1. Introduction
The capacity to adapt to environmental challenges is as fundamental a feature of the living as is the ability to reproduce. Abscisic acid (ABA) is a terpenoid signal molecule present from unicellular organisms to higher plants and mammals, with a conserved, cross-kingdom role as a “stress signal”, allowing organisms to respond to environmental stimuli as diverse as water and nutrient availability, blood glucose levels, UV irradiation [
1].
Abscisic acid is produced in response to water and nutrient availability by microalgae, as well as by higher plants [
2], to UV light by plant stomatal cells, as well as by human granulocytes and keratinocytes [
3], to hypoxia by higher plants, as well as by mammalian cardiomyocytes [
4,
5]. Such diversity of cell types and of functional responses is obviously mediated by cell-specific signaling pathways and molecular effectors, however ABA emerges as a hormonal signal orchestrating the response to environmental stress of organisms as distant in evolution as marine sponges [
6] and mammalian cardiomyocytes [
5]. It should be noted that the environmental stimuli eliciting ABA-mediated responses are very “basic”: soil humidity, nutrient and oxygen availability affecting plant growth, water temperature regulating marine sponge filtration, UV light inducing plant antioxidant defenses and human keratinocyte inflammatory response.
ABA perception occurs through different receptors in plants [
7] and in animals. In mammals, two ABA receptors are known so far, LANCL1 and LANCL2, belonging to the lanthionine synthetase C-like (LanCL) gene family, most likely the result of an ancestral gene duplication, which also contains LANCL3, transcribed at very low levels and probably a pseudogene. LANCL proteins show a significant sequence homology with bacterial lanthionine synthetase, which produces cysteine-derived natural antibiotics (lantibiotics). Although mammalian LANCL proteins do not synthesize lantibiotics [
8], they evidently play an essential role in animal physiology since triple LANCL knock-out (KO) mice die prematurely [
9]. LANCL1 and LANCL2 share several structural and functional features which make them a redundant system for ABA sensing and signaling: i) both are attached to the inner side of the plasma membrane, through which ABA is transported by non-specific anion transporters [
10]; ii) they are similarly and ubiquitously expressed in mammalian tissues and organs (
https://www.proteinatlas.org/); iii) both bind ABA, albeit with a somewhat different affinity, with a Kd in the submicromolar and in the low micromolar range, respectively [
11,
12]; iv) they both activate the AMPK/PGC-1α/ERRα/Sirt1 axis, a signaling pathway which controls mitochondrial mass and function. Interestingly, silencing of LANCL2 in cells or its genetic ablation in mice induce the spontaneous overexpression of LANCL1, while silencing of LANCL1 results in overexpression of LANCL2 in adipose tissue and muscle cells [
12,
13]. Receptor redundancy and a reciprocal “compensatory” transcriptional control again point to the physiological relevance of the ABA-LANCL1/2 hormone-receptor system in mammals.
Indeed, the ABA/LANCL system has been shown to stimulate insulin-independent glucose uptake and its oxidative metabolism in adipocytes and muscle cells, which, combined, represent approx. 50% of mammalian body weight. As a consequence of this ABA/LANCL-mediated increase of glucose uptake and metabolism by muscle and adipose tissue, blood glucose levels are reduced in ABA-treated rodents and humans as compared with untreated controls [
14,
15]. The AMPK/PGC-1α/ERRα axis activated by the ABA-LANCL1/2 hormone-receptors system not only controls glycemia, by stimulating adipose and muscle cells to drain glucose from the blood, but also stimulates mitochondrial respiration in muscle and adipose cells [
12,
13]. Mitochondria are the power plant of cells, where enzymatic oxidation of metabolic substrates produces reduced NAD and FAD coenzymes, which in turn power the respiratory chain, generating the mitochondrial proton gradient (ΔΨ) between the inner mitochondrial membrane (IMM) and the mitosol that eventually allows ADP phosphorylation to ATP by ATP synthase. The partial dissipation of the ΔΨ by specific proton-channels allows thermogenesis, arguably as essential a feature of the animal kingdom as respiration itself. These proton channels are referred to as uncoupling proteins, because they partly uncouple proton flux through the inner mitochondrial membrane from ATP synthesis. They are under the transcriptional control of specific hormones which regulate thermogenesis, i.e., T3 and catecholamines, acting via specific receptors (thyroid hormone and β-adrenergic receptors, respectively). Recently, overexpression of LANCL1 and/or of LANCL2 was shown to increase, while their combined silencing conversely reduced, mitochondrial number, respiration and uncoupling both in human brown adipocytes [
13] and in rat cardiomyocytes [
5]. Notably, overexpression of LANCL1/2
per se (without addition of ABA) induced these significant effects on mitochondrial function, by activating a transcriptional response leading to activation of the AMPK/PGC-1α/Sirt1 axis, which in turn controls mitochondrial biogenesis and function [
16].
The significantly higher mitochondrial mass, O2 consumption and oxidative phoshorylation (oxphos) uncoupling observed in LANCL1/2 overexpressing rat cardiomyocytes should result in a higher heat production of the overexpressing compared with the double-silenced cells under the same conditions; however, a direct temperature or heat production measurement on cells has never been performed until now.
The aim of this study was to directly measure heat production by LANCL1/2-overexpressing vs. double-silenced H9c2 rat cardiomyocytes with super-sensitive temperature probes on relatively small cell samples. These measures were compared with those obtained with a differential scanning calorimeter (DSC) on the same cell types. Results obtained indicate an almost double heat production by LANCL1/2-overexpressing vs. double-silenced cells, and a further 100% increase of heat production in ABA-treated vs. –untreated LANCL1/2-overexpressing cells. These results provide the first direct demonstration that the ABA-LANCL1/2 hormone-receptors system controls thermogenesis in mammalian cells.
2. Materials and Methods
2.1. Cell Transduction
H9c2 rat cardiomyoblasts, obtained from ATCC (LGC Standards s.r.l. Milan, Italy), were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 25 mM glucose (DMEM-high glucose) (Sigma-Aldrich, Milan, Italy) supplemented with 10% fetal bovine serum (FBS) (Sigma-Aldrich, Milan, Italy), penicillin (62.5 μg/ml) and streptomycin (100 μg/ml) (Sigma-Aldrich, Milan, Italy) (complete medium) in a humidified atmosphere containing 5% CO2 at 37 °C.
LANCL1 and LANCL2 silencing (sh), LANCL1 and LANCL2 overexpression (ov) and cell transductions were performed as described in [12-Spinelli 2021 Mol Met].
The protein expression levels of hLANCL1 and hLANCL2 in over-expressing cells were approx. 10 and 40 times higher than in control cells, transfected with the empty vector, while protein levels were both reduced by approx. 90% in double-silenced cells, as detected by Western blot and qPCR [
5].
2.2. Cell Culture
Twentyfour hours before each experiment, cells were seeded at a density of 1x106 in 75 cm2 flasks in DMEM containing 5 mM glucose (DMEM low glucose), without FBS. “Starved” cells were harvested by trypsin treatment, washed twice in PBS containing Ca2+/Mg2+ and resuspended in the same buffer at 12.5x106/ml (for DSC experiments) or at 2.5x106 cells/ml for measures with the temperature probes. Cells were kept at room temperature in the immediate vicinity of the instrument to be used for temperature recording (DSC or temperature probes set-up) for thermalization until use.
The final concentrations of the nutrients used to start oxidative metabolism in the starved cells were the same in all experiments: 2 mM glutamine, 1 mM pyruvate and 17.5 mM glucose in PBS with Ca2+/Mg2+. To inhibit oxidative phosphorylation, cells were pre-incubated for 2h with 0.03 mg/ml digitonin, 0.5 µM rotenone and 0.5 µM antimycin A prior to experimental measures. To inhibit glycolysis and mitochondrial respiration, 3 mM KCN and 10 mM 2′-deoxy-glucose were added together with the nutrients immediately before measurements.
2.3. Temperature Probes
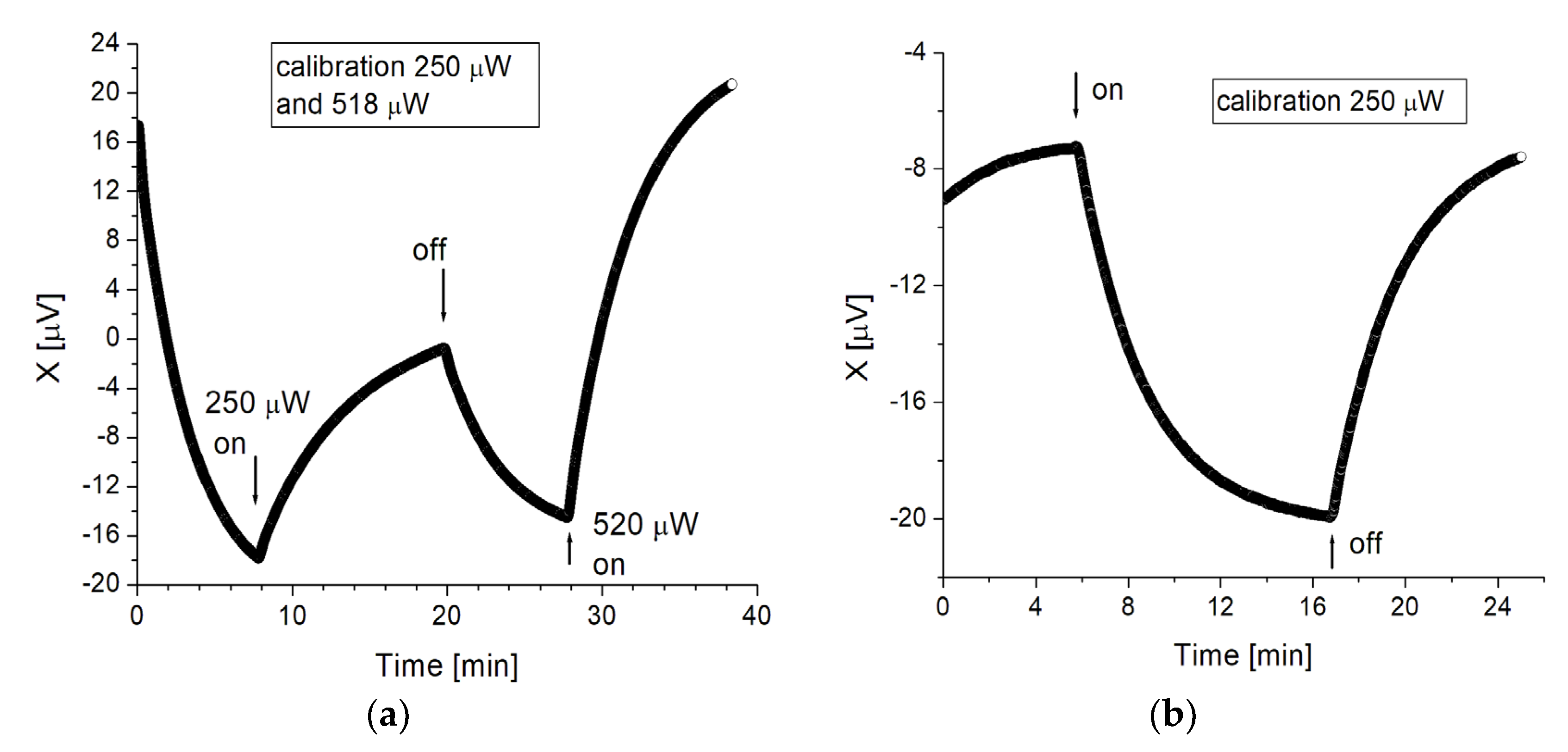
NTC type BR series, glass encapsulated bead thermistors cat n. BR55KA622K, produced by Amphenol, were obtained from Mouser Electronics, Inc. Milano, Italy. The glass bead has nominal diameter × length of 1.4 × 3 mm and a thermal time constant of 0.2 s in water. The temperature coefficient (% resistance change / °C) is α = −4.0 at 25 °C, and we used probes with resistance R ≈ 6.2 kΩ at room temperature. Some measurements were also obtained with epoxy encapsulated NTC type thermistors produced by Vishay (cat. No. NTCLE305E4502SB) obtained from Newark Electronics (cat. 86R9240). These have similar characteristics: α = −3.7 at 25 °C, R ≈ 5 kΩ at room temperature. After carefully soldering the leads to insulated copper wire, the former were mounted on a 1 mL type disposable pipette tip, cut to size to allow the wires to run inside the pipette tip, with the glass bulb encapsulating the thermistor protruding at the end. The thermistor leads were glued to the pipette tip, for mechanical stability and electrical insulation, using 5 min epoxy. This insulation proved unreliable in water after about 20 minutes, which restricted our measurements to time scales below 10 min, the probe assemblies being air dried in between measurements. The epoxy encapsulated probes were suspended directly by their (factory insulated) leads from the screw cap of a 1.5 mL vial. For the measurements, the two probes (corresponding to sample and reference) were wired as two arms of a Wheatstone bridge configuration, thus automatically taking the difference signal between the probes. The other two arms of the bridge were formed by 6.7 kΩ resistances and a 1 kΩ, 10 turns potentiometer which allowed to balance the bridge so the difference signal could be amplified. We used lock-in detection, the bridge being powered by the sinusoidal reference signal from a lock-in amplifier (Stanford Research Systems, model SR530).
The signal was acquired by a PC running National Instruments Labview software via a PCI-6321 data acquisition board.). Typical settings for the lock-in amplifier were: reference signal Vin = 140 mV rms amplitude at v ~ 100 Hz frequency, 500 μV sensitivity, 1 s time constant. The potentiometer on the bridge then allowed the signal to be zeroed within a few μV before starting the measurements. From the bridge configuration and the temperature coefficient of the probes, with Vin = 100 mV, a 10 μV signal corresponded to a temperature difference of 0.01 °C.
2.4. Experimental Procedure with the Temperature Probes
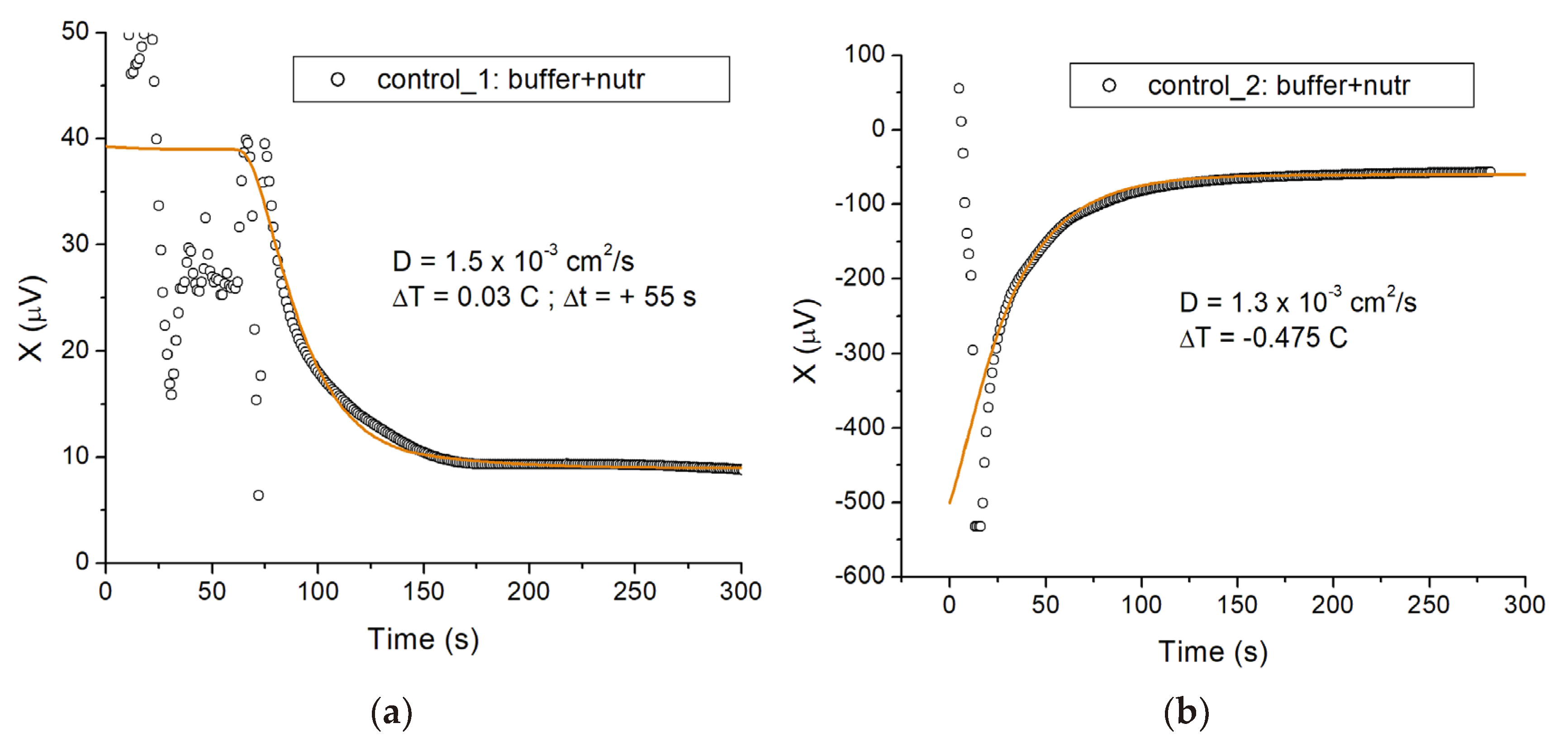
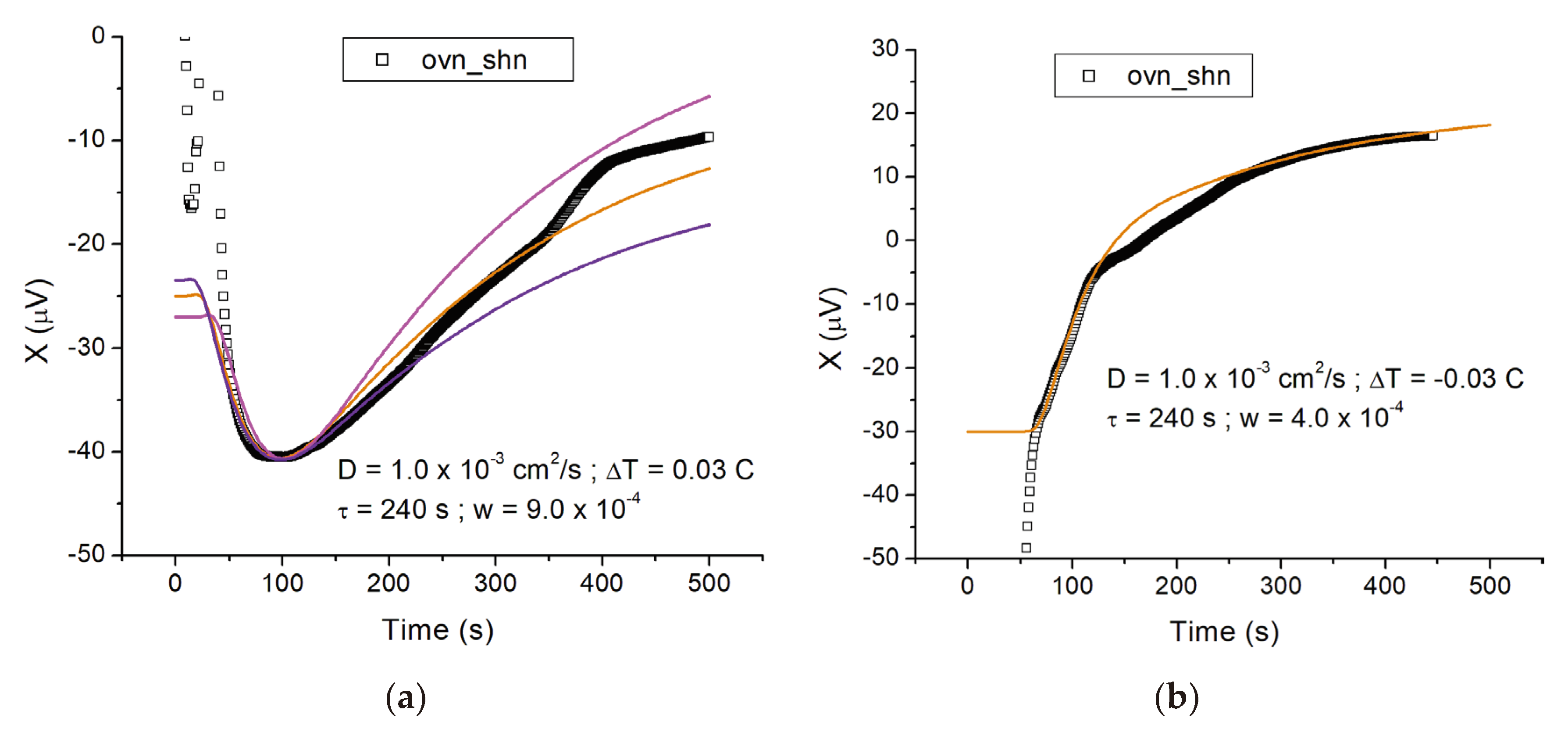
All parts of the set-up were pre-thermalized at room temperature for at least 30 min before starting the experiments. In a typical experiment, 1.0 mL of a cell suspension containing 2.5 × 106 cells in PBS with Ca2+/Mg2+ was added to each one of two Eppendorf tubes, held in a holder, in air. With the probes in air, the signal was zeroed by means of the potentiometer. Then a temperature probe assembly was inserted in each Eppendorf, the micropipette tip being held in position at the center of the suspension by a removable Styrofoam lid. Recording was started immediately after insertion of the probes in the cell suspension; after thermalization of the probes, the probes were removed, a volume of 10 µl of PBS, containing or not the nutrient mixture to allow cell oxidative metabolism, was added to the Eppendorf tubes, the cell suspension was mixed once by gentle pipetting, the probes were inserted again and fixed in the central position of the suspension and recordings were started again.
2.5. DSC
Measurements were performed using a heat flux Differential Scanning Calorimetry (DSC 111 by Setaram) designed as a Calvet-type calorimeter. The sample and reference sensors are inserted in a calorimetric block and are composed of 120 thermocouples mounted in a cylinder surrounding the measurement zone and providing a highly sensitivity sensor.
Briefly, 200 µl of the cell suspensions to be compared were added to each one of two “home-made” Ta crucibles and thermalized at ambient temperature for at least 30 min. After addition of the nutrient mixture (10 µl), or of an equal volume of PBS with Ca
2+/Mg
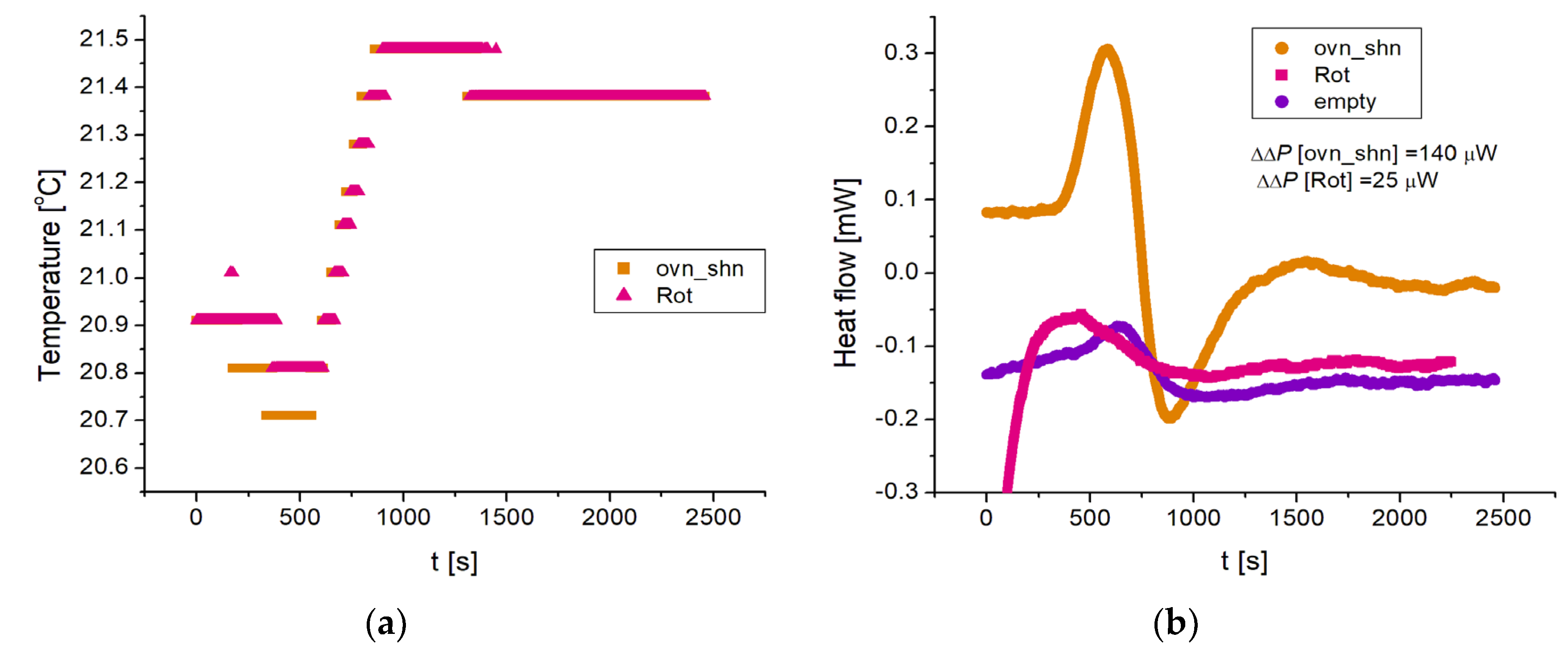
2+ (controls) the crucibles were capped, placed inside the DSC chamber and the temperature ramp program was started. A typical temperature recording is shown in
Figure 5. Recordings were acquired for 2450s.
4. Discussion
Monitoring temperature fluctuations and/or heat production in living cells is of great interest to the biologist, as it informs on cell metabolism, mitochondrial respiration and uncoupling and may allow to identify molecular controllers of heat generation. Temperature monitoring on isolated cells has been obtained in the recent past mostly by indirect methods, via temperature-sensitive fluorescent or luminescent probes [19-23]. These methods suffer some limitations due to the short life-span of the fluorescent signal and its sensitivity to changing environmental cellular conditions. A recent and innovative study by Han et al. introduces an entirely different approach to temperature measurement (and not just “sensing”) by means of a sensor containing a Pd-Cr thin-film thermocouple, a freestanding Si3N4 platform, and a dual-temperature control system: with this chip, the authors measured temperature fluctuations in human epithelial tumor cells (HeLa) (
in the order of magnitude of 0.3-1.5 K) [
24].
In our study, we measure the heating power produced by a population of cultured cells (
N = 2.5 × 10
6) by two different methods: directly by calorimetry, and indirectly by following the time course of the temperature fluctuations. Both methods are differential, the quantity measured being the difference (of heating power, or temperature, respectively) between a sample and a reference. We were able to compare samples of genetically modified cells, and draw conclusions on the corresponding physiology. Thus, we obtained a direct measurement of the heating power generated by the mitochondrial activity of rat H9c2 cardiomyocytes, demonstrating a
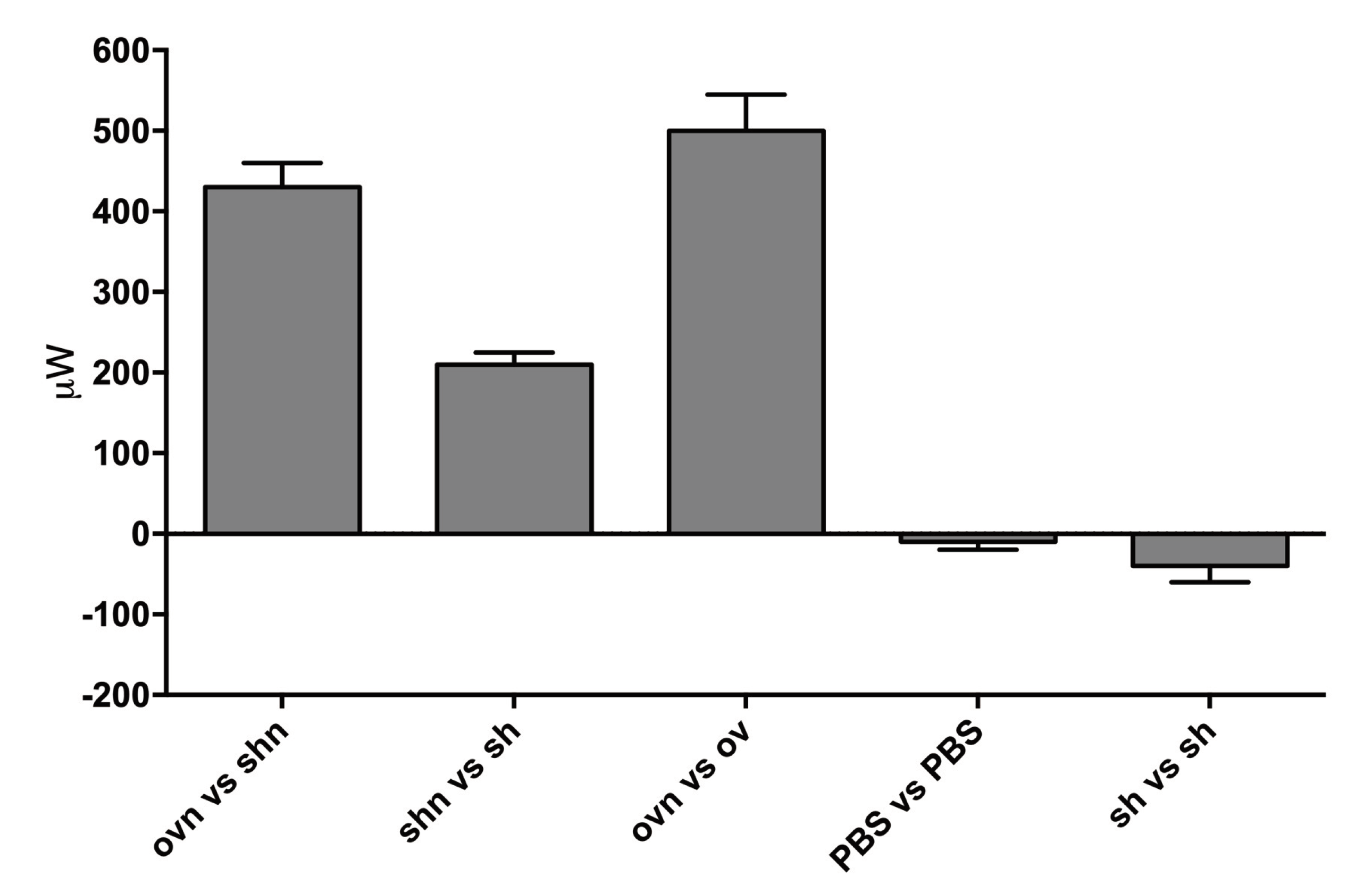
hitherto unknown role for the ABA/LANCL1-2 hormone-receptors system in (cardio)myocyte thermogenesis. Cells overexpressing human LANCL1 and LANCL2 showed a significantly higher heat generation than cells double-silenced for the expression of endogenous LANCL1/2 and this difference was almost completely abolished by treatment with the electron transfer chain inhibitors rotenone and antimycin A (
Figure 7).
On the experimental side, a novel aspect of this study lies in the demonstration that a relatively simple apparatus based on low-cost, commercially available thermistor probes has sufficient sensitivity to allow measurement of heat production by a limited number of cells (~2 × 106 cells), easily attainable not only with cultured cells, as in this study, but also with explanted tissues.
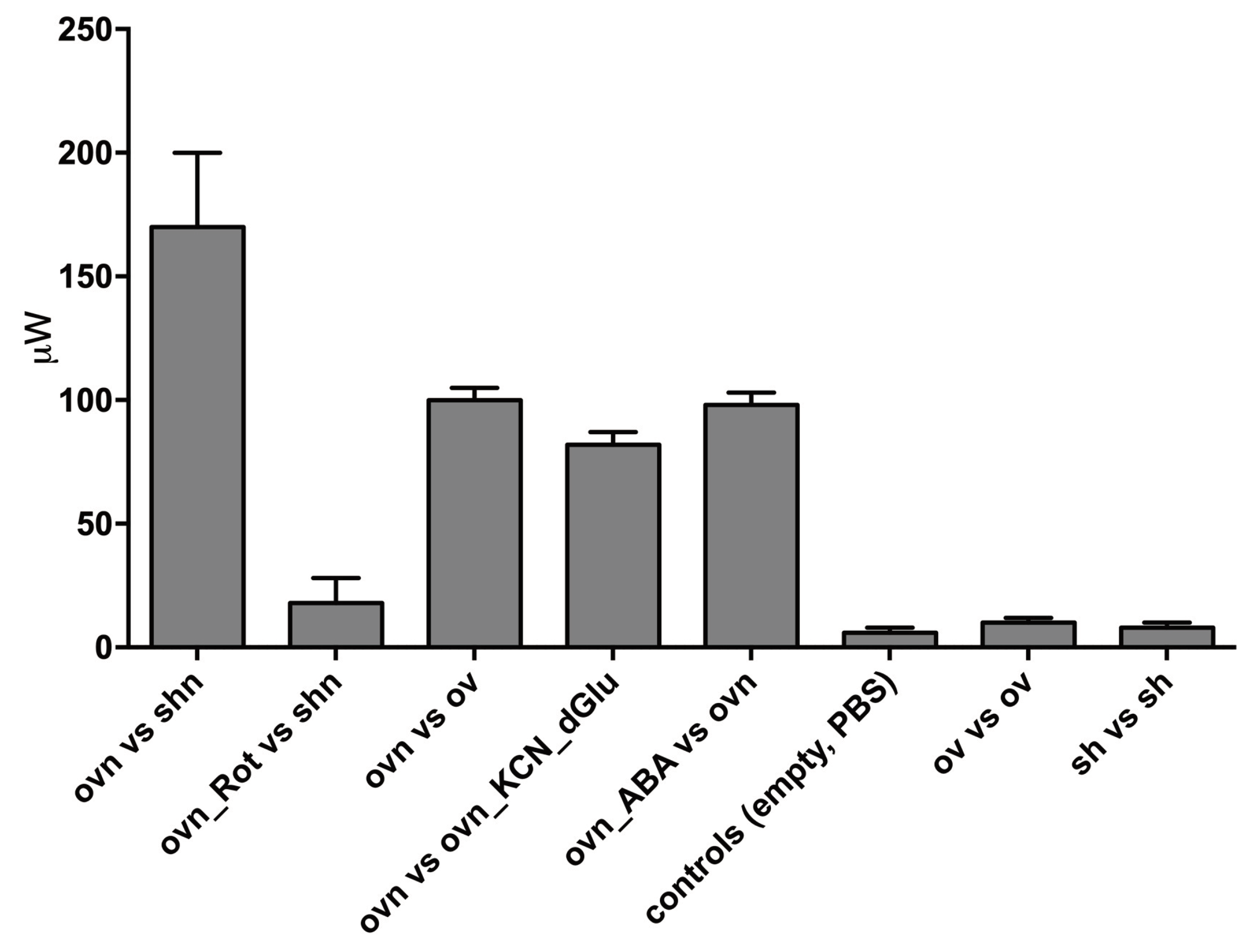
Qualitative and quantitative confirmation of the experimental results obtained with the thermistor probes connected to a lock-in amplifier was obtained with measurements taken on the same cell types with a differential scanning calorimeter set on an isotherm mode of analysis. While the probes allowed to measure a temperature variation between two samples starting from a few seconds after nutrient addition to the starved cells, the DSC measurements had an approx. 15 min delay from the addition of nutrients, due to the time required for thermalization of the chamber containing the crucibles. This fact may explain the approx. 3-fold higher heat production measured with the temp probes as compared with the DSC (~400 μW vs ~150 μW, respectively) when comparing over-expressing vs. double-silenced cells, both with nutrients. In the future, measurements with the temperature probes will allow to study the kinetics of how cell thermogenesis reacts to changing external conditions, and the dynamics of its regulation. Already the present measurements hint at an increased heating power in the first minutes after nutrients are added, compared to the long time steady state.
Both the temperature probes and the DSC measurements agree that heat generation by over-expressing cells is twice that of double-silenced cells. The calorimetry measurements show that ABA further increases heat production in over-expressing cells by approx. 100%.
How do these numbers
compare with an estimate of the thermal energy produced by myocytes? In human muscle cells at rest, the ATP concentration is ~4
mM and that of ADP ~9
μM. These values result in a free energy of ATP hydrolysis ∆
G = −64
kJ/mol. The average cell produces (and hydrolyses) ~10
7 – 10
8 ATP/s. Taking the upper value, one cell hydrolyzes ~1.7 × 10
−16 mole/s of ATP, generating 1.7 × 64 × 10
−13 W ≈ 10
pW of heating power. For 2.5 × 10
6 cells, the number used in all experiments in our study, we thus find ~25
μM of heating power generated. This amount of power is approx. 6 and 16 times less than the values measured in this study on LANCL1/2-overexpressing H9c2 cardiomyocytes by means of the DSC or the temp probes, respectively. However, over-expressing cells are particularly well-performing, as they produce approx. twice the heat generated by double-silenced cells (
Figure 3).
The
heart is obviously the “
pumping central” of the circulatory system, imparting a pressure sufficient to allow blood cells to reach its utmost periphery. Anatomy could suggest also a heating function of the heart: i) it pumps between 5 and 5,5 L/min of blood, i.e., the entire blood volume flows within the heart every minute; ii) it lies deep inside the thoracic cavity, in between the lungs, which provide an effective thermal insulation, as they are full of air. However, based upon the thermal energy produced by cardiomyocytes in this study, it appears that the heating capacity of approx. 2.5 billion cardiomyocytes, the total number of cardiomyocytes in the heart, would amount to approx. 9
J/min ≈ 2
cal/min, if they showed a heating capacity similar to the LANCL1/2-overexpressing cells used in this study. In fact, striated muscle, both cardiac and skeletal, shows the highest levels of LANCL1/2 expression among non-nervous tissues [
12]. As approx. 5L of blood are pumped through the heart each min, heating of this blood volume would amount to only ~0.4 × 10
−3 °C. Thus, rather than heating the blood, the heart is more likely to be itself warmed by the blood flowing through its cavities every minute. Of the approx. 290-3800 kJ of thermal energy dissipated per hour by the human body (depending on activity and environmental conditions) we can estimate from our measures that around 0.540
kJ (9
J/min × 60
min) are produced by the heart.
The main source of heat in mammals is obviously the skeletal muscle: indeed, the total muscle mass accounts for approx. 40% of body weight in males and 30% in females, i.e., approx. 100 times the percentage body weight of the heart. Shivering represents a reaction to cold and a means to increase muscle heat production through its involuntary contraction, sustained by an increased mitochondrial respiration and the “proton leak” through the inner mitochondrial membrane, which is in part “basal” but can be increased, by so-called “uncoupling” proteins. Physical exertion increases body temperature through the same mechanism. A doubling of heat generation, as observed here in LANCL1/2-overexpressing vs. –silenced myocytes, or in ABA-treated vs. –untreated myocytes could result in a significantly increased muscle heat production in vivo.
As compared with the heat produced by 2-3 billion cardiomyocytes (the total number of cardiomyocytes in the heart), i.e., 2 cal/min, if muscle myocytes behaved similarly to LANCL1/2-overexpressing H9c2 (i.e., producing ~150 μW/2.5 × 106 cells = 60 pW/cell of heating power), total muscle myocytes (approx. 1013) would produce 600 W = 2160 kJ/hr of heating power. Brown adipose tissue (BAT) also produces heat, by means of the expression of tissue-specific “uncoupling” proteins, which increase the amount of energy dissipated as heat in mitochondria. However, the contribution by BAT to human body heat production is limited by its reduced mass (compared with muscle) and by the fact that it is confined to discrete areas in the body, interestingly predominantly located in the mediastinum, around the heart and the major arterial vessels.
ABA-induced stimulation of the LANCL1 and LANCL2 hormone receptors as well as LANCL1/2 expression levels
per se have been shown to stimulate mitochondrial biogenesis, O
2 consumption, and proton gradient dissipation in rodent skeletal myocytes [
12], in human brown adipocytes [
13] and in rat cardiomyocytes [
25]. The approx. doubling of heat generation reported here for LANCL1/2-overexpressing vs. double-silenced cardiomyocytes will likely be observed also on LANCL1/2-overexpressing vs. double-silenced skeletal myocytes and brown adipocytes. In fact, transcription of several uncoupling proteins is significantly increased in LANCL1/2-overexpressing cells and further increases upon incubation of the cells with ABA: sarcolipin and UCP-3 in skeletal myocytes [
12], the ADP/ATP translocator and UCP-1/3 in cardiomyocytes [
25] and UCP-1 in brown adipocytes [
13], indicating a possible systemic, whole-body increase of proton gradient dissipation controlled by tissue expression levels of the LANCL1/2 proteins.
Why would oxphos uncoupling be beneficial? By reducing reactive oxygen species (ROS) generation, an essentially unavoidable by-product of respiration, mild uncoupling of mitochondrial oxidative phosphorylation shows potential to combat ROS-induced pathological conditions including obesity, neurodegenerative diseases, non-alcoholic fatty liver disease (NAFLD), diabetes and the metabolic syndrome and cardiovascular diseases [
26]. Accumulating experimental evidence supports the conclusion that mild mitochondrial uncoupling, which naturally occurs in all tissues but can be increased pharmacologically or hormonally, is beneficial to the heart and prolongs rodents’ lifespan [
27], which is arguably the sum of a combined beneficial effect on several organs and systems. Indeed, the mechanisms that alleviate proton and/or electron “jamming” at the respiratory complexes, i.e., backwards electron transfer from reduced respiratory complexes to coenzymes/substrates [
28,
29] and proton leak through the inner mitochondrial membrane through “uncoupling” proteins, allow the orderly flux of charges, maximize ATP production while at the same time minimizing ROS generation.
In conclusion, the present study provides to our knowledge the first demonstration that the ABA/LANCL system is involved in thermogenesis in mammals and opens a new area of investigation into its interaction with hypothalamic-controlled thermal regulation and with thyroid hormones. We already observed that overexpression of LANCL1/2 in human brown adipocytes significantly increases, while the double silencing conversely reduces, mRNA levels of β-adrenergic and of thyroid receptors [
13].
Interestingly, ABA administration to honeybee larvae has been shown to improve their survival at low temperatures, a clue that ABA, along with its conserved role as a stress hormone from plants to mammals, has other, as yet unexplored, functions in animal (and insect) physiology to surprise the open-minded researcher [
30].
Author Contributions
Conceptualization, GZ, FF, SD, EZ; methodology, SS, LS, MP, JCGU; software, FF; investigation, GZ, FF, SD, EZ, SS, LS, MP, JCGU; data curation, GZ; EZ; SS; writing—original draft preparation, GZ, EZ; writing—review and editing, GZ, FF, SD, EZ; visualization, GZ, SS; supervision, GZ, EZ. All authors have read and agreed to the published version of the manuscript.