Submitted:
17 February 2024
Posted:
19 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2.0. Methods
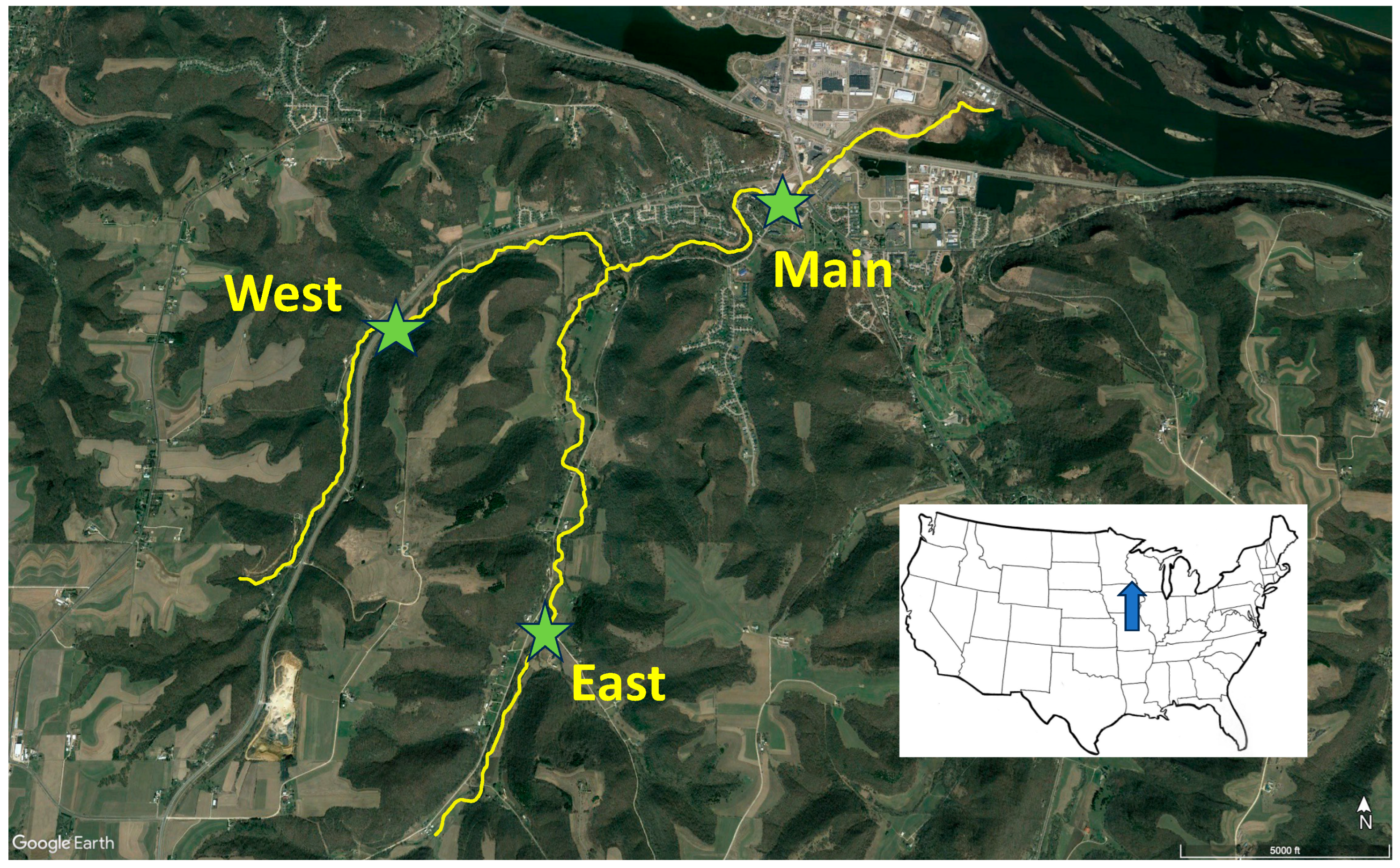
2.1. Study area
2.2. Physical variables
2.3. Benthic invertebrate sampling
2.4. Production estimates
2.5. Behavioral observations
3. Results
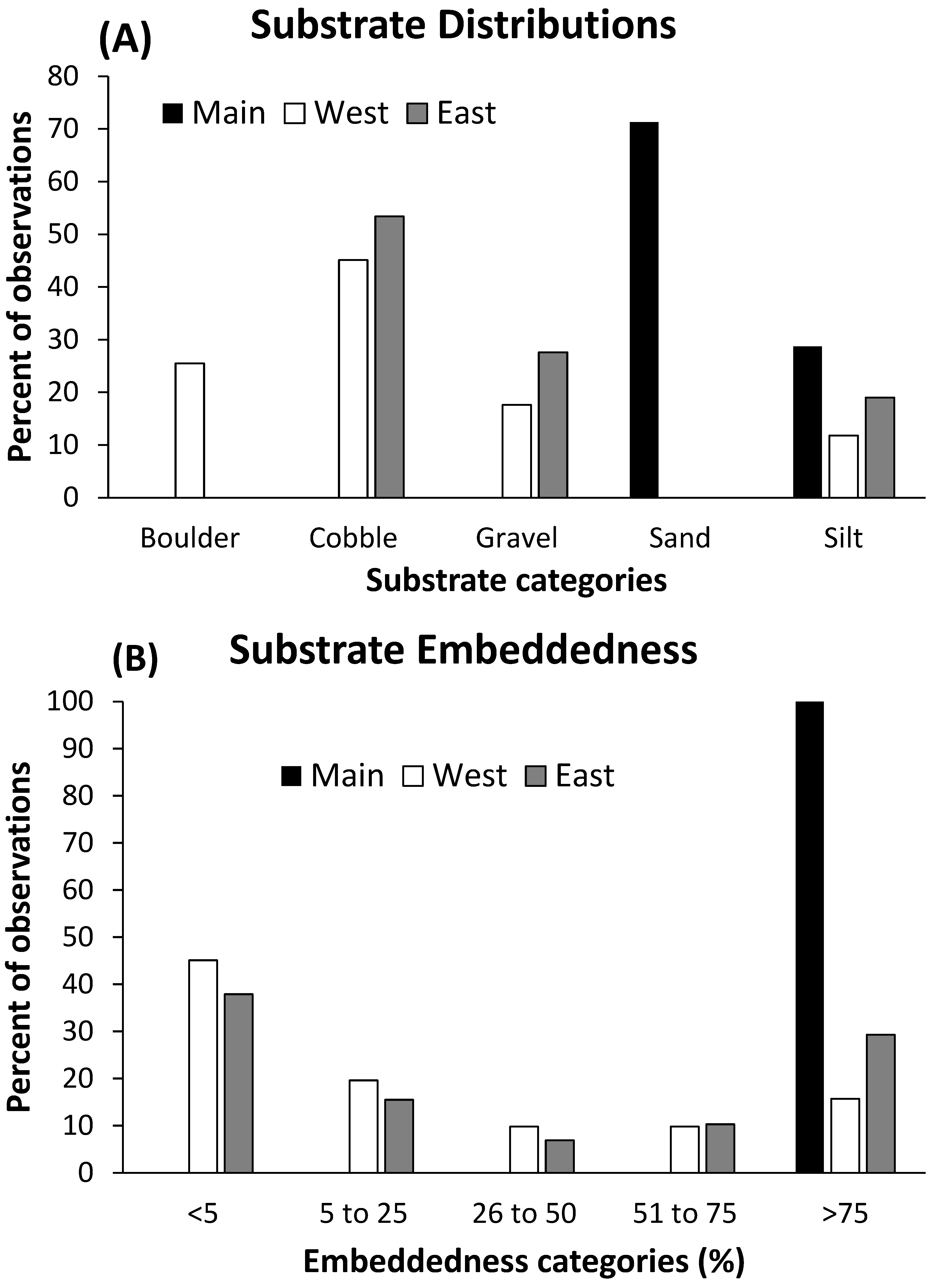
3.1. Physical environment
3.2. Invertebrate communities
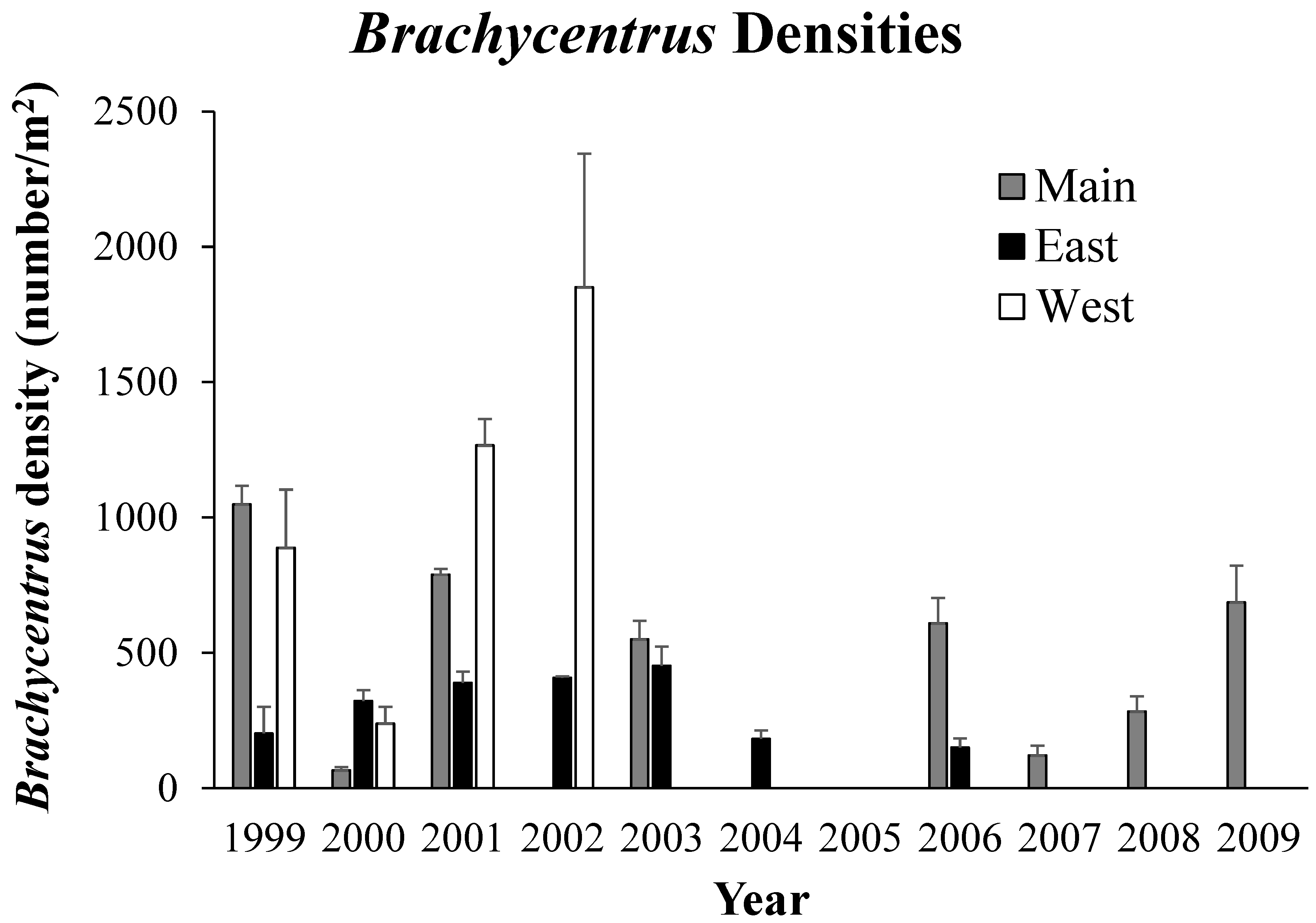
3.3. Densities of Brachycentrus larvae
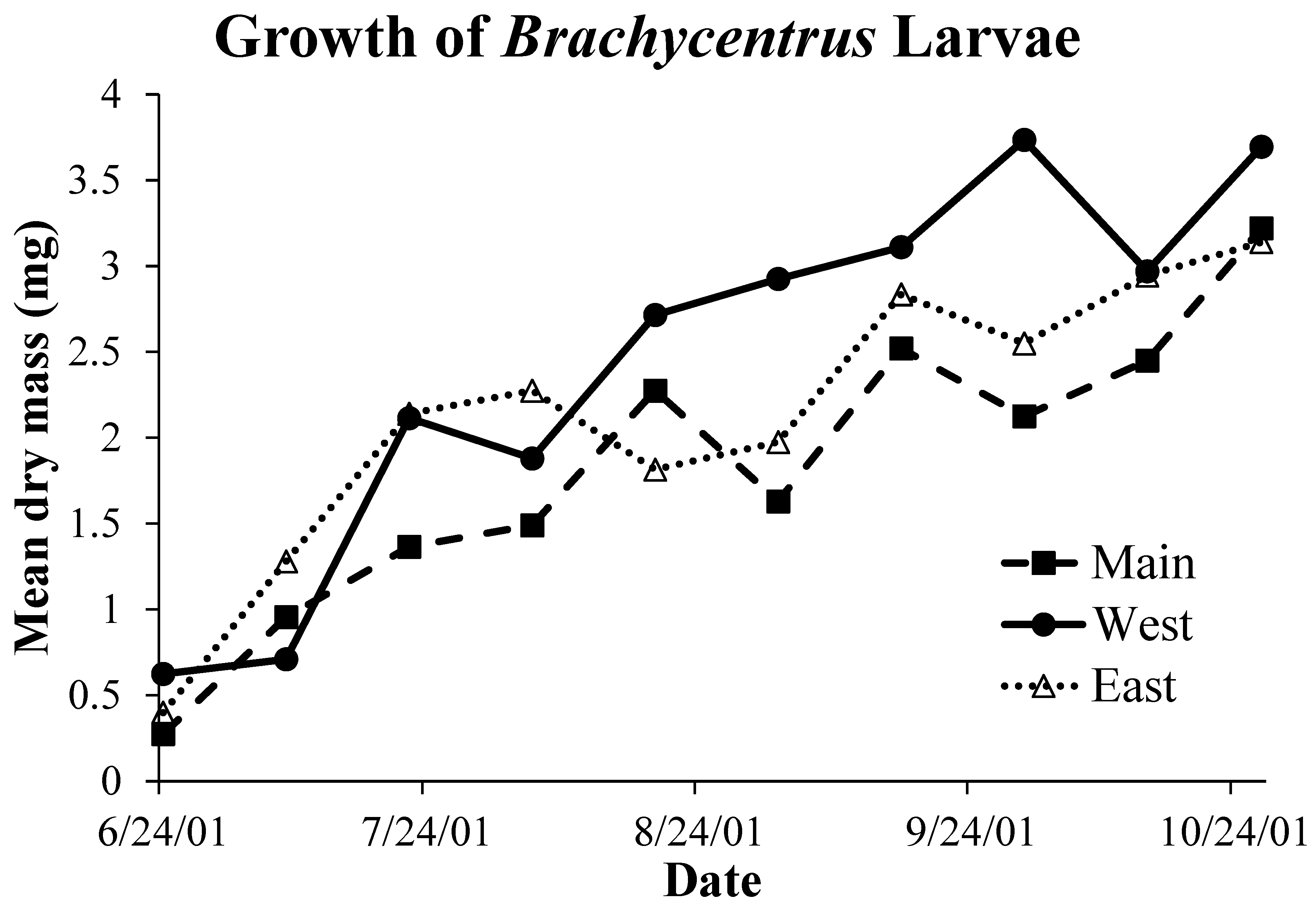
3.4. Brachycentrus production
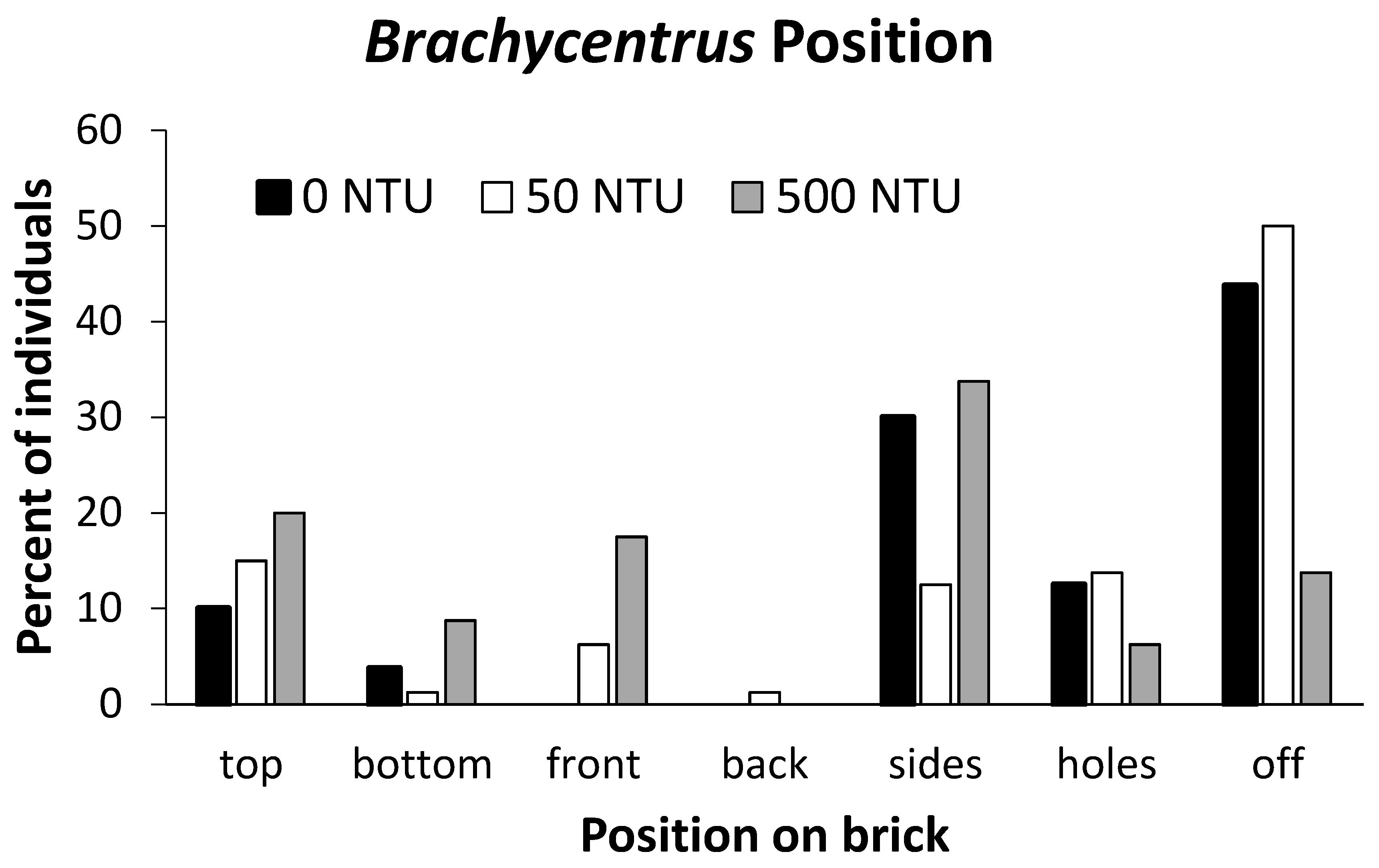
3.5. Behavioral response to suspended sediments
4. Discussion
5. Conclusions
Funding
Author contributions
Ethics approval - Compliance with ethical standards
Data Availability Statement
Acknowledgements
Conflict of interest
References
- Doppelt, B. : Scurlock, M.; Frissell C, Karr J. Entering the watershed: a new approach to save America’s river ecosystems. Island Press: Washington, D.C., USA, 1993. [Google Scholar]
- Allan, J.D.; Castillo, M.M.; Capps, K.A. Stream ecology: structure and function of running waters, 3rd edition. Springer Nature: Switzerland, 2021.
- Simon, T.P. 1998. Assessing the sustainability and biological integrity of water resources using fish communities. CRC Press: Boca Raton, Florida, USA, 1998.
- Karr, J.R.; Chu, E.W. Restoring life in running waters: better biological monitoring. Island Press: Washington, D.C., USA, 1999.
- Trimble, S.W. Historical agriculture and soil erosion in the Upper Mississippi Valley hill country. CRC Press: Boca Raton, Florida, USA, 2013.
- Waters, T.F. The streams and rivers of Minnesota. University of Minnesota Press: Minneapolis, Minnesota, USA, 1980.
- Waters, T.F. Sediment in streams: sources, biological effects and control. American Fisheries Society Monograph 7: Bethesda, MD, USA, 1995.
- Thorn, W.; Anderson, C.; Lorenzen, W.; Hendrickson, D.; Wagner, J. A review of trout management in southeast Minnesota streams. N. Am. J. Fish. Manag., 1997, 17, 860–872. [Google Scholar] [CrossRef]
- Minnesota Department of Natural Resources. 2011. Fisheries long-range plan for trout stream resource management in southeast Minnesota 2010–2015 and progress report. Minnesota Department of Natural Resources, Division of Fisheries and Wildlife, Section of Fisheries: Saint Paul, MN, USA, 2011. Available online: https://files.dnr.state.mn.us/areas/fisheries/lanesboro/setrout_mgtplan/full_report.pdf (accessed on 9 February 2024).
- Stout, J.C.; Belmot, P.; Schottler, S.P.; Willenbring, J.K. 2014. Identifying sediment sources and sinks in the Root River, southeastern Minnesota. Ann. Am. Assoc. Geogr., 2014, 104, 20–39. [Google Scholar] [CrossRef]
- Mundahl, N.D.; Mundahl, E.D. Aquatic community structure and stream habitat in a karst agricultural landscape. Ecol. Process., 2022, 11, 18. [Google Scholar] [CrossRef]
- Varela, W.L.; Mundahl, N.D.; Bergen, S.; Staples, D.F.; Cochran-Biederman, J.; Weaver, C.R. Physical and biological stream health in an agricultural watershed after 30+ years of targeted conservation practices. Water, 2023, 15, 3475. [Google Scholar] [CrossRef]
- Weaver, C.R.; Brockman, M.; Mundahl, N.D.; Arnold, W.A.; Blumentritt, D.; Varela, W.L.; Franz, J.L. 2024. Detection of strobilurin fungicides in trout streams within an agricultural watershed. Hydrology, 2024, 11, 13. [Google Scholar] [CrossRef]
- Anderson, D. Economic impact of recreational trout angling in the Driftless Area. Report to Trout Unlimited, 2016. Available online: https://bloximages.chicago2.vip.townnews.com/lacrossetribune.com/content/tncms/assets/v3/editorial/7/e4/7e46cd72-74b0-5c00-b640-079c58168870/5908d7f129b2d.pdf.pdf (accessed on 9 February 2024).
- Troelstrup, N.H. Jr.; Perry, J.A. 1989. Water quality in southeastern Minnesota streams: observations along a gradient of land use and geology. J. Minn. Acad. Sci., 1989, 55, 6–13. [Google Scholar]
- Mundahl, N.D.; Hunt, A.M. Recovery of stream invertebrates after catastrophic flooding in southeastern Minnesota, USA. J. Freshw. Ecol. 2011, 26, 445–457. [Google Scholar] [CrossRef]
- Gaufin, A.R.; Clubb, R.; Newell, R. Studies on the tolerance of aquatic insects to low oxygen concentrations. Great Basin Nat., 1974, 34, 45–59. [Google Scholar]
- Dunkel, F.V.; Richards, D.C. Effect of an azadirachtin formulation on six nontarget aquatic invertebrates. Environ. Entomol., 1998, 27, 66–674. [Google Scholar] [CrossRef]
- Mangum, F.A.; Madrigal, J.L. Rotenone effects on aquatic macroinvertebrates of the Strawberry River, Utah: a five-year summary. J Freshw. Ecol., 1999, 14, 125–135. [Google Scholar] [CrossRef]
- Krueger, C.C.; Cook, E.F. Life cycles and drift of Trichoptera from a woodland stream in Minnesota. Can. J. Zool., 1984, 62, 1479–1484. [Google Scholar] [CrossRef]
- Johnson, K.R.; Jepson, P.C.; Jenkins, J.J. Esfenvalerate-induced case-abandonment in the larvae of the caddisfly (Brachycentrus americanus). Environ. Toxicol. Chem., 2008, 27, 397–403. [Google Scholar] [CrossRef]
- Relyea, C.D.; Minshall, G.W.; Danehy, R.J. Development and validation of an aquatic fine sediment biotic index. Environ. Manag., 2012, 49, 242–252. [Google Scholar] [CrossRef]
- Nebeker, A.V.; Lemke, A.E. 1968. Preliminary studies on the tolerance of aquatic insects to heated waters. J. Kans. Entomol., 1968, 41, 413–418. [Google Scholar]
- Bell, H.L. , Nebeker, A.V. Preliminary studies on the tolerance of aquatic insects to low pH. J. Kans. Entomol., 1969, 42, 230–236. [Google Scholar]
- Clubb, R.W.; Gaufin, A.R.; Lords, J.L. Acute cadmium toxicity studies upon nine species of aquatic insects. Environ. Res., 1975, 9, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Houghton, D.C. 2015. Regional caddisfly (Trichoptera) indicator species for mid-order Michigan and Minnesota streams. Great Lakes Entomol., 2015, 48, 93–97. [Google Scholar]
- Flint, O.S. Jr. The genus Brachycentrus in North America, with a proposed phylogeny of the genera of Brachycentridae (Trichoptera). Smithsonian Contributions to Zoology Number 398, Smithsonian Institution Press: Washington, D.C., USA, 1984.
- Hilsenhoff, W.L. 1985. The Brachycentridae (Trichoptera) of Wisconsin. Great Lakes Entomol., 1985, 18, 5. [Google Scholar]
- Gallepp, G.W. Responses of caddisfly larvae (Brachycentrus spp.) to temperature, food availability and current velocity. Am. Midl. Nat., 1977, 98, 59–84. [Google Scholar] [CrossRef]
- Hauer, F.R.; Stanford, J.A. Ecology and coexistence of two species of Brachycentrus (Trichoptera) in a Rocky Mountain river. Can. J. Zool., 1986, 64, 1469–1474. [Google Scholar] [CrossRef]
- Miller, S.W.; Wooster, D.; Li, J. Developmental, growth, and population biomass responses of a river-dwelling caddisfly (Brachycentrus occidentalis) to irrigation water withdrawals. Hydrobiologia, 2012, 679, 187–203. [Google Scholar] [CrossRef]
- Veldboom, J.A.; Haro, R.J. Stoichiometric relationship between suspension-feeding caddisfly (Trichoptera: Brachycentridae) and seston. Hydrobiologia, 2011, 675, 129–141. [Google Scholar] [CrossRef]
- Dodds, W.K. Community interactions between the filamentous alga Cladophora glomerata (L.) Kuetzing, its epiphytes, and epiphyte grazers. Oecologia, 1991, 85, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Angradi, T.R.; Griffith, J.S. Diel feeding chronology and diet selection of rainbow trout (Oncorhynchus mykiss) in the Henrys Fork of the Snake River, Idaho. Can. J. Fish. Aquat. Sci., 1990, 47, 199–209. [Google Scholar] [CrossRef]
- Cochran-Biederman, J.L.; Vondracek, B. Seasonal feeding selectivity of brown trout Salmo trutta in five groundwater-dominated streams. J. Freshw. Ecol., 2017, 32, 653–673. [Google Scholar] [CrossRef]
- Mundahl, N.D.; Varela, W.L.; Weaver, C.; Mundahl, E.D.; Cochran-Biederman, J.L. Stream habitat and aquatic communities in an agricultural watershed: changes related to a mandatory riparian buffer law. Environ. Manag., 2023, 72, 945–958. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency, Causal analysis/diagnosis decision information system (CADDIS), volume 2 – sources, stressors, and responses: sediments. United States Environmental Protection Agency: USA, 2024; Available online: https://www.epa.gov/caddis-vol2/sediments (accessed on 8 February 2024).
- Armour, C.L.; Burnham, K.P.; Platts, W.S. Field methods and statistical analyses for monitoring small salmonid streams. FWS/OBS-83/33. United States Fish and Wildlife Service: Washington, D.C., USA, 1983.
- Ross, D.H.; Wallace, J.B. Production of Brachycentrus spinae Ross (Trichoptera: Brachycentridae) and its role in seston dynamics of a southern Appalachian stream (USA). Environ. Entomol., 1981, 10, 240–246. [Google Scholar] [CrossRef]
- Benke, A.C. Secondary production of macroinvertebrates. Pages 557–578 in Hauer, F.R.; Lamberti, G.A. (eds.) Methods in stream ecology. Academic Press: San Diego, CA, USA, 1996. [Google Scholar]
- Hauer, F.R.; Benke, A.C. Influence of temperature and river hydrograph on blackfly growth rates in a subtropical blackwater river. J North Am. Benthol. Soc., 1987, 6, 251–261. [Google Scholar] [CrossRef]
- Hauer, F.R.; Resh, V.H. Benthic macroinvertebrates. Pages 339-369 in Hauer, F.R.; Lamberti, G.A. (eds.) Methods in stream ecology. Academic Press: San Diego, CA, USA, 1996. [Google Scholar]
- Olsen, J.R.; Stedinger, J.R.; Matalas, N.C.; Stakhiv, E.Z. Climate variability and flood frequency estimation for the upper Mississippi and lower Missouri rivers. J. Am. Water Res. Assoc., 1999, 35, 1509–1523. [Google Scholar] [CrossRef]
- Andresen, J.; Hilberg, S.; Kunkel, K. Historical climate and climate trends in the midwestern USA. In Winkler, J.; Andresen, J.; Hatfield, J.; Bidwell, D.; Brown, D. (eds) U.S. global change research program, national climate assessment, midwest technical input report. Great Lakes Integrated Sciences and Assessments Center: Ann Arbor, MI, USA, 2012. Available online: http://glisa.msu.edu/docs/NCA/MTIT_Historical.pdf (accessed on 1 June 2023).
- Mallakpour, I.; Villarini, G. 2015. The changing nature of flooding across the central United States. Nat. Clim. Change, 2015, 5, 250–254. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Atlas of America’s polluted waters. EPA Report 840-B-00-002. Office of Water, U.S. Environmental Protection Agency: Washington, D.C., USA, 2000. W.
- Kemp, P.; Sear, D.; Collins, A.; Naden, P.; Jones, I. The impacts of fine sediment on riverine fish. Hydrol. Process., 2011, 25, 1800–1821. [CrossRef]
- Descloux, S.; Datry, T.; Marmonier, P. Benthic and hyporheic invertebrate assemblages along a gradient of increasing streambed colmation by fine sediment. Aquat. Sci., 2013, 75, 493–507. [Google Scholar] [CrossRef]
- Lenat, D.R.; Penrose, D.L.; Eagleson, K.W. Variable effects of sediment addition on stream benthos. Hydrobiologia, 1981, 79, 187–194. [Google Scholar] [CrossRef]
- Akamagwuna, F.C.; Odume, O.N. Ephemeroptera, Plecoptera and Trichoptera (EPT) functional feeding group responses to fine grain sediment stress in a river in the Eastern Cape, South Africa. Environ. Monit. Assess., 2020, 192, 214. [Google Scholar] [CrossRef] [PubMed]
- Mecom, J.O.; Cummins, K.W. A preliminary study of the trophic relationships of the larvae of Brachycentrus americanus (Banks) (Trichoptera: Brachycentridae). Trans. Am. Microsc., 1964, 83, 233–243. [Google Scholar] [CrossRef]
- Wallace, J.B.; Merritt, R.W. Filter-feeding ecology of aquatic insects. Annu. Rev. Entomol., 1980, 25, 103–132. [Google Scholar] [CrossRef]
- Broekhuizen, N.; Parkyn, S.; Miller, D. Fine sediment effects on feeding and growth in invertebrate grazers. Hydrobiologia, 2001, 457, 125–132. [Google Scholar] [CrossRef]
- Doretto, A.; Piano, E.; Bona, F.; Fenoglio, S. How to assess the impact of fine sediments on the macroinvertebrate communities of alpine streams? A selection of the best metrics. Ecol. Indic., 2018, 84, 60–69. [Google Scholar] [CrossRef]
- Wetmore, S.H.; Mackay, R.J. Characterization of the hydraulic habitat of Brachycentrus occidentalis, a filter-feeding caddisfly. J. North Am. Benthol. Soc., 1990, 9, 157–169. [Google Scholar] [CrossRef]
- Krueger, C.C.; Waters, T.F. Annual production of macroinvertebrates in three streams of different water quality. Ecology, 1983, 64, 840–850. [Google Scholar] [CrossRef]
- Voelz, N.J.; Poff, N.L.; Ward, J.V. Differential effects of a brief disturbance on caddisflies (Trichoptera) in a regulated river. Am. Midl. Nat., 1994, 132, 173–182. [Google Scholar] [CrossRef]
- Wallace, J.B.; Eggert, S.L. Benthic invertebrate fauna, small streams. Pages 98–115 in Likens, G.E. (ed.). River ecosystem ecology: a global perspective – a derivative of encyclopedia of inland waters. Academic Press: San Diego, California, USA, 2010.
- Sweeney, B.W. Bioenergetic and developmental response of a mayfly to thermal variation. Limnol. Oceanogr., 1978, 23, 461–477. [Google Scholar] [CrossRef]
- Gallepp, G.W. Behavioral ecology of Brachycentrus occidentalis Banks during the pupation period. Ecology, 1974, 55, 1283–1294. [Google Scholar] [CrossRef]
- Gallepp, G.W.; Hasler, A.D. Behavior of larval caddisflies (Brachycentrus spp.) as influences by marking. Am. Midl. Nat., 1975, 93, 247–254. [Google Scholar] [CrossRef]
| Variable | East Burns | West Burns | Main Burns |
| Water temperature | 13.6 | 15.4 | 16.2 |
| (°C) | (7.4-17.3) | (5.9-18.6) | (6.4-21.6) |
| Discharge | 0.069 | 0.025 | 0.275 |
| (m3/sec) | (0.025-0.332) | (0.009-0.216) | (0.100-2.186) |
| Turbidity | 2.2 | 5.1 | 11.5 |
| (NTU) | (1.2-346) | (1.8-1245) | (3.6-1085) |
| Total suspended | 11 | 22 | 38 |
| solids (mg/L) | (0-5086) | (0-5448) | (0-5548) |
| pH | 8.07 | 8.18 | 8.2 |
| (7.45-8.46) | (7.56-8.56) | (7.57-8.69) | |
| Rock surface area | 322 | 309 | 654 |
| (cm2) | (154-880) | (90-759) | (152-1486) |
| Embeddedness score | 2.8 (1.7) | 2.3 (1.5) | 5.0 (0.0) |
| Year | East Burns | West Burns | Main Burns |
| 2000 | 51,808 | 42,743 | 999,534 |
| 2001 | 19,734 | 17,149 | 185,759 |
| 2002 | 51,965 | 23,387 | 321,185 |
| Average | 41,169 | 27,760 | 502,159 |
| Taxa | East Burns | West Burns | Main Burns | |||||||
| NON-INSECTS | ||||||||||
| Asellus | X | X | X | |||||||
| Gammarus | X | X | X | |||||||
| Oligochaeta | X | X | ||||||||
| Hirudinea | X | |||||||||
| Physella | X | X | X | |||||||
| Amnicola | X | |||||||||
| Sphaeriidae | X | |||||||||
| Acari | X | |||||||||
| Dugesia | X | |||||||||
| Nematomorpha | X | |||||||||
| INSECTS | ||||||||||
| Ephemeroptera | ||||||||||
| Baetis | X | X | X | |||||||
| Ephemerella | X | |||||||||
| Trichoptera | ||||||||||
| Brachycentrus | X | X | X | |||||||
| Glossosoma | X | X | X | |||||||
| Hydropsyche | X | X | X | |||||||
| Cheumatopsyche | X | |||||||||
| Hesperophylax | X | X | ||||||||
| Hydroptila | X | |||||||||
| Chimarra | X | |||||||||
| Rhyacophila | X | |||||||||
| Limnephilus | X | X | ||||||||
| Micrasema | X | X | ||||||||
| Coleoptera | ||||||||||
| Optioservus | X | X | X | |||||||
| Macronychus | X | X | X | |||||||
| Gyrinus | X | X | ||||||||
| Agabus | X | X | ||||||||
| Megaloptera | ||||||||||
| Sialis | X | |||||||||
| Diptera | ||||||||||
| Simulium | X | X | X | |||||||
| Dicranota | X | X | ||||||||
| Tipula | X | X | X | |||||||
| Hexatoma | X | |||||||||
| Antocha | X | X | ||||||||
| Limonia | X | |||||||||
| Chrysops | X | |||||||||
| Chironomidae | X | X | ||||||||
| Empididae | X | X | ||||||||
| Total taxa | 26 | 29 | 13 |
| Variable | East Burns | West Burns | Main Burns |
| Density (larvae/m2) | 389 (166) | 1266 (392) | 789 (83) |
| Biomass (dry mg/m2) | 723 (60) | 2739 (438) | 1390 (479) |
| Daily P (mg/m2/day) | 6.71 (2.60) | 30.29 (8.51) | 14.82 (1.42) |
| Annual P (mg/m2/year) | 2,458 | 11,089 | 5,415 |
| P/B ratio | 3.4 | 4.05 | 3.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





