1. Introduction
The main thoracic malignancies in Japan, in terms of number of deaths and morbidity, are primary lung cancer (75,394/126,548: 2019) and malignant pleural mesothelioma (1,466/1,835: 2019) [
1,
2]. Both of these cancers are on the rise, with about a 70-fold difference in their total number of cases (
Table 1).
Nevertheless, in clinical trials aimed at the development of new drugs, mesothelioma has been treated in the same way as lung cancer. Treatments for lung cancer have been developed, based on new cell-killing anticancer agents, various molecularly targeted therapeutics, immune checkpoint inhibitors (ICIs), etc. While the treatment of lung cancer had been rapidly advancing and fragmented, no new anticancer drugs for mesothelioma were developed for about 15 years, a trend commonly recognized in Japan and globally. Under these circumstances, in 2018, Japan led the world in the advancement of drug therapy for mesothelioma when the Pharmaceuticals and Medical Devices Agency (PMDA) approved nivolumab, designated as an orphan drug, as monotherapy for previously treated malignant pleural mesotheliomas (MPMs) [
3].
In this article, we review the unique evolution of drug therapy for MPM, in light of the historical background, focusing on the true benefits of ICIs in the treatment of this condition.
2. Chemotherapy for MPM
Prior to 2003, various cytotoxic anticancer agents, with a median survival of approximately 6‒8 months, had been tested as single agents or in combination, but none were considered a standard treatment for MPM [
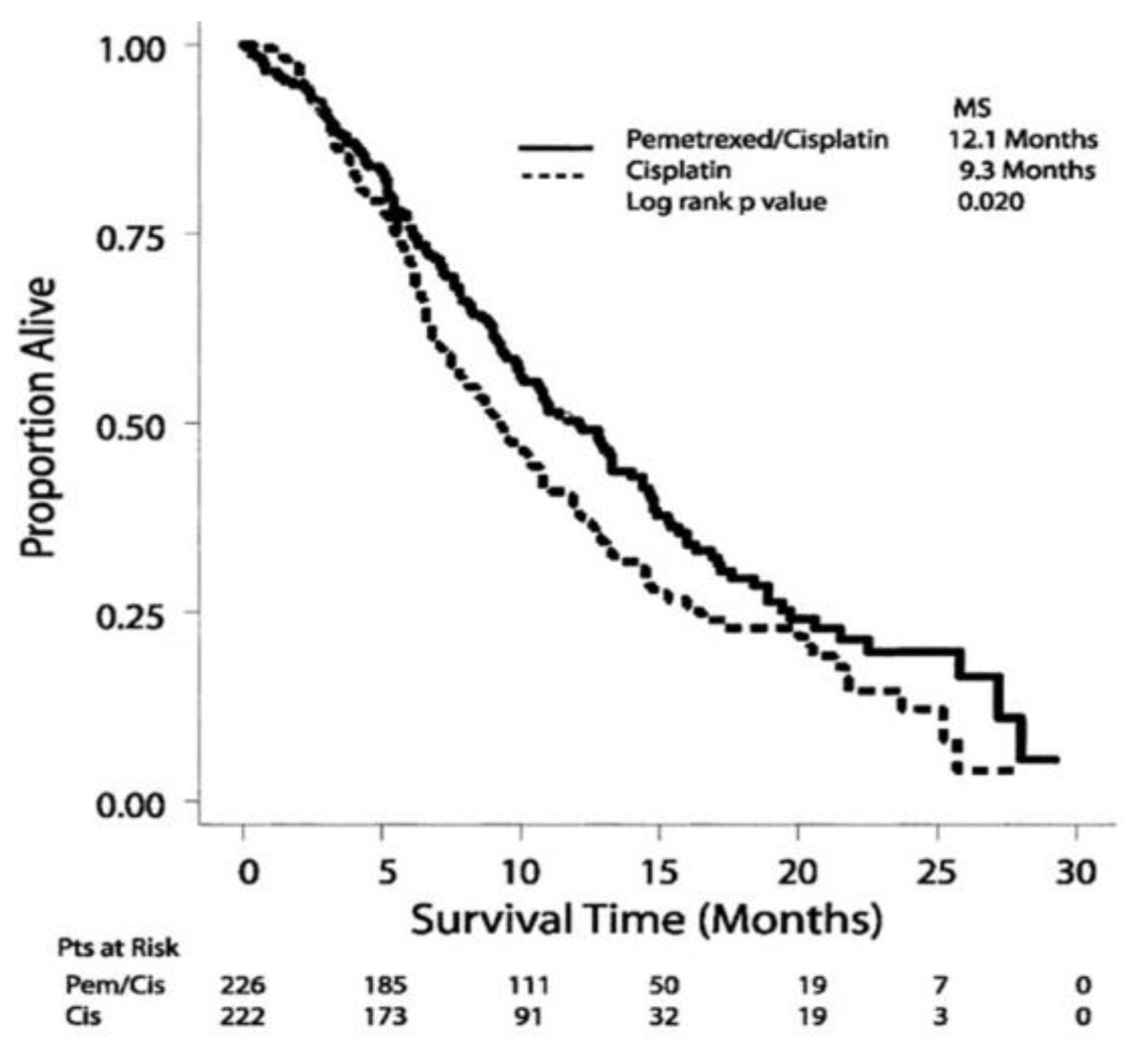
4]. Subsequently, in February 2004, the FDA became the first regulatory agency in the world to approve cisplatin (CDDP) plus pemetrexed (PEM) combination therapy as a standard first-line treatment for untreated and unresectable MPM. This was based on the results of a phase III clinical trial (EMPHACIS trial: Evaluation of Mesothelioma in a Phase III Trial of Pemetrexed with Cisplatin) published in 2003 by Vogelzang et al., which compared CDDP monotherapy with CDDP plus PEM in 456 patients with MPM [
5]. In a comparative study, the median survival values of CDDP monotherapy and CDDP plus PEM were 9.3 months and 12.1 months, respectively, with response rates of 16.7% and 41.3%, respectively. The 1-year survival rate was 50.3% in the combination group compared with 38.0% in the CDDP-only group (
Figure 1). Based on these results, CDDP plus PEM combination therapy was approved in Japan by the PMDA in January 2007, and has been administered as the only approved first-line treatment for untreated MPM to date. We also confirmed, by analyzing all 953 cases registered as post-marketing surveillance, that treatment with CDDP plus PEM for MPM has the same safety and efficacy as preclinical studies and is generally tolerable [
6].
However, it is difficult to say that CDDP plus PEM has a sufficient effect on survival, and while there was a great desire for more effective drugs, no new drug for MPM had been approved since 2004.
One of the reasons for this is that clinical trials focused on developing new drugs for mesothelioma have been conducted following similar protocols as those for lung cancers, which affect approximately 70 times more patients. As a result, there has been a significant delay in advancing the treatment options for mesothelioma.
3. Angiogenesis Inhibitors Against MPM
The 14 years from 2004 to 2018 were not without progress in the medical treatment of MPM. In fact, positive data on bevacizumab, a monoclonal antibody against VEGF-A, were reported in a study titled "Phase 3 study of the effect of Bev added to CDDP/PEM on unresectable MPM (MAPS trial)” [
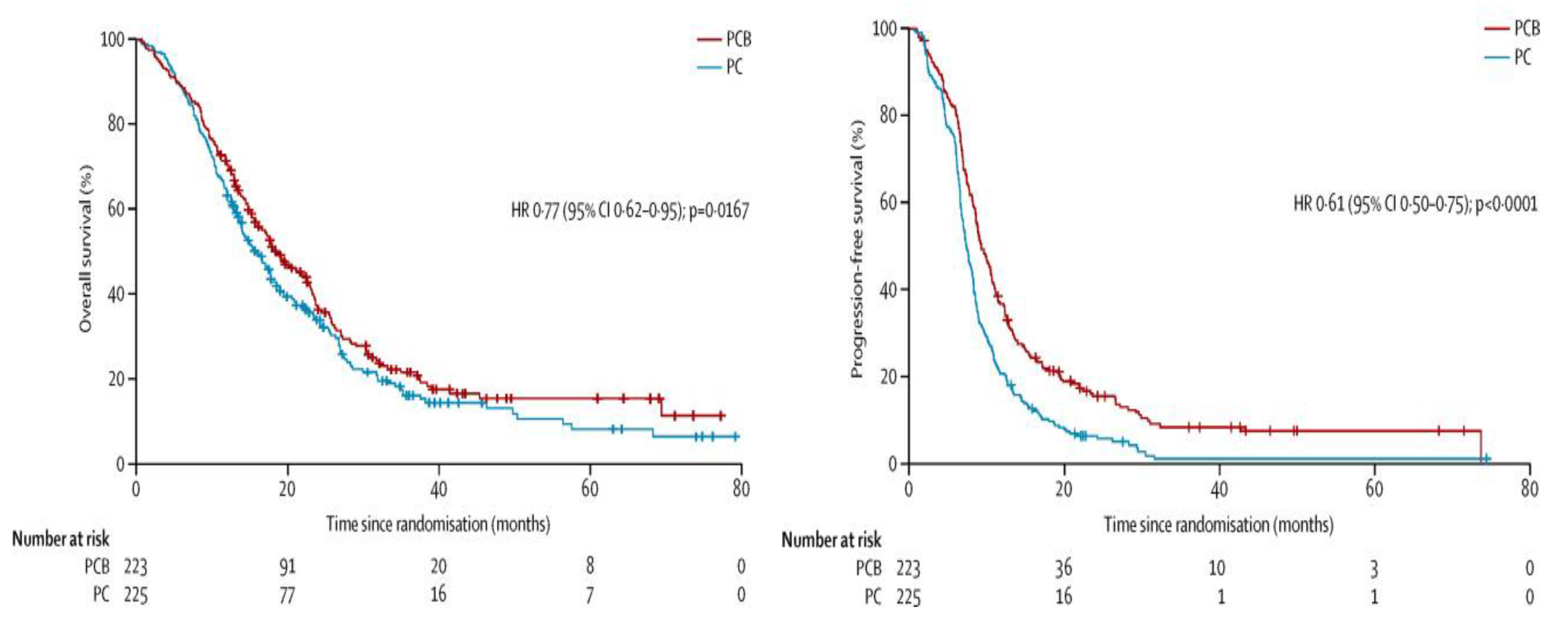
7], published in The Lancet in December 2015. The study was conducted over a period of approximately 6 years, from February 2008 to January 2014, and a total of 448 patients with MPM were randomized (223 patients (50%) in the CPB (CDDP/PEM/bevacizumab) group, 225 patients (50%) in the CP (CDDP/PEM) group): 75% were men, the median age was 65.7 years, 81% had the epithelial type, 97% had an Eastern Cooperative Oncology Group performance status (PS) score 0‒1, and 57% were smokers.
Treatment consisted of CDDP (75 mg/m2) plus PEM (500 mg/m2) (+/- bevacizumab (15 mg/kg)) administered once every 3 weeks for a maximum of 6 cycles. Only 74.9% of the CPB group and 76.0% of the CP group completed 6 cycles, with a median follow-up of 39.4 months and no difference between the two groups.
The median overall survival (OS) for the primary endpoint was significantly longer in the CPB group (18.8 months, 15.9‒22.6) than in the CP group (16.1 months, 95% CI: 14.0‒17.9) (Hazard ratio (HR): 0.77, 95% CI: 0.62‒0.95, p = 0.0167). Progression-free survival (PFS) also improved significantly in the CPB group (9.2 months vs. 7.3 months, HR: 0.61, 95% CI: 0.50‒0.71, p < 0.0001) (
Figure 2).
The results of the MAPS trial prompted the entire world to move toward acquiring additional adaptations of bevacizumab for MPM. In July 2015, CDDP/PEM/bevacizumab was recommended as the first-line treatment for MPM in the National Comprehensive Cancer Network (NCCN) Guideline Version 2. In Japan, bevacizumab (Avastin ®) received orphan drug designation for the treatment of MPM from the Ministry of Health, Labour and Welfare in December 2016, and an expanded access program phase II clinical trial (Study JO 39183) [
8] was conducted to investigate the tolerability and safety of a 3-drug combination of CDDP, PEM, and bevacizumab in previously untreated unresectable MPM. However, starting from 2017, a corporate decision was made that posed challenges in using the MAPS trial data as an application for regulatory approval from the FDA and the European Medicines Agency (EMA). The detailed reasons for this decision have not been disclosed. In Japan, a notice of termination of bevacizumab studies and other activities on MPM was submitted to the Ministry of Health, Labour and Welfare in June 2017, which led to the revocation of bevacizumab's orphan drug designation. As a result, the MAPS trial results did not lead to the first new drug application in nearly 10 years since CDDP/PEM.
This series of events has prompted researchers to suggest that the acceptance of a paper in a top-tier journal and pharmaceutical approval of a new drug have similarities and differences, and highlights the importance of adherence to good clinical practice.
4. Immune Checkpoint Inhibitors Against MPM
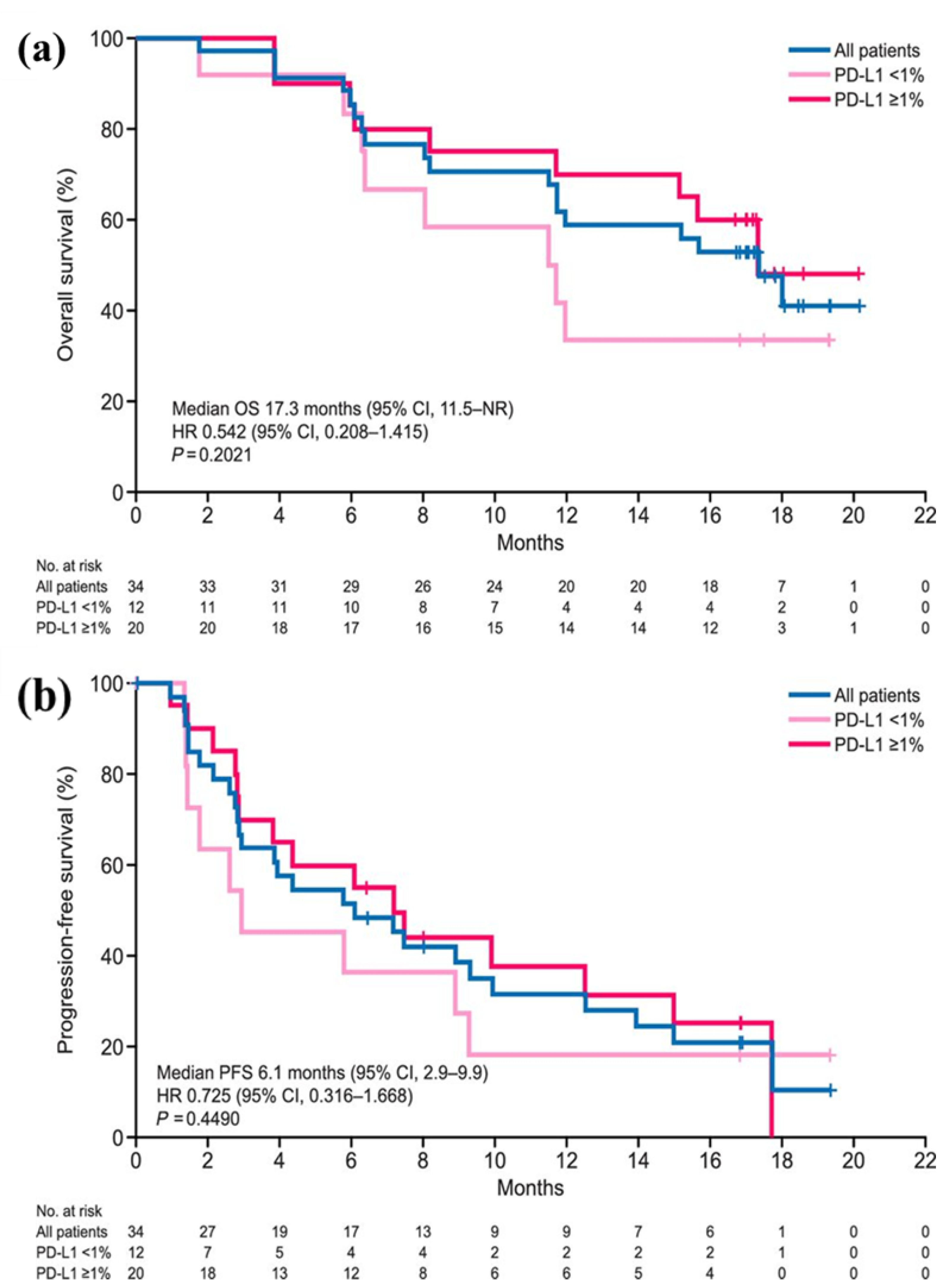
After nivolumab was designated as an orphan drug by the Ministry of Health, Labour and Welfare of Japan in December 2017, it was approved by the PMDA in August 2018 for previously treated, advanced or recurrent MPM. Until then, no drug had received regulatory approval for previously treated, advanced, or relapsed MPM. The MERIT trial was based on the open-label, single-arm phase II study conducted in Japanese patients with unresectable, advanced, or recurrent MPM (34 patients: 24 after primary treatment and 10 after secondary treatment), referred to as the MERIT trial [
9]. The results achieved an objective response rate (expected 19.2%) as the primary endpoint at 29.4% (10/34 patients) (95% CI: 16.8‒46.2%) with a median PFS of 6.1 months (95% CI: 2.9‒not reached) and a median OS that had not been reached (
Figure 3).
The fact that nivolumab received additional approval from the results of the MERIT study, which had only 34 cases, caused controversy in Japan. The fragility of the evidence was one of its limitations. However, the fact that the PMDA approved nivolumab for MPM was highly appreciated by other countries and was truly an epoch-making event in the development of drugs for mesothelioma treatment, which had been performed in accordance with the treatment for lung cancer [
3]. In addition, the usefulness of nivolumab in previously-treated progressive/recurrent MPM was verified in the CONFIRM study. Thus, the PMDA's decision to approve nivolumab ahead of the world was decisive [
10].
In light of the Japanese decision and based on the results of the CheckMate 743 trial [
11], in October 2020, the FDA approved the combination of nivolumab and ipilimumab as the first and only immunotherapeutic agents for untreated, unresectable MPM. The combination therapy was recognized worldwide as the first new systemic therapy approved by the FDA for first-line treatment of MPM in 16 years and was recommended by the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology-Malignant Pleural Mesothelioma (Version 1.2022) [
12,
13].
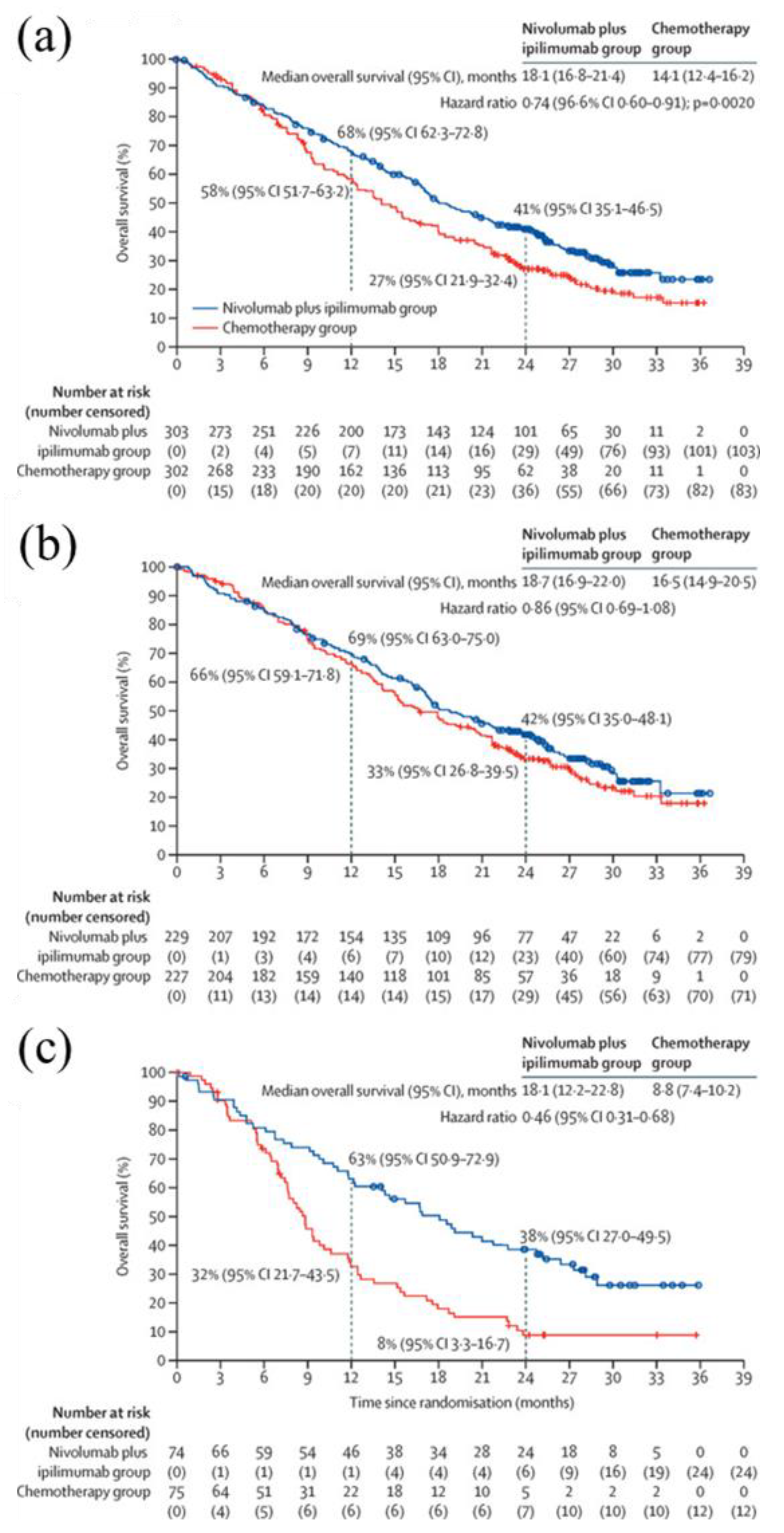
The results of the CheckMate 743 trial (in which Japan and China were Asian participants), an open-label, multicenter, randomized phase III trial in patients with untreated MPM (n = 605), showed that the combination of nivolumab and ipilimumab significantly prolonged survival compared with conventional standard chemotherapy (platinum and PEM), with a primary endpoint of a median OS of 18.1 months (95% CI: 16.8–21.4) in the nivolumab-ipilimumab group, 14.1 months (95% CI: 12.4–16.2) in the standard chemotherapy group, HR 0.74 (95% CI: 0.60–0.91), p = 0.0020, and significant prolongation in the nivolumab plus ipilimumab group (
Figure 4) [
13]. The results of the CheckMate 743 study were submitted to The Lancet for early publication almost immediately after FDA approval [
11].
In addition, follow-up data from the CheckMate 743 trial have provided new insights into the time to end ICI therapy, which is now one of the challenges of ICI therapy for thoracic malignancies. While it is clear that the development of ICIs that inhibit the PD-1 pathway has dramatically changed the treatment of patients with advanced/recurrent non-small cell lung cancer, the prognostic significance of their continued use is controversial and inconclusive, especially for patients who have not progressed over a set period of time [
14]. Patient-reported outcome results, one of the follow-up data from the MPM CheckMate 743 trial, revealed that continuation of ICI combination therapy for 2 years was beneficial [
15]. The patient-reported outcome results that stemmed from the CheckMate 743 trial demonstrated that the combination of nivolumab and ipilimumab in MPM provides a virtuous cycle that preserves activities of daily living and quality of life and, as a result, allows for the continuation of ICI therapy.
In this way, treatment for MPM, which has been used in the past as a treatment for lung cancer, has come to rival that for lung cancer. Moreover, the question of the best time to end ICI led MPM to gain a certain perspective ahead of lung cancer treatment. The potential to catch up from nearly 20 years of lung cancer treatment may also be one of the benefits that ICIs have brought to the treatment of MPM.
5. Critical Challenges to Resolve in ICI Treatment for MPM
There is a scientific basis for the combination of nivolumab and ipilimumab in the treatment of MPM. Nivolumab and ipilimumab both target immune checkpoint proteins, which are distinct and even complementary [
16]. The clinical benefits of nivolumab and ipilimumab have also been demonstrated in multiple other tumor types, and this combination has been approved for the treatment of melanoma, renal cell carcinoma, hepatocellular carcinoma, colorectal cancer with high microsatellite instability or mismatch repair deficiency, and non-small cell lung cancer with a confirmed clinical response [
17].
Messori et al. indirectly compared four current novel treatments for inoperable MPM based on individual patient data retrospectively reconstructed from each of the four trials [
18]. This study compared PEM plus CDDP, previously considered the standard of care for inoperable pleural mesothelioma, as a control against four new treatments (nivolumab + ipilimumab, bevacizumab + PEM + CDDP, pembrolizumab monotherapy, and durvalumab + PEM + CDDP). The Shiny method (or IPDfromKM: the individual patient data from published Kaplan-Meier curve method), which has been proven to be the best in the field for extracting highly reliable data, was employed using a novel technique to reconstruct individual patient data from the current Kaplan and Meier curves [
19]. The results were as follows:
Nivolumab + ipilimumab and bevacizumab + PEM + CDDP showed a better OS compared with controls (HR, 0.79 and 0.79, respectively; p < 0.05). Pembrolizumab demonstrated only a numerical improvement (p > 0.05). In contrast, OS worsened with durvalumab + PEM + CDDP [
18].
The study showed that new treatments for inoperable mesothelioma have similar efficacy and generally provide a small but significant survival benefit compared to standard treatments. There remains no consensus on which of these treatment options is superior with regard to efficacy. Therefore, further research needs to be conducted in this area.
Generally, clinical trials aimed at drug approval for an indication, such as the CheckMate 743 trial, are the result of several large, well-designed clinical trials with extremely high levels of evidence [
20]. However, the eligibility criteria for such clinical trials are very strict, and the evidence is based on carefully selected cases. For many cases “unfit” for clinical trial that are seen in clinical practice, such as patients with poor PS, complications of interstitial pneumonia and organ damage, and patients with multiple cancers, it is not possible to extrapolate the evidence as it is.
Kerrigan et al. used a propensity score-matched, weighted analysis of MPM, and their study found that there was no difference in OS by choice of 1st line PEM + CDDP, 2nd line immunotherapy or chemotherapy, or by receipt of maintenance therapy [
21].
Therefore, it is still unclear how nivolumab plus ipilimumab will impact real-world cases of MPM or if this immunotherapy will truly be a paradigm shift for the treatment of patients with MPM.
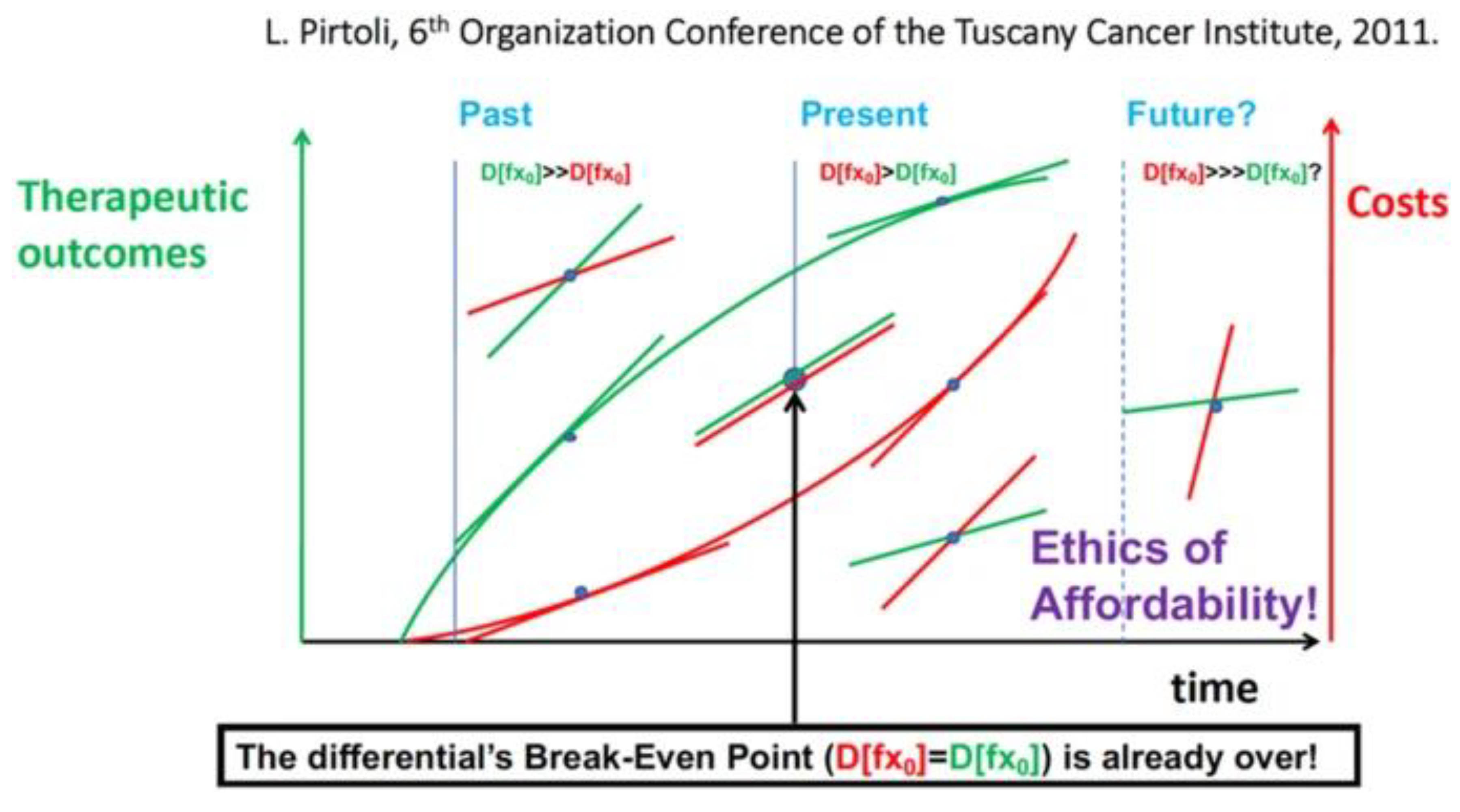
When these new advances in cancer treatment emerged, including those for MPM, the beneficial results justified the high patient costs. Recently, the profit/cost ratio has gradually reversed to the break-even point, and we are approaching a situation in which the cost of new cancer treatments is out of reach for many patients. This trend has been recognized by the medical and scientific communities, and as a result, many attempts have been made to address the ethical issue of setting fair prices for future cancer treatments (
Figure 5) [
22].
In 2017, a Markov model comparing the cost-effectiveness of adding bevacizumab to PEM plus CDDP was established for Chinese payers, and the model's output indicators included the patients' quality-adjusted life years, lifetime costs, and incremental cost-effectiveness ratio [
23]. Further, a study extrapolating this Markov model and evaluating the cost-effectiveness of nivolumab plus ipilimumab and PEM plus CDDP/carboplatin as first-line agents for unresectable MPM from a payer's point of view in the United States found that nivolumab plus ipilimumab had no economic advantage over CDDP/carboplatin in patients with unresectable MPM [
24]. The study used
$150,000 as the willingness-to-pay threshold and concluded that immunotherapy had no economic advantage over conventional chemotherapy for first-line treatment of unresectable MPM [
24].
Another study suggested that the incremental cost-effectiveness ratio of nivolumab plus ipilimumab as a first-line treatment for unresectable MPM exceeds the theoretical willingness-to-pay threshold in the United States, suggesting that nivolumab plus ipilimumab may not be a cost-effective option [
25].
Therefore, Ye et al. suggested that changing the price of nivolumab and ipilimumab is a valid and viable strategy for the efficient use of nivolumab and ipilimumab, and health insurance authorities should negotiate with pharmaceutical companies to ensure fair drug prices and adjust health insurance lists to reduce the burden of patient care [
25].
This conclusion seems to be valid, and, the conclusion is one of the issues to be discussed in the near future, even after ignoring the fact that there may be some problems with the comparison, for example regarding chemotherapy (which has already expired, and many cheaper generics are now available) and the most expensive drugs in the United States (the drug price before the patent expired).
6. Circumstances surrounding medical treatment for MPM in different countries
The history of pharmaceutical approval for MPM in various countries is shown in
Table 2. The regulatory approval of treatments for MPM by national authorities stagnated worldwide for about 15 years after 2004, but the actual circumstances surrounding drug treatment for MPM during this period vary widely from country to country.
In this article, we have focused on the high cost of several recently proposed front-line therapies for MPM; however, it should be noted that in Japan, the pharmaceutical approval by a drug regulatory agency (e.g. US-FDA, EMA, PMDA, etc.) is just as critical an issue as the financial toxicity of drugs.
There are controversial opinions that pharmaceutical approval by drug regulatory agencies is not an important issue, especially considering that the combination of CDDP/PEM/bevacizumab (Avastin) is not currently approved by the FDA but was approved by the NCCN Guidelines and is commonly used in the United States and in various places in Europe and Asia [
26].
However, in Japan, which has a universal health insurance system, it is nearly impossible to administer drugs not approved by the drug regulatory agency, and as a result, almost no patients with MPM receive MAPS treatment.
Therefore, while we can agree that the effort [
27] to use the existing clinical trial data and FDA approval to propose that the appropriate balance between the economic impact of new cancer drugs and the actual benefit to patients is important, the findings [
28] suggesting that the statistical robustness of the three randomized clinical trials (the Mesothelioma Cisplatin Pemetrexed Study (MPS) of cisplatin plus pemetrexed vs cisplatin; the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS) of cisplatin plus pemetrexed plus bevacizumab vs cisplatin plus pemetrexed; and the CheckMate 743 study of nivolumab plus ipilimumab vs cisplatin plus pemetrexed) of front-line treatments recommended for MPM since 2004, using the Survival-Inferred Fragility Index, make it impossible to draw conclusions about the survival benefit of the three trials from virtual comparison, posing a significant obstacle to understanding in Japan.
Therefore, we can agree the appropriate balance between the economic impact of new cancer drugs approved by regulatory agencies and the actual benefit to patients is now very important.
7. Conclusions
In this review article, we summarized the history of the development of drugs, including ICIs, for the treatment of MPM, compared multiple treatment regimens, and discussed the current issue of pharmacoeconomics. As there had been no drugs approved for the treatment of MPM since 2004, the FDA approval of nivolumab plus ipilimumab in 2020 was a great advancement in the treatment of MPM. However, there are no data directly comparing nivolumab plus ipilimumab, including unapproved regimens, with or without chemotherapy, nor with a real-world patient population, so further investigation is needed.
Currently, it is a global concern that the combination of nivolumab and ipilimumab is expensive, and that it is very difficult for individuals and families to pay for it. Therefore, the economic burden should be distributed among citizens through public funds.
Many of the causes of MPM are known to be from exposure to asbestos. Considering that in the past societies benefited from asbestos use, consideration should be given to sharing the financial burden of treating patients with MPM, even if only partially.
8. Future Directions
In Japan, the high price of nivolumab has sparked a public debate, and multiple drug price revisions have resulted in lower nivolumab prices. We believe that it is necessary to examine the cost-effectiveness according to the actual circumstances of each country.
It should be taken into account that in Japan, and other countries where there is an absolute advantage in pharmaceutical approval by a drug regulatory agency over guidelines, the financial toxicity of a drug cannot be discussed unless the drug is approved by a drug regulatory agency and appears in clinical practice.
Author Contributions
Conceptualization, K.K.; validation, J.H.; writing—original draft preparation, K.K.; writing—review and editing, H.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We would like to thank Editage (
www.editage.com) for English language editing.
Conflicts of Interest
The co-author, JH, is an employee of ONO Pharmaceuticals, Co. Ltd. developing nivolumab for cancers including MPM. The corresponding author, KK, declares no conflicts of interest.
References
- National Cancer Center Japan, Cancer Information Service. Cancer registry and statistics, (National Cancer Registry) (2023) [in Japanese]. Available online: https://ganjoho.jp/reg_stat/statistics/data/dl/en.html (accessed on 30 January 2023).
- Cancer Incidence of Japan 2019, Cancer and Disease Control Division, Ministry of Health, Labour and Welfare. https://www.mhlw.go.jp/content/10900000/000942181.pdf (accessed on 30 January 2023).
- Scherpereel, A.; Wallyn, F.; Albelda, S.M.; Munck, C. Novel therapies for malignant pleural mesothelioma. Lancet Oncol 2018, 19, e161–e172. [Google Scholar] [CrossRef]
- Kuribayashi, K.; Fukuoka, K. Advances in the Medical Treatment of Malignant Mesothelioma. J Cancer Biol Res 2014, 2, 1037. [Google Scholar]
- Vogelzang, N.J.; Rusthoven, J.J.; Symanowski, J.; Denham, C.; Kaukel, E.; Ruffie, P.; Gatzemeier, U.; Boyer, M.; Emri, S.; Manegold, C.; et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003, 21, 2636–2644. [Google Scholar] [CrossRef]
- Kuribayashi, K.; Voss, S.; Nishiuma, S.; Arakawa, K.; Nogi, Y.; Mikami, K.; Kudoh, S. Safety and effectiveness of pemetrexed in patients with malignant pleural mesothelioma based on all-case drug-registry study. Lung Cancer 2012, 75, 353–359. [Google Scholar] [CrossRef]
- Zalcman, G.; Mazieres, J.; Margery, J.; Greillier, L.; Audigier-Valette, C.; Moro-Sibilot, D.; Molinier, O.; Corre, R.; Monnet, I.; Gounant, V.; et al. Bevacizumab for newly diagnosed pleural mesothelioma in the mesothelioma Avastin cisplatin pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet 2016, 387, 1405–1414. [Google Scholar] [CrossRef]
- Nakano, T.; Kuribayashi, K.; Kondo, M.; Morise, M.; Tada, Y.; Hirano, K.; Hayashi, M.; Tanaka, M.; Hirabayashi, M. Bevacizumab plus cisplatin/pemetrexed then bevacizumab alone for unresectable malignant pleural mesothelioma: a Japanese safety study. Asia Pac J Clin Oncol 2021, 17, 264–272. [Google Scholar] [CrossRef]
- Okada, M.; Kijima, T.; Aoe, K.; Kato, T.; Fujimoto, N.; Nakagawa, K.; Takeda, Y.; Hida, T.; Kanai, K.; Imamura, F.; et al. Clinical efficacy and safety of nivolumab: results of a multicenter, open-label, single-arm, Japanese phase II study in malignant pleural mesothelioma (MERIT). Clin Cancer Res 2019, 25, 5485–5492. [Google Scholar] [CrossRef]
- Fennell, D.A.; Ewings, S.; Ottensmeier, C.; Califano, R.; Hanna, G.G.; Hill, K.; Danson, S.; Steele, N.; Nye, M.; Johnson, L.; et al. Nivolumab versus placebo in patients with relapsed malignant mesothelioma (CONFIRM): a multicentre, double-blind, randomised, phase 3 trial. Lancet Oncol 2021, 22, 1530–1540. [Google Scholar] [CrossRef]
- Baas, P.; Scherpereel, A.; Nowak, A.K.; Fujimoto, N.; Peters, S.; Tsao, A.S.; Mansfield, A.S.; Popat, S.; Jahan, T. Antonia, S. et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial’. Lancet 2021, 397, 375–386. [Google Scholar] [CrossRef]
- Drugs@FDA: FDA-Approved Drugs, HIGHLIGHTS OF PRESCRIBING INFORMATION, OPDIVO (nivolumab). Available online: www.accessdata.fda.gov/drugsatfda_docs/label/2022/125554s114lbl.pdf (accessed on: 12 February 2024).
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Mesothelioma: Pleural, Version 1.2023 — December 15, 2022. Available online: www.nccn.org/professionals/physician_gls/pdf/meso_pleural.pdf(accessed on: 12 February 2024).
- Nomura, S.; Goto, Y.; Mizutani, T.; Kataoka, T.; Kawai, S.; Okuma, Y.; Murakami, H.; Tanaka, K.; Ohe, Y. A randomized phase III study comparing continuation and discontinuation of PD-1 pathway inhibitors for patients with advanced non-small-cell lung cancer (JCOG1701, SAVE study). Jpn J Clin Oncol 2020, 50, 821–825. [Google Scholar] [CrossRef]
- Scherpereel, A.; Antonia, S.; Bautista, Y.; Grossi, F.; Kowalski, D.; Zalcman, G.; Nowak, A.K.; Fujimoto, N.; Peters, S.; Tsao, A.S.; et al. First-line nivolumab plus ipilimumab versus chemotherapy for the treatment of unresectable malignant pleural mesothelioma: patient-reported outcomes in CheckMate 743. Lung Cancer 2022, 167, 8–16. [Google Scholar] [CrossRef]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol 2016, 39, 98–106. [Google Scholar] [CrossRef]
- Nakajima, E.C.; Vellanki, P.J.; Larkins, E.; Chatterjee, S.; Mishra-Kalyani, P.S.; Bi, Y.; Qosa, H.; Liu, J.; Zhao, H.; Biable, M.; et al. FDA approval summary: nivolumab in combination with ipilimumab for the treatment of unresectable malignant pleural mesothelioma. Clin Cancer Res 2022, 28, 446–451. [Google Scholar] [CrossRef]
- Messori, A.; Trippoli, S. Current treatments for inoperable mesothelioma: indirect comparisons based on individual patient data reconstructed retrospectively from 4 trials. J Chemother 2022, 35, 1–5. [Google Scholar] [CrossRef]
- Liu, N.; Zhou, Y.; Lee, J.J. IPDfromKM: reconstruct individual patient data from published Kaplan-Meier survival curves. BMC Med Res Methodol 2021, 21, 111m. [Google Scholar] [CrossRef]
- Peters, S.; Scherpereel, A.; Cornelissen, R.; Oulkhouir, Y.; Greillier, L.; Kaplan, M.A.; Talbot, T.; Monnet, I.; Hiret, S.; Baas, P.; et al. First-line nivolumab plus ipilimumab versus chemotherapy in patients with unresectable malignant pleural mesothelioma: 3-year outcomes from CheckMate 743. Ann Oncol 2022, 33, 488–499. [Google Scholar] [CrossRef]
- Kerrigan, K.; Jo, Y.; Chipman, J.; Haaland, B.; Puri, S.; Akerley, W.; Patel, S. A real-world analysis of the use of systemic therapy in malignant pleural mesothelioma and the differential impacts on overall survival by practice pattern. JTO Clin Res Rep 2022, 3, 100280. [Google Scholar] [CrossRef]
- Pirtoli, L.; Alia, L.; Zacchini, S. Oncology and a time of crisis. Science, complexity, ethic values, and incertitude. An argumentative essay. Medicus 2021, 5, 104–117. [Google Scholar] [CrossRef]
- Zhan, M.; Zheng, H.; Xu, T.; Yang, Y.; Li, Q. Cost-effectiveness analysis of additional bevacizumab to pemetrexed plus cisplatin for malignant pleural mesothelioma based on the MAPS trial. Lung Cancer 2017, 110, 1–6. [Google Scholar] [CrossRef]
- Yang, L.; Cao, X.; Li, N.; Zheng, B.; Liu, M.; Cai, H. Cost-effectiveness analysis of nivolumab plus ipilimumab versus chemotherapy as the first-line treatment for unresectable malignant pleural mesothelioma. Ther Adv Med Oncol 2022, 14, 17588359221116604. [Google Scholar] [CrossRef]
- Ye, Z.M.; Tang, Z.Q.; Xu, Z.; Zhou, Q.; Li, H. Cost-effectiveness of nivolumab plus ipilimumab as first-line treatment for American patients with unresectable malignant pleural mesothelioma. Front Public Health 2022, 10, 947375. [Google Scholar] [CrossRef]
- Tsao, A.S.; Pass, H.I.; Rimner, A.; Mansfield, A.S. New era for malignant pleural mesothelioma: updates on therapeutic options. J Clin Oncol 2022, 40, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Meirson, T.; Nardone, V.; Pentimalli, F.; Markel, G.; Bomze, D.; D’Apolito, M.; Correale, P.; Giordano, A.; Pirtoli, L.; Porta, C.; et al. Analysis of new treatments proposed for malignant pleural mesothelioma raises concerns about the conduction of clinical trials in oncology. J Transl Med 2022, 20, 593. [Google Scholar] [CrossRef] [PubMed]
- Meirson, T.; Pentimalli, F.; Cerza, F.; Baglio, G.; Gray, S.G.; Correale, P.; Krstic-Demonacos, M.; Markel, G.; Giordano, A.; Bomze, D.; et al. Comparison of 3 randomized clinical trials of frontline therapies for malignant pleural mesothelioma. JAMA Netw Open 2022, 5, e221490. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).