Introduction
Contact allergy represents a significant consideration in evaluating scalp diseases, mainly when attributed to the use of hair care products (HCP). The typical erythema, peripheral spread, pruritus, and clinical history aid the differential diagnosis. When allergens are administered to the scalp, they frequently produce dermatitis in the eyelids, ears, and neck, given the scalp’s notable resistance to contact dermatitis. Nevertheless, the scalp can manifest severe reactions when exposed to potent allergens like paraphenylenediamine (PPD). The primary culprits for contact allergy on the scalp encompass bleaches and dyes, shampoos and conditioners, perming and straightening products, as well as topical medications 1. Vehicles and preservatives are other allergens in addition to active ingredients or drugs. The use of topical steroids and oral antihistamines usually resolves dermatitis rapidly, with systemic steroids required only in severe cases. Patch test confirms the diagnosis and facilitates allergen avoidance and the selection of alternative products based on available series combined with the ingredients of the suspected elicitors2–5.
Although cosmetic and hair product safety has improved, increased hair care consumption and allergen exposure have raised patient safety concerns. Frequently encountered allergens, including PPD6, preservatives, nickel, cobalt, balsam of Peru, fragrance mix7, and carba mix, have been documented in scientific literature as contributors to the development of allergic contact dermatitis (ACD) of the scalp. Commercially available hair products often contain these allergens. The most common sources of allergens are hair dyes8, shampoos9, and conditioners, but wigs, headbands, hats, masks, and spectacles may also be allergenic1. Dermatitis confined solely to the scalp, without affecting the neck, face, or other body regions, is infrequent, making this subgroup of patients with isolated scalp symptoms intriguing. Predominant indicators encompass eczematous lesions, pruritus, and a sensation of burning on the scalp. Isolated scalp dermatitis, exclusive of involvement of the neck, face, or other body parts, is an uncommon presentation but warrants special attention. Identifying this specific group of patients exhibiting distinctive symptoms, including eczematous lesions, itching, and a burning sensation, is crucial for healthcare professionals. This recognition enhances their capacity to deliver personalized patient education, counseling, and treatment 10,11.
This article explores the spectrum of allergens causing scalp ACD, emphasizing the prevalence of common triggers. Despite improvements in cosmetics and hair product safety, the literature reports a continued association between these allergens and scalp dermatitis. The aim of this review is to furnish clinicians with a thorough comprehension of contact dermatitis triggered by hair care products. This aims to enable prompt diagnosis, successful avoidance of allergens, and the informed selection of alternative products, ultimately contributing to improved patient care.
Clinical patterns
Contact allergens able to cause allergic contact dermatitis are commonly present in hair cosmetics, including shampoos, hair dyes, bleaches, and straightening creams. This dermatological condition is notably prevalent among hairdressers, who encounter these products regularly and may develop dermatitis on their hands. In contrast, clients and individuals using these products at home are more prone to experiencing dermatitis on their head, neck, and face. Recognizing the potential risks associated with these products is crucial, and appropriate precautions should be taken to prevent allergic reactions. The robust barrier offered by the thick epidermis, the lack of creases and folds, and the numerous pilosebaceous glands all contribute significantly to restricting the entry of allergens 1,11,12.
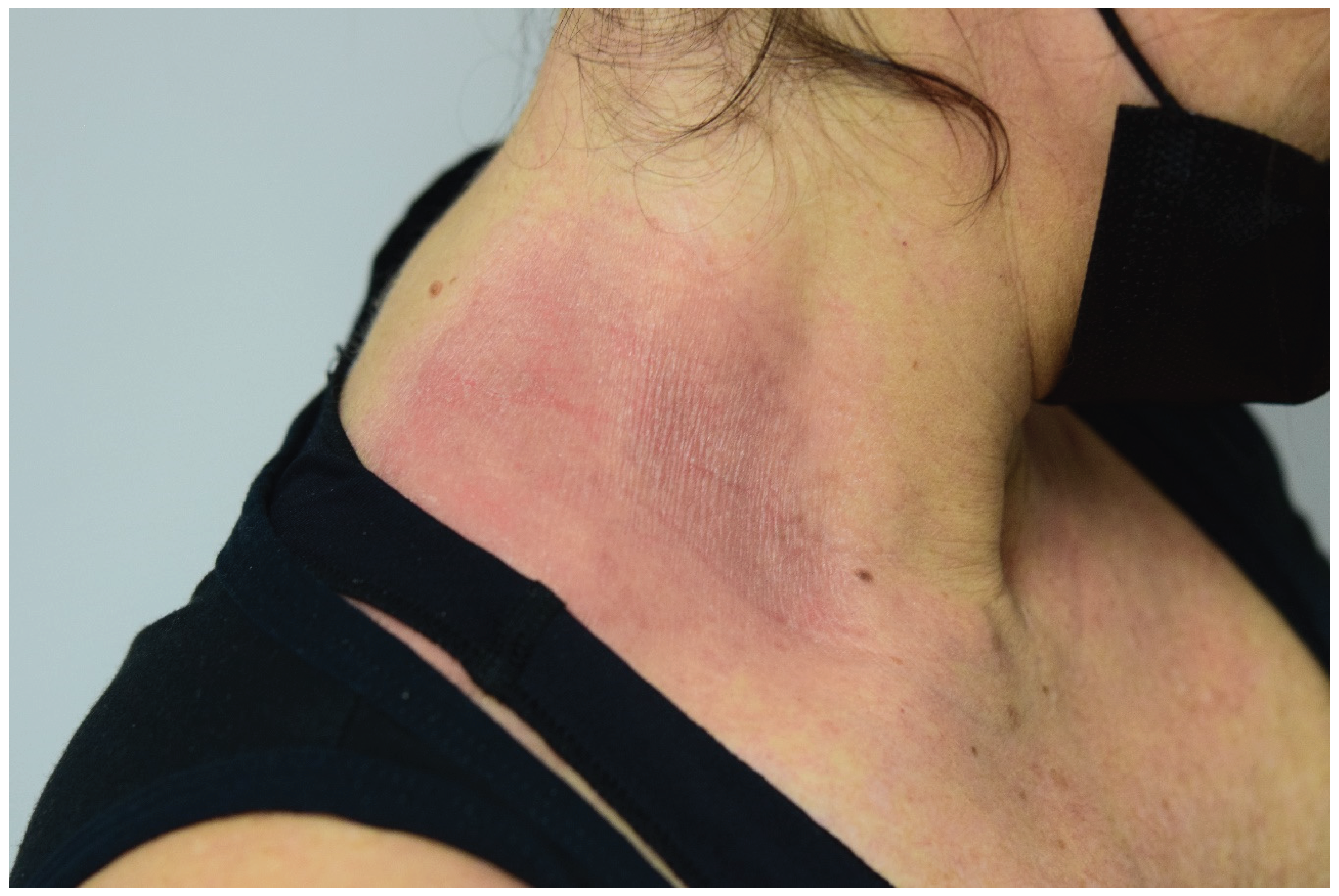
Washing patterns. Lesions are caused by the drainage of allergens from the lateral areas of the face (
Figure 1). These situations often result from the use of shampoos, conditioners, or comparable products that are applied temporarily to the scalp and have brief but repeated contact with the facial skin.
1.
Hairline pattern. Contact dermatitis displays a distinctive distribution along the hairline, characterized by eczema plaques at the junction of the hairline and the facial skin (
Figure 2). Regions frequently impacted include the frontal area, retroauricular region, nape of the neck, and the upper ear region. This pattern is often observed in cases linked to using hair dyes and perms
1.
Geographical pattern. The reaction often presents as eczematous plaques that delimit the contact area with the allergen (
Figure 3). This pattern is typical of responses to objects such as hair bands/clips, wigs, hats, and masks
1.
Patch test
The patch test is acknowledged as the gold standard for investigating allergic contact dermatitis. Concerning hair cosmetics, we already have allergens in most working groups’ basal batteries, such as paraphenylenediamine (PPD) or preservatives such as isothiazolinones. However, hair products contain other molecules with sensitizing power grouped in a specific hairdressing battery. The first step will be to combine both batteries and, depending on the case, associate others, such as the fragrance battery or gums. We must remember that testing one’s products can be vital in confirming the diagnosis, given that sometimes the problem allergen is not marketed but is present at the source of sensitization. Patch testing with patients’ products is usually recommended, that is, applied in a well when we talk about leave-on products.
In contrast, rinse-off products must be diluted, usually 10% in water vehicles, to be patched in a standard way in a well. Another alternative can be its application in semi-open or open without diluting. We must patch at least ten healthy controls to rule out the irritative reaction. The methodology used to perform the patch test correctly is included in the guide published in 201513. Occlusion of the allergens studied is recommended for 48 hours, with a subsequent reading 15 minutes after lifting the occlusion dressing and a reading at 96 hours. In doubtful cases, a late reading can be performed after 7 or 10 days, especially for weak reactions after 96 hours or when there is significant doubt about an irritative reaction.
Classic and emerging allergens are listed in
Table 1, with the recommended concentration and vehicle. Not all are commercialized, but they can be prepared on the workstation if necessary.
The dermoscopic patterns observable in patients with ACD located on the scalp have recently been published. Erythema and scales are present in all patients, while the vascular pattern is characterized by arboriform vessels and red loops14.
Allergens
In cases of ACD affecting the scalp, dyes emerge as the most frequently cited culprits, followed by shampoos and conditioners. Within dyes, PPD stands out as the predominant allergen15–17. PPD, an oxidative dye found in numerous coloring products18, exhibits its highest concentration in dark shades, although it is also present in brighter colors. Notably, some manufacturers may omit to declare their presence on product labels. The clinical manifestation specific to PPD-induced ACD is marked by acute edematous dermatitis, predominantly affecting the eyelids, face, and neck, with minimal impact on the scalp 5,19.
Contrastingly, conditioners and shampoos are deemed unlikely instigators of ACD due to their brief contact duration and the rinsing-off process, rendering them well-tolerated even in sensitized individuals. Among the prevalent allergens in these hair care products (HCP) are fragrances, cocamidopropyl betaine, and preservatives such as quaternium-15. Lotions, including propylene glycol20 as a vehicle and minoxidil, commonly harbor these key allergens. Clinical manifestations require differential diagnosis with seborrheic dermatitis or psoriasis, including erythema, pruritus, scaling, and dryness of the scalp1,3.
Surfactants
Surfactants serve as cleansing agents by disrupting the physicochemical bonds that bind impurities and residues to the hair 21. Substances like non-soluble fats, such as sebum, do not dissolve easily in water. Upon encountering water, surfactants promote the formation of a micelle structure. The ionic ends of the surfactant are drawn to the surrounding water molecules, leading to the emulsification or suspension of particles in the water. In this emulsified state, these particles can be effectively rinsed off22.
In cosmetic applications, formulations traditionally incorporate sodium dodecyl sulfate (SDS), valued for its effectiveness as a detergent and foaming agent. Nevertheless, considering its recognized irritant properties to the skin and mucous membranes, its utilization has waned. Presently, formulations commonly opt for blends of surfactants possessing diverse properties, a strategic approach to optimizing performance while fostering ecological sustainability 11,23,24.
Classified according to the the electric charge of their polar ends, surfactants are grouped into four categories: anionic, cationic, amphoteric, and non-ionic. The primary cleaning agents fall under the anionic category. The key cleaning agents are predominantly found in the anionic category. Although anionic surfactants are efficient in eliminating sebum and dirt, their potent cleansing characteristics can heighten negative electrical charges on the hair’s surface, leading to an escalation in frizz and friction22,25–27. Secondary surfactants are integrated into formulations to minimize potential harm and offset the repercussions of static electricity caused by anionic surfactants. These additional surfactants encompass cationic types, amphoteric surfactants, and non-ionic surfactants. Cationic surfactants, possessing a positive charge, promptly adhere to negatively charged strands induced by anionic surfactants, effectively diminishing the frizz effect. We will delve into the specific types of surfactants within each classification 22,28.
Anionic surfactants: Anionic surfactants such as sodium laureth sulfate (SLES), sodium lauroyl methyl isethionate (SLMI), or sodium methyl lauroyl taurate (SLMT) are often found in commercial shampoos22.
Cationic surfactants: Benzalkonium chloride, trimethylalkylammonium chloride, and cetrimonium chloride are cationic surfactants that are cosmetically acceptable for hair conditioning products. These surfactants, notably employed as hair softeners, contribute to effective conditioning22.
Nonionic surfactants. Nonionic surfactants, notably the ethoxylated surfactants based on ethylene oxides, represent the most prevalent type in this category. Another significant group of nonionic surfactants includes ‘multihydroxy’ compounds, such as glycol esters, glycerol and polyglycerol esters, glycosides and polyglicosides, as well as sucrose esters. They are commonly used for mild cleansing purposes22,27.
Amphoteric surfactants. Predominantly represented by N-alkyl betaines derived from trimethylglycine (betaine), these surfactants find application in mild cleansing formulations22.
Preservatives
Preservatives are often used to avoid the biological degradation of cosmetics by the micro-organisms that frequently contaminate them. However, the ideal preservative with excellent antimicrobial, stable, stable, effective over a wide pH range, and non-toxic, non-irritant, and non-sensitizing properties has not yet been identified 29–32. Preservatives are extensively used in a variety of consumer products, such as hair cosmetics, resulting in widespread exposure. Parabens33 are the most utilized preservatives in cosmetic products, followed by formaldehyde releasers and isothiazolinones. The preservatives associated with the highest rates of sensitization include methyldibromoglutaronitrile, formaldehyde, and Kathon CG, while parabens exhibit the lowest prevalence 29,32.
Parabens: Parabens constitute a group of alkyl esters, including methyl-, ethyl-, propyl-, butyl-, and benzyl paraben, derived from para-hydroxybenzoic acid. Renowned for their exceptional properties, they stand out as one of the most extensively utilized preservatives in cosmetic, pharmaceutical, and food products. In cosmetic formulations, parabens rank as the most prevalent preservative, with the Food and Drug Administration designating them as the second most common ingredient, surpassed only by water. When used as cosmetic preservatives on healthy skin, their desensitization capacity is relatively low, at approximately 1%, representing one of the lowest rates among preservatives. However, instances of sensitization are markedly higher when therapeutic preparations are applied to damaged skin 34-36.
Formaldehyde and formaldehyde releasers: Formaldehyde is an allergen that is widely present. Employed as a preservative in numerous cosmetic, household, and industrial items, formaldehyde also serves as a contaminant in various products. In the European Union, the permissible maximum concentration of formaldehyde in cosmetics is 0.2% (0.1% in oral hygiene products), yet there is no explicit regulation regarding this in the United States 37,38. Despite a reduction in the utilization of formaldehyde and its replacement with safer preservatives like formaldehyde releasers, leading to a decrease in sensitization occurrences, a recent upswing in cases has been identified. Formaldehyde releasers are substances that, when exposed to water, release formaldehyde in different quantities based on factors like the type of preservative, its concentration, and the water content in the product. While more than 40 substances of this kind have been identified, only a restricted number are utilized in dermatology units. Examples of formaldehyde releasers used in cosmetic products, listed in terms of formaldehyde release, include 2-bromo-2-nitropropane-1,3-diol (bronopol), imidazolidinyl urea (IU), dimethylol-dimethyl hydantoin (DMDMH), diazolidinyl urea (DU), and quaternium-1537,39–43.
Isothiazolinones: Isothiazolinones stand out as one of the most utilized preservatives, present in approximately 23% of cosmetic products. Kathon CG is a mixture in a 3:1 ratio of methylchloroisothiazolinone (MCI) and methylisothiazolinone (MI), where MCI represents the more allergenic component. Alongside formaldehyde and quaternium-15, MCI/MI is a prevalent cause of allergic contact reactions with preservatives, with MCI/MI demonstrating the highest clinical significance among them. 44–46. Owing to its sensitizing potential, the maximum permissible concentration of isothiazolinones in Europe has been regulated at 15 ppm for both rinse-off and leave-on cosmetics. There is a conjecture that the heightened use of methylisothiazolinone (MI) in isolation, without methylchloroisothiazolinone (MCI) as a preservative, has contributed to an increased sensitivity to MI. Consequently, this has led to a rise in the prevalence of positive MCI/MI reactions through cross-reaction. If there is a suspicion of developing sensitivity to isothiazolinones, it is advised to conduct patch tests using both the MCI/MI mixture and MI alone. This is essential because patch testing with MCI/MI alone captures only around 40% of diagnosed MI allergies. The concentration of MI in the Kathon CG patch (25 ppm) is considerably lower than in the patch containing the isolated preservative (75 ppm) 44,46,47.
Fragrances
Considering the widespread inclusion of fragrances in various items, including shampoos, conditioners, and hair tonics, there is a substantial likelihood of the scalp being exposed to these fragrances. A minimum of 1% of the adult population is impacted by fragrance allergy. The aromatic sap known as Balsam of Peru is obtained from the Myroxylon balsamum tree, native to Central and South America 48,49.
Balsam of Peru comprises numerous potential allergens, such as benzoic acid, benzyl acetate, benzyl benzoate, and cinnamic acid 50. It is a naturally occurring, sweet-smelling substance frequently present in perfumes and fragrances. Patients should exercise caution when using products related to aromatherapy, scented oils, candles, air fresheners, deodorizers, or incense with scents like cinnamon, vanilla, or clove. Even products labeled as ‘unscented’ might contain a masking fragrance, so opting for truly unscented products is advisable48,49,51,52. Balsam of Peru is present in various products, including hair tonics, pomades, shampoos, conditioners, shaving lotions, aftershave perfumes, colognes, and scented cosmetics. Additionally, it is used in foods, beverages, and medicines, apart from its role in fragrances. Conducting patch tests for fragrance mix and balsam of Peru has the potential to detect up to 90% of cases involving fragrance allergies. Specifically, testing for balsam of Peru alone can identify 50% of these cases. Given the high diagnostic yield, it is advisable to employ patch testing to identify fragrance allergies.48,49,51.
Conditioners
Conditioners serve to minimize friction, untangle hair, reduce frizz, and enhance combability. Their mechanism entails neutralizing the negative electrical charge present on the hair fiber by introducing positive charges, while concurrently lubricating the cuticle to reduce the hydrophilicity of the fiber22. These formulations include substances designed for antistatic and lubricating purposes, classified into five primary groups: polymers, oils, waxes, hydrolyzed amino acids, and cationic molecules. Notably, silicone emerges as the most active and widely employed conditioning agent. Cationic ingredients are frequently incorporated into various shampoo formulations alongside anionic surfactants to counteract charges and form a cationic-anionic complex, resulting in a neutral hydrophobic ingredient. Hair subjected to bleaching or chemical treatments demonstrates an increased affinity for conditioning agents due to its low isoelectric point (higher concentration of negative sites) and heightened porosity compared to untreated hair 22,53,54.
Protein hydrolysates (PHs) are incorporated into hair conditioners to mend damaged hair and impart a fuller appearance to the hair. Interestingly, instances have been documented where the inclusion of PHs in hair conditioners led to immediate skin reactions, such as urticaria, and, in some cases, more severe reactions like angioedema and bronchospasm55.
Antidandruff
Zinc pyrithione, an ingredient commonly found in shampoos, has demonstrated both safety and efficacy in addressing dandruff and scalp psoriasis. Research indicates its potential to reduce cell turnover rates in hyperproliferative dermatoses like psoriasis. Additionally, the compound exhibits fungistatic and antimicrobial properties, although the precise mechanism of its action remains uncertain. Notably, irritant or allergic responses may rarely contribute to psoriatic flares and köbnerization. Instances have been documented where psoriasis exacerbation occurred due to the induction of ACD resulting from the use of antidandruff shampoos including zinc pyrithione56.
Dyes
Although most documented cases of allergic contact dermatitis (ACD) linked to hair dyes are linked to PPD-phenylenediamine (PPD), there are few other compounds in hair dyes recognized for their strong sensitizing potential. PPD is used as a component in both permanent and semi-permanent hair dyes
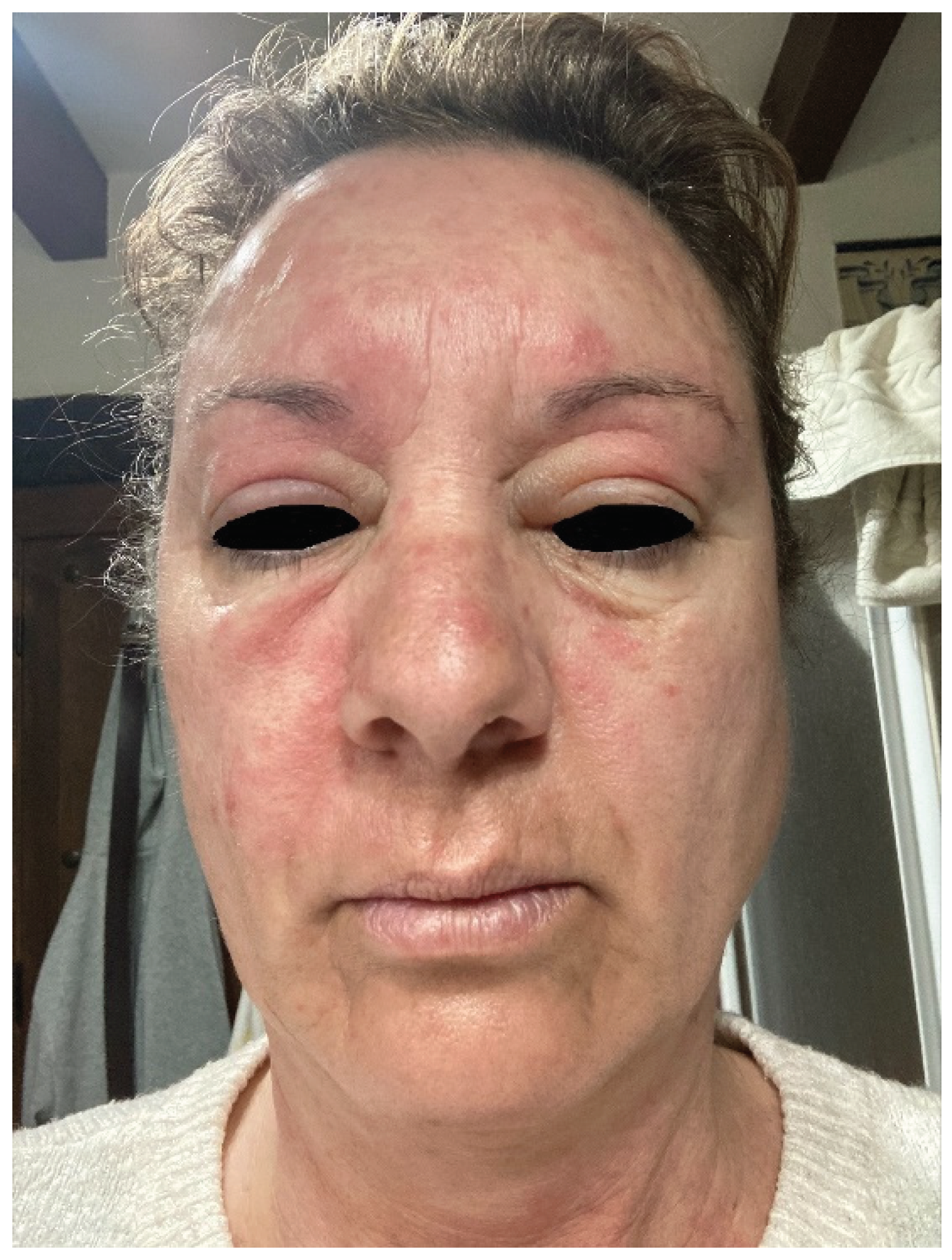
15,38. While it serves as a coloring agent in various cosmetic products, the main source of sensitization to PPD is exposure through the use of hair dyes. Although this component is employed as a coloring agent in various cosmetic products, the primary cause of sensitization to PPD arises from exposure to hair dyes. There is a notable correlation between PPD sensitivity and individuals in the hairdressing profession. Individuals sensitized to PPD who use permanent hair dye may experience severe reactions characterized by eyelid (
Figure 4), ear, or full-face edema. PPD derivatives, including para-amino diphenylamine (PAD), o-nitro-p-phenylenediamine (ONPPD), and para-toluene diamine (PTD), are fewer common triggers of cutaneous allergy in hair dye formulations, and potential cross-reactions among them may contribute to sensitization. Since various hair dyes fall into categories like permanent, semi-permanent, and temporary, positive patch test results for different dyes may be misconstrued as initial sensitization. However, it is essential to also contemplate the possibility of cross-sensitization to distinct azo dyes and para-amino compounds, as reported in some instances, or concurrent contact allergy
15,30,57.
Paradoxically, several studies suggest that the occurrence of allergic reactions to PPD appears to have decreased in recent years, despite the worldwide increase in hair dye use. Also, there has been a decrease in the contact rate between hairdressing professionals, probably due to the use of gloves as protection. Nevertheless, the surge in body art trends, coupled with the utilization of temporary tattoos containing PPD dyes like black ink, seems to be a significant factor in initiating allergic contact reactions to hair dyes. The Cosmetic Ingredient Expert Panel asserts the safety of 2-amino-4-hydroxyethylaminoanisole and its sulfate derivative, utilized as coupling agents in oxidative hair dyes, but cautions against their use in other cosmetic products due to the potential formation of N-nitroso compounds. Periodically, allergic contact dermatitis cases have been documented for certain components of temporary dyes, including quinine15,30,57.
Some researchers have investigated alternative hair dye options for hairdressers already sensitized to paraphenylenediamine, in search of safer alternatives. Hair dyes like Disperse Yellow 9, Disperse Red 11, and Disperse Blue 3 may be considered for patients with contact allergy to PPD. Other constituents present in hair dye formulations, such as resorcinol, m-aminophen, and 4-amino-2-hydroxytoluene, could potentially be responsible for allergic contact dermatitis. Additionally, hair contact sensitization may also be induced by various ingredients in coloring products (persulphate salts), permanent products (thioglycolate of glycerine), preservatives, perfumes, shampoo/conditioning surfactants (CAPB, hydrolyzed animal proteins), or photoprotectants 15,38.
Anti-loss hair products
Accessible in solution, gel, and foam formulations, topical minoxidil stands out as the most prescribed treatment for androgenetic alopecia. Approved for this indication, topical minoxidil is available in both 2% and 5% formulations 58. Topical minoxidil proves to be a secure and efficient remedy for individuals with androgenetic alopecia; however, on occasion, it might cause itching, irritant dermatitis, or allergic contact dermatitis. Allergic contact dermatitis from topical minoxidil can present as pustular dermatosis on the scalp, eczematous lesions, erythema, itching, or scaling of the scalp.59. The primary cause of allergic contact dermatitis in response to topical minoxidil is often attributed to the solvents, namely propylene glycol, butylene glycol, and glycerin, with minoxidil itself being an exceptionally rare culprit. In cases where allergic contact dermatitis is suspected, patch testing becomes imperative to discern whether the reaction is triggered by minoxidil or the solvents 60–64.
Antioxidants and ecological cosmetics
Technology and advances in cosmetics introduce new molecules every year with specific properties that add value to the cosmetic product and improve hair conditions. Antioxidants are an increasingly enhanced ingredient in shampoos, which usually eliminate surfactants like sodium lauryl sulfate to improve the quality of the product66. Antioxidants obtained from plants such as Hancornia speciosa67, Cyclea peltata68, or Withania frutescens69 have been reported with interesting results. Polyphenols are potent antioxidants that also behave as antibacterial and antifungal, making them an interesting molecule in this field.
However, although this attractive field is developing and new formulations appear every time, the reality is that the field of contact dermatitis can pose a serious problem given that we do not have allergens marketed for the study of hypersensitivity reactions to these haptens. Therefore, a definitive diagnosis of ACD will not be reached in most cases.
Frontal fibrosing alopecia and ACD
In recent years, new hypotheses have been reported trying to relate frontal fibrosing alopecia (FFA) with ACD. The reality is that the majority are female patients with a personal history of use/abuse of both facial and hair cosmetics. Dyes, hairsprays, and other hair cosmetics contain ingredients with a high sensitization potential. The most prominent allergens are ethylhexyl salicylate70, drometrizole trisiloxane71, diethylamino hydroxybenzoyl hexyl benzoate72, and benzyl salicylate73.
This topic is controversial given that we also found publications that have yet to establish a causal relationship between FFA and the use of shampoos74 or even benzyl salicylate75. The debate is served, where new works will shed light on the existing relationship between FFA and ACD.
These patients sometimes use hair prostheses that are usually fixed with adhesives containing acrylates76. Sensitization to acrylates has been reported due to the use of these wig fixatives in patients with FFA but also with alopecia areata77.
Occupational dermatosis: hairdressing
Certainly, it stands out as one of the prevalent occupations encountered in contact dermatitis consultations. Exposure to the different allergens discussed throughout the manuscript is a potentially relevant cause in hairdressing professionals78. PPD, nickel, isothiazolinones, fragrances, and formaldehyde/formaldehyde releasers are the most common happens. Most of these allergens are encompassed in the standard panels adopted by nearly all contact dermatitis research teams.
A very interesting review of sensitization rates in hairdressers has recently been reported79, where PPD showed a high rate, reaching 4.3%. Other relevant haptens such as toluene 2,5-diamine, ammonium persulfate, or glycerin thioglycolate showed global prevalence rates above 1.5%, which is why these allergens are included in the specific hairdressing battery.
It has been reported that these professionals have up to a five-fold increased risk of developing sensitization to haptens such as ammonium persulfate or glyceryl thioglycolate. Apart from the manifestation of skin issues, exposure to these allergens has also been linked to the onset of other conditions like asthma or allergic rhinitis.
A relevant aspect that must always be considered is the false safety of some products labeled as hypoallergenic, especially when we talk about PPD. In a published study, it was observed that some of the “hypoallergenic” hair dyes contained PPD among their ingredients80. Educating the patient to avoid haptens appropriately is critical to therapeutic success in ACD, so teaching how to interpret product labeling is the foundation of any patch testing study.
Hairdressing is a business that has grown significantly and has incorporated aesthetic procedures where acrylates and glues are manipulated. This scenario must be considered when the problem case is being studied, given that these allergens must also be incorporated into the patch test in cases where the patient confirms exposure to haptens such as 2-hydroxyethyl methacrylate and ethyl cyanoacrylate81.
Treatment
Detection of the allergen or allergens responsible for the eczematous/non-eczematous rash is the key to success since their avoidance will lead to a complete or significant improvement in the skin lesions. Sometimes, patients show diseases overlapping with ACD, prompting the use of topical anti-inflammatory therapies such as corticosteroids or topical calcineurin inhibitors. Those situations that are not controlled with topical treatment may require systemic treatment with cyclosporine, methotrexate, or advanced therapies with dupilumab82. Cases of scarring alopecia secondary to hair dyes have been reported83. Cases of telogen effluvium following scalp rash secondary to ACD have also been reported 84. We must be careful with the recommendation of hair restorers since cases of sensitization to glycyrrhizic acid85 present in this type of product have been reported.
Conclusions
In conclusion, this comprehensive exploration of contact dermatitis induced HCP underscores the evolving landscape of allergen exposure in cosmetic dermatology. The intricate patterns of scalp reactions, ranging from washing and hairline distribution to geographical manifestations, serve as valuable clinical indicators for healthcare professionals. Recognition of the diverse allergens, including prevalent culprits like paraphenylenediamine (PPD), fragrances, and surfactants, is crucial for timely diagnosis and targeted patient care.
Despite advancements in cosmetic safety, the persistence of common allergens in hair care products poses ongoing challenges, necessitating vigilant awareness among healthcare providers and consumers. The introduction of alternative, bio-based ingredients is a promising avenue to mitigate the impact of irritant substances on the skin and mucous membranes.
As the dermatological community strives for enhanced patient safety, identifying emerging allergens and continuously refining diagnostic approaches, such as patch testing, remain imperative. Collaborative efforts between clinicians, researchers, and the cosmetics industry are essential to advance our understanding of contact dermatitis, enabling the formulation of safer and more tolerable hair care products. By prioritizing informed allergen avoidance and promoting eco-friendly practices, we can contribute to a healthier and more sustainable future for dermatological care in hair cosmetics.
Funding Sources
This article has no funding source.
Informed Consent
Written informed consent was obtained from the patient for publication of her case details.
IRB Approval Status
Does not apply.
Data Availability Statement
The data that support the findings are available from the corresponding author, FJNT, upon reasonable request. The corresponding author had full access to all the data in this manuscript and takes responsibility for the integrity of the data. The patients in this manuscript have given written informed consent to publication of their case details
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- Rozas-Muñoz E, Gamé D, Serra-Baldrich E. Allergic Contact Dermatitis by Anatomical Regions: Diagnostic Clues. Actas Dermosifiliogr 2018, 109, 485–507. [CrossRef]
- Contact_dermatitis_to_hair_cosmetics_Current_diagnostic_recommendations.
- Pham CT, Juhasz M, Lin J, et al. Allergic Contact Dermatitis of the Scalp Associated with Scalp Applied Products: A Systematic Review of Topical Allergens. Dermatitis 2022, 33, 235–248. [CrossRef]
- Warshaw EM, Ruggiero JL, DeKoven JG, et al. Contact Dermatitis Associated With Hair Care Products: A Retrospective Analysis of the North American Contact Dermatitis Group Data, 2001-2016. Dermatitis 2022, 33, 91–102. [CrossRef]
- Thomas ZRM, Jamiolkowski D, Chantraine S, et al. Contact dermatitis to hair cosmetics: Current diagnostic recommendations. JDDG - Journal of the German Society of Dermatology 2021, 19, 1729–1734. [CrossRef]
- Vogel TA, Heijnen RW, Coenraads P-J, et al. Two decades of p-phenylenediamine and toluene-2,5-diamine patch testing - focus on co-sensitizations in the European baseline series and cross-reactions with chemically related substances. Contact Dermatitis 2017, 76, 81–88.
- Karim M, Klein EJ, Nohria A, et al. Potential for Allergic Contact Dermatitis in Popular Hair Care Practices and Ingredients. Dermatitis 2023, 34, 484–491. [CrossRef]
- Guerra-Tapia A, Gonzalez-Guerra E. Hair cosmetics: dyes. Actas Dermosifiliogr 2014, 105, 833–9.
- Zirwas M, Moennich J. Shampoos. Dermatitis 2009, 20, 106–10.
- Ojo EO, Gowda A, Nedorost S. Scalp Dermatitis in Patients Sensitized to Components of Hair Products. Dermatitis 2019, 30, 264–267. [CrossRef]
- Dias MFRG. Hair cosmetics: An overview. International Journal of Trichology 2015, 7, 2–15.
- Uter W, Johansen JD, Macan J, et al. Diagnostics and Prevention of Occupational Allergy in Hairdressers. Current Allergy and Asthma Reports 2023, 23, 267–275. [CrossRef]
- Johansen JD, Aalto-Korte K, Agner T, et al. European Society of Contact Dermatitis guideline for diagnostic patch testing - recommendations on best practice. Contact Dermatitis 2015, 73, 195–221. [CrossRef]
- Starace M, Bruni F, Marcondes MT, et al. The identification of trichoscopic features of allergic scalp contact dermatitis: a pilot-study of a single center. Italian journal of dermatology and venereology 2023, 158, 334–340. [CrossRef]
- Fernández-Vozmediano JM, Padilla-Moreno M, Armario-Hita JC, et al. Patrón de sensibilización por contacto a parafenilendiamina y su detección en tintes capilares. Actas Dermosifiliogr 2011, 102, 206–211.
- Warshaw EM, Peterson MY, Atwater AR, et al. Patch Testing to Paraphenylenediamine: The North American Contact Dermatitis Group Experience (1994-2018). Dermatitis 2023, 34, 536–546. [CrossRef]
- Jenkins D, Chow ET. Allergic contact dermatitis to paraphenylenediamine. Australas J Dermatol 2015, 56, 40–3. [CrossRef]
- Encabo Durán B, Romero-Pérez D, Silvestre Salvador JF. Allergic Contact Dermatitis Due to Paraphenylenediamine: An Update. Actas Dermosifiliogr 2018, 109, 602–609.
- Hillen U, Grabbe S, Uter W. Patch test results in patients with scalp dermatitis: analysis of data of the Information Network of Departments of Dermatology. Contact Dermatitis. 2007, 56, 87-93. [CrossRef]
- Scheman A, Roszko K. Contact Allergy to Propylene Glycol and Cross-Reactions. Dermatitis 2018, 29, 350–351. [CrossRef]
- Weinhammer AP, Scheman A, Reeder MJ. Prevalence of Surfactant in the Contact Allergen Management Program. Dermatitis 2019, 30, 358–362. [CrossRef]
- Coderch L, Alonso C, García MT, et al. Hair Lipid Structure: Effect of Surfactants. Cosmetics 2023, 10, 107. [CrossRef]
- Fernández-Peña L, Guzmán E. Physicochemical aspects of the performance of hair-conditioning formulations. Cosmetics 2020, 7, 1–21. [CrossRef]
- Draelos ZD. Essentials of hair care often neglected: Hair cleansing. International Journal of Trichology 2010, 2, 24–29. [CrossRef]
- Luengo GS, Fameau AL, Léonforte F, Greaves AJ. Surface science of cosmetic substrates, cleansing actives and formulations. Adv Colloid Interface Sci. 2021, 290, 102383. [CrossRef]
- Fernandes C, Medronho B, Alves L, Rasteiro MG. On Hair Care Physicochemistry: From Structure and Degradation to Novel Biobased Conditioning Agents. Polymers (Basel). 2023, 15, 608. [CrossRef]
- Presley CL, Militello M, Barber C, et al. The History of Surfactants and Review of Their Allergic and Irritant Properties. Dermatitis : contact, atopic, occupational, drug 2021, 32, 289–297. [CrossRef]
- Weinhammer AP, Scheman A, Reeder MJ. Prevalence of Surfactant in the Contact Allergen Management Program. Dermatitis 2019, 30, 358–362. [CrossRef]
- Lundov MD, Moesby L, Zachariae C, et al. Contamination versus preservation of cosmetics: A review on legislation, usage, infections, and contact allergy. Contact Dermatitis 2009, 60, 70–78. [CrossRef]
- Yazar K, Boman A, Lidén C. P-Phenylenediamine and other hair dye sensitizers in Spain. Contact Dermatitis 2012, 66, 27–32.
- Yazar K, Johnsson S, Lind ML, et al. Preservatives and fragrances in selected consumer-available cosmetics and detergents. Contact Dermatitis 2011, 64, 265–272. [CrossRef]
- Svedman C, Andersen KE, Brandão FM, et al. Follow-up of the monitored levels of preservative sensitivity in Europe. Overview of the years 2001-2008. Contact Dermatitis 2012, 67, 312–314.
- Fransway AF, Fransway PJ, Belsito D V, et al. Parabens. Dermatitis 2019, 30, 3–31.
- De Groot AC. Fatal attractiveness: the shady side of cosmetics. Clin Dermatol. 1998, 16, 167-79.
- Orton DI, Wilkinson JD. Cosmetic allergy: incidence, diagnosis, and management. Am J Clin Dermatol. 2004, 5, 327-37.
- Ley BD, Mendaza FH, Conde-Salazar Gómez L. Parabenos: ¿mito o realidad? 2006. Piel. 2006, 21, 231-40.
- Latorre N, Silvestre JF, Monteagudo AF. Dermatitis de contacto alérgica por formaldehído y liberadores de formaldehído. Actas Dermosifiliogr 2011, 102, 86–97.
- Latorre N, Borrego L, Fernández-Redondo V, et al. Patch testing with formaldehyde and formaldehyde-releasers: Multicentre study in Spain (2005-2009). Contact Dermatitis 2011, 65, 286–292. [CrossRef]
- de Groot AC, Maibach HI. Frequency of sensitization to common allergens: comparison between Europe and the USA. Contact Dermatitis. 2010, 62, 325-9. [CrossRef]
- de Groot AC, Veenstra M. Formaldehyde-releasers in cosmetics in the USA and in Europe. Contact Dermatitis. 2010, 62, 221-4. [CrossRef]
- de Groot AC, White IR, Flyvholm MA, Lensen G, Coenraads PJ. Formaldehyde-releasers in cosmetics: relationship to formaldehyde contact allergy. Part 1. Characterization, frequency and relevance of sensitization, and frequency of use in cosmetics. Contact Dermatitis. 2010, 62, 2-17. [CrossRef]
- Kireche M, Gimenez-Arnau E, Lepoittevin JP. Preservatives in cosmetics: reactivity of allergenic formaldehyde-releasers towards amino acids through breakdown products other than formaldehyde. Contact Dermatitis. 2010, 63, 192-202. [CrossRef]
- Agner T, Flyvholm MA, Menné T. Formaldehyde allergy: A follow-up study. Am J Contact Dermat. 1999, 10, 12-7.
- Castanedo-Tardana MP, Zug KA. Methylisothiazolinone. Dermatitis 2013, 24, 2–6.
- Cuesta L, Silvestre JF, Toledo F, Ballester I, Betlloch I. Delayed hypersensitivity to methylchloroisothiazolinone/methylisothiazolinone not detected by the baseline series of the Spanish group. Contact Dermatitis. 2010, 62, 250-1. [CrossRef]
- Geier J, Lessmann H, Schnuch A, et al. Recent increase in allergic reactions to methylchloroisothiazolinone/ methylisothiazolinone: Is methylisothiazolinone the culprit? Contact Dermatitis 2012, 67, 334–341.
- García-Gavín J, Vansina S, Kerre S, Naert A, Goossens A. Methylisothiazolinone, an emerging allergen in cosmetics? Contact Dermatitis. 2010, 63, 96-101.
- Bordel-Gómez MT, Miranda-Romero A, Castrodeza-Sanz J. Epidemiology of contact dermatitis: Prevalence of sensitization to different allergens and associated factors. Actas Dermosifiliogr 2010, 101, 59–75. [CrossRef]
- Arribas MP, Soro P, Silvestre JF. Contact dermatitis to fragrances. Part 1. Actas Dermo-Sifiliograficas 2012, 103, 874–879. [CrossRef]
- de Groot AC. Myroxylon pereirae resin (balsam of Peru) - A critical review of the literature and assessment of the significance of positive patch test reactions and the usefulness of restrictive diets. Contact Dermatitis 2019, 80, 335–353.
- Arribas MP, Soro P, Silvestre JF. Allergic contact dermatitis to fragrances: Part 2. Actas Dermo-Sifiliograficas 2013, 104, 29–37. [CrossRef]
- Goossens A. Contact-Allergic Reactions to Cosmetics. J Allergy (Cairo) 2011, 2011, 1–6. [CrossRef]
- Robbins CR. Interactions of Shampoo and Conditioner Ingredients with Hair. In: Chemical and Physical Behavior of Human Hair. Springer Berlin Heidelberg, 2012, pp. 329–443.
- Nazir H, Wang L, Lian G, et al. Multilayered silicone oil droplets of narrow size distribution: Preparation and improved deposition on hair. Colloids Surf B Biointerfaces 2012, 100, 42–49. [CrossRef]
- Niinimäki A, Niinimäki M, Mäkinen-Kiljunen S, Hannuksela M. Contact urticaria from protein hydrolysates in hair conditioners. Allergy. 1998, 53, 1078-82. [CrossRef]
- Jo JH, Jang HS, Ko HC, Kim MB, Oh CK, Kwon YW, Kwon KS. Pustular psoriasis and the Kobner phenomenon caused by allergic contact dermatitis from zinc pyrithione-containing shampoo. Contact Dermatitis. 2005, 52, 142-4. [CrossRef]
- Thyssen JP, White JML. Epidemiological data on consumer allergy to p-phenylenediamine. Contact Dermatitis 2008, 59, 327–343.
- Dias MFRG, Loures AF, Ekelem C. Hair Cosmetics for the Hair Loss Patient. Indian Journal of Plastic Surgery 2021, 54, 507–513. [CrossRef]
- El Anzi O, Hassam B. Pustular dermatosis of the scalp due to topical minoxidil 5. Pan Afr Med J 2018, 30, 83. [CrossRef]
- Ebner H, Müller E. Allergic contact dermatitis from minoxidil. Contact Dermatitis. 1995, 32, 316-7. [CrossRef]
- Nagarajan H, Rai R. Contact dermatitis to minoxidil. Contact Dermatitis 2021, 84, 57. [CrossRef]
- BinJadeed H, Almudimeegh AM, Alomran SA, Alshathry AH. A Case of Contact Allergic Dermatitis to Topical Minoxidil. Cureus. 2021, 13, e12510. [CrossRef]
- Friedman ES, Friedman PM, Cohen DE, et al. Allergic contact dermatitis to topical minoxidil solution: Etiology and treatment. J Am Acad Dermatol 2002, 46, 309–312. [CrossRef]
- Navarro-Triviño FJ, Pegalajar-García MD, Gil-Villalba A, et al. Allergic Contact Dermatitis Due to Minoxidil in a Patient with Alopecia Areata. Actas Dermosifiliogr 2022, 113, S8–S9.
- Kim MS, Chung BY, Chang SE, et al. Pigmented contact dermatitis and hair dyes: A retrospective case-control multicentre study in Korea. J Eur Acad Dermatol Venereol 2023, 37, 2543–2549. [CrossRef]
- Panontin JF, Rambo MKD, Isaac V, et al. New antioxidant lauryl-free herbal shampoo formulation with a Brazilian plant extract. Braz J Biol 2022, 82, e264677. [CrossRef]
- Santos UP, Campos JF, Torquato HF V., et al. Antioxidant, Antimicrobial and Cytotoxic Properties as Well as the Phenolic Content of the Extract from Hancornia speciosa Gomes. PLoS One 2016, 11, e0167531. [CrossRef]
- Saripalla DD, Khokhani ND, Kamath A, et al. Organoleptic and physicochemical properties of natural-based herbal shampoo formulations with Cyclea peltata as a key ingredient. J Cosmet Dermatol 2022, 21, 1666–1674. [CrossRef]
- El Moussaoui A, Jawhari FZ, Almehdi AM, et al. Antibacterial, antifungal and antioxidant activity of total polyphenols of Withania frutescens.L. Bioorg Chem 2019, 93, 103337. [CrossRef]
- Pastor-Nieto MA, Gatica-Ortega ME, Borrego L. Sensitisation to ethylhexyl salicylate: Another piece of the frontal fibrosing alopecia puzzle. Contact Dermatitis. 2023 Nov 27. [CrossRef]
- Pastor-Nieto MA, Gatica-Ortega M. Allergic contact dermatitis to drometrizole trisiloxane in a woman thereafter diagnosed with frontal fibrosing alopecia. Contact Dermatitis 2023, 89, 215–217. [CrossRef]
- Gatica-Ortega ME, Vergara-de-la-Campa L, Alonso-Naranjo L, et al. Relevant sensitization to diethylamino hydroxybenzoyl hexyl benzoate and fragrances in a patient with frontal fibrosing alopecia and acquired dermal macular hyperpigmentation. Contact Dermatitis 2022, 87, 287–289. [CrossRef]
- Pastor-Nieto MA, Gatica-Ortega ME, Sánchez-Herreros C, et al. Sensitization to benzyl salicylate and other allergens in patients with frontal fibrosing alopecia. Contact Dermatitis 2021, 84, 423–430. [CrossRef]
- Ramos PM, Anzai A, Duque-Estrada B, et al. Risk factors for frontal fibrosing alopecia: A case-control study in a multiracial population. J Am Acad Dermatol 2021, 84, 712–718. [CrossRef]
- Rayinda T, McSweeney SM, McFadden JP, et al. There is no proven association between sensitization to benzyl salicylate and frontal fibrosing alopecia. Contact Dermatitis 2021, 85, 483–484. [CrossRef]
- Ródenas-Herranz T, Navarro-Triviño FJ, Linares-González L, et al. Acrylate allergic contact dermatitis caused by hair prosthesis fixative. Contact Dermatitis 2020, 82, 62–64. [CrossRef]
- Torchia D, Giorgini S, Gola M, et al. Allergic contact dermatitis from 2-ethylhexyl acrylate contained in a wig-fixing adhesive tape and its ‘incidental’ therapeutic effect on alopecia areata. Contact Dermatitis 2008, 58, 170–171.
- Uter W, Johansen JD, Macan J, et al. Diagnostics and Prevention of Occupational Allergy in Hairdressers. Curr Allergy Asthma Rep 2023, 23, 267–275. [CrossRef]
- Uter W, Strahwald J, Hallmann S, et al. Systematic review on skin adverse effects of important hazardous hair cosmetic ingredients with a focus on hairdressers. Contact Dermatitis 2023, 88, 93–108. [CrossRef]
- Lee H-J, Kim W-J, Kim J-Y, et al. Patch tests with commercial hair dye products in patients with allergic contact dermatitis to paraphenylenediamine. Indian J Dermatol Venereol Leprol 2016, 82, 645. [CrossRef]
- Symanzik C, Weinert P, Babić Ž, et al. Allergic contact dermatitis caused by 2-hydroxyethyl methacrylate and ethyl cyanoacrylate contained in cosmetic glues among hairdressers and beauticians who perform nail treatments and eyelash extension as well as hair extension applications: A systematic review. Contact Dermatitis 2022, 86, 480–492. [CrossRef]
- Jin P, Yang C, Bai J, et al. Successfully treatment with Dupilumab for systemic contact dermatitis following hair dye in a patient with dermatomyositis. J Cosmet Dermatol 2022, 21, 6468–6469. [CrossRef]
- Dev T, Khan E, Patel U, et al. Cicatricial alopecia following allergic contact dermatitis from hair dyes: A rare clinical presentation. Contact Dermatitis 2022, 86, 59–61. [CrossRef]
- Tosti A, Piraccini BM, van Neste DJ. Telogen effluvium after allergic contact dermatitis of the scalp. Arch Dermatol 2001, 137, 187–90.
- Cabrita SF, Silva R, Correia MP. Allergic contact dermatitis due to glycyrrhizic acid as an ingredient of a hair restorer. Contact Dermatitis 2003, 49, 46–46. [CrossRef]
- Uter W, Geier J, Pirker C, et al. Ammonium thiolactate and thiolactic acid: important hairdressers’ allergens? Contact Dermatitis 2002, 46, 242–3.
- Geier J, Lessmann H, Schnuch A, et al. Diagnostic quality of the patch test preparation monoethanolamine 2% pet. Contact Dermatitis 2005, 52, 171–3. [CrossRef]
- Kanerva L, Jolanki R, Riihimäki V, et al. Patch test reactions and occupational dermatoses caused by hydrogen peroxide. Contact Dermatitis 1998, 39, 146. [CrossRef]
- Fautz R, Fuchs A, van der Walle H, et al. Hair dye-sensitized hairdressers: the cross-reaction pattern with new generation hair dyes. Contact Dermatitis 2002, 46, 319–24. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).