1. Introduction
Plasma electrolytic processes have attracted the attention of metal finishing industry due to their ability to significantly improve surface properties [
1]. Among them, plasma electrolytic polishing (PeP) is a special case of electrochemical machining that requires high voltage and uses environmentally friendly aqueous salt solutions [
2]. Material removal is a consequence of a special case of anodic dissolution [
1,
3,
4]. The PeP process, first described in 1979 [
5], is a surface treatment technology that leads to very smooth, high-gloss surfaces with improved corrosion resistance [
6] and is a promising post-processing technology for additively manufactured parts [
7].
The process takes place in a vat containing a material-specific aqueous electrolyte solution with low viscosity. Its conductivity is adjusted from 4 to 30 S m
−1 by adding up to 12 % of various salts and suitable complexing agents. The workpiece in the vat is anodically polarised; the voltages used are in the range of 180 to 400 V. In addition to the workpiece, there is a cathode electrode in the electrolytic cell. The surface ratio between the anode and cathode should be greater than 1:10 and the cathode does not have to resemble the geometry of the part; a ring or plate shape is common. The ratio is necessary to ensure the required current density for plasma formation on the anode surface. The relationship between current density and applied electrical potential must be set to adjust the process window to the electro-hydrodynamic area for the PeP process. There, caused by the process conditions, a vapour skin forms around the workpiece and a plasma layer is created. In combination with the
electrochemical reactions such as (anodic) metal dissolution, (anodic) oxide formation, hydrogen formation and alkalisation, the
plasma reactions such as ionisation of the vapour skin and hydrothermal reactions such as metal dissolution by metal-water reaction, lead to a removal of surface peaks and thus to a polishing of the part. The vapour skin results in the process surface temperature not significantly exceeding the electrolyte boiling temperature; hence during the process, the part reaches a maximum temperature of
[
8].
A variety of electrically conductive materials have been successfully polished, namely a variety of steels [
9], copper alloys [
8,
10,
11] including metallic glass [
12], aluminium alloys [
13], and other materials such as cobalt-chromium alloy [
14], titanium alloy [
15], nitinol [
16], etc. Regardless of which material is to be polished, the current peak and the power that occur during the initialisation phase of the process must be taken into account. Namely, when a voltage is applied between the two electrodes submerged in an electrolyte, a high current peak is generated due to the high electrical conductivity of the electrolyte. When the vapour skin forms, the conductivity decreases considerably and the current drops to several amperes depending on the size of the workpiece surface [
8]. The larger the workpiece surface, the higher the current and the power. This is also the case in the process initialisation phase, when a vapour skin forms around the workpiece. In this phase, it is advantageous not to exceed the power required after the vapour skin has completely formed, i.e. during
stable polishing. In extreme situations, the electrical power may be too high and fuses will switch off the power supply to prevent damage to the power supply and/or electrical wiring. The most common solution in practise is to initiate the process by slowly submerging a workpiece in an electrolyte to allow time for the vapour skin to form and to avoid a large surface area being in direct contact with the electrolyte. The influence of the submerging velocity on the electrical quantities and phenomena during the initialisation phase has not yet been systematically investigated.
In this paper, the effect of submerging velocity (the workpiece velocity in
z direction) on the peak current and average power is investigated in the case of a workpiece size corresponding to a microreactor baseplate [
17] to be manufactured by laser powder bed fusion (PBF-LB/M) and polished by PeP [
18]. We formulate the hypothesis that the highest power occurs when the workpiece comes into contact with an electrolyte and forms a short circuit on a large surface before the vapour skin forms. The peak current occurring there is very important. We also try to determine the highest submerging velocity at which the electrical power during process initialisation does not exceed the power required for stable polishing. Based on the results, the hypothesis is rejected but the appropriate submerging velocity is successfully defined.
2. Materials and Methods
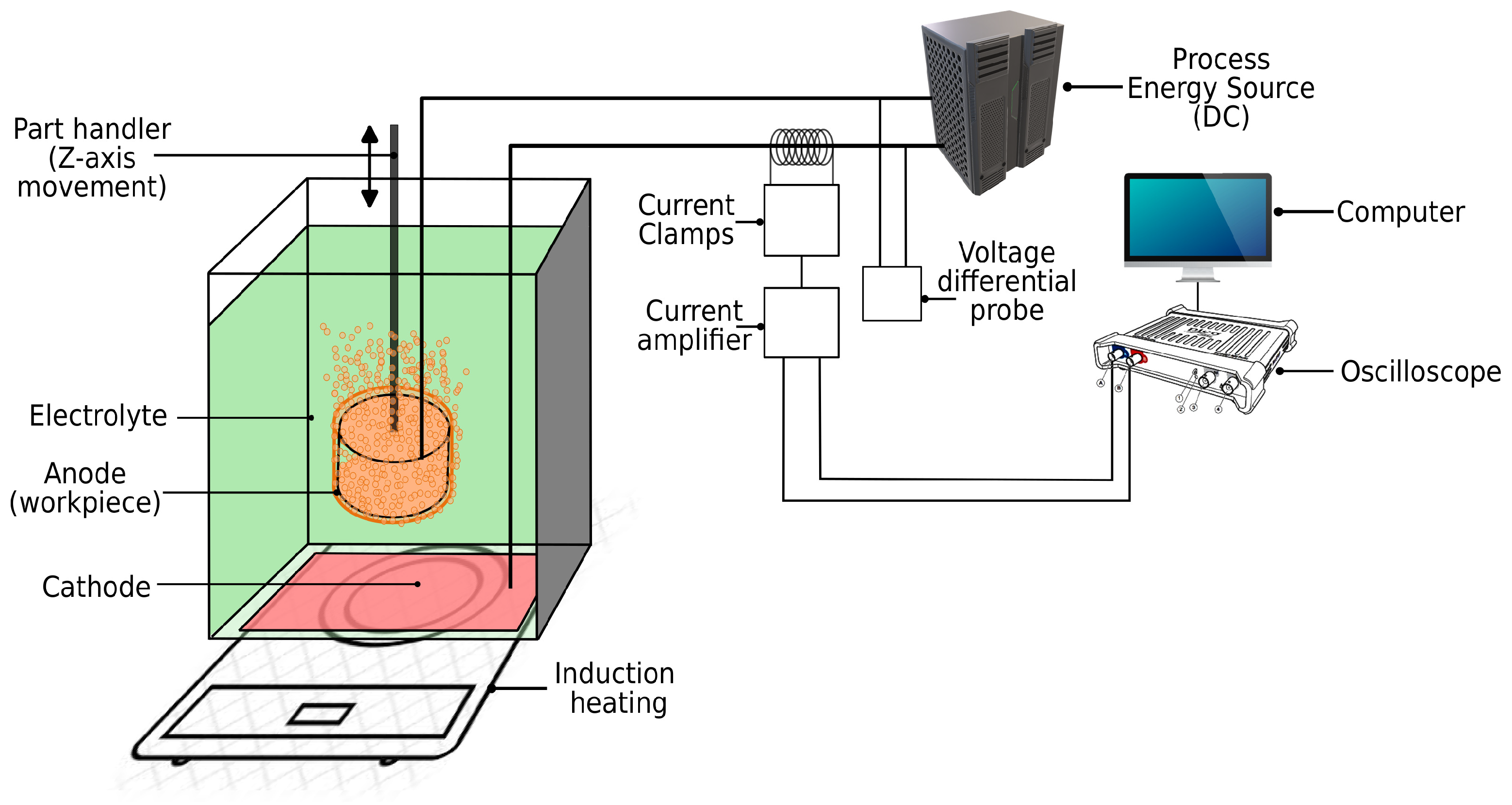
The experiments were carried out with the 80 kW PeP plant (Leukhardt Schaltanlagen GmbH, Germany), which can supply 150 A and voltages in the range from 150 to 380 V. The machine has a z stage and enables the control of the workpiece movement in z direction and thus the setting of the submerging velocity in the range from 0 to 500 mm s−1. The temperature and concentration of the electrolyte play an important role in the PeP process. To create the same conditions in all experiments, sodium carbonate () dissolved in water was used as electrolyte to avoid material removal on the workpiece. We kept the temperature of the electrolyte at 80 C and the concentration of sodium carbonate at 0.55 M. The temperature was controlled by the Caso design TC2400 induction heating hob (Caso Gmbh, Germany). Due to water evaporation, the electrical conductivity was frequently monitored and maintained at S m−1; fresh water was added when needed. The workpiece used for the experiments was the size of a mould insert for the mass production of microreactor units. It was a plate with a diameter of 40 mm and a thickness of 10 mm. The bottom surface are was therefore 1256 mm2 and the total surface area was 3768 mm2. The material was stainless steel AISI 316L and its bottom surface was mechanical polished to mirror surface (Ra = ). Thus, a repeatable effective surface area as well as the wetting characteristics such as contact angle of the workpiece were maintained, making the experiments more repeatable and more under control. The workpiece bottom surface was parallel to the electrolyte surface when submerging.
To acquire the voltage and current signals, the Picoscope
® 3205D oscilloscope (Pico Technology, UK) with a resolution of 8 bits at 1 GS s
−1 and a bandwidth of 100 MHz was connected to a PC (Linux OS with PicoScope
® 7 oscilloscope software) via a USB cable. The voltage differential probe Testec TT-SI9101 (Testec Elektronik GmbH, Germany) with a bandwidth of 100 MHz was used with an attenuation ratio of 1:100 to reduce the input voltage to the oscilloscope. The current was measured with a Tektronix TCP303 current probe (Tektronix Inc., USA) with 15 MHz bandwidth and the ability to measure currents up to 150 A. The probe was connected to a Tektronix TCPA300 amplifier (Tektronix Inc., USA) with 100 MHz bandwidth. The entire setup is shown in
Figure 1.
The current and voltage waveforms were recorded in an oscilloscope buffer and transferred to the PC after acquisition. They were analysed in a Matlab
® programming environment. Due to the relatively low resolution of the oscilloscope in the
y-axis (8 bits), the voltage and current signals were first filtered using a Savitzky-Golay smoothing filter [
19] with second-degree polynomial function, taking into account
ms before and after the observed sample on the signal (see Supplement material for the effect of filtering). The filtered voltage and current signals were used to calculate the power signal.
Two current peaks were identified in the initialisation phase of the process, namely when the workpiece and the electrolyte make electrical
contact and when the electrolyte
splashes onto the workpiece top surface. Within these two time domains, the average power
was calculated as the energy within the time domain divided by the duration of the time domain
t according to the following equation
where
is the voltage,
the current,
i a sample number and
N is the number of samples in the time domain. The average current and power during a
stable polishing were also calculated for the last
ms of the signals.
The experiments were carried out at seven submerging velocities, namely 5, 20, 100, 200, 300, 400, and 500 mm s−2. Two additional experiments were carried out. In the first, the bottom surface of the workpiece was touching the electrolyte surface and the contact area was 1256 mm2. In the second, the workpiece was fully submerged in the electrolyte and the contact area was 3768 mm2. Most of the experiments were performed five times. The exceptions are: experiments with velocity 500 mm s−1 were performed three times, velocity 400 mm s−1 ten times and with submerged workpiece only two times.
3. Results and discussion
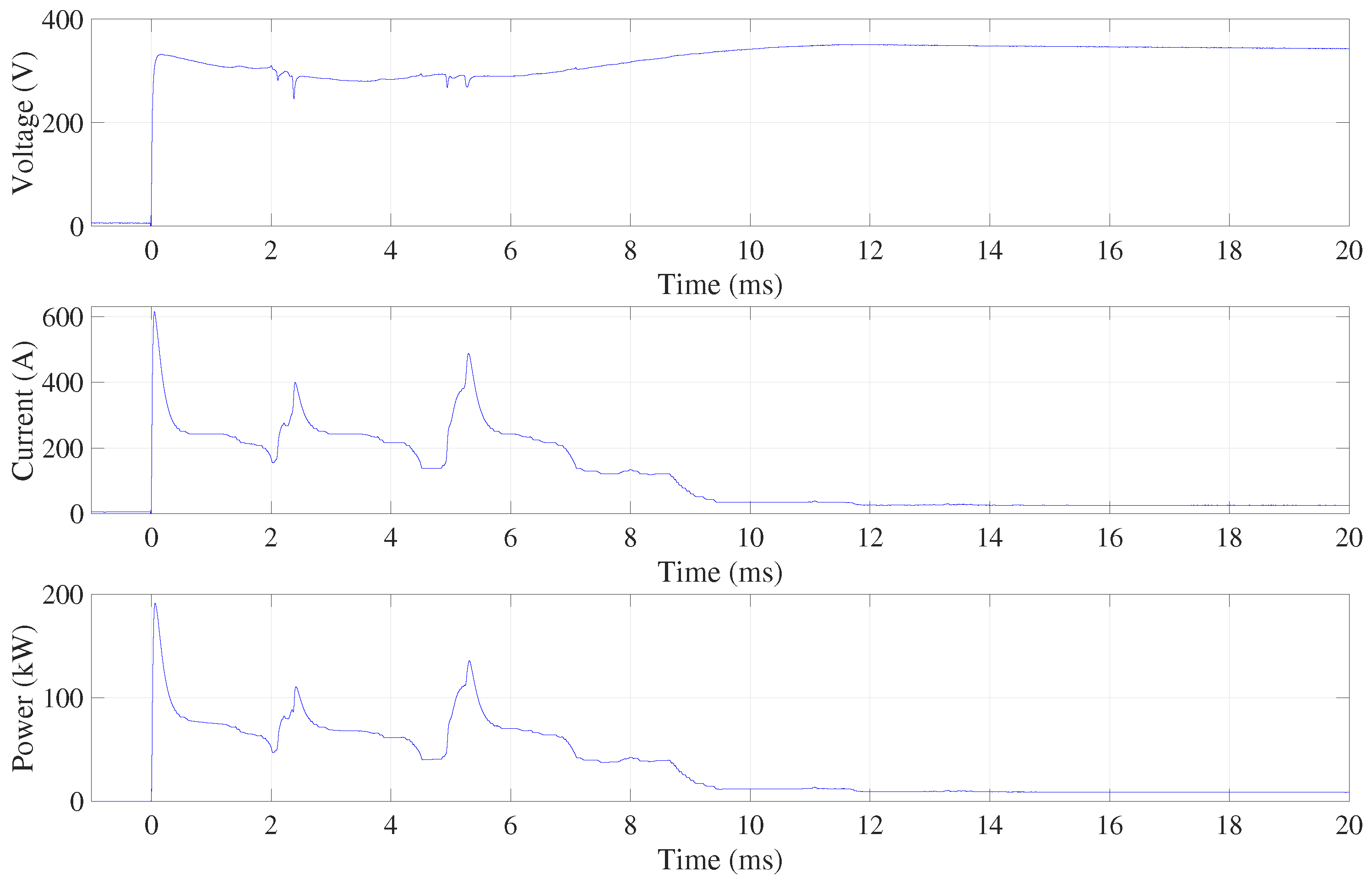
The polishing process begins with a short circuit due to the high conductivity of the electrolyte liquid. Once the vapour skin has formed, the resistivity is increased and the current is reduced. When polishing starts with the workpiece fully submerged, the peak current is up to 600 A and the peak power is up to 200 kW, as shown in
Figure 2. There is also a significant voltage drop until the vapour skin has formed around the entire workpiece. Hence, even in the case of relatively small workpieces to be polished, like mould inserts for manufacturing of microreactors, the process should be initiated by submerging the workpiece and not by starting the process when the workpiece is already submerged in the electrolyte. The three consecutive peaks occur during this initialisation phase. These peaks are present in all acquired signals when the workpiece is submerged. This high repeatability is also observed in the case the electrode bottom surface only touches the electrolyte (see Supplementary material to check repeatability). Both experiments with a fixed workpiece position in the
z direction show that the formation of the vapour skin is a dynamic but relatively repeatable process.
To avoid high current peaks during process initialisation, the workpiece is slowly submerged in the electrolyte.
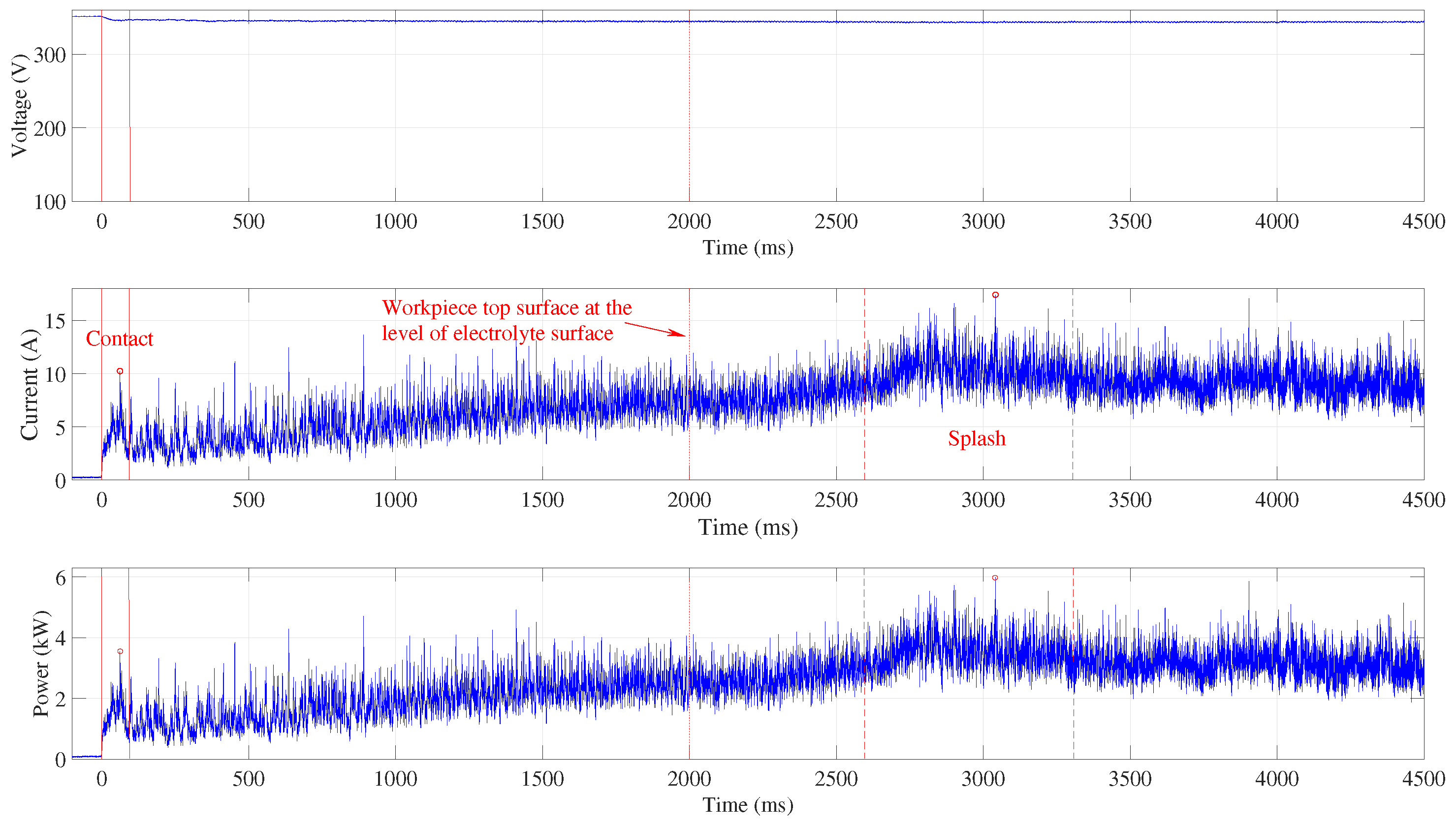
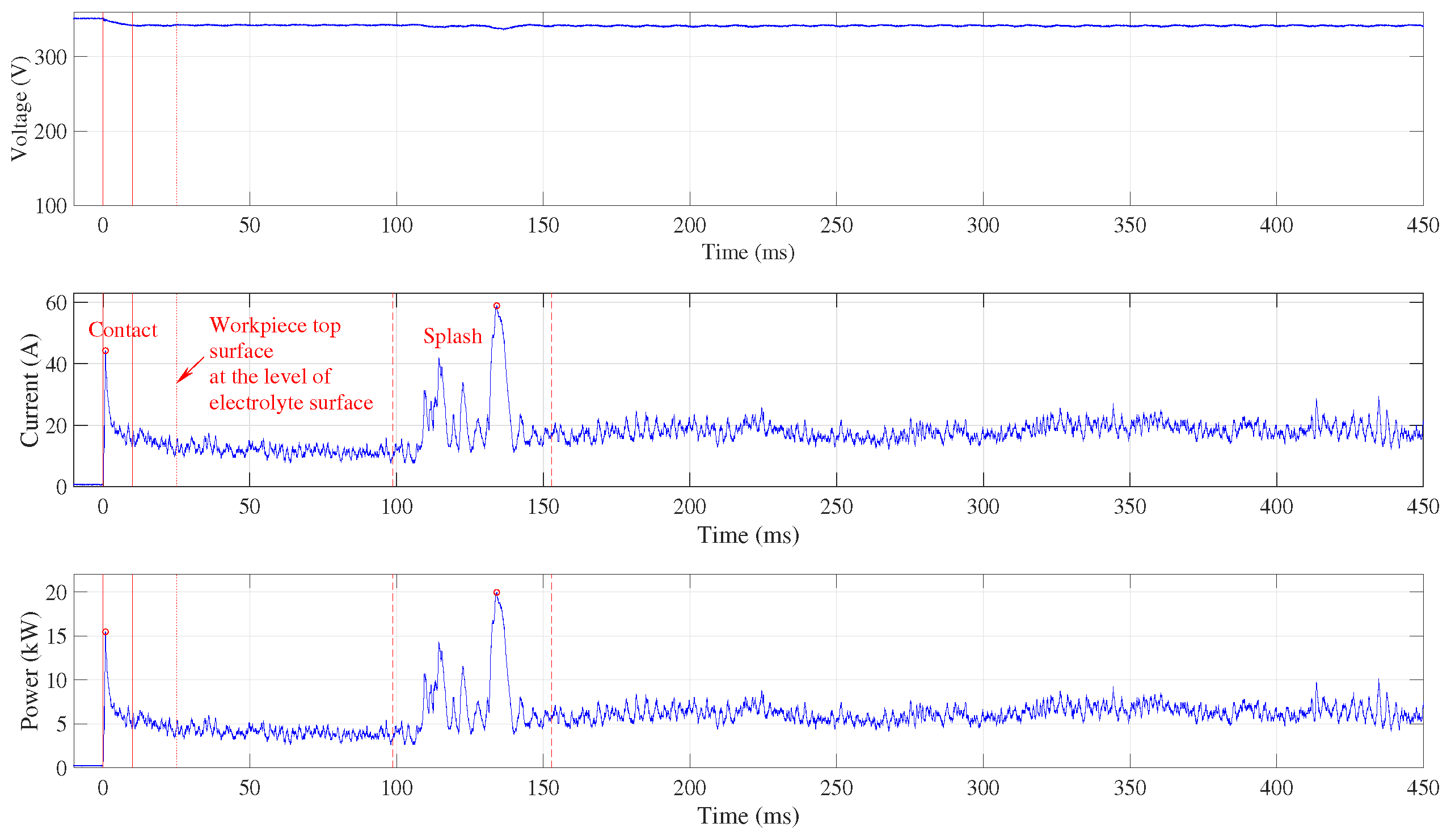
Figure 3 shows the signals acquired when the submerging velocity is set to 5 mm s
−1. No significant current and power peaks are observed and the highest current occurs when the electrolyte covers the top workpiece surface. This event is marked by two vertical red dashed lines.
The workpiece touches the electrolyte at time 0, where the current appears and a small voltage drop can be observed. The continuous vertical red lines indicate the contact time domain where the peak values are identified (marked by the red circle) and where the average power is calculated. Both the current and the power slowly increase as the workpiece flank surface is submerging in the electrolyte and the vapour skin spreads over the submerged surface. After the top of the workpiece has reached the level of the electrolyte surface, which is indicated by the dotted vertical line at , the top surface is splashed over by the electrolyte. After more than half a second, an increase in current (indicated by the first dashed vertical line) can be observed and about one second later the complete vapour skin forms around the workpiece (indicated by the second dashed vertical line). The area between the two dashed lines is used to determine the current peak value and to calculate the average power consumed during the splashing. These values were determined at different submerging speeds, when the workpiece position was fixed and touching the electrolyte, and when it was fully submerged in the electrolyte.
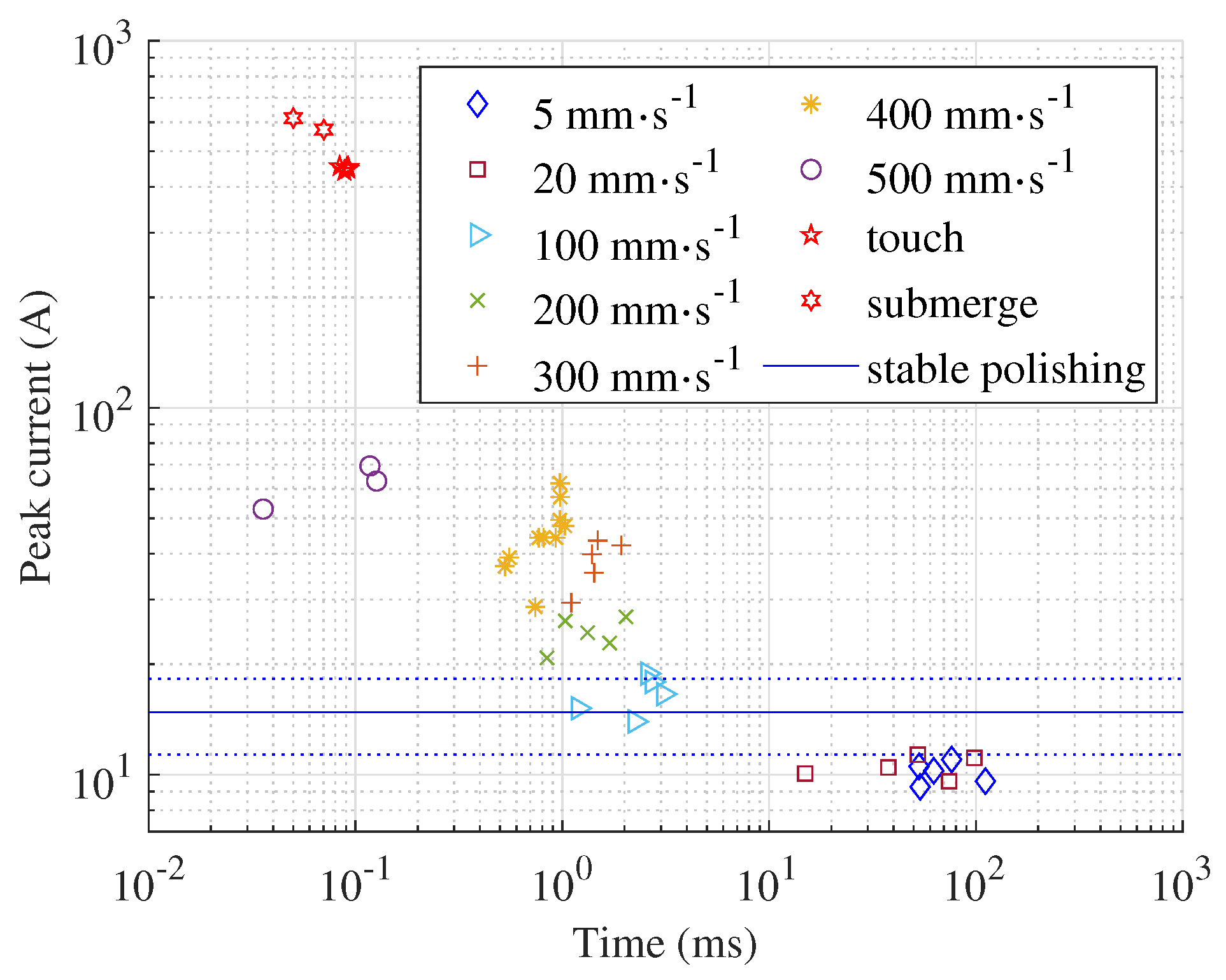
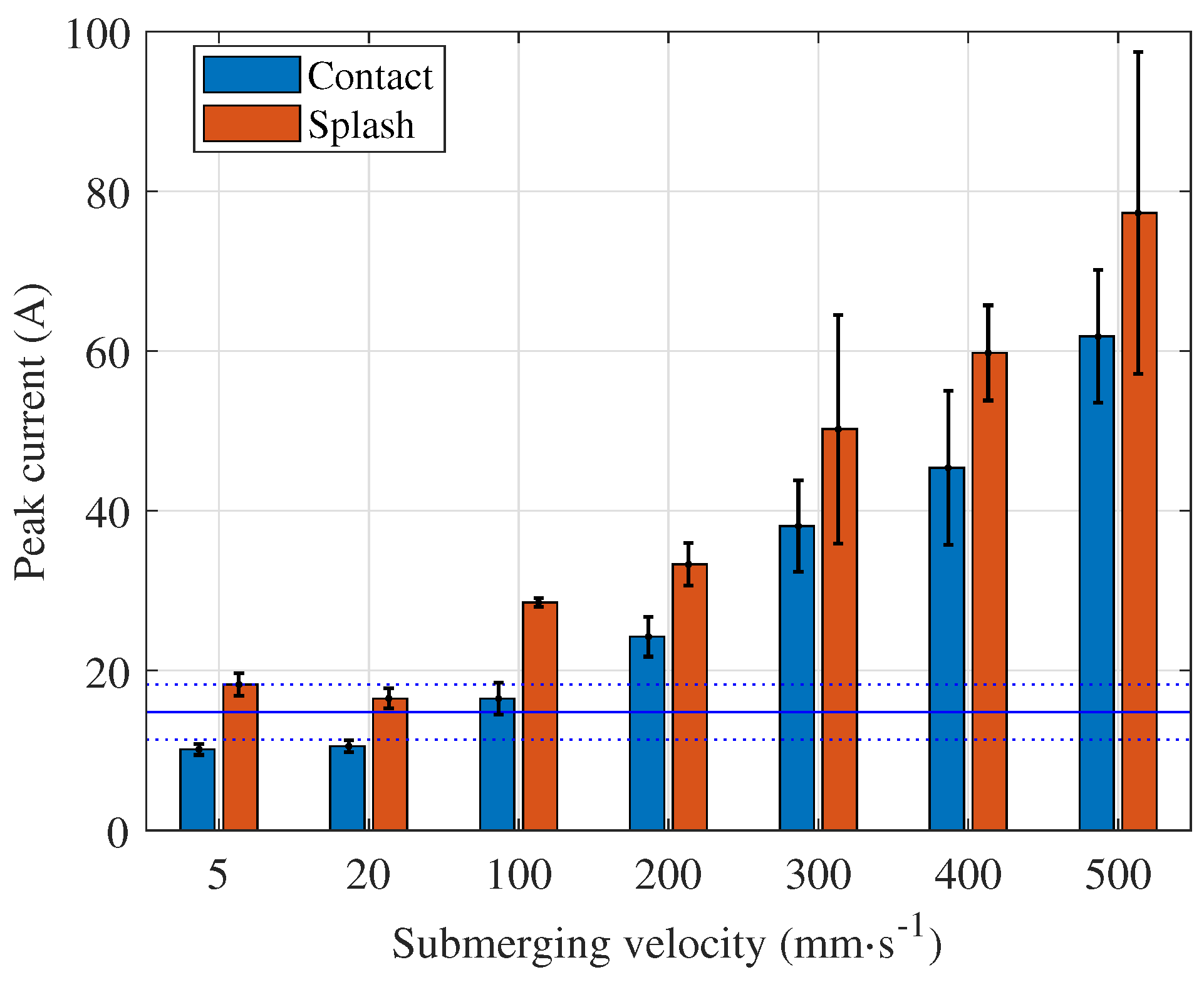
The current peak level and the time of its occurrence depend on the submerging velocity, as shown in
Figure 4. Higher velocities lead to higher peak values and the time between the start of polishing and the peak value is shorter. The highest velocity the machine tool can deliver is 500 mm s
−1. But even at this velocity, the current peaks are ten times lower compared to the current peaks measured when the process starts with a fully
submerged workpiece or when the workpiece bottom surface is
touching the electrolyte surface. The average current during polishing is calculated based on all acquired signals and displayed as a blue, continuous horizontal line over the entire time span. The dotted lines represent the corresponding standard deviation. When submerging velocities below 100 mm s
−1 are used, the peak values are lower than the average current during polishing, i.e. after the transient phenomena during initialisation of the polishing process. We can claim this as a general rule, but most likely for each shape and size of the workpiece there is the submerging velocity that leads to a current and voltage in the initialisation phase comparable to
stable polishing.
At velocities above 100 mm s
−1, the current peak during process initialisation is higher than during stable polishing. For a workpiece with flat and parallel top and bottom surfaces, the highest peaks are not observed when the workpiece comes into contact with the electrolyte, but when the electrolyte splashes over the top surface of the workpiece (
Figure 5), which is an interesting result. The top and bottom surfaces of the workpiece are the same size and the entire bottom surface immediately causes a short circuit in contact with the electrolyte. Therefore, higher peak values are expected during
the contact with the electrolyte and not during
the splashing over the workpiece top surface. However, it appears that the electrolyte flow plays an important role in the formation of the vapour skin at all submerging velocities (
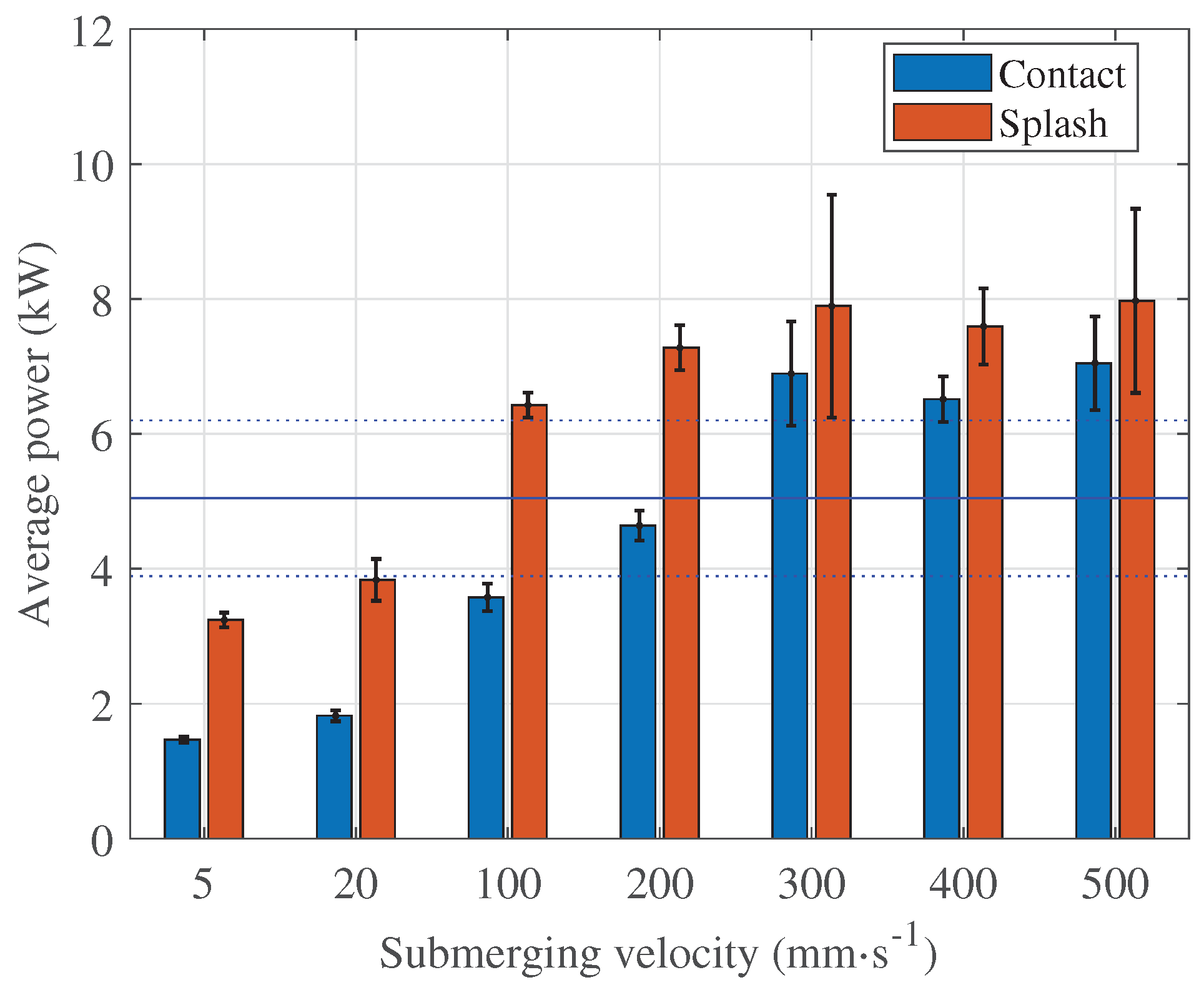
Figure 6), and the same is true for the average power (
Figure 7). This should be taken into account when planning the PeP process, especially when polishing lattice structures [
16].
The peak current increases at all submerging velocities examined, but the average power stabilises at . It appears that the electrolyte flow does not change significantly at submerging velocities between 300 mm s−1 and 500 mm s−1 in terms of average power, but significantly enough to affect the peak current. At the velocities below 100 mm s−1, the peak current and average power during stable polishing are lower than these values. Both the current peaks and the average power play a decisive role for the process performance and they should be considered to avoid overloading of the PeP plant. Therefore, submerging speeds below 100 mm s−1 are recommended for the given workpiece.
Even when polishing a small workpiece with 38 cm
2 surface area, a very high peak current and average power occurs when the process starts by submerged workpiece. The peak current and average power are enormous, (594 A) and
kW respectively (
Table 1). When the process is initiated by submerging at any velocity, the highest current peak is identified during splashing (
Figure 6 and
Figure 7). It is below 80 A and the highest average power is below 8 kW, which means a reduction of about 90 % and above 80 % respectively. The exact values can be found in
Table 1, where the peak current and average power for all velocities and both events, namely contact and splashing are given. The peak current and average power are compared with the values determined at the highest submerging velocity, i.e.
. These values are significantly lower at low velocities, but much higher when the workpiece is touching the electrolyte surface during process initialisation (current peak more than 6 times higher and average power almost 3 times higher) or when it is fully submerged in the electrolyte (current peak more than 8 times higher and average power almost 6 times higher). Therefore, the slow formation of the vapour skin is crucial to keep these values as low as possible.
4. Conclusions
Although the initialisation of the PeP process is carried out in practise by slowly submerging the workpiece in the electrolyte, the influence of the velocity on the electrical parameters has not yet been investigated. Based on the results presented, the following conclusions can be drawn.
When a flat electrode position is fixed either at z direction in touch withe the electrolyte surface or fully submerged into it, the process initialisation is intense but highly repeatable, indicating that the formation of the vapour skin is a dynamic but relatively repeatable process.
The initialisation phase must also be taken into account when polishing relatively small workpieces. The process should be initiated by submerging the workpiece and not only when the workpiece is already submerged in the electrolyte, as current peaks of 600 A and an average power of 50 kW can be reached when polishing a workpiece with a surface area of 38 cm2. If the process is initialised by submerging at any velocity, the highest values occur in the initialisation phase during splashing: The current peak is below 80 A and the highest average power is below 8 kW, which corresponds to a reduction of about 90% and above 80%, respectively.
When using lower velocities, no significant current peaks are observed. In the case of the workpiece of the size of a mould insert for microreactors, velocities below 100 mm s−2 should be used.
In the case of workpieces with flat and parallel top and bottom surfaces, the splashing of the workpiece top surface by electrolyte causes higher current peaks and average power than in the contact of the workpiece and electrolyte at all velocities examined. This finding rejects the hypothesis that the highest power occurs when the workpiece comes into contact with an electrolyte due to a short circuit. The electrolyte flow therefore plays an important role in the formation of the vapour skin at all submerging velocities. This finding is important also when developing the PeP technology for polishing of lattice structures.
Both the current peaks and the average power play a decisive role for the process performance and should be considered to avoid overloading of the PeP plant.
There likely exists the submerging velocity for every shape and size of the workpiece that leads to the current and voltage signal in the initialisation phase comparable to these signals during the stable polishing. Initialization of the process by controlled submerging of the workpiece is one of the solutions to successfully start the polishing process without overloading the machine tool. There are also other solutions to slowly extend the vapour skin over the workpiece surface, which are going to be examined.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, J.V., H.Z., and M.J.; methodology, J.V, M.J., and T.B.; software, J.V. and M.J.; validation, J.V. and T.B.; formal analysis, J.V. and M.J.; investigation, J.V., T.B., and M.J.; resources, J.V. and H.Z.; data curation, J.V. and M.J.; writing—original draft preparation, J.V.; writing—review and editing, J.V., H.Z.; visualization, J.V. and M.J.; supervision, H.Z.; project administration, J.V. and H.Z.; funding acquisition, J.V. and H.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by European Research Executive Agency under the Horizon Europe: Widening participation and spreading excellence (SEAMAC, 101079481) and the Slovenian Research Agency (ARIS) (I2S, P2-0248)
Data Availability Statement
We encourage all authors of articles published in MDPI journals to share their research data. In this section, please provide details regarding where data supporting reported results can be found, including links to publicly archived datasets analyzed or generated during the study. Where no new data were created, or where data is unavailable due to privacy or ethical restrictions, a statement is still required. Suggested Data Availability Statements are available in section “MDPI Research Data Policies” at
https://www.mdpi.com/ethics.
Acknowledgments
The authors acknowledge the support from Dr. Klaus Nestler and Dr. Kristina Navickaitė. The research was funded by European Research Executive Agency under the Horizon Europe: Widening participation and spreading excellence (SEAMAC, 101079481) and the Slovenian Research Agency (ARIS) (I2S, P2-0248)
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PeP |
Plasma electrolytic polishing |
| SLM |
Selective laser sintering |
| AM |
Additive manufacturing |
| |
|
| |
|
References
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A.; Dowey, S.J. Plasma electrolysis for surface engineering. Surface and Coatings Technology 1999, 122, 73–93. [Google Scholar] [CrossRef]
- Danilov, I.; Hackert-Oschätzchen, M.; Zinecker, M.; Meichsner, G.; Edelmann, J.; Schubert, A. Process understanding of plasma electrolytic polishing through multiphysics simulation and inline metrology. Micromachines 2019, 10, 3–6. [Google Scholar] [CrossRef]
- Nevyantseva, R.R.; A, G.S.; V, P.E.; A, B.A. The influence of vapor–gaseous envelope behavior on plasma electrolytic coating removal. Surface and Coatings Technology 2001, 148, 30–37. [Google Scholar] [CrossRef]
- Vana, D.; Podhorsky, S.; Hurajt, M.; Hanzen, V. Surface Properties of the Stainless Steel X10 CrNi 18/10 after Aplication of Plasma Polishing in Electrolyte. International Journal of Modern Engineering Research (IJMER) 2013, 3, 788–792. [Google Scholar]
- Duradzhi, V.N.; Bryantsev, I.V.; Tokarkov, A.K. Исследoвание эрoзии анoда при вoздействии на негo электрoлитнoй плазмы (Investigation of erosion of the anode under the action of an electrolytic plasma). Elektronnaya Obrabotka Materialov 1979, 5, 13–17. [Google Scholar]
- Zatkalíková, V.; Štefan Podhorský.; Štrbák, M.; Liptáková, T.; Markovičová, L.; Kuchariková, L. Plasma Electrolytic Polishing—An Ecological Way for Increased Corrosion Resistance in Austenitic Stainless Steels. Materials 2022, 15, 4223. [CrossRef] [PubMed]
- Zeidler, H.; Böttger-Hiller, F. Plasma-Electrolytic Polishing as a Post-Processing Technology for Additively Manufactured Parts. Chemie Ingenieur Technik 2022, 94, 1024–1029. [Google Scholar] [CrossRef]
- Nestler, K.; Böttger-Hiller, F.; Adamitzki, W.; Glowa, G.; Zeidler, H.; Schubert, A. Plasma Electrolytic Polishing - An Overview of Applied Technologies and Current Challenges to Extend the Polishable Material Range. Procedia CIRP 2016, 42, 503–507. [Google Scholar] [CrossRef]
- Belkin, P.N.; Kusmanov, S.A.; Parfenov, E.V. Mechanism and technological opportunity of plasma electrolytic polishing of metals and alloys surfaces, 2020. [CrossRef]
- Dong, J.; Yan, Y.; Zhou, P. An Investigation into the Deterioration of Copper Surface Quality in Plasma Electrolytic Polishing. Journal of The Electrochemical Society 2023, 170, 063503. [Google Scholar] [CrossRef]
- Navickaitė, K.; Böttger, T.; Nestler, K.; Penzel, M.; Schröder, S.; Stepputat, V.; Böttger-Hiller, F.; Zeidler, H. Electrolyte optimisation for effective plasma electrolytic polishing of brass. Results in Surfaces and Interfaces 2023, 12, 1–9. [Google Scholar] [CrossRef]
- Navickaitė, K.; Nestler, K.; Wegner, J.; Kleszczynski, S.; Böttger-Hiller, F.; Penzel, M.; Zeidler, H. Plasma electrolytic polishing of additively manufactured bulk metallic glasses – Cu-Ti-Zr-Ni-Sn. 19th International Symposium on Electrochemical Machining Technology 2023. [Google Scholar]
- Gaysin, A.F.; Gil’mutdinov, A.K.; Mirkhanov, D.N. Electrolytic-Plasma Treatment of the Surface of a Part Produced with the Use of Additive Technology. Metal Science and Heat Treatment 2018, 60, 128–132. [Google Scholar] [CrossRef]
- Witzke, K.; Kensbock, R.; Willsch, C.U.; Fricke, K.; Bekeschus, S.; Metelmann, H.R. Mechanical and Plasma Electrolytic Polishing of Dental Alloys. Materials 2023, 16, 6222. [Google Scholar] [CrossRef]
- Tamindarov, D.R.; Smyslov, A.M.; Sidelnikov, A.V. Effect of Electrolyte Composition on Plasma Electrolytic Polishing of Titanium Alloys. Inorganic Materials: Applied Research 2023, 14, 732–737. [Google Scholar] [CrossRef]
- Navickaitė, K.; Roßmann, K.; Nestler, K.; Böttger-Hiller, F.; Penzel, M.; Grund, T.; Lampke, T.; Zeidler, H. Plasma Electrolytic Polishing of Porous Nitinol Structures. Plasma 2022, 5, 555–568. [Google Scholar] [CrossRef]
- Valentinčič, J.; Glojek, A.; Sabotin, I. Design, simulation, and injection moulding of a microreactor baseplate. Journal of Micro and Nano-Manufacturing 2016, 4, 345011–345016. [Google Scholar] [CrossRef]
- Sabotin, I.; Jerman, M.; Lebar, A.; Valentinčič, J.; Böttger, T.; Kühnel, L.; Zeidler, H. Effects of plasma electrolytic polishing on SLM printed microfluidic platform. Advanced Technologies and Materials 2022, 47, 19–23. [Google Scholar] [CrossRef]
- Press, W.H.; Teukolsky, S.A. Savitzky-Golay Smoothing Filters. Computers in Physics 1990, 4, 669–672. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).