Submitted:
16 February 2024
Posted:
19 February 2024
You are already at the latest version
Abstract
Keywords:
INTRODUCTION
METHODS
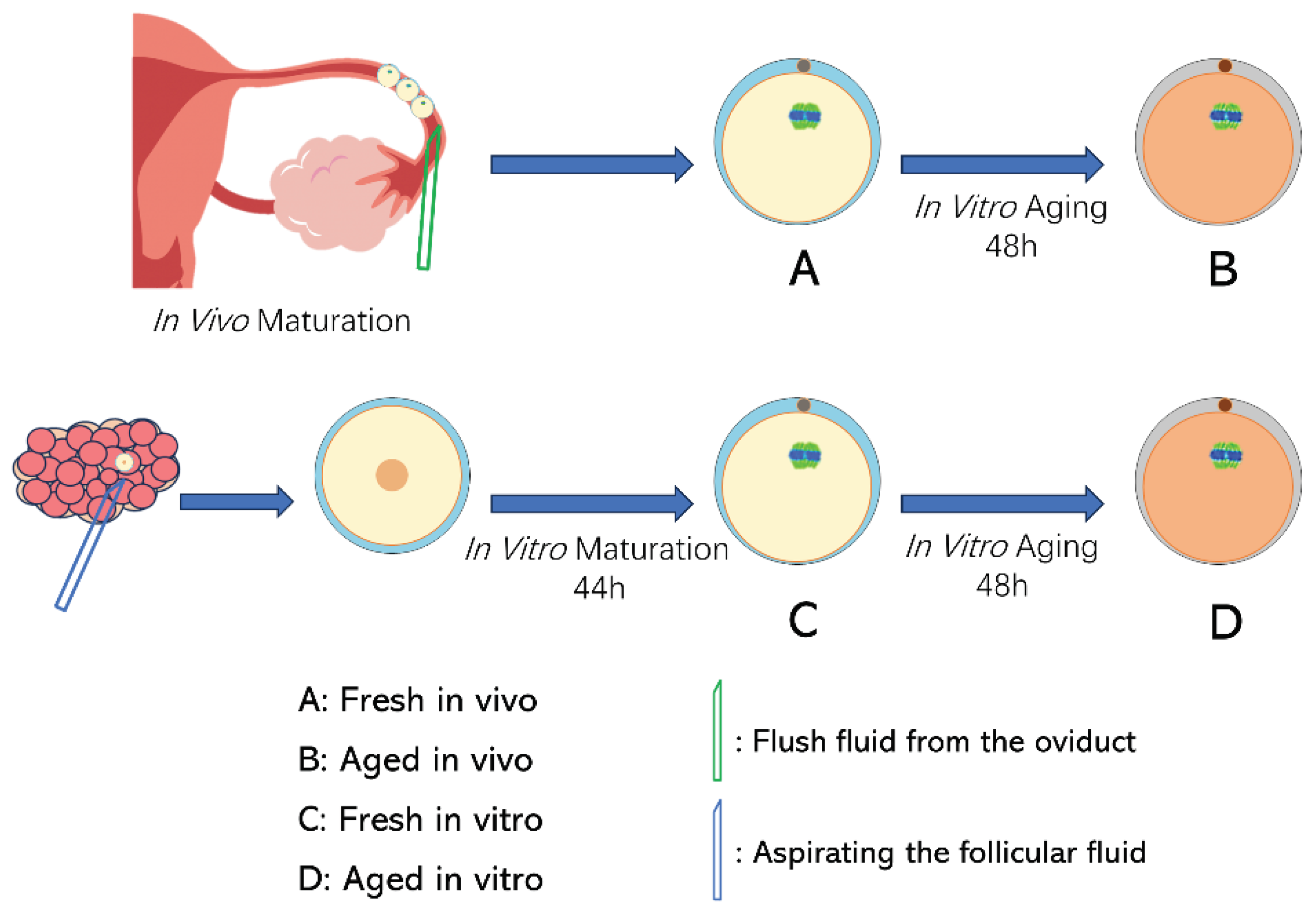
1. Collection of porcine cumulus–oocyte complexes (COCs) and in vitro maturation (IVM)
2. In vitro aging
3. In vivo- fresh and aged-oocyte collection
4. Quantitative reverse transcription PCR (qRT-PCR)
5. RNA sequencing and data analysis
RESULTS
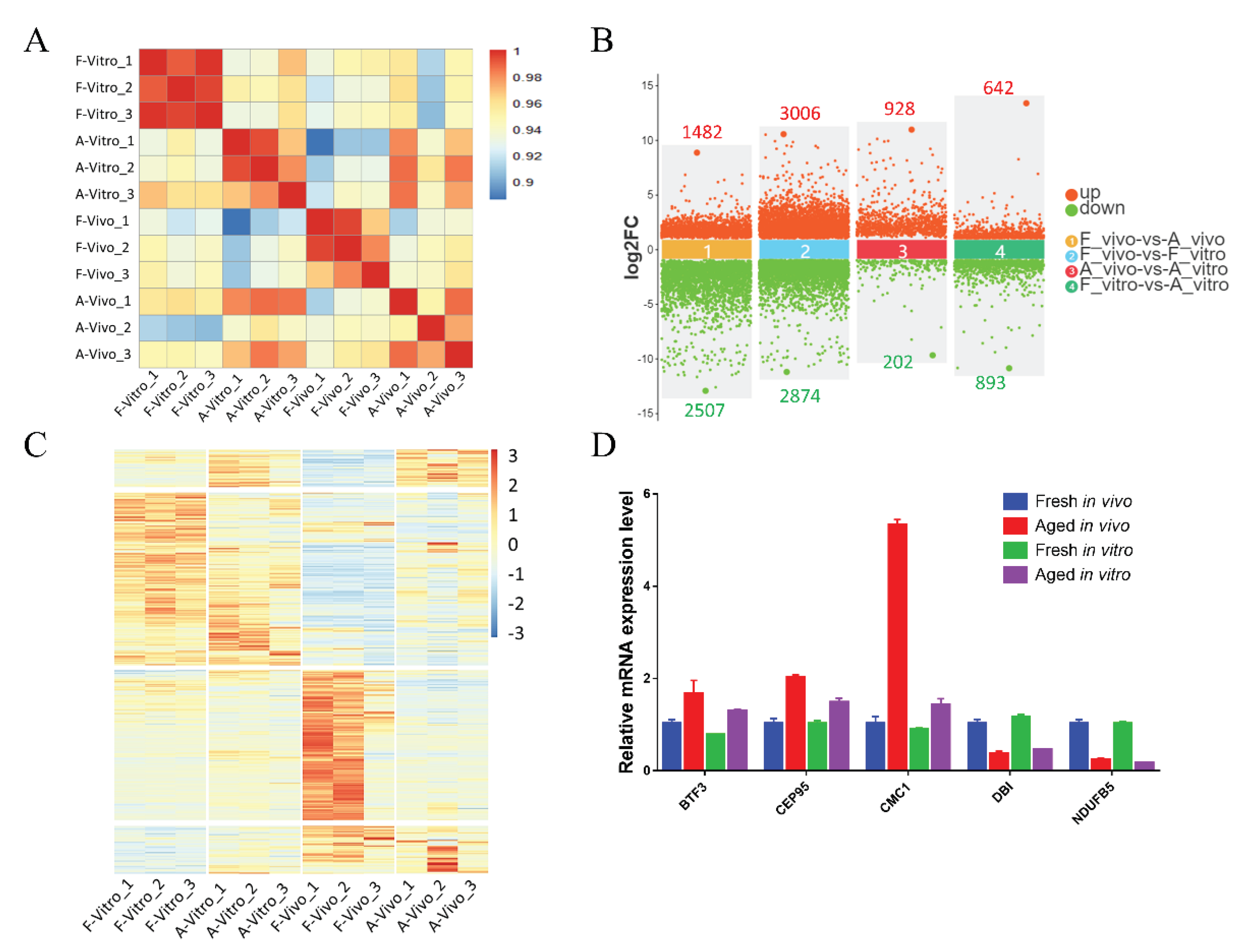
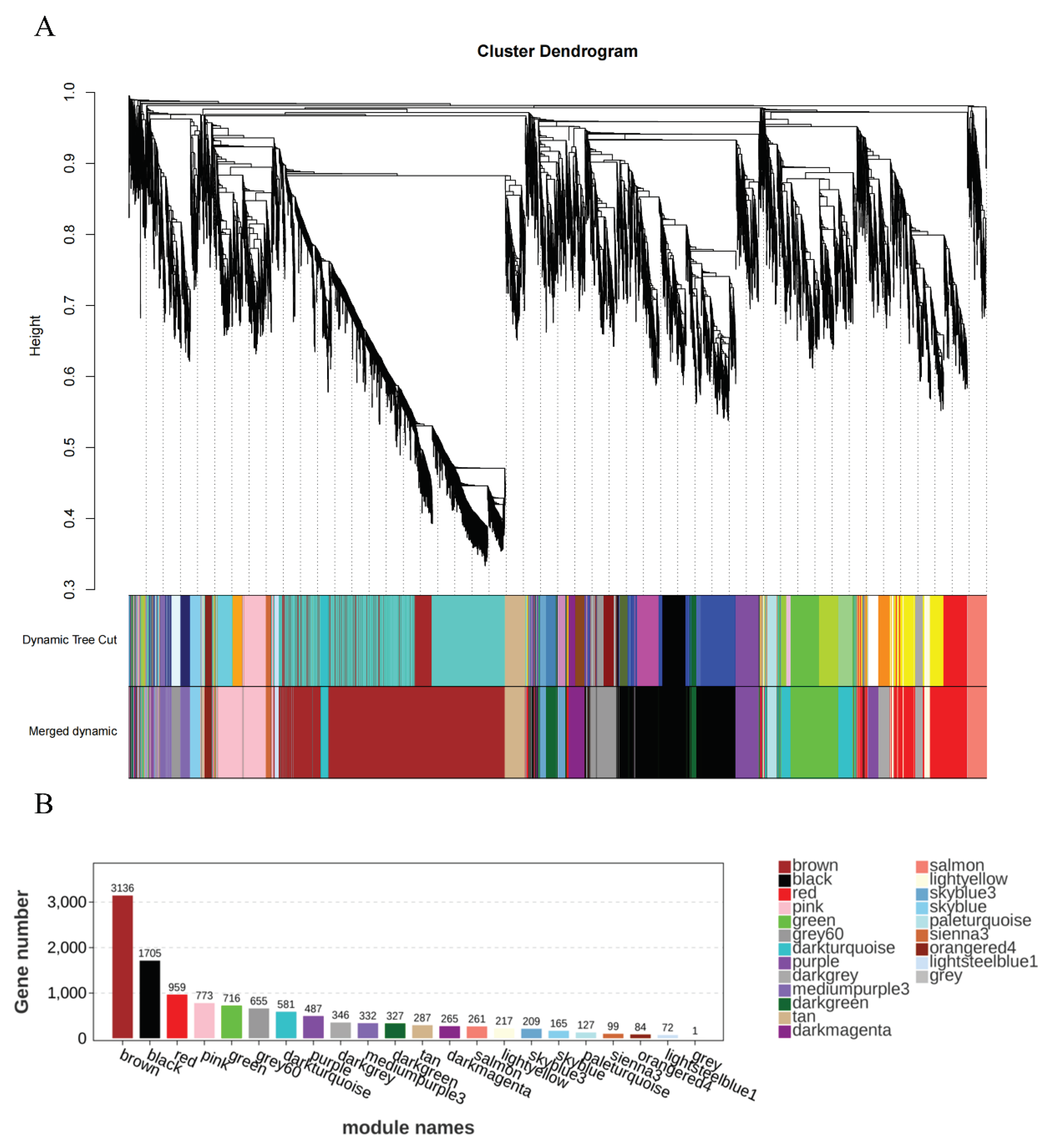
1. Global transcriptome analysis of fresh and aged in vivo and vitro-matured porcine oocytes.
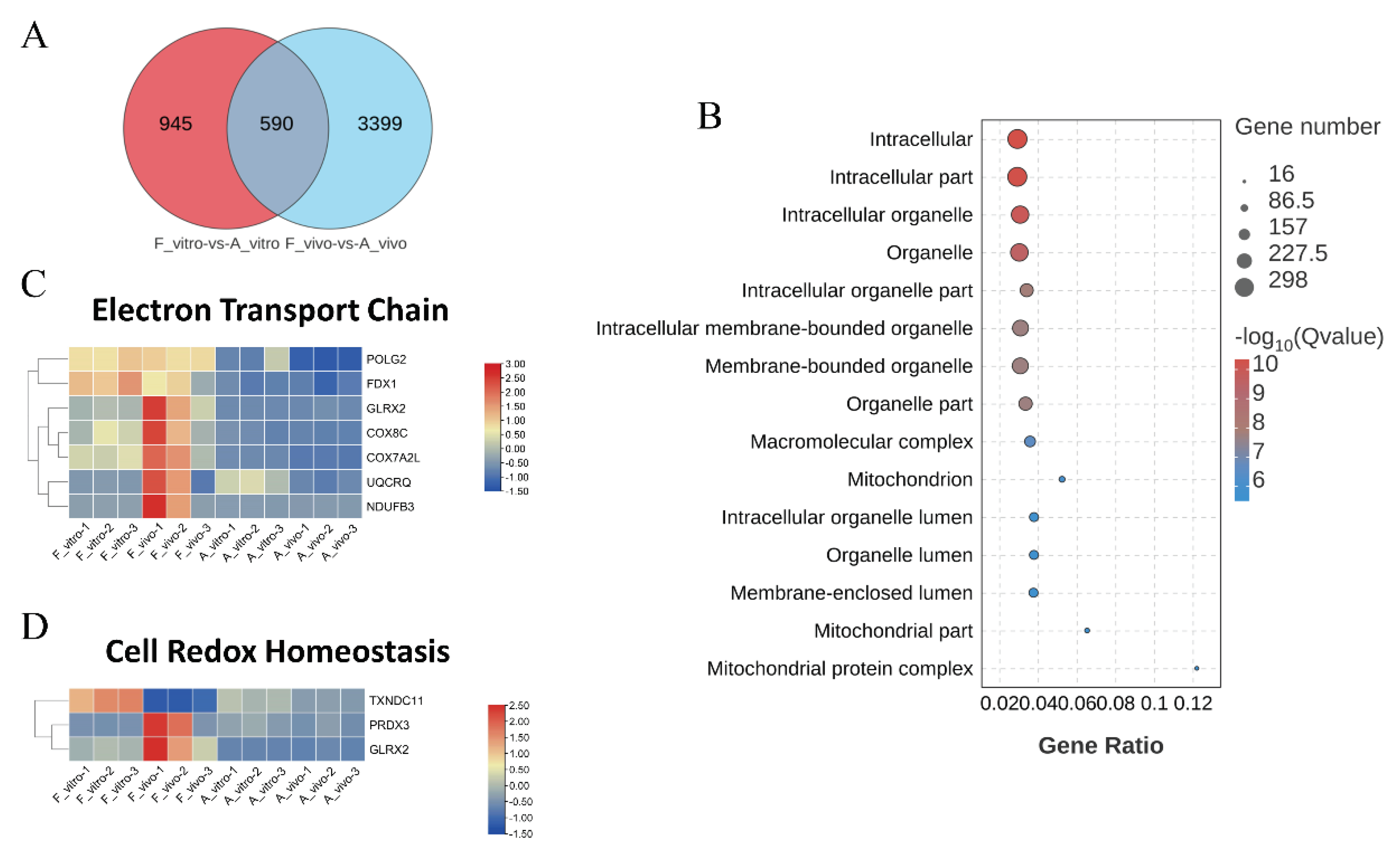
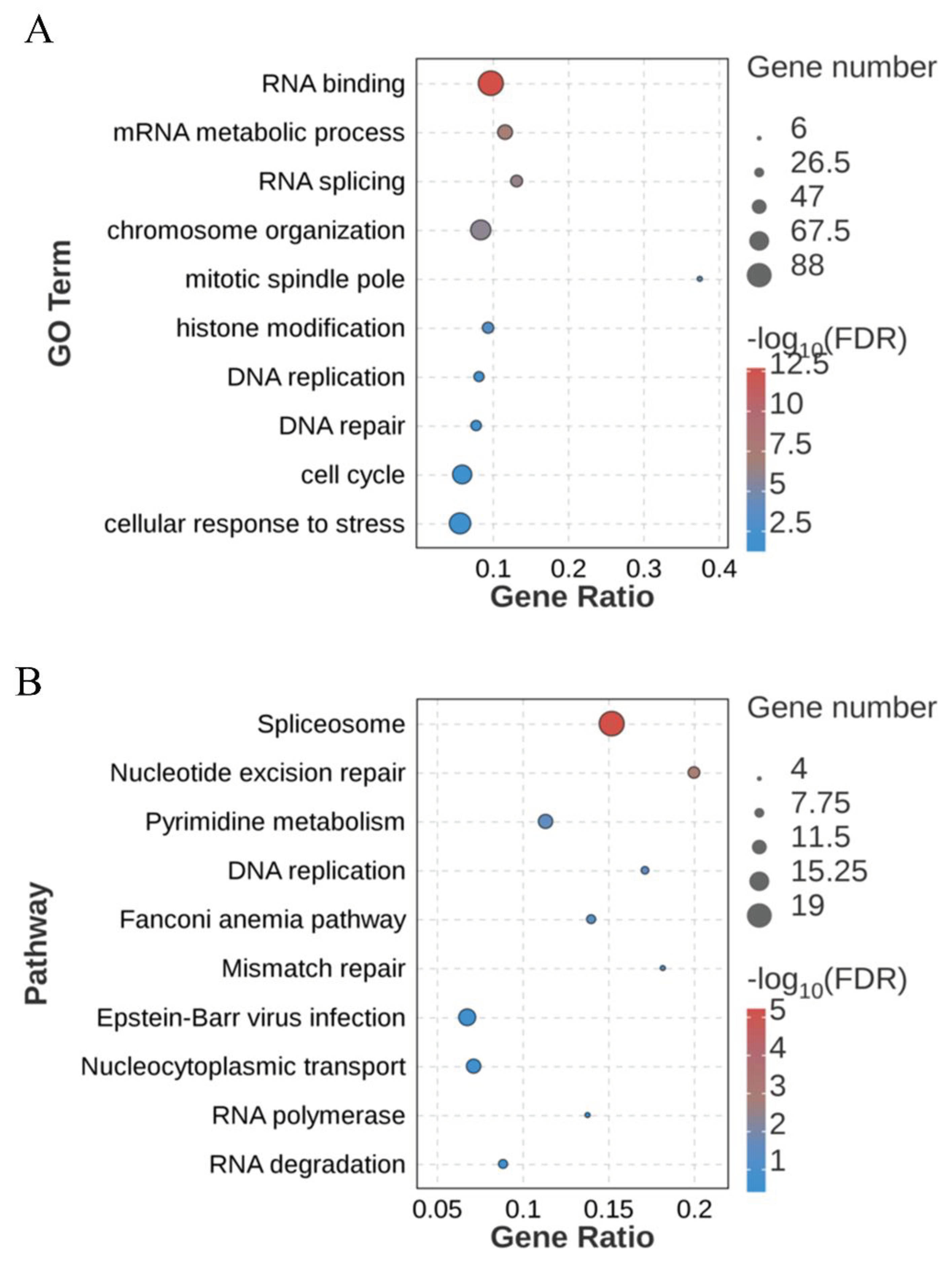
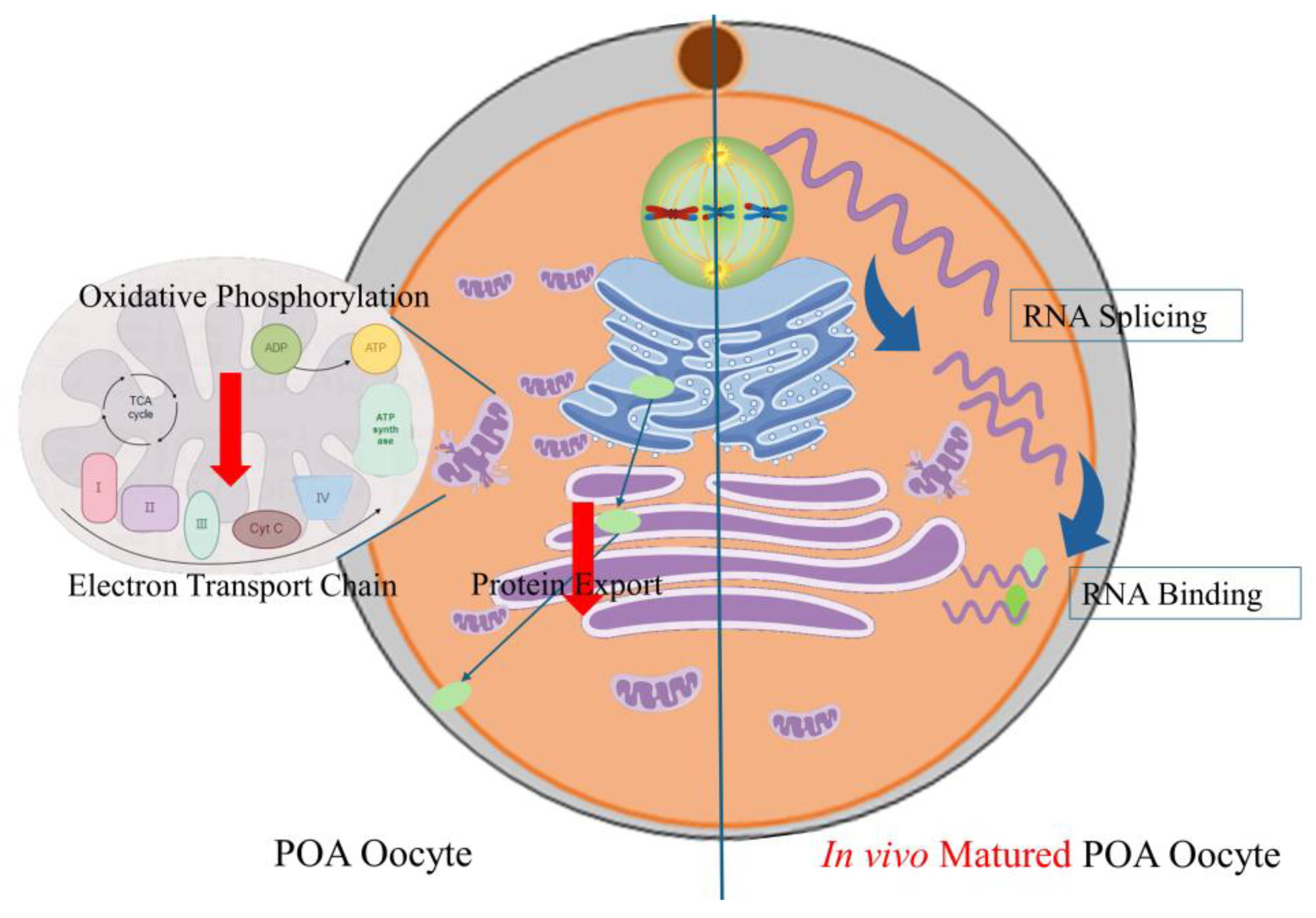
2. POA interferes with the mitochondrial function in both in vivo and vitro-matured porcine oocytes
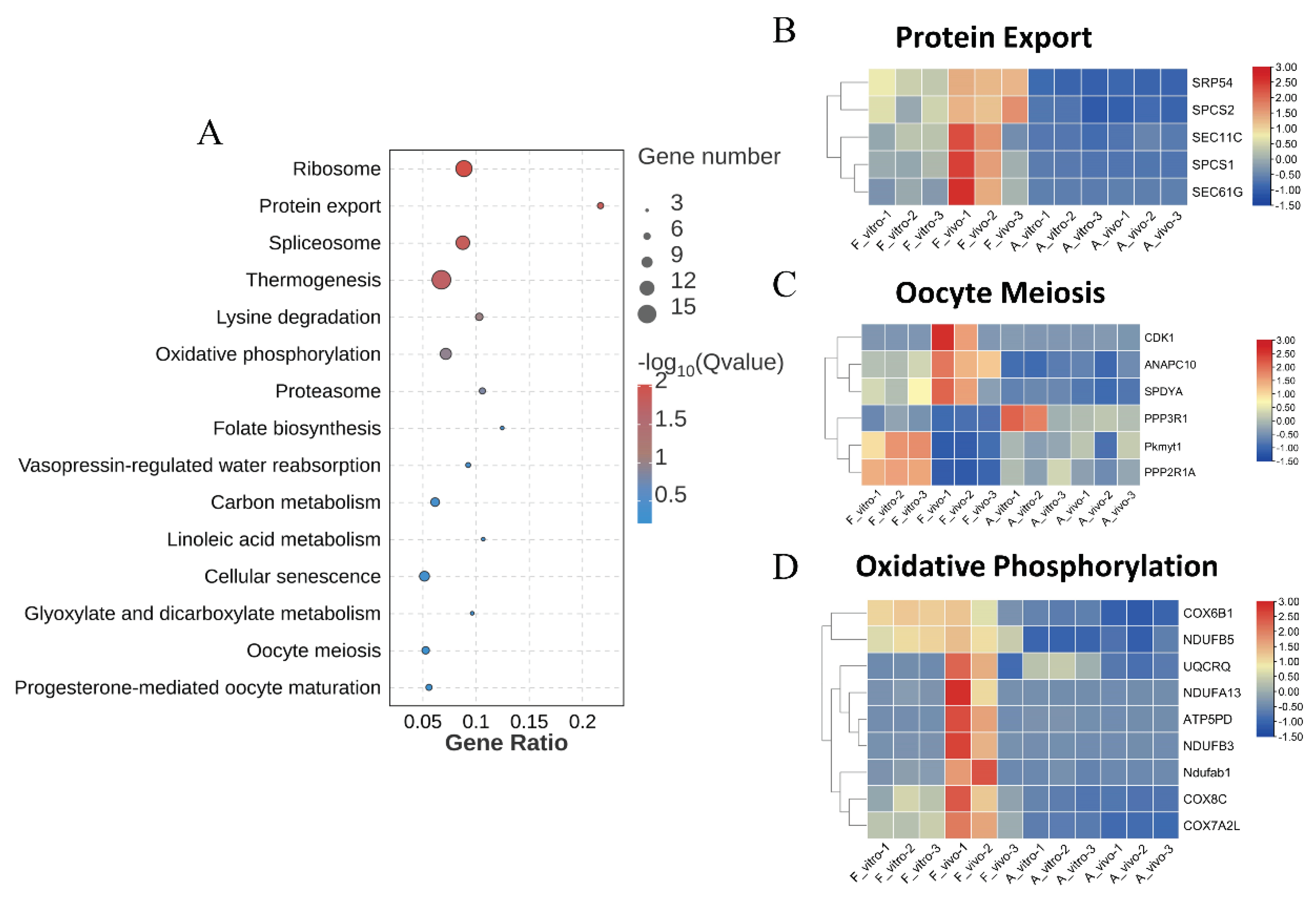
3. POA affects protein export and oocyte meiotic in both in vivo and vitro-matured porcine oocytes
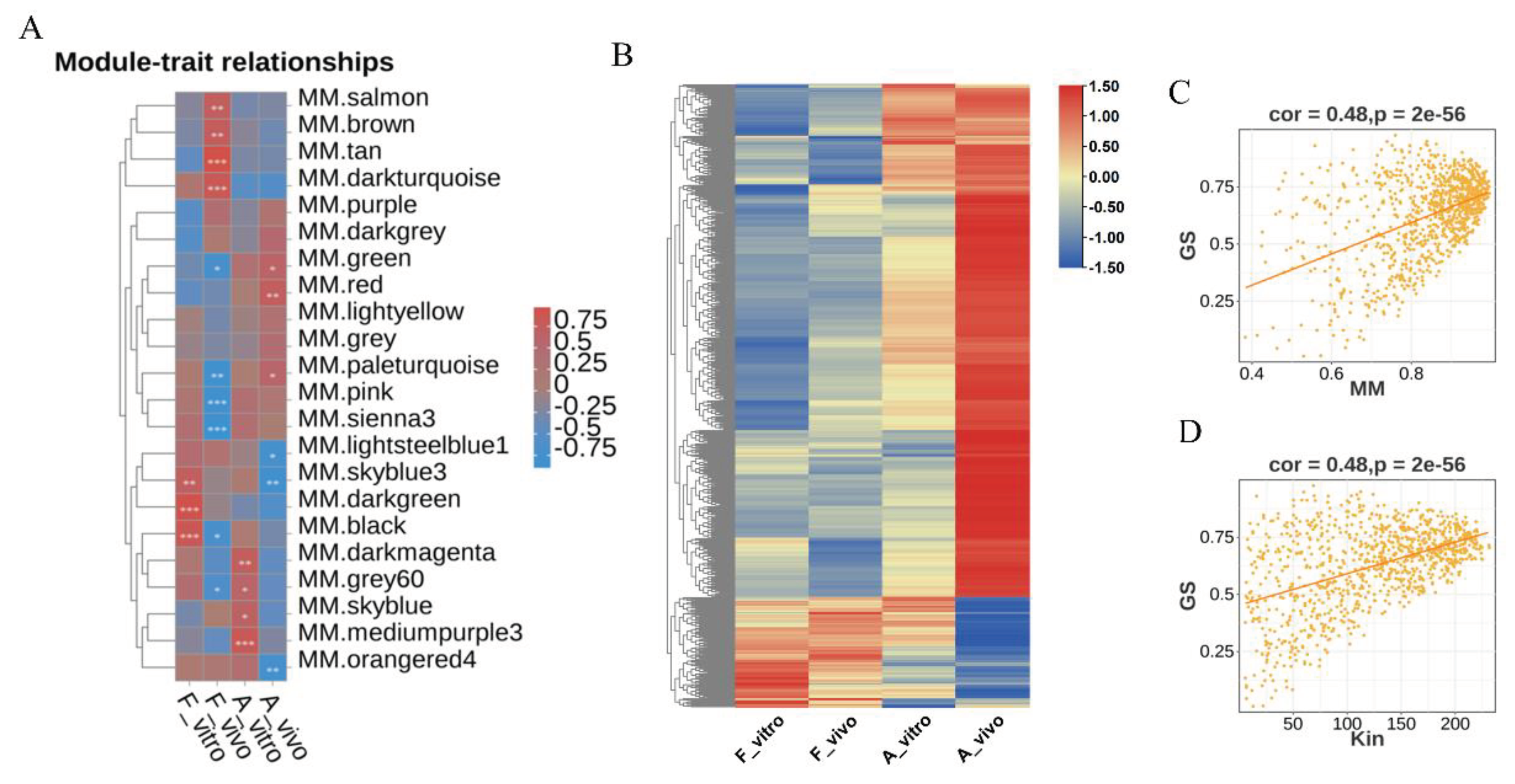
4. Selection of specific target modules for aged in vivo matured porcine oocytes
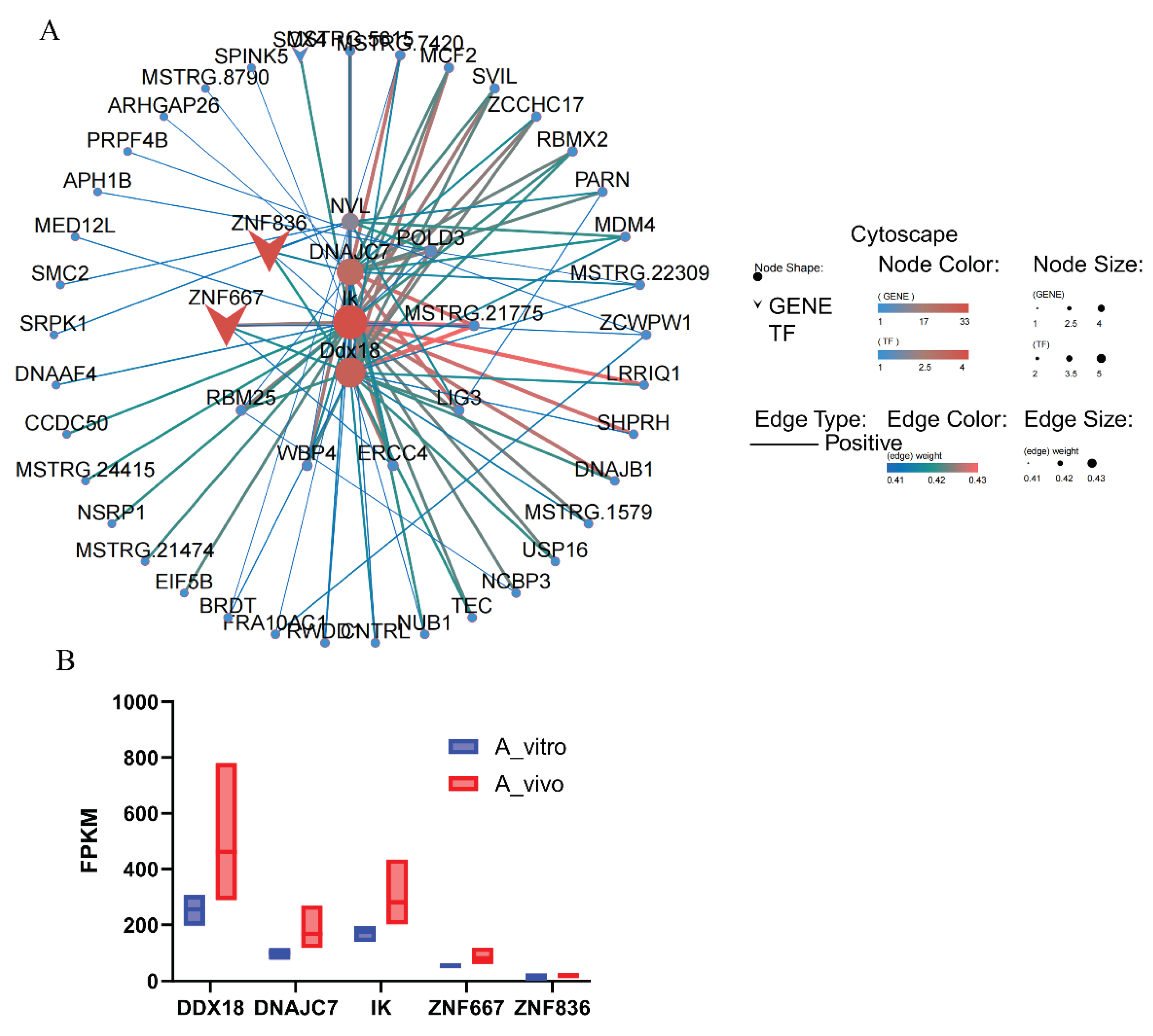
5. DNAJC7, DDX18 and IK were the hub genes in the gene regulation network in aged in vivo porcine oocytes.
DISCUSSION
CONCLUSION
Author Contributions
Acknowledgements
Conflict of Interest Statement
Data Availability Statement
References
- Lord, T.; Aitken, R.J. Oxidative stress and ageing of the post-ovulatory oocyte. Reproduction 2013, 146, R217–227. [Google Scholar] [CrossRef] [PubMed]
- Di Nisio, V.; Antonouli, S.; Damdimopoulou, P.; Salumets, A.; Cecconi, S. In vivo and in vitro postovulatory aging: when time works against oocyte quality? J Assist Reprod Genet 2022, 39, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; ShiYang, X.; Zhang, Y.; Miao, Y.; Chen, Y.; Cui, Z.; Xiong, B. Coenzyme Q10 ameliorates the quality of postovulatory aged oocytes by suppressing DNA damage and apoptosis. Free Radic Biol Med 2019, 143, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Ducibella, T.; Duffy, P.; Reindollar, R.; Su, B. Changes in the distribution of mouse oocyte cortical granules and ability to undergo the cortical reaction during gonadotropin-stimulated meiotic maturation and aging in vivo. Biol Reprod 1990, 43, 870–876. [Google Scholar] [CrossRef]
- Xu, Z.; Abbott, A.; Kopf, G.S.; Schultz, R.M.; Ducibella, T. Spontaneous activation of ovulated mouse eggs: time-dependent effects on M-phase exit, cortical granule exocytosis, maternal messenger ribonucleic acid recruitment, and inositol 1,4,5-trisphosphate sensitivity. Biol Reprod 1997, 57, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Naito, K.; Noguchi, J.; Kaneko, H.; Tojo, H. Maturation/M-phase promoting factor regulates aging of porcine oocytes matured in vitro. Cloning Stem Cells 2002, 4, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Wakayama, S.; Thuan, N.V.; Kishigami, S.; Ohta, H.; Mizutani, E.; Hikichi, T.; Miyake, M.; Wakayama, T. Production of offspring from one-day-old oocytes stored at room temperature. J Reprod Dev 2004, 50, 627–637. [Google Scholar] [CrossRef]
- Lord, T.; Nixon, B.; Jones, K.T.; Aitken, R.J. Melatonin prevents postovulatory oocyte aging in the mouse and extends the window for optimal fertilization in vitro. Biol Reprod 2013, 88, 67. [Google Scholar] [CrossRef]
- Zhang, N.; Wakai, T.; Fissore, R.A. Caffeine alleviates the deterioration of Ca(2+) release mechanisms and fragmentation of in vitro-aged mouse eggs. Mol Reprod Dev 2011, 78, 684–701. [Google Scholar] [CrossRef]
- Tatone, C.; Di Emidio, G.; Barbaro, R.; Vento, M.; Ciriminna, R.; Artini, P.G. Effects of reproductive aging and postovulatory aging on the maintenance of biological competence after oocyte vitrification: insights from the mouse model. Theriogenology 2011, 76, 864–873. [Google Scholar] [CrossRef]
- Guérin, P.; El Mouatassim, S.; Ménézo, Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum Reprod Update 2001, 7, 175–189. [Google Scholar] [CrossRef]
- Liang, X.W.; Zhu, J.Q.; Miao, Y.L.; Liu, J.H.; Wei, L.; Lu, S.S.; Hou, Y.; Schatten, H.; Lu, K.H.; Sun, Q.Y. Loss of methylation imprint of Snrpn in postovulatory aging mouse oocyte. Biochem Biophys Res Commun 2008, 371, 16–21. [Google Scholar] [CrossRef]
- Nagy, Z.P.; Rienzi, L.F.; Ubaldi, F.M.; Greco, E.; Massey, J.B.; Kort, H.I. Effect of reduced oocyte aging on the outcome of rescue intracytoplasmic sperm injection. Fertil Steril 2006, 85, 901–906. [Google Scholar] [CrossRef]
- Gupta, S.; Sekhon, L.; Kim, Y.; Agarwal, A. The role of oxidative stress and antioxidants in assisted reproduction. Current Women's Health Reviews 2010, 6, 227–238. [Google Scholar] [CrossRef]
- Reis Soares, S.; Rubio, C.; Rodrigo, L.; Simón, C.; Remohí, J.; Pellicer, A. High frequency of chromosomal abnormalities in embryos obtained from oocyte donation cycles. Fertil Steril 2003, 80, 656–657. [Google Scholar] [CrossRef]
- Babayev, E.; Seli, E. Oocyte mitochondrial function and reproduction. Curr Opin Obstet Gynecol 2015, 27, 175–181. [Google Scholar] [CrossRef]
- Santos, R.R.; Schoevers, E.J.; Roelen, B.A. Usefulness of bovine and porcine IVM/IVF models for reproductive toxicology. Reprod Biol Endocrinol 2014, 12, 117. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, Y.; Yang, J.Y.; Cheng, D.; Liu, X.; Ma, X.; West, F.D.; Wang, H. Comparative gene expression signature of pig, human and mouse induced pluripotent stem cell lines reveals insight into pig pluripotency gene networks. Stem Cell Rev Rep 2014, 10, 162–176. [Google Scholar] [CrossRef]
- Jarrell, V.L.; Day, B.N.; Prather, R.S. The transition from maternal to zygotic control of development occurs during the 4-cell stage in the domestic pig, Sus scrofa: quantitative and qualitative aspects of protein synthesis. Biol Reprod 1991, 44, 62–68. [Google Scholar] [CrossRef]
- Haggarty, P.; Wood, M.; Ferguson, E.; Hoad, G.; Srikantharajah, A.; Milne, E.; Hamilton, M.; Bhattacharya, S. Fatty acid metabolism in human preimplantation embryos. Hum Reprod 2006, 21, 766–773. [Google Scholar] [CrossRef]
- McEvoy, T.G.; Coull, G.D.; Broadbent, P.J.; Hutchinson, J.S.; Speake, B.K. Fatty acid composition of lipids in immature cattle, pig and sheep oocytes with intact zona pellucida. J Reprod Fertil 2000, 118, 163–170. [Google Scholar] [CrossRef]
- Baek, S.Y.; Sa, S.J.; Jeong, Y.D.; Cho, E.S.; Hong, J.G.; YS, K.I.; Cho, K.H.; Park, S.H.; Kim, K.W.; Lee, H.C.; et al. Altrenogest affects expression of galectin-3 and fibroblast growth factor 9 in the reproductive tract of sows. Anim Biotechnol 2021, 32, 537–543. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 2014, 15, 550. [Google Scholar] [CrossRef]
- Grøndahl, M.L.; Yding Andersen, C.; Bogstad, J.; Nielsen, F.C.; Meinertz, H.; Borup, R. Gene expression profiles of single human mature oocytes in relation to age. Hum Reprod 2010, 25, 957–968. [Google Scholar] [CrossRef]
- Reyes, J.M.; Silva, E.; Chitwood, J.L.; Schoolcraft, W.B.; Krisher, R.L.; Ross, P.J. Differing molecular response of young and advanced maternal age human oocytes to IVM. Hum Reprod 2017, 32, 2199–2208. [Google Scholar] [CrossRef]
- Kirillova, A.; Smitz, J.E.J.; Sukhikh, G.T.; Mazunin, I. The Role of Mitochondria in Oocyte Maturation. Cells 2021, 10. [Google Scholar] [CrossRef]
- Van Blerkom, J.; Davis, P.W.; Lee, J. ATP content of human oocytes and developmental potential and outcome after in-vitro fertilization and embryo transfer. Hum Reprod 1995, 10, 415–424. [Google Scholar] [CrossRef]
- Zhang, Q.; Hao, J.X.; Liu, B.W.; Ouyang, Y.C.; Guo, J.N.; Dong, M.Z.; Wang, Z.B.; Gao, F.; Yao, Y.Q. Supplementation of mitochondria from endometrial mesenchymal stem cells improves oocyte quality in aged mice. Cell Prolif 2023, 56, e13372. [Google Scholar] [CrossRef]
- Khan, S.A.; Reed, L.; Schoolcraft, W.B.; Yuan, Y.; Krisher, R.L. Control of mitochondrial integrity influences oocyte quality during reproductive aging. Mol Hum Reprod 2023, 29. [Google Scholar] [CrossRef]
- Xue, Y.; Meng, T.G.; Ouyang, Y.C.; Liu, S.L.; Guo, J.N.; Wang, Z.B.; Schatten, H.; Song, C.Y.; Guo, X.P.; Sun, Q.Y. Miro1 regulates mitochondrial homeostasis and meiotic resumption of mouse oocyte. J Cell Physiol 2022, 237, 4477–4486. [Google Scholar] [CrossRef]
- Piazzon, N.; Schlotter, F.; Lefebvre, S.; Dodré, M.; Méreau, A.; Soret, J.; Besse, A.; Barkats, M.; Bordonné, R.; Branlant, C.; et al. Implication of the SMN complex in the biogenesis and steady state level of the signal recognition particle. Nucleic Acids Res 2013, 41, 1255–1272. [Google Scholar] [CrossRef]
- Evans, E.A.; Gilmore, R.; Blobel, G. Purification of microsomal signal peptidase as a complex. Proc Natl Acad Sci U S A 1986, 83, 581–585. [Google Scholar] [CrossRef]
- Alzahrani, N.; Wu, M.J.; Sousa, C.F.; Kalinina, O.V.; Welsch, C.; Yi, M. SPCS1-Dependent E2-p7 processing determines HCV Assembly efficiency. PLoS Pathog 2022, 18, e1010310. [Google Scholar] [CrossRef]
- Fang, H.; Mullins, C.; Green, N. In addition to SEC11, a newly identified gene, SPC3, is essential for signal peptidase activity in the yeast endoplasmic reticulum. J Biol Chem 1997, 272, 13152–13158. [Google Scholar] [CrossRef]
- Böhni, P.C.; Deshaies, R.J.; Schekman, R.W. SEC11 is required for signal peptide processing and yeast cell growth. J Cell Biol 1988, 106, 1035–1042. [Google Scholar] [CrossRef]
- Antonin, W.; Meyer, H.A.; Hartmann, E. Interactions between Spc2p and other components of the endoplasmic reticulum translocation sites of the yeast Saccharomyces cerevisiae. J Biol Chem 2000, 275, 34068–34072. [Google Scholar] [CrossRef]
- Mullins, C.; Meyer, H.A.; Hartmann, E.; Green, N.; Fang, H. Structurally related Spc1p and Spc2p of yeast signal peptidase complex are functionally distinct. J Biol Chem 1996, 271, 29094–29099. [Google Scholar] [CrossRef]
- García-Aguirre, I.; Alamillo-Iniesta, A.; Rodríguez-Pérez, R.; Vélez-Aguilera, G.; Amaro-Encarnación, E.; Jiménez-Gutiérrez, E.; Vásquez-Limeta, A.; Samuel Laredo-Cisneros, M.; Morales-Lázaro, S.L.; Tiburcio-Félix, R.; et al. Enhanced nuclear protein export in premature aging and rescue of the progeria phenotype by modulation of CRM1 activity. Aging Cell 2019, 18, e13002. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 2008, 9, 559. [Google Scholar] [CrossRef]
- Schieweck, R.; Ninkovic, J.; Kiebler, M.A. RNA-binding proteins balance brain function in health and disease. Physiol Rev 2021, 101, 1309–1370. [Google Scholar] [CrossRef]
- Lenzken, S.C.; Achsel, T.; Carrì, M.T.; Barabino, S.M. Neuronal RNA-binding proteins in health and disease. Wiley Interdiscip Rev RNA 2014, 5, 565–576. [Google Scholar] [CrossRef]
- Gebauer, F.; Schwarzl, T.; Valcárcel, J.; Hentze, M.W. RNA-binding proteins in human genetic disease. Nat Rev Genet 2021, 22, 185–198. [Google Scholar] [CrossRef]
- Varesi, A.; Campagnoli, L.I.M.; Barbieri, A.; Rossi, L.; Ricevuti, G.; Esposito, C.; Chirumbolo, S.; Marchesi, N.; Pascale, A. RNA binding proteins in senescence: A potential common linker for age-related diseases? Ageing Res Rev 2023, 88, 101958. [Google Scholar] [CrossRef]
- Conlon, E.G.; Manley, J.L. RNA-binding proteins in neurodegeneration: mechanisms in aggregate. Genes Dev 2017, 31, 1509–1528. [Google Scholar] [CrossRef]
- Cookson, M.R. RNA-binding proteins implicated in neurodegenerative diseases. Wiley Interdiscip Rev RNA 2017, 8. [Google Scholar] [CrossRef]
- Salvato, I.; Ricciardi, L.; Dal Col, J.; Nigro, A.; Giurato, G.; Memoli, D.; Sellitto, A.; Lamparelli, E.P.; Crescenzi, M.A.; Vitale, M.; et al. Expression of targets of the RNA-binding protein AUF-1 in human airway epithelium indicates its role in cellular senescence and inflammation. Front Immunol 2023, 14, 1192028. [Google Scholar] [CrossRef]
- Yoon, D.S.; Lee, K.M.; Choi, Y.; Ko, E.A.; Lee, N.H.; Cho, S.; Park, K.H.; Lee, J.H.; Kim, H.W.; Lee, J.W. TLR4 downregulation by the RNA-binding protein PUM1 alleviates cellular aging and osteoarthritis. Cell Death Differ 2022, 29, 1364–1378. [Google Scholar] [CrossRef]
- Kwon, S.M.; Min, S.; Jeoun, U.W.; Sim, M.S.; Jung, G.H.; Hong, S.M.; Jee, B.A.; Woo, H.G.; Lee, C.; Yoon, G. Global spliceosome activity regulates entry into cellular senescence. Faseb j 2021, 35, e21204. [Google Scholar] [CrossRef]
- Li, J.; Lu, M.; Zhang, P.; Hou, E.; Li, T.; Liu, X.; Xu, X.; Wang, Z.; Fan, Y.; Zhen, X.; et al. Aberrant spliceosome expression and altered alternative splicing events correlate with maturation deficiency in human oocytes. Cell Cycle 2020, 19, 2182–2194. [Google Scholar] [CrossRef]
- Kubo, N.; Wu, D.; Yoshihara, Y.; Sang, M.; Nakagawara, A.; Ozaki, T. Co-chaperon DnaJC7/TPR2 enhances p53 stability and activity through blocking the complex formation between p53 and MDM2. Biochem Biophys Res Commun 2013, 430, 1034–1039. [Google Scholar] [CrossRef]
- Lin, W.L.; Chen, J.K.; Wen, X.; He, W.; Zarceno, G.A.; Chen, Y.; Chen, S.; Paull, T.T.; Liu, H.W. DDX18 prevents R-loop-induced DNA damage and genome instability via PARP-1. Cell Rep 2022, 40, 111089. [Google Scholar] [CrossRef]
- Ka, H.I.; Seo, H.; Choi, Y.; Kim, J.; Cho, M.; Choi, S.Y.; Park, S.; Han, S.; An, J.; Chung, H.S.; et al. Loss of splicing factor IK impairs normal skeletal muscle development. BMC Biol 2021, 19, 44. [Google Scholar] [CrossRef]
| Genes | Primer sequences(5’-3’)F | Primer sequences(5’-3’)R |
|---|---|---|
| BTF3 | GTGTGTGCGCCTTATCTCAG | GTTTGGCGAGTTTCTCCTGG |
| CEP95 | AGAGGGCAGGAGAGAGGTTA | ACATCCTCCTCTTCACAGCC |
| CMC1 | CGCAGAACAGCATCTCAGAC | TCCAGAGTCCTTGCAGCATT |
| DBI | ACAGCCACTACAAACAAGCG | ACGCTTTCATGGCATCTTCC |
| NDUFB5 | GCTTTGCCCTCAGTCAACAT | CATGGCTACTATGGGCGAGA |
| 18s | CGCGGTTCTATTTTGTTGGT | AGTCGGCATCGTTTATGGTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




