Submitted:
19 February 2024
Posted:
19 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and discussion
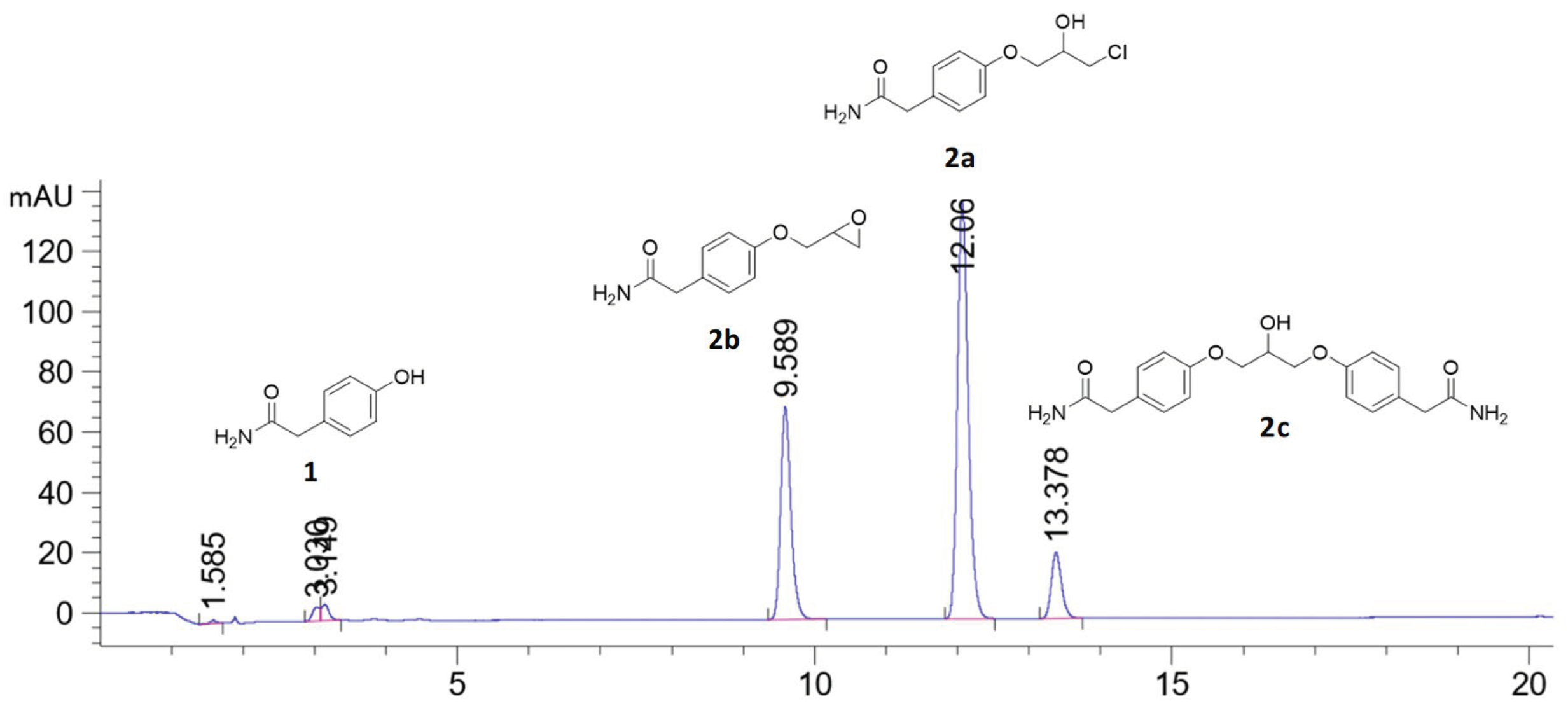
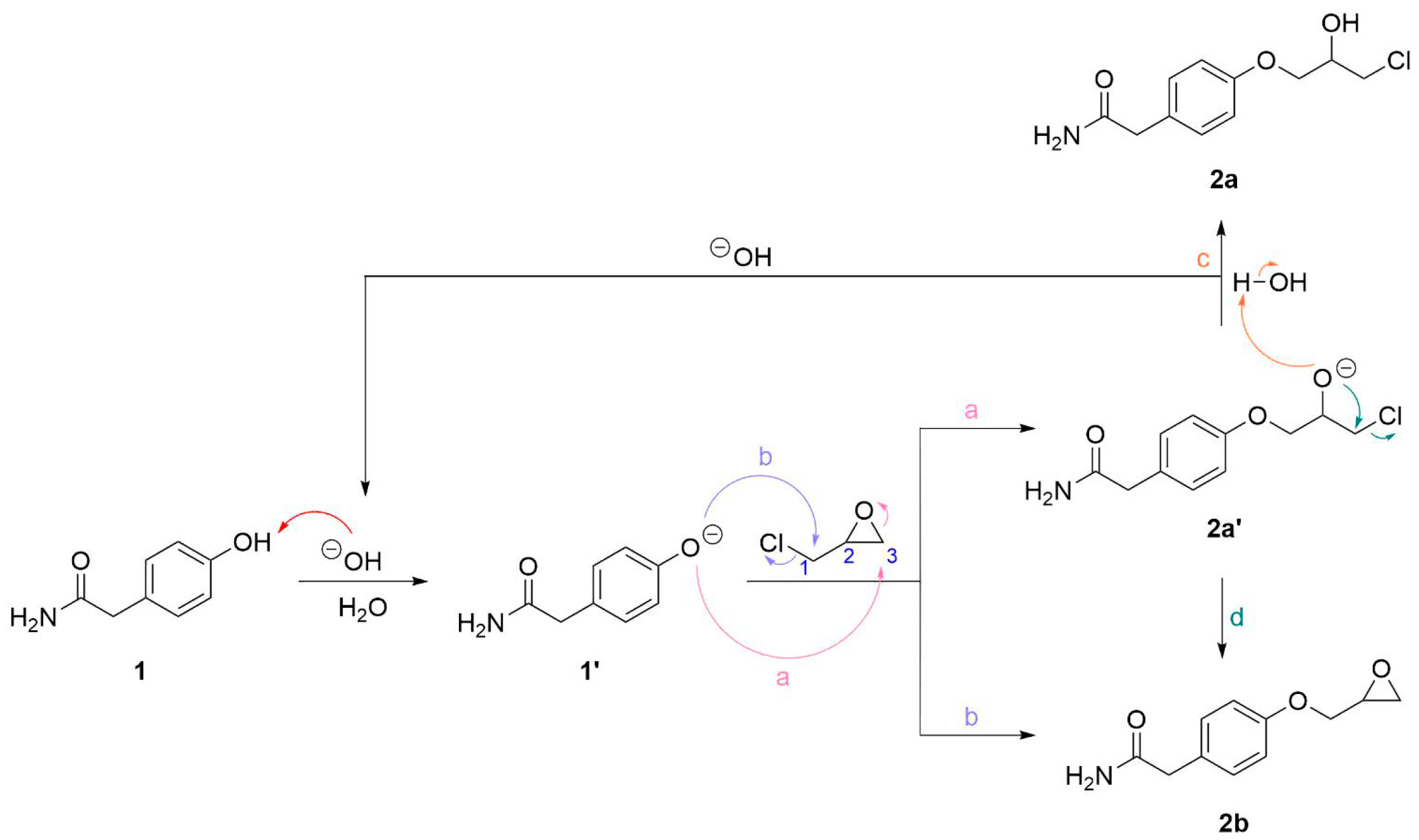
2.1. Deprotection of the phenol proton
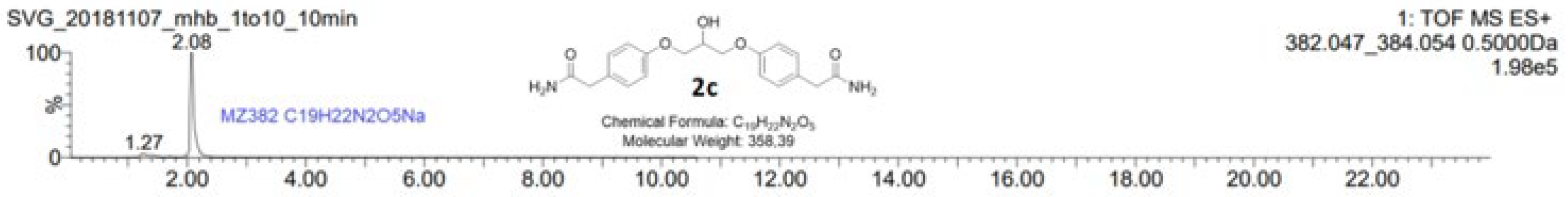
2.2. Dimer Formation With Use of Stoichiometric Amount of Base
2.3. Base Catalyzed Deprotection of Phenol 1
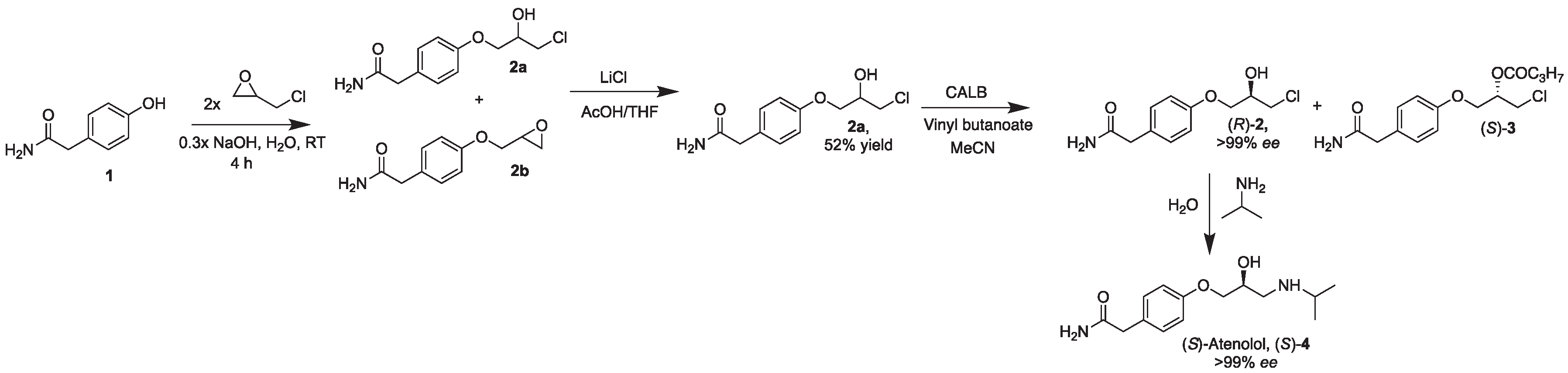
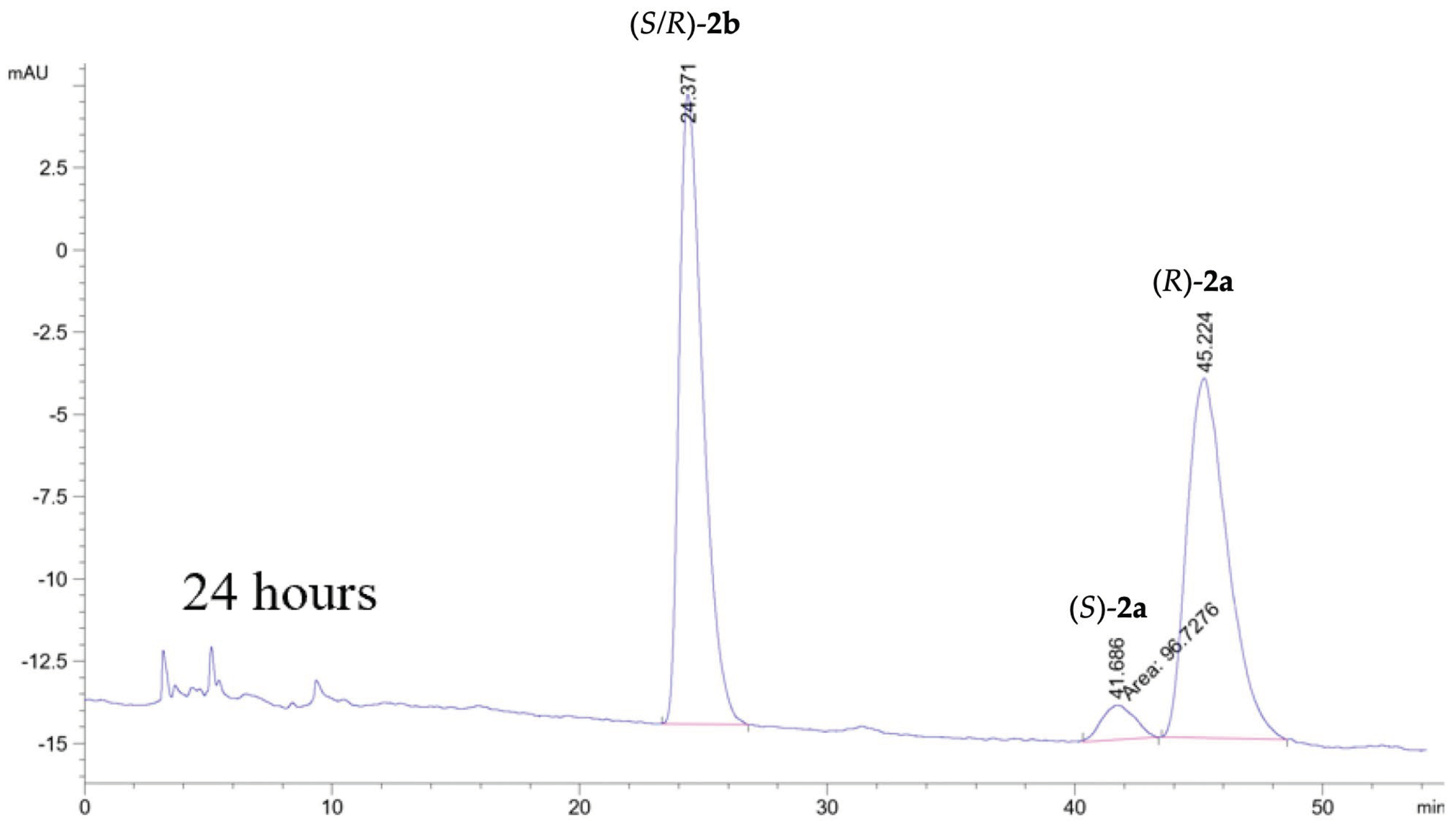
2.4. Lipase Catalyzed Kinetic Resolution of Chlorohydrin 2a
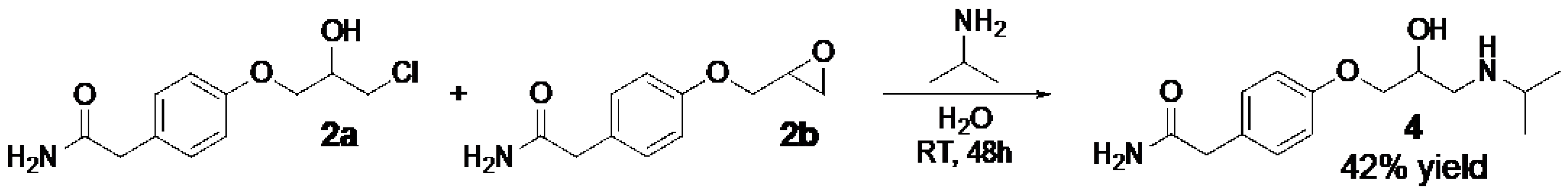
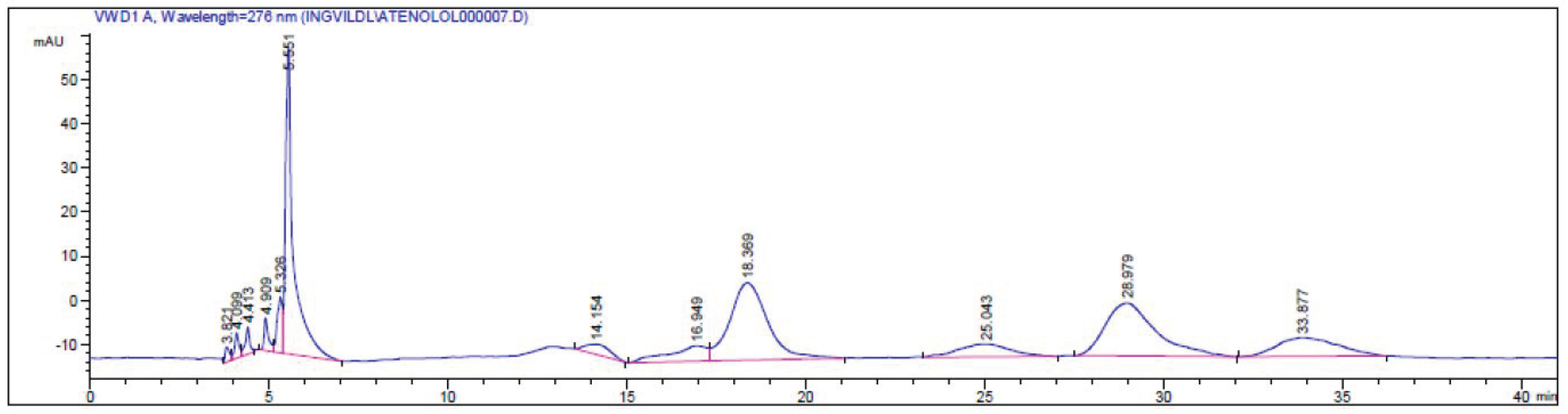
2.5. Synthesis of racemic atenolol (4) and attempt to resolve 4 with CALA
3. Materials and methods
3.1. Chemicals
3.2. Enzymes
3.3. General Analyses
3.4. High-Performance Liquid Chromatography (HPLC)
3.5. NMR Analyses
3.6. Mass Spectroscopy Analyses
3.7. Infrared Spectroscopy Analyses
3.8. Specific Rotation Analyses
3.9. Assignment of Absolute Configurations
3.10. Synthesis Protocols
3.10.1. Synthesis of chlorohydrin 2a and epoxide 2b
3.10.2. Synthesis of chlorohydrin 2a by ring-opening of epoxide 2b
3.10.3. Synthesis of Racemic Atenolol (4) Directly from Phenol 1
3.10.4. Synthesis of (R)-2-(4-(3-chloro-2 hydroxypropoxy)phenyl)acetamide, (R)-2a
3.10.5. Synthesis of (S)-Atenolol, (S)-4
3.10.6. Kinetic resolution of 2-(4-(2-hydroxy-3 (isopropylamino)propoxy)phenyl)acetamide (4)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benjamin, E.J., Muntner, P., Alonso, A., Bittencourt, M.S., Callaway, C.W., Carson, A.P., Chamberlain, A.M., Chang, A.R., Cheng, S., Das, S.R., Delling, F.N., Djousse, L., Elkind, M.S.V., Ferguson, J.F., Fornage, M., Jordan, L.C., Khan, S.S., Kissela, B.M., Knutson, K.L., Kwan, T.W., Lackland, D.T., Lewis, T.T., Lichtman, J.H., Longenecker, C.T., Loop, M.S., Lutsey, P.L., Martin, S.S, Matsushita, K., Moran, A.E., Mussolino, M.E., O’Flaherty, M., Pandey, A., Perak, A.M., Rosamond, W.D., Roth, G.A., Sampson, U.K.A., Satou, G.M., Schroeder, E.B., Shah, S.H., Spartano, N.L., Stokes, A., Tirschwell, D.L., Tsao, C.W., Turakhia, M.P., VanWagner, L.B., Wilkins, J.T., Wong, S.S., Virani, S.S. Heart Disease and Stroke Statistics—2019 Update: A Report From the American Heart Association. Circulation 2019, 139 (10), e56-e528. [CrossRef]
- Dansie, L.S., Bakken, G.V., Berg, C.L., Blix, H.S., Ilic, M., Litleskare, I., Sharikabad, M.N., Skoufa, I.I. , Torheim S. and Granum. T. Drug Consumption in Norway 2017-2021. Norwegian Institute of Public Health, Sept. 2022, pp 172, ISBN electronic ed.: 978-82-8406-313-3.
- Norwegian Health Information. www.nhi.no Accessed Feb. 11, 2024.
- https://www.databridgemarketresearch.com/reports/global-beta-blockers-market. Accessed Feb. 11, 2024.
- Carlberg, B., Samuelsson, O., Lindholm. L.J. Atenolol in hypertension: is it a wise choice? Lancet 2004, 364, 1684-1689. [CrossRef]
- Stoschitzky K, Egginger G, Zernig G, Klein W, Lindner W. Stereoselective features of (R)- and (S)-atenolol. Stereoselective Features of (R)- and (S)-Atenolol: Clinical, Pharmacological, Pharmacokinetic, and Radioligand Binding Studies. Chirality 1993, 5, 15-24. [CrossRef]
- Stoschitzky, K.; Lindner, W.; Zernig, G. Racemic beta-blockers – fixed combinations of different drugs. J. Clin. Bas. Cardiol. 1998, 1, 15–19. [Google Scholar]
- Mehta, S.R., Bhawal, B.M., Deshpande, V.H., Gurjar, M.K. PROCESS FOR PRODUCING ATENOLOL OF HIGH OPTICAL PURITY. Emcure Pharmaceuticals Limited, Pune, India, US patent 6,982,349 B1, Jan. 2006.
- Dwivedee, B.P., Gosh, S., Bhaumik, J., Banoth, L. Banerjee, U.C. Lipase-catalyzed green synthesis of enantiopure atenolol. RSC Adv. 2015, 5 (21), 15850-15860. [CrossRef]
- Agustian, J.; Kamaruddin, A.H.; Aboul-Enein, H.Y. Enantio-conversion and -selectivity of racemic atenolol kinetic resolution using free Pseudomonas fluorescens lipase (Amano) conducted via ransesterification reaction. Rsc. Adv. 2016, 6, 26077–26085. [Google Scholar] [CrossRef]
- Sikora, A.; Chałupka, J.; Marszałł, M.P. The Use of Ion Liquids as a Trojan Horse Strategy in Enzyme-Catalyzed Biotransformation of (R,S)-Atenolol. Catalysts 2020, 10((7)), 787–798. [Google Scholar] [CrossRef]
- Chałupka, J., Sikora, A., Marszałł, M. P. The Utilization of Two-Phase Catalytic System in Enantioselective Biotransformation of Racemic Atenolol. Catalysts 2022, 12, 1068-1080. [CrossRef]
- Sikora, A., Chelminiak-Dudkiewicz, D., Ziegler-Borowska, M., Marszałł, M.P. Enantioseparation of (RS)-atenolol with the use of lipases immobilized onto new-synthesized magnetic nanoparticles. Tetrahedron-Asymmetry 2017, 28, 374-380. [CrossRef]
- Sikora, A., Chełminiak-Dudkiewicz, D., Siódmiak, T., Tarczykowska, A., Sroka, W.D., Ziegler-Borowska, M., Marszałł, M.P. Enantioselective acetylation of (R,S)-atenolol: The use of Candida rugosa lipases immobilized onto magnetic chitosan nanoparticles in enzyme-catalyzed biotransformation. J. Mol. Catal. B Enzym. 2016, 134, 43-50. [CrossRef]
- Sikora, A., Sroka, W.D., Siodmiak, T., Marszałł, M.P. Kinetic Resolution of (R, S)-atenolol with the Use of Lipases in Various Organic Solvents. Curr. Org. Synth. 2017, 14, 747-754. [CrossRef]
- Subhas Bose, D., Venkat Narsaiah, A. An efficient asymmetric synthesis of (S)-atenolol: using hydrolytic kinetic resolution. Bioorg. & Med. Chem. 2005, 13 (3), 627-630. [CrossRef]
- Darnle, S.V., Patil, P.N., Salunkhe, M.M. Chemoenzymatic Synthesis of (R) - and (S) -Atenolol and Propranolol employing Lipase Catalyzed Enantioselective Esterification and Hydrolysis. Synth. Comm. 1999, 29 (22), 3855-3862. [CrossRef]
- Lund, I.T., Bøckmann, P.L., Jacobsen, E.E. Highly enantioselective CALB-catalyzed kinetic resolution of building blocks for b-blocker atenolol. Tetrahedron, 2016, 72, (46), 7288-7292. [CrossRef]
- Troøyen, S.H., Tennfjord, A.L., Klungseth, K., Bocquin, L.H.Y., Jacobsen, E.E. Green Chemo-Enzymatic Protocols for the Synthesis of Enantiopure b-Blockers (S)-Esmolol and (S)-Penbutolol. Catalysts 2022, 12, 980-992. [CrossRef]
- Bøckmann, P.L., Jacobsen, E.E. Chemo-Enzymatic Synthesis of Enantiopure β-Blocker (S)-Metoprolol and Derivatives. Top. Catal. 2023, 1-9. [CrossRef]
- Tjosaas, F., Anthonsen, T., Jacobsen, E.E. Biocatalytic resolution of saphenic acid. Substrate preferences for lipases A and B from Candida antarctica. ARKIVOC 2008, vi, 81-90. DOI: http://hdl.handle.net/11250/2430662.
- Hoff, B.H. Anthonsen, T. Gas chromatographic enantiomer separation of C-3 and C-4 synthons: Prediction of absolute configuration from elution order and enzymatic resolution. Chirality 1999, 11, 760-767. [CrossRef]
- Lystvet, S., Hoff, B. H., Anthonsen, T., Jacobsen, E. E., Chemoenzymatic synthesis of enantiopure 1-phenyl-2-haloethanols and their esters. Biocatal. Biotransform., 2010, 28 (4), 272-278. [CrossRef]
- Anthonsen, H.W, Hoff, B.H., Anthonsen, T. Calculation of Enantiomer Ratio and Equilibrium Constants in Biocatalytic Ping-Pong Bi-Bi Resolutions. Tetrahedron: Asymmetry 1996, 7, 2633-2639. [CrossRef]
- Verho, O., Bäckvall, J.-E. Chemoenzymatic Dynamic Kinetic Resolution: A Powerful Tool for the Preparation of Enantiomerically Pure Alcohols and Amines. J. Am. Chem. Soc. 2015 137, 3996-4009. [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




