Submitted:
17 February 2024
Posted:
19 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and methods
2.1. Apparatus
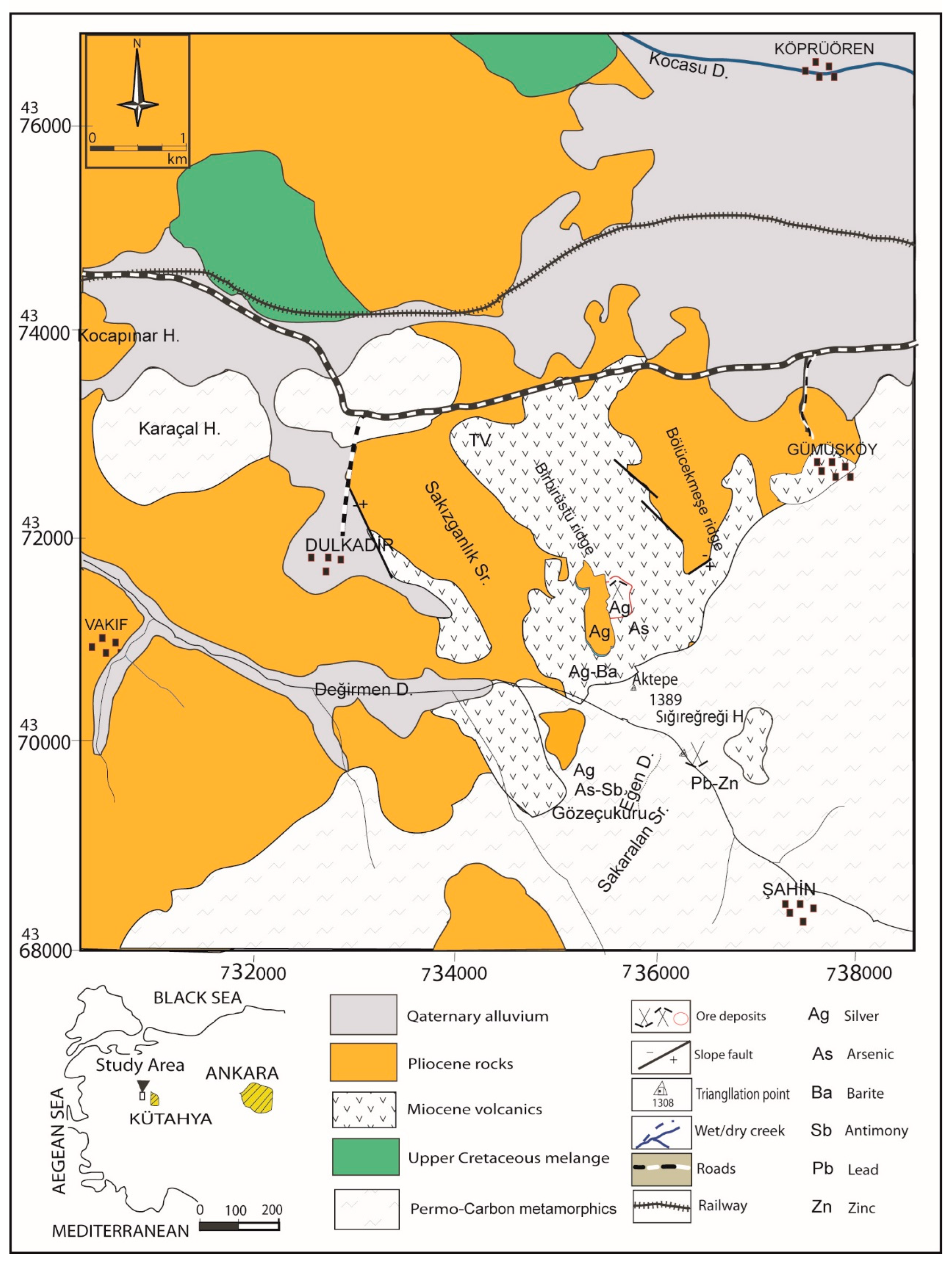
2.2. The study area
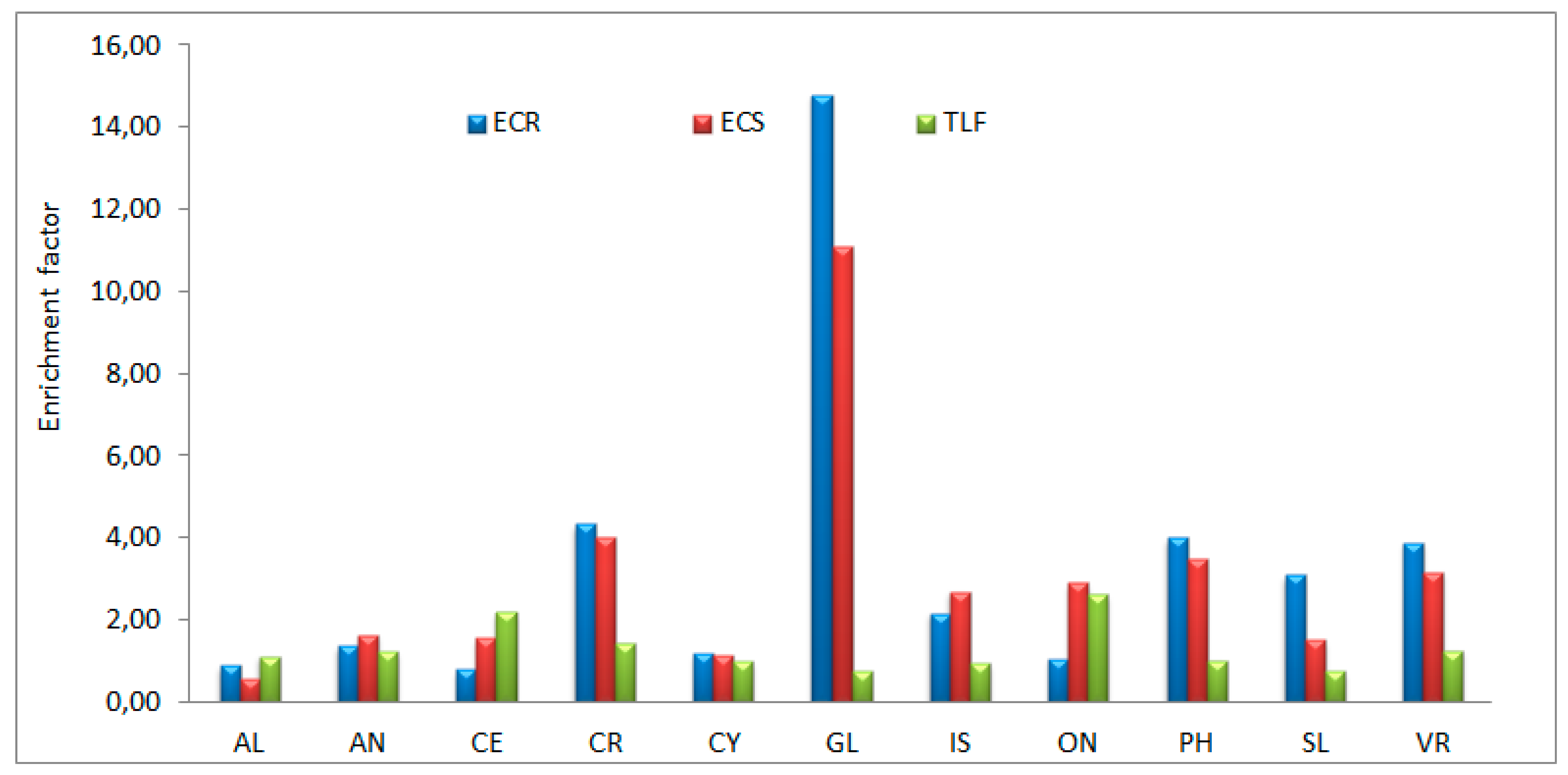
2.4. ECR
2.5. ECS
2.6. TLF
2.7. Analytical statistics
3. Results and Discussion
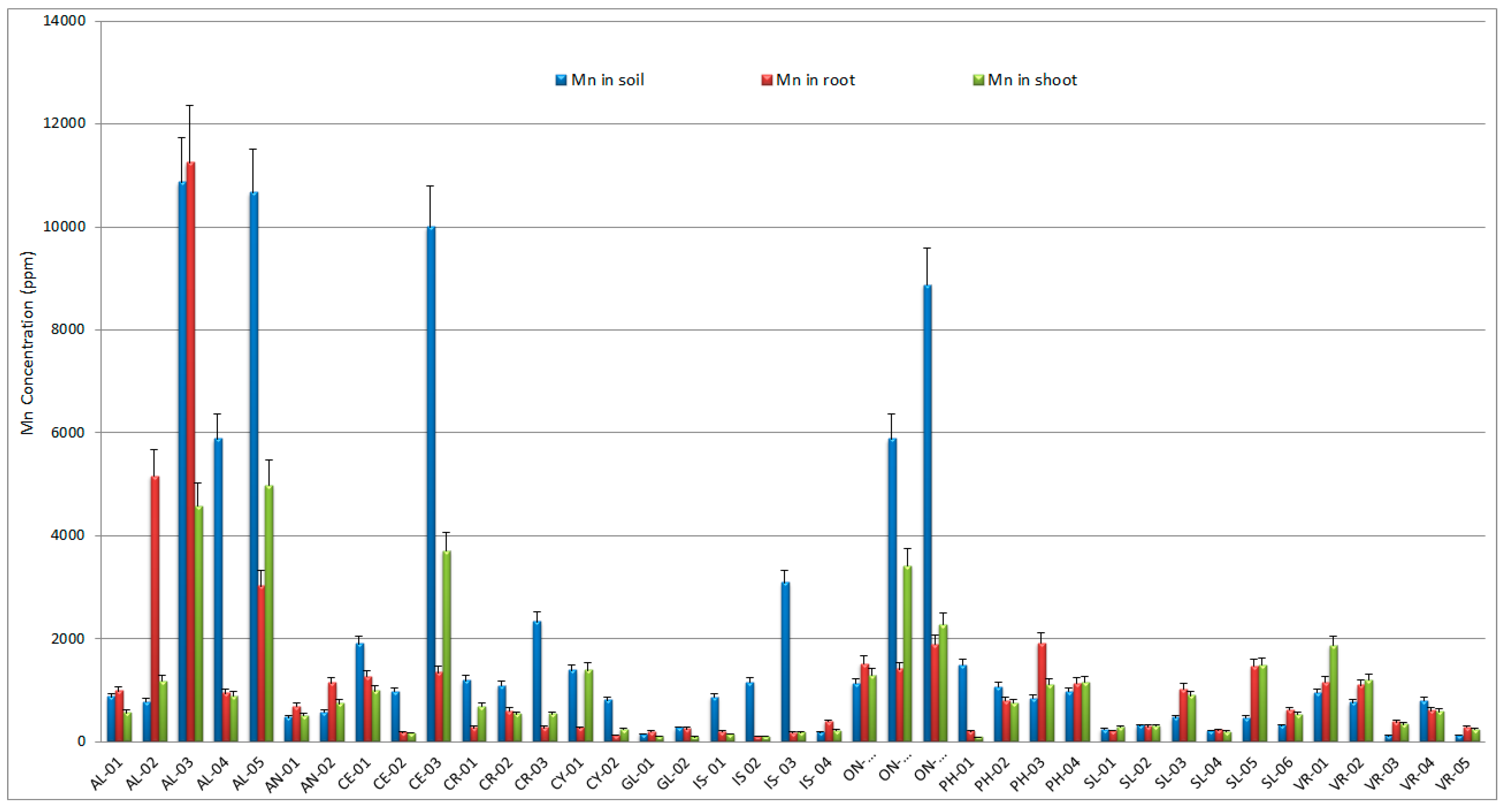
3.1. Manganese in soil
3.2. Manganese in native plants
CONCLUSIONS
Acknowledgements
References
- [1] Peng K.J., Luo C.L., You W.X., et al. 2008. Manganese uptake and interactions with cadmium in the hyperaccumlator. Phytolacca Americana L. J. Hazard. Mater. 154:674 − 681.
- [2] Kabata-Pendias A. 2011. Trace Elements in soils and plants. CRC Press, Boca Raton.
- [3]Khan K, Khan H, Lu Y, Ihsanullah I, Nawab J, Khan S, Shah NS, Shamshad I, Maryam A. 2014. Evaluation of toxicological risk of foodstuffs contaminated with heavy metals in Swat, Pakistan. Ecotox Environ Saf. 108, 224–232. [CrossRef]
- [4] Iqbal, M., 2016. Vicia faba bioassay for environmental toxicity monitoring: a review. Chemosphere 144, 785–802. [CrossRef]
- [5] Iqbal, M., Iqbal, N., Bhatti, I.A., Ahmad, N., Zahid, M. 2016. Response surface methodology application in optimization of cadmium adsorption by shoe waste: a good option of waste mitigation by waste. Ecol. Eng. 88, 265–275. [CrossRef]
- [6]Mani D, Kumar C, Patel NK. 2016. Integrated micro-biochemical approach for phytoremediation of cadmium and lead contaminated soils using Gladiolus grandiflorus L cut flower. Ecotox Env Saf 124, 435-446. [CrossRef]
- [7]US EPA, 2000. Introduction to phytoremediation. National Risk Management Research Laboratory, Office of Research and Development, EPA/600/R-99/107.
- [8]Wong MH. 2003. Ecological restoration of mine degraded soils, with emphasis on metal contaminated soils, Chemosphere 50,775–80.
- [9] Skinner W.M., Martin R.R., Naftel S.J., et al. 2005. Multi-technique studies of the distribution of metals between the soil, rhizosphere and roots of Populus tremuloides growing in forest soil. ICOBTE, 8 Int. Conf. Book Abstr. 488–489, Adelaide.
- [10] Ansari AA, Gill SS, Gill R, Lanza GR, Newman L. 2015. Phytoremediation. Management of Environmental Contaminants, Springer International Publishing Switzerland. 366 p.
- [11] Sasmaz M, Akgül B, Yıldırım D, Sasmaz A. 201a. Mercury uptake and phytotoxicity in terrestrial plants grown naturally in the Gumuskoy (Kutahya) mining area, Turkey. Int J Phytorem 18 (1), 69-76. [CrossRef]
- [12] Sasmaz A. 2011. As, Ag, Pb, Sb and Tl levels in soil and plants around Gumuskoy mining area (Kutahya) and their possible effects on the environment. TUBİTAK project (CAYDAG-110Y003). Ankara, 83 pp.
- [13] Liu XH, Gao YT, Sardar K, Duan G, Chen AK, Ling L, Zao L, Zhonghan L, Xuecan W. 2008. Accumulation of Pb, Cu, and Zn in terrestrial plants growing on contaminated sites and their potential accumulation capacity in Heqing, Yunnan. J Environ Sci 20:1469-1474.
- [14] Yoon J, Cao X, Zhou Q, Ma LQ. 2006. Accumulation of Pb, Cu, and Zn in terrestrial plants growing on a contaminated Florida site. Sci. Total Environ. 368, 456–464.
- [15] Arik F. 2002. Gümüsköy (Kütahya, Turkey) gümüş yataginin jeokimyasal modellemesi, Selçuk Üniv. Fen Bilimleri Enst. Doktora Tezi, p 318.
- [16] Arik F. 2012. Genetic characteristics of the Gozecukuru As-Sb deposits near Kutahya, Turkey. J. Geol. Soc. India 80:855-868. [CrossRef]
- [17] Arık F, Yaldız T. 2010. Heavy Metal Determination and Pollution of the Soil and Plants of Southeast Tavşanlı (Kütahya, Turkey). Clean Soil Air Water 38, 1017–1030.
- [18] Kartalkanat A. 2008. Anadolu’da madenciliginin tarihçesi; Kütahya-Gümüsköy’de 3500 Yıldır süren madencilik çalışmaları. MTA Dergisi 137, 91-97.
- [19] Tüysüz, N., 2000. Geology, lithogeochemistry and genesis of the Murgul massive sulfide deposit, NE Turkey. Chemie der Erde, 60: 231-250.
- [20] Unal, E., Gökçe, A., 2007. Geology and fluid inclusion characteristics of the Akgüney (Kabadüz-Ordu) copper–lead–zinc deposits. Geological Bulletin of Turkey, 50 (3) : 158–175.
- [21] Helvacı C. 2015. Geological features of Neogene basins hosting borate deposits: an overview of deposits and future forecast, Turkey. Bulletin of the Mineral Research and Exploration 151, 169-215. [CrossRef]
- [22] Azizi, M.R. Abedeni A, Alipour S, Niroomand S, Sasmaz A, Talaei B. 2017. Rare earth element geochemistry and tetrad effect in fluorites: A case study from the Qahr-Abad deposit, Iran. N.Jb. Palont.Abh 283/3, 255-273. [CrossRef]
- [23] Kuşcu, I., 2019. Skarn and skarn deposits of Turkey. In: Pirajno, F., et al., eds., Mineral Resources of Turkey. Springer, Swirzerland. 283-336.
- [24] Sipahi, F., Akpınar, I., Saydam Eker, Ç., Kaygusuz, A., Vural, A., Yılmaz, M. 2017. Formation of the Eğrikar (Gümüşhane) Fe–Cu skarn type mineralization in NE Turkey: U–Pb zircon age, lithogeochemistry, mineral chemistry, fluid inclusion, and O–H–C–S isotopic compositions. Journal of Geochemical Exploration, 182: 32–52.
- [25] Sasmaz, A. 2020. The Atbara porphyry gold–copper systems in the Red Sea Hills, Neoproterozoic Arabian–Nubian Shield, NE Sudan. Journal of Geochemical Exploration 214, 106539.
- [26] Konakci, N., Sasmaz, A. 2021. Geochemical approach to the genesis of the Buyukkizilcik (Afsin) barite deposit, SE Turkey. Italian Journal of Geosciences 140 (3), 422-437. [CrossRef]
- [27] Chen SB, Zhu YG, Hu QH. 2005. Soil to plant transfer of 238U, 226Ra and 232Th on U mining-impacted soil from southeastern China. J. Environ. Rad 82, 223-236.
- [28] Wei, C.Y., Chen, T.B., Huang, Z.C., 2002. Cretan bake (Pteris cretica L.): An arsenic-accumulating plant. Acta Ecol. Sin. 22, 777–782.
- [29] Zhao FJ, Lombi E, Mc Grath SP. 2003. Assessing the potential for zinc and cadmium phytoremediation with the hyperaccumulator Thlaspi caerulescens. Plant Soil 249, 37-43. [CrossRef]
- [30] Sasmaz, M., Uslu Senel, G., Obek, E. 2021. Strontium accumulation by the terrestrial and aquatic plants affected by mining and municipal wastewaters (Elazig, Turkey). Environ Geochem Health 43, 2257–2270. [CrossRef]
- [31] Zu, Y.Q., Li, Y., Chen, J.J., Chen, H.Y., Qin, L., Schvartz, C. 2005. Hyperaccumulation of Pb, Zn and Cd in herbaceous grown on lead-zinc mining area in Yunnan, China. Environ Int. 31, 755-762.
- [32] Ferrando D. 2010. Manganese hyperaccumulation by plants. http://www.botany.unimelb.edu.au/botany.
- [32] Sasmaz M, Akgul B, Sasmaz A. 2015. Distribution and accumulation of selenium in wild plants growing naturally in the Gumuskoy (Kutahya) Mining Area, Turkey. Bull Environ Contam Toxic 94 (5), 598-603. [CrossRef]
- [33] Pais I, Jones JB. 2000. The handbook of trace elements. St. Lucie Press. Florida, 222 p.
- [34] Curlik J., Šefcik P. 1999. Geochemical atlas of the Slovak Republic. Soils. Ministry of the Environment of the Slovak Republic.
- [35] Mielke H.W., Gonzales C.R., Powell E., et al. 2002. Natural and anthropogenic processes that concentrate Mn in rural and urban environments of the Lowe Mississippi River Delta. Environ. Res. Sec. A 90:157–168.
- [36] Shanahan J.O., Brummer J.E., Leininger W.C., Paschke M.W. 2007. Manganese and zinc toxicity thresholds for mountain and Greyer willow. Int. J. Phytoremediat. 9:437–452.
- [37] Min, Y., Tie, B., Tang, M., Aoyama, I. 2007. Accumulation and uptake of manganese in a hyperaccumulator Phytolacca Americana. Miner Eng 20:188–190.
- [38] Green C.H., Heil D.M., Cardon G.E., et al. 2003. Solubility of manganese and trace metals in soils affected by acid mine runoff. J. Environ. Qual. 32:1323–1334.
- [39] Bihanic, C., Petit, E., Perrot, R. et al. 2021. Manganese distribution in the Mn-hyperaccumulator Grevillea meisneri from New Caledonia. Sci Rep 11, 23780. [CrossRef]
- [40] Losfeld, G., L’Huillier, L., Fogliani, B. et al. 2015. Leaf-age and soil-plant relationships: key factors for reporting trace-elements hyperaccumulation by plants and design applications. Environ Sci Pollut Res 22, 5620–5632. [CrossRef]
- [41] van der Ent A, Baker AJM, Reeves RD, Pollard AJ, Schat H (2013a) Hyperaccumulators of metal and metalloid trace elements: facts and fiction. Plant Soil 362:319–334.
- [42] Yang, S.X., Deng, H., Li, M.S. 2008. Manganese uptake and accumulation in a woody hyperaccumulator, Schima superba. Plant Soil & Env. 54 (10), 441-446. [CrossRef]
- [43] Kula, E., Wildova, E. Hrdlicka, P. 2018. Accumulation and dynamics of manganese content in bilberry (Vaccinium myrtillus). Environ Monit Assess 190, 224. [CrossRef]
| Mn in soil | Mn in root | Mn in shoot | ECR | ECS | TLF | |
|---|---|---|---|---|---|---|
| AL-01 | 141±8.3 | 86±5.4 | 114±9 | 0,61 | 0,81 | 1,33 |
| AL-02 | 270±17 | 572±42 | 113±7 | 2,12 | 0,42 | 0,20 |
| AL-03 | 479±35 | 305±25 | 172±12 | 0,64 | 0,36 | 0,56 |
| AL-04 | 252±14 | 240±14 | 206±14 | 0,95 | 0,82 | 0,86 |
| AL-05 | 500±32 | 98±8.2 | 261±16 | 0,20 | 0,52 | 2,66 |
| Averega | 328 | 260 | 173 | 0,90 | 0,59 | 1,12 |
| AN-01 | 2018±125 | 2425±132 | 3612±232 | 1,20 | 1,79 | 1,49 |
| AN-02 | 173±12 | 268±18 | 260±15 | 1,55 | 1,50 | 0,97 |
| Average | 1096 | 1347 | 1936 | 1,38 | 1,65 | 1,23 |
| CE-01 | 107±8 | 166±12 | 246±20 | 1,56 | 2,30 | 1,48 |
| CE-02 | 51±4.4 | 19±1.4 | 28±2.2 | 0,36 | 0,55 | 1,50 |
| CE-03 | 207±16 | 105±7 | 381±24 | 0,51 | 1,84 | 3,64 |
| Average | 122 | 97 | 218 | 0,81 | 1,56 | 2,20 |
| CR-01 | 55±3.2 | 218±13 | 145±12 | 3,94 | 2,62 | 0,67 |
| CR-02 | 26±1.7 | 38±2.8 | 114±10 | 1,49 | 4,42 | 2,98 |
| CR-03 | 29±2.2 | 218±14 | 145±11 | 7,64 | 5,08 | 0,67 |
| Average | 36 | 158 | 134 | 4,35 | 4,04 | 1,44 |
| CY-01 | 40±3.1 | 44±2.6 | 72±5.2 | 1,11 | 1,81 | 1,64 |
| CY-02 | 14±1.2 | 18±1.4 | 7±0.5 | 1,26 | 0,45 | 0,36 |
| Average | 27 | 31 | 39 | 1,18 | 1,13 | 1,00 |
| GL-01 | 181±14 | 2288±188 | 1722±144 | 12,61 | 9,49 | 0,75 |
| GL-02 | 168±13 | 2864±164 | 2118±182 | 17,05 | 12,61 | 0,74 |
| Average | 175 | 2576 | 1920 | 14,83 | 11,05 | 0,75 |
| IS- 01 | 26±1.6 | 84±6.2 | 76±6.8 | 3,21 | 2,93 | 0,91 |
| IS 02 | 143±11 | 190±14 | 49±4.4 | 1,32 | 0,34 | 0,26 |
| IS- 03 | 132±12 | 139±12 | 36±2.6 | 1,05 | 0,27 | 0,26 |
| IS- 04 | 86±7.3 | 261±14 | 621±52 | 3,05 | 7,26 | 2,38 |
| Average | 97 | 168 | 196 | 2,16 | 2,70 | 0,95 |
| ON-01 | 76±4.2 | 79±3.5 | 183±12 | 1,03 | 2,42 | 2,34 |
| ON-02 | 152±12 | 188±14 | 730±52 | 1,24 | 4,79 | 3,88 |
| ON-03 | 116±10 | 105±8.5 | 178±15 | 0,91 | 1,54 | 1,69 |
| Average | 115 | 124 | 364 | 1,06 | 2,92 | 2,63 |
| PH-01 | 75±5.1 | 563±32 | 299±22 | 7,56 | 4,00 | 0,53 |
| PH-02 | 28±1.6 | 149±13 | 228±15 | 5,25 | 8,01 | 1,53 |
| PH-03 | 194±17 | 565±27 | 299±18 | 2,91 | 1,54 | 0,53 |
| PH-04 | 560±44 | 149±13 | 228±10 | 0,27 | 0,41 | 1,53 |
| Average | 214 | 357 | 263 | 4,00 | 3,49 | 1,03 |
| SL-01 | 133±9 | 376±23 | 693±52 | 2,82 | 5,21 | 1,85 |
| SL-02 | 10±0.6 | 66±4.5 | 15±1.2 | 6,87 | 1,52 | 0,22 |
| SL-03 | 16±1.1 | 123±11 | 24±1.6 | 7,65 | 1,50 | 0,20 |
| SL-04 | 226±15 | 124±10 | 116±10 | 0,55 | 0,51 | 0,94 |
| SL-05 | 426±28 | 218±18 | 98±7.3 | 0,51 | 0,23 | 0,45 |
| SL-06 | 875±66 | 124±9 | 116±10 | 0,14 | 0,13 | 0,94 |
| Average | 281 | 172 | 177 | 3,09 | 1,52 | 0,76 |
| VR-01 | 15±1.3 | 81±6.2 | 145±12 | 5,35 | 9,56 | 1,79 |
| VR-02 | 56±4.4 | 183±13 | 407±38 | 3,26 | 7,24 | 2,22 |
| VR-03 | 129±11 | 2134±166 | 525±27 | 16,49 | 4,06 | 0,25 |
| VR-04 | 78±5.5 | 160±13 | 128±10 | 2,04 | 1,63 | 0,80 |
| VR-05 | 60±4.3 | 1682±132 | 762±66 | 28,00 | 12,69 | 0,45 |
| Average | 221 | 469 | 430 | 11,03 | 7,13 | 1,10 |
| Cu | Pb | Zn | Ag | Mn | Fe | As | U | Sr | Sb | Ca | P | Ba | Hg | Na | K | Sc | Tl | Hg | Se | Cd | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cu | 1.00 | ||||||||||||||||||||
| Pb | 0.76 | 1.00 | |||||||||||||||||||
| Zn | 0.76 | 0.71 | 1.00 | ||||||||||||||||||
| Ag | 0.55 | 0.40 | 0.63 | 1.00 | |||||||||||||||||
| Mn | 0.83 | 0.77 | 0.79 | 0.49 | 1.00 | ||||||||||||||||
| Fe | 0.62 | 0.65 | 0.64 | 0.14 | 0.70 | 1.00 | |||||||||||||||
| As | -0.07 | 0.31 | -0.08 | -0.42 | -0.01 | 0.42 | 1.00 | ||||||||||||||
| U | -0.35 | 0.12 | -0.21 | -0.44 | -0.26 | 0.25 | 0.79 | 1.00 | |||||||||||||
| Sr | -0.46 | -0.01 | -0.39 | -0.55 | -0.35 | 0.09 | 0.81 | 0.90 | 1.00 | ||||||||||||
| Sb | -0.12 | 0.31 | -0.01 | -0.37 | -0.09 | 0.31 | 0.92 | 0.78 | 0.76 | 1.00 | |||||||||||
| Ca | -0.19 | 0.06 | 0.17 | -0.15 | -0.03 | 0.06 | 0.30 | 0.17 | 0.19 | 0.48 | 1.00 | ||||||||||
| P | -0.33 | -0.03 | -0.36 | -0.43 | -0.30 | 0.05 | 0.76 | 0.77 | 0.90 | 0.68 | 0.00 | 1.00 | |||||||||
| Ba | 0.04 | 0.42 | 0.25 | -0.07 | 0.28 | 0.37 | 0.37 | 0.29 | 0.23 | 0.39 | 0.50 | 0.01 | 1.00 | ||||||||
| Hg | -0.01 | 0.48 | -0.07 | -0.12 | 0.06 | 0.09 | 0.51 | 0.48 | 0.36 | 0.52 | 0.25 | 0.19 | 0.54 | 1.00 | |||||||
| Na | 0.02 | -0.18 | 0.00 | -0.27 | -0.03 | 0.01 | -0.09 | -0.24 | -0.19 | -0.13 | 0.23 | -0.19 | 0.02 | -0.18 | 1.00 | ||||||
| K | 0.19 | -0.03 | 0.39 | 0.41 | 0.14 | -0.17 | -0.75 | -0.59 | -0.66 | -0.58 | 0.07 | -0.65 | 0.03 | -0.35 | 0.30 | 1.00 | |||||
| Sc | -0.14 | -0.40 | -0.41 | -0.37 | -0.20 | -0.01 | 0.26 | 0.06 | 0.20 | 0.04 | -0.33 | 0.42 | -0.44 | -0.33 | 0.16 | -0.47 | 1.00 | ||||
| Tl | -0.41 | 0.11 | -0.26 | -0.48 | -0.30 | 0.10 | 0.80 | 0.87 | 0.90 | 0.81 | 0.30 | 0.81 | 0.25 | 0.52 | -0.11 | -0.57 | 0.05 | 1.00 | |||
| Hg | -0.01 | 0.48 | -0.07 | -0.12 | 0.06 | 0.09 | 0.51 | 0.48 | 0.36 | 0.52 | 0.25 | 0.19 | 0.54 | 1.00 | -0.18 | -0.35 | -0.33 | 0.52 | 1.00 | ||
| Se | 0.73 | 0.72 | 0.76 | 0.40 | 0.67 | 0.75 | 0.29 | 0.08 | -0.02 | 0.25 | 0.12 | 0.06 | 0.14 | 0.04 | 0.02 | 0.02 | -0.12 | 0.11 | 0.04 | 1.00 | |
| Cd | 0.76 | 0.82 | 0.72 | 0.34 | 0.79 | 0.79 | 0.22 | -0.04 | -0.13 | 0.13 | 0.07 | -0.14 | 0.55 | 0.19 | 0.02 | 0.06 | -0.25 | -0.08 | 0.19 | 0.76 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





