1. Introduction
Platinum- and taxane-based chemotherapy is the standard of care for patients with advanced gynecologic cancers [
1,
2,
3,
4]. However, multiple treatments with the same drug, such as platinum and taxanes, may result in oncologic resistance and hypersensitivity reactions (HSR). This has an impact on further treatment and outcome by necessitating a switch to a chemotherapy regimen that is less effective and more toxic [
5,
6,
7,
8]. Pre-medication with antihistamines and corticosteroids is usually recommended for mild HSR and routinely administered for taxane and platinum-based chemotherapy [
6,
7,
8,
9]. However, premedication is not effective in preventing more severe allergic reactions, particularly those to platinum salts [
10,
11].
Desensitization is the establishment of a temporary tolerance to a substance that previously triggered HSR [
12]. It should be considered in patients with HSR as a safe alternative to platinum salts and taxanes in the use of standard chemotherapy, aiming at the best therapeutic results according to international standards [
13,
14,
15,
16].
Platinum hypersensitivity affects approximately 5% of the general oncologic population and 8 to 16% of women with gynecologic cancers, and taxane hypersensitivity affects 10 to 13% of the general oncologic population [
13,
14,
17,
18,
19]. This is of clinical importance and justifies an optimal strategy for the maintenance of treatment. At present, desensitization techniques are well established and there are international guidelines for their administration [
20]. However, clinical effects of desensitization are rarely studied and few specialized centers offer desensitization as part of standard practice.
Therefore, the aims of our cross-sectional study were to 1) reveal the current management of HSRs to platinum and/or taxane chemotherapy in patients with gynecologic cancer and 2) determine if there is a need for standardizing the management of HSRs for best patient care.
2. Materials and Methods
Study Design
We conducted a cross-sectional survey among gynecological and medical oncologists. SurveyMonkey software was used to create and distribute the questionnaire. The survey was posted online on the European Network of Young Gynaecological Oncologists (ENYGO), European Society of Gynaecological Oncology (ESGO), and Oncoalert social media channels, Furthermore, we sent emails to the ENYGO members and subscribers of the Oncoalert Newsletter. We collected data between April 2023 to September 2023.
Variables
The survey consisted of 33 questions in English, prepared and based on the expertise of gynecological and medical oncologists treating patients with gynecological cancers (
Supplementary file S1). The questionnaire contained four main sections including 1) general demographic information (nine questions), 2) HSR and platinum-based chemotherapy (11 questions), 3) HSR and chemotherapy with taxane (11 questions), and 4) future direction of HSR and desensitization in gynecological cancer (two questions). HSR Grade was defined as Common Terminology for Adverse Events (CTCAE) Grade 1-4. Briefly, CTCAE 1, 2, 3, and 4 refer to mild, moderate, severe, and life-threatening adverse events, respectively.
Data Sources/Measurement
The software SurveyMonkey was used for questionnaire creation and distribution. Data were collated from members and followers of European Network of ENYGO and ESGO (Newsletter including HSR Survey received=1938 and opened=106). Additionally, the survey was put online on ENYGO and Oncoalert social media channels.
Statistical Analyses
Descriptive statistics are presented as counts and frequencies for categorical data and medians (range) for metric or ordinal variables. In case of medians p-values correspond to the Kruskall-Wallis tests, in case of categorical data p-values correspond to Fisher's exact tests. P-values of group comparisons correspond to log-rank tests. A P value < 0.05 was considered significant. All analyses were performed using the Statistical Package for the Social Sciences (SPSS) for Macintosh, Version 28.0 (IBM Corp., Armonk, NY, USA).
3. Results
Demographic Data
A total of 144 respondents from 33 countries completed the survey. The final analysis included 133 respondents, of whom 79 administered platinum-based and 67 taxane-based chemotherapy. We excluded respondents who were only involved in the treatment of breast cancer (n=6), respondents who did not treat gynecological cancers (n=5), and one respondent who was a nurse. The countries with the highest participation were Switzerland (10.5%), Italy (9%) and Germany (8.3%) (
Table 1,
Supplementary file S1). The gender of the participants was balanced with 54.9% female and 45.1% male with a mean age of 38 years. The majority of participants were gynecological oncologists (43.6%) and medical oncologists (33.8%). Seventy-six participants (57.1%) were working mainly in a university hospital and 103 participants (77.4%) were involved in chemotherapy treatment. The clinical experience of the participants was well balanced, with 32.3% having less than 5 years, 33.8% 5-10 years, and 33.8% more than 10 years. Detailed demographic characteristics of these participants were shown in
Table 1.
HSR and Platinum-Based Chemotherapy
Out of the 79 participants who administered platinum-based chemotherapy, more than half of them treated more than 100 gynecological cancer patients per year with platinum-based chemotherapy (
Table 2). The majority (73.4%) of participants experienced more than five platinum HSRs per year. In 84.8% of Grade 1 and 2 HSRs (according to CTCAE), participants used pre-medication with antihistamines/steroids and made a new attempt with standard infusion of platinum-based chemotherapy. However, 41.8% used desensitization in these patients, and only 15.2% stopped chemotherapy and 8.9% changed the treatment regimen, respectively . In contrast, in cases with Grade 3-4 HSR 35.4% of the participants suspended chemotherapy while 34.1% changed the regimen. There was a minimal increase in the use of desensitization in patients with Grade 3-4 HSR compared to patients with Grade 1-2 HSR (49.4% vs. 41.8%). Desensitization was mainly performed by medical oncologists (40.5%) and allergologists (27.8%) in their own clinics. Sixty-seven percent of the participants were able to continue platinum-based chemotherapy after tolerance was achieved in more than 50% of the cases. However, 45.6% of participants experienced one or more critical events during tolerance induction, mainly due to recurrent HSR CTCAE Grade 1-2 (47.2%) and Grade 3-4 (66.7%).
HSR and Taxane-Based Chemotherapy
A total of 67 participants administered taxane-based chemotherapy, with the majority treating more than 100 gynecological cancer patients per year and regularly experiencing HSR in their patients (
Table 2). Pre-medication with antihistamines and steroids together with a retry of the standard infusion was the main mode of action for Grade 1-2 HSR (92.5%). In contrast, in Grade 3-4 HSR, the mode of action was balanced (
Table 2). When desensitization was used, the majority were able to continue with standard taxane treatment. Desensitization was performed by medical oncologists (34.3%), allergologists (23.9%), and gynecological oncologists (10.4%). Fifty-two percent never experienced a critical incident during tolerance induction, but 25.4% experienced it more than once. The main reasons for critical incidents are HSR to taxane CTCAE Grade 1 and 2 (34.5%) and Grade 3-4 (62.1%).
Desensitization of HSR in Gynecological Cancers
The majority (53.3%) of participants without experience in chemotherapy treatment were not aware of desensitization of HSR to platinum and taxane based chemotherapy, whereas the majority of participants involved in chemotherapy treatment were aware of desensitization. However, participants strongly emphasized the need to standardise the management of platinum and taxane HSR in gynecological cancer and to develop international guidelines, regardless of their involvement in chemotherapy treatment (
Table 3).
Management of Hypersensitivity Reactions Based on Length of Clinical Practice Experience
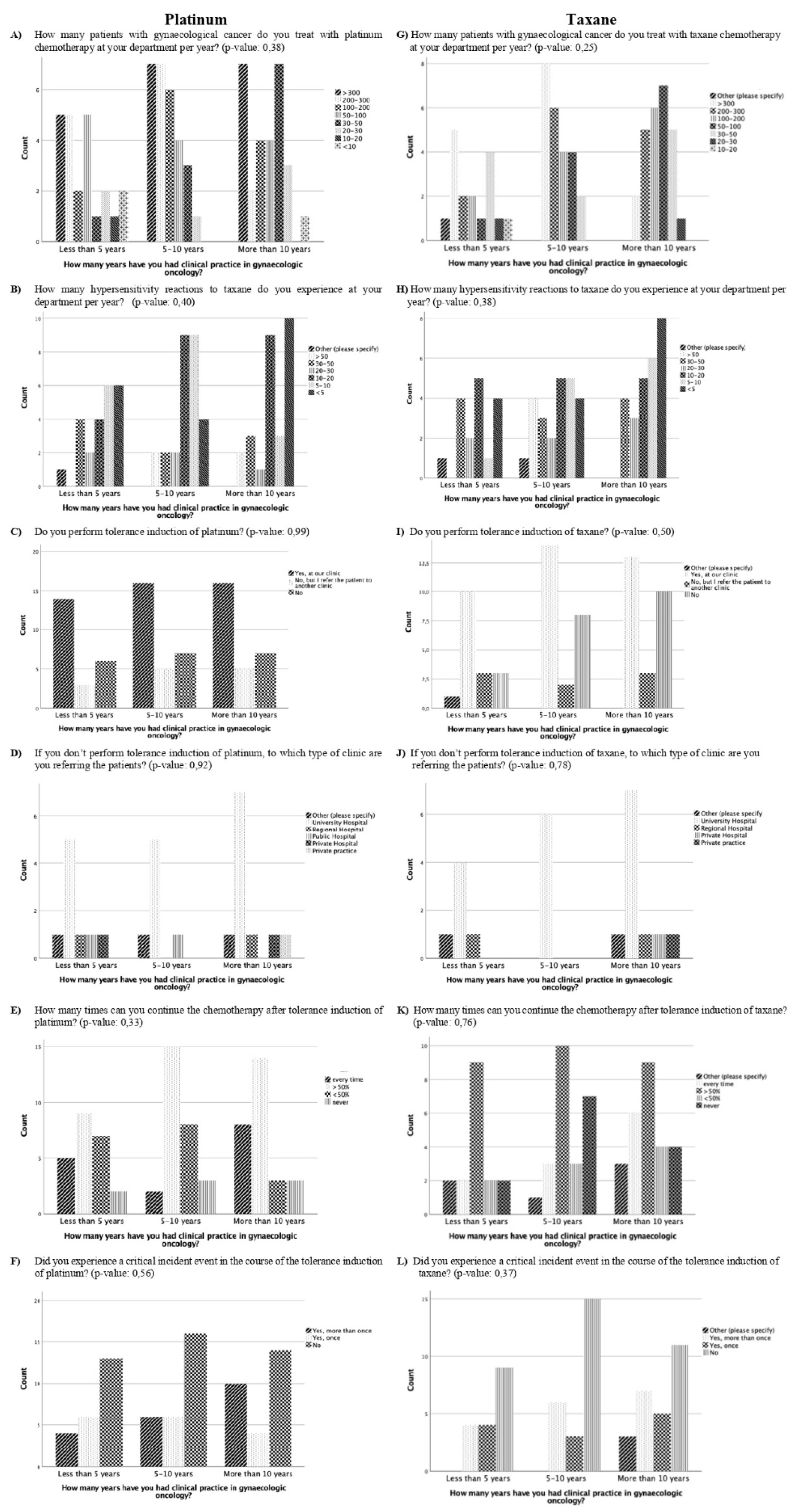
No significant difference was evident in the results of the questions comparing participants with less than five years, five to ten years, or more than ten years of clinical practice experience (
Figure 1). However, participants with more than ten year’s experience were more likely to report experiencing <5 or 5-10 HSR to taxane per year than the participants with five to ten year’s experience or less than 5 year’s experience (
p=0.38, 8 vs. 6 vs. 4 and 5 vs. 4 vs 1, respectively) (
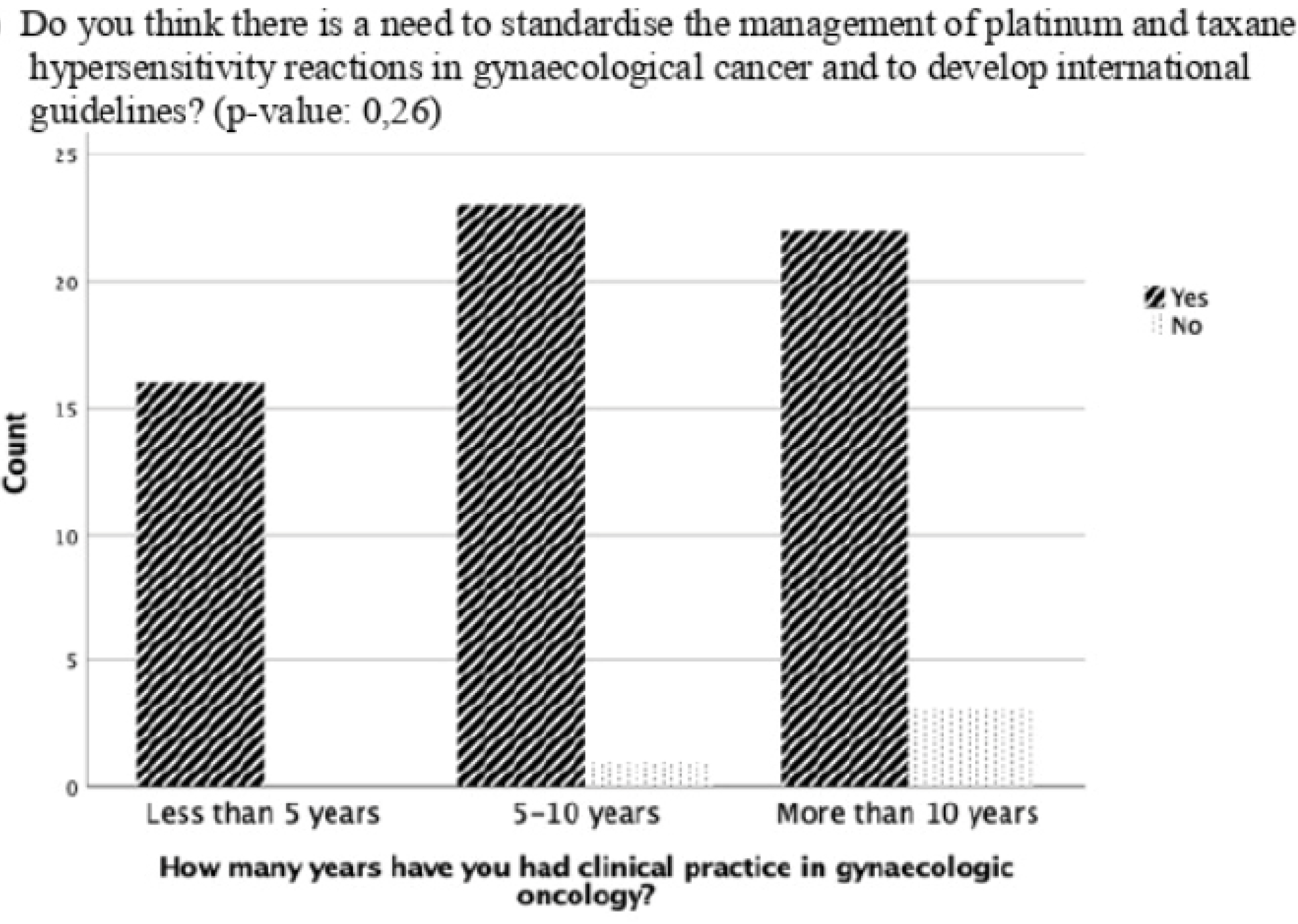
Figure 1). In addition, there was a trend that the longer the experience in clinical practice, the more often the participants did not think there was a need for standardisation and guidelines for managing HSR with taxane and platinum (
p=0.26) (
Figure 2).
4. Discussion
The majority of the participants had a high frequency of HSR to taxane and platinum in their clinical practice, with more than five HSRs per year. Management of HSR is heterogeneous and depends on the grade of HSR. Overall, we found that the use of desensitization for HSR to taxane and platinum in clinical practice is low at less than 50% and guidelines for the treatment of HSR with taxane and platinum in gynecological cancers were of great interest to clinicians, regardless of their experience with chemotherapy.
Clinicians treating gynecological cancers regularly experience HSR to platinum- and taxane-based chemotherapy (8-16% and 10-13%, respectively) [
7,
8,
13,
14,
17,
18,
19], which is also due to the fact that more lines of treatment are being used in gynecological cancer than 1-2 decades ago [
21]. Additionally, real HSR rates are likely to be underestimated, as oncologists often report only severe reactions [
6,
22]. Our study showed a high frequency of HSR, with more than 73% of the participants treating more than five patients with HSR to platinum and taxane-based chemotherapy, which emphasizes the need to find a strategy to maintain the optimal treatment regimen in this large group of patients.
The treatment strategy for HSR to taxanes and platinum often depends on its Grade. For mild HSR, pre-medication with antihistamines and corticosteroids is typically recommended and routinely used [
6,
7,
8,
9]. This is well represented in our study, with more than 84.8% and 92% of patients with Grade 1-2 HSR receiving platinum and taxane, respectively, regularly pre-medicated with antihistamines and corticosteroids. However, pre-medication is ineffective in preventing more severe HSR (Grade 3-4) to platinum and taxane, and therefore desensitization should be considered to continue standard chemotherapy for the best therapeutic outcome in these patients [
10,
11,
13,
14,
15,
16].
The results of our survey showed a higher use of desensitization in Grade 3-4 HSR compared to Grade 1-2 HSR with platinum and taxane (49.4% and 48.8% vs 41.8% and 20.9%, respectively). Moreover, 31.6% and 44.8% of the participants regularly premedicate their patients with antihistamines and corticosteroids for Grade 3-4 HSR to platinum and taxane, respectively. In addition, there is a high rate of switching to another treatment regimen with 34.1% for platinum and 44.8% for taxane-based chemotherapy. This may be explained by the finding that only 67.1% of patients after platinum desensitization and 58.2% of patients after taxane desensitization had a high likelihood (>50%) of continuing platinum- or taxane-based chemotherapy. In addition, there was a high incidence of critical events (40%) during the desensitization process. This is in contrast to what is known about the safety of desensitization procedures and their management [
13,
14,
15,
16,
19,
20], This is important to address as an improved outcome for overall survival has been demonstrated in hypersensitive patients receiving carboplatin desensitization compared to non-hypersensitive patients in relapsed ovarian cancer, independent of germline
BRCA status [
23]. One way to address this important issue is to standardise the management of platinum and taxane HSR in gynecological cancer by developing international guidelines. This was particularly emphasized by participants with (59.2%) and without (83.3%) experience of medical treatment of gynecological cancer patients.
This survey provides a global representation of participants and their current management of platinum and taxane HSR in gynecological cancer. An advantage is the direct feedback from clinicians regularly confronted with HSR in their daily clinical practice on their awareness and views on this topic. However, the small cohort size is a weakness of this study and limits the statistical power of the results. This is an anticipated problem with survey studies, especially when the target group are clinicians with a heavy workload and limited time to complete a survey. However, a variety of methods were used to distribute the survey, including the official social media channels of ENYGO, ESGO, and Oncoalert, and distribution of the survey via an email system to ENYGO, ESGO, and Oncoalert databases. However, the variety of methods makes it impossible to know the response rate to this survey. Additionally, the fact that 23% of the respondents were not involved in chemotherapy treatment is a major limitation, as this could bias the results. To account for this, we analysed the results of the questionnaire on HSR to platinum and taxane-based chemotherapy only for those respondents involved in chemotherapy treatment.
Currently, only a limited number of cancer centres have established desensitization as part of their standard practice. However, desensitization protocols for patients with taxane and platinum HSRs are available and recommended [
6,
7,
8,
19,
20]. Knowledge of desensitization procedures in gynecological oncology could be optimized by regular analysis and management of successful tolerance induction to platinum and taxanes in patients with HSR. This is important to achieve an optimal treatment in accordance with international standards [
10,
11]. However, since the goal is to provide the best treatment within the recommended timeframe, it is also important not to delay planned chemotherapy for desensitization. For this reason, patients who develop an HSR should be seen and tested within one to two weeks of the reaction. Our study shows the willingness of the participants to use the guidelines for the treatment of HSR when they become available.
5. Conclusions
Our cross-sectional survey showed that HSR in gynecological cancer is common, but management is variable with low use of desensitization. In addition, clinical practitioners emphasized the need for standardization and guidelines for the management of HSR to platinum and taxane in gynecological cancer.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org, Supplementary file S1: Survey with thirty-three questions, prepared and based on the expertise of gynecological and medical oncologists treating patients with gynecological cancers.
Author Contributions
Conceptualization, Tibor Zwimpfer, Esra Bilir, Nicolò Bizzarri, Céline Montavon and Viola Heinzelmann-Schwarz; Data curation, Tibor Zwimpfer, Esra Bilir, Khayal Gasimli, Andrej Cokan, Joanna Kacperczyk-Bartnik, Tanja Nikolova, Ilker Kahramanoglu, Aleksander Shushkevich and Tereza Cicakova; Formal analysis, Tibor Zwimpfer, Esra Bilir, Khayal Gasimli and Joanna Kacperczyk-Bartnik; Funding acquisition, Tibor Zwimpfer; Investigation, Tibor Zwimpfer, Esra Bilir, Khayal Gasimli, Zoia Razumova, Tanja Nikolova, Andrei Pletnev, Ilker Kahramanoglu, Aleksander Shushkevich, Aleksandra Strojna, Charalampos Theofanakis and Tereza Cicakova; Methodology, Tibor Zwimpfer, Khayal Gasimli, Andrej Cokan, Zoia Razumova, Tanja Nikolova, Céline Montavon and Gilberto Morgan; Project administration, Tibor Zwimpfer, Nicolò Bizzarri, Zoia Razumova, Ilker Kahramanoglu, Aleksander Shushkevich, Tereza Cicakova and Viola Heinzelmann-Schwarz; Resources, Tibor Zwimpfer, Nicolò Bizzarri, Zoia Razumova, Aleksandra Strojna, Charalampos Theofanakis, Tereza Cicakova, Marcus Vetter, Gilberto Morgan and Viola Heinzelmann-Schwarz; Software, Esra Bilir and Tereza Cicakova; Supervision, Tibor Zwimpfer, Nicolò Bizzarri, Andrei Pletnev, Aleksandra Strojna, Marcus Vetter, Céline Montavon, Gilberto Morgan and Viola Heinzelmann-Schwarz; Visualization, Esra Bilir; Writing – original draft, Tibor Zwimpfer, Esra Bilir, Khayal Gasimli, Andrej Cokan, Charalampos Theofanakis and Viola Heinzelmann-Schwarz; Writing – review & editing, Tibor Zwimpfer, Esra Bilir, Khayal Gasimli, Andrej Cokan, Nicolò Bizzarri, Zoia Razumova, Joanna Kacperczyk-Bartnik, Tanja Nikolova, Andrei Pletnev, Ilker Kahramanoglu, Aleksander Shushkevich, Aleksandra Strojna, Charalampos Theofanakis, Marcus Vetter, Céline Montavon and Viola Heinzelmann-Schwarz.
Funding
This work was supported by the Swiss National Foundation [P500PM_20726/1, 2021]; Bangerter-Rhyner Stiftung [0297, 2021]; and Freie Gesellschaft Basel [2022].
Institutional Review Board Statement
All study activities were conducted in accordance with Institutional Review Board (IRB) guidelines for exempt studies. All methods were carried out in accordance with relevant guidelines and regulations. A formal IRB certification of exemption (Req-2021-00692) was provided by the ethics committee of Northwest and Central Switzerland (EKNZ), on the 24th of June 2021. The EKNZ can confirm that the research project (Req-2021-00692) fulfilled the general ethical and scientific standards for research with human subjects. The anonymization of personal data was guaranteed.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets that have been used and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors would like to thank all the participants of the survey. We also acknowledge the ESGO office's support in all project stages.
Conflicts of Interest
The authors declare that they have no conflicts of interest or financial ties to disclose.
References
- Oaknin, A, Bosse TJ, Creutzberg CL, Giornelli, G, Harter, P, Joly, F, et al. Endometrial cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022, 33, 860–877. [CrossRef] [PubMed]
- Ledermann JA, Matias-Guiu, X, Amant, F, Concin, N, Davidson, B, Fotopoulou, C, et al. ESGO-ESMO-ESP consensus conference recommendations on ovarian cancer: pathology and molecular biology and early, advanced and recurrent disease. Ann Oncol. 2024. [CrossRef]
- Gonzalez-Martin, A, Harter, P, Leary, A, Lorusso, D, Miller RE, Pothuri, B, et al. Newly diagnosed and relapsed epithelial ovarian cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol, 2023; 34, 833–848. [CrossRef]
- Marth, C, Landoni, F, Mahner, S, McCormack, M, Gonzalez-Martin, A, Colombo, N, Committee EG. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018, 29 (Suppl 4), iv262. [CrossRef]
- Castells, M, Sancho-Serra Mdel, C, Simarro M. Hypersensitivity to antineoplastic agents: mechanisms and treatment with rapid desensitization. 2012, 61, 1575–1584. [CrossRef]
- O'Malley DM, Vetter MH, Cohn DE, Khan, A, Hays JL. Outpatient desensitization in selected patients with platinum hypersensitivity reactions. Gynecol Oncol. 2017, 145, 603–610. [CrossRef] [PubMed]
- Moon DH, Lee JM, Noonan AM, Annunziata CM, Minasian, L, Houston, N, et al. Deleterious BRCA1/2 mutation is an independent risk factor for carboplatin hypersensitivity reactions. Br J Cancer. 2013, 109, 1072–1078. [CrossRef] [PubMed]
- Pagani, M, Bavbek, S, Alvarez-Cuesta, E, Berna Dursun, A, Bonadonna, P, Castells, M, et al. Hypersensitivity reactions to chemotherapy: an EAACI Position Paper. Allergy. 2022, 77, 388–403. [CrossRef] [PubMed]
- Boulanger, J, Boursiquot JN, Cournoyer, G, Lemieux, J, Masse MS, Almanric, K, et al. Management of hypersensitivity to platinum- and taxane-based chemotherapy: cepo review and clinical recommendations. Curr Oncol. 2014, 21, e630–41. [CrossRef] [PubMed]
- Pagani, M. The complex clinical picture of presumably allergic side effects to cytostatic drugs: symptoms, pathomechanism, reexposure, and desensitization. Med Clin North Am. 2010, 94, 835–852. [Google Scholar] [CrossRef] [PubMed]
- Dizon DS, Sabbatini PJ, Aghajanian, C, Hensley ML, Spriggs DR. Analysis of patients with epithelial ovarian cancer or fallopian tube carcinoma retreated with cisplatin after the development of a carboplatin allergy. Gynecol Oncol. 2002, 84, 378–382. [CrossRef] [PubMed]
- Cernadas JR, Brockow, K, Romano, A, Aberer, W, Torres MJ, Bircher, A, et al. General considerations on rapid desensitization for drug hypersensitivity - a consensus statement. Allergy. 2010, 65, 1357–1366. [CrossRef] [PubMed]
- Vetter MH, Khan, A, Backes FJ, Bixel, K, Cohn DE, Copeland LJ, et al. Outpatient desensitization of patients with moderate (high-risk) to severe platinum hypersensitivity reactions. Gynecol Oncol. 2019, 152, 316–321. [CrossRef] [PubMed]
- Tsao LR, Young FD, Otani IM, Castells MC. Hypersensitivity Reactions to Platinum Agents and Taxanes. Clin Rev Allergy Immunol. Clin Rev Allergy Immunol. 2022, 62, 432–448. [CrossRef] [PubMed]
- Sloane, D, Govindarajulu, U, Harrow-Mortelliti, J, Barry, W, Hsu FI, Hong, D, et al. Safety, Costs, and Efficacy of Rapid Drug Desensitizations to Chemotherapy and Monoclonal Antibodies. J Allergy Clin Immunol Pract. 2016, 4, 497–504. [CrossRef] [PubMed]
- Rosello, S, Blasco, I, Garcia Fabregat, L, Cervantes, A, Jordan, K, Committee EG. Management of infusion reactions to systemic anticancer therapy: ESMO Clinical Practice Guidelines. Ann Oncol. 2017; 28, (Suppl 4), iv100–iv118. [CrossRef]
- Koshiba, H, Hosokawa, K, Kubo, A, Miyagi, Y, Oda, T, Miyagi, Y, et al. Incidence of Carboplatin-related hypersensitivity reactions in Japanese patients with gynecologic malignancies. Int J Gynecol Cancer. 2009, 19, 460–465. [CrossRef] [PubMed]
- Sendo, T, Sakai, N, Itoh, Y, Ikesue, H, Kobayashi, H, Hirakawa, T, et al. Incidence and risk factors for paclitaxel hypersensitivity during ovarian cancer chemotherapy. Cancer Chemother Pharmacol. Cancer Chemother Pharmacol. 2005, 56, 91–96. [CrossRef] [PubMed]
- Zwimpfer TA, Scherer, K, Schotzau, A, Heinzelmann-Schwarz, V, Hartmann, K, Vetter, M, Montavon C. Desensitization in patients with hypersensitivity to platinum and taxane in gynecological cancers. Cancer Med. 2023, 13, e6840. [CrossRef]
- Scherer, K, Brockow, K, Aberer, W, Gooi JH, Demoly, P, Romano, A, et al. Desensitization in delayed drug hypersensitivity reactions -- an EAACI position paper of the Drug Allergy Interest Group. Allergy. 2013, 68, 844–852. [CrossRef] [PubMed]
- Kessous, R, Wissing MD, Laskov, I, Abitbol, J, Bitharas, J, Agnihotram VR, et al. Multiple lines of chemotherapy for patients with high-grade ovarian cancer: Predictors for response and effect on survival. Int J Cancer. 2021, 148, 2304–2312. [CrossRef] [PubMed]
- Shepherd, GM. Hypersensitivity reactions to chemotherapeutic drugs. Clin Rev Allergy Immunol. 2003, 24, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Altwerger, G, Florsheim EB, Menderes, G, Black, J, Schwab, C, Gressel GM, et al. Impact of carboplatin hypersensitivity and desensitization on patients with recurrent ovarian cancer. J Cancer Res Clin Oncol. 2018, 144, 2449–2456. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).