Submitted:
20 February 2024
Posted:
21 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental
2.1. Materials
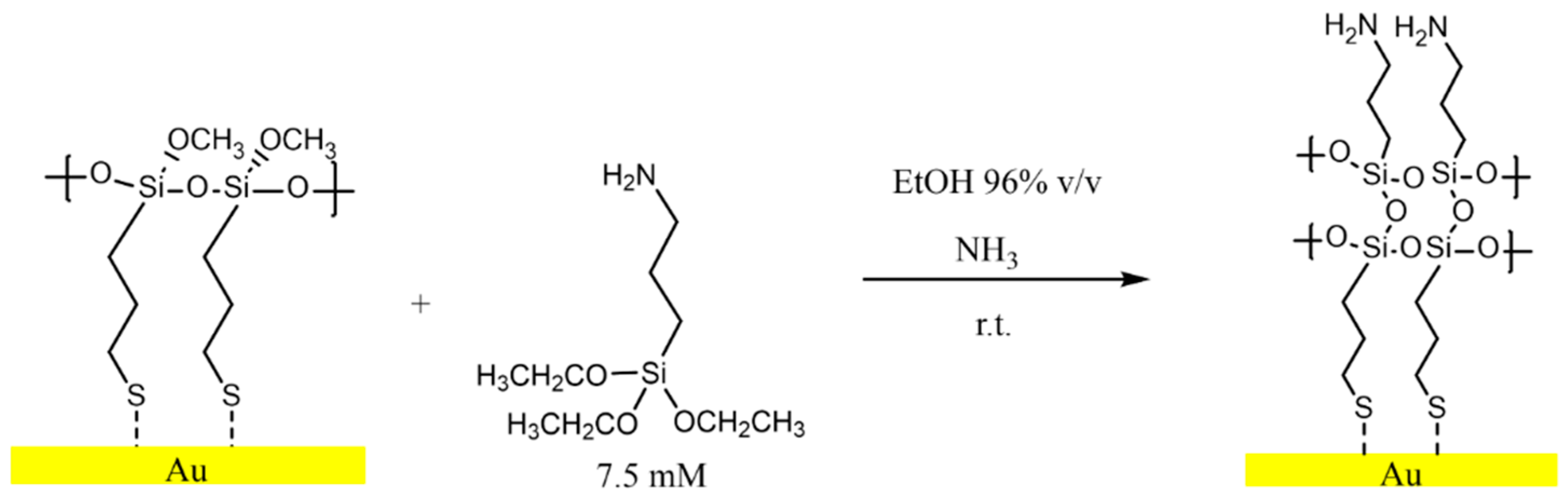
2.2. MPTMS Functionalization
2.3. APTES Functionalization
2.4. X-ray Photoelectron Spectroscopy (XPS) and Angle-Resolved X-ray Photoelectron Spectroscopy (AR-XPS)
3. Results
3.1. X-ray Photoelectron Spectroscopy of Freshly Cleaved Gold before Functionalization
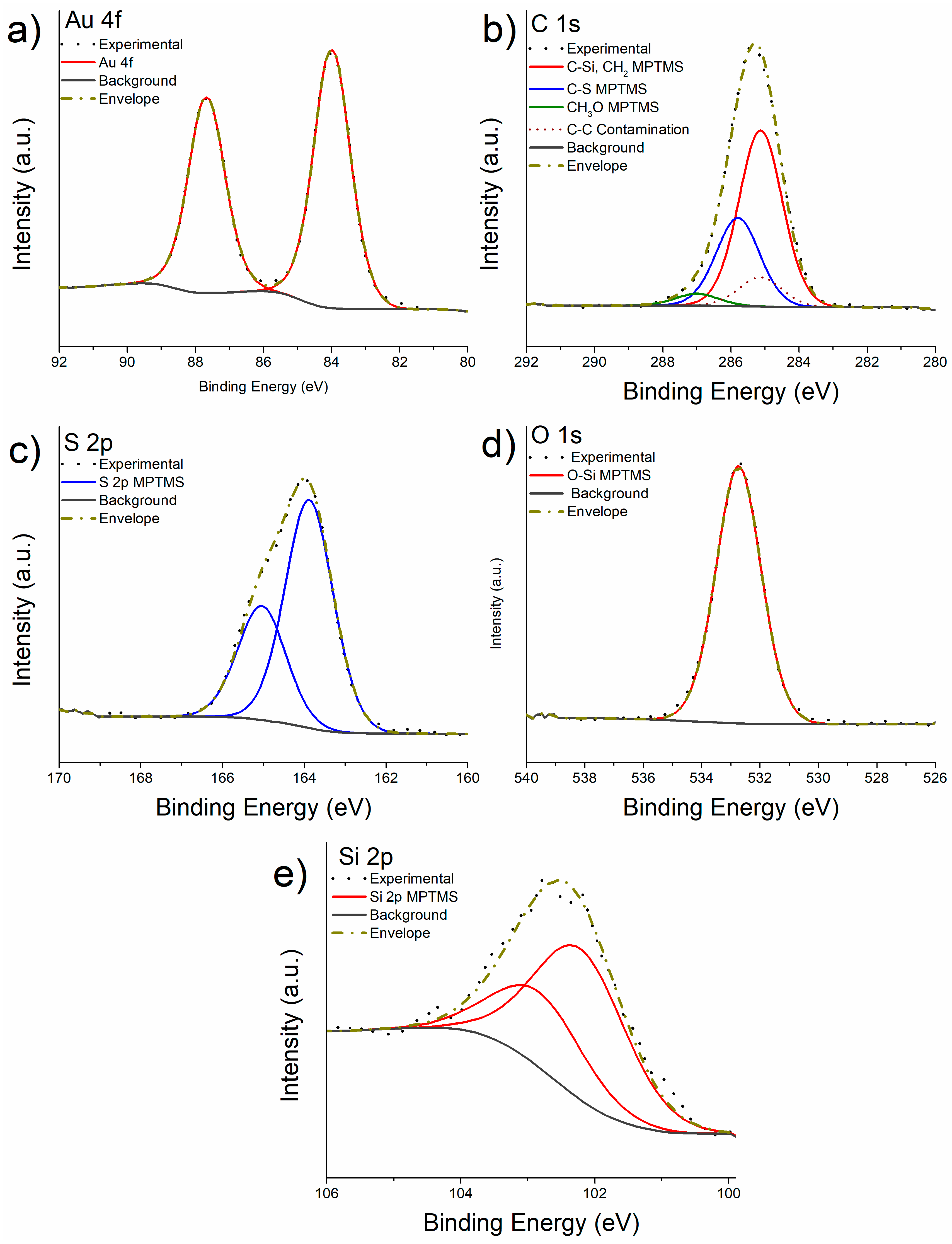
3.2. X-ray Photoelectron Spectroscopy after Functionalization with MPTMS
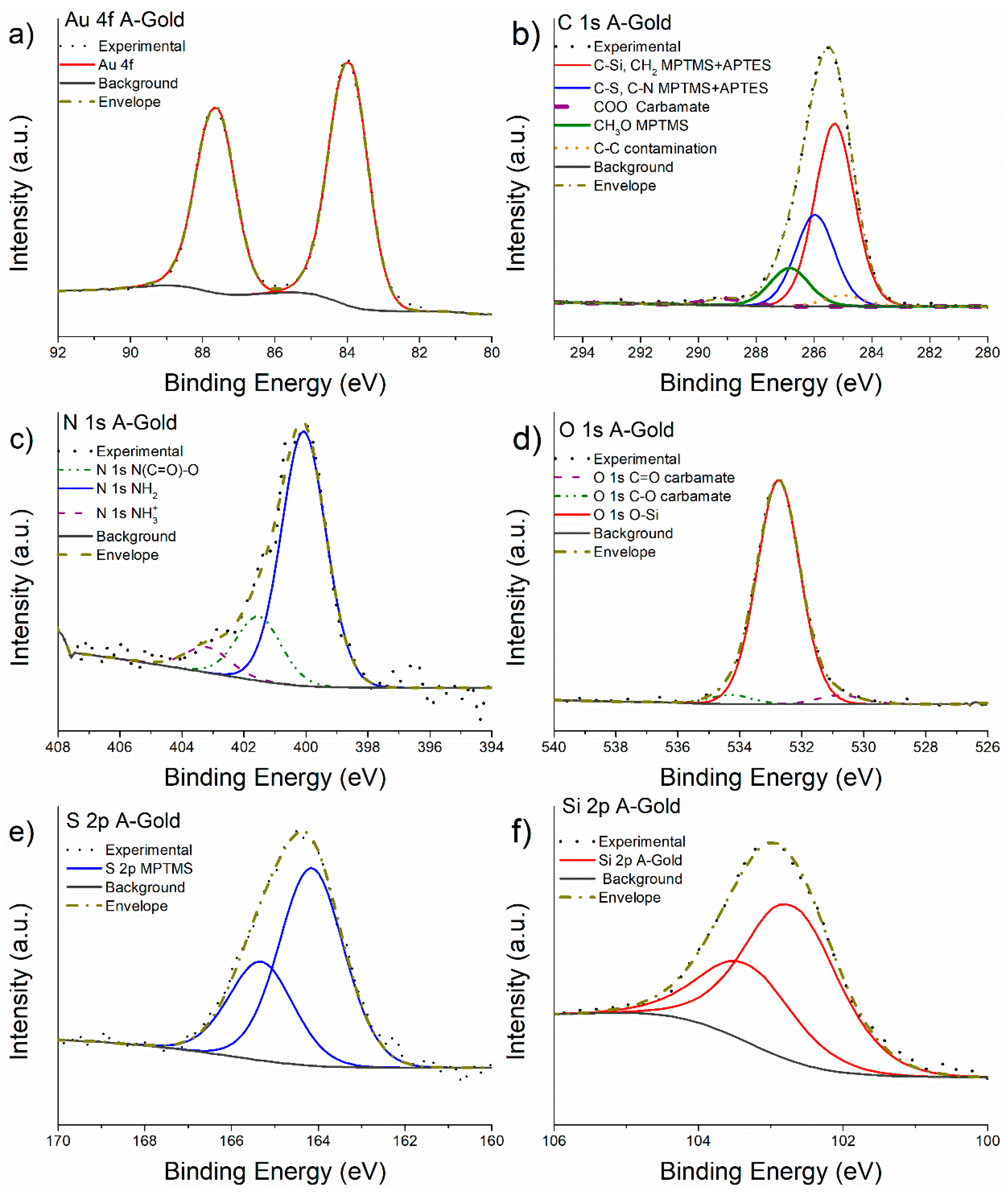
3.3. X-ray Photoelectron Spectroscopy of MPTMS Functionalized Gold after Grafting with APTES
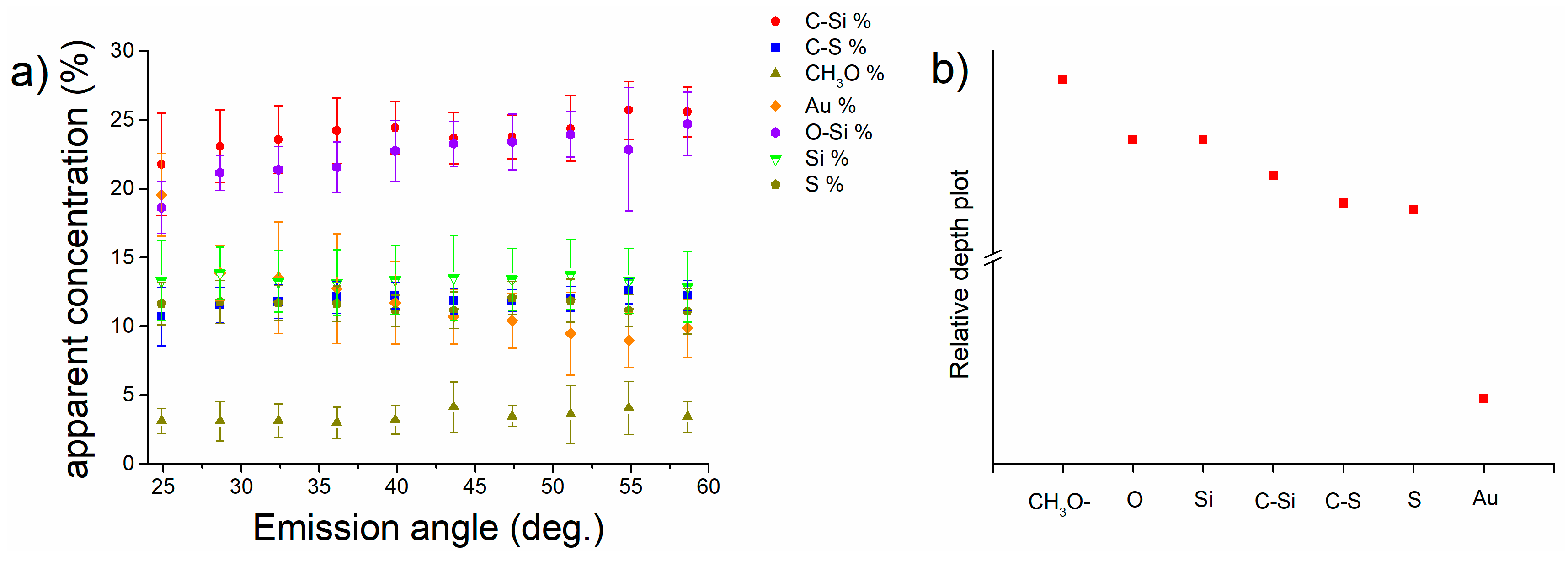
3.4. Angle-Resolved X-ray Photoelectron Spectroscopy on MPTMS Functionalized Gold M-Gold
3.5. Angle-Resolved X-ray Photoelectron Spectroscopy of A-Gold
3.6. Calculation of the Layer Thickness
4. Discussion
4.1. Understanding APTES Functionalization Mechanisms
4.2. Models for the Functionalized M-Gold and A-Gold Surfaces
4.3. Thickness and Composition of the Functionalized Layers
4.3.1. Layer Thickness
4.3.2. Composition of the Functionalized Layers
| Functional group | M-gold (at%) | A-gold (at %) | ||
| Experimental | Calculated | Experimental | Calculated | |
| C-Si, C-C | 27 (2) | 24 | 27 (1) | 30 |
| C-S | 13 (1) | 12 | 13 (1) | 15 |
| C Methoxide | 4 (1) | 12 | 4 (1) | - |
| O-C=O carbamate | - | - | 1.3 (0.2) | - |
| Si-O-Si | 29 (2) | 29 | 23 (2) | 23 |
| S | 13 (2) | 12 | 10 (2) | 10 |
| Si | 13 (2) | 12 | 15 (1) | 15 |
| -NH2 | - | - | 3 (1) | 5 |
| NHCOO | - | - | 1.3 (0.4) | - |
| -NH3+ | - | - | 0.5 (0.1) | - |
4.4. Parameter Identification for Developing a Transferable Analytical Strategy in Polymeric Systems
5. Conclusions
- The detailed analysis of the XPS high-resolution spectra allowed understanding the overlayer formation of MPTMS first, and MPTMS+APTES onto freshly cleaved gold surfaces. The quantitative analysis suggests the functionalization of the gold surface with MPTMS was successful and homogeneous. The at% of the CH3O was found to be 4% instead of 12% expected from the stoichiometry suggesting a three-dimensional cross-linking of the Si-O-Si bond. After the grafting of APTES onto the M-gold surface, quantitative analysis was in good agreement with the stoichiometry considering a ratio MPTMS:APTES of 2:1.
- Angle-resolved XPS allowed understanding the in-depth distribution of the overlayer functional groups after the two functionalization steps. Based on these results a model of the functionalized surface was proposed and consists of a first layer of MPTMS (after the first step) with the Si-O groups facing the outer part of the surface. Following the grafting of the APTES onto the M-gold surface ARXPS results indicate that APTES react with MPTMS through Si-O groups facing the amino groups in the outer part of the functionalization layer. Furthermore, through the ARXPS results have been possible to a 1 nm layer of MPTMS+APTES.
- The analytical strategy presented in this work based on XPS and ARXPS by using gold as a carbon-free substrate represents a useful tool for a proper curve fitting of C 1s signal and layer thickness estimation to be adopted for similar systems with carbon-based substrate such as polymer.
Supplementary Materials
Acknowledgements
References
- Vericat, C.; Vela, M.E.; Benitez, G.; Carro, P.; Salvarezza, R.C. Self-Assembled Monolayers of Thiols and Dithiols on Gold: New Challenges for a Well-Known System. Chem. Soc. Rev. 2010, 39, 1805–1834. [Google Scholar] [CrossRef] [PubMed]
- Spencer, N.D. Tailoring Surfaces: Modifying Surface Composition and Structure for Applications in Tribology, Biology and Catalysis; Co-Published with Indian Institute of Science (IISc), Bangalore, India, 2011; ISBN 978-981-4289-42-9.
- Xia, Y.; Zhao, X.-M.; Whitesides, G.M. Pattern Transfer: Self-Assembled Monolayers as Ultrathin Resists. Microelectronic Engineering 1996, 32, 255–268. [Google Scholar] [CrossRef]
- Villanueva, M.E.; González, J.A.; Rodríguez-Castellón, E.; Teves, S.; Copello, G.J. Antimicrobial Surface Functionalization of PVC by a Guanidine Based Antimicrobial Polymer. Materials Science and Engineering: C 2016, 67, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology. Chem. Rev. 2005, 105, 1103–1170. [Google Scholar] [CrossRef]
- Gothe, P.K.; Gaur, D.; Achanta, V.G. MPTMS Self-Assembled Monolayer Deposition for Ultra-Thin Gold Films for Plasmonics. J. Phys. Commun. 2018, 2, 035005. [Google Scholar] [CrossRef]
- Piwoński, I.; Grobelny, J.; Cichomski, M.; Celichowski, G.; Rogowski, J. Investigation of 3-Mercaptopropyltrimethoxysilane Self-Assembled Monolayers on Au(111) Surface. Applied Surface Science 2005, 242, 147–153. [Google Scholar] [CrossRef]
- Kumar, S.; Soni, S.; Danowski, W.; van Beek, C.L.F.; Feringa, B.L.; Rudolf, P.; Chiechi, R.C. Correlating the Influence of Disulfides in Monolayers across Photoelectron Spectroscopy Wettability and Tunneling Charge-Transport. J. Am. Chem. Soc. 2020, 142, 15075–15083. [Google Scholar] [CrossRef]
- Lees, W.J.; Whitesides, G.M. Equilibrium Constants for Thiol-Disulfide Interchange Reactions: A Coherent, Corrected Set. J. Org. Chem. 1993, 58, 642–647. [Google Scholar] [CrossRef]
- Azzam, W.; Bashir, A.; Biedermann, P.U.; Rohwerder, M. Formation of Highly Ordered and Orientated Gold Islands: Effect of Immersion Time on the Molecular Adlayer Structure of Pentafluorobenzenethiols (PFBT) SAMs on Au(111). Langmuir 2012, 28, 10192–10208. [Google Scholar] [CrossRef]
- Cortés, E.; Rubert, A.A.; Benitez, G.; Carro, P.; Vela, M.E.; Salvarezza, R.C. Enhanced Stability of Thiolate Self-Assembled Monolayers (SAMs) on Nanostructured Gold Substrates. Available online: https://pubs.acs.org/doi/full/10.1021/la804251a (accessed on 12 February 2024).
- Yang, Y.; Qing, Y.; Hao, X.; Fang, C.; Ouyang, P.; Li, H.; Wang, Z.; Liao, Y.; Fang, H.; Du, J. APTES-Modified Remote Self-Assembled DNA-Based Electrochemical Biosensor for Human Papillomavirus DNA Detection. Biosensors 2022, 12, 449. [Google Scholar] [CrossRef]
- Villanueva, M.E.; Salinas, A.; González, J.A.; Teves, S.; Copello, G.J. Dual Antibacterial Effect of Immobilized Quaternary Ammonium and Aliphatic Groups on PVC. New J. Chem. 2015, 39, 9200–9206. [Google Scholar] [CrossRef]
- Krishna, D.N.G.; Philip, J. Review on Surface-Characterization Applications of X-Ray Photoelectron Spectroscopy (XPS): Recent Developments and Challenges. Applied Surface Science Advances 2022, 12, 100332. [Google Scholar] [CrossRef]
- Watts, J.F.; Wolstenholme, J. An Introduction to Surface Analysis by XPS and AES; John Wiley & Sons, 2019; ISBN 978-1-119-41758-3.
- Briggs, D. Surface Analysis of Polymers by XPS and Static SIMS; Cambridge Solid State Science Series; Cambridge University Press: Cambridge, 1998; ISBN 978-0-521-35222-2. [Google Scholar]
- Afanas’ev, V.P.; Selyakov, D.N.; Ridzel, O.Y.; Semenov-Shefov, M.A.; Strukov, A.N. Investigation of Monolayer and Submonolayer Films Using X-Ray Photoelectron Spectroscopy. J. Phys.: Conf. Ser. 2020, 1713, 012002. [Google Scholar] [CrossRef]
- Parry, K.L.; Shard, A.G.; Short, R.D.; White, R.G.; Whittle, J.D.; Wright, A. ARXPS Characterisation of Plasma Polymerised Surface Chemical Gradients. Surface and Interface Analysis 2006, 38, 1497–1504. [Google Scholar] [CrossRef]
- Spori, D.M.; Venkataraman, N.V.; Tosatti, S.G.P.; Durmaz, F.; Spencer, N.D.; Zürcher, S. Influence of Alkyl Chain Length on Phosphate Self-Assembled Monolayers. Langmuir 2007, 23, 8053–8060. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Heng, C.K.; Puiu, P.D.; Zhou, X.D.; Lee, A.C.; Lim, T.M.; Tan, S.N. Detection of Saccharomyces Cerevisiae Immobilized on Self-Assembled Monolayer (SAM) of Alkanethiolate Using Electrochemical Impedance Spectroscopy. Analytica Chimica Acta 2005, 554, 52–59. [Google Scholar] [CrossRef]
- Gates, B.D.; Xu, Q.; Stewart, M.; Ryan, D.; Willson, C.G.; Whitesides, G.M. New Approaches to Nanofabrication: Molding, Printing, and Other Techniques. Chem. Rev. 2005, 105, 1171–1196. [Google Scholar] [CrossRef] [PubMed]
- Piwoński, I.; Grobelny, J.; Cichomski, M.; Celichowski, G.; Rogowski, J. Investigation of 3-Mercaptopropyltrimethoxysilane Self-Assembled Monolayers on Au(111) Surface. Applied Surface Science 2005, 242, 147–153. [Google Scholar] [CrossRef]
- Penna, A.; Careri, M.; Spencer, N.D.; Rossi, A. Effects of Tailored Surface Chemistry on Desorption Electrospray Ionization Mass Spectrometry: A Surface-Analytical Study by XPS and AFM. J. Am. Soc. Mass Spectrom. 2015, 26, 1311–1319. [Google Scholar] [CrossRef]
- Graf, N.; Yegen, E.; Gross, T.; Lippitz, A.; Weigel, W.; Krakert, S.; Terfort, A.; Unger, W.E.S. XPS and NEXAFS Studies of Aliphatic and Aromatic Amine Species on Functionalized Surfaces. Surface Science 2009, 603, 2849–2860. [Google Scholar] [CrossRef]
- Walton, J.; Alexander, M.R.; Fairley, N.; Roach, P.; Shard, A.G. Film Thickness Measurement and Contamination Layer Correction for Quantitative XPS. Surface and Interface Analysis 2016, 48, 164–172. [Google Scholar] [CrossRef]
- Naik, V.V.; Crobu, M.; Venkataraman, N.V.; Spencer, N.D. Multiple Transmission-Reflection IR Spectroscopy Shows That Surface Hydroxyls Play Only a Minor Role in Alkylsilane Monolayer Formation on Silica. J. Phys. Chem. Lett. 2013, 4, 2745–2751. [Google Scholar] [CrossRef]
- Passiu, C.; Rossi, A.; Bernard, L.; Paul, D.; Hammond, J.; Unger, W.E.S.; Venkataraman, N.V.; Spencer, N.D. Fabrication and Microscopic and Spectroscopic Characterization of Planar, Bimetallic, Micro- and Nanopatterned Surfaces. Langmuir 2017, 33, 5657–5665. [Google Scholar] [CrossRef] [PubMed]
- Passiu, C.; Rossi, A.; Weinert, M.; Tysoe, W.; Spencer, N.D. Probing the Outermost Layer of Thin Gold Films by XPS and Density Functional Theory. Applied Surface Science 2020, 507, 145084. [Google Scholar] [CrossRef]
- Tougaard, S. Practical Guide to the Use of Backgrounds in Quantitative XPS. Journal of Vacuum Science & Technology A 2021, 39, 011201. [Google Scholar] [CrossRef]
- Scofield, J.H. Hartree-Slater Subshell Photoionization Cross-Sections at 1254 and 1487 eV. Journal of Electron Spectroscopy and Related Phenomena 1976, 8, 129–137. [Google Scholar] [CrossRef]
- Zommer, L. Determination of the Spectrometer Transmission Function for XPS Quantitative Analysis. Vacuum 1995, 46, 617–620. [Google Scholar] [CrossRef]
- Seah, M.P.; Dench, W.A. Quantitative Electron Spectroscopy of Surfaces: A Standard Data Base for Electron Inelastic Mean Free Paths in Solids. Surface and Interface Analysis 1979, 1, 2–11. [Google Scholar] [CrossRef]
- Gunter, P.L.J.; Gijzeman, O.L.J.; Niemantsverdriet, J.W. Surface Roughness Effects in Quantitative XPS: Magic Angle for Determining Overlayer Thickness. Applied Surface Science 1997, 115, 342–346. [Google Scholar] [CrossRef]
- Engelhard, M.H.; Baer, D.R.; Herrera-Gomez, A.; Sherwood, P.M.A. Introductory Guide to Backgrounds in XPS Spectra and Their Impact on Determining Peak Intensities. Journal of Vacuum Science & Technology A: Vacuum, Surfaces, and Films 2020, 38, 063203. [Google Scholar] [CrossRef]
- Fairley, N.; Fernandez, V.; Richard-Plouet, M.; Guillot-Deudon, C.; Walton, J.; Smith, E.; Flahaut, D.; Greiner, M.; Biesinger, M.; Tougaard, S.; et al. Systematic and Collaborative Approach to Problem Solving Using X-Ray Photoelectron Spectroscopy. Applied Surface Science Advances 2021, 5, 100112. [Google Scholar] [CrossRef]
- Fracassi, A.; Ray, A.; Nakatsuka, N.; Passiu, C.; Tanriver, M.; Schauenburg, D.; Scherrer, S.; Ouald Chaib, A.; Mandal, J.; Ramakrishna, S.N.; et al. KAT Ligation for Rapid and Facile Covalent Attachment of Biomolecules to Surfaces. ACS Appl. Mater. Interfaces 2021, 13, 29113–29121. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-S.; Lu, W.; Wang, Y.; Tran, T. Studies of (3-Mercaptopropyl)Trimethoxylsilane and Bis(Trimethoxysilyl)Ethane Sol–Gel Coating on Copper and Aluminum. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2009, 73, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.S. da; Melo, H.G. de; Benedetti, A.V.; Suegama, P.H. Influence of Ce(IV) Ions Amount on the Electrochemical Behavior of Organic-Inorganic Hybrid Coatings in 0.1 Mol L-1 NaCl Solution. Eclética Química 2019, 44, 27–56. [Google Scholar]
- Ederer, J.; Janoš, P.; Ecorchard, P.; Tolasz, J.; Štengl, V.; Beneš, H.; Perchacz, M.; Pop-Georgievski, O. Determination of Amino Groups on Functionalized Graphene Oxide for Polyurethane Nanomaterials: XPS Quantitation vs. Functional Speciation. RSC Adv. 2017, 7, 12464–12473. [Google Scholar] [CrossRef]
- Stegmeier, S.; Fleischer, M.; Tawil, A.; Hauptmann, P.; Egly, K.; Rose, K. Mechanism of the Interaction of CO2 and Humidity with Primary Amino Group Systems for Room Temperature CO2 Sensors. Procedia Chemistry 2009, 1, 236–239. [Google Scholar] [CrossRef]
- Hill, J.M.; Royce, D.G.; Fadley, C.S.; Wagner, L.F.; Grunthaner, F.J. Properties of Oxidized Silicon as Determined by Angular-Dependent X-Ray Photoelectron Spectroscopy. Chemical Physics Letters 1976, 44, 225–231. [Google Scholar] [CrossRef]
- Brundle, C.R.; Conti, G.; Mack, P. XPS and Angle Resolved XPS, in the Semiconductor Industry: Characterization and Metrology Control of Ultra-Thin Films. Journal of Electron Spectroscopy and Related Phenomena 2010, 178–179, 433–448. [Google Scholar] [CrossRef]
- XPS and NEXAFS Studies of Aliphatic and Aromatic Amine Species on Functionalized Surfaces | Elsevier Enhanced Reader. Available online: https://reader.elsevier.com/reader/sd/pii/S0039602809005068?token=F8A8BC084615658A974BD13927A0D9267C67AEF99FD405F53A180FB8D52092A0CE7105DCF3DF3BC1C5B9A0FB7034191E&originRegion=eu-west-1&originCreation=20220922072105 (accessed on 22 September 2022).
- Sui, W.; Zhao, W.; Zhang, X.; Peng, S.; Zeng, Z.; Xue, Q. Comparative Anti-Corrosion Properties of Alkylthiols SAMs and Mercapto Functional Silica Sol–Gel Coatings on Copper Surface in Sodium Chloride Solution. J Sol-Gel Sci Technol 2016, 80, 567–578. [Google Scholar] [CrossRef]
- Sypabekova, M.; Hagemann, A.; Rho, D.; Kim, S. Review: 3-Aminopropyltriethoxysilane (APTES) Deposition Methods on Oxide Surfaces in Solution and Vapor Phases for Biosensing Applications. Biosensors 2023, 13, 36. [Google Scholar] [CrossRef]
- Casula, G.; Fantauzzi, M.; Elsener, B.; Rossi, A. Surface Modification of Food-Grade PVC Monitored by Angle-Resolved XPS. Vacuum 2024, 113010. [Google Scholar] [CrossRef]
- Castner, D.G.; Hinds, K.; Grainger, D.W. X-Ray Photoelectron Spectroscopy Sulfur 2p Study of Organic Thiol and Bisulfide Binding Interactions with Gold Surfaces. Langmuir 1996, 12, 5083–5086. [Google Scholar] [CrossRef]
- Cristina, L.J.; Ruano, G.; Salvarezza, R.; Ferrón, J. Thermal Stability of Self-Assembled Monolayers of n-Hexanethiol on Au(111)-(1 × 1) and Au(001)-(1 × 1). J. Phys. Chem. C 2017, 121, 27894–27904. [Google Scholar] [CrossRef]
- Watcharinyanon, S.; Nilsson, D.; Moons, E.; Shaporenko, A.; Zharnikov, M.; Albinsson, B.; Mårtensson, J.; Johansson, L.S.O. A Spectroscopic Study of Self-Assembled Monolayer of Porphyrin-Functionalized Oligo(Phenyleneethynylene)s on Gold: The Influence of the Anchor Moiety. Phys. Chem. Chem. Phys. 2008, 10, 5264–5275. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Kara, A.; Pasquali, L.; Bendounan, A.; Sirotti, F.; Esaulov, V.A. On Sulfur Core Level Binding Energies in Thiol Self-Assembly and Alternative Adsorption Sites: An Experimental and Theoretical Study. J Chem Phys 2015, 143, 104702. [Google Scholar] [CrossRef] [PubMed]
- Inkpen, M.S.; Liu, Z.-F.; Li, H.; Campos, L.M.; Neaton, J.B.; Venkataraman, L. Non-Chemisorbed Gold–Sulfur Binding Prevails in Self-Assembled Monolayers. Nat. Chem. 2019, 11, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Peng, B.; Yan, Z.; Zhao, X. Gold Nanoparticles Supported on Mesoporous Silica: Origin of High Activity and Role of Au NPs in Selective Oxidation of Cyclohexane. Scientific Reports 2016, 6, 18817. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, P.M.; Glamsch, S.; Ehlert, C.; Lippitz, A.; Kulak, N.; Unger, W.E.S. Synchrotron-Radiation XPS Analysis of Ultra-Thin Silane Films: Specifying the Organic Silicon. Applied Surface Science 2016, 363, 406–411. [Google Scholar] [CrossRef]







| FWHM (eV) | Background | Line shape | Constrains | |
| Au 4f | 1.2 (0.1) | U 3 Tougaard | GL(50) T(5.5) | Area Au 4f5/2 = Area Au 4f7/2 x 0.75 FWHM Au 4f5/2 = FWHM Au 4f7/2 x 1 |
| C 1s contamination | 1.6 (0.1) | GL(30) | FWHM C 1s Contamination = FWHM C 1s C-Si, C-C x 1 | |
| C 1s C-Si, C-C | 1.6 (0.1) | GL(30) | ||
| C 1s C-S | 1.6 (0.1) | GL(30) | FWHM C 1s C-S = FWHM C 1s C-Si, C-C x 1 Area C 1s C-S=Area C-Si, C-C x 0.5 |
|
| C 1s methoxide | 1.6 (0.1) | GL(30) | FWHM C 1s methoxide = FWHM C 1s C-Si, C-C x 1 | |
| C 1s of the NH(C*O)O group | 1.6 (0.1) | GL(30) | FWHM C 1s of the NH(C*O)O group = FWHM C 1s of C-Si, and of C-C x 1 | |
| O 1s Si-O-Si | 1.8 (0.1) | GL(30) | ||
| O 1s of the NH(CO)O* carbamate group | 1.8 (0.1) | GL(30) | FWHM O 1s NH(CO)O* = FWHM O 1s Si-O-Si x 1 | |
| O 1s of the NH(CO*)O carbamate group | 1.8 (0.1) | GL(30) | Area O 1s NH(CO*)O = Area O 1s NH(CO)O* x 1 FWHM O 1s NH(CO)O* = FWHM O1s Si-O-Si x 1 |
|
| S 2p | 1.6 (0.1) | GL(30) | Area S 2p1/2 = Area S 2p3/2 x 0.5 FWHM S 2p1/2 = FWHM S 2p3/2 x 1 BE 2p1/2 = BE 2p3/2 + 1.16 |
|
| Si 2p | 1.5 (0.1) | GL(30) | Area Si 2p1/2 = Area Si 2p3/2 x 0.5 FWHM Si 2p1/2 = FWHM Si 2p3/2 x 1 BE 2p1/2 = BE 2p3/2 + 0.6 |
|
| N 1s -NH2 | 1.6 (0.1) | GL(30) | ||
| N 1s -NH3+ | 1.6 (0.1) | GL(30) | FWHM N 1s -NH3+ = FWHM N 1s -NH2 x 1 | |
| N 1s -N*H(CO)O | 1.6 (0.1) | GL(30) | FWHM -N*H(CO)O = FWHM N 1s -NH2 x 1 |
| Binding energy (eV) | M-Gold 10% at % |
|
| C 1s C-Si, C-C | 284.9 (0.1) | 27 (2) |
| C 1s C-S | 285.9 (0.2) | 13 (1) |
| C 1s Methoxide (-OCH3) |
287.0 (0.1) | 4 (1) |
| O 1s Si-O-Si | 532.6 (0.1) | 29 (3) |
| S 2p | 163.7 (0.1) | 13 (2) |
| Si 2p | 102.3 (0.2) | 13 (2) |
| Binding energy (eV) | A-Gold at % |
|
| C 1s C-Si, C-C | 285.3 (0.1) | 27 (1) |
| C 1s C-S | 286.0 (0.2) | 13 (1) |
| C 1s Methoxide | 287.0 (0.2) | 4 (1) |
| C 1s O-C=O carbamate | 289.2 (0.2) | 1.2 (0.2) |
| O 1s C=O* carbamate | 531.1 (0.2) | 1.3 (0.5) |
| O 1s Si-O-Si | 532.7 (0.1) | 23 (2) |
| O 1s O*-C carbamate | 534.2 (0.1) | 1.3 (0.5) |
| S 2p | 164.1 (0.1) | 10 (2) |
| Si 2p | 102.7 (0.1) | 15 (1) |
| N 1s -NH2 | 399.8 (0.3) | 3 (1) |
| N 1s NHCOO | 400.8 (0.3) | 1.2 (0.4) |
| N 1s -NH3+ | 402.2 (0.3) | 0.6 (0.1) |
| Element | Au | S | Si | N |
| IMFP l (nm) | 2.02 | 3.16 | 3.24 | 3.06 |
| Thickness M-Gold (nm) | 1.9 (0.3) | 1.4 (0.3) | 1.1 (0.2) | - |
| Thickness A-Gold (nm) | 1.8 (0.3) | 1.2 (0.4) | 0.6 (0.2) | 0.3 (0.1) |
| Layer | Freshly cleaved gold | MPTMS on PVC [46] | MPTMS on gold | MPTMS + APTES on gold |
| Contamination lc | 0.4 nm | 1.1 nm | 0.9 nm | 0.3 nm |
| Layer thickness t | - | 0.5 nm | 0.5 nm | 1.0 nm |
| Avogadro calculation | - | 0.7 nm | 0.47 nm | 1.0 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





