1. Introduction
An accurate prediction of the progression, regression, or persistence of cervical lesions is critical for guiding appropriate clinical management decisions and ensuring optimal patient outcomes. While several screening methods, such as cytology and human papillomavirus (HPV) testing, have been effective in identifying individuals at risk, the development of more accurate predictive tools remains a priority.
The natural history of cervical lesions is influenced by HPV characteristics such as type, viral load, and persistence [
1]. Beside the viral characteristics, environmental or exogenous factors have been recognized as modifiers of the natural progression of HPV infections that can lead to cervical cancer, and they include smoking, multiple pregnancies, prolonged use of hormonal contraceptives, and co-infection with sexually transmitted infections such as
Chlamydia trachomatis or Human Immunodeficiency Virus (HIV) [
2,
3,
4,
5].
Depending on the institution, the results of a colposcopic biopsy may be reported as cervical intraepithelial neoplasia (CIN) 1, 2, or 3 according to the Richart histopathological grading system, or as histologic low-grade squamous intraepithelial lesion (LSIL) or high-grade squamous intraepithelial lesion (HSIL) according to the Lower Anogenital Squamous Terminology system [
6,
7]. As a general rule, histologic LSIL is equivalent to CIN1, while histologic HSIL is equivalent to CIN2 and CIN3. CIN3 is considered to be a more reliable diagnosis compared to CIN2, as expert pathologists show a remarkable agreement of over 80% on CIN3 diagnoses, while their agreement on CIN2 diagnoses is less than 30% [
8]. CIN3 is also more likely to indicate a histological correlation with cellular transformation, carrying a significant risk of progressing to cervical cancer. It is frequently linked to highly carcinogenic HPV genotypes [
9].
Treatment is recommended for non-pregnant patients, who have been diagnosed with CIN3 (HSIL), or adenocarcinoma in situ (AIS) [
10]. On the other hand, patients with CIN2 can be referred to a specific treatment or can undergo further follow-up due to the risk of preterm birth after excisional approaches, although this risk is questionable [
11].
However, it is important to point out that CIN1 lesions have a natural history that is marked by a high rate of spontaneous regression (approximately 80%) [
12], but can evolve to more severe cervical lesions (CIN2/3) in more than 10% of the cases [
13]. A recent systematic review and meta-analysis conducted by Loopik et al., which included 89 studies, evaluated the regression, persistence, and progression rates of conservatively managed CIN 1-3 [
14]. The results from this meta-analysis indicated that in women with CIN 1 conservatively treated, the rates of regression, persistence, and progression to CIN 2-3 or worse were 60%, 25%, and 11%, respectively. For CIN 2, the overall rates of regression were 55%, persistence were 23%, and progression were 19%. Lastly, in regards to CIN 3, the corresponding percentages were 28%, 67%, and 2%. Less than 1% of women with CIN1 or 2 progressed to cervical cancer in this study.
Recent literature has outlined several tests that could improve the detection and prognosis of cervical lesions progression. These tests include methylation assays of viral and host markers, immune cytodiagnosis, proteomic or transcriptomics panels [
15,
16,
17,
18]. The p16/Ki67 dual stain test (CINtecPlus) is a cutting-edge technology that has been approved for the precise detection of cell transformation [
19]. The presence of p16 and Ki67 indicates potential cellular transformation caused by HPV, as p16 demonstrates the disruption of the retinoblastoma pathway caused by E7 oncoproteins, while Ki67 serves as a marker of cellular proliferation [
20]. Multiple studies have demonstrated that utilizing dual stain instead of Papanicolaou-stain cytology can enhance the accuracy of identifying precancerous conditions and distinguishing them from low-grade abnormalities in patients with positive HPV test results [
20,
21,
22,
23].
The risk stratification of progression, regression, or persistence of cervical lesions using the p16/Ki67 dual stain test was poorly studied in the literature. Moreover, many reports evaluated the predictive performance of the p16/Ki67 dual stain test in previously stained slides. Thus, the aim of this prospective study was to determine and compare the predictive accuracy of cytology, HPV genotyping, and p16/Ki67 dual staining (performed on fresh collected samples) taken individually or combined with personal risk factors for the progression, regression of persistence of cervical lesions in patients with a proven HPV infection.
2. Materials and Methods
This prospective study was conducted at the Avicena Profertis Clinic in Iasi, Romania, between October 2022 and December 2023, and included patients infected with HPV with or without cervical lesions that underwent follow-up or were programmed for excisional treatments (Ethical approval No 728/01.10.2022). The inclusion criteria comprised the following: patients with HPV genotyping positive for at least one of the HPV strains, who had indication for CINtecPlus testing, and who gave their informed consent for participating in this study. The exclusion criteria were represented by: patients with concomitant vaginal or vulvar precancerous lesions, a negative HPV genotyping test, personal history of genital cancer, pregnancy, lack of informed consent.
In the first stage of the evaluation, patients underwent Pap testing and HPV genotyping. If the patients had a positive HPV genotyping result, they underwent CINtecPlus testing, and the results were recorded in the database.
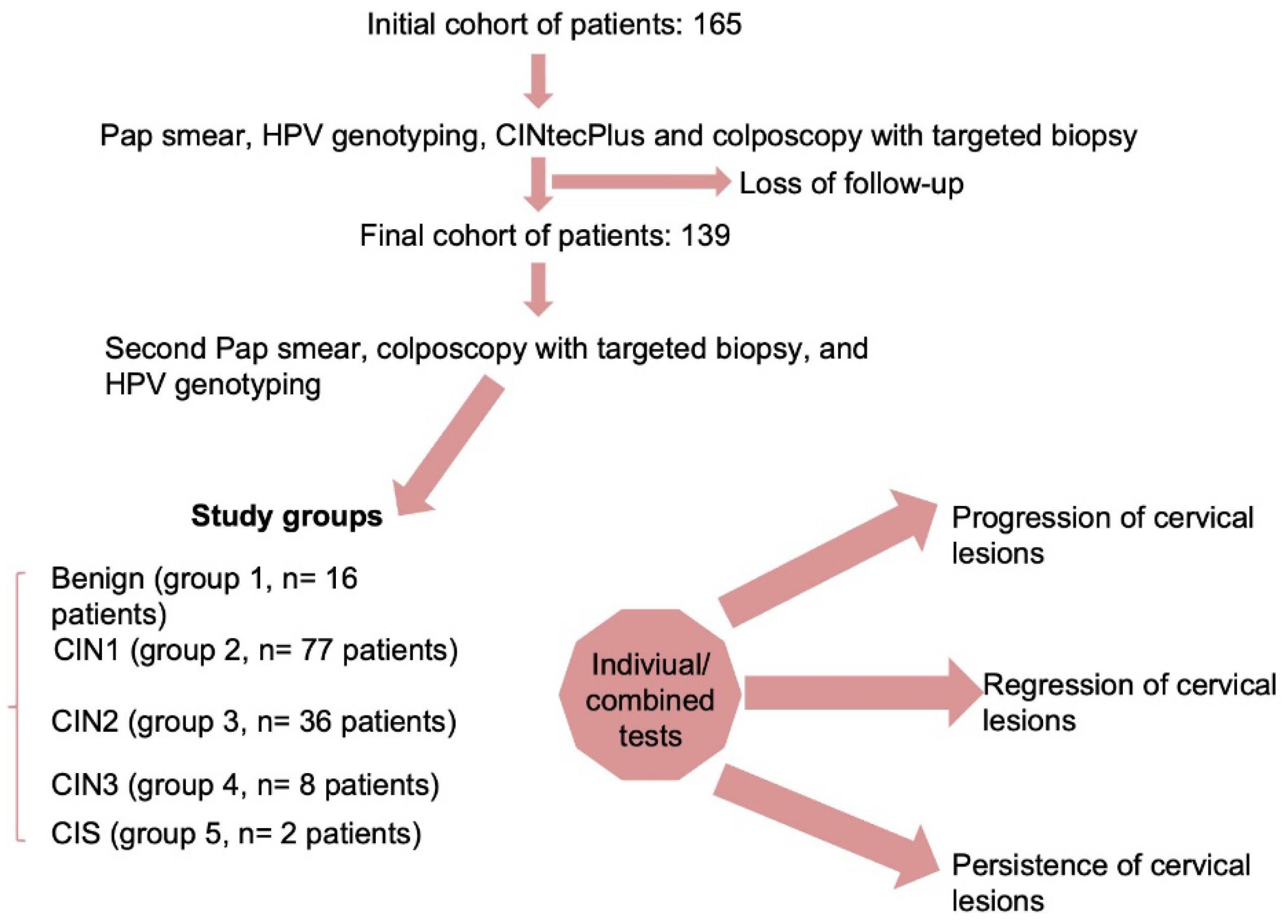
Patients with abnormal Pap smear results such as ASC-US, LSIL, and HSIL underwent colposcopy examination and a targeted cervical biopsy of the suspected cervical lesions. Pathologists classified the histology findings on the cervical biopsy or conization probes as either benign, CIN1, CIN2, CIN3 or microinvasive cervical cancer at the first examination and at the follow-up visit. The histopathological report was correlated with both cytology and HPV test results. Patients who had a histopathological diagnosis of CIN3 or microinvasive cervical cancer in this stage were excluded from the follow-up and underwent standard protocol, while patients with no documented histopathological lesions or with CIN1 or CIN2 were further included in the follow-up. These histology results were considered the gold standard. The initial cohort of patients comprised 165 subjects, but only 139 patients completed the follow-up program (
Figure 1).
Patients were followed up in a one year time-frame, and at the second visit, they underwent a second Pap smear, colposcopy with targeted biopsy, and HPV genotyping. The results from these tests were also recorded.
Our database also included clinical data of the patients, such as age, BMI, clinical risk factors for cervical cancer, vaccination, and contraceptive history, as well as personal obstetrical and gynecological history.
We defined the progression of the lesions in the following situations:
- -
if the initial histopathological diagnosis was CIN1 or CIN2 and at follow-up the histopathological diagnosis was CIN2/CIN3 or microinvasive cervical cancer;
- -
if the initial histopathological diagnosis was CIN1 or CIN2 and at follow-up the histopathological diagnosis was a combination of CIN1/ CIN2/CIN3 or microinvasive cervical cancer;
We defined regression of the lesions if the initial histopathological diagnosis was CIN1/ CIN2 and at follow-up the histopathological diagnosis was a lower grade, such as negative/ CIN1. The persistence of the lesions was documented by the same histopathological result at the follow-up.
The cases that tested negative for HPV or showed signs of new infections with different strains of HPV compared to baseline were considered to have a transitory HPV infection. In contrast, cases where the same genotype was discovered as a follow-up were considered to have a persistent HPV infection.
The collected cervical samples were sent in a ThinPrep
® vial and processed on a ThinPrep 5000 (Hologic, Marlborough, Massachusetts, USA), stained by the Papanicolaou technique, microscopically observed by an experienced cytotechnician, and reviewed by the pathologists. The results were interpreted according to the Bethesda system from 2001, revised in 2014 [
24] and they consisted of: NILM (negative for intraepithelial lesions or malignancy), ASC-US (atypical squamous cell—undetermined significance), ASC-H (atypical squamous cell—cannot exclude HSIL), LSIL (low-grade intraepithelial lesion), HSIL (high-grade intraepithelial lesion), AGC (atypical glandular cells), other.
HPV testing was performed on cervical samples using Allplex™ PCR System (Seegene Inc., Songpa-gu, Seoul, Republic of Korea) for detection of human papillomavirus - 19 high-risk HPV types (16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 69, 73, 82) and 9 low-risk HPV types (6, 11, 40, 42, 43, 44, 54, 61, 70).
Using the ThinPrep 5000 Processor (Hologic, Marlborough, Massachusetts, USA), a slide was produced retrospectively from the remaining cytology material for p16/Ki-67 immunostaining. Based on the instructions provided by the manufacturer, the CINtec PLUS Cytology kit (Roche mtm Laboratories AG, Mannheim, Germany) was used to assess the slides. If one cervical epithelial cell per slide was stained with both brown cytoplasmic (p16) and red nuclear (Ki-67) stain, the sample was categorized as positive; otherwise, it was termed negative if only one stain was visible. Some of the results from CINtecPlus testing are presented as
supplementary materials (Supplementary Figures S1–S6).
The colposcopy was performed using a photo/video colposcope 3MLS LED 1”- LEISEGANG (Feinmechanik-Optik GmbH, Berlin, Germany). Colposcopists conducted the biopsies in accordance with established clinical protocol. As is standard procedure, hematoxylin and eosin (H&E) staining was applied to three sections of every biopsy sample.
Data description and univariate analysis comprised a chi-squared test for categorical variables, which were presented as frequencies with corresponding percentages, and an analysis of variance (ANOVA) with Bonferroni posthoc test for continuous variables, which were presented as means and standard deviations (SD). The univariate analysis was performed for the following groups considering the second histopathological results: benign (group 1, n= 16 patients), CIN1 (group 2, n= 77 patients), CIN2 (group 3, n= 36 patients), CIN3 (group 4, n= 8 patients), and in situ carcinoma (CIS) (group 5, n= 2 patients).
The primary outcomes were the progression, regression and persistence of the cervical lesions. We performed a sensitivity analysis using clinical risk factors for cervical cancer (smokers, immunosuppression, long-term use of oral contraceptives, multiple sexual partners, early debut of sexual activity, poor socioeconomic status, lack of HPV vaccination, family history of cervical cancer), the results from HPV genotyping, Pap smear, and CINtecPlus as predictors taken individually or in combination, and considering the histopathological results of cervical biopsies as gold standards. The statistical analyses were carried out using STATA SE (version 17, StataCorp LLC, College Station, TX, USA). A p-value of less than 0.05 was considered statistically significant.
3. Results
The database used for this analysis included 139 patients with HPV infections, which were segregated according to the second histopathological results in the following groups: benign (group 1, n= 16 patients), CIN1 (group 2, n= 77 patients), CIN2 (group 3, n= 36 patients), and CIN3 (group 4, n= 8 patients), and in situ carcinoma (CIS) (group 5, n= 2 patients). Their demographic and clinical characteristics are presented in
Table 1.
The mean age for patients who had a CIN2, CIN3, or CIS was significantly higher compared to the age of the patients with benign histopathological results or CIN1 (p= 0.0003). Moreover, the proportion of patients who had multiple sexual partners (> 5) was significantly higher for CIN2, CIN3, and CIS groups compared to the other groups (p= 0.03).
Regarding Pap smear results, the first and second groups had a significantly higher proportion of NILM and ASC-US lesions, while the fourth and fifth groups presented with a significantly higher proportion of HSIL results. The second group also had the highest proportion of LSIL results, while the fifth group had a 50% rate of carcinoma based on the results of cervical cytology.
The results of HPV genotyping indicated that patients with CIN2, CIN3, and CIS had significantly higher rates of infection with high-risk HPV strains such as HPV 16 and 18 (p< 0.001). On the other hand, we could not find any statistically significant difference between groups regarding other high-risk (p= 0.05) or low-risk (p= 0.45) HPV strains.
A positive CINtecPlus result was significantly more frequently encountered for patients with CIN2, CIN3, and CIS (p< 0.001) compared with patients with a benign histopathological result or CIN1.
Finally, we could not find any statistically significant difference between groups regarding clinical risk factors for cervical cancer such as BMI, smoking status, early debut of sexual activity, prolonged use of oral contraceptives, a positive personal history of HPV vaccination, or invasive procedures on the cervix (p> 0.05).
In the second stage of our analysis, we evaluated the predictive accuracy of cytology, HPV genotyping, and p16/Ki67 dual staining (CINtecPlus) taken individually or combined with personal risk factors for the progression, regression of persistence of cervical lesions in patients with a proven HPV infection.
In
Table 2 is presented the predictive performance of all evaluated index tests and combined models considering the progression of cervical lesions (n = 34 patients, 24.46%). Our results indicated that the presence of at least 3 clinical risk factors (accuracy: 67.13%), a cervical cytology suggestive of HSIL (accuracy: 64.03%), the presence of 16/18 HPV strains (accuracy: 66.19%), and a positive result of CINtecPlus (accuracy: 53.73%) had the highest performance in terms of accuracy.
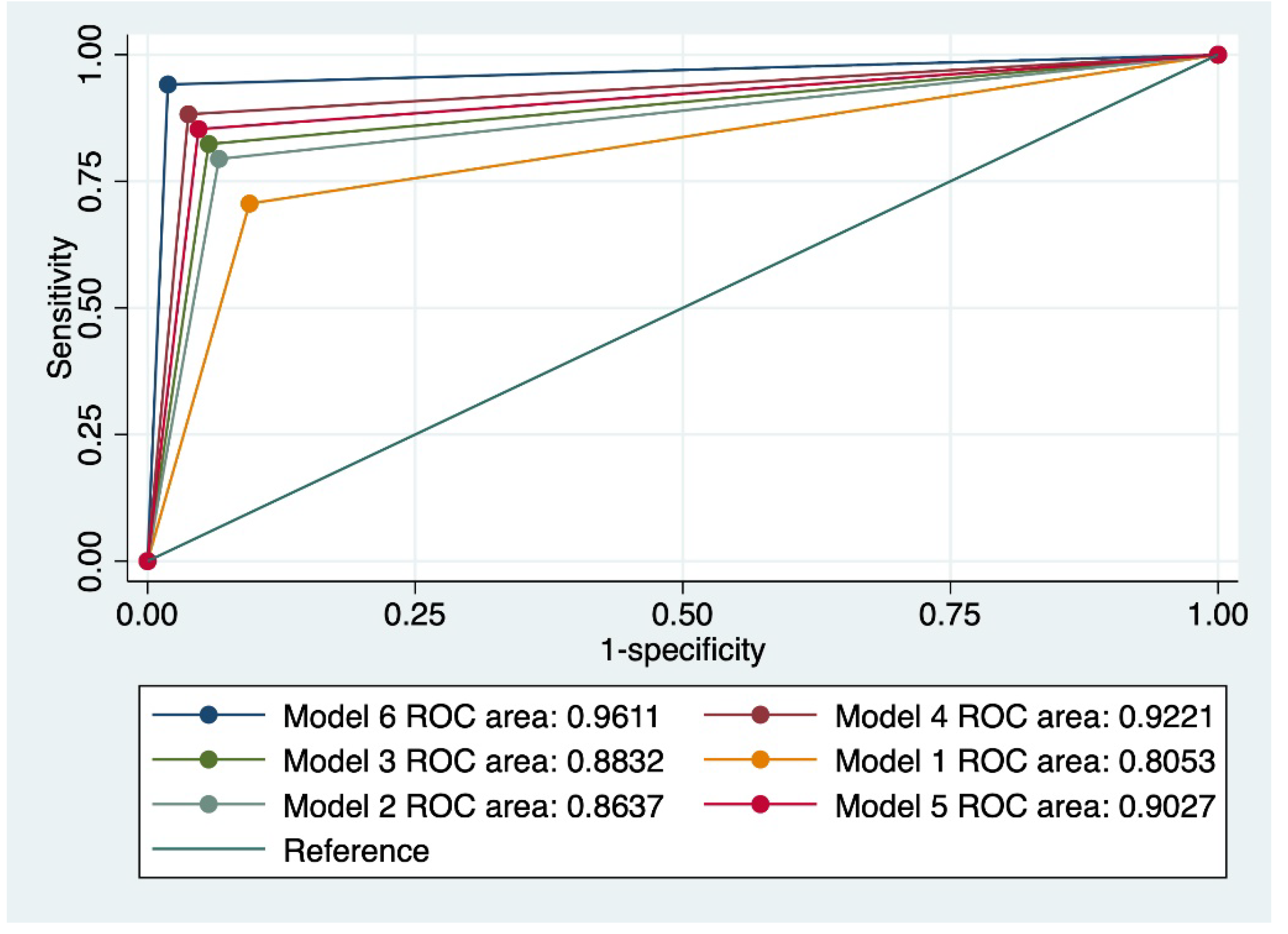
We also evaluated various combinations of index tests which resulted in 6 combined models. The highest predictive performance for the progression of cervical lesions was achieved by a model that comprised a Pap smear suggestive of HSIL, the presence of 16/18 HPV strains, a positive CINtecPlus result along with the presence of at least 3 clinical risk factors (model 6). This model was characterized by a sensitivity (Se) of 74.42%, a specificity of 97.92%, an area under the receiver operating curve (AUC) of 0.961, and an accuracy of 90.65%.
Figure 2 outlines a comparison of all evaluated models taking into account the value of the ROC curve.
Table 3 presents the predictive performance of all evaluated index tests and combined models considering the regression of cervical lesions (n = 19 patients, 13.66%). The presence of low-risk HPV strains (accuracy: 73.72%), a Pap smear suggestive of ASC-US (accuracy: 66.19%), a negative CINtecPlus (accuracy: 40.3%), and a personal history of less than 3 risk factors for cervical cancer (accuracy: 64.03%) best predicted the regression of cervical abnormalities.
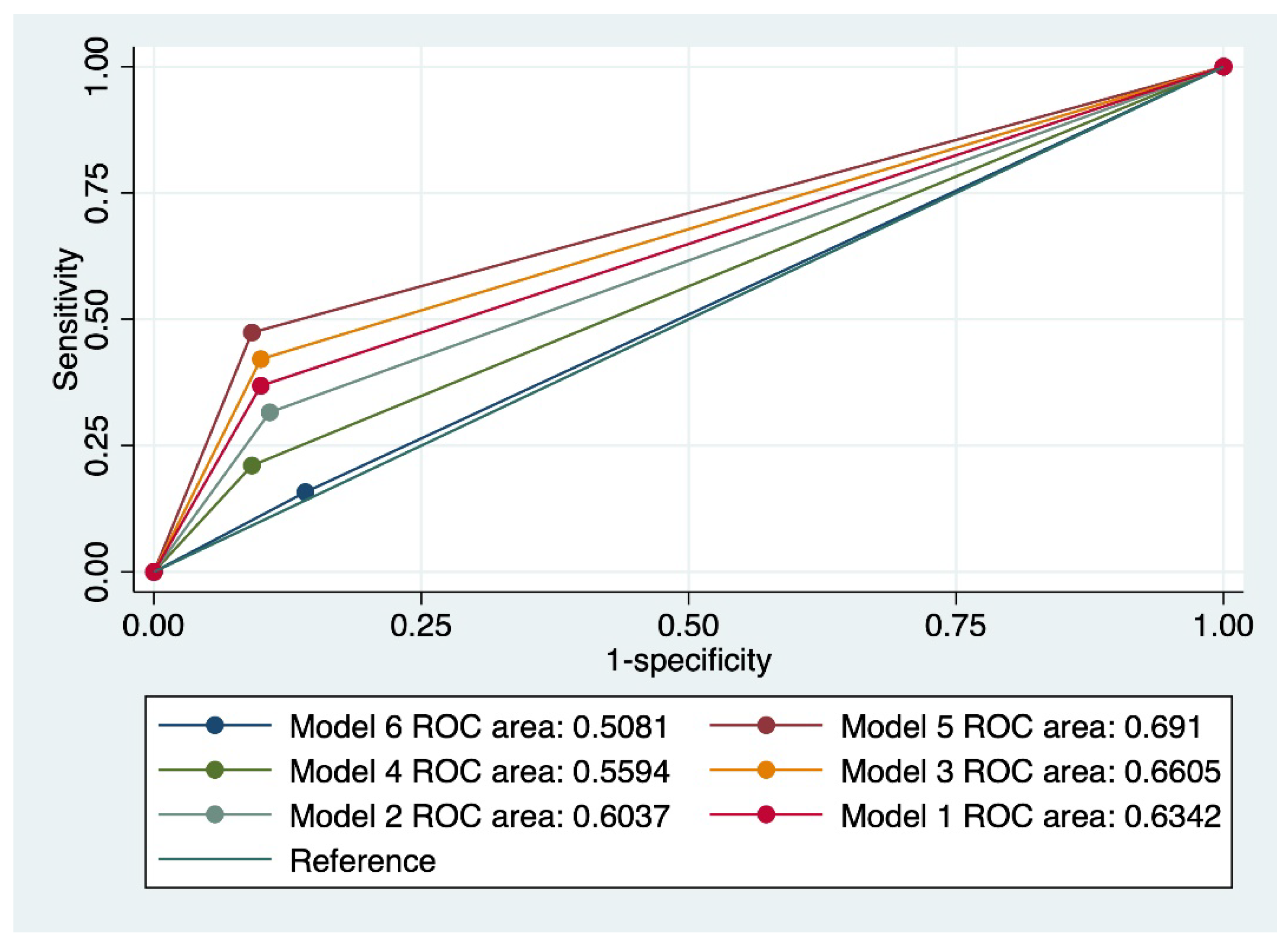
Also, a model that comprised a cervical cytology suggestive of LSIL, in the absence of HPV 16/18, a negative CINtecPlus result, and a personal history of less than 3 risk factors for cervical cancer (model 5) achieved the best results in terms of predicting the regression of cervical abnormalities (Se-33.3%, Sp-88%, AUC- 0.691, and accuracy: 82.14%).
Figure 3 outlines a comparison of all evaluated models, taking into account the value of the ROC curve.
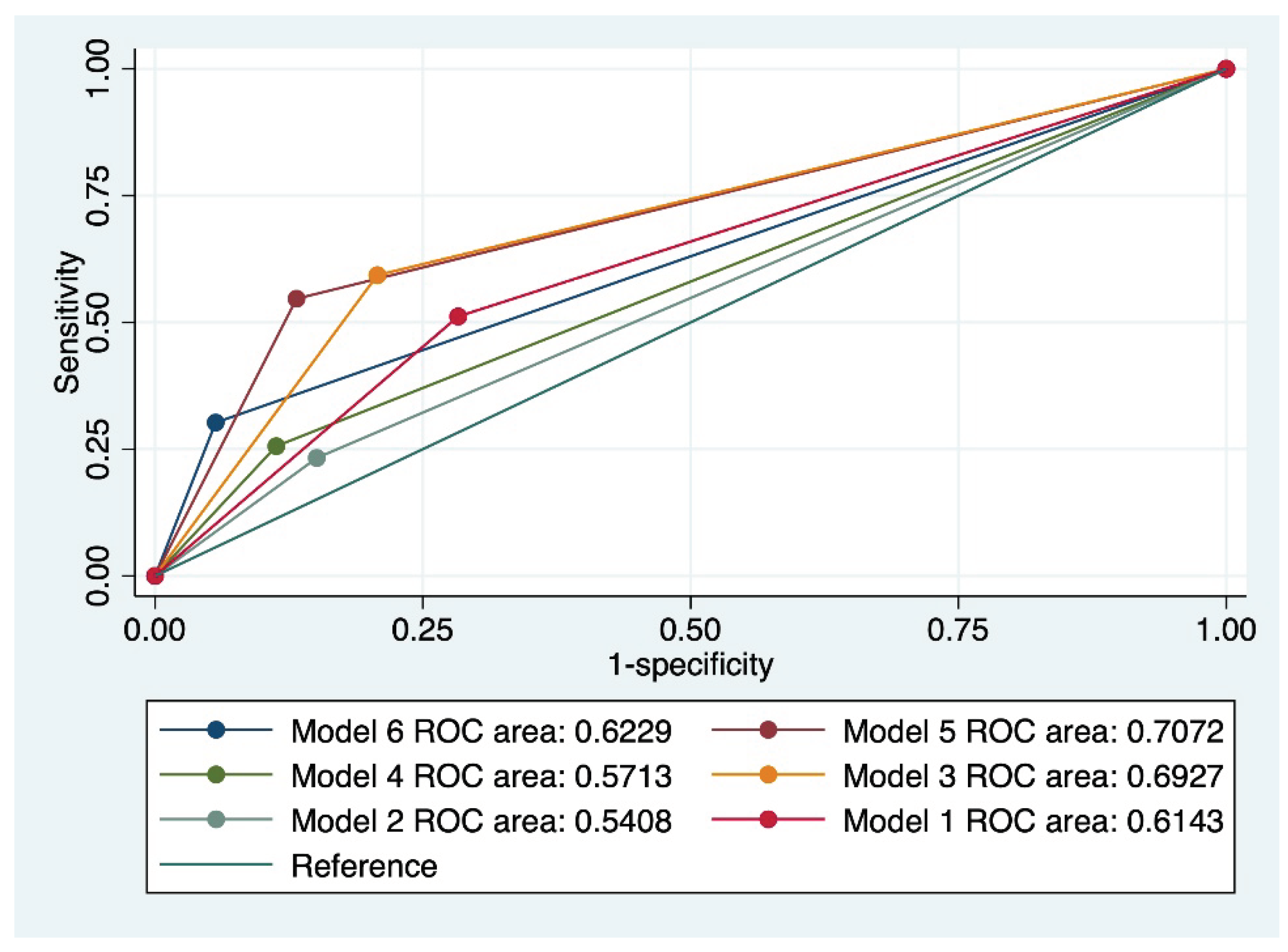
The final analysis evaluated the persistence of cervical abnormalities and the results are presented in
Table 4 and
Figure 3. The overall accuracy of individual index tests for the prediction of cervical abnormalities persistence was low to moderate, between 38.13 and 58.27%, the latter accuracy being attributed to a cervical cytology suggestive of LSIL. Moreover, all models that included LSIL outperformed models that included HSIL when used for the prediction of cervical abnormalities persistence. The best predictive performance for this outcome was achieved by model 5, which comprised a cervical cytology suggestive of LSIL, the presence of HPV 16/18 strains, a positive CINtecPlus result, and at least 3 clinical risk factors for cervical cancer, with a Sensitivity of 61.04%, Specificity of 88.71%, an AUC value of 0.707, and an accuracy of 73.38%.
4. Discussion
The risk stratification of cervical abnormalities represents a challenge for clinicians, especially for patients with CIN2 who have not completed their family planning due to the controversial risk of preterm birth after excisional therapies. On the other hand, it is important to note that the follow-up of patients with cervical abnormalities relies on tests characterized by variable sensitivity and specificities depending on the studied population.
This study was concentrated on HPV positive patients who underwent follow-up during a one-year time frame, and we evaluated the predictive accuracy of various index tests (cytology, HPV genotyping, and CINtecPlus) taken individually or combined with clinical risk factors for the progression, regression, or persistence of cervical abnormalities.
Our results indicated that the progression of cervical abnormalities was most accurately predicted by the presence of at least 3 clinical risk factors (accuracy: 67.13%), a cervical cytology suggestive of HSIL (accuracy: 64.03%), the presence of 16/18 HPV strains (accuracy: 66.19%), and a positive result of CINtecPlus (accuracy: 53.73%). Most of the evaluated index tests had high specificity and low to moderate sensitivity. A model that reunited all these individual index tests achieved a Se of 74.42%, a specificity of 97.92%, an AUC of 0.961, and an accuracy of 90.65%.
Indeed, a high-grade index cytology, the presence of 16/18 HPV strains, and a positive CINtecPlus result have been associated with a higher risk of progression of cervical lesions [
25,
26,
27]. Comparative literature data regarding the accuracy of the combined model for the prediction of cervical lesions progression is missing, but there are several studies that investigated the predictive performance of various combinations of index tests for disease detection. Thus, in a recent observational prospective study, the authors found out that a combination of p16/Ki67 dual staining and HPV 16/18 had a sensitivity of 42.8% and a specificity of 92.8% for the detection of high-grade CIN2 lesions [
28]. Moreover, the same authors outlined a sensitivity of 44% and a specificity of 91% for the detection of high-grade CIN3 lesions.
When we evaluated the performance of individual index tests for the prediction of cervical abnormalities regression, we found out that the presence of low-risk HPV strains (accuracy: 73.72%), a Pap smear suggestive of ASC-US (accuracy: 66.19%), a negative CINtecPlus (accuracy: 40.3%), and a personal history of less than 3 risk factors for cervical cancer (accuracy: 64.03%) achieved the best results. Follow-up studies confirmed a higher rate of cervical lesions regression in the presence of low-grade lesions (ASC-US/LSIL), infection by HPV other than HPV-16, or a negative CINtecPlus result [
29,
30]. Our model that reunited all these index tests achieved the best results for the prediction of cervical lesions regression (Se-33.3%, Sp-88%, AUC- 0.691, and accuracy: 82.14%).
Finally, a model that comprised a cervical cytology suggestive of LSIL, the presence of HPV 16/18 strains, a positive CINtecPlus result, and at least 3 clinical risk factors for cervical cancer achieved a Se of 61.04%, Sp of 88.71%, an AUC value of 0.707, and an accuracy of 73.38% for the prediction of cervical lesions persistence.
The prediction of cervical lesions regression or persistence was modest in terms of sensitivity and overall accuracy, even though it included factors associated with regression or persistence of cervical lesions [
31,
32], thus, leaving treatment algorithms dependent on repeated examinations and testing. Moreover, these results indicate the need for inclusion of more sensitive tests that can outperform the classical approaches. This finding is supported by recent studies that outline a higher predictive performance of various molecular or methylation markers [
33,
34].
Louvanto et al., investigated the predictive performance of pyrosequencing methylation and HPV genotyping for the prediction of regression, persistence or progression of HSIL (CIN2) in a 2-year surveillance period [
34]. The authors demonstrated that the S5 classifier outperformed cytology and HPV genotyping when used for the prediction of regression
versus progression of cervical lesions. Moreover, a combination of the S5 classifier and cytology had an AUC value of 0.735 in comparing regression
versus progression of cervical lesions, whereas HPV genotyping did not provide additional prognostic benefit.
Several risk factors for the recurrence of cervical lesions have been proposed in the literature [
35]. One study conducted by Bogani et al., investigated the impact of persistent HPV infection on the recurrence risk of CIN2+ in a cohort of 545 patients who underwent primary conization [
36]. Their results indicated that patients with persistent HPV infection after 6 months had a risk of recurrence of 7.46%, while patients with persistent HPV infection at 12 months after conization had a risk of cervical lesions recurrence of 13.1%. On the other hand, they found out that the persistence of HPV infection for more than 12 months did significantly increase the risk of recurrence. These results point out the need to thoroughly follow-up patients with persistent HPV infection in order to timely detect cervical lesions recurrence after primary conization.
The limitations of this study are represented by the small cohort of patients included in the follow-up, the limited time-frame, and inclusion of patients with a cytology positive for only ASC-US, LSIL, or HSIL. We hypothesize that a longer follow-up period would allow us better understand the dynamics of cervical lesions progression, regression, or persistence, especially in patients with a high-risk profile. The strengths of this study stem from the use of histopathology results as gold standards both at the inclusion in the study and at the follow-up, the assessment of the predictive accuracy of various models for the prediction of progression, regression, and persistence of cervical lesions, as well as a CINtecPlus assay performed on fresh cytology samples. We advocate for the use of CINtecPlus assay on fresh cytology samples, and not on already colored slides because this approach could offer better insight on the markers’ positivity, and limit the false-negative results. Moreover, this study has a prospective design and it was conducted during a one-year time frame. We will follow-up these patients for another year, and report the results in another paper.
Our results outline the limited performance of current individual screening assays for the prediction of cervical lesions regression or persistence, thus pointing out the need for performing multiple tests during the patient’s follow-up. Our results clearly indicated that the combined screening approaches have superior predictive performance in comparison with individual screening test for the prediction of cervical lesions progression, persistence, or regression. Thus, the clinicians should integrate data obtained from multiple screening tests and corroborate it with literature data that indicates a high or a low risk of cervical lesions progression, regression or persistence. Moreover, we hypothesize that the inclusion of new biomarkers could improve the overall prognostic accuracy and reduce the long-term costs of the HPV-positive patient surveillance program. Also, deep neural networks could be employed for improvement of cervical lesions detection and classification [
37,
38].
Finally, this study indicated low HPV vaccinal rates and raised awareness about the need to implement national policies that will increase the acceptability of HPV vaccination, which is low in Romania [
39].
5. Conclusions
Cervical lesion progression was best predicted by a model that included the presence of at least 3 clinical risk factors, a cervical cytology suggestive of HSIL, the presence of 16/18 HPV strains, and a positive result of CINtecPlus.
The prediction of cervical lesions regression or persistence was modest using individual index tests or combined models, thus outlining the need to improve current surveillance of HPV positive patients wither by multiple testing or by including new biomarkers.
Further follow-up studies on various populations of HPV positive patients could offer us a better insight into specific prediction models.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Figure S1: CINtec plus positive. Co-expression of p16 (brown) signals and Ki-67 (red) signals in the same atypical squamous cells (x20); Figure S2: CINtec plus negative. Expression of Ki-67 (red) signals in atypical squamous cells (x40); Figure S3: CINtec plus negative. No expression of Ki-67 and p16 in squamous cells (x20); Figure S4: CINtec plus positive. Co-expression of p16 (brown) signal (weak) and Ki-67 (red) signal in the same squamous cells (x20); Figure S5: CINtec plus positive. Co-expression of p16 and Ki-67 in the same atypical squamous cells (x20); Figure S6: CINtec plus positive. Co-expression of p16 and Ki-67 in the same atypical squamous cell (x40).
Author Contributions
Conceptualization, D.S., T.G., I-V.M., A-S.M-P., S.V., P.V., and I-A.V.; methodology, I-S.S., R-A.B., R.S., A.C., C.V. and I.P; software, M.M-P., A-M.A, and G.A.; validation, M.M-P., A-M.A, and G.A; formal analysis, I-S.S., R-A.B., R.S., A.C., C.V., and I.P.; investigation, D.S., T.G., I-V.M., A-S.M-P., S.V., P.V., and I-A.V.; resources, M.M-P., A-M.A, and G.A; data curation, I-S.S., R-A.B, R.S.,. A.C., C.V., and I.P; writing—original draft preparation, D.S., T.G., I-V.M., A-S.M-P., S.V., P.V., and I-A.V.; writing—review and editing, D.S., T.G., I-V.M., A-S.M-P., S.V., P.V., and I-A.V.; visualization, M.M-P., A-M.A, and G.A; supervision, D.S.; project administration, D.S.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of Avicena Profertis Clinic, Iasi, Romania (No. 728/01.10.2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets are available from the correspondent authors upon a reasonable request due to local policies.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ronco G, Dillner J, Elfström KM, Tunesi S, Snijders PJF, Arbyn M; et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: Follow-up of four European randomised controlled trials. Lancet 2014, 383, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Roura E, Travier N, Waterboer T, de Sanjosé S, Bosch FX, Pawlita M; et al. The Influence of Hormonal Factors on the Risk of Developing Cervical Cancer and Pre-Cancer: Results from the EPIC Cohort. PLoS ONE 2016, 11, e0147029. [Google Scholar] [CrossRef]
- Zhu H, Shen Z, Luo H, Zhang W, Zhu X. Chlamydia Trachomatis Infection-Associated Risk of Cervical Cancer: A Meta-Analysis. Medicine 2016, 95, e3077. [Google Scholar] [CrossRef]
- Pérez-González A, Cachay E, Ocampo A, Poveda E. Update on the Epidemiological Features and Clinical Implications of Human Papillomavirus Infection (HPV) and Human Immunodeficiency Virus (HIV) Coinfection. Microorganisms 2022, 10. [Google Scholar] [CrossRef]
- Iliescu M, Cărăuleanu A. The Portrait of a Good Doctor: Conclusions from a Patients and Medical Students Survey. Revista de Cercetare si Interventie Sociala 2014, 47, 261–271. [Google Scholar]
- Davey DD, Neal MH, Wilbur DC, Colgan TJ, Styer PE, Mody DR. Bethesda 2001 implementation and reporting rates: 2003 practices of participants in the College of American Pathologists Interlaboratory Comparison Program in Cervicovaginal Cytology. Arch Pathol Lab Med. 2004, 128, 1224–1229. [CrossRef] [PubMed]
- Darragh TM, Colgan TJ, Cox JT, Heller DS, Henry MR, Luff RD; et al. The Lower Anogenital Squamous Terminology Standardization Project for HPV-Associated Lesions: Background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Arch Pathol Lab Med. 2012, 136, 1266–1297. [Google Scholar] [CrossRef]
- Carreon JD, Sherman ME, Guillén D, Solomon D, Herrero R, Jerónimo J; et al. CIN2 is a much less reproducible and less valid diagnosis than CIN3: Results from a histological review of population-based cervical samples. Int J Gynecol Pathol. 2007, 26, 441–446. [Google Scholar] [CrossRef]
- Demarco M, Egemen D, Raine-Bennett TR, Cheung LC, Befano B, Poitras NE; et al. A Study of Partial Human Papillomavirus Genotyping in Support of the 2019 ASCCP Risk-Based Management Consensus Guidelines. J Low Genit Tract Dis. 2020, 24, 144–147. [Google Scholar] [CrossRef]
- Perkins RB, Guido RS, Castle PE, Chelmow D, Einstein MH, Garcia F; et al. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J Low Genit Tract Dis. 2020, 24, 102–131. [Google Scholar] [CrossRef]
- Conner SN, Frey HA, Cahill AG, Macones GA, Colditz GA, Tuuli MG. Loop electrosurgical excision procedure and risk of preterm birth: A systematic review and meta-analysis. Obstet Gynecol. 2014, 123, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Stoler MH, Schiffman M. Interobserver reproducibility of cervical cytologic and histologic interpretations: Realistic estimates from the ASCUS-LSIL Triage Study. Jama. 2001, 285, 1500–1505. [Google Scholar] [CrossRef] [PubMed]
- Gurumurthy M, Cotton SC, Sharp L, Smart L, Little J, Waugh N; et al. Postcolposcopy management of women with histologically proven CIN 1: Results from TOMBOLA. J. Low. Genit. Tract Dis. 2014, 18, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Loopik DL, Bentley HA, Eijgenraam MN, IntHout J, Bekkers RLM, Bentley JR. The Natural History of Cervical Intraepithelial Neoplasia Grades 1, 2, and 3: A Systematic Review and Meta-analysis. J Low Genit Tract Dis. 2021, 25, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Berggrund M, Enroth S, Lundberg M, Assarsson E, Stålberg K, Lindquist D; et al. Identification of Candidate Plasma Protein Biomarkers for Cervical Cancer Using the Multiplex Proximity Extension Assay. Mol Cell Proteomics. 2019, 18, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Guo C, Qu X, Tang X, Song Y, Wang J, Hua K; et al. Spatiotemporally deciphering the mysterious mechanism of persistent HPV-induced malignant transition and immune remodelling from HPV-infected normal cervix, precancer to cervical cancer: Integrating single-cell RNA-sequencing and spatial transcriptome. Clin Transl Med. 2023, 13, e1219. [Google Scholar] [CrossRef] [PubMed]
- Güzel C, van Sten-Van’t Hoff J, de Kok I, Govorukhina NI, Boychenko A, Luider TM; et al. Molecular markers for cervical cancer screening. Expert Rev Proteom. 2021, 18, 675–691. [Google Scholar] [CrossRef]
- El-Zein M, Gotlieb W, Gilbert L, Hemmings R, Behr MA, Franco EL. Dual staining for p16/Ki-67 to detect high-grade cervical lesions: Results from the Screening Triage Ascertaining Intraepithelial Neoplasia by Immunostain Testing study. Int J Cancer. 2021, 148, 492–501. [Google Scholar] [CrossRef]
- Perkins RB, Wentzensen N, Guido RS, Schiffman M. Cervical Cancer Screening: A Review. JAMA 2023, 330, 547–558. [Google Scholar] [CrossRef]
- Abbas M, Erduran I, De Jonge J, Bettendorf O. Evaluation of P16/Ki67 (CINtecPlus) and L1-capsid compared with HPV-genotyping in cervical cytology in women ≥35 years old focusing on patients with atypical squamous cells of undetermined significance. Oncol Lett. 2022, 24, 242. [Google Scholar] [CrossRef]
- Rokita W, Kedzia W, Pruski D, Friebe Z, Nowak-Markwitz E, Spaczyński R; et al. Comparison of the effectiveness of cytodiagnostics, molecular identification of HPV HR and CINtecPLUS test to identify LG SIL and HG SIL. Ginekol Pol. 2012, 83, 894–898. [Google Scholar]
- Yu L, Fei L, Liu X, Pi X, Wang L, Chen S. Application of p16/Ki-67 dual-staining cytology in cervical cancers. J Cancer. 2019, 10, 2654–2660. [Google Scholar] [CrossRef]
- Ungureanu C, Socolov D, Anton G, Mihailovici M, Teleman S. Immunocytochemical expression of p16INK4a and HPV L1 capsid proteins as predictive markers of the cervical lesions progression risk. Rom. J. Morphol. Embryol. Rev. Roum. Morphol. Embryol. 2010, 51, 497–503. [Google Scholar]
- Wilbur DC, Nayar R. Bethesda 2014: Improving on a paradigm shift. Cytopathology. 2015, 26, 339–342. [CrossRef] [PubMed]
- Lycke KD, Kahlert J, Damgaard RK, Eriksen DO, Bennetsen MH, Gravitt PE; et al. Clinical course of cervical intraepithelial neoplasia grade 2: A population-based cohort study. Am J Obstet Gynecol. 2023, 229, 656.e1–e15. [Google Scholar] [CrossRef]
- Rodríguez-Trujillo A, Martí C, Angeles MA, Sierra A, Esteve R, Saco A; et al. Value of HPV 16/18 Genotyping and p16/Ki-67 Dual Staining to Predict Progression to HSIL/CIN2+ in Negative Cytologies From a Colposcopy Referral Population. Am J Clin Pathol. 2018, 150, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Li Y, Liu J, Gong L, Sun X, Long W. Combining HPV DNA load with p16/Ki-67 staining to detect cervical precancerous lesions and predict the progression of CIN1-2 lesions. Virol J. 2019, 16, 117. [Google Scholar] [CrossRef] [PubMed]
- Øvestad IT, Dalen I, Andersland MS, Vintermyr OK, Moltu P, Berland JM; et al. Triaging HPV-Positive Cervical Samples with p16 and Ki-67 Dual Stained Cytology within an Organized Screening Program-A Prospective Observational Study from Western Norway. Int J Mol Sci. 2023, 24. [Google Scholar] [CrossRef]
- Nourrisson A, Lepetit H, Marty M, Garrigue I, Brun JL. Regression of cervical high-grade squamous intraepithelial lesions (HSIL/CIN2) managed expectantly. J Gynecol Obstet Hum Reprod. 2022, 51, 102442. [Google Scholar] [CrossRef]
- White C, Bakhiet S, Bates M, Keegan H, Pilkington L, Ruttle C; et al. Triage of LSIL/ASC-US with p16/Ki-67 dual staining and human papillomavirus testing: A 2-year prospective study. Cytopathology 2016, 27, 269–276. [Google Scholar] [CrossRef]
- Sykes PH, Simcock BJ, Innes CR, Harker D, Williman JA, Whitehead M; et al. Predicting regression of cervical intraepithelial neoplasia grade 2 in women under 25 years. Am. J. Obstet. Gynecol. 2022, 226, 222.e1–e13. [Google Scholar] [CrossRef]
- Huică I, Iancu IV, Botezatu A, Pleşa A, Socolov D, Teleman S; et al. Factors Associated with Persistence of Hpv Genital Infection in a Small Cohort of Romanian Women. Acta Clin Croat. 2019, 58, 410–416. [Google Scholar] [CrossRef]
- Bumrungthai S, Ekalaksananan T, Kleebkaow P, Pongsawatkul K, Phatnithikul P, Jaikan J; et al. Mathematical Modelling of Cervical Precancerous Lesion Grade Risk Scores: Linear Regression Analysis of Cellular Protein Biomarkers and Human Papillomavirus E6/E7 RNA Staining Patterns. Diagnostics 2023, 13, 1084. [Google Scholar] [CrossRef] [PubMed]
- Louvanto K, Aro K, Nedjai B, Bützow R, Jakobsson M, Kalliala I; et al. Methylation in Predicting Progression of Untreated High-grade Cervical Intraepithelial Neoplasia. Clinical Infectious Diseases. 2019, 70, 2582–2590. [Google Scholar] [CrossRef]
- Bogani G, Sopracordevole F, Ciavattini A, Vizza E, Vercellini P, Ghezzi F; et al. HPV persistence after cervical surgical excision of high-grade cervical lesions. Cancer Cytopathol. 2023. [CrossRef]
- Bogani G, Sopracordevole F, Ciavattini A, Vizza E, Vercellini P, Giannini A; et al. Duration of human papillomavirus persistence and its relationship with recurrent cervical dysplasia. Eur J Cancer Prev. 2023, 32, 525–532. [Google Scholar] [CrossRef]
- Huang P, Tan X, Chen C, Lv X, Li Y. AF-SENet: Classification of Cancer in Cervical Tissue Pathological Images Based on Fusing Deep Convolution Features. Sensors 2020, 21. [Google Scholar] [CrossRef]
- Huang P, Zhang S, Li M, Wang J, Ma C, Wang B; et al. Classification of Cervical Biopsy Images Based on LASSO and EL-SVM. IEEE Access. 2020, 8, 24219–24228. [Google Scholar] [CrossRef]
- Grigore M, Vasilache IA, Cianga P, Constantinescu D, Duma O, Matasariu RD; et al. Acceptability of Human Papilloma Virus Self-Sampling for Cervical Cancer Screening in a Cohort of Patients from Romania (Stage 2). J Clin Med. 2022, 11. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).