubMed]
- Whyte, D.A.; Al-Hammadi, S.; Balhaj, G.; Brown, O.M.; Penefsky, H.S.; Souid, A.K. Cannabinoids inhibit cellular respiration of human oral cancer cells. Pharmacology 2010, 85, 328–335. [Google Scholar] [CrossRef]
- Semlali, A.; Beji, S.; Ajala, I.; Rouabhia, M. Effects of tetrahydrocannabinols on human oral cancer cell proliferation, apoptosis, autophagy, oxidative stress, and DNA damage. Arch. Oral Biol. 2021, 129, 105200. [Google Scholar] [CrossRef]
- Munson, A.E.; Harris, L.S.; Friedman, M.A.; Dewey, W.L.; Carchman, R.A. Antineoplastic Activity of Cannabinoids2. JNCI: J. Natl. Cancer Inst. 1975, 55, 597–602. [Google Scholar] [CrossRef]
- Friedman, M.A. In vivo effects of cannabinoids on macromolecular biosynthesis in Lewis lung carcinomas. Cancer Biochem. Biophys. 1977, 2, 51–54. [Google Scholar]
- Nahas, G.G.; Morishima, A.; Desoize, B. Effects of cannabinoids on macromolecular synthesis and replication of cultured lymphocytes. Fed. Proc. 1977, 36, 1748–1752. [Google Scholar] [PubMed]
- Schwarz, H.; Blanco, F.J.; Lotz, M. Anadamide, an endogenous cannabinoid receptor agonist inhibits lymphocyte proliferation and induces apoptosis. J. Neuroimmunol. 1994, 55, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Yamaori, S.; Ishii, H.; Chiba, K.; Yamamoto, I.; Watanabe, K. Δ-Tetrahydrocannabinol induces cytotoxicity in macrophage J774-1 cells: involvement of cannabinoid receptor 2 and p38 MAPK. Toxicology 2013, 314, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Loveless, S.; Harris, L.; Munson, A. Hyporesponsiveness to the immunosuppressant effects of delta-8-tetrahydrocannabinol. J. Immunopharmacol. 1981, 3, 371–384. [Google Scholar] [CrossRef]
- Watson, E.S.; Murphy, J.C.; Turner, C.E. Allergenic properties of naturally occurring cannabinoids. J. Pharm. Sci. 1983, 72, 954–955. [Google Scholar] [CrossRef] [PubMed]
- Wirguin, I.; Mechoulam, R.; Breuer, A.; Schezen, E.; Weidenfeld, J.; Brenner, T. Suppression of experimental autoimmune encephalomyelitis by cannabinoids. Immunopharmacol. 1994, 28, 209–214. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Shoyama, Y.; Watanabe, S.; Yamamoto, T. Behavioral suppression induced by cannabinoids is due to activation of the arachidonic acid cascade in rats. Brain Res. 2001, 889, 149–154. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Kubota, T.; Watanabe, S.; Yamamoto, T. Activation of brain prostanoid EP3 receptors via arachidonic acid cascade during behavioral suppression induced by Delta8-tetrahydrocannabinol. J. Neurochem. 2004, 88, 148–154. [Google Scholar] [CrossRef]
- Chen, Y.; Buck, J. Cannabinoids protect cells from oxidative cell death: a receptor-independent mechanism. J. Pharmacol. Exp. Ther. 2000, 293, 807–812. [Google Scholar]
- Thapa, D.; Cairns, E.A.; Szczesniak, A.-M.; Toguri, J.T.; Caldwell, M.D.; Kelly, M.E. The cannabinoids Δ8THC, CBD, and HU-308 act via distinct receptors to reduce corneal pain and inflammation. Cannabis cannabinoid Res. 2018, 3, 11–20. [Google Scholar] [CrossRef]
- Punyamurthula, N.S.; Adelli, G.R.; Gul, W.; Repka, M.A.; ElSohly, M.A.; Majumdar, S. Ocular disposition of ∆8-tetrahydrocannabinol from various topical ophthalmic formulations. AAPS PharmSciTech 2017, 18, 1936–1945. [Google Scholar] [CrossRef]
- Muchtar, S.; Almog, S.; Torracca, M.; Saettone, M.; Benita, S. A submicron emulsion as ocular vehicle for delta-8-tetrahydrocannabinol: effect on intraocular pressure in rabbits. Ophthalmic Res. 1992, 24, 142–149. [Google Scholar] [CrossRef]
- Elsohly, M.; Harland, E.; Murphy, J.; Wirth, P.; Waller, C. Cannabinoids in glaucoma: a primary screening procedure. J. Clin. Pharmacol. 1981, 21, 472S–478S. [Google Scholar] [CrossRef] [PubMed]
- Elsohly, M.A.; Harland, E.C.; Benigni, D.A.; Waller, C.W. Cannabinoids in glaucoma II: the effect of different cannabinoids on intraocular pressure of the rabbit. Curr. Eye Res. 1984, 3, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Green, K.; Symonds, C.M.; Oliver, N.W.; Elijah, R.D. Intraocular pressure following systemic administration of cannabinoids. Curr. Eye Res. 1982, 2, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Badawy, Z.S.; Chohan, K.R.; Whyte, D.A.; Penefsky, H.S.; Brown, O.M.; Souid, A.-K. Cannabinoids inhibit the respiration of human sperm. Fertil. Steril. 2009, 91, 2471–2476. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Elbracht, C. Effect of tetrahydrocannabinols on pubertal body weight spurt and sex hormones in developing male rats. Res. Exp. Med. 1983, 182, 95–104. [Google Scholar] [CrossRef]
- Knopf, A. CDC and FDA warn of delta-8 THC harms. Alcoholism & Drug Abuse Weekly 2021, 33, 7–7. [Google Scholar]
- Erickson, B.E. Delta-8-THC craze concerns chemists. Chem. Eng. News 2021, 99, 25–28. [Google Scholar]
- Leas, E.C.; Harati, R.M.; Satybaldiyeva, N.; Morales, N.E.; Huffaker, S.L.; Mejorado, T.; Grant, I. Self-reported adverse events associated with∆ 8-Tetrahydrocannabinol (Delta-8-THC) Use. J Cannabis Res. 2023, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.J.; Swenson, C.; Fishkin, I.; Malanga, A. Now I know my CBDs; cases of psychiatric admissions after delta-8-tetrahydrocannabinol (delta-8-THC, Δ⁸-THC) product usage. Psychiatry Res. Case Rep. 2023, 2, 100166. [Google Scholar] [CrossRef]
- Shaker, K.; Nillas, A.; Ellison, R.; Martin, K.; Trecki, J.; Gerona, R.; Aldy, K. Delta-8-tetrahydrocannabinol exposure and confirmation in four pediatric patients. J Med Toxicol. 2023, 19, 190–195. [Google Scholar] [CrossRef]
- Wrenn, J.; Friedman, M. Effects of chronic administration of delta8-and delta9-tetrahydro-cannabinol on hepatic tyrosine aminotransferase activity in mice. Arch. Int. Pharmacodyn. Ther. 1978, 235, 4–8. [Google Scholar]
- Bozman, M.E.; Manoharan, S.V.R.R.; Vasavada, T. Marijuana variant of concern: delta 8-tetrahydrocannabinol (delta-8-THC, Δ8-THC). Psychiatry Res. Case Rep. 2022, 1, 100028. [Google Scholar] [CrossRef]
- Watanabe, K.; Narimatsu, S.; Yamamoto, I.; Yoshimura, H. Difference in tolerance development to hypothermia and pentobarbital-induced sleep prolongating effect of 11-hydroxy-Δ8-tetrahydrocannabinol and 11-oxo-Δ8-tetrahydrocannabinol in mice. Eur. J. Pharmacol. 1982, 77, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Yamamoto, I.; Yoshimura, H. Development of tolerance and cross-tolerance to the cataleptogenic effects of Δ8-tetrahydrocannabinol and 11-hydroxy-Δ8-tetrahydrocannabinol in mice. Eur. J. Pharmacol. 1983, 94, 349–351. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, I.; Watanabe, K.; Narimatsu, S.; Hamajima, K.; Yoshimura, H. Cross-tolerance to the hypothermic effect of Δ8-tetrahydrocannabinol 11-hydroxy-Δ8-tetrahydrocannabinol and chlorpromazine in the mouse. Eur. J. Pharmacol. 1985, 111, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Narimatsu, S.; Yamamoto, I.; Watanabe, K.; Yoshimura, H. Change in hypothermia and catalepsy induced by cannabinoids or morphine in mice tolerant to these substances. Eur. J. Pharmacol. 1987, 141, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Sjödén, P.-O.; Järbe, T.U.; Henriksson, B.G. Effects of long-term administration and withdrawal of tetrahydrocannabinols (Δ8-THC and Δ9-THC) on open-field behavior in rats. Pharm. Biochem. Behav. 1973, 1, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Hagiwara, Y.; Tanaka, H.; Sugiura, T.; Waku, K.; Shoyama, Y.; Watanabe, S.; Yamamoto, T. Endogenous cannabinoid, 2-arachidonoylglycerol, attenuates naloxone-precipitated withdrawal signs in morphine-dependent mice. Brain Res. 2001, 909, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, H.N. Time course of the effects of naturally occurring cannabinoids on morphine abstinence syndrome. Pharm. Biochem. Behav. 1978, 8, 7–11. [Google Scholar] [CrossRef]
- Nakamura-Palacios, E.M.; Bueno, O.F.A.; Takahashi, R.N.; Tufik, S. Acute or chronic effects of cannabinoids on spontaneous or pharmacologically induced yawning in rats. Pharm. Biochem. Behav. 2002, 74, 205–212. [Google Scholar] [CrossRef]
- Anggadiredja, K.; Yamaguchi, T.; Tanaka, H.; Shoyama, Y.; Watanabe, S.; Yamamoto, T. Decrease in prostaglandin level is a prerequisite for the expression of cannabinoid withdrawal: a quasi abstinence approach. Brain Res. 2005, 1066, 201–205. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





