Submitted:
20 February 2024
Posted:
21 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Microbial Biosynthesis of CLA
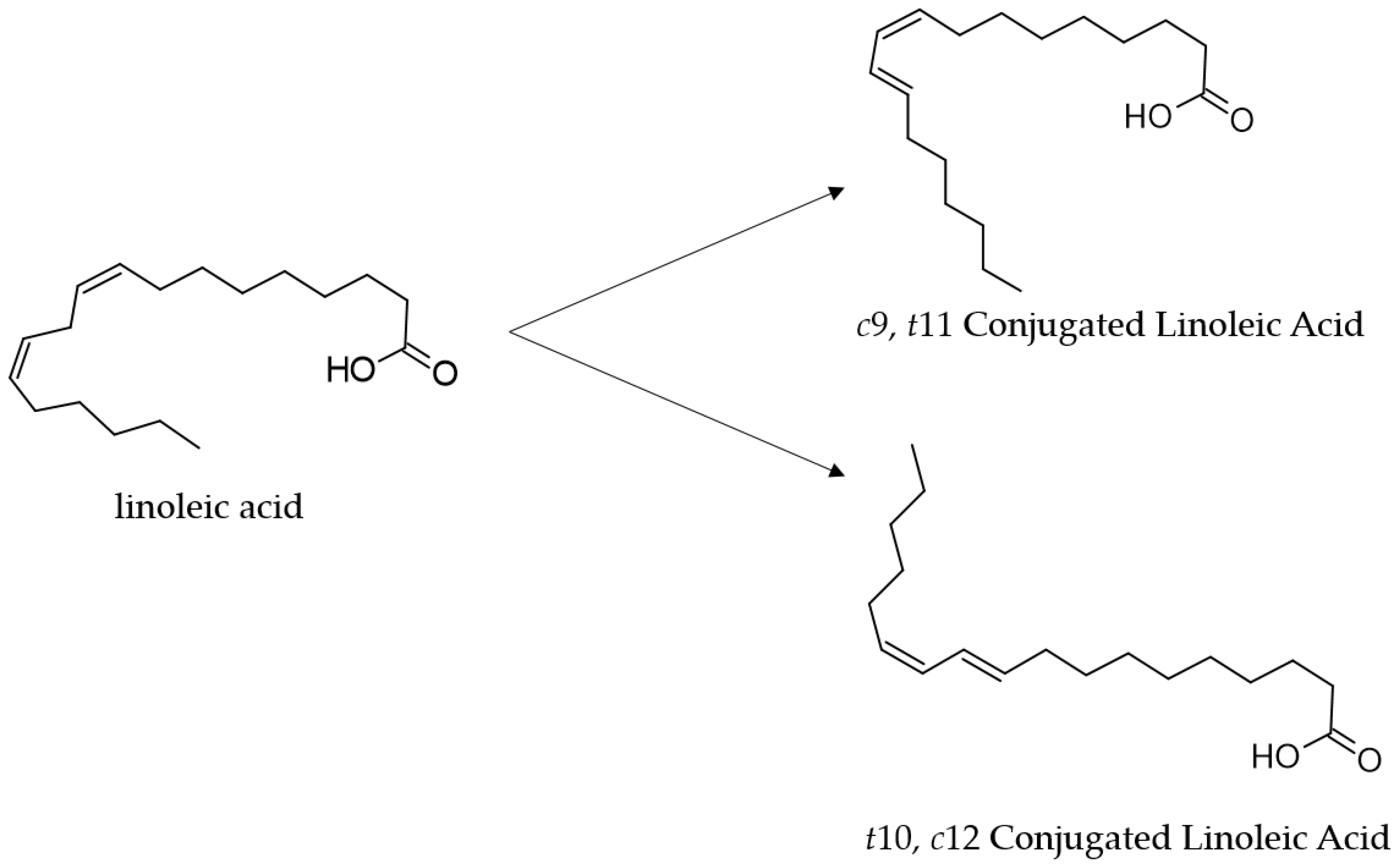
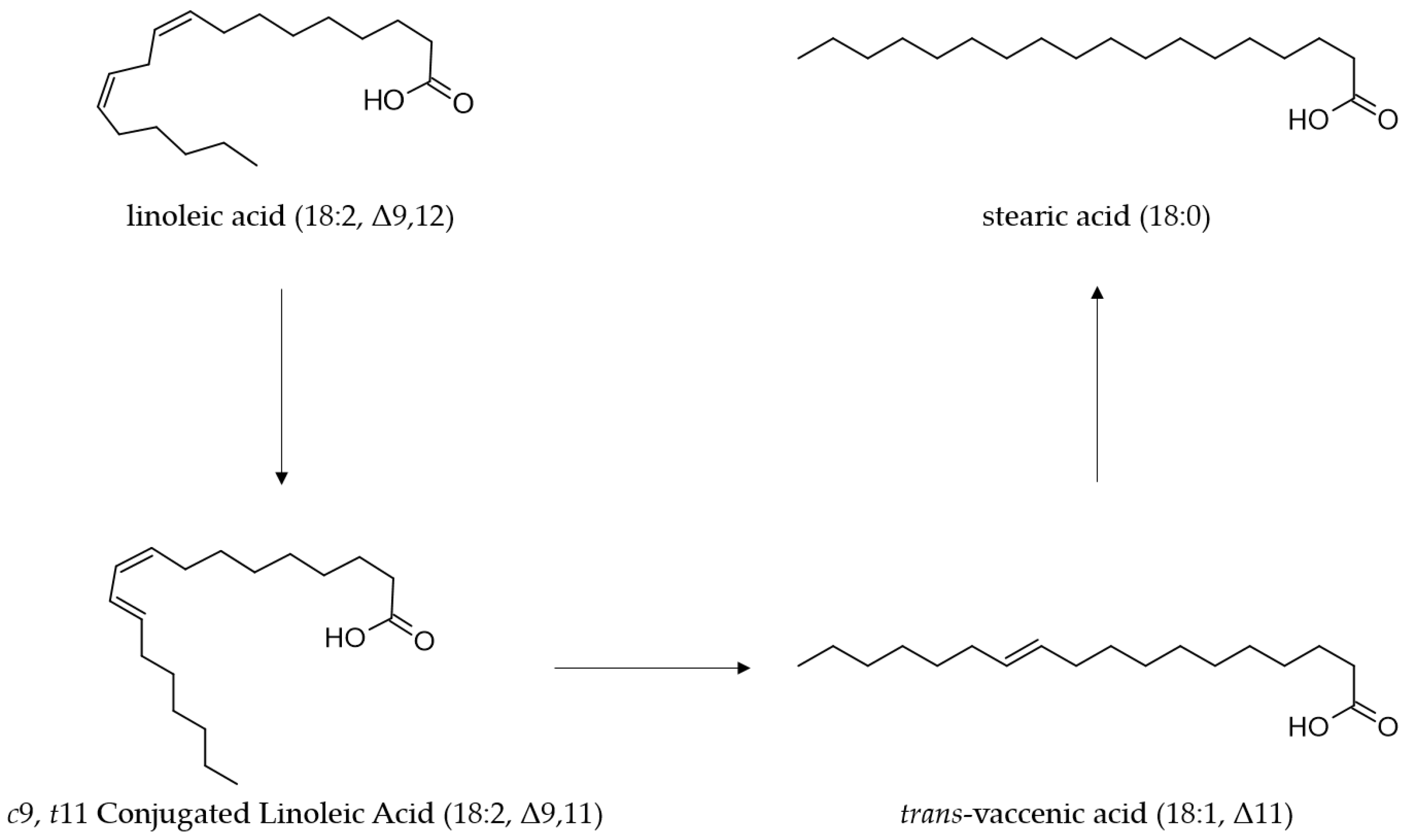
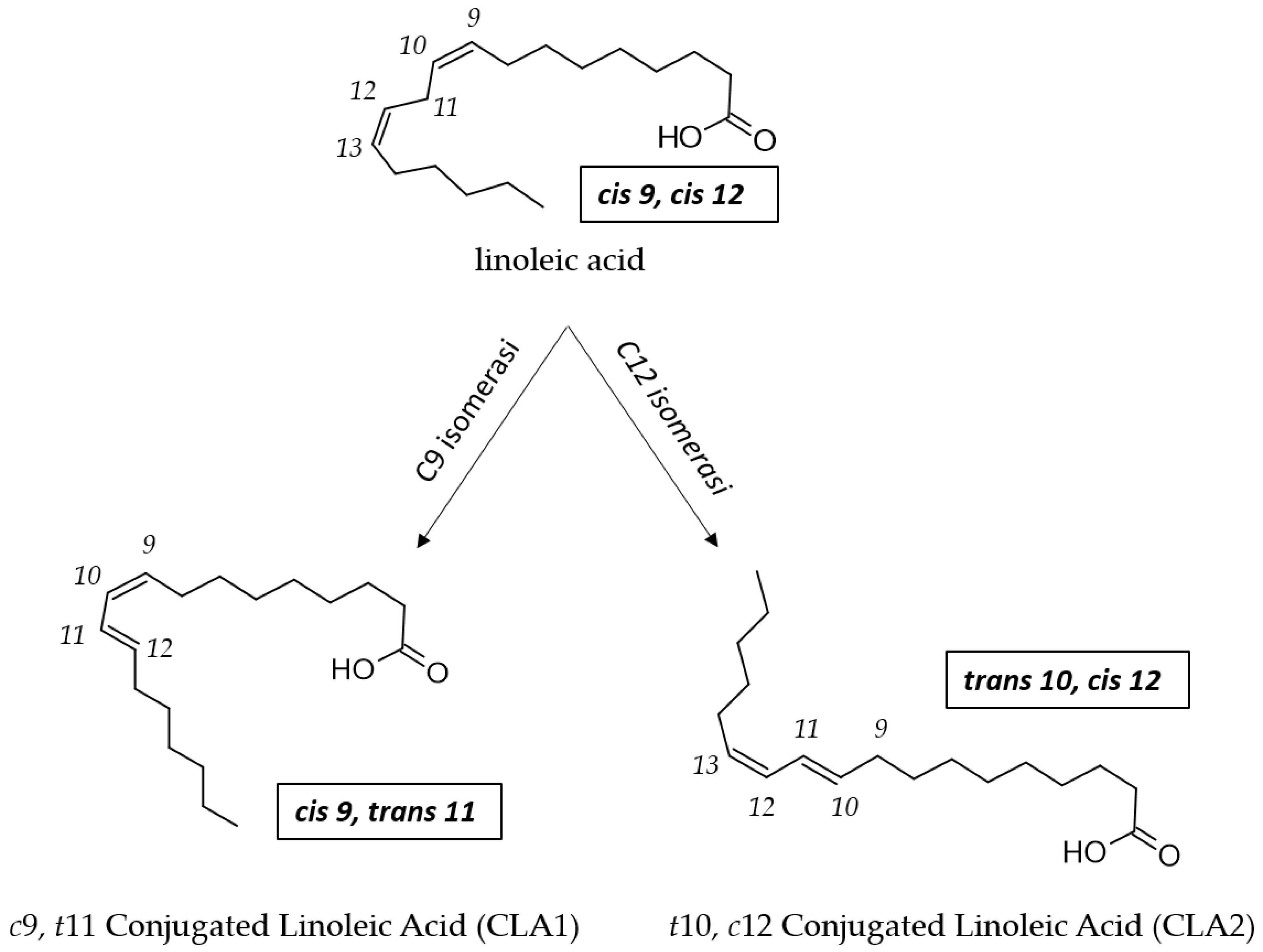
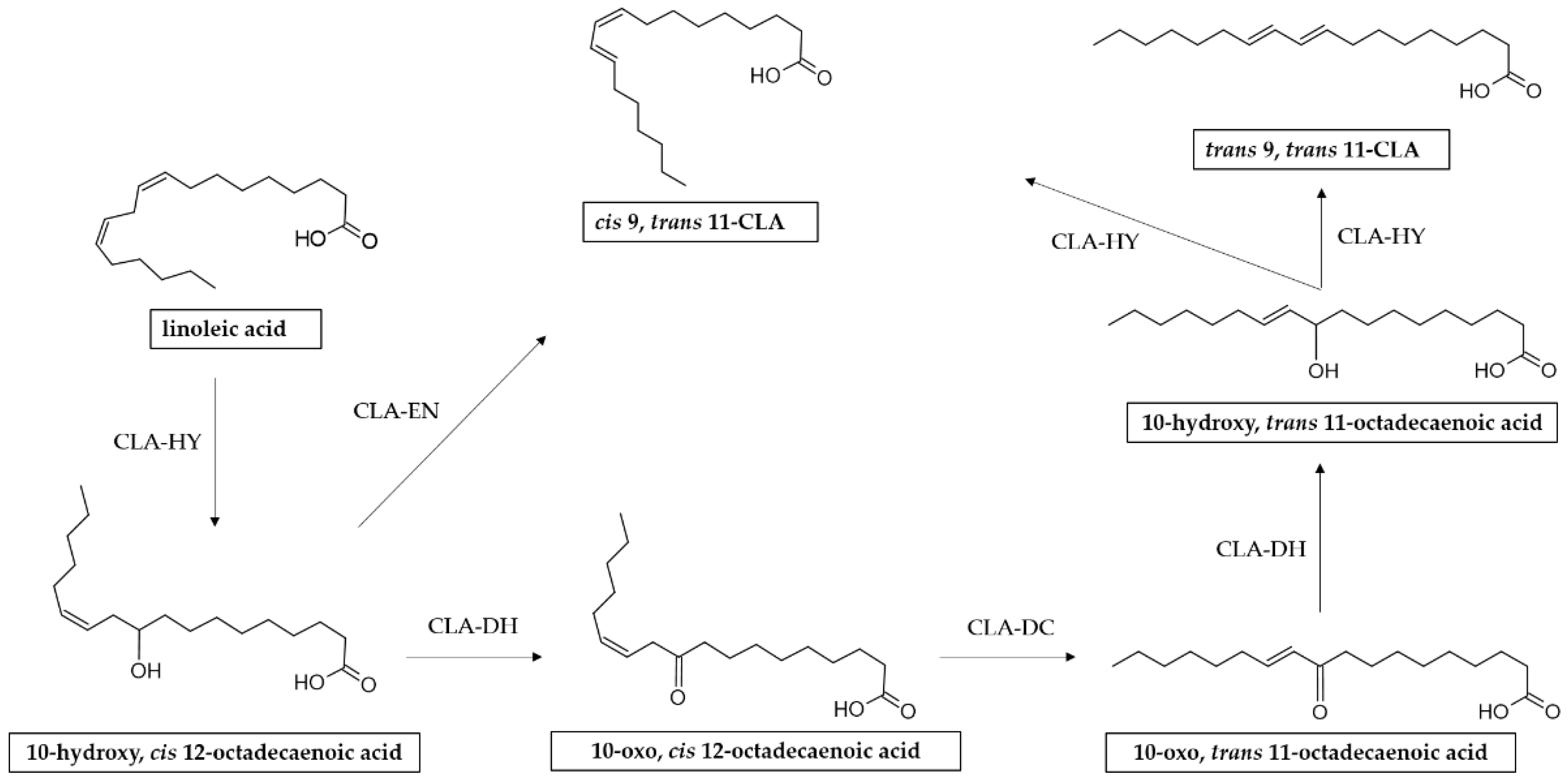
3. Molecular Mechanism of CLA Synthesis by L. plantarum
4. In vitro CLA Production by L. plantarum
5. CLA Production from Vegetable Oils by L. plantarum
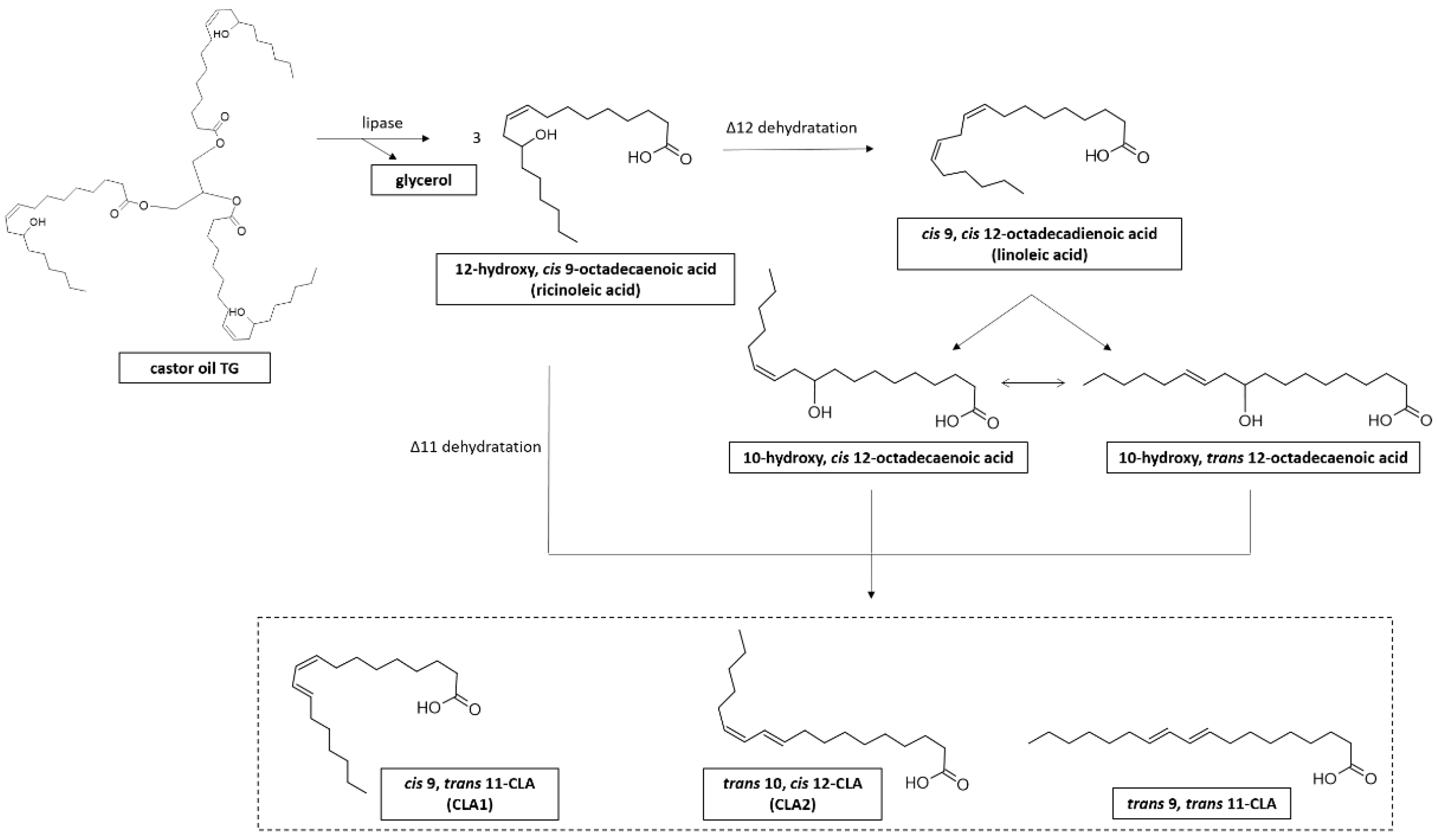
6. CLA-Producing L. plantarum Strains in Fermented Food
7. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kim, J.H.; Kim, Y.; Kim, Y.J.; Park, Y. Conjugated linoleic acid: potential health benefits as a functional food ingredient. Annu. Rev. Food Sci. Technol. 2016, 7, 221–244. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Meng, X.; Tong, P.; Liu, X. Biosynthesis of c9,t11-conjugated linoleic acid and the effect on characteristics in fermented soy milk. Food Chem. 2022, 368, 130866. [Google Scholar] [CrossRef]
- de Carvalho, E.B.T.; de Melo, I.L.P.; Mancini-Filho, J. Chemical and physiological aspects of isomers of conjugated fatty acids. Cienc. e Tecnol. Aliment. 2010, 30, 295–307. [Google Scholar] [CrossRef]
- Salsinha, A.S.; Machado, M.; Rodríguez-Alcalá, L.M.; Gomes, A.M.; Pintado, M. Bioactive lipids: Chemistry, biochemistry, and biological properties. In Bioactive Lipids; 2022; pp. 1–35. ISBN 9780128240434. [Google Scholar]
- Niezgoda, N.; Gliszczyńska, A.; Gładkowski, W.; Chojnacka, A.; Kiełbowicz, G.; Wawrzeńczyk, C. Production of concentrates of CLA obtained from sunflower and safflower and their application to the lipase-catalyzed acidolysis of egg yolk phosphatidylcholine. Eur. J. Lipid Sci. Technol. 2016, 118, 1566–1578. [Google Scholar] [CrossRef]
- Choi, B.-D.; Kang, S.-J.; Ha, Y.-L.; Ackman, R.G. Accumulation of conjugated linoleic acid (CLA) in tissues of fish fed diets containing various levels of CLA. In Quality Attributes of Muscle Foods; Springer US: Boston, MA, 1999; pp. 61–71. [Google Scholar]
- Dachev, M.; Bryndová, J.; Jakubek, M.; Moučka, Z.; Urban, M. The effects of conjugated linoleic acids on cancer. Processes 2021, 9, 454. [Google Scholar] [CrossRef]
- Malinska, H.; Hüttl, M.; Oliyarnyk, O.; Bratova, M.; Kazdova, L. Conjugated linoleic acid reduces visceral and ectopic lipid accumulation and insulin resistance in chronic severe hypertriacylglycerolemia. Nutrition 2015, 31, 1045–1051. [Google Scholar] [CrossRef]
- Stachowska, E.; Siennicka, A.; Baśkiewcz-Hałasa, M.; Bober, J.; Machalinski, B.; Chlubek, D. Conjugated linoleic acid isomers may diminish human macrophages adhesion to endothelial surface. Int. J. Food Sci. Nutr. 2012, 63, 30–35. [Google Scholar] [CrossRef] [PubMed]
- DeGuire, J.R.; Makarem, N.; Vanstone, C.A.; Morin, S.; Duque, G.; Weiler, H.A. Conjugated linoleic acid is related to bone mineral density but does not affect parathyroid hormone in men. Nutr. Res. 2012, 32, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Lehnen, T.E.; da Silva, M.R.; Camacho, A.; Marcadenti, A.; Lehnen, A.M. A review on effects of conjugated linoleic fatty acid (CLA) upon body composition and energetic metabolism. J. Int. Soc. Sports Nutr. 2015, 12. [Google Scholar] [CrossRef] [PubMed]
- Derakhshande-Rishehri, S.-M.; Mansourian, M.; Kelishadi, R.; Heidari-Beni, M. Association of foods enriched in conjugated linoleic acid (CLA) and CLA supplements with lipid profile in human studies: a systematic review and meta-analysis. Public Health Nutr. 2015, 18, 2041–2054. [Google Scholar] [CrossRef] [PubMed]
- Viladomiu, M.; Hontecillas, R.; Bassaganya-Riera, J. Modulation of inflammation and immunity by dietary conjugated linoleic acid. Eur. J. Pharmacol. 2016, 785, 87–95. [Google Scholar] [CrossRef]
- Benjamin, S.; Prakasan, P.; Sreedharan, S.; Wright, A.-D.G.; Spener, F. Pros and cons of CLA consumption: an insight from clinical evidences. Nutr. Metab. 2015, 12. [Google Scholar] [CrossRef]
- Rastgoo, S.; Shimi, G.; Shiraseb, F.; Karbasi, A.; Ashtary-Larky, D.; Yousefi, M.; Golalipour, E.; Asbaghi, O.; Zamani, M. The effects of conjugated linoleic acid supplementation on inflammatory cytokines and adipokines in adults: a GRADE-assessed systematic review and dose–response meta-analysis. Front. Immunol. 2023, 14. [Google Scholar] [CrossRef]
- Asbaghi, O.; Shimi, G.; Hosseini Oskouie, F.; Naseri, K.; Bagheri, R.; Ashtary-Larky, D.; Nordvall, M.; Rastgoo, S.; Zamani, M.; Wong, A. The effects of conjugated linoleic acid supplementation on anthropometrics and body composition indices in adults: A systematic review and dose-response meta-analysis. Br. J. Nutr. 2023. [Google Scholar] [CrossRef]
- Basak, S.; Duttaroy, A.K. Conjugated linoleic acid and its beneficial effects in obesity, cardiovascular disease, and cancer. Nutrients 2020, 12, 1913. [Google Scholar] [CrossRef]
- Hajihashemi, P.; Feizi, A.; Heidari, Z.; Haghighatdoost, F. Association of omega-6 polyunsaturated fatty acids with blood pressure: A systematic review and meta-analysis of observational studies. Crit. Rev. Food Sci. Nutr. 2023, 63, 2247–2259. [Google Scholar] [CrossRef] [PubMed]
- Badawy, S.; Liu, Y.; Guo, M.; Liu, Z.; Xie, C.; Marawan, M.A.; Ares, I.; Lopez-Torres, B.; Martínez, M.; Maximiliano, J.-E.; et al. Conjugated linoleic acid (CLA) as a functional food: is it beneficial or not? Food Res. Int. 2023, 172, 113158. [Google Scholar] [CrossRef] [PubMed]
- den Hartigh, L.J. Conjugated linoleic acid effects on cancer, obesity, and atherosclerosis: A review of pre-clinical and human trials with current perspectives. Nutrients 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Yuce, M.; Gumuskaptan, C.; Con, A.H.; Yazici, F. Conjugated linoleic acid strengthens the apoptotic effect of cisplatin in A549 cells. Prostaglandins Other Lipid Mediat. 2023, 166. [Google Scholar] [CrossRef] [PubMed]
- Słowikowski, B.K.; Drzewiecka, H.; Malesza, M.; Mądry, I.; Sterzyńska, K.; Jagodziński, P.P. The influence of conjugated linoleic acid on the expression of peroxisome proliferator-activated receptor-γ and selected apoptotic genes in non-small cell lung cancer. Mol. Cell. Biochem. 2020, 466, 65–82. [Google Scholar] [CrossRef]
- Maki, K.C.; Eren, F.; Cassens, M.E.; Dicklin, M.R.; Davidson, M.H. ω-6 polyunsaturated fatty acids and cardiometabolic health: current evidence, controversies, and research gaps. Adv. Nutr. 2018, 9, 688–700. [Google Scholar] [CrossRef]
- Sellem, L.; Flourakis, M.; Jackson, K.G.; Joris, P.J.; Lumley, J.; Lohner, S.; Mensink, R.P.; Soedamah-Muthu, S.S.; Lovegrove, J.A. Impact of replacement of individual dietary SFAs on circulating lipids and other biomarkers of cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials in humans. Adv. Nutr. 2022, 13, 1200–1225. [Google Scholar] [CrossRef]
- Kim, H.K.; Kang, E.Y.; Go, G. Recent insights into dietary ω-6 fatty acid health implications using a systematic review. Food Sci. Biotechnol. 2022, 31, 1365–1376. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Hooper, L.; Martin, N.; Jimoh, O.F.; Kirk, C.; Foster, E.; Abdelhamid, A.S. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst. Rev. 2020, 2020. [Google Scholar] [CrossRef]
- Scientific Advisory Committee on Nutrition (SACN) satured fats and health. 2019, 443.
- MacDonald, H.B. Conjugated linoleic acid and disease prevention: a review of current knowledge. J. Am. Coll. Nutr. 2000, 19, 111S–118S. [Google Scholar] [CrossRef]
- Ritzenthaler, K.L.; McGuire, M.K.; Falen, R.; Shultz, T.D.; Dasgupta, N.; McGuire, M.A. Estimation of conjugated linoleic acid intake by written dietary assessment methodologies underestimates actual intake evaluated by food duplicate methodology. J. Nutr. 2001, 131, 1548–1554. [Google Scholar] [CrossRef]
- Gorissen, L.; Leroy, F.; De Vuyst, L.; De Smet, S.; Raes, K. Bacterial production of conjugated linoleic and linolenic acid in foods: a technological challenge. Crit. Rev. Food Sci. Nutr. 2015, 55, 1561–1574. [Google Scholar] [CrossRef] [PubMed]
- Nunes, J.C.; Torres, A.G. Fatty acid and CLA composition of Brazilian dairy products, and contribution to daily intake of CLA. J. Food Compos. Anal. 2010, 23, 782–789. [Google Scholar] [CrossRef]
- Wu, C.; Chen, H.; Mei, Y.; Yang, B.; Zhao, J.; Stanton, C.; Chen, W. Advances in research on microbial conjugated linoleic acid bioconversion. Prog. Lipid Res. 2024, 93, 101257. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.B.; Gao, H.H.; Stanton, C.; Ross, R.P.P.; Zhang, H.; Chen, Y.Q.Y.Q.; Chen, H.; Chen, W. Bacterial conjugated linoleic acid production and their applications. Prog. Lipid Res. 2017, 68, 26–36. [Google Scholar] [CrossRef]
- Gong, M.; Hu, Y.; Wei, W.; Jin, Q.; Wang, X. Production of conjugated fatty acids: a review of recent advances. Biotechnol. Adv. 2019, 37. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, T.R.; Nam, S.-H.; Ure, A.L. Factors affecting conjugated linoleic acid content in milk and meat. Crit. Rev. Food Sci. Nutr. 2005, 45, 463–482. [Google Scholar] [CrossRef] [PubMed]
- Siurana, A.; Calsamiglia, S. A metaanalysis of feeding strategies to increase the content of conjugated linoleic acid (CLA) in dairy cattle milk and the impact on daily human consumption. Anim. Feed Sci. Technol. 2016, 217, 13–26. [Google Scholar] [CrossRef]
- Salamon, R.; Vargáné-Visi, É.; András, C.D.; Csapóné Kiss, Z.; Csapó, J. Synthetic methods to obtain conjugated linoleic acids (CLAs) by catalysis – A review. Acta Aliment. 2015, 44, 229–234. [Google Scholar] [CrossRef]
- Kuhl, G.; De Dea Lindner, J. Biohydrogenation of linoleic acid by lactic acid bacteria for the production of functional cultured dairy products: a review. Foods 2016, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, A.; Mollaei Tavani, S.; Arjeh, E.; Jafari, S.M.S.M. Production of conjugated linoleic acid by lactic acid bacteria; important factors and optimum conditions. Food Chem. X 2023, 20, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Terán, V.; Pizarro, P.L.; Zacarías, M.F.; Vinderola, G.; Medina, R.; Van Nieuwenhove, C. Production of conjugated dienoic and trienoic fatty acids by lactic acid bacteria and bifidobacteria. J. Funct. Foods 2015, 19, 417–425. [Google Scholar] [CrossRef]
- Kishino, S.; Ogawa, J.; Omura, Y.; Matsumura, K.; Shimizu, S. Conjugated linoleic acid production from linoleic acid by lactic acid bacteria. J. Am. Oil Chem. Soc. 2002, 79, 159–163. [Google Scholar] [CrossRef]
- Khan, A.; Nadeem, M.; Al-Asmari, F.; Imran, M.; Ambreen, S.; Rahim, M.A.; Oranab, S.; Esatbeyoglu, T.; Bartkiene, E.; Rocha, J.M. Effect of Lactiplantibacillus plantarum on the conversion of linoleic acid of vegetable oil to conjugated linoleic acid, lipolysis, and sensory properties of cheddar cheese. Microorganisms 2023, 11, 2613. [Google Scholar] [CrossRef]
- Filannino, P.; De Angelis, M.; Di Cagno, R.; Gozzi, G.; Riciputi, Y.; Gobbetti, M. How Lactobacillus plantarum shapes its transcriptome in response to contrasting habitats. Environ. Microbiol. 2018, 20, 3700–3716. [Google Scholar] [CrossRef]
- Siezen, R.J.; van Hylckama Vlieg, J.E.T. Genomic diversity and versatility of Lactobacillus plantarum, a natural metabolic engineer. Microb. Cell Fact. 2011, 10. [Google Scholar] [CrossRef]
- Testa, B.; Lombardi, S.J.; Tremonte, P.; Succi, M.; Tipaldi, L.; Pannella, G.; Sorrentino, E.; Iorizzo, M.; Coppola, R. Biodiversity of Lactobacillus plantarum from traditional Italian wines. World J. Microbiol. Biotechnol. 2014, 30, 2299–2305. [Google Scholar] [CrossRef]
- Iorizzo, M.; Albanese, G.; Testa, B.; Ianiro, M.; Letizia, F.; Succi, M.; Tremonte, P.; D’andrea, M.; Iaffaldano, N.; Coppola, R. Presence of lactic acid bacteria in the intestinal tract of the mediterranean trout (Salmo macrostigma) in its natural environment. Life 2021, 11. [Google Scholar] [CrossRef]
- Iorizzo, M.; Pannella, G.; Lombardi, S.J.; Ganassi, S.; Testa, B.; Succi, M.; Sorrentino, E.; Petrarca, S.; De Cristofaro, A.; Coppola, R.; et al. Inter-and intra-species diversity of lactic acid bacteria in Apis mellifera ligustica colonies. Microorganisms 2020, 8, 1–17. [Google Scholar] [CrossRef]
- Fidanza, M.; Panigrahi, P.; Kollmann, T.R. Lactiplantibacillus plantarum–nomad and ideal probiotic. Front. Microbiol. 2021, 12. [Google Scholar] [CrossRef]
- Letizia, F.; Albanese, G.; Testa, B.; Vergalito, F.; Bagnoli, D.; Di Martino, C.; Carillo, P.; Verrillo, L.; Succi, M.; Sorrentino, E.; et al. In Vitro assessment of bio-functional properties from Lactiplantibacillus plantarum strains. Curr. Issues Mol. Biol. 2022, 44, 2321–2334. [Google Scholar] [CrossRef]
- Iorizzo, M.; Testa, B.; Ganassi, S.; Lombardi, S.J.; Ianiro, M.; Letizia, F.; Succi, M.; Tremonte, P.; Vergalito, F.; Cozzolino, A.; et al. Probiotic properties and potentiality of Lactiplantibacillus plantarum strains for the biological control of chalkbrood disease. J. Fungi 2021, 7. [Google Scholar] [CrossRef] [PubMed]
- Iorizzo, M.; Albanese, G.; Letizia, F.; Testa, B.; Tremonte, P.; Vergalito, F.; Lombardi, S.J.; Succi, M.; Coppola, R.; Sorrentino, E. Probiotic potentiality from versatile Lactiplantibacillus plantarum strains as resource to enhance freshwater fish health. Microorganisms 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gonzalez, N.; Battista, N.; Prete, R.; Corsetti, A. Health-promoting role of Lactiplantibacillus plantarum isolated from fermented foods. Microorganisms 2021, 9, 349. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gonzalez, N.; Comas, J.C.; Harris, H.M.B.; Strain, C.; Stanton, C.; Hill, C.; Corsetti, A.; Gahan, C.G.M. Impact of food origin Lactiplantibacillus plantarum strains on the human intestinal microbiota in an in vitro system. Front. Microbiol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Iorizzo, M.; Ganassi, S.; Albanese, G.; Letizia, F.; Testa, B.; Tedino, C.; Petrarca, S.; Mutinelli, F.; Mazzeo, A.; De Cristofaro, A. Antimicrobial activity from putative probiotic lactic acid bacteria for the biological control of american and european foulbrood diseases. Vet. Sci. 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Iorizzo, M.; Testa, B.; Lombardi, S.J.; Ganassi, S.; Ianiro, M.; Letizia, F.; Succi, M.; Tremonte, P.; Vergalito, F.; Cozzolino, A.; et al. Antimicrobial activity against Paenibacillus larvae and functional properties of Lactiplantibacillus plantarum strains: potential benefits for honeybee health. Antibiotics 2020, 9, 1–18. [Google Scholar] [CrossRef]
- Behera, S.S.S.S.; Ray, R.C.R.C.R.C.; Zdolec, N. Lactobacillus plantarum with functional properties: an approach to increase safety and shelf-life of fermented foods. Biomed Res. Int. 2018, 2018, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Bangar, S.P.S.P.; Echegaray, N.; Suri, S.; Tomasevic, I.; Lorenzo, J.M.J.M.; Melekoglu, E.; Rocha, J.M.J.M.; Ozogul, F.; Manuel Lorenzo, J.; et al. The impacts of Lactiplantibacillus plantarum on the functional properties of fermented foods: a review of current knowledge. Microorganisms 2022, 10, 826. [Google Scholar] [CrossRef] [PubMed]
- De Leonardis, A.; Testa, B.; Macciola, V.; Lombardi, S.J.; Iorizzo, M. Exploring enzyme and microbial technology for the preparation of green table olives. Eur. Food Res. Technol. 2016, 242, 363–370. [Google Scholar] [CrossRef]
- Lombardi, S.J.; Pannella, G.; Iorizzo, M.; Testa, B.; Succi, M.; Tremonte, P.; Sorrentino, E.; Di Renzo, M.; Strollo, D.; Coppola, R. Inoculum strategies and performances of malolactic starter Lactobacillus plantarum M10: impact on chemical and sensorial characteristics of fiano wine. Microorganisms 2020, 8. [Google Scholar] [CrossRef]
- Arena, M.; Caggianiello, G.; Russo, P.; Albenzio, M.; Massa, S.; Fiocco, D.; Capozzi, V.; Spano, G. Functional starters for functional yogurt. Foods 2015, 4, 15–33. [Google Scholar] [CrossRef]
- Seddik, H.A.; Bendali, F.; Gancel, F.; Fliss, I.; Spano, G.; Drider, D. Lactobacillus plantarum and its probiotic and food potentialities. Probiotics Antimicrob. Proteins 2017, 9, 111–122. [Google Scholar] [CrossRef]
- Russo, P.; de Chiara, M.L.V.; Vernile, A.; Amodio, M.L.; Arena, M.P.; Capozzi, V.; Massa, S.; Spano, G. fresh-cut pineapple as a new carrier of probiotic lactic acid bacteria. Biomed Res. Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Iorizzo, M.; Paventi, G.; Di Martino, C. Biosynthesis of gamma-aminobutyric acid (GABA) by Lactiplantibacillus plantarum in fermented food production. Curr. Issues Mol. Biol. 2023, 46, 200–220. [Google Scholar] [CrossRef]
- Iorizzo, M.; Testa, B.; Lombardi, S.J.; García-Ruiz, A.; Muñoz-González, C.; Bartolomé, B.; Moreno-Arribas, M.V. Selection and technological potential of Lactobacillus plantarum bacteria suitable for wine malolactic fermentation and grape aroma release. LWT 2016, 73, 557–566. [Google Scholar] [CrossRef]
- Ferlay, A.; Bernard, L.; Meynadier, A.; Malpuech-Brugère, C. Production of trans and conjugated fatty acids in dairy ruminants and their putative effects on human health: A review. Biochimie 2017, 141, 107–120. [Google Scholar] [CrossRef]
- Zhang, L.; Shen, H.; Zhang, J.; Mao, S. Variety of rumen microbial populations involved in biohydrogenation related to individual milk fat percentage of dairy cows. Front. Vet. Sci. 2023, 10. [Google Scholar] [CrossRef]
- Klieve, A.V.; Hennessy, D.; Ouwerkerk, D.; Forster, R.J.; Mackie, R.I.; Attwood, G.T. Establishing populations of Megasphaera elsdenii YE 34 and Butyrivibrio fibrisolvens YE 44 in the rumen of cattle fed high grain diets. J. Appl. Microbiol. 2003, 95, 621–630. [Google Scholar] [CrossRef]
- Liavonchanka, A.; Feussner, I. Biochemistry of PUFA double bond isomerases producing conjugated linoleic acid. ChemBioChem 2008, 9, 1867–1872. [Google Scholar] [CrossRef] [PubMed]
- Or-Rashid, M.M.; Wright, T.C.; McBride, B.W. Microbial fatty acid conversion within the rumen and the subsequent utilization of these fatty acids to improve the healthfulness of ruminant food products. Appl. Microbiol. Biotechnol. 2009, 84, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Lashkari, S.; Bonefeld Petersen, M.; Krogh Jensen, S. Rumen biohydrogenation of linoleic and linolenic acids is reduced when esterified to phospholipids or steroids. Food Sci. Nutr. 2020, 8, 79–87. [Google Scholar] [CrossRef]
- Maia, M.R.G.; Chaudhary, L.C.; Figueres, L.; Wallace, R.J. Metabolism of polyunsaturated fatty acids and their toxicity to the microflora of the rumen. Antonie Van Leeuwenhoek 2007, 91, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Salsinha, A.S.; Pimentel, L.L.; Fontes, A.L.; Gomes, A.M.; Rodríguez-Alcalá, L.M. Microbial production of conjugated linoleic acid and conjugated linolenic acid relies on a multienzymatic system. Microbiol. Mol. Biol. Rev. 2018, 82. [Google Scholar] [CrossRef]
- Wang, K.; Xin, Z.; Chen, Z.; Li, H.; Wang, D.; Yuan, Y. Progress of conjugated linoleic acid on milk fat metabolism in ruminants and humans. Animals 2023, 13, 3429. [Google Scholar] [CrossRef] [PubMed]
- Song, N.-E.N.-E.N.-E.E.; Kim, N.-J.J.N.-J.; Kim, Y.-H.Y.-H.H.; Baik, S.-H.H.S.-H. Probiotic properties of lactic acid bacteria with high conjugated linoleic acid converting activity isolated from jeot-gal, high-salt fermented seafood. Microorganisms 2021, 9, 2247. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Kumar, S.; Choudhury, P.K.; Tyagi, B.; Tyagi, N. Conjugated linoleic acid producing potential of lactobacilli isolated from goat (AXB) rumen fluid samples. Asian-Australasian J. Anim. Sci. 2020, 33, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Tapia, A.M.; Bautista, J.A.N.; Mendoza, B.C.; Pham, L.J.; Sarmago, I.G.; Oliveros, M.C.R. Production of conjugated linoleic acid by lactic acid bacteria: screening and optimization. Philipp. J. Sci. 2019, 148, 457–464. [Google Scholar]
- Hosseini, E.S.; Kermanshahi, R.K.; Hosseinkhani, S.; Shojaosadati, S.A.; Nazari, M. Conjugated linoleic acid production from various substrates by probiotic Lactobacillus plantarum. Ann. Microbiol. 2015, 65, 27–32. [Google Scholar] [CrossRef]
- Senizza, A.; Rocchetti, G.; Callegari, M.L.; Lucini, L.; Morelli, L. Linoleic acid induces metabolic stress in the intestinal microorganism Bifidobacterium breve DSM 20213. Sci. Rep. 2020, 10, 5997. [Google Scholar] [CrossRef]
- Maia, M.R.; Chaudhary, L.C.; Bestwick, C.S.; Richardson, A.J.; McKain, N.; Larson, T.R.; Graham, I.A.; Wallace, R.J. Toxicity of unsaturated fatty acids to the biohydrogenating ruminal bacterium, Butyrivibrio fibrisolvens. BMC Microbiol. 2010, 10, 52. [Google Scholar] [CrossRef]
- Kim, Y.J.; Liu, R.H. Increase of conjugated linoleic acid content in milk by fermentation with lactic acid bacteria. J. Food Sci. 2002, 67, 1731–1737. [Google Scholar] [CrossRef]
- Andrade, J.C.; Ascencao, K.; Gullòn, P.; Henriques, S.M.S.; Pinto, J.M.S.; Rocha-Santos, T.A.P.; Freitas, A.C.; Gomes, A.M. Production of conjugated linoleic acid by food-grade bacteria: A review. Int. J. Dairy Technol. 2012, 65, 467–481. [Google Scholar] [CrossRef]
- Liavonchanka, A.; Hornung, E.; Feussner, I.; Rudolph, M.G. Structure and mechanism of the Propionibacterium acnes polyunsaturated fatty acid isomerase. Proc. Natl. Acad. Sci. 2006, 103, 2576–2581. [Google Scholar] [CrossRef]
- Fontes, A.L.; Pimentel, L.L.; Soares, A.M.S.; Domingues, M.D.R.; Rodríguez-Alcalá, L.M.; Gomes, A.M. Study of the viability of using lipase-hydrolyzed commercial vegetable oils to produce microbially conjugated linolenic acid-enriched milk. Food Chem. 2023, 413. [Google Scholar] [CrossRef]
- Aziz, T.; Sarwar, A.; Naveed, M.; Shahzad, M.; Aqib Shabbir, M.; Dablool, A.S.; ud Din, J.; Ali Khan, A.; Naz, S.; Cui, H.; et al. Bio-Molecular analysis of selected food derived Lactiplantibacillus strains for CLA production reveals possibly a complex mechanism. Food Res. Int. 2022, 154, 111031. [Google Scholar] [CrossRef]
- Kishino, S.; Ogawa, J.; Yokozeki, K.; Shimizu, S. Linoleic acid isomerase in Lactobacillus plantarum AKU1009a proved to be a multi-component enzyme system requiring oxidoreduction cofactors. Biosci. Biotechnol. Biochem. 2011, 75, 318–22. [Google Scholar] [CrossRef]
- Yang, B.; Qi, H.; Gu, Z.; Zhang, H.; Chen, W.; Chen, H.; Chen, Y.Q. Characterization of the triple-component linoleic acid isomerase in Lactobacillus plantarum ZS2058 by genetic manipulation. J. Appl. Microbiol. 2017, 123, 1263–1273. [Google Scholar] [CrossRef]
- Takeuchi, M.; Kishino, S.; Park, S.-B.; Hirata, A.; Kitamura, N.; Saika, A.; Ogawa, J. Efficient enzymatic production of hydroxy fatty acids by linoleic acid Δ9 hydratase from Lactobacillus plantarum AKU 1009a. J. Appl. Microbiol. 2016, 120, 1282–1288. [Google Scholar] [CrossRef]
- Yang, B.; Chen, H.; Gao, H.; Ren, Q.; Zhang, H.; Chen, W. Genetic determinates for conjugated linolenic acid production in Lactobacillus plantarum ZS2058. J. Appl. Microbiol. 2020, 128, 191–201. [Google Scholar] [CrossRef]
- Yang, B.; Chen, H.; Tian, F.; Zhao, J.; Gu, Z.; Zhang, H.; Chen, Y.Q.; Chen, W. Complete genome sequence of Lactobacillus plantarum ZS2058, a probiotic strain with high conjugated linoleic acid production ability. J. Biotechnol. 2015, 214, 212–213. [Google Scholar] [CrossRef]
- Liu, X.-X.; Zhang, H.-Y.; Song, X.; Yang, Y.; Xiong, Z.-Q.; Xia, Y.-J.; Ai, L.-Z. Reasons for the differences in biotransformation of conjugated linoleic acid by Lactobacillus plantarum. J. Dairy Sci. 2021, 104, 11466–11473. [Google Scholar] [CrossRef]
- Liu, X.-X.; Liu, L.; Song, X.; Wang, G.-Q.; Xiong, Z.-Q.; Xia, Y.-J.; Ai, L.-Z. The arginine repressor ArgR2 controls conjugated linoleic acid biosynthesis by activating the cla operon in Lactiplantibacillus plantarum. Microbiol. Spectr. 2022, 10. [Google Scholar]
- Maddocks, S.E.; Oyston, P.C.F. Structure and function of the LysR-type transcriptional regulator (LTTR) family proteins. Microbiology 2008, 154, 3609–3623. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Anaya, J.; Hernández-Santoyo, A. Production of bioactive conjugated linoleic acid by the multifunctional enolase from Lactobacillus plantarum. Int. J. Biol. Macromol. 2016, 91, 524–535. [Google Scholar] [CrossRef]
- Dahiya, D.K.; Puniya, A.K. Isolation, molecular characterization and screening of indigenous lactobacilli for their abilities to produce bioactive conjugated linoleic acid (CLA). J. Food Sci. Technol. 2017, 54, 792–801. [Google Scholar] [CrossRef]
- Özer, C.O.; Kılıç, B. Optimization of pH, time, temperature, variety and concentration of the added fatty acid and the initial count of added lactic acid Bacteria strains to improve microbial conjugated linoleic acid production in fermented ground beef. Meat Sci. 2021, 171. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.W.; Lv, J.P.; Li, S.R. Production of conjugated linoleic acid by whole-cell of Lactobacillus plantarum A6-1F. Biotechnol. Biotechnol. Equip. 2011, 25, 2266–2272. [Google Scholar] [CrossRef]
- Özer, C.O.; Kılıç, B. Conjugated linoleic acid production by L. plantarum AB20-961 and L. plantarum DSM2601 in fermented sausage. In Proceedings of the 63rd International Congress of Meat Science and Technology; Troy, D., McDonnell, C., Hinds, L., Kerry, J., Eds.; 2017; Vol. 0, pp. 566–567. [Google Scholar]
- Özer, C.O.; Kılıç, B.; Kılıç, G.B. In-vitro microbial production of conjugated linoleic acid by probiotic L. plantarum strains: utilization as a functional starter culture in sucuk fermentation. Meat Sci. 2016, 114, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Kishino, S.; Ogawa, J.; Ando, A.; Omura, Y.; Shimizu, S. Ricinoleic acid and castor oil as substrates for conjugated linoleic acid production by washed cells of Lactobacillus plantarum. Biosci. Biotechnol. Biochem. 2002, 66, 2283–2286. [Google Scholar] [CrossRef]
- Devi, P.U.M.; Rashmi, H.K. Effect of physico-chemical factors on the production of conjugated linoleic acid by Lactobacillus plantarum. Asian J. Microbiol. Biotechnol. Environ. Sci. 2017, 19, 412–418. [Google Scholar]
- Chen, D.-J.; Yan, L.-H.; Li, Q.; Zhang, C.; Si, C.-L.; Li, Z.-Y.; Song, Y.-J.; Zhou, H.; Zhang, T.-C.; Luo, X.-G. Bioconversion of conjugated linoleic acid by Lactobacillus plantarum CGMCC8198 supplemented with Acer truncatum bunge seeds oil. Food Sci. Biotechnol. 2017, 26, 1595–1611. [Google Scholar] [CrossRef]
- Khosravi, A.; Safari, M.; Khodaiyan, F.; Gharibzahedi, S.M.T.S.M.T. Bioconversion enhancement of conjugated linoleic acid by Lactobacillus plantarum using the culture media manipulation and numerical optimization. J. Food Sci. Technol. 2015, 52, 5781–5789. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Bao, Y.; Liu, X.; Zhang, H. Conjugated linoleic acid conversion by six Lactobacillus plantarum strains cultured in mrs broth supplemented with sunflower oil and soymilk. J. Food Sci. 2012, 77. [Google Scholar] [CrossRef]
- Sosa-Castañeda, J.; Hernández-Mendoza, A.; Astiazarán-García, H.; Garcia, H.S.; Estrada-Montoya, M.C.; González-Córdova, A.F.; Vallejo-Cordoba, B. Screening of Lactobacillus strains for their ability to produce conjugated linoleic acid in milk and to adhere to the intestinal tract. J. Dairy Sci. 2015, 98, 6651–6659. [Google Scholar] [CrossRef]
- Ando, A.; Ogawa, J.; Kishino, S.; Shimizu, S. Conjugated linoleic acid production from castor oil by Lactobacillus plantarum JCM 1551. Enzyme Microb. Technol. 2004, 35, 40–45. [Google Scholar] [CrossRef]
- Ando, A.; Ogawa, J.; Kishino, S.; Shimizu, S. CLA production from ricinoleic acid by lactic acid bacteria. JAOCS, J. Am. Oil Chem. Soc. 2003, 80, 889–894. [Google Scholar] [CrossRef]
- Ares-Yebra, A.; Garabal, J.I.; Carballo, J.; Centeno, J.A. Formation of conjugated linoleic acid by a Lactobacillus plantarum strain isolated from an artisanal cheese: evaluation in miniature cheeses. Int. Dairy J. 2019, 90, 98–103. [Google Scholar] [CrossRef]
- Ribeiro, S.C.; Stanton, C.; Yang, B.; Ross, R.P.; Silva, C.C.G. Conjugated linoleic acid production and probiotic assessment of Lactobacillus plantarum isolated from Pico cheese. LWT 2018, 90, 403–411. [Google Scholar] [CrossRef]
- Liu, P.; Shen, S.; Ruan, H.; Zhou, Q.; Ma, L.; He, G. Production of conjugated linoleic acids by Lactobacillus plantarum strains isolated from naturally fermented Chinese pickles. J. Zhejiang Univ. Sci. B 2011, 12, 923–930. [Google Scholar] [CrossRef]
- Ye, S.; Yu, T.; Yang, H.; Li, L.; Wang, H.; Xiao, S.; Wang, J. Optimal culture conditions for producing conjugated linoleic acid in skim-milk by co-culture of different Lactobacillus strains. Ann. Microbiol. 2013, 63, 707–717. [Google Scholar] [CrossRef]
- Khaskheli, A.A.; Talpur, F.N.; Demir, A.S.; Cebeci, A.; Jawaid, S. A highly selective whole cell biocatalysis method for the production of two major bioactive conjugated linoleic acid isomers. Biocatal. Agric. Biotechnol. 2013, 2, 328–332. [Google Scholar] [CrossRef]
- Khaskheli, A.A.; Talpur, F.N.; Cebeci Aydin, A.; Jawaid, S.; Surhio, M.A.; Afridi, H.I. One-pot conjugated linoleic acid production from castor oil by Rhizopus oryzae lipase and resting cells of Lactobacillus plantarum. Biosci. Biotechnol. Biochem. 2017, 81, 2002–2008. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Lin, J.; Gong, D. Identification of lactic acid bacterial strains with high conjugated linoleic acid-producing ability from natural sauerkraut fermentations. J. Food Sci. 2009, 74. [Google Scholar] [CrossRef]
- Al-Saman, M.A.E.-H.; Elsanhoty, R.M.; Elhadary, A.E. The impact of oil type and lactic acid bacteria on conjugated linoleic acid production. J. Biochem. Microbiol. Biotechnol. 2016, 4, 25–29. [Google Scholar] [CrossRef]
- Kim, B.; Lee, B.W.; Hwang, C.E.; Lee, Y.Y.; Lee, C.; Kim, B.J.; Park, J.Y.; Sim, E.Y.; Haque, M.A.; Lee, D.H.; et al. Screening of conjugated linoleic acid (CLA) producing Lactobacillus plantarum and production of CLA on soy-powder milk by these stains. Korean J. Microbiol. 2015, 51, 231–240. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, B.; Hwang, C.E.; Haque, M.A.; Kim, S.C.; Lee, C.S.; Kang, S.S.; Cho, K.M.; Lee, D.H. Changes in conjugated linoleic acid and isoflavone contents from fermented soymilks using Lactobacillus plantarum P1201 and screening for their digestive enzyme inhibition and antioxidant properties. J. Funct. Foods 2018, 43, 17–28. [Google Scholar] [CrossRef]
- Hwang, C.E.; Lee, D.H.; Kim, B.; Joo, O.S.; Kim, S.C.; Lee, J.H.; Hong, S.Y.; Choi, A.R.; Cho, K.M. Enhanced digestive enzyme activity and anti-adipogenic of fermented soy-powder milk with probiotic Lactobacillus plantarum P1201 through an increase in conjugated linoleic acid and isoflavone aglycone content. Korean J. Food Preserv. 2018, 25, 461–470. [Google Scholar] [CrossRef]
- Kouchak Yazdi, Z.; Alemzadeh, I.; Vossoughi, M. Comparison and optimization of conjugated linoleic acid production by Lactobacillus plantarum and Lactobacillus plantarum subsp. plantarum. Sci. Iran. 2017, 24, 1272–1280. [Google Scholar] [CrossRef]
- Palachum, W.; Choorit, W.; Chisti, Y. Accumulation of conjugated linoleic acid in Lactobacillus plantarum WU-P19 is enhanced by induction with linoleic acid and chitosan treatment. Ann. Microbiol. 2018, 68, 611–624. [Google Scholar] [CrossRef]
- Aziz, T.; Sarwar, A.; Fahim, M.; Al Dalali, S.; Ud Din, Z.; Ud Din, J.; Xin, Z.; Jian, Z.; Pacheco Fill, T.; Zhennai, Y. In silico characterization of linoleic acid biotransformation to rumenic acid in food derived Lactobacillus plantarum YW11. Acta Biochim. Pol. 2020, 67, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xu, Q.; Ye, Q.; Chen, W.; Zhang, H. Bioconversion kinetics of conjugated linoleic acid by Lactobacillus plantarum ZS2058. Wei Sheng Wu Xue Bao 2009, 49, 174–179. [Google Scholar]
- Niu, X.Y.; Chen, W.; Tian, F.W.; Zhao, J.X.; Zhang, H. Bioconversion of conjugated linoleic acid by resting cells of Lactobacillus plantarum ZS2058 in potassium phosphate buffer system. Wei Sheng Wu Xue Bao 2007, 47, 244–248. [Google Scholar]
- Yang, B.; Chen, H.; Gu, Z.; Tian, F.; Ross, R.P.; Stanton, C.; Chen, Y.Q.; Chen, W.; Zhang, H. Synthesis of conjugated linoleic acid by the linoleate isomerase complex in food-derived lactobacilli. J. Appl. Microbiol. 2014, 117, 430–439. [Google Scholar] [CrossRef]
- Vera Pingitore, E.; Pessione, A.; Fontana, C.; Mazzoli, R.; Pessione, E. Comparative proteomic analyses for elucidating metabolic changes during EPS production under different fermentation temperatures by Lactobacillus plantarum Q823. Int. J. Food Microbiol. 2016, 238, 96–102. [Google Scholar] [CrossRef]
- Guerzoni, M.E.; Lanciotti, R.; Cocconcelli, P.S. Alteration in cellular fatty acid composition as a response to salt, acid, oxidative and thermal stresses in Lactobacillus helveticus. Microbiology 2001, 147, 2255–2264. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, D.K.; Puniya, A.K. Optimisation of fermentation variables for conjugated linoleic acid bioconversion by Lactobacillus fermentum DDHI 27 in modified skim milk. Int. J. Dairy Technol. 2018, 71, 46–55. [Google Scholar] [CrossRef]
- Troegeler-Meynadier, A.; Nicot, M.C.; Bayourthe, C.; Moncoulon, R.; Enjalbert, F. Effects of pH and Concentrations of linoleic and linolenic acids on extent and intermediates of ruminal biohydrogenation in vitro. J. Dairy Sci. 2003, 86, 4054–4063. [Google Scholar] [CrossRef]
- Matejčeková, Z.; Spodniaková, S.; Dujmić, E.; Liptáková, D.; Valík, Ľ. Modelling growth of Lactobacillus plantarum as a function of temperature: Effects of media. J. Food Nutr. Res. 2019, 58, 125–134. [Google Scholar]
- Takeuchi, M.; Kishino, S.; Hirata, A.; Park, S.-B.; Kitamura, N.; Ogawa, J. Characterization of the linoleic acid Δ9 hydratase catalyzing the first step of polyunsaturated fatty acid saturation metabolism in Lactobacillus plantarum AKU 1009a. J. Biosci. Bioeng. 2015, 119, 636–641. [Google Scholar] [CrossRef]
- Lv, H.; Ren, D.; Yan, W.; Wang, Y.; Liu, H.; Shen, M. Linoleic acid inhibits Lactobacillus activity by destroying cell membrane and affecting normal metabolism. J. Sci. Food Agric. 2020, 100, 2057–2064. [Google Scholar] [CrossRef]
- Plengvidhya, V.; Breidt, F.; Lu, Z.; Fleming, H.P. DNA Fingerprinting of lactic acid bacteria in sauerkraut fermentations. Appl. Environ. Microbiol. 2007, 73, 7697–7702. [Google Scholar] [CrossRef]
- Xiong, T.; Guan, Q.; Song, S.; Hao, M.; Xie, M. Dynamic changes of lactic acid bacteria flora during Chinese sauerkraut fermentation. Food Control 2012, 26, 178–181. [Google Scholar] [CrossRef]
- Oh, D.-K.; Hong, G.-H.; Lee, Y.; Min, S.; Sin, H.-S.; Cho, S.K. Production of conjugated linoleic acid by isolated Bifidobacterium strains. World J. Microbiol. Biotechnol. 2003, 19, 907–912. [Google Scholar] [CrossRef]
- Aziz, T.; Sarwar, A.; Al-Dalali, S.; Din, Z.U.; Megrous, S.; ud Din, J.; Zou, X.; Zhennai, Y. Production of linoleic acid metabolites by different probiotic strains of Lactobacillus plantarum. Prog. Nutr. 2019, 21, 693–701. [Google Scholar]
- Min, Z.; Xiaona, H.; Aziz, T.; Jian, Z.; Zhennai, Y. Exopolysaccharides from Lactobacillus plantarum YW11 improve immune response and ameliorate inflammatory bowel disease symptoms. Acta Biochim. Pol. 2020, 67, 487–493. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, J.; Yang, Z. Immunomodulatory and antitumor activities of the exopolysaccharide produced by potential probiotic Lactobacillus plantarum YW11 in a HT-29 tumor-burdened nude mouse model. Food Sci. Technol. 2022, 42. [Google Scholar] [CrossRef]
- Ogawa, J.; Kishino, S.; Ando, A.; Sugimoto, S.; Mihara, K.; Shimizu, S. Production of conjugated fatty acids by lactic acid bacteria. J. Biosci. Bioeng. 2005, 100, 355–364. [Google Scholar] [CrossRef]
- Nitbani, F.O.; Tjitda, P.J.P.; Wogo, H.E.; Detha, A.I.R. Preparation of ricinoleic acid from castor oil: a review. J. Oleo Sci. 2022, 71, ess21226. [Google Scholar] [CrossRef] [PubMed]
- Okwunodulu, I.N.; Agha, E.F. Nutritional properties of indigenous fermented condiment (ogiri) produced from partial substitution of castor oil bean (Ricinus communis) with soybean (Glycine max) seeds. Niger. J. Biotechnol. 2021, 37, 32–46. [Google Scholar] [CrossRef]
- Achi, O.K. The potential for upgrading traditional fermented foods through biotechnology. African J. Biotechnol. 2005, 4, 375–380. [Google Scholar]
- Worbs, S.; Köhler, K.; Pauly, D.; Avondet, M.-A.; Schaer, M.; Dorner, M.B.; Dorner, B.G. Ricinus communis intoxications in human and veterinary medicine—a summary of real cases. Toxins (Basel). 2011, 3, 1332–1372. [Google Scholar] [CrossRef]
- Liu, G.; Liao, M.; Guo, B.; Kan, Q.; Zhou, S.; Feng, K.; Lin, W.; Huang, Y.; Miao, J.; Cao, Y. Detoxification of three toxins in castor meal by a novel continuous phase-transition extraction in a pilot-scale. Ind. Crops Prod. 2021, 172, 114076. [Google Scholar] [CrossRef]
- Ulanova, R.; Kravchenko, I. Lactic acid bacteria fermentation for detoxification of castor bean meal and processing of novel protein feeds supplement. Int. J. Eng. Sci. Innov. Technol. 2013, 2, 618–624. [Google Scholar]
- Ngene, A.C.; Onwuakor, C.E.; Aguiyi, J.C.; Ifeanyi, V.O.; Ohaegbu, C.G.; Okwuchukwu, C.P.; Kim, E.G.; Egbere, J.O. Screening of some lactic acid bacteria isolated from selected nigerian fermented foods for vitamin production. Adv. Microbiol. 2019, 09, 943–955. [Google Scholar] [CrossRef]
- Uzoh, C.V.; Orji, J.O.; Okeh, C.O.; Nworie, C.O.; Igwe, P.C.; Uwanta, L.I. Assessment of probiotic potentials of lactic acid bacteria isolated from some locally fermented foods. Asian J. Food Res. Nutr. 2022, 1, 11–16. [Google Scholar]
- Ojinnaka, M.C.; Ojimelukwe, P.C.; Ezeama, C.F. An assessment of the microbial and amino acid contents of ogiri produced by fermenting oil bean seeds of Ricinus communis. Sky J. Food Sci. 2013, 2, 10–18. [Google Scholar]
- Adesulu-Dahunsi, A.T.; Dahunsi, S.O.; Ajayeoba, T.A. Co-occurrence of Lactobacillus species during fermentation of african indigenous foods: impact on food safety and shelf-life extension. Front. Microbiol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Akkaya, M.R. Prediction of fatty acid composition of sunflower seeds by near-infrared reflectance spectroscopy. J. Food Sci. Technol. 2018, 55, 2318–2325. [Google Scholar] [CrossRef]
- Zhao, W.-H.; Zhang, J.-F.; Zhe-Wang; Zhang, Y.-X.; Tian, W.-X. The extract of leaves of Acer truncatum Bunge: a natural inhibitor of fatty acid synthase with antitumor activity. J. Enzyme Inhib. Med. Chem. 2006, 21, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Lin, F.; Zhang, R.; Wang, M.; Gu, R.; Long, C. Acer truncatum Bunge: a comprehensive review on ethnobotany, phytochemistry and pharmacology. J. Ethnopharmacol. 2022, 282, 114572. [Google Scholar] [CrossRef]
- Bach, A.; Calsamiglia, S.; Stern, M.D. Nitrogen metabolism in the rumen. J. Dairy Sci. 2005, 88, E9–E21. [Google Scholar] [CrossRef]
- Jutzeler van Wijlen, R.P.; Colombani, P.C. Grass-based ruminant production methods and human bioconversion of vaccenic acid with estimations of maximal dietary intake of conjugated linoleic acids. Int. Dairy J. 2010, 20, 433–448. [Google Scholar] [CrossRef]
- Schmid, A.; Collomb, M.; Sieber, R.; Bee, G. Conjugated linoleic acid in meat and meat products: a review. Meat Sci. 2006, 73, 29–41. [Google Scholar] [CrossRef]
- Bauman, D.E.; Lock, A.L. Conjugated linoleic acid: biosynthesis and nutritional significance. In Advanced Dairy Chemistry Volume 2 Lipids; Springer US: Boston, MA; pp. 93–136.
- Gutiérrez, L.F. Conjugated linoleic acid in milk and fermented milks: variation and effects of the technological processes. Rev. Vitae 2016, 23, 134–145. [Google Scholar] [CrossRef]
- Collomb, M.; Schmid, A.; Sieber, R.; Wechsler, D.; Ryhänen, E.-L. Conjugated linoleic acids in milk fat: variation and physiological effects. Int. Dairy J. 2006, 16, 1347–1361. [Google Scholar] [CrossRef]
- Garabal, J.I.; Rodríguez-Alonso, P.; Centeno, J.A. Characterization of lactic acid bacteria isolated from raw cows’ milk cheeses currently produced in Galicia (NW Spain). LWT - Food Sci. Technol. 2008, 41, 1452–1458. [Google Scholar] [CrossRef]
- Rule, D.C.; Broughton, K.S.; Shellito, S.M.; Maiorano, G. Comparison of muscle fatty acid profiles and cholesterol concentrations of bison, beef cattle, elk, and chicken1. J. Anim. Sci. 2002, 80, 1202–1211. [Google Scholar] [CrossRef]
- Marco, A.; Juarez, M.M.; Brunton, N.; Wasilewski, P.D.; Lynch, B.; Moon, S.-S.; Troy, D.J.; Mullen, A.M. Enriching breakfast sausages by feeding pigs with CLA supplemented diets. Food Chem. 2009, 114, 984–988. [Google Scholar] [CrossRef]
- Zdolec, N.; Mikuš, T.; Kiš, M. Lactic acid bacteria in meat fermentation: dry sausage safety and quality. In Lactic Acid Bacteria in Food Biotechnology; Elsevier, 2022; pp. 145–159. [Google Scholar]
- Wang, Y.; Han, J.; Wang, D.; Gao, F.; Zhang, K.; Tian, J.; Jin, Y. Research update on the impact of lactic acid bacteria on the substance metabolism, flavor, and quality characteristics of fermented meat products. Foods 2022, 11, 2090. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Pateiro, M.; Tomasevic, I.; Domínguez, R.; da Silva Barretto, A.C.; Santos, E.M.; Lorenzo, J.M. Functional fermented meat products with probiotics—A review. J. Appl. Microbiol. 2022, 133, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Özer, C.O.; Kılıç, B. Utilization of optimized processing conditions for high yield synthesis of conjugated linoleic acid by L. plantarum AB20–961 and L. plantarum DSM2601 in semi-dry fermented sausage. Meat Sci. 2020, 169. [Google Scholar] [CrossRef] [PubMed]
- Ozer, C.O.; Kilic, B. Optimization of enhanced conjugated linoleic acid production by L. plantarum AB20-961 and L. plantarum DSM2601 in meat model system using response surface methodology. In 63rd International Congress of Meat Science and Technology; Troy, D., McDonnell, C., Hinds, L., Kerry, J., Eds.; 2017; pp. 88–89. ISBN 9789086868605. [Google Scholar]
- Kim, I.-S.; Kim, C.-H.; Yang, W.-S. Physiologically active molecules and functional properties of soybeans in human health—a current perspective. Int. J. Mol. Sci. 2021, 22, 4054. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, G.; Diao, J.; Wang, C. Recent advances in exploring and exploiting soybean functional peptides—a review. Front. Nutr. 2023, 10. [Google Scholar] [CrossRef] [PubMed]
- Peñalvo, J.L.; Castilho, M.C.; Silveira, M.I.N.; Matallana, M.C.; Torija, M.E. Fatty acid profile of traditional soymilk. Eur. Food Res. Technol. 2004, 219, 251–253. [Google Scholar] [CrossRef]
- Xie, C.L.; Hwang, C.E.; Oh, C.K.; Yoon, N.A.; Ryu, J.H.; Jeong, J.Y.; Roh, G.S.; Kim, H.J.; Cho, G.J.; Choi, W.S.; et al. Fermented soy-powder milk with Lactobacillus plantarum P1201 protects against high-fat diet-induced obesity. Int. J. Food Sci. Technol. 2017, 52, 1614–1622. [Google Scholar] [CrossRef]
| L. plantarum strain | Culture medium, environmental conditions |
CLA source | Total CLA | CLA isomers (%) | Ref. |
| A6-1F | PBS, pH 7.0, 37 °C 15% (w/v) cell concentration |
1.5 mg/mL LA | 260.1-275.7 μg/mL | CLA1 mainly | [97] |
| AB20-961 (DSM2601) |
sausage fermentation 79 h (73 h) |
5% SAO | 4.1 mg/g fat (7.5 mg/g fat) in sausage |
n. d. | [98] |
| AB20-961 (DSM2601) |
pH 7.9, 79 h (73 h) 8 log CFU/g fermented ground beef |
5% FA source | 7.9 mg/g fat (38.3 mg/g fat) in sausage |
60% CLA1 40% CLA2 |
[96] |
| AB20-961 | subculture in MRS, 37 °C, 24 h sucuk fermentation 12 h |
2% HSO | 6.1 mg/g fat in sucuk |
69% CLA1 31% CLA2 in sucuk |
[99] |
| AKU1009a | KPB, pH 6.5, 33 % (wet w/v) washed cells, 108 h | 0.12 mg/mL LA | 40 mg/mL | 38% CLA1 62% t9t11-CLA |
[42] |
| AKU1009a | KPB, pH 6.5, 37 °C, washed cells, 24 h, |
4.0 mg/mL CO (88% RA, 5% LA, 7% other) | 1.14 mg/mL | 17% CLA1 83% t9t11-CLA |
[100] |
| ATCC8014 | MRS, 40 °C, pH of 6.5, 48 h |
0.1 mg/mL LA | 37.5 μg/mL | expressed as CLA1 | [101] |
| ATCC8014 | MRS, 37 °C, pH of 6.5, 72 h, 2% washed cells |
8 mg/mL SO | 0.8 mg/mL | 48% CLA1 52% CLA2 |
[78] |
| CGMCC8198 | MRS, 30 °C, 24 h 1% inoculum |
0.5 mg/mL ATB | 5.8 mg/mL | 35% CLA1 65% CLA2 |
[102] |
| CRL1920 (CRL1935) |
MRS, 37 °C, 48 h | 0.5 mg/mL LA | 17.3 μg/mL (17.5 μg/mL) |
40% CLA1 30% CLA2 30% t9t11-CLA |
[41] |
| DSM 20179 | MRS (4.1% v/v inoculum, 4.0 g/L yeast extract), 37 °C, 24 h | 3.0 mg/mL LA | 240.7 μg/mL | 90% CLA1 10% CLA2 |
[103] |
| HIF15 | MRS + 0.05% L-cys-HCl, 37 °C, 48 h | 0.5 mg/mL LA | 46.2 μg/mL | 75% CLA1 11% CLA2 13% t9t11-CLA |
[95] |
| HIF15 | SM, 10 mg/ml yeast extracts, 0.3% glucose | 0.5 mg/mL LA | 52.6 μg/mL | 68% CLA1 9% CLA2 16% t9t11-CLA |
[95] |
| IMAU60042 | MRS, 37 °C, 20 h | 1.2 μg/mL SO (LA 67.3% of total FA) | 48.7 μg/g |
n.d. | [104] |
| IMAU60042 | soy milk, 42 °C, 48 h 2.0 × 107 CFU/mL inoculum |
122.4 μg/g | 10% CLA1 90% CLA2 |
[104] | |
| J25 | Skim milk, pH 6.4, 36 °C, 48 h 5% inoculum |
2% LA | 23.5 μg/mL | 58% CLA1 42% CLA2 |
[105] |
| JBCC105683 | MRS, 30 °C 48 h |
0.6 mg/mL LA | 748.8 μg/mL | 86% CLA1 14% CLA2 |
[75] |
| JBCC105675 | MRS, 30 °C 48 h |
0.2 mg/mL LA | 427.2 μg/mL | 85% CLA1 15% CLA2 |
[75] |
| JBNU105645 | MRS, 30 °C 48 h |
0.1 mg/mL LA | 227.4 μg/mL | 52% CLA1 48% CLA2 |
[75] |
| JCM 1551 | 1.0 M citrate buffer 37 °C, pH of 6.0, 99 h | 5.0 mg/mL CO | 2.7 mg/mL (up to 7.5 mg/mL with 30 mg/mL CO, 171 h) |
26% CLA1 74% t9t11-CLA |
[106] |
| JCM 1551 | 0.1 M KPB, pH 6.5, 37 °C 12% (w/v) wet cell |
3.4 mg/mL RA | 2.4 mg/mL | 21% CLA1 79% t9t11-CLA |
[107] |
| L200 | MRS, 30 °C, 48 h 2% inoculum |
0.25 mg/mL LA + 0.1 mg/mL BSA | 34.7 μg/mL | 93% CLA1 2% CLA2 5% t9t11-CLA |
[108] |
| L2C21E8 L3C1E8 |
MRS (or SM), 30 °C, 48 h | 0.5 mg/mL LA | 17.9 μg/mL 15.4 μg/mL |
expr. as CLA1 | [109] |
| lp15 | MRS, 30 °C 48 h |
0.1 mg/mL LA | 26.1 μg/mL | 76% CLA1 24% CLA2 |
[110] |
| Lp in co-culture with L. acidophilus |
Skim milk, pH 6.4, 36 °C, 48 h 5% inoculum |
5% SAO | 316.5 μg/mL | n.d. | [111] |
| L. plantarum from buffalo milk | MRS; 37 °C, pH of 5.5, 120 h |
1.6 mg/mL LA with BSA (5 mg/mg LA) |
272 μg/mL | 51% CLA1 49% CLA2 |
[112] |
| L. plantarum from buffalo milk | KPB, pH 6.5, 37 °C, 20 h 12% (w/v) washed cells, lipase |
8 mg/mL CO | 406 μg/mL | 56% CLA1 44% CLA2 |
[113] |
| NCUL005 | MRS, 30 °C, 24 h | 2.5 mL/L LA | 623 μg/mL | 32% CLA1 68% CLA2 |
[114] |
| P1 | MRS, 37 °C, 24 h | 10 mg/mL SO | 400 μg/mL | n.d. | [115] |
| P1201 | 10% soy-powder milk, 37 °C, 48 h | 198.7 μg/mL | 90% CLA1 | [116] | |
| P1201 | soy-powder hydrolyzed milk 35 °C, 48 h |
1% SAO | 1.3 mg/g | 92% CLA1 8% CLA2 |
[117] [118] |
| PTCC1058 | KPB, pH 6.5, 37 °C, 121 h 15% (w/v) washed cells, lipase |
4.6 mg/mL CO | 1.7 mg/mL | 44% CLA1 46% CLA2 |
[119] |
| PTCC1745 | KPB, pH 6.5, 37 °C, 121 h 15% (w/v) washed cells, lipase |
9.6 mg/mL CO | 1.6 mg/mL | 41% CLA1 55% CLA2 |
[119] |
| S48 |
10% soy-powder milk, 37 °C, 48 h | 183.6 μg/mL |
90% CLA1 |
[116] | |
| UALp-05 in co-culture with of L. lactis ssp. lactis and L. lactis ssp. cremoris |
91% milk fat to produce cheddar cheese |
9% SAO | 75 μg/g in 90 days ripened cheese | 25% CLA1 20% CLA2 20% c9c11-CLA 18% t9c11-CLA 16% c10t12-CLA |
[43] |
| WU-P19 | MRS; 37 °C, pH of 6.0, 36 h (10% v/v inoculum) + chitosan |
0.6 mg/mL LA |
21 mg/g biomass | 48% CLA1 52% CLA2 |
[120] |
| YW11 | MRS, 24-36 h | 10% (v/v) LA | n.d | CLA1 (only with 10% LA) and other not conjugated LA metabolites | [121] |
| ZS2058 | 40 °C, pH of 6.5, 5 x 1010 CFU/mL | 0.4 mg/mL LA | 16.0 μg/mL (x h) | CLA1 | [122] |
| ZS2058 | MRS, 37°C, 24 h |
0.5 mg/mL LA | 312.4 μg/mL | 96.4% CLA1 3.6% CLA2 |
[123] |
| ZS2058 | MRS, 37°C, 48 h | 0.55 mg/mL LA | 0.313 mg/mL | 66% CLA1 4.4% CLA2 29% t9t11-CLA |
[124] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





