Submitted:
19 February 2024
Posted:
20 February 2024
You are already at the latest version
Abstract
Keywords:
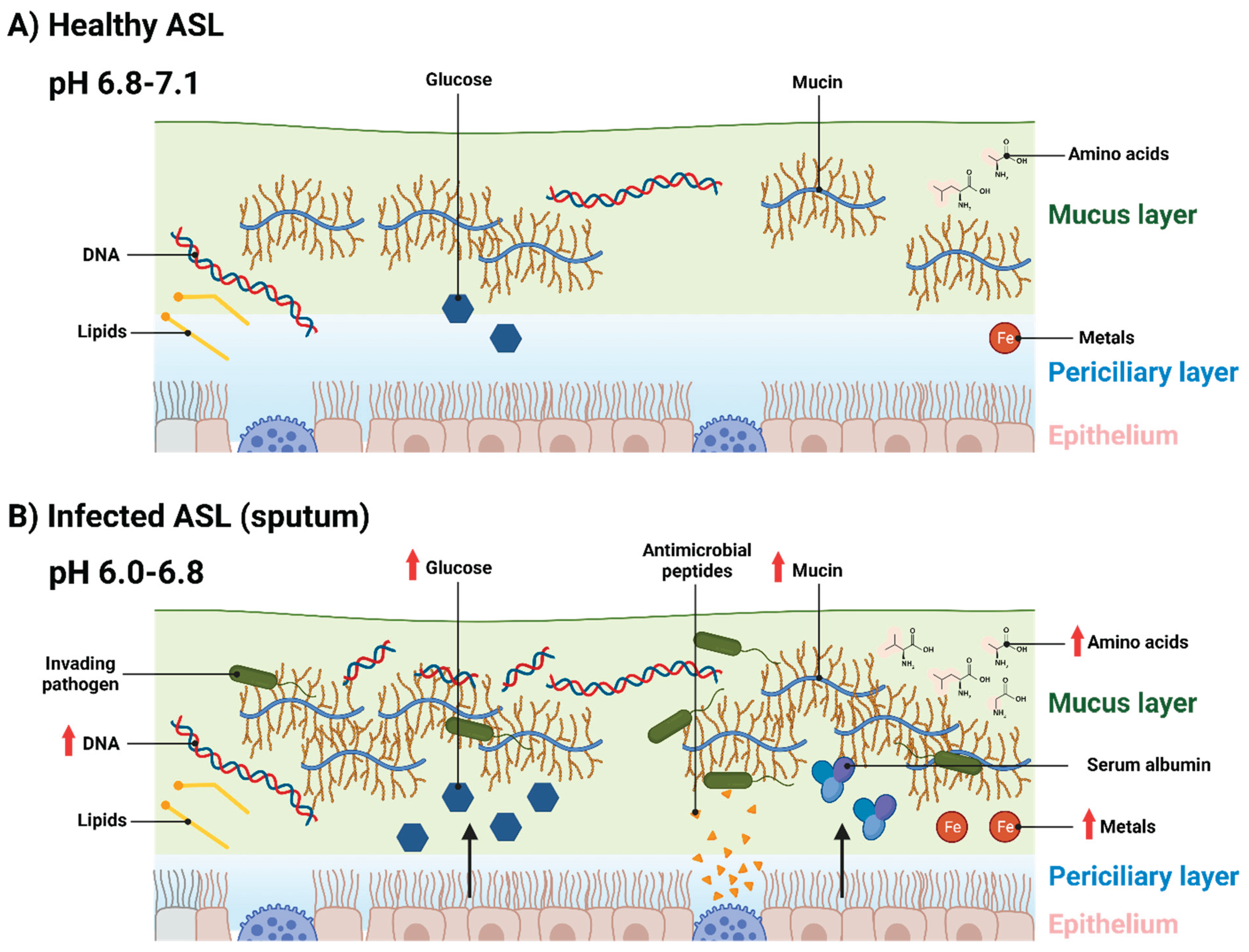
Introduction:
Mucin
DNA
pH and ion concentrations
Sugars and other carbon sources
Amino acids
Lipids
Antimicrobial peptides and enzymes
Metals
Polyamines
Serum albumin
Future developments and conclusions
| Component | Average concentrations | Conditions | Detection method |
|---|---|---|---|
| Mucin | 2.7 mg/mL (61), 6.5 mg/mL (61), ~5 mg/mL (15), ~20 mg/mL (15). | Healthy (61), CF exacerbation (61), newly intubated elective laryngoscopy patients (15), ICU patients mechanically ventilated for at least 4 days (15). | Size exclusion chromatography/differential refractive index (61), enzyme-linked immunosorbent assay of subglottic samples (15). |
| DNA | 0.96 mg/mL (55), 6.7mg/mL (55), 5.2 mg/mL (55), 20 ng/mL (15, 85), 40 ng/mL (15), ~100 ng/mL (85), ~250 ng/mL (85), 0.7 µg/mL (96), 3.2 µg/mL (96), 5.4 µg/mL (96), 2 µg/mL (194), 10 µg/mL (194), 416% more DNA by area in CF sputum compared to asthma and chronic bronchitis sputum (60). | Healthy (55, 85, 194), stable CF (55, 194), CF exacerbation (55), non-ICU patients (15), ICU patients (15), VAP (85), ARDS (85), VAP and ARDS (85), non-CF patients (96), infants with CF (96), older CF patients (96). | Microfluorimetry (55), fluorometric assays (15), colorimetric assays (85), Hoechst dye-binding assay (96), Quant-iT PicoGreen assay (194), confocal microscopy (60). |
| pH | 6.2-7 (nasal) (98), 7.1 (lower airways) (98), 6.78 (99), 7.18 (101), 6.57 (101), 6.97 (102), 6.58 (106), 6.62 (106), 6.72 (104), 6.61 (106), 6.89 (105). | Non-CF (98, 99, 101, 102, 106), CF (98, 101), pneumonia (104, 106), chronic lung disease (106), acute exacerbation of COPD (105). | Monocrystalline antimony catheter (98), in-gold combined pH-glass electrode (98), fluorescent indicators on freshly excised human bronchi (99, 102), fluorescent indicators on nasal biopsies (101), pH electrode (106), pH strips (105). |
| Glucose | 0.4 mM (114), 1 mM (113), 4 mM (113), 1.2 mM (114), 2 mM (114), 3.5 mM (120). | Healthy (114), viral infection (113), hyperglycaemic diabetes (113), CF (114), CF and diabetes (114), mechanically ventilated patients (120). | High-performance anion-exchange chromatography with pulsed amperometric detection (114), glucose oxidase sticks (120). |
| Amino acids | 2.52 mg/mL; total (143), 5.7 mg/mL; total (143), 12.3 mM; total (144), 18.2 mM; total (144), 0.42 nmol/mg; alanine (47), 2.2 nmol/mg; asparagine (47), 0.42 nmol/mg; glutamine (47), 1.06 nmol/mg; glycine (47), 0.43 nmol/mg; lysine (47), 0.13 nmol/mg; valine (47). | Healthy (143), CF (143, 144), CF exacerbation (144), Healthy tissue from lobectomies of lung cancer patients (47). | Thin layer chromatography (143), high-performance liquid chromatography (144), nuclear magnetic resonance (47). |
| Lysozyme | 3.9 µg/mL (161), 9.1 µg/mL (161). | Culture-negative CF patients (161), culture-positive CF patients (161). | Lysozyme activity assay (161). |
| Lactoferrin | 5 µg/mL (61), 9 µg/mL (61), 3.0 µg/mL (161), 22.3 µg/mL (161). | Non-CF (61), CF (61), culture-negative CF patients (161), culture-positive CF patients (161). | Immunologic techniques (61), enzyme-linked immunosorbent assay (161). |
| Ferritin | 0.2 µg/mL (185), 2.4 µg/mL (185), 3.6 µg/mL (185), 0.6 µg/mL (185). | Healthy (185), CF (185), CF exacerbation (185), COPD (185). | Microparticle enzyme immunoassay (185). |
| Putrescine | 11.91 µmol/L (176), 6.18 µmol/L (176), 96.02 µmol/L (176), 20.59 µmol/L (176). | Healthy (176), CF stable (176), CF exacerbation pre-antibiotic treatment (176), CF exacerbation post antibiotic treatment (176). | High-performance liquid chromatography (176). |
| Spermine | 0.22 µmol/L (176), 1.71 µmol/L (176), 7.32 µmol/L (176), 1.35 µmol/L (176). | Healthy (176), CF stable (176), CF exacerbation pre-antibiotic treatment (176), CF exacerbation post antibiotic treatment (176). | High-performance liquid chromatography (176). |
| Spermidine | 0.88 µmol/L (176), 1.62 µmol/L (176), 0.78 µmol/L (176), 0.62 µmol/L (176). | Healthy (176), CF stable (176), CF exacerbation pre-antibiotic treatment (176), CF exacerbation post antibiotic treatment (176). | High-performance liquid chromatography (176). |
| Serum albumin | 0.1 dg/L (185), 0.4 dg/L (185), 0.7 dg/L (185), 0.2 dg/mL (185). 127.4 µg/mL (193), 244.4 µg/mL (193). | Healthy (185, 193), CF (185, 193), CF exacerbation (185), COPD (185). | Rate immunophelometry (185), competitive radioimmunoassay (193). |
Author contributions
Acknowledgements
Abbreviation
| ARDS | Acute respiratory distress syndrome |
| ASL | Airway surface liquid |
| ASM | Artificial sputum medium |
| BALF | Bronchoalveolar lavage fluid |
| BSA | Bovine serum albumin |
| BSM | Bovine submaxillary mucin |
| CF | Cystic fibrosis |
| CFTR | cystic fibrosis transmembrane conductance regulator |
| COPD | Chronic obstructive pulmonary disease |
| COVID-19 | Coronavirus disease 2019 |
| ETT | Endotracheal tube |
| GalNAc | N-acetylgalactose |
| GlcNAc | N-acetylglucosamine |
| HNE | Human neutrophil elastase |
| ICU | Intensive care unit |
| MCL | mucus layer |
| MMP | Matrix metalloproteases |
| MRSA | Methicillin-resistant S. aureus |
| NET | Neutrophil extracellular trap |
| NTHi | Nontypable Haemophilus influenzae |
| PCL | Periciliary layer |
| PGM | Porcine gastric mucin |
| SCFM | Synthetic CF mucus media |
| VAP | Ventilated associated pneumonia |
References
- Widdicombe JH. Regulation of the depth and composition of airway surface liquid. J Anat. 2002;201(4):313-8. [CrossRef]
- Zajac M, Dreano E, Edwards A, Planelles G, Sermet-Gaudelus I. Airway Surface Liquid pH Regulation in Airway Epithelium Current Understandings and Gaps in Knowledge. Int J Mol Sci. 2021;22(7). [CrossRef]
- Thiagarajah JR, Song Y, Derichs N, Verkman AS. Airway surface liquid depth imaged by surface laser reflectance microscopy. J Gen Physiol. 2010;136(3):353-62. [CrossRef]
- Atanasova KR, Reznikov LR. Strategies for measuring airway mucus and mucins. Respir Res. 2019;20(1):261. [CrossRef]
- Bhaskar KR, O'Sullivan DD, Seltzer J, Rossing TH, Drazen JM, Reid LM. Density gradient study of bronchial mucus aspirates from healthy volunteers (smokers and nonsmokers) and from patients with tracheostomy. Exp Lung Res. 1985;9(3-4):289-308. [CrossRef]
- Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest. 2002;109(5):571-7. [CrossRef]
- Rostami MR, LeBlanc MG, Strulovici-Barel Y, Zuo W, Mezey JG, O'Beirne SL, et al. Smoking shifts human small airway epithelium club cells toward a lesser differentiated population. NPJ Genom Med. 2021;6(1):73. [CrossRef]
- Wanner A, Salathe M, O'Riordan TG. Mucociliary clearance in the airways. Am J Respir Crit Care Med. 1996;154(6 Pt 1):1868-902. [CrossRef]
- Roomans GM, Kozlova I, Nilsson H, Vanthanouvong V, Button B, Tarran R. Measurements of airway surface liquid height and mucus transport by fluorescence microscopy, and of ion composition by X-ray microanalysis. J Cyst Fibros. 2004;3 Suppl 2:135-9. [CrossRef]
- King M, Zahm JM, Pierrot D, Vaquez-Girod S, Puchelle E. The role of mucus gel viscosity, spinnability, and adhesive properties in clearance by simulated cough. Biorheology. 1989;26(4):737-45. [CrossRef]
- Amatngalim GD, Hiemstra PS. Airway Epithelial Cell Function and Respiratory Host Defense in Chronic Obstructive Pulmonary Disease. Chin Med J (Engl). 2018;131(9):1099-107. [CrossRef]
- Mager S, Sloan J. Possible role of amino acids, peptides, and sugar transporter in protein removal and innate lung defense. Eur J Pharmacol. 2003;479(1-3):263-7. [CrossRef]
- Pezzulo AA, Gutiérrez J, Duschner KS, McConnell KS, Taft PJ, Ernst SE, et al. Glucose Depletion in the Airway Surface Liquid Is Essential for Sterility of the Airways. PLoS One. 2011;6(1). [CrossRef]
- Vargas Buonfiglio LG, Borcherding JA, Frommelt M, Parker GJ, Duchman B, Vanegas Calderon OG, et al. Airway surface liquid from smokers promotes bacterial growth and biofilm formation via iron-lactoferrin imbalance. Respir Res. 2018;19(1):42. [CrossRef]
- Powell J, Garnett JP, Mather MW, Cooles FAH, Nelson A, Verdon B, et al. Excess Mucin Impairs Subglottic Epithelial Host Defense in Mechanically Ventilated Patients. Am J Respir Crit Care Med. 2018;198(3):340-9. [CrossRef]
- Palmer KL, Aye LM, Whiteley M. Nutritional cues control Pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum. J Bacteriol. 2007;189(22):8079-87. [CrossRef]
- Stickler DJ, Morris NS, Winters C. Simple physical model to study formation and physiology of biofilms on urethral catheters. Methods Enzymol. 1999;310:494-501. [CrossRef]
- Pratten J, Smith AW, Wilson M. Response of single species biofilms and microcosm dental plaques to pulsing with chlorhexidine. J Antimicrob Chemother. 1998;42(4):453-9. [CrossRef]
- Werthen M, Henriksson L, Jensen PO, Sternberg C, Givskov M, Bjarnsholt T. An in vitro model of bacterial infections in wounds and other soft tissues. APMIS. 2010;118(2):156-64. [CrossRef]
- Maierl M, Jorger M, Rosker P, Reisner A. In vitro Dynamic Model of a Catheterized Bladder and Biofilm Assay. Bio Protoc. 2015;5(2). [CrossRef]
- Millhouse E, Jose A, Sherry L, Lappin DF, Patel N, Middleton AM, et al. Development of an in vitroperiodontal biofilm model for assessing antimicrobial and host modulatory effects of bioactive molecules. BMC Oral Health. 2014;14. [CrossRef]
- Furner-Pardoe J, Anonye BO, Cain R, Moat J, Ortori CA, Lee C, et al. Anti-biofilm efficacy of a medieval treatment for bacterial infection requires the combination of multiple ingredients. Scientific Reports. 2020;10. [CrossRef]
- Harrington NE, Sweeney E, Harrison F. Building a better biofilm - Formation of in vivo-like biofilm structures by Pseudomonas aeruginosa in a porcine model of cystic fibrosis lung infection. Biofilm. 2020;2:100024. [CrossRef]
- Fung C, Naughton S, Turnbull L, Tingpej P, Rose B, Arthur J, et al. Gene expression of Pseudomonas aeruginosa in a mucin-containing synthetic growth medium mimicking cystic fibrosis lung sputum. J Med Microbiol. 2010;59(Pt 9):1089-100. [CrossRef]
- Hare NJ, Soe CZ, Rose B, Harbour C, Codd R, Manos J, et al. Proteomics of Pseudomonas aeruginosa Australian epidemic strain 1 (AES-1) cultured under conditions mimicking the cystic fibrosis lung reveals increased iron acquisition via the siderophore pyochelin. J Proteome Res. 2012;11(2):776-95. [CrossRef]
- Ruhluel D, O'Brien S, Fothergill JL, Neill DR. Development of liquid culture media mimicking the conditions of sinuses and lungs in cystic fibrosis and health. F1000Research. 2022. [CrossRef]
- Dinesh SD. Artificial Sputum Medium. Protocol Exchange. 2010. [CrossRef]
- Ghani M, Soothill JS. Ceftazidime, gentamicin, and rifampicin, in combination, kill biofilms of mucoid Pseudomonas aeruginosa. Can J Microbiol. 1997;43:999-1004. [CrossRef]
- Kirchner S, Fothergill JL, Wright EA, James CE, Mowat E, Winstanley C. Use of artificial sputum medium to test antibiotic efficacy against Pseudomonas aeruginosa in conditions more relevant to the cystic fibrosis lung. J Vis Exp. 2012. [CrossRef]
- Quinn RA, Whiteson K, Lim YW, Salamon P, Bailey B, Mienardi S, et al. A Winogradsky-based culture system shows an association between microbial fermentation and cystic fibrosis exacerbation. ISME. 2015. [CrossRef]
- Sriramulu DD, Lunsdorf H, Lam JS, Romling U. Microcolony formation: a novel biofilm model of Pseudomonas aeruginosa for the cystic fibrosis lung. J Med Microbiol. 2005;54(Pt 7):667-76. [CrossRef]
- Serisier DJ, Carroll MP, Shute JK, Young SA. Macrorheology of cystic fibrosis, chronic obstructive pulmonary disease & normal sputum. Respir Res. 2009;10(1):63. [CrossRef]
- Fahy JV, Kim KW, Liu J, Boushey HA. Prominent neutrophilic inflammation in sputum from subjects with asthma exacerbation. J Allergy Clin Immunol. 1995;95(4):843-52.
- Imam JS, Duarte, A. G. Non-CF bronchiectasis: Orphan disease no longer. Respir Med. 2020;166. [CrossRef]
- Eickmeier O, Huebner, M., Herrmann, E., Zissler, U., Rosewich, M., Baer, P. C., Buhl, R., Schmitt-Grohé, S., Zielen, S., Schubert, R. Sputum biomarker profiles in cystic fibrosis (CF) and chronic obstructive pulmonary disease (COPD) and association between pulmonary function. Cytokine. 2010;50(2). [CrossRef]
- Rubin BK. Mucus, phlegm, and sputum in cystic fibrosis. Respir Care. 2009;54(6):726-32; discussion 32.
- Koenig SM, Truwit JD. Ventilator-associated pneumonia: diagnosis, treatment, and prevention. Clin Microbiol Rev. 2006;19(4):637-57. [CrossRef]
- Charles PMV, Kali A, Easow JM, Joseph NM, Ravishankar M, Srinivasan S, et al. Ventilator-associated pneumonia. Australas Med J. 2014;7(8):334-44. [CrossRef]
- Mehta A, Bhagat R. Preventing Ventilator-Associated Infections. Clin Chest Med. 2016;37(4):683-92. [CrossRef]
- Nora D, Povoa P. Antibiotic consumption and ventilator-associated pneumonia rates, some parallelism but some discrepancies. Ann Transl Med. 2017;5(22):450. [CrossRef]
- Camara M, Green W, MacPhee CE, Rakowska PD, Raval R, Richardson MC, et al. Economic significance of biofilms: a multidisciplinary and cross-sectoral challenge. NPJ Biofilms Microbiomes. 2022;8(1):42. [CrossRef]
- Gragueb-Chatti I, Hyvernat H, Leone M, Agard G, Peres N, Guervilly C, et al. Incidence, Outcomes and Risk Factors of Recurrent Ventilator Associated Pneumonia in COVID-19 Patients: A Retrospective Multicenter Study. J Clin Med. 2022;11(23). [CrossRef]
- Alves D, Grainha T, Pereira MO, Lopes SP. Antimicrobial materials for endotracheal tubes: A review on the last two decades of technological progress. Acta Biomater. 2023;158:32-55. [CrossRef]
- Papazian L, Klompas M, Luyt CE. Ventilator-associated pneumonia in adults: a narrative review. Intensive Care Med. 2020;46(5):888-906. [CrossRef]
- Yamada K, Yamamoto Y, Yanagihara K, Araki N, Harada Y, Morinaga Y, et al. In vivo efficacy and pharmacokinetics of biapenem in a murine model of ventilator-associated pneumonia with Pseudomonas aeruginosa. J Infect Chemother. 2012;18(4):472-8. [CrossRef]
- Luna CM, Sibila O, Agusti C, Torres A. Animal models of ventilator-associated pneumonia. Eur Respir J. 2009;33(1):182-8. [CrossRef]
- Benahmed MA, Elbayed K, Daubeuf F, Santelmo N, Frossard N, Namer IJ. NMR HRMAS spectroscopy of lung biopsy samples: Comparison study between human, pig, rat, and mouse metabolomics. Magn Reson Med. 2014;71(1):35-43. [CrossRef]
- Kozlova I, Vanthanouvong V, Almgren B, Hogman M, Roomans GM. Elemental composition of airway surface liquid in the pig determined by x-ray microanalysis. Am J Respir Cell Mol Biol. 2005;32(1):59-64. [CrossRef]
- Cowley EA, Govindaraju K, Guilbault C, Radzioch D, Eidelman DH. Airway surface liquid composition in mice. Am J Physiol Lung Cell Mol Physiol. 2000;278(6):L1213-20. [CrossRef]
- Robinson NP, Kyle H, Webber SE, Widdicombe JG. Electrolyte and other chemical concentrations in tracheal airway surface liquid and mucus. J Appl Physiol (1985). 1989;66(5):2129-35. [CrossRef]
- Boucher RC, Stutts MJ, Bromberg PA, Gatzy JT. Regional differences in airway surface liquid composition. J Appl Physiol Respir Environ Exerc Physiol. 1981;50(3):613-20. [CrossRef]
- Song Y, Thiagarajah J, Verkman AS. Sodium and chloride concentrations, pH, and depth of airway surface liquid in distal airways. J Gen Physiol. 2003;122(5):511-9. [CrossRef]
- Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev. 2006;86(1):245-78. [CrossRef]
- Boat TF, Cheng PW, Iyer RN, Carlson DM, Polony I. Human respiratory tract secretion: mucous glycoproteins of nonpurulent tracheobronchial secretions, and sputum of patients with bronchitis and cystic fibrosis. Arch Biochem Biophys. 1977;177:97-104. [CrossRef]
- Henke MO, John G, Germann M, Lindemann H, Rubin BK. MUC5AC and MUC5B mucins increase in cystic fibrosis airway secretions during pulmonary exacerbation. Am J Respir Crit Care Med. 2007;175(8):816-21. [CrossRef]
- Kirkham S, Sheehan JK, Knight D, Richardson PS, Thornton DJ. Heterogeneity of airways mucus: variations in the amounts and glycoforms of the major oligomeric mucins MUC5AC and MUC5B. Biochem J. 2002;361(Pt 3):537-46. [CrossRef]
- Wickstrom C, Davies JR, Eriksen GV, Veerman EC, Carlstedt I. MUC5B is a major gel-forming, oligomeric mucin from human salivary gland, respiratory tract and endocervix: identification of glycoforms and C-terminal cleavage. Biochem J. 1998;334:685-93. [CrossRef]
- Xia B, Royall JA, Damera G, Sachdev GP, Cummings RD. Altered O-glycosylation and sulfation of airway mucins associated with cystic fibrosis. Glycobiology. 2005;15(8):747-75. [CrossRef]
- Patarin J, Ghiringhelli E, Darsy G, Obamba M, Bochu P, Camara B, et al. Rheological analysis of sputum from patients with chronic bronchial diseases. Sci Rep. 2020;10(1):15685. [CrossRef]
- Henke MO, Renner A, Huber RM, Seeds MC, Rubin BK. MUC5AC and MUC5B Mucins Are Decreased in Cystic Fibrosis Airway Secretions. Am J Respir Cell Mol Biol. 2004;31(1):86-91. [CrossRef]
- Henderson AG, Ehre C, Button B, Abdullah LH, Cai LH, Leigh MW, et al. Cystic fibrosis airway secretions exhibit mucin hyperconcentration and increased osmotic pressure. J Clin Invest. 2014;124(7):3047-60. [CrossRef]
- Hellyer TP, Morris AC, McAuley DF, Walsh TS, Anderson NH, Singh S, et al. Diagnostic accuracy of pulmonary host inflammatory mediators in the exclusion of ventilator-acquired pneumonia. Thorax. 2015;70(1):41-7. [CrossRef]
- Gernez Y, Tirouvanziam R, P. C. Neutrophils in chronic inflammatory airway diseases: can we target them and how? Eur Respir J. 2010;35:467-9. [CrossRef]
- Kirkham S, Kolsum U, Rousseau K, Singh D, Vestbo J, Thornton DJ. MUC5B Is the Major Mucin in the Gel Phase of Sputum in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2008;178(10):1033-9. [CrossRef]
- Bassi GL, Zanella A, Cressoni M, Stylianou M, Kolobow T. Following tracheal intubation, mucus flow is reversed in the semirecumbent position: possible role in the pathogenesis of ventilator-associated pneumonia. Crit Care Med. 2008;36(2):518-25. [CrossRef]
- Hoffman CL, Lalsiamthara J, Aballay A. Host Mucin Is Exploited by Pseudomonas aeruginosa To Provide Monosaccharides Required for a Successful Infection. mBio. 2020;11(2). [CrossRef]
- Yesilkaya H, Manco S, Kadioglu A, Terra VS, Andrew PW. The ability to utilize mucin affects the regulation of virulence gene expression in Streptococcus pneumoniae. FEMS Microbiol Lett. 2008;278(2):231-5. [CrossRef]
- Venkatakrishnan V, Packer NH, Thaysen-Andersen M. Host mucin glycosylation plays a role in bacterial adhesion in lungs of individuals with cystic fibrosis. Expert Rev Respir Med. 2013;7(5):553-76. [CrossRef]
- Wheeler KM, Carcamo-Oyarce G, Turner BS, Dellos-Nolan S, Co JY, Lehoux S, et al. Mucin glycans attenuate the virulence of Pseudomonas aeruginosa in infection. Nat Microbiol. 2019;4(12):2146-54. [CrossRef]
- Wang BX, Wheeler, K. M., Cady, K. C., Lehoux, S., Cummings, R. D., Laub, M. T., Ribbeck, K. Mucin Glycans Signal through the Sensor Kinase RetS to Inhibit Virulence-Associated Traits in Pseudomonas aeruginosa. Curr Biol. 2021;31(1). [CrossRef]
- Takagi J, Aoki K, Turner BS, Lamont S, Lehoux S, Kavanaugh N, et al. Mucin O-glycans are natural inhibitors of Candida albicans pathogenicity. Nat Chem Biol. 2022;18(7):762-73. [CrossRef]
- Weiser JN, Ferreira DM, Paton JC. Streptococcus pneumoniae: transmission, colonization and invasion. Nat Rev Microbiol. 2018;16(6):355-67. [CrossRef]
- Ahearn CP, Gallo MC, Murphy TF. Insights on persistent airway infection by non-typeable Haemophilus influenzae in chronic obstructive pulmonary disease. Pathog Dis. 2017;75(4). [CrossRef]
- Turner KH, Wessel AK, Palmer GC, Murray JL, Whiteley M. Essential genome of Pseudomonas aeruginosa in cystic fibrosis sputum. Proc Natl Acad Sci U S A. 2015;112(13):4110-5. [CrossRef]
- Padra M, Adamczyk B, Benktander J, Flahou B, Skoog EC, Padra JT, et al. Helicobacter suis binding to carbohydrates on human and porcine gastric mucins and glycolipids occurs via two modes. Virulence. 2018;9(1):898-918. [CrossRef]
- Barmpatsalou V, Dubbelboer IR, Rodler A, Jacobson M, Karlsson E, Pedersen BL, et al. Physiological properties, composition and structural profiling of porcine gastrointestinal mucus. Eur J Pharm Biopharm. 2021;169:156-67. [CrossRef]
- Rousseau K, Kirkham S, Johnson L, Fitzpatrick B, Howard M, Adams EJ, et al. Proteomic analysis of polymeric salivary mucins: no evidence for MUC19 in human saliva. Biochem J. 2008;413(3):545-52. [CrossRef]
- Lieleg O, Lieleg C, Bloom J, Buck CB, Ribbeck K. Mucin biopolymers as broad-spectrum antiviral agents. Biomacromolecules. 2012;13(6):1724-32. [CrossRef]
- Harris G, Holbein BE, Zhou H, Xu HH, Chen W. Potential Mechanisms of Mucin-Enhanced Acinetobacter baumannii Virulence in the Mouse Model of Intraperitoneal Infection. Infect Immun. 2019;87(11). [CrossRef]
- Neve RL, Carrillo BD, Phelan VV. Impact of Artificial Sputum Medium Formulation on Pseudomonas aeruginosa Secondary Metabolite Production. J Bacteriol. 2021;203(21):e0025021. [CrossRef]
- Nisizawa K, Pigman W. The composition and properties of the mucin clot from cattle submaxillary glands. Arch Oral Biol. 1959;1:161-70. [CrossRef]
- Tettamanti G, Pigman W. Purification and characterization of bovine and ovine submaxillary mucins. Arch Biochem Biophys. 1968;124(1):41-50. [CrossRef]
- Schömig VJ, Käsdorf BT, Scholz C, Bidmon K, Lieleg O, Berensmeier S. An optimized purification process for porcine gastric mucin with preservation of its native functional properties. RSC Adv. 2016;6:44932-43. [CrossRef]
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532-5. [CrossRef]
- Mikacenic C, Moore R, Dmyterko V, West TE, Altemeier WA, Liles WC, et al. Neutrophil extracellular traps (NETs) are increased in the alveolar spaces of patients with ventilator-associated pneumonia. Crit Care. 2018;22(1):358. [CrossRef]
- Radermecker C, Detrembleur N, Guiot J, Cavalier E, Henket M, d'Emal C, et al. Neutrophil extracellular traps infiltrate the lung airway, interstitial, and vascular compartments in severe COVID-19. J Exp Med. 2020;217(12). [CrossRef]
- Barnes BJ, Adrover JM, Baxter-Stoltzfus A, Borczuk A, Cools-Lartigue J, Crawford JM, et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J Exp Med. 2020;217(6). [CrossRef]
- Porto BN, Stein RT. Neutrophil Extracellular Traps in Pulmonary Diseases: Too Much of a Good Thing? Front Immunol. 2016;7:311. [CrossRef]
- Yildiz C, Palaniyar N, Otulakowski G, Khan MA, Post M, Kuebler WM, et al. Mechanical ventilation induces neutrophil extracellular trap formation. Anesthesiology. 2015;122(4):864-75. [CrossRef]
- De Rose V. Mechanisms and markers of airway inflammation in cystic fibrosis. Eur Respir J. 2002;19(2):333-40. [CrossRef]
- Rosenecker J, Naundorf S, Rudolph C. Airway surface liquid contains endogenous DNase activity which can be activated by exogenous magnesium. Eur J Med Res. 2009;14(7):304-8. [CrossRef]
- Lethem MI, James SL, Marriott C, Burke JF. The origin of DNA associated with mucus glycoproteins in cystic fibrosis sputum. Eur Respir J. 1990;3(1):19-23. [CrossRef]
- Lewenza S, Johnson L, Charron-Mazenod L, Hong M, Mulcahy-O'Grady H. Extracellular DNA controls expression of Pseudomonas aeruginosa genes involved in nutrient utilization, metal homeostasis, acid pH tolerance and virulence. J Med Microbiol. 2020;69(6):895-905. [CrossRef]
- Wilton M, Charron-Mazenod L, Moore R, Lewenza S. Extracellular DNA Acidifies Biofilms and Induces Aminoglycoside Resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2016;60(1):544-53. [CrossRef]
- Brandt T, Breitenstein S, von der Hardt H, Tummler B. DNA concentration and length in sputum of patients with cystic fibrosis during inhalation with recombinant human DNase. Thorax. 1995;50(8):880-2. [CrossRef]
- Kirchner KK, Wagener JS, Khan TZ, Copenhaver SC, Accurso FJ. Increased DNA levels in bronchoalveolar lavage fluid obtained from infants with cystic fibrosis. Am J Respir Crit Care Med. 1996;154(5). [CrossRef]
- Olivença DV, Fonseca LL, Voit EO, Pinto FR. Thickness of the airway surface liquid layer in the lung is affected in cystic fibrosis by compromised synergistic regulation of the ENaC ion channel. J R Soc Interface. 2019;16(157). [CrossRef]
- McShane D, Davies JC, Davies MG, Bush A, Geddes DM, Alton EW. Airway surface pH in subjects with cystic fibrosis. Eur Respir J. 2003;21(1):37-42. [CrossRef]
- Jayaraman S, Song Y, Vetrivel L, Shankar L, Verkman AS. Noninvasive in vivo fluorescence measurement of airway-surface liquid depth, salt concentration, and pH. J Clin Invest. 2001;107(3):317-24. [CrossRef]
- Berkebile AR, McCray PB, Jr. Effects of airway surface liquid pH on host defense in cystic fibrosis. Int J Biochem Cell Biol. 2014;52:124-9. [CrossRef]
- Song Y, Salinas D, Nielson DW, Verkman AS. Hyperacidity of secreted fluid from submucosal glands in early cystic fibrosis. Am J Physiol Cell Physiol. 2006;290(3):C741-9. [CrossRef]
- Jayaraman S, Joo NS, Reitz B, Wine JJ, Verkman AS. Submucosal gland secretions in airways from cystic fibrosis patients have normal [Na(+)] and pH but elevated viscosity. Proc Natl Acad Sci U S A. 2001;98(14). [CrossRef]
- Yoon SS, Coakley R, Lau GW, Lymar SV, Gaston B, Karabulut AC, et al. Anaerobic killing of mucoid Pseudomonas aeruginosa by acidified nitrite derivatives under cystic fibrosis airway conditions. J Clin Invest. 2006;116(2):436-46. [CrossRef]
- Karnad DR, Mhaisekar DG, Moralwar KV. Respiratory mucus pH in tracheostomized intensive care unit patients: Effects of colonization and pneumonia. Crit Care Med. 1990;18(7):699-701.
- Lozo Vukovac E, Mise K, Gudelj I, Peric I, Duplancic D, Vukovic I, et al. Bronchoalveolar pH and inflammatory biomarkers in patients with acute exacerbation of chronic obstructive pulmonary disease. J Int Med Res. 2019;47(2):791-802. [CrossRef]
- Bodem CR, Lampton LM, Miller DP, Tarka EF, Everett ED. Endobronchial pH. Relevance of aminoglycoside activity in gram-negative bacillary pneumonia. Am Rev Respir Dis. 1983;127(1):39-41. [CrossRef]
- Clary-Meinesz C, Mouroux J, Cosson J, Huitorel P, Blaive B. Influence of external pH on ciliary beat frequency in human bronchi and bronchioles. Eur Respir J. 1998;11(2):330-3. [CrossRef]
- Simonin J, Bille E, Crambert G, Noel S, Dreano E, Edwards A, et al. Airway surface liquid acidification initiates host defense abnormalities in Cystic Fibrosis. Sci Rep. 2019;9(1):6516. [CrossRef]
- Liu Y, Xie YZ, Shi YH, Yang L, Chen XY, Wang LW, et al. Airway acidification impaired host defense against Pseudomonas aeruginosa infection by promoting type 1 interferon beta response. Emerg Microbes Infect. 2022;11(1):2132-46. [CrossRef]
- Joris L, Dab I, Quinton PM. Elemental composition of human airway surface fluid in healthy and diseased airways. Am Rev Respir Dis. 1993;148(6 Pt 1):1633-7. [CrossRef]
- Goldman MJ, Anderson GM, Stolzenberg ED, Kari UP, Zasloff M, Wilson JM. Human beta-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell. 1997;88(4):553-60. [CrossRef]
- Garnett JP, Nguyen TT, Moffatt JD, Pelham ER, Kalsi KK, Baker EH, et al. Pro-inflammatory mediators disrupt glucose homeostasis in airway surface liquid. J Immunol. 2012;189(1):373-80. [CrossRef]
- Philips BJ, Meguer JX, Redman J, Baker EH. Factors determining the appearance of glucose in upper and lower respiratory tract secretions. Intensive Care Med. 2003;29(12):2204-10. [CrossRef]
- Baker EH, Clark N, Brennan AL, Fisher DA, Gyi KM, Hodson ME, et al. Hyperglycemia and cystic fibrosis alter respiratory fluid glucose concentrations estimated by breath condensate analysis. J Appl Physiol (1985). 2007;102(5):1969-75. [CrossRef]
- Baker EH, Janaway CH, Philips BJ, Brennan AL, Baines DL, Wood DM, et al. Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease. Thorax. 2006;61(4). [CrossRef]
- Mirabella S, Gomez-Paz S, Lam E, Gonzalez-Mosquera L, Fogel J, Rubinstein S. Glucose dysregulation and its association with COVID-19 mortality and hospital length of stay. Diabetes Metab Syndr. 2022;16(3):102439. [CrossRef]
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-9. [CrossRef]
- Wang W, Shen M, Tao Y, Fairley CK, Zhong Q, Li Z, et al. Elevated glucose level leads to rapid COVID-19 progression and high fatality. BMC Pulm Med. 2021;21(1):64. [CrossRef]
- Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934-43. [CrossRef]
- Philips BJ, Redman J, Brennan A, Wood D, Holliman R, Baines D, et al. Glucose in bronchial aspirates increases the risk of respiratory MRSA in intubated patients. Thorax. 2005;60(9):761-4. [CrossRef]
- Baker EH, Baines DL. Airway Glucose Homeostasis: A New Target in the Prevention and Treatment of Pulmonary Infection. Chest. 2018;153(2):507-14. [CrossRef]
- Gill SK, Hui K, Farne H, Garnett JP, Baines DL, Moore LS, et al. Increased airway glucose increases airway bacterial load in hyperglycaemia. Sci Rep. 2016;6:27636. [CrossRef]
- McAlister FA, Majumdar SR, Blitz S, Rowe BH, Romney J, Marrie TJ. The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community-acquired pneumonia. Diabetes Care. 2005;28(4):810-5. [CrossRef]
- Brennan AL, Gyi KM, Wood DM, Johnson J, Holliman R, Baines DL, et al. Airway glucose concentrations and effect on growth of respiratory pathogens in cystic fibrosis. J Cyst Fibros. 2007;6(2):101-9. [CrossRef]
- Garnett JP, Braun D, McCarthy AJ, Farrant MR, Baker EH, Lindsay JA, et al. Fructose transport-deficient Staphylococcus aureus reveals important role of epithelial glucose transporters in limiting sugar-driven bacterial growth in airway surface liquid. Cell Mol Life Sci. 2014;71(23):4665-73. [CrossRef]
- Dennesen P, Veerman E, van Nieuw Amerongen A, Jacobs J, Kessels A, van der Keybus P, et al. High levels of sulfated mucins in bronchoalveolar lavage fluid of ICU patients with ventilator-associated pneumonia. Intensive Care Med. 2003;29(5):715-9. [CrossRef]
- Davril M, Degroote S, Humbert P, Galabert C, Dumur V, Lafitte J, et al. The sialylation of bronchial mucins secreted by patients suffering from cystic fibrosis or from chronic bronchitis is related to the severity of airway infection. Glycobiology. 1999;9(3):311-21. [CrossRef]
- Aprianto R, Slager J, Holsappel S, Veening JW. High-resolution analysis of the pneumococcal transcriptome under a wide range of infection-relevant conditions. Nucleic Acids Res. 2018;46(19):9990-10006. [CrossRef]
- Blanchette KA, Shenoy AT, Milner J, 2nd, Gilley RP, McClure E, Hinojosa CA, et al. Neuraminidase A-Exposed Galactose Promotes Streptococcus pneumoniae Biofilm Formation during Colonization. Infect Immun. 2016;84(10):2922-32. [CrossRef]
- Korgaonkar AK, Whiteley M. Pseudomonas aeruginosa enhances production of an antimicrobial in response to N-acetylglucosamine and peptidoglycan. J Bacteriol. 2011;193(4):909-17. [CrossRef]
- Ding X, Robbe-Masselot C, Fu X, Leonard R, Marsac B, Dauriat CJG, et al. Airway environment drives the selection of quorum sensing mutants and promote Staphylococcus aureus chronic lifestyle. Nat Commun. 2023;14(1):8135. [CrossRef]
- Lucas SK, Villarreal AR, Ahmad MM, Itabiyi A, Feddema E, Boyer HC, et al. Anaerobic Microbiota Derived from the Upper Airways Impact Staphylococcus aureus Physiology. Infect Immun. 2021;89(9):e0015321. [CrossRef]
- Mukherjee K, Khatua B, Mandal C. Sialic Acid-Siglec-E Interactions During Pseudomonas aeruginosa Infection of Macrophages Interferes With Phagosome Maturation by Altering Intracellular Calcium Concentrations. Front Immunol. 2020;11:332. [CrossRef]
- Khatua B, Bhattacharya, K., Mandal, C. Sialoglycoproteins adsorbed by Pseudomonas aeruginosa facilitate their survival by impeding neutrophil extracellular trap through siglec-9. J Leukoc Biol. 2012;91(4). [CrossRef]
- Jackson MD, Wong SM, Akerley BJ. Sialic Acid Protects Nontypeable Haemophilus influenzae from Natural IgM and Promotes Survival in Murine Respiratory Tract. Infect Immun. 2021;89(6). [CrossRef]
- Wong SM, Jackson MD, Akerley BJ. Suppression of Alternative Lipooligosaccharide Glycosyltransferase Activity by UDP-Galactose Epimerase Enhances Murine Lung Infection and Evasion of Serum IgM. Front Cell Infect Microbiol. 2019;9:160. [CrossRef]
- Rose MC, Nickola TJ, Voynow JA. Airway mucus obstruction: mucin glycoproteins, MUC gene regulation and goblet cell hyperplasia. Am J Respir Cell Mol Biol. 2001;25(5):533-7. [CrossRef]
- Green AE, Pottenger, S., Monshi, M. S., Barton, T. E., Phelan, M., Neill, D. R.. Airway metabolic profiling during Streptococcus pneumoniae infection identifies branched chain amino acids as signatures of upper airway colonisation. PLoS Pathog. 2023;19(9). [CrossRef]
- Rojo F. Carbon catabolite repression in Pseudomonas : optimizing metabolic versatility and interactions with the environment. FEMS Microbiol Rev. 2010;34(5):658-84. [CrossRef]
- La Rosa R, Johansen HK, Molin S. Adapting to the Airways: Metabolic Requirements of Pseudomonas aeruginosa during the Infection of Cystic Fibrosis Patients. Metabolites. 2019;9(10). [CrossRef]
- La Rosa R, Johansen HK, Molin S. Convergent Metabolic Specialization through Distinct Evolutionary Paths in Pseudomonas aeruginosa. mBio. 2018;9(2):10.1128/mbio.00269-18. [CrossRef]
- Ren X, Palmer LD. Acinetobacter Metabolism in Infection and Antimicrobial Resistance. Infect Immun. 2023;91(6):e0043322. [CrossRef]
- Barth AL, Pitt TL. The high amino-acid content of sputum from cystic fibrosis patients promotes growth of auxotrophic Pseudomonas aeruginosa. J Med Microbiol. 1996;45(2):110-9. [CrossRef]
- Thomas SR, Ray A, Hodson ME, Pitt TL. Increased sputum amino acid concentrations and auxotrophy of Pseudomonas aeruginosa in severe cystic fibrosis lung disease. Thorax. 2000;55(9):795-7. [CrossRef]
- Hallman M, Merritt TA, Akino T, Bry K. Surfactant protein A, phosphatidylcholine, and surfactant inhibitors in epithelial lining fluid. Correlation with surface activity, severity of respiratory distress syndrome, and outcome in small premature infants. Am Rev Respir Dis. 1991;144(6):1376-84. [CrossRef]
- Robinson MJ, Krasnodembskaya AD. Therapeutic targeting of metabolic alterations in acute respiratory distress syndrome. Eur Respir Rev. 2020;29(156). [CrossRef]
- Aiyer A, Manos J. The Use of Artificial Sputum Media to Enhance Investigation and Subsequent Treatment of Cystic Fibrosis Bacterial Infections. Microorganisms. 2022;10(7):1269. [CrossRef]
- Schwab U, Abdullah LH, Perlmutt OS, Albert D, Davis CW, Arnold RR, et al. Localization of Burkholderia cepacia complex bacteria in cystic fibrosis lungs and interactions with Pseudomonas aeruginosa in hypoxic mucus. Infect Immun. 2014;82(11):4729-45. [CrossRef]
- Liaqat A, Mason M, Foster BJ, Kulkarni S, Barlas A, Farooq AM, et al. Evidence-Based Mechanical Ventilatory Strategies in ARDS. J Clin Med. 2022;11(2). [CrossRef]
- Farooqui AA. Lipid mediators in the neural cell nucleus: their metabolism, signaling, and association with neurological disorders. Neurosci Rev J Bring Neurobiol Neurol Psychiatry. 2009;15(4):392-407. [CrossRef]
- Zehethofer N, Bermbach S, Hagner S, Garn H, Muller J, Goldmann T, et al. Lipid Analysis of Airway Epithelial Cells for Studying Respiratory Diseases. Chromatographia. 2015;78(5-6):403-13. [CrossRef]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1(1):31-9. [CrossRef]
- Sahu S, Lynn WS. Lipid composition of sputum from patients with asthma and patients with cystic fibrosis. Inflammation. 1978;3(1):27-36. [CrossRef]
- Sohlenkamp C, Lopez-Lara IM, Geiger O. Biosynthesis of phosphatidylcholine in bacteria. Prog Lipid Res. 2003;42(2):115-62. [CrossRef]
- Cundell DR, Gerard NP, Gerard C, Idanpaan-Heikkila I, Tuomanen EI. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature. 1995;377(6548):435-8. [CrossRef]
- Weiser JN, Pan N, McGowan KL, Musher D, Martin A, Richards J. Phosphorylcholine on the lipopolysaccharide of Haemophilus influenzae contributes to persistence in the respiratory tract and sensitivity to serum killing mediated by C-reactive protein. J Exp Med. 1998;187(4):631-40. [CrossRef]
- Sun Z, Kang, Y., Norris, M. H., Troyer, R. M., Son, M. S., Schweizer, H. P., Dow, S. W., Hoang, T. T. Blocking Phosphatidylcholine Utilization in Pseudomonas aeruginosa, via Mutagenesis of Fatty Acid, Glycerol and Choline Degradation Pathways, Confirms the Importance of This Nutrient Source In Vivo. PLOS ONE. 2014;9(7). [CrossRef]
- Willsey GG, Ventrone S, Schutz KC, Wallace AM, Ribis JW, Suratt BT, et al. Pulmonary Surfactant Promotes Virulence Gene Expression and Biofilm Formation in Klebsiella pneumoniae. Infect Immun. 2018;86(7). [CrossRef]
- Wei J, Zhao X, Wang S, Zhang M, Yao W, Yuan Y. Determination of related substances in egg yolk lecithin by HPLC-CAD and characterization of its profiling by HPLC-Q-TOF-MS. J Pharm Biomed Anal. 2022;221:115079. [CrossRef]
- Ganz T. Antimicrobial polypeptides in host defense of the respiratory tract. J Clin Invest. 2002;109(6):693-7. [CrossRef]
- Sagel SD, Sontag MK, Accurso FJ. Relationship Between Antimicrobial Proteins and AirwayInflammation and Infection in Cystic Fibrosis. Pediatr Pulmonol. 2009;44(4):402-9. [CrossRef]
- Feingold DS, Goldman JN, Kuritz HM. Locus of the action of serum and the role of lysozyme in the serum bactericidal reaction. J Bacteriol. 1968;96(6):2118-26. [CrossRef]
- Arnold RR, Cole MF, McGhee JR. A bactericidal effect for human lactoferrin. Science. 1977;197(4300):263-5. [CrossRef]
- Drago-Serrano ME, de la Garza-Amaya M, Luna JS, Campos-Rodríguez R. Lactoferrin-lipopolysaccharide (LPS) binding as key to antibacterial and antiendotoxic effects. Int Immunopharmacol. 2012;12(1):1-9. [CrossRef]
- Aratani Y. Myeloperoxidase: Its role for host defense, inflammation, and neutrophil function. Arch Biochem Biophys. 2018;640:47-52. [CrossRef]
- Wilkinson TS, Morris AC, Kefala K, O'Kane CM, Moore NR, Booth NA, et al. Ventilator-Associated Pneumonia Is Characterized by Excessive Release of Neutrophil Proteases in the Lung. Chest. 2012;142(6):1425-32. [CrossRef]
- Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283(2-3):65-87. [CrossRef]
- Kinnula VL, Crapo JD. Superoxide dismutases in the lung and human lung diseases. Am J Respir Crit Care Med. 2003;167(12):1600-19. [CrossRef]
- Reid DW, Carroll V, O'May C, Champion A, Kirov SM. Increased airway iron as a potential factor in the persistence of Pseudomonas aeruginosa infection in cystic fibrosis. Eur Respir J. 2007;30:286-92. [CrossRef]
- Smith DJ, Anderson GJ, Bell SC, Reid DW. Elevated metal concentrations in the CF airway correlate with cellular injury and disease severity. J Cyst Fibros. 2014;13(3):289-95. [CrossRef]
- Sunder-Plassmann G, Patruta SI, Horl WH. Pathobiology of the role of iron in infection. Am J Kidney Dis. 1999;34(4 Suppl 2):S25-9. [CrossRef]
- Bussiere FI, Gueux E, Rock E, Girardeau JP, Tridon A, Mazur A, et al. Increased phagocytosis and production of reactive oxygen species by neutrophils during magnesium deficiency in rats and inhibition by high magnesium concentration. Br J Nutr. 2002;87(2):107-13. [CrossRef]
- Gray RD, Duncan A, Noble D, Imrie M, O'Reilly DS, Innes JA, et al. Sputum trace metals are biomarkers of inflammatory and suppurative lung disease. Chest. 2010;137(3):635-41. [CrossRef]
- Stites SW, Walters B, O'Brien-Ladner AR, Bailey K, Wesselius LJ. Increased iron and ferritin content of sputum from patients with cystic fibrosis or chronic bronchitis. Chest. 1998;114(3):814-9. [CrossRef]
- Stites SW, Plautz MW, Bailey K, O'Brien-Ladner AR, Wesselius LJ. Increased concentrations of iron and isoferritins in the lower respiratory tract of patients with stable cystic fibrosis. Am J Respir Crit Care Med. 1999;160(3):796-801. [CrossRef]
- Grasemann H, Shehnaz D, Enomoto M, Leadley M, Belik J, Ratjen F. L-ornithine derived polyamines in cystic fibrosis airways. PLoS One. 2012;7(10):e46618. [CrossRef]
- Pegg AE. Functions of Polyamines in Mammals. J Biol Chem. 2016;291(29):14904-12. [CrossRef]
- Shah P, Swiatlo E. A multifaceted role for polyamines in bacterial pathogens. Mol Microbiol. 2008;68(1):4-16. [CrossRef]
- Chou HT, Li J, Peng Y, Lu C. Molecular characterization of PauR and its role in control of putrescine and cadaverine catabolism through the γ-glutamylation pathway in Pseudomonas aeruginosa PAO1. J Bacteriol. 2013;195(17):3906-13.
- Twomey KB, Alston M, An SQ, O'Connell OJ, McCarthy Y, Swarbreck D, et al. Microbiota and metabolite profiling reveal specific alterations in bacterial community structure and environment in the cystic fibrosis airway during exacerbation. PLoS One. 2013;8(12):e82432. [CrossRef]
- Pathak KV, McGilvrey MI, Hu CK, Garcia-Mansfield K, Lewandoski K, Eftekhari Z, et al. Molecular Profiling of Innate Immune Response Mechanisms in Ventilator-associated Pneumonia. Mol Cell Proteomics. 2020;19(10):1688-705. [CrossRef]
- Holz O, DeLuca DS, Roepcke S, Illig T, Weinberger KM, Schudt C, et al. Smokers with COPD Show a Shift in Energy and Nitrogen Metabolism at Rest and During Exercise. Int J Chron Obstruct Pulmon Dis. 2020;15:1-13. [CrossRef]
- Hasan CM, Pottenger S, Green AE, Cox AA, White JS, Jones T, et al. Pseudomonas aeruginosa utilises the host-derived polyamine spermidine to facilitate antimicrobial tolerance. JCI Insight. 2022. [CrossRef]
- Kwon DH, Lu CD. Polyamine effects on antibiotic susceptibility in bacteria. Antimicrob Agents Chemother. 2007;51(6):2070-7. [CrossRef]
- Reid DW, Lam QT, Schneider H, Walters EH. Airway iron and iron-regulatory cytokines in cystic fibrosis. Eur Respir J. 2004;24(2):286-91. [CrossRef]
- Innes AL, Carrington SD, Thornton DJ, Kirkham S, Rousseau K, Dougherty RH, et al. Ex vivo sputum analysis reveals impairment of protease-dependent mucus degradation by plasma proteins in acute asthma. Am J Respir Crit Care Med. 2009;180(3):203-10. [CrossRef]
- Sankar S, Yamaguchi M, Kawabata S, Ponnuraj K. Streptococcus pneumoniae Surface Adhesin PfbA Exhibits Host Specificity by Binding to Human Serum Albumin but Not Bovine, Rabbit and Porcine Serum Albumins. Protein J. 2020;39(1):1-9. [CrossRef]
- Egesten A, Frick IM, Mörgelin M, Olin AI, Björck L. Binding of Albumin Promotes Bacterial Survival at the Epithelial Surface. J Biol Chem. 2011;286(4):2469-76. [CrossRef]
- Teevan-Hanman C, O'Shea, P.. Candida albicans exhibit two classes of cell surface binding sites for serum albumin defined by their affinity, abundance and prospective role in interkingdom signalling. PLOS ONE. 2021;16(7). [CrossRef]
- Austermeier S, Pekmezovic M, Porschitz P, Lee S, Kichik N, Moyes DL, et al. Albumin Neutralizes Hydrophobic Toxins and Modulates Candida albicans Pathogenicity. mBio. 2021;12(3):e0053121. [CrossRef]
- Kruczek C, Wachtel M, Alabady MS, Payton PR, Colmer-Hamood JA, Hamood AN. Serum albumin alters the expression of iron-controlled genes in Pseudomonas aeruginosa. Microbiology (Reading). 2012;158(Pt 2):353-67. [CrossRef]
- Smith AC, Rice A, Sutton B, Gabrilska R, Wessel AK, Whiteley M, et al. Albumin Inhibits Pseudomonas aeruginosa Quorum Sensing and Alters Polymicrobial Interactions. Infect Immun. 2017;85(9). [CrossRef]
- Sagel SD, Kapsner R, Osberg I, Sontag MK, Accurso FJ. Airway inflammation in children with cystic fibrosis and healthy children assessed by sputum induction. Am J Respir Crit Care Med. 2001;164(8 Pt 1):1425-31. [CrossRef]
- Marcos V, Zhou-Suckow Z, Onder Yildirim A, Bohla A, Hector A, Vitkov L, et al. Free DNA in cystic fibrosis airway fluids correlates with airflow obstruction. Mediators Inflamm. 2015;2015:408935. [CrossRef]
| Metal | Healthy | Asthma | COPD | CF | Bronchiectasis | Smoker |
|---|---|---|---|---|---|---|
| Zn | 15.35 (10.4-25.6)a 179 (103-597)b 40.45 (20.99)c |
12.7 (7.2-41.4) a | 25.4 (9.8-50.7) a | 135.3 (54.2-209.6) a 1285 (678-1811) b |
111.3 (46.1-150.7) a 537 (401-838) b |
48.16 (35.06) c |
| Fe | 13.5 (8.6-21.5) a 0 (0-37) b 6.38 (9.12) c |
30 (6.9-35.3) a | 21.3 (3.1-35.6) a | 56.9 (24.3-115.3) a 797 (398 – 1292) b |
54.2 (22.7-91.6) a 1075 (862-1324) b |
23.37 (28.47) c |
| Mn | 0 (0-0.25) a 5 (2-9) b 0.12 (0.16) c |
0.8 (0.2-1.7) a | 0 (0-0.7) a | 0.3 (0.1-0.8) a 6 (4-17) b |
0.6 (0.2-1.3) a 6 (4-10) b |
0.21 (0.24) c |
| Cu | 8.6 (3-16.4) a 106 (55.3-196) b 4.77 (4.9) c |
15.2 (8.6-29.5) a | 15.2 (12.2-22) a | 19.5 (14.5-30.1) a 173 (128-257) b |
15.7 (10.9-33.3) a 226 (130-314) b |
4 (2.26) c |
| Ca | 45000 (28000-58000) b 811.7 (181.61) c |
102000 (76000-123000) b | 124000 (78000-156000) b | 856.8 (183.34) c | ||
| Mg | 4000 (2000-7000) b 389.06 (66.93) c |
30000 (19000-44000) b | 33000 (27000-39000) b | 428.76 (110.42) c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




