1. Introduction
Dry eye (DE), is a multifactorial disease of the ocular surface caused by impairment of tear production and cornea damage, which affects 5–40% of adults over 40 years of age [
1]. The prevalence of DE is steadily increasing, and DE is one of three major eye diseases rapidly increasing in the elderly [
2]. The primary symptoms of DE are stiffness, vision blurring, eye fatigue, and eye congestion. Dysfunction of tear film can cause damage to the cornea and other epithelial eye tissue, preventing them from functioning correctly [
3]. Also, DE is closely related to inflammation-induced tear film instability and ocular surface [
4]. Because DE can result epithelial lesions or local inflammation reaction, leading to a deterioration of ocular surface defense mechanisms, dysfunction, and cellular degeneration of conjunctiva and cornea apart [
5]. Therefore, it is meaningful to find attempts to therapeutic approach to DE treatments. One of approaches, artificial tears containing hyaluronic acid or cyclosporine A are used to treat DE, but provide only temporary symptomatic relief [
6]. Long-term application of these agents can have adverse effects such as an corneal and ocular hypertension, infection, and inflammation [
7]. Benzalkonium chloride (BAC) is the most commonly used preservative in topical artificial tears products. Although BAC have amphiphilic, high water-soluble characters, and antimicrobial effect, long-term use can occur side effect including DE and ocular inflammation [
8,
9]. Even recent studies reported that BAC can reach the posterior eye and optic nerve [
10,
11].
Cornea injury is a representative feature of dry eyes, is characterized by ocular surface inflammation and destruction of the tear film due to up-regulation of inflammatory cytokines [
12]. Also, ocular inflammation is considered the hallmarks of DE. Production of pro-inflammatory triggers, including interleukin-20 (IL-20) and tumor necrosis factor-α onto the ocular epithelial surface and subsequent damage causes tear film dysfunction and ultimately DE [
13]. Metalloproteinases (MMPs) are positively associated with the severity of inflammation in the conjunctiva tissue, and recent studies have identified MMPs as a potential therapeutic target for DE [
14]. MMPs also disrupt tight junctions essential for maintenance of the corneal barrier. In DE, matrix metalloproteinase-9 (MMP-9) levels are increased in the tears and ocular epithelial surface [
15]. Exposure of the cornea to desiccating stress increases corneal epithelial permeability, which is regulated in part by increased MMP-9 level [
16,
17,
18]. Also, the pro-inflammatory cytokine IL-20 is involved in the pathogenesis of inflammatory DE disease. A recent study identified that circulating level is significantly increased in DE patient tears and corneas, and in induced DE models [
19].
The gut microbiota regulates host physiological processes via strengthening gut tight junctions and regulating the intestinal epithelium, with improved function associated with increased microbial diversity [
20]. The composition and activity of the gut microbiota affect host health by causing changes in metabolic activity or changes in local distribution [
21]. For instance, certain symbiotic bacteria such as Bifidobacterium can prevent colonization of pathogenic bacteria by reducing the intestinal pH [
22]. Also, with recent enhanced understanding of the important role of gut microbiome, the host inflammation response and the pathogenesis of ocular disease also has been brought to attention [
23]. Recent studies have reported an association between ocular disease and the microbiota profile of the host intestine. This is called the 'gut-eye axis', which indicates that changes in the gut microbiome alter host immunity, with consequential influence for ocular health and disease [
24,
25]. Furthermore, the gut microbiome is associated with myriad pathophysiological processes in the host, especially chronic inflammatory diseases [
26,
27,
28,
29]. Therefore, probiotics have recently attracted attention as a potential dietary supplement to prevent inflammation. One of the major mechanisms of immune system-related benefits of probiotics studied
in vitro and
in vivo studies is to enhance the epithelial barrier and regulate inflammatory cytokine production. Indeed, numerous studies have reported that
Lactobacillus and
Bifidobacterium have the ability to accelerate the anti-inflammatory process and reduce the production of the pro-inflammatory cytokines IL-1b or IL-6.
Bacteria of the phylum Firmicutes are some of the most important probiotic bacteria of the gut microbiome. Firmicutes are widely distributed in nature, and include
Limosilactobacillus fermetum,
Limosilactobacillus ruuteri, Lactobacillus acidophilus,
Lacticaseibacillus casei, and
Lactobacillus delbrueckii subsp. Bulgaricus. Especially,
Limosilactobacillus fermentum (
L. fermentum) is an obligately heterofermentative microbiota that ferments carbohydrates to produce lactic acid, ethanol, acetic acid, and carbon dioxide [
30]. Recent studies have reported beneficial effects of
L. fermentum in obesity, cardiovascular disease, metabolic mellitus, and gastrointestinal barrier dysfunction [
31,
32,
33,
34]. It is also known through animal experiments that
L. fermentum acts as an antimicrobial and antioxidant modulator [
35]. In our previous study, we identified that oral administration of
Limosilactobacillus fermentum HY7302 (HY7302) improves DE symptoms in the mice model [
36]. However, the molecular mechanisms of the effects of probiotic intake on ocular tissues have not yet been elucidated. Therefore, in this study, to understand the efficacy and molecular mechanisms of probiotics on DE-induced corneal damage, we determined the signaling regulation of inflammatory and apoptotic factors in ocular tissues after HY7302 intake in BAC-induced DE mice. To understand the mechanisms for the therapeutic effects of HY7302 in DE, it is essential to delineate the role of the gut–eye axis and microbiome in regulating DE pathology. Thus, we investigated the correlation effect of HY7302 probiotics with the intestinal microbiome of corneal damaged mice in this study.
2. Materials and Methods
2.1. Animal Study
Six-week-old male Balb/c mice (ORIENT, Seongnam-si, Republic of Korea) were used in the study. All in vivo studies were approved by the Institutional Animal Care and Use Committee (IACUC number P235002) of NDIC Co., Ltd in Korea and adhered to the guidelines of code of practice for the housing and care of Animals used in scientific procedures. Prior to experiments, mice were acclimatized for 1 week with ad libitum access to water and food on a 12 h light/12 h dark cycle. Room temperature was 23 ± 2°C and relative humidity was 40–70%. Mice were randomly allocated to one of five groups: CON, non-DE control group; DE, topical 0.1% BAC; DE + Omega3, topical 0.1% BAC + oral Omega3 (200 mg/kg/day); DE + HY7302L, topical 0.1% BAC + oral HY7302 (108 CFU /kg/day); DE + HY7302H, topical 0.1% BAC + oral HY7302 (109 CFU/kg/day). (n = 8 mice/group). To induce DE, mouse eyes were exposed to 0.1% BAC (Sigma-Aldrich, St. Louis, MI, USA) dissolved in phosphate buffered saline, with 5 μL/eye applied twice daily to the ocular surface for 14 days. During the same period, HY7302 (1 × 108 CFU/kg/day or 1 × 109 CFU/kg/day) dissolved in 0.5% aqueous carboxymethylcellulose solution were administered orally. Omega-3 fatty acids in 0.5% aqueous carboxymethylcellulose solution was orally gavaged (200 mg/kg/day) in a positive control group.
2.2. Blood parameter analysis
Blood was collected via the abcominal vein immediately after euthanasia, and serum was prepared by centrifuging at 3,000 RPM for 20 min. Serum concentrations of MMP-9 (ab253227) and IL-20 (ab235645) were measured using commercial assay kits (Abcam, Cambridge, UK).
2.3. Measurement of tear volume, corneal fluorescein score, and tear break-up time
Tear volume was measured in each mouse following abdominal injection of 10 mg/kg xylazine/100 mg/kg ketamine. Tear amounts were measured using Schirmer’s test strips (Bio Color Tear Test, Bio Optics, Seongnam-si, Republic of Korea). Tear break-up time (TBUT) was determined using a cobalt blue slit lamp after corneas were treated with 0.5% fluorescein solution on day 14 of BAC treatment. The time (sec) until appearance of the first crack line on the dryed tear film layer was recorded. Subsequently, corneas were washed with a troterin antidote treatment (Alcon, Seoul, Republic of Korea). Flouorescein sodium solution (0.1%) was appied to the cornea, and corneal images were captured under blue light (Micron-IV, Phoenix, Kawasaki, Japan). The corneal fluorescein staining (CFS) score was caculated using a 0–3 point scale. The cornea was divided into into five areas, which were scored individually, and the values were added to obtain the combined score.
2.4. Histological analysis
Eyes were fixed with 4% paraformaldehyde and embedded in paraffin. Sections were obtained, stained with hematoxylin and eosin (H&E), and analyzed by light microscopy (Nikon Eclipse E600 microscope, Nikon Corporation, Tokyo, Japan). The number of corneal epithelium detachments per area was calculated using Image J software.
2.5. Western blotting
Mice eye tissues or cells were lysed using pro-prep buffer (iNtRON Biotechnology Inc., Seoul, Korea) containing proteinase and phosphatase inhibitors. Homogenates were centrifuged at 10,000 × g for 15 min at 4°C and supernatants were collected. Total protein concentration was measured using a Bio-Rad protein assay kit (Bio-Rad, Hercules, CA, USA). Protein lysates (18 µg) were seperated on 4–15% Precast gradient SDS-PAGE gels and transferred to PVDF membranes. Membranes were incubated at 4°C overnight with primary antibodies in Tris-buffered saline containing 0.05% Tween-20 (TBS-T) and 5% skimmilk. After washing with TBS-T, membranes were incubated in 5% non-fat dried milk containing secondary antibody conjugated to IgG horseradish peroxidase for 1 h. Protein bands were visualized using EZ-Western Lumi Femto (DoGenBio, Seoul, Korea) and an LAS-4000 imager (GE Healthcare Life Sciences, Marlborough, MA, USA), which was also used to quantify band density. B-cell lymphoma 2 (Bcl-2 D17C4, cs3498), Bcl-2-associated X protein (BAX, cs2772), Cleaved Caspase-3 (Cleaved Cas-3 Asp175, 5A1E, cs9664), Glyceraldehyde 3-phosphate dehydrogenase (GAPDH 14C10, cs2118), phospho-p44/42 MAPK (p-ERK Erk1/2 137F5, cs4695), p44/42 MAPK (ERK Erk1/2 Thr202/Tyr204, cs4370), phospho-c-Jun N-terminal kinases (p-JNK Thr183/Tyr185 81E11, cs4668), c-Jun N-terminal kinases (JNK, cs9252), Interleukin 1 beta (IL-1β D3H1Z, cs12507), Matrix Metalloproteinase-9 (MMP-9 E7N3Y, cs24317), and Anti-rabbit IgG HRP-linked secondary antibodies were purchased from Cell signaling (Cell Signaling Technology, Massachusetts, USA).
2.6. Microbiome 16s rRNA gene amplification and sequencing
Sequencing libraries with amplified V3-V4 regions were generated according to the Illumina 16s Metagenomic Sequencing Library Preparation Guide (Illumina, San Diego, CA, USA). Input gDNA (2 ng) was PCR-amplified with 5× reaction buffer, 1 mM dNTP mix, 500 nM of each universal F/R PCR primer, and Herculase II fusion DNA polymerase (Agilent Technologies, Santa Clara, CA, USA). Thermal cycling for the first PCR step included 3 mins denaturation at 95°C, 25 cycles of 30 s at 55°C and 30 s at 72°C, followed by a 5 min final extension at 72°C. For sequencing, the V3-V4 regions of the bacterial 16S rRNA gene were amplified using primer set 341F (5′-TCGTC GGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG-3′) and 806R (5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC-3′). PCR products were purified using AMPure beads (Agencourt Bioscience, Beverly, MA, USA). Following purification, 2 µL PCR product from the first step was PCR-amplified for final library construction using NexteraXT Indexed Primer (Illumina, San Diego, CA, USA). Thermal cycling of the second PCR step was performed as described for the first step with 10 cycles. qPCR was conducted according to the qPCR Quantification Protocol Guide (KAPA Library Quantification kits for Illumina Sequencing platforms) and quantified using a TapeStation D1000 ScreenTape (Agilent Technologies, Waldbronn, Germany). All datasets have been deposited in NCBI Gene Expression Omnibus under accession number PRJNA1065618.
2.7. Microbiome Bioinformatic Analysis of 16s rRNA Sequencing Data
The 16s rRNA amplicon sequence data were analyzed using the QIIME2 platform (version 2023.9) [
37]. Sequences were demultiplexed using the q2-demux plugin and sequences with low quality scores were removed using the DADA2 plugin, generating an amplicon sequence variants (ASVs) table [
38]. ASVs were aligned using the MAFFT plugin, and were used to generate a rooted phylogenetic tree for phylogenetic diversity analysis using FastTree 2 [
39,
40]. Taxonomy analysis was performed using the QIIME2 feature classifier plugin, with a 99% identity threshold to the Silva 138 database [
41,
42]. Alpha diversity metrics of observed features were calculated to measure microbial diversity. A non-parametric Kruskal-Wallis test was used to determine the statistical significances of differences in microbial diversity. Unweighted UniFrac distance metrics were analyzed using Principle Coordinate Analysis (PCoA) [
43,
44], and the statistical significance of differences in PCoA plots between groups was assessed using permutational multivariate analysis of variance (PERMANOVA). Furthermore, using the Linear discriminant analysis effect size (LEfSe) method, significant differences in the relative abundance of bacterial composition between the HY7302 Low and High groups and DE groups were identified (LDA > 3.0). Correlations between gut microbiota and DE parameters (MMP9 IL-20, TV, TBUT, and Detachment)were calculated by using the Spearman’s rank correlation coefficient in the R software package.
2.8. Cell culture
The mouse CMT-93 (CCL-223) colon epithelial cell line was purchased from ATCC (Manassas, VA, USA). CMT-93 cells were cultured in DMEM/F12 containing 10% FBS and 1% penicillin/streptomycin (P/S) in a humidified 5% CO2 incubator at 37°C. HY7302 probiotics were prepared as a 1 × 1010 CFU/mL stock solution in distilled water and then diluted in medium to final concentrations of 1 × 106 CFU/mL or 1 × 107 CFU/mL. Cells were treated with 100 ng/mL TNFα to increase epithelial tight junction permeability.2.9. RNA isolation and quantitative polymerase chain reaction (q-PCR) analysis
2.9. RNA isolation and quantitative polymerase chain reaction (q-PCR) analysis
RNA was extracted using Trizol and 2 μg total RNA was reverse-transcribed to cDNA using a commercial kit (Maxime RT PreMix Kit, Intronm Seongnam, Korea). cDNA was analyzed by qPCR (Applied Biosystems, Carlsbad, CA, USA) using the TaqMan Probe-Based Gene Expression analysis system in combination with the TaqMan Gene Expression Master Mix (Applied Biosystems, Waltham, MA, USA). Tight juction protein-1 (TJP-1, Mm01320638_m1), tight juction protein-2 (TJP-2, Hs00910543_m1), Ocludin (Mm00500910_m1), Claudin-4 (CLDN-4, Mm00515514_s1), tumor necrosis factor (TNF, Mm00443258_m1), MMP-9 (Mm00442991_m1), and Interleukin-6 (IL-6, Mm00446190_m1) transcripts were quantified using gene-specific primers. Target gene mRNA levels were normalized against the corresponding level of GAPDH mRNA (Mm99999915_g1). To compare gene expression levels between groups, relative mRNA levels were calculated using the 2(−ΔΔCT) method.
2.10. Statistical analyses
mRNA and protein data are expressed as means ± standard deviations (SD). Data were analyzed and compared statistically with an unpaired two-tailed Student’s t-test using SPSS version 26.0 (IBM, Somers, NY, USA).
4. Discussion
DE, a multifactorial ocular surface disease, is characterized by tear film instability that correlates with symptoms and pathologies such as blurred vision, eye pain, and disruption of the ocular surface [
47]. The etiology of DE is complex, and damage caused by increased ocular surface inflammation or apoptosis and corneal and conjunctival abnormalities contribute to DE pathogenesis [
48]. Ocular surface desiccation is an important DE trigger factor. Prior studies have used mice, rats, and rabbits as animal models to investigate disease mechanisms of DE. In the BAC model, the ocular preservative BAC is administered to the ocular surface twice daily for 14 days. In the atropine model, DE is induced by administration of 1% atropine sulfate to the ocular surface three times daily for 5 days. Mouse models of Sjὃgren syndrome are used to study spontaneous inflammatory DE. Desiccating stress, in which eyes are exposed to a constant low-humidity air flow for 4 h daily, can be used to induce DE. Aging animal models are also used to study DE [
49]. In addition, the severity of DE in experimental animal studies is evaluated with DE tests, including the Schirmer tear test, corneal fluorescein staining, rose Bengal staining, and corneal sensitivity measured by esthesiometry.
In a prior study [
36], we determined the effects of the
Limosilactobacillus fermentum strain HY7302 in the DE mice model. We identified that oral administration of 1 × 10
9 CFU/kg/day HY7302 improves CFS score and TV. The effects of HY7302 on inflammatory signaling pathways in ocular tissue in BAC-treated cornea damaged mice has not been investigated. It is very important to assess the correlation of DE severity with potential changes in the gut microbiome mediated by HY7302 administration with the analysis of affected signaling pathways of eye tissue. Therefore, in the present study, we aimed to investigate the association between microbiome changes and severity of DE parameters correlated to inflammation, and to delineate the physiological and molecular mechanisms for HY3702 alleviation of DE.
In the present study, DE was induced with daily exposure to 0.1% BAC for 14 days. The efficacy of L. fermentum HY7302 at low and high doses (1 × 108 CFU/kg/day and 1 × 109 CFU/kg/day) was evaluated. As shown in our data, BAC-induced DE significantly decreased tear secretion, as measured by TV. Furthermore, DE increased CFS score and decreased TBUT. Treatment with HY7302 probiotics alleviated BAC-induced DE. Corneal epitheliums were dramatically detached in the BAC-induced DE group, while oral administration of HY7302 or omega3, used as a positive control, significantly alleviated detachment of the corneal epithelium. Together, these findings suggested that HY7302 probiotics significantly improved the phenotypes of BAC-induced DE.
Impaired tear production function in DE is related to environmental stressors of the ocular surface, which eventually cause chronic corneal epithelial damage and inflammation [
50]. Inflammation contributes significantly to chronic ocular surface damage, and can impair tear film homeostasis, perpetuating a vicious cycle [
51,
52]. Destruction of the eye barrier by corneal dryness induces an inflammatory response to external pathogens and increases production of inflammatory cytokines, including TNF, IL-6, IL-8, and chemokines [
25]. In the present study, p-JNK/JNK ratio and IL-1β protein level were significantly increased in the conjunctiva of the DE group relative to the CON group, as measured by Western blotting. However, these changes to phosphoprotein ratios and protein levels were alleviated in HY7302-treated groups relative to the DE group. Contrastingly, no differences in p-JNK/JNK ratio or IL-1β level were detected in the Omega3 positive control group relative to the DE group. The effect of HY7302 on MMP-9, which disrupts corneal epithelial barrier function, was also investigated, identifying that HY7302 treatment significantly decreased serum MMP-9 levels and the protein MMP-9 level in DE mice ocular tissue. Interestingly, according to our prior study [
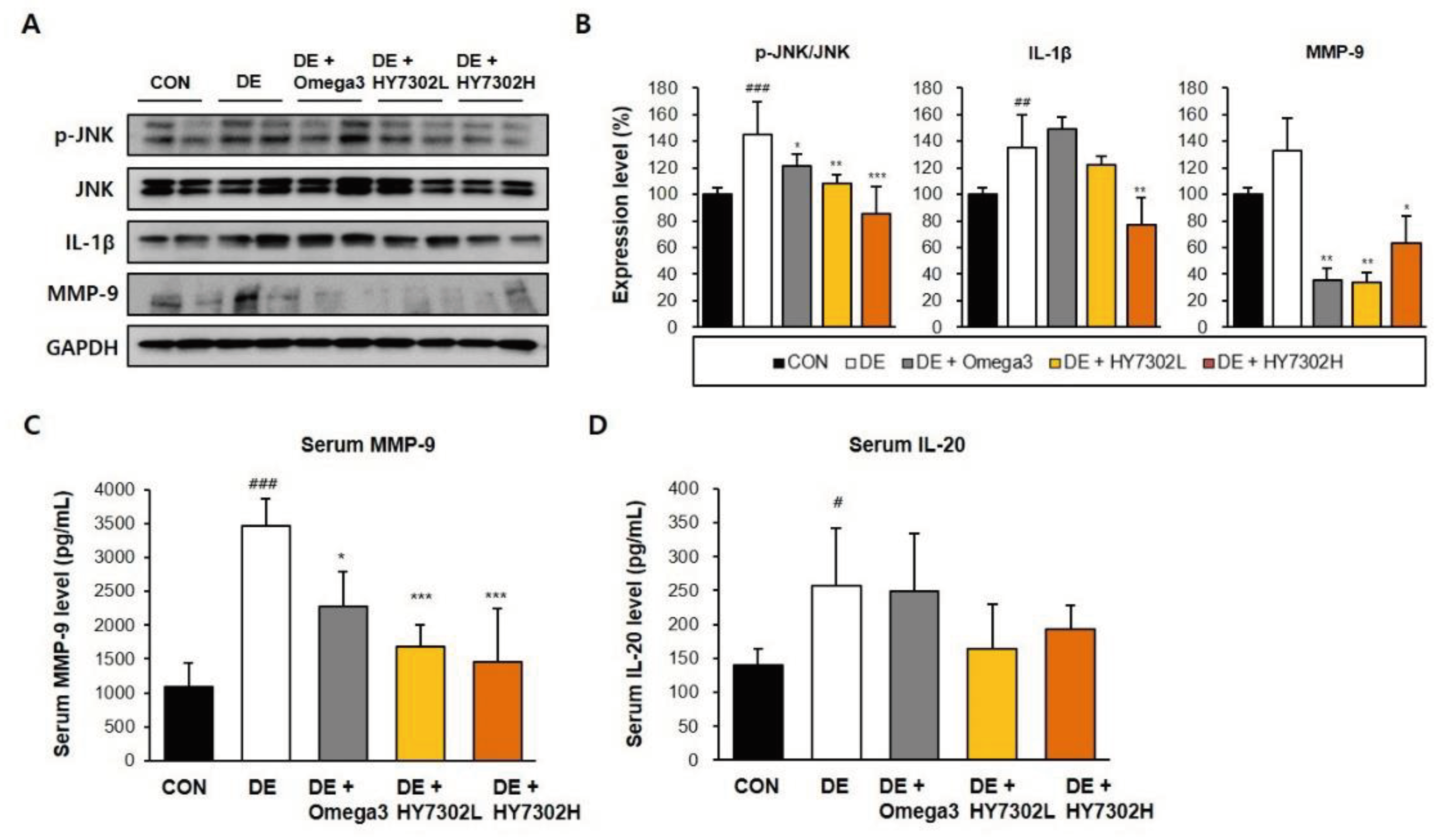
36], MMP-9 protein level of eye tissue significantly correlates with disease severity in BAC-induced DE. Consistently, in the present study, BAC-induced DE increased serum IL-20 levels, which was alleviated by HY7302 treatment but not significantly different. Therefore, our findings suggest that HY7302 probiotics inhibit the be systemic inflammatory response in tissue by regulating expression of MMP-9 and pro-inflammatory cytokines.
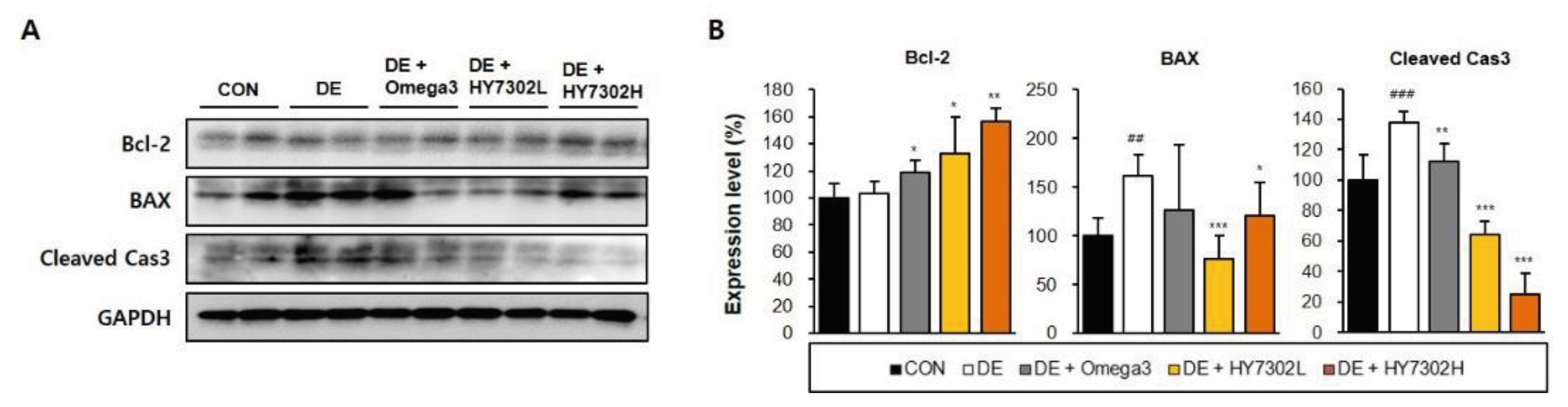
Importantly, DE is often accompanied by asymptomatic epithelial disease and abnormal inflammatory responses in the cornea and conjunctiva, which could be related to increased apoptosis in ocular tissues [
53]. In Sjogren syndrome, a systemic inflammatory disease associated with DE, pathological cell death is an important disease mechanism [
54]. Apoptosis is induced by activation of caspases, such as caspase-3, and is a normal homeostatic process that functions as a defense mechanism when cells are exposed to diverse noxious stimuli [
55]. We identified that protein levels of apoptotic factors were affected in BAC-induced DE mice, with increased BAX and cleaved caspase 3 and decreased Bcl2. HY7302 treatment increased bcl-2 levels and decreased BAX and cleaved caspase 3 levels, suggesting decreased apoptosis. Interestingly, low-dose HY7302 treatment decreased BAX levels in DE mice more significantly than did high-dose HY7302 treatment. Thus, HY7302 intake could be more likely to have therapeutic effects in forms of DE associated with ocular epithelial inflammation and apoptosis.
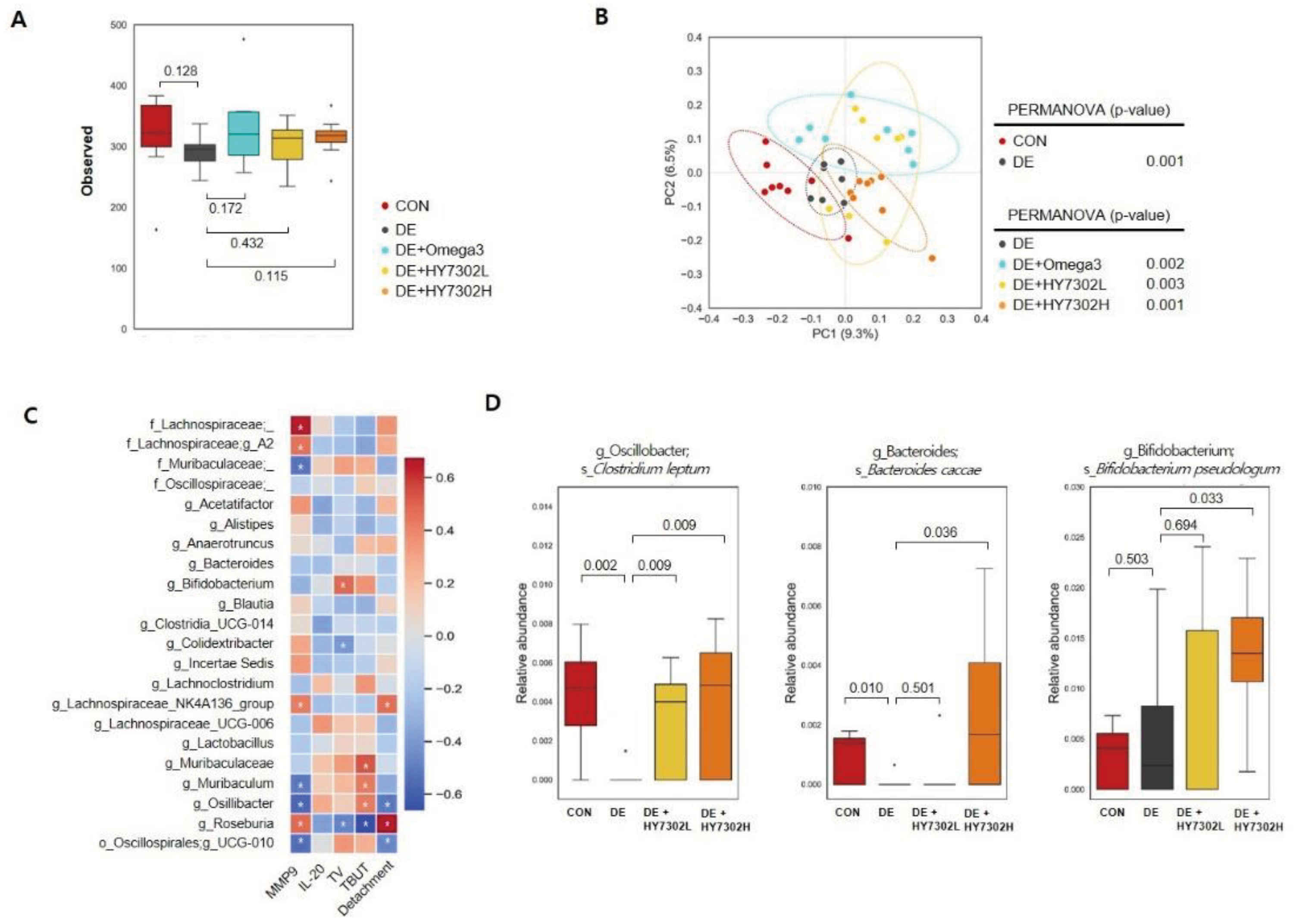
Healthy aging is associated with changes to the gut-eye axis. Since the ocular surface environment and intestines are primary interfaces with the external environment, maintenance of inflammatory homeostasis in both organs is important for ocular function health related with tear secretion. The relationship between the gut microbiota and eye health has been underscored in recent findings. For example, studies of the gut–eye axis have demonstrated that the microbiota affects the pathogenesis of multiple eye diseases, including DE, age-related ocular disease, uveitis, and glaucoma [
56,
57]. Therefore, understanding the gut-ocular axis and role of the microbiome in eye disease is important for the development of new therapeutic approaches related to ingestion of functional probiotics to control the microbiome. Therefore, in the present study, we evaluated the effect of HY7302 intake on gut microbial communities by analyzing gut microbiota profiles. Alpha diversity, which represents changes to diversity in experimental groups, was assessed by determining the observed number of ASVs (Observed features). Also, beta diversity, which measures changes in diversity between groups, was determined by calculating the unweighted UniFrac distance to examine differences in the microbial community composition and structure. HY7302 modestly increased gut microbiota alpha diversity, but this change was not statistically significant. However, the beta diversity index significantly decreased between the CON and DE groups. Further, beta diversity index significantly increased in HY7302-treated groups relative to the DE group, suggesting improvement of gut microbiota diversity compared with the DE group. In addition, genus-level changes in the microbiomes of BAC-induced DE mice were partially reversed by HY7302 administration. The abundances of family Lachnospiraceae and family Muribaculaceae increased, while Oscillibacter decreased in HY7302-treated DE mice relative to untreated DE mice. Lachnospiraceae, Muribaculaceae, and Oscillibacter are cores of the gut microbiota community, and influence the overall health of the host. These populations affect immune system regulation and natural defenses against external infection [
58].
In a recent study, gut microbiome analysis revealed compositional changes in Sjogren’s syndrome, which is associated with decreased tear secretion and TBUT. Abundance of the genus Bifidobacterium is significantly decreased by Sjogren’s syndrome, and overall beta diversity is decreased in Sjogren’s syndrome which correlated with relative DE severity [
59]. Likewise, in the present study, in species-level analyses, the relative abundances of
Clostridium leptum and
Bacteroides caccae were significantly decreased in the DE group relative to the CON group (
p = 0.002 and
p = 0.010, respectively). However, these populations were significantly increased in HY7302H-treated DE mice relative to untreated DE mice (
p = 0.009 and
p = 0.036, respectively). Recent studies have identified that Bacteroides spp. comprise a major fraction of the gut bacteriome and are important for maintenance of the gut microbial food web [
60]. However, changes to normal dietary probiotics could induce hyperproliferation of Bacteroides spp., which could cause microbiome changes induced by other intestinal symbionts. Bacteroides caccae promotes mucus degradation, which reduces intestinal inflammation by decreasing bacterial interactions with epithelial cells of the large intestine. In addition, Colidextribacter abundance is significantly correlated with pro-inflammatory metabolites generated by the gut microbiome, suggesting that Colidextribacter could produce inflammatory metabolites [
61]. We also identified that
Bifidobacterium pseudologum abundance was significantly higher in the HY7302H-treated DE group than in the untreated DE group (
p = 0.033). A recent study reported that
B. pseudolongum is closely related to intestinal barrier enhance, regulating inflammation, oxidative stress, and tight junction protein levels in this context [
62]. This suggests that in the present study, HY7302 treatment could have normalized the intestinal inflammatory response by increasing
B. pseudolongum abundance. Together, microbiome analyses demonstrated that HY7302 could alleviate BAC-induced DE pathologies in ocular tissue by increasing the abundances of species associated with inflammation or other chronic ocular diseases. Future studies will aim to determine the physiological effects and metabolic profile of
L. fermentum HY7302.
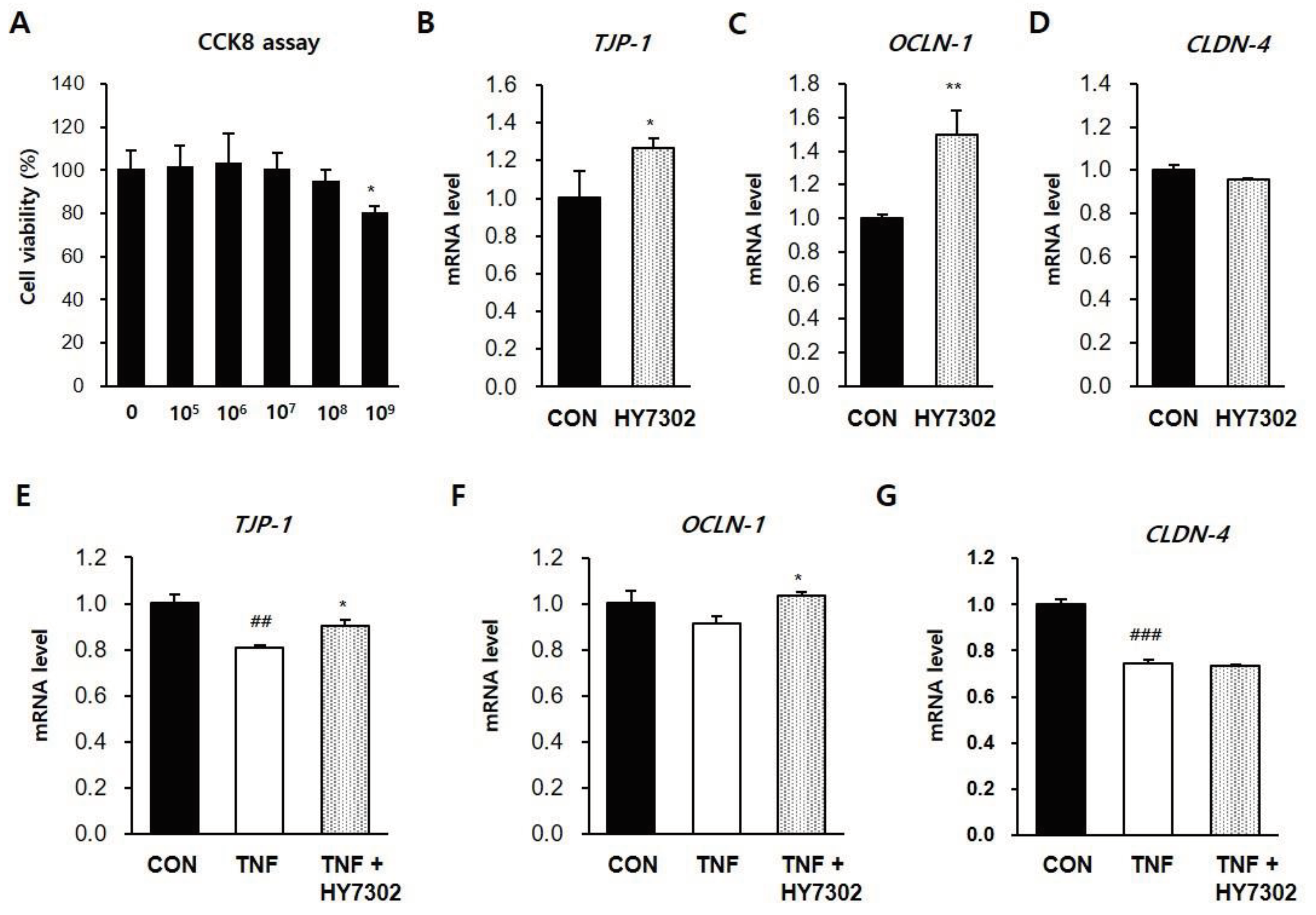
Recent studies have identified that inflammatory bowel disease (IBD), a common chronic intestinal inflammatory disease, is associated with DE. IBD has detrimental effects on extraintestinal systems such as the eye due to intestinal wall damage [
36]. A significant relationship between IBD, ocular surface damage, and recurrent corneal erosion was also identified. Regarding on this, finally, in relation to the pathological microbiota mechanisms of DE and the immunomodulatory effects of HY7302 probiotics, we determined if HY7302 could improve tight junction function in the CMT-93 mouse colon epithelial cell line. Tight junction protein 1 (TJP-1), occludin-1 (OCLN-1), and claudin-4 (CLDN-4) maintain barrier function by regulating the permeability of intestinal epithelial cells. Further, pro-inflammatory cytokines such as IL-1β and IL-6 are released when tight junctions are damaged and permeability increases. The pro-inflammatory factor TNFα is an important regulator of the inflammatory process in this context, and affects MMP-9 production. We identified that treatment of CMT-93 cells with HY7302 increased mRNA levels of
TJP-1 and
OCLN-1 but did not affect
CLDN-4 mRNA levels. HY7302 treatment also increased
TJP-1 and
OCLN-1 mRNA levels in cells stimulated with TNFα. Also, HY7302 suppressed the inflammatory response to TNFa stimulation by decreasing
TNF, MMP-9, and
IL-6 mRNA levels, p-ERK/ERK and p-p38/p38 ratios, and MMP-9 and IL-1 β protein levels in cells stimulated with TNFα. This suggests that HY7302 ingestion could alleviate the intestinal inflammatory response by increasing tight junction protein expression, which could indirectly decrease ocular tissue inflammation.
In conclusion, oral intake of L. fermentum HY7302 probiotics alleviated BAC-induced cornea damage. TV and TBUT increased, while CFS scores and corneal detachment injury index decreased, in DE mice treated with HY7302 relative to untreated DE mice. Moreover, HY7302 decreased DE-induced ocular inflammation and apoptosis and alleviated corneal epithelial detachment in this context. HY7302 treatment decreased inflammatory cytokine production and MMP-9 secretion in ocular tissue, which are important DE regulators. Further, HY732 increased microbiota beta-diversity and altered microbiome composition in the context of DE. Taken together, these findings suggest HY7302 could alleviate DE by regulating gut–eye axis communication via the inflammatory response. Therefore, HY7302 probiotics could potentially improve eye health by controlling intestinal health and immune regulation.
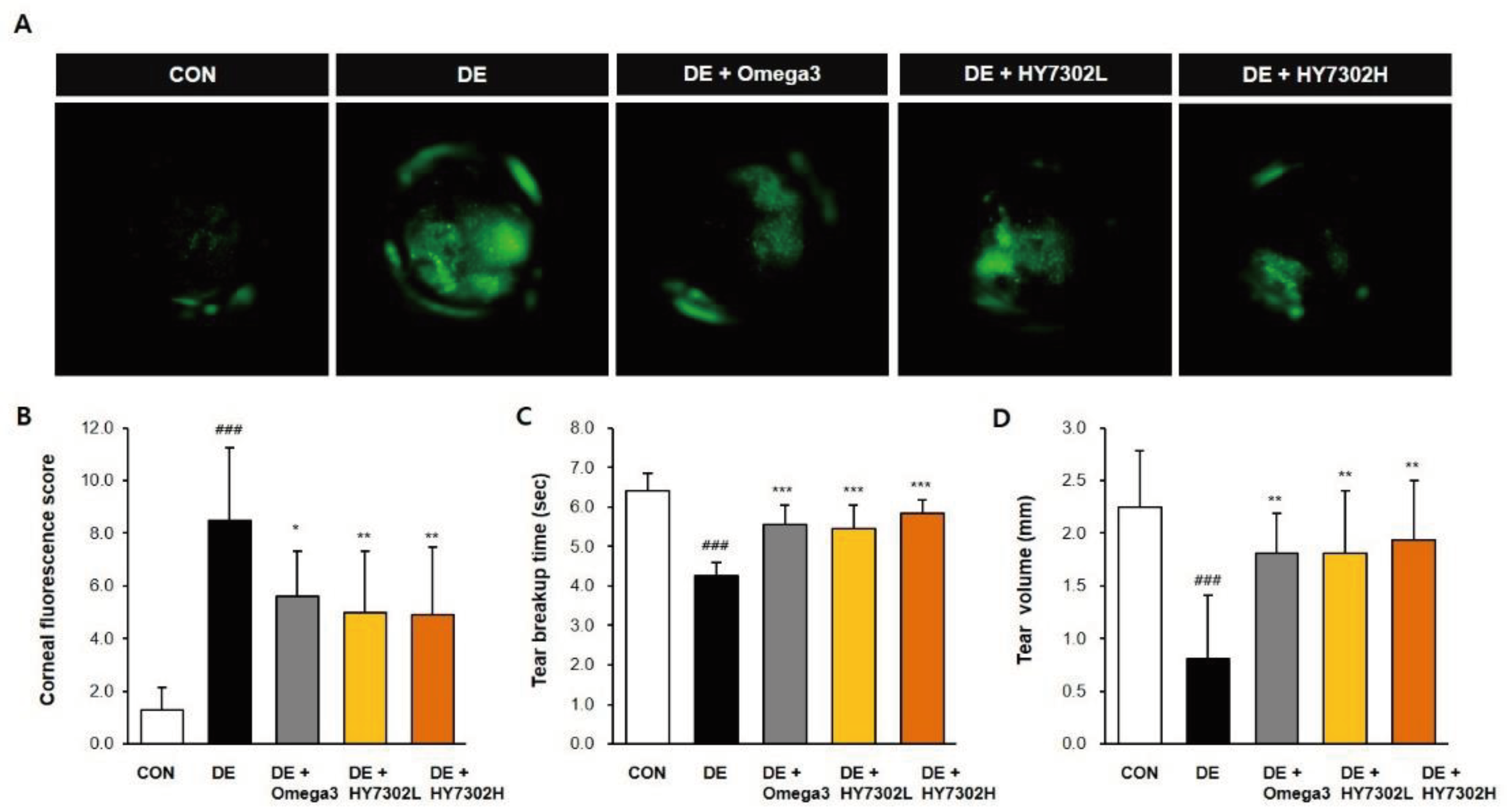
Figure 1.
Effect of HY7302 on corneal fluorescein score, tear break-up time, and tear volume. (A-B) Corneal fluorescein images and corneal fluorescein sodium staining (CFS) scores in DE mice. (C) Tear break-up time as detected with commercial fluorescein strips. (D) Tear volume as measured by Schirmer’s test. CON, non-DE control group; DE, topical 0.1% BAC; DE + Omega3, topical 0.1% BAC + oral Omega3 (200 mg/kg/day); DE + HY7302L, topical 0.1 % BAC + oral HY7302 (108 CFU/kg/day); DE + HY7302H, topical 0.1% BAC + oral HY7302 (109 CFU/kg/day). ### p< 0.001 relative to CON. *p < 0.05, **p < 0.01, and ***p<0.001 relative to DE.
Figure 1.
Effect of HY7302 on corneal fluorescein score, tear break-up time, and tear volume. (A-B) Corneal fluorescein images and corneal fluorescein sodium staining (CFS) scores in DE mice. (C) Tear break-up time as detected with commercial fluorescein strips. (D) Tear volume as measured by Schirmer’s test. CON, non-DE control group; DE, topical 0.1% BAC; DE + Omega3, topical 0.1% BAC + oral Omega3 (200 mg/kg/day); DE + HY7302L, topical 0.1 % BAC + oral HY7302 (108 CFU/kg/day); DE + HY7302H, topical 0.1% BAC + oral HY7302 (109 CFU/kg/day). ### p< 0.001 relative to CON. *p < 0.05, **p < 0.01, and ***p<0.001 relative to DE.
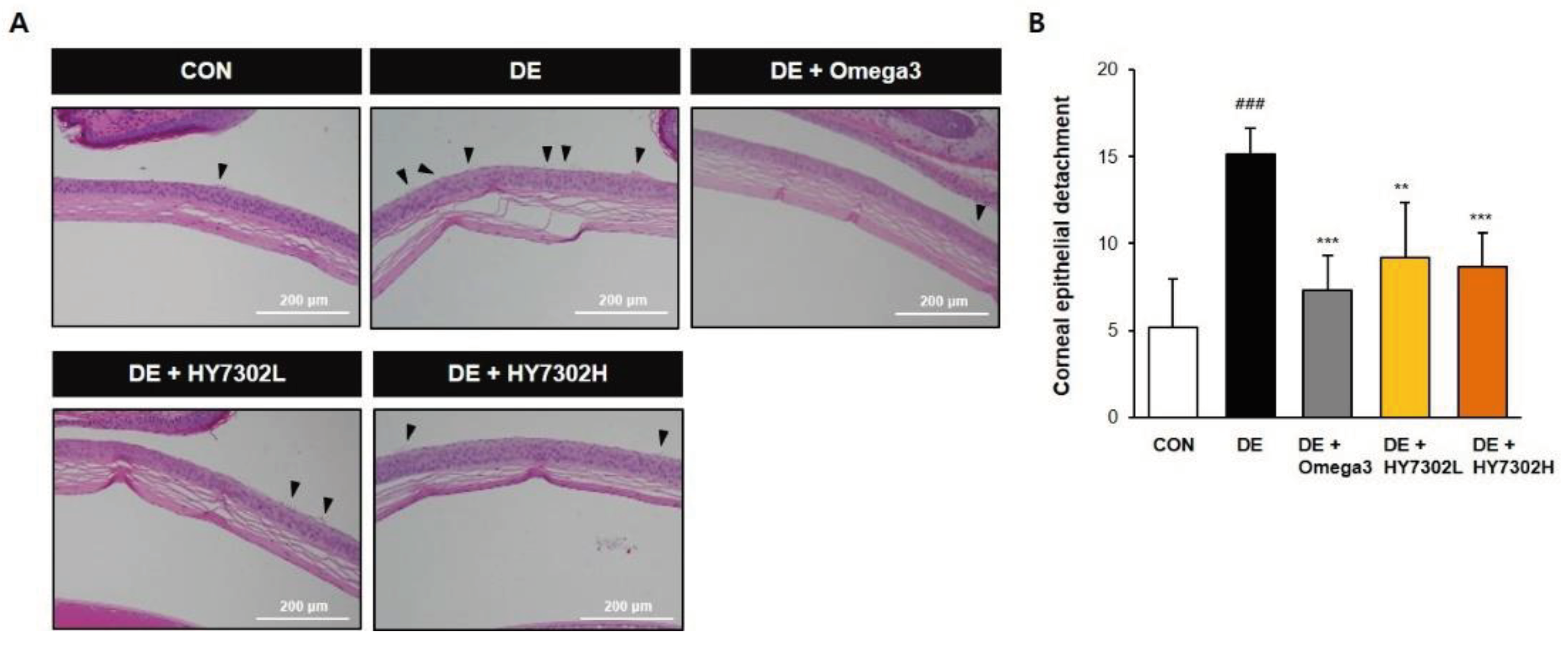
Figure 2.
Effect of HY7302 on corneal epithelial detachment. (A) Representative hematoxylin and eosin images of DE mouse corneas. Arrows indicate detaching damaged apical tissue. (B) Quantification of corneal epithelial detachment, expressed as means ± SD (N = 4 eyes/group). ###p < 0.001 relative to CON. **p < 0.01 and ***p < 0.001 relative to DE.
Figure 2.
Effect of HY7302 on corneal epithelial detachment. (A) Representative hematoxylin and eosin images of DE mouse corneas. Arrows indicate detaching damaged apical tissue. (B) Quantification of corneal epithelial detachment, expressed as means ± SD (N = 4 eyes/group). ###p < 0.001 relative to CON. **p < 0.01 and ***p < 0.001 relative to DE.
Figure 3.
Effect of HY7302 on serum and eye tissue pro-inflammatory factor levels in BAC-induced DE mice. (A) Western blot images of extracellular signal-regulated kinase (ERK), phospho-ERK (p-ERK), c-Jun N-Terminal kinase (JNK), phospho-JNK (p-JNK), matrix metalloproteinase-9 (MMP-9), inteleukin-1 beta (IL-1β), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). (B) quantification of phosphoprotein ratios or protein levels relative to CON. Serum concentrations of (C) matrix metalloproteinase-9 (MMP-9) and (D) interleukin-20 (IL-20) were measured by ELISA. #p < 0.05 and ###p < 0.001 relative to CON. *p< 0.05, **p < 0.01, and ***p < 0.001 relative to DE.
Figure 3.
Effect of HY7302 on serum and eye tissue pro-inflammatory factor levels in BAC-induced DE mice. (A) Western blot images of extracellular signal-regulated kinase (ERK), phospho-ERK (p-ERK), c-Jun N-Terminal kinase (JNK), phospho-JNK (p-JNK), matrix metalloproteinase-9 (MMP-9), inteleukin-1 beta (IL-1β), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). (B) quantification of phosphoprotein ratios or protein levels relative to CON. Serum concentrations of (C) matrix metalloproteinase-9 (MMP-9) and (D) interleukin-20 (IL-20) were measured by ELISA. #p < 0.05 and ###p < 0.001 relative to CON. *p< 0.05, **p < 0.01, and ***p < 0.001 relative to DE.
Figure 5.
Effect of HY7302 on gut microbiota diversity, Spearman’s correlation, and relative species-level abundances in BAC-induced DE mice. (A) Box plots of microbial alpha diversities of each group were calculated using observed features. (B) PCoA plots of the bacterial community using unweighted UniFrac distance and PERMANOVA were used to measure dissimilarity between groups. (C) Spearman’s correlation analysis between genus-level taxonomy profiles and DE indicators (serum MMP-9, serum IL-20, TV, TBUT, and corneal epithelial detachment). In the heatmap, red squares indicate positive correlations and blue squares indicate negative correlations. (*p < 0.05). (D) Relative changes in abundances of microbiota species (Clostridium leptum, Bacteroides caccae, Bifidobacterium pseudolongum) at baseline and after HY7302 treatment.
Figure 5.
Effect of HY7302 on gut microbiota diversity, Spearman’s correlation, and relative species-level abundances in BAC-induced DE mice. (A) Box plots of microbial alpha diversities of each group were calculated using observed features. (B) PCoA plots of the bacterial community using unweighted UniFrac distance and PERMANOVA were used to measure dissimilarity between groups. (C) Spearman’s correlation analysis between genus-level taxonomy profiles and DE indicators (serum MMP-9, serum IL-20, TV, TBUT, and corneal epithelial detachment). In the heatmap, red squares indicate positive correlations and blue squares indicate negative correlations. (*p < 0.05). (D) Relative changes in abundances of microbiota species (Clostridium leptum, Bacteroides caccae, Bifidobacterium pseudolongum) at baseline and after HY7302 treatment.
Figure 6.
Effect of HY7302 on mRNA levels of tight junction components in TNFα-stimulated CMT93 cells. (A) Cell viability of CMT93 cells following 24 h HY7302 treatment was detected using a Cell Counting Kit-8 (CCK-8) assay. (B-D) mRNA levels of (B) tight junction protein-1 (TJP-1), (C) occuludin-1 (OCLD-1), and (D) Claudin-4 (CLDN-4) in CMT93 cells treated with vehicle (CON group) or 107 CFU/mL HY7302 (HY7302 group) were measured by quantitative-PCR. (E-G) mRNA levels of (E) TJP-1, (F) OCLD-1, and (G) CLDN-4 were measured by qPCR in CMT93 cells treated with vehicle (CON group), 100 ng/mL TNFα (TNF group) or 100 ng/mL TNFα + 107 CFU/mL HY7302 (TNF + HY3702 group). ##p < 0.01 and ###p < 0.001 relative to CON group. *p < 0.05 and **p < 0.01 relative to TNF treated group.
Figure 6.
Effect of HY7302 on mRNA levels of tight junction components in TNFα-stimulated CMT93 cells. (A) Cell viability of CMT93 cells following 24 h HY7302 treatment was detected using a Cell Counting Kit-8 (CCK-8) assay. (B-D) mRNA levels of (B) tight junction protein-1 (TJP-1), (C) occuludin-1 (OCLD-1), and (D) Claudin-4 (CLDN-4) in CMT93 cells treated with vehicle (CON group) or 107 CFU/mL HY7302 (HY7302 group) were measured by quantitative-PCR. (E-G) mRNA levels of (E) TJP-1, (F) OCLD-1, and (G) CLDN-4 were measured by qPCR in CMT93 cells treated with vehicle (CON group), 100 ng/mL TNFα (TNF group) or 100 ng/mL TNFα + 107 CFU/mL HY7302 (TNF + HY3702 group). ##p < 0.01 and ###p < 0.001 relative to CON group. *p < 0.05 and **p < 0.01 relative to TNF treated group.
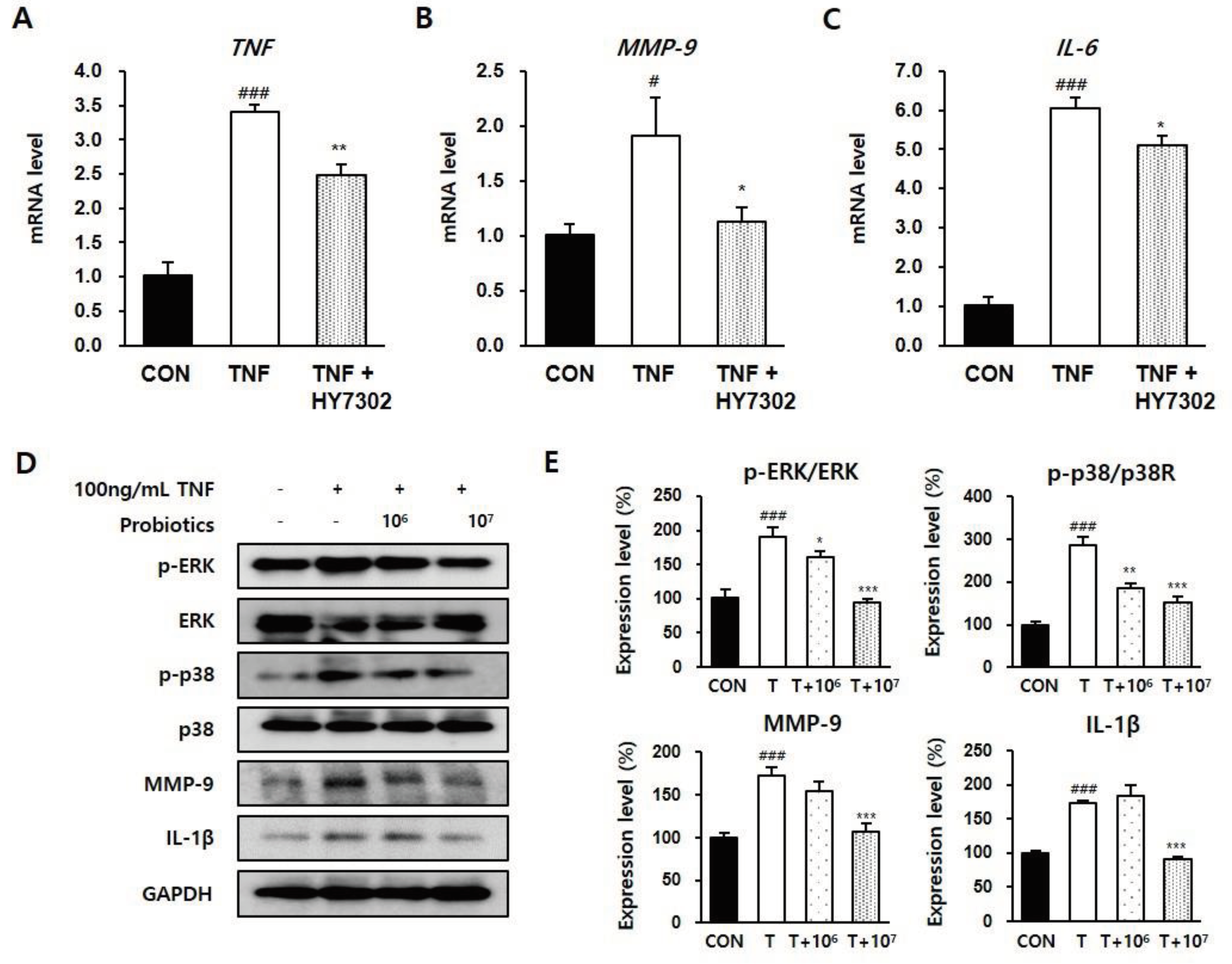
Figure 7.
Effect of HY7302 on pro-inflammatory factors in TNFα-stimulated CMT93 cells. (A-C) mRNA levels of (A) tumor necrosis factor (TNF), (B) matrix metalloproteinase-9 (MMP-9), and (C) inteleukin-6 (IL-6) were measured by qPCR in CMT93 treated with vehicle (CON group), 100 ng/mL TNFα (TNF group), or 100 ng/mL TNFα + 107 CFU/mL HY7302 (TNF + HY7302 group). (D) Western blot images of extracellular signal-regulated kinase (ERK), phospho-ERK (p-ERK), p38 mitogen-activated protein kinases (p38), phospho-p38 (p-p38), matrix metalloproteinase-9 (MMP-9), inteleukin-1 beta (IL-1β) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and (E) phosphoprotein ratios or protein levels relative to vehicle-treated cells (CON group). For experimental groups, cells were treated with 100 ng/mL TNFα (T group), 100 ng/mL TNFα + 106 CFU/mL HY7302 (T + 106 group) or 100 ng/mL TNFα + 107 CFU/mL HY7302 (T + 107 group). # p < 0.05 and ###p < 0.001 relative to CON group. *p < 0.05, **p < 0.01, and ***p < 0.001 relative to TNFα-only groups (TNF group in A-C and T group in D-E).
Figure 7.
Effect of HY7302 on pro-inflammatory factors in TNFα-stimulated CMT93 cells. (A-C) mRNA levels of (A) tumor necrosis factor (TNF), (B) matrix metalloproteinase-9 (MMP-9), and (C) inteleukin-6 (IL-6) were measured by qPCR in CMT93 treated with vehicle (CON group), 100 ng/mL TNFα (TNF group), or 100 ng/mL TNFα + 107 CFU/mL HY7302 (TNF + HY7302 group). (D) Western blot images of extracellular signal-regulated kinase (ERK), phospho-ERK (p-ERK), p38 mitogen-activated protein kinases (p38), phospho-p38 (p-p38), matrix metalloproteinase-9 (MMP-9), inteleukin-1 beta (IL-1β) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and (E) phosphoprotein ratios or protein levels relative to vehicle-treated cells (CON group). For experimental groups, cells were treated with 100 ng/mL TNFα (T group), 100 ng/mL TNFα + 106 CFU/mL HY7302 (T + 106 group) or 100 ng/mL TNFα + 107 CFU/mL HY7302 (T + 107 group). # p < 0.05 and ###p < 0.001 relative to CON group. *p < 0.05, **p < 0.01, and ***p < 0.001 relative to TNFα-only groups (TNF group in A-C and T group in D-E).