Submitted:
21 February 2024
Posted:
22 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Materials
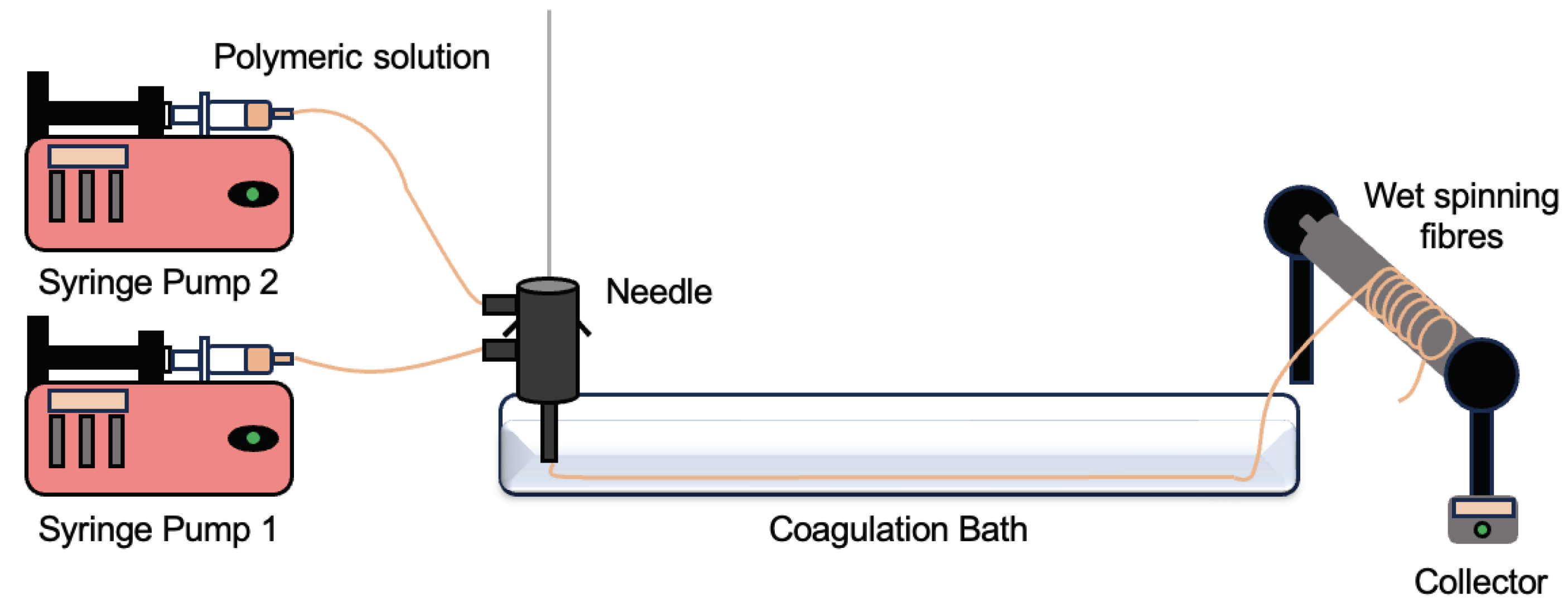
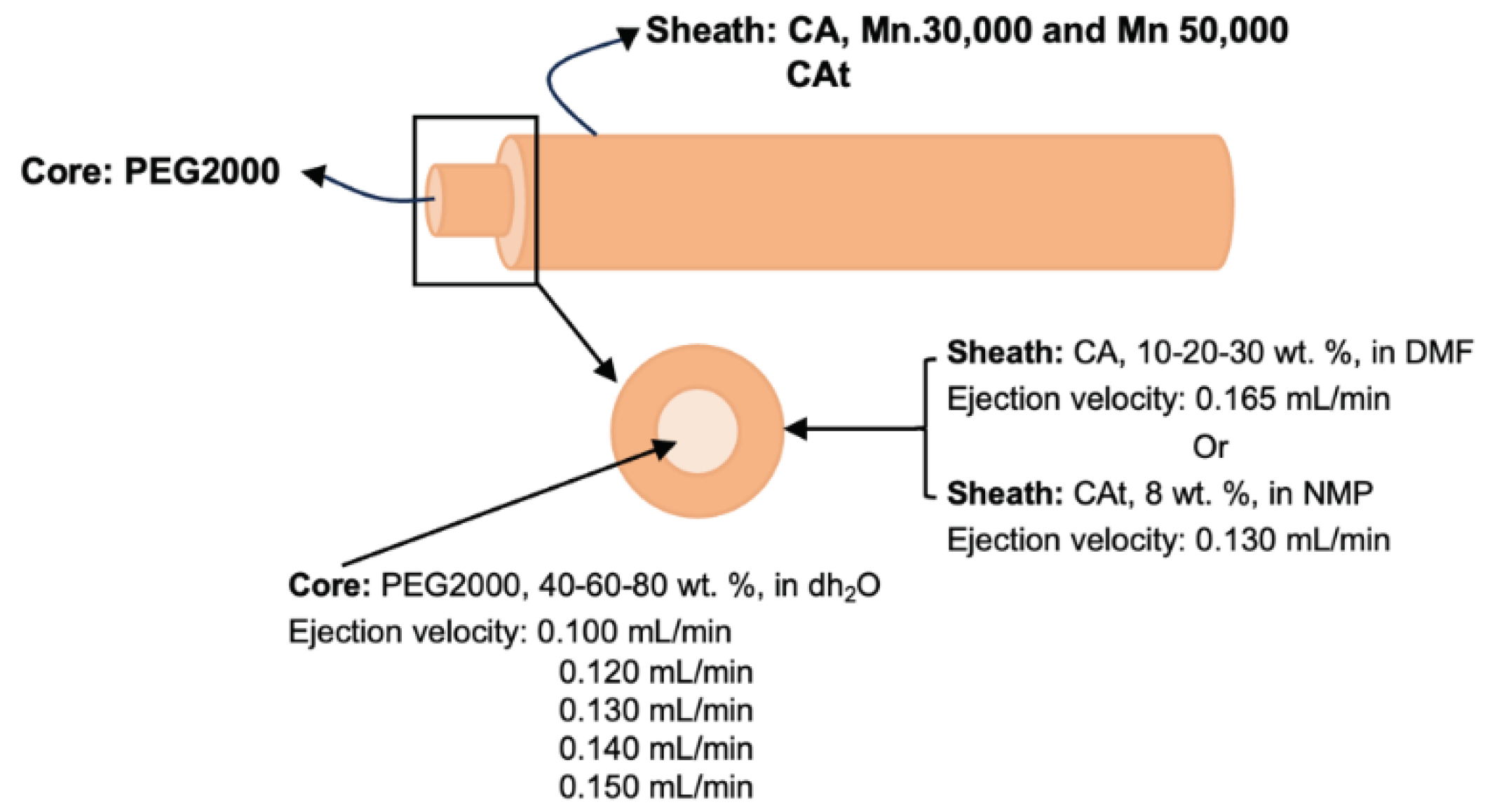
2.3. Fibres production
2.4. Fibres physical, chemical, thermal and mechanical characterization
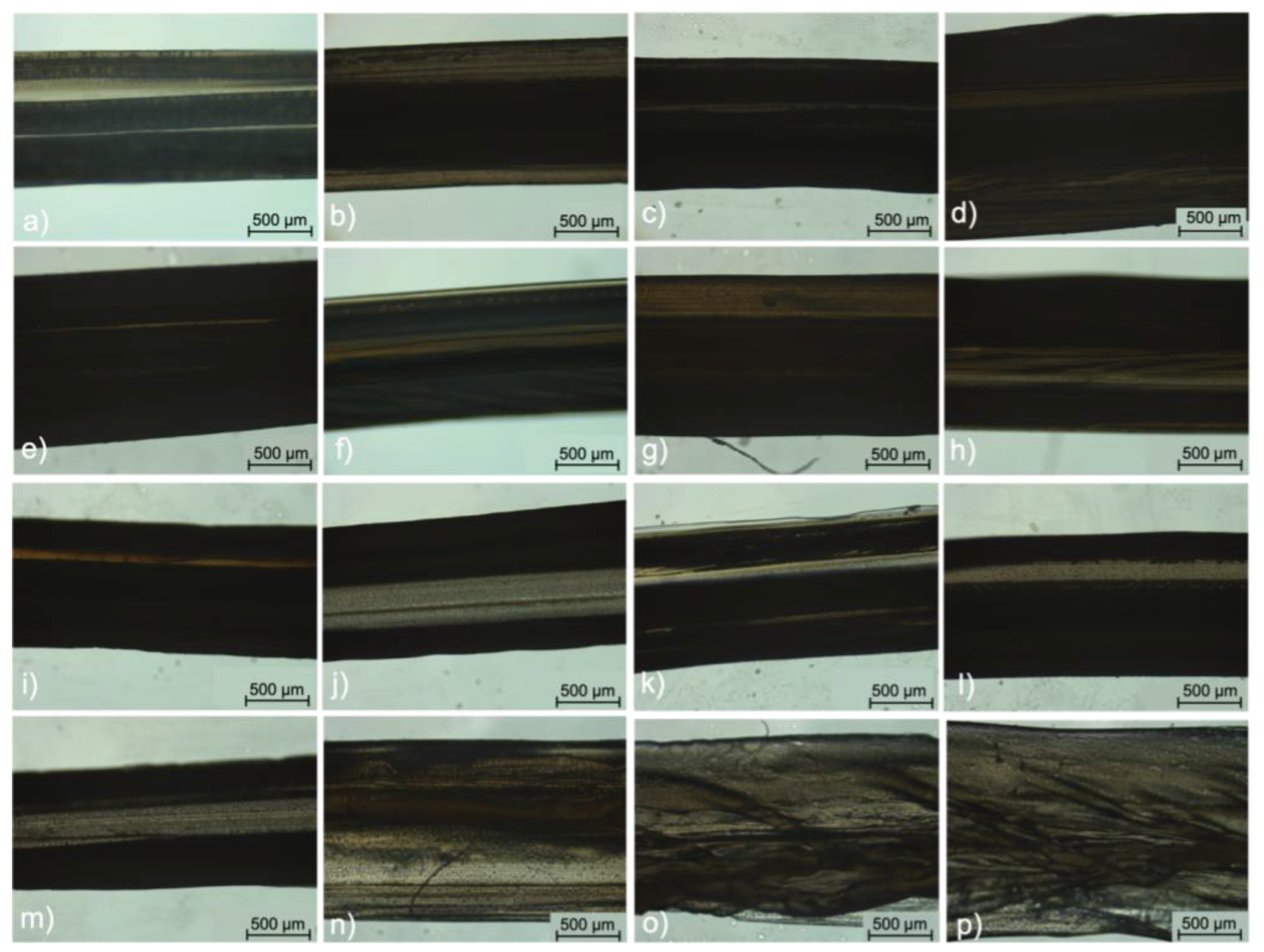
2.4.1. Bright-field microscopy
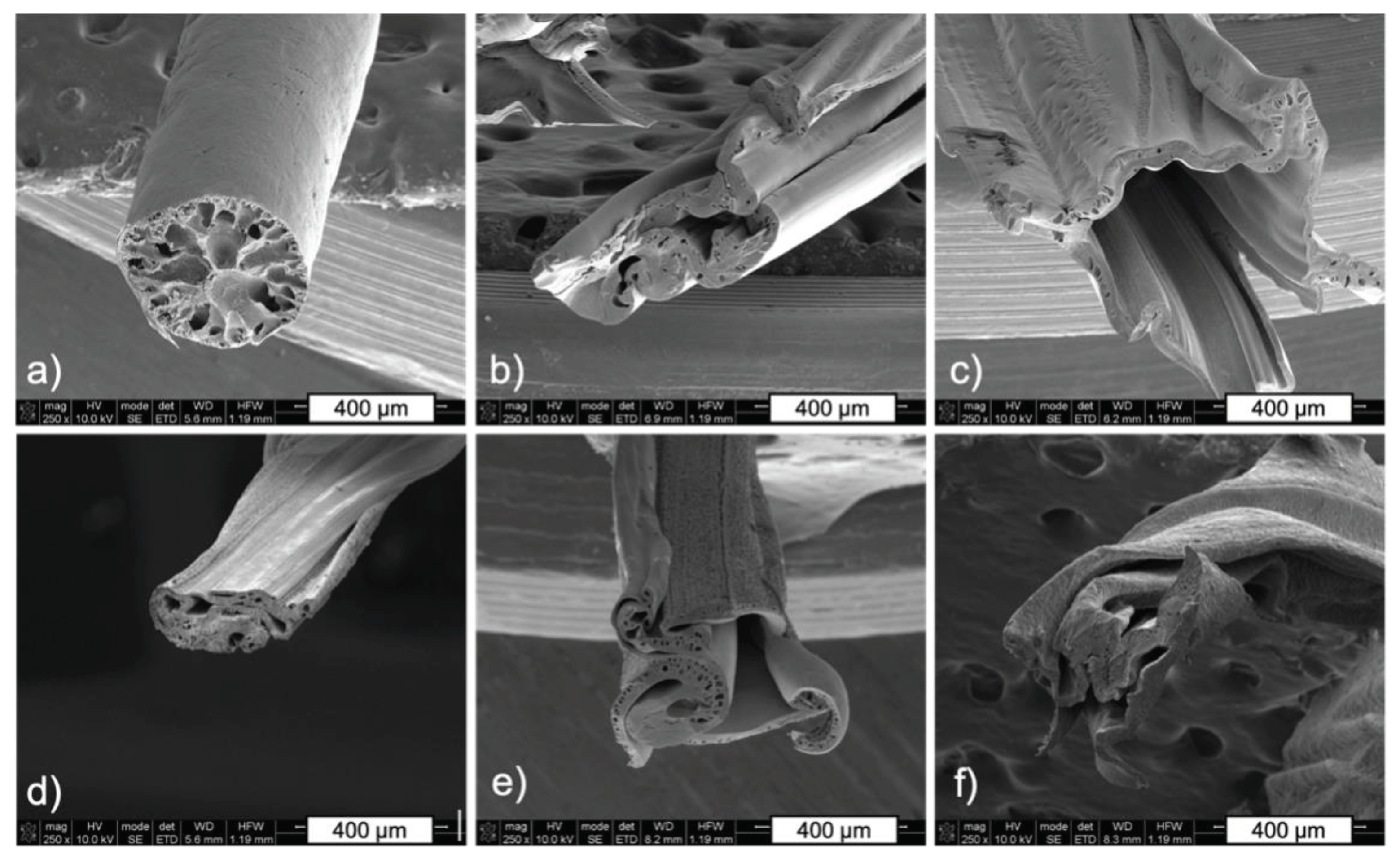
2.4.2. Scanning Electron Microscopy (SEM)
2.4.3. Attenuated Total Reflectance-Fourier Transform Infrared spectroscopy (ATR-FTIR)
2.4.4. Thermogravimetric Analysis (TGA)
2.4.5. Differential Scanning Calorimetry (DSC)
2.4.6. Mechanical Performance – Dynamometer
3. Results
3.1. Degree of substitution for CAt
3.2. Fibres production
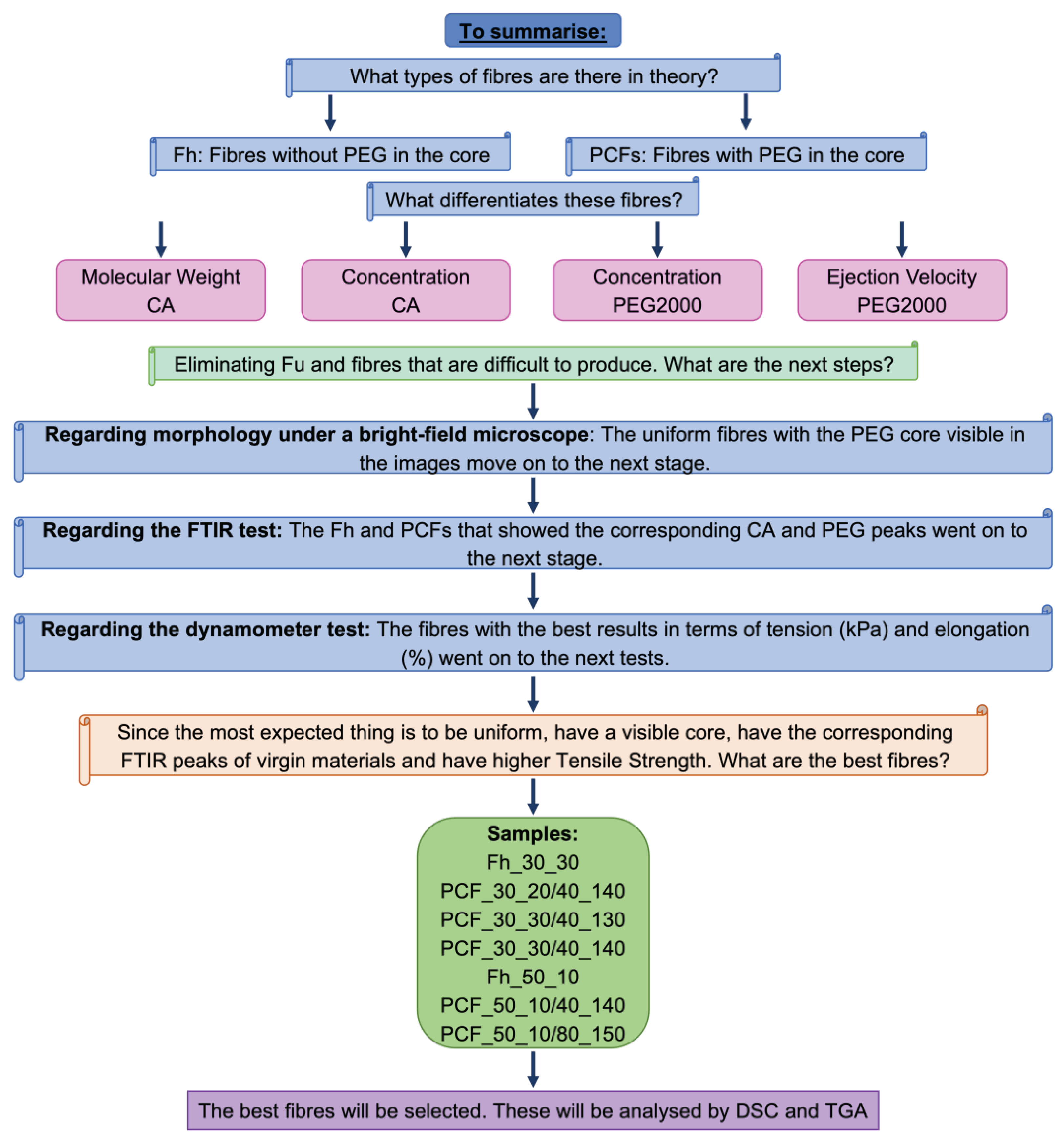
3.3. Sample selection
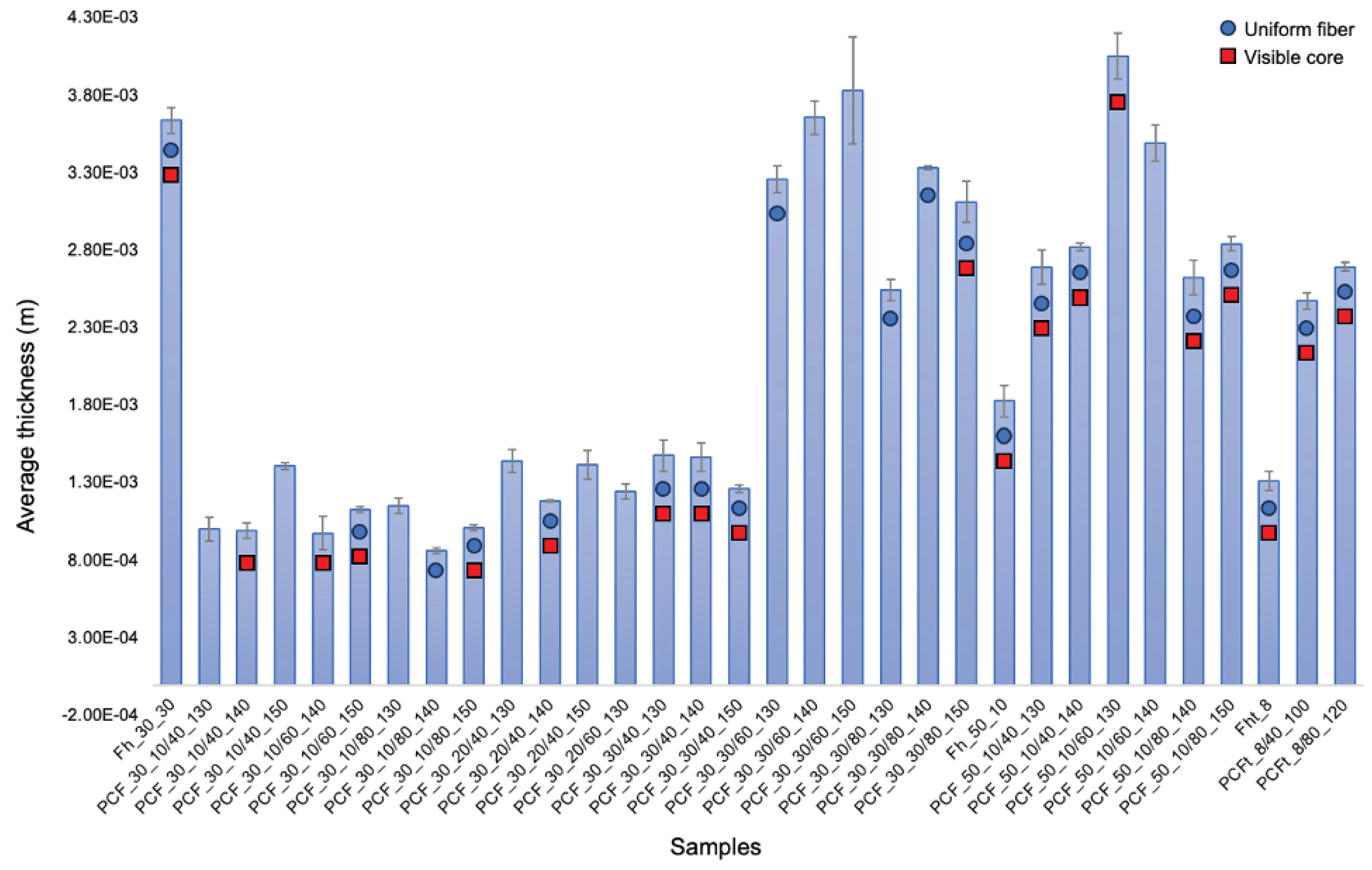
3.4. Morphology
3.5. Chemical Composition (ATR-FTIR)
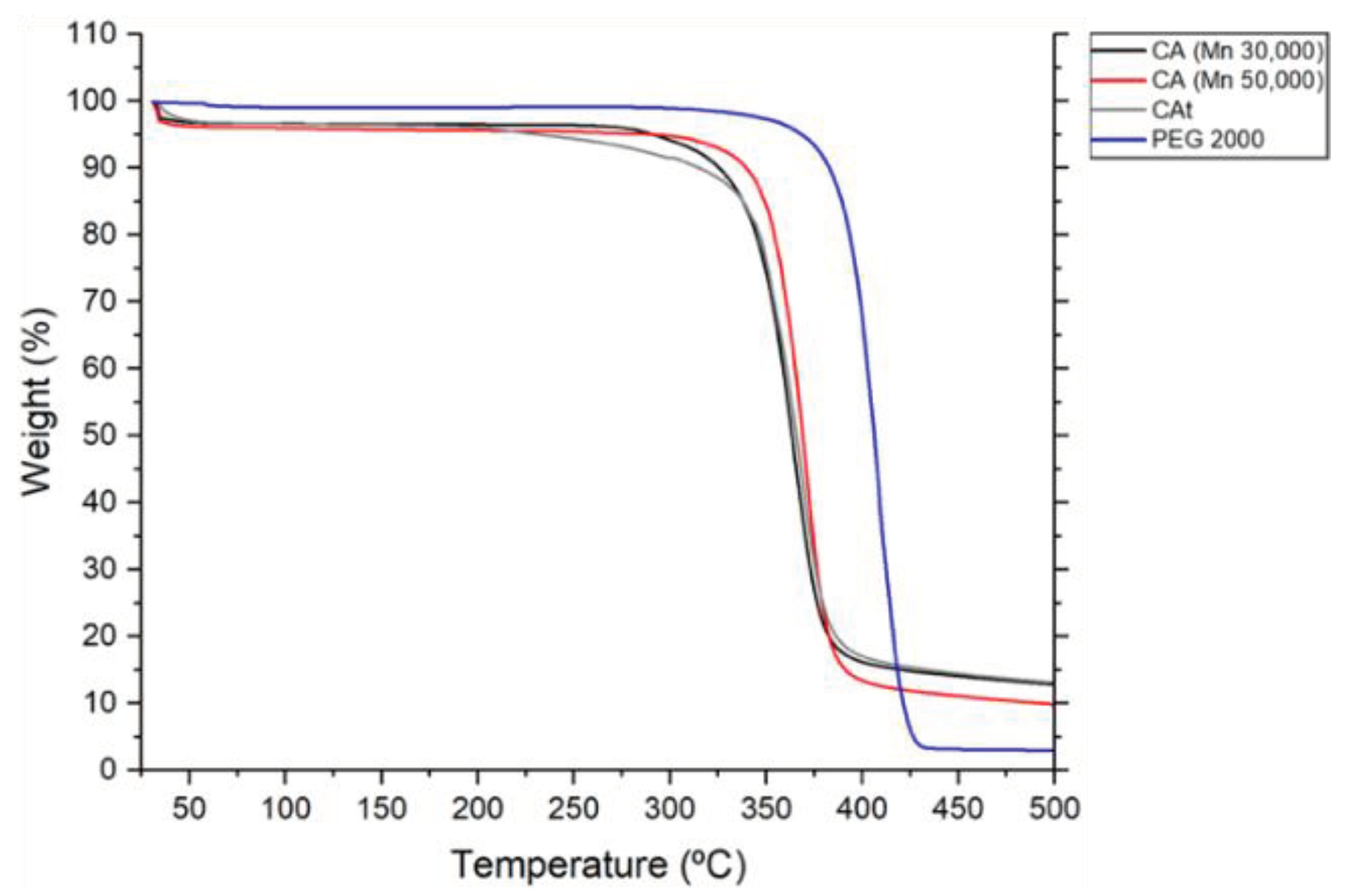
3.6. Thermogravimetric Analysis (TGA)
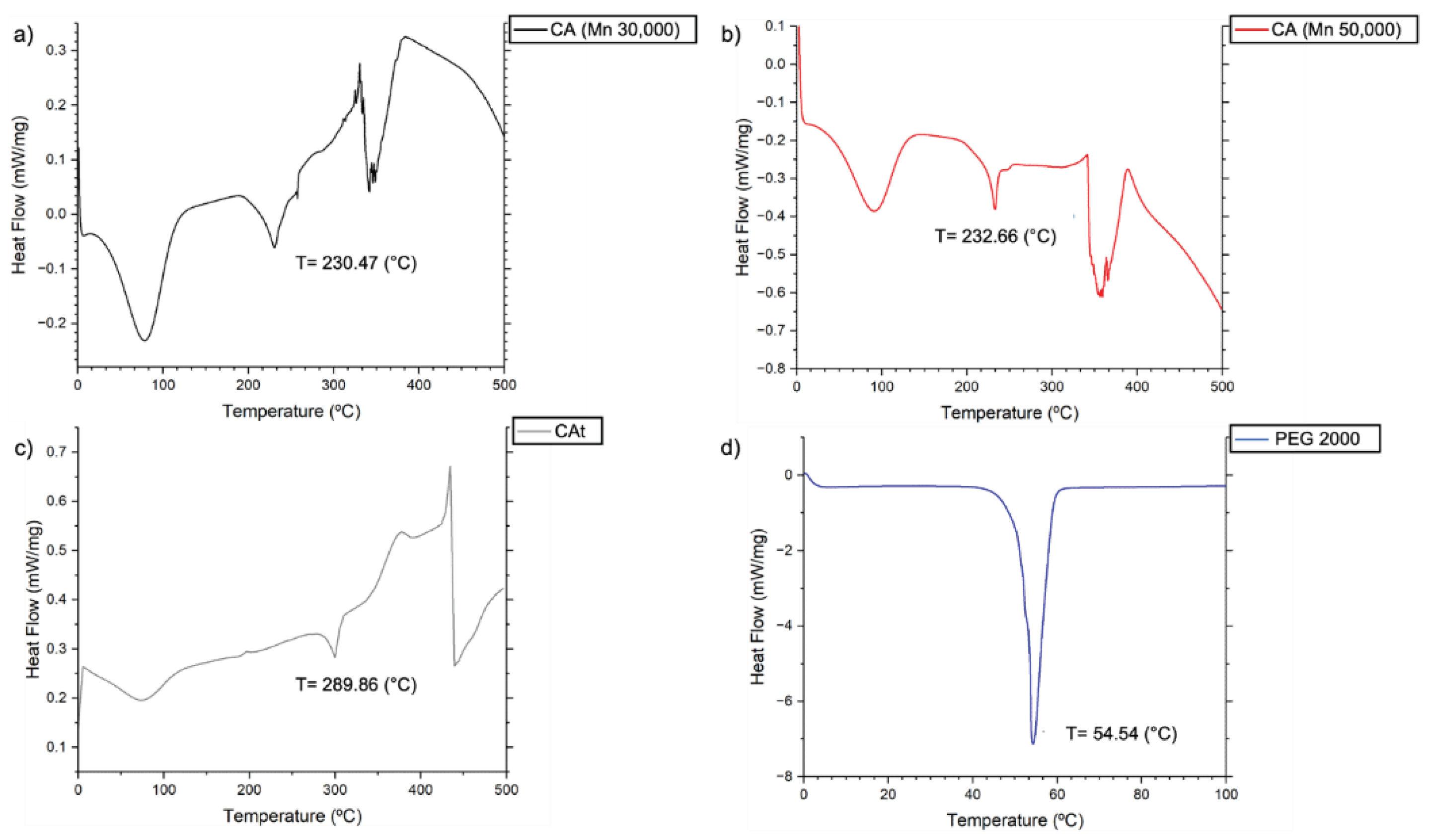
3.7. Differential Scanning Calorimetry (DSC)
3.8. Mechanical behavior
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgements
References
- Segundo, I.R.; Freitas, E.; Branco, V.T.F.C.; Landi, S.; Costa, M.F.; Carneiro, J.O. Review and Analysis of Advances in Functionalized, Smart, and Multifunctional Asphalt Mixtures. Renew. Sustain. Energy Rev. 2021, 151, 111552. [Google Scholar] [CrossRef]
- Ibrahim, S.H.; Ibrahim, N.I.A.; Wahid, J.; Goh, N.A.; Koesmeri, D.R.A.; Nawi, M.N.M. The Impact of Road Pavement on Urban Heat Island (UHI) Phenomenon. Int. J. Technol. 2018, 9, 1597. [Google Scholar] [CrossRef]
- Piracha, A.; Chaudhary, M.T. Urban Air Pollution, Urban Heat Island and Human Health: A Review of the Literature. Sustainability 2022, 14, 9234. [Google Scholar] [CrossRef]
- Chen, G.; Chen, Y.; Tan, X.; Zhao, L.; Cai, Y.; Li, L. Assessing the Urban Heat Island Effect of Different Local Climate Zones in Guangzhou, China. Build. Environ. 2023, 244, 110770. [Google Scholar] [CrossRef]
- Andrade, C.; Fonseca, A.; Santos, J.A. Climate Change Trends for the Urban Heat Island Intensities in Two Major Portuguese Cities. Sustainability 2023, 15, 3970. [Google Scholar] [CrossRef]
- Vardhu, V.A.K.; Sharma, Dr.A. Classification, Mitigations and Methods to Detect UHI: A Review. INTERANTIONAL J. Sci. Res. Eng. Manag. 2023, 07. [Google Scholar] [CrossRef]
- Mondal, S. Phase Change Fibers. In Handbook of Fibrous Materials; Hu, J., Kumar, B., Lu, J., Eds.; Wiley, 2020; pp. 263–279. ISBN 978-3-527-34220-4. [Google Scholar]
- Betancourt-Jimenez, D.; Montoya, M.; Haddock, J.; Youngblood, J.P.; Martinez, C.J. Regulating Asphalt Pavement Temperature Using Microencapsulated Phase Change Materials (PCMs). Constr. Build. Mater. 2022, 350, 128924. [Google Scholar] [CrossRef]
- Chou, H.-M.; Chen, C.-R.; Nguyen, V.-L. A New Design of Metal-Sheet Cool Roof Using PCM. Energy Build. 2013, 57, 42–50. [Google Scholar] [CrossRef]
- Osterman, E.; Tyagi, V.V.; Butala, V.; Rahim, N.A.; Stritih, U. Review of PCM Based Cooling Technologies for Buildings. Energy Build. 2012, 49, 37–49. [Google Scholar] [CrossRef]
- Pinheiro, C.; Hammes, N.; Lima, O.; Landi, S.; Homem, N.; Rocha Segundo, I.; Felgueiras, H.P.; Freitas, E.; Costa, M.F.M.; Carneiro, J. Reducing the Effects of Low Albedo of Asphalt Materials Incorporating Polyethylene Glycol (PEG) 1000, 2000 and 4000 as Phase Change Materials (PCM). EPJ Web Conf. 2023, 287, 09024. [Google Scholar] [CrossRef]
- Pinheiro, C.; Landi, S.; Lima, O.; Ribas, L.; Hammes, N.; Segundo, I.R.; Homem, N.C.; Castelo Branco, V.; Freitas, E.; Costa, M.F.; et al. Advancements in Phase Change Materials in Asphalt Pavements for Mitigation of Urban Heat Island Effect: Bibliometric Analysis and Systematic Review. Sensors 2023, 23, 7741. [Google Scholar] [CrossRef]
- Baetens, R.; Jelle, B.P.; Gustavsen, A. Phase Change Materials for Building Applications: A State-of-the-Art Review. Energy Build. 2010, 42, 1361–1368. [Google Scholar] [CrossRef]
- Diaconu, B.M.; Varga, S.; Oliveira, A.C. Experimental Assessment of Heat Storage Properties and Heat Transfer Characteristics of a Phase Change Material Slurry for Air Conditioning Applications. Appl. Energy 2010, 87, 620–628. [Google Scholar] [CrossRef]
- Gil, A.; Oró, E.; Peiró, G.; Álvarez, S.; Cabeza, L.F. Material Selection and Testing for Thermal Energy Storage in Solar Cooling. Renew. Energy 2013, 57, 366–371. [Google Scholar] [CrossRef]
- Yinfei, D.; Pusheng, L.; Jiacheng, W.; Hancheng, D.; Hao, W.; Yingtao, L. Effect of Lightweight Aggregate Gradation on Latent Heat Storage Capacity of Asphalt Mixture for Cooling Asphalt Pavement. Constr. Build. Mater. 2020, 250, 118849. [Google Scholar] [CrossRef]
- Cunha, S.; Leite, P.; Aguiar, J. Characterization of Innovative Mortars with Direct Incorporation of Phase Change Materials. J. Energy Storage 2020, 30, 101439. [Google Scholar] [CrossRef]
- Kulkarni, P.; Muthadhi, A. Thermal Energy Storage Cement Mortar with Direct Incorporation of Organic and Inorganic Phase Change Materials. Innov. Infrastruct. Solut. 2021, 6, 30. [Google Scholar] [CrossRef]
- Pérez-Silva, I.; Ibarra, I.S.; Castañeda-Ovando, A.; Galán-Vidal, C.A.; Páez-Hernández, Ma.E. Development of Cellulose Acetate Microcapsules with Cyanex 923 for Phenol Removal from Aqueous Media. J. Chem. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Gbekou, F.K.; Benzarti, K.; Boudenne, A.; Eddhahak, A.; Duc, M. Mechanical and Thermophysical Properties of Cement Mortars Including Bio-Based Microencapsulated Phase Change Materials. Constr. Build. Mater. 2022, 352, 129056. [Google Scholar] [CrossRef]
- Drissi, S.; Ling, T.-C.; Mo, K.H. Development of Leak-Free Phase Change Material Aggregates. Constr. Build. Mater. 2020, 230, 117029. [Google Scholar] [CrossRef]
- Rao, V.V.; Parameshwaran, R.; Ram, V.V. PCM-Mortar Based Construction Materials for Energy Efficient Buildings: A Review on Research Trends. Energy Build. 2018, 158, 95–122. [Google Scholar] [CrossRef]
- Abdullah, M.F.; Andriyana, A.; Muhamad, F.; Ang, B.C. Effect of Core-to-Shell Flowrate Ratio on Morphology, Crystallinity, Mechanical Properties and Wettability of Poly(Lactic Acid) Fibers Prepared via Modified Coaxial Electrospinning. Polymer 2021, 237, 124378. [Google Scholar] [CrossRef]
- Felgueiras, H.P.; Homem, N.C.; Teixeira, M.A.; Ribeiro, A.R.M.; Antunes, J.C.; Amorim, M.T.P. Physical, Thermal, and Antibacterial Effects of Active Essential Oils with Potential for Biomedical Applications Loaded onto Cellulose Acetate/Polycaprolactone Wet-Spun Microfibers. Biomolecules 2020, 10, 1129. [Google Scholar] [CrossRef]
- Ozipek, B.; Karakas, H. Wet Spinning of Synthetic Polymer Fibers. In Advances in filament yarn spinning of textiles and polymers; Elsevier, 2014; pp. 174–186. [Google Scholar]
- Rohani Shirvan, A.; Nouri, A.; Sutti, A. A Perspective on the Wet Spinning Process and Its Advancements in Biomedical Sciences. Eur. Polym. J. 2022, 181, 111681. [Google Scholar] [CrossRef]
- Tang, Z.; Jia, S.; Wang, F.; Bian, C.; Chen, Y.; Wang, Y.; Li, B. Highly Stretchable Core–Sheath Fibers via Wet-Spinning for Wearable Strain Sensors. ACS Appl. Mater. Interfaces 2018, 10, 6624–6635. [Google Scholar] [CrossRef]
- Zhang, J.; Song, S.; Zhang, C.; Li, C.; Xu, J.; Xia, L.; Liu, X.; Xu, W. Fabrication of Leather-like Yarns Using Waste Leather for Textile Application. Prog. Org. Coat. 2024, 186, 108053. [Google Scholar] [CrossRef]
- Quan, Q.; Zhang, Y.; Piao, H.; Zhang, H.; Zhao, J. Polybutyrolactam (PBY) Fiber: A Promising Biobased and Biodegradable Fiber Fabricated by Dry-Jet-Wet Spinning. Polymer 2022, 260, 125392. [Google Scholar] [CrossRef]
- Teo, W.E.; Ramakrishna, S. A Review on Electrospinning Design and Nanofibre Assemblies. Nanotechnology 2006, 17, R89–R106. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, X.; Xu, Y.; Xu, Y.; Qiao, J.; Shi, T.; Huang, Z.; Liu, Y.; Fang, M.; Min, X.; et al. Flexible Polyethylene Glycol/Polyvinylpyrrolidone Composite Phase Change Fibres: Preparation, Characterization, and Thermal Conductivity Enhancement. Polymer 2021, 214, 123258. [Google Scholar] [CrossRef]
- Mirabedini, A. Developing Novel Spinning Methods to Fabricate Continuous Multifunctional Fibres for Bioapplications; Springer Theses; Springer International Publishing: Cham, 2018; ISBN 978-3-319-95377-9. [Google Scholar]
- Chen, C.; Zhao, Y.; Liu, W. Electrospun Polyethylene Glycol/Cellulose Acetate Phase Change Fibers with Core–Sheath Structure for Thermal Energy Storage. Renew. Energy 2013, 60, 222–225. [Google Scholar] [CrossRef]
- Swapnil, S.I.; Datta, N.; Mahmud, M.M.; Jahan, R.A.; Arafat, M.T. Morphology, Mechanical, and Physical Properties of Wet-spun Cellulose Acetate Fiber in Different Solvent-coagulant Systems and In-situ Crosslinked Environment. J. Appl. Polym. Sci. 2021, 138, 50358. [Google Scholar] [CrossRef]
- Santos-Sauceda, I.; Castillo-Ortega, M.M.; Del Castillo-Castro, T.; Armenta-Villegas, L.; Ramírez-Bon, R. Electrospun Cellulose Acetate Fibers for the Photodecolorization of Methylene Blue Solutions under Natural Sunlight. Polym. Bull. 2021, 78, 4419–4438. [Google Scholar] [CrossRef]
- Babapoor, A.; Karimi, G.; Golestaneh, S.I.; Mezjin, M.A. Coaxial Electro-Spun PEG/PA6 Composite Fibers: Fabrication and Characterization. Appl. Therm. Eng. 2017, 118, 398–407. [Google Scholar] [CrossRef]
- Homem, N.C.; Amorim, M.T.P. Synthesis of Cellulose Acetate Using as Raw Material Textile Wastes. Mater. Today Proc. 2020, 31, S315–S317. [Google Scholar] [CrossRef]
- Puleo, A.C.; Paul, D.R.; Kelley, S.S. The Effect of Degree of Acetylation on Gas Sorption and Transport Behavior in Cellulose Acetate. J. Membr. Sci. 1989, 47, 301–332. [Google Scholar] [CrossRef]
- Homem, N.C.; Tavares, T.D.; Miranda, C.S.; Antunes, J.C.; Amorim, M.T.P.; Felgueiras, H.P. Functionalization of Crosslinked Sodium Alginate/Gelatin Wet-Spun Porous Fibers with Nisin Z for the Inhibition of Staphylococcus Aureus-Induced Infections. Int. J. Mol. Sci. 2021, 22, 1930. [Google Scholar] [CrossRef]
- Kramar, A.; González-Benito, F.J. Cellulose-Based Nanofibers Processing Techniques and Methods Based on Bottom-Up Approach—A Review. Polymers 2022, 14, 286. [Google Scholar] [CrossRef]
- Liu, L.; Gong, D.; Bratasz, L.; Zhu, Z.; Wang, C. Degradation Markers and Plasticizer Loss of Cellulose Acetate Films during Ageing. Polym. Degrad. Stab. 2019, 168, 108952. [Google Scholar] [CrossRef]
- Ahn, Y.-H.; DeWitt, S.J.A.; McGuire, S.; Lively, R.P. Incorporation of Phase Change Materials into Fibers for Sustainable Thermal Energy Storage. Ind. Eng. Chem. Res. 2021, 60, 3374–3384. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Fahmy, T.Y.; Salaheldin, E.I.; Mobarak, F.; Youssef, M.A.; Mabrook, M.R. Role of Tosyl Cellulose Acetate as Potential Carrier for Controlled Drug Release. Life Sci. J. 2015, 10, 127–133. [Google Scholar]
- Nikoomanesh, N.; Zandi, M.; Ganjloo, A. Development of Eco-Friendly Cellulose Acetate Films Incorporated with Burdock (Arctium Lappa L.) Root Extract. Prog. Org. Coat. 2024, 186, 108009. [Google Scholar] [CrossRef]
- Fei, P.; Liao, L.; Cheng, B.; Song, J. Quantitative Analysis of Cellulose Acetate with a High Degree of Substitution by FTIR and Its Application. Anal. Methods 2017, 9, 6194–6201. [Google Scholar] [CrossRef]
- Frisoni, G.; Baiardo, M.; Scandola, M.; Lednická, D.; Cnockaert, M.C.; Mergaert, J.; Swings, J. Natural Cellulose Fibers: Heterogeneous Acetylation Kinetics and Biodegradation Behavior. Biomacromolecules 2001, 2, 476–482. [Google Scholar] [CrossRef]
- Huda, E.; Rahmi. Khairan Preparation and Characterization of Cellulose Acetate from Cotton. IOP Conf. Ser. Earth Environ. Sci. 2019, 364, 012021. [Google Scholar] [CrossRef]
- Zhuang, J.; Li, M.; Pu, Y.; Ragauskas, A.; Yoo, C. Observation of Potential Contaminants in Processed Biomass Using Fourier Transform Infrared Spectroscopy. Appl. Sci. 2020, 10, 4345. [Google Scholar] [CrossRef]
- Zhu, Q.; Wu, H.; Ma, Z.; Liu, Y.; Li, J.; Zhu, L.; Zhang, X.; Wang, C.; Chen, D.; Zhu, D. Micro-Volume Blood Separation Membrane for In-Situ Biosensing. Biosensors 2022, 12, 712. [Google Scholar] [CrossRef]
- Quan, S.; Li, S.; Wang, Z.; Yan, X.; Guo, Z.; Shao, L. A Bio-Inspired CO 2 -Philic Network Membrane for Enhanced Sustainable Gas Separation. J. Mater. Chem. A 2015, 3, 13758–13766. [Google Scholar] [CrossRef]
- Snyder, R.G.; Hsut, S.L.; Krimm, S. Vibrational Spectp in the C-H Stretching Region and the Structure of the Polymethylene Chain.
- Hospodarova, V.; Singovszka, E.; Stevulova, N. Characterization of Cellulosic Fibers by FTIR Spectroscopy for Their Further Implementation to Building Materials. Am. J. Anal. Chem. 2018, 09, 303–310. [Google Scholar] [CrossRef]
- Das, A.M.; Ali, A.A.; Hazarika, M.P. Synthesis and Characterization of Cellulose Acetate from Rice Husk: Eco-Friendly Condition. Carbohydr. Polym. 2014, 112, 342–349. [Google Scholar] [CrossRef]
- Chen, J.; Xu, J.; Wang, K.; Cao, X.; Sun, R. Cellulose Acetate Fibers Prepared from Different Raw Materials with Rapid Synthesis Method. Carbohydr. Polym. 2016, 137, 685–692. [Google Scholar] [CrossRef]
- Lopes, S.; Bueno, L.; Aguiar Júnior, F.D.; Finkler, C. Preparation and Characterization of Alginate and Gelatin Microcapsules Containing Lactobacillus Rhamnosus. An. Acad. Bras. Ciênc. 2017, 89, 1601–1613. [Google Scholar] [CrossRef]
- Zhu, S.; Ji, T.; Niu, D.; Yang, Z. Investigation of PEG/Mixed Metal Oxides as a New Form-Stable Phase Change Material for Thermoregulation and Improved UV Ageing Resistance of Bitumen. RSC Adv. 2020, 10, 44903–44911. [Google Scholar] [CrossRef]
- Sundararajan, S.; Samui, A.B.; Kulkarni, P.S. Shape-Stabilized Poly(Ethylene Glycol) (PEG)-Cellulose Acetate Blend Preparation with Superior PEG Loading via Microwave-Assisted Blending. Sol. Energy 2017, 144, 32–39. [Google Scholar] [CrossRef]
- Faradilla, R.F.; Lee, G.; Sivakumar, P.; Stenzel, M.; Arcot, J. Effect of Polyethylene Glycol (PEG) Molecular Weight and Nanofillers on the Properties of Banana Pseudostem Nanocellulose Films. Carbohydr. Polym. 2019, 205, 330–339. [Google Scholar] [CrossRef]
- Shaikh, H.M.; Anis, A.; Poulose, A.M.; Al-Zahrani, S.M.; Madhar, N.A.; Alhamidi, A.; Aldeligan, S.H.; Alsubaie, F.S. Synthesis and Characterization of Cellulose Triacetate Obtained from Date Palm (Phoenix Dactylifera L.) Trunk Mesh-Derived Cellulose. Molecules 2022, 27, 1434. [Google Scholar] [CrossRef]
- Ribeiro, S.D.; Meneguin, A.B.; Barud, H. da S.; Silva, J.M.; Oliveira, R.L.; Asunção, R.M.N. de; Tormin, T.F.; Muñoz, R.A.A.; Filho, G.R.; Ribeiro, C.A. Synthesis and Characterization of Cellulose Acetate from Cellophane Industry Residues. Application as Acetaminophen Controlled-Release Membranes. J. Therm. Anal. Calorim. 2022, 147, 7265–7275. [Google Scholar] [CrossRef]
- Vinodhini, P.A.; K., S.; Thandapani, G.; P.N., S.; Jayachandran, V.; Sukumaran, A. FTIR, XRD and DSC Studies of Nanochitosan, Cellulose Acetate and Polyethylene Glycol Blend Ultrafiltration Membranes. Int. J. Biol. Macromol. 2017, 104, 1721–1729. [Google Scholar] [CrossRef]
- Ansu, A.K.; Pandya, M.; Sharma, R.K.; Tripathi, D. Experimental Investigation of Hybrid PCM Polyethylene Glycol with Al 2 O 3 and CuO Nanoparticles, In Review. 2022.
- Chen, C.; Wang, L.; Huang, Y. Electrospun Phase Change Fibers Based on Polyethylene Glycol/Cellulose Acetate Blends. Appl. Energy 2011, 88, 3133–3139. [Google Scholar] [CrossRef]
- Cai, Y.; Gao, C.; Xu, X.; Fu, Z.; Fei, X.; Zhao, Y.; Chen, Q.; Liu, X.; Wei, Q.; He, G.; et al. Electrospun Ultrafine Composite Fibers Consisting of Lauric Acid and Polyamide 6 as Form-Stable Phase Change Materials for Storage and Retrieval of Solar Thermal Energy. Sol. Energy Mater. Sol. Cells 2012, 103, 53–61. [Google Scholar] [CrossRef]
- Gonçalves, S.M.; Dos Santos, D.C.; Motta, J.F.G.; Santos, R.R.D.; Chávez, D.W.H.; Melo, N.R.D. Structure and Functional Properties of Cellulose Acetate Films Incorporated with Glycerol. Carbohydr. Polym. 2019, 209, 190–197. [Google Scholar] [CrossRef]

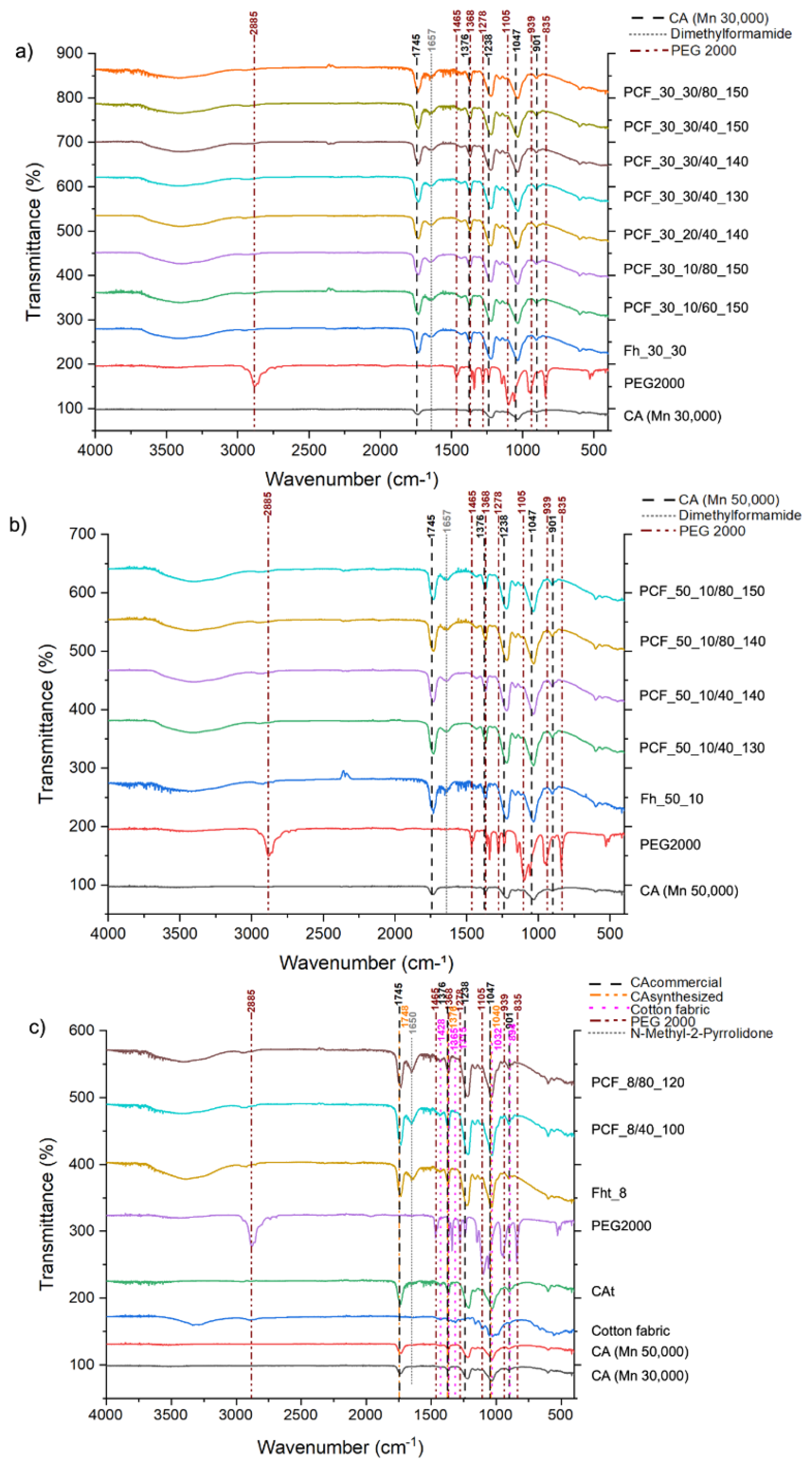
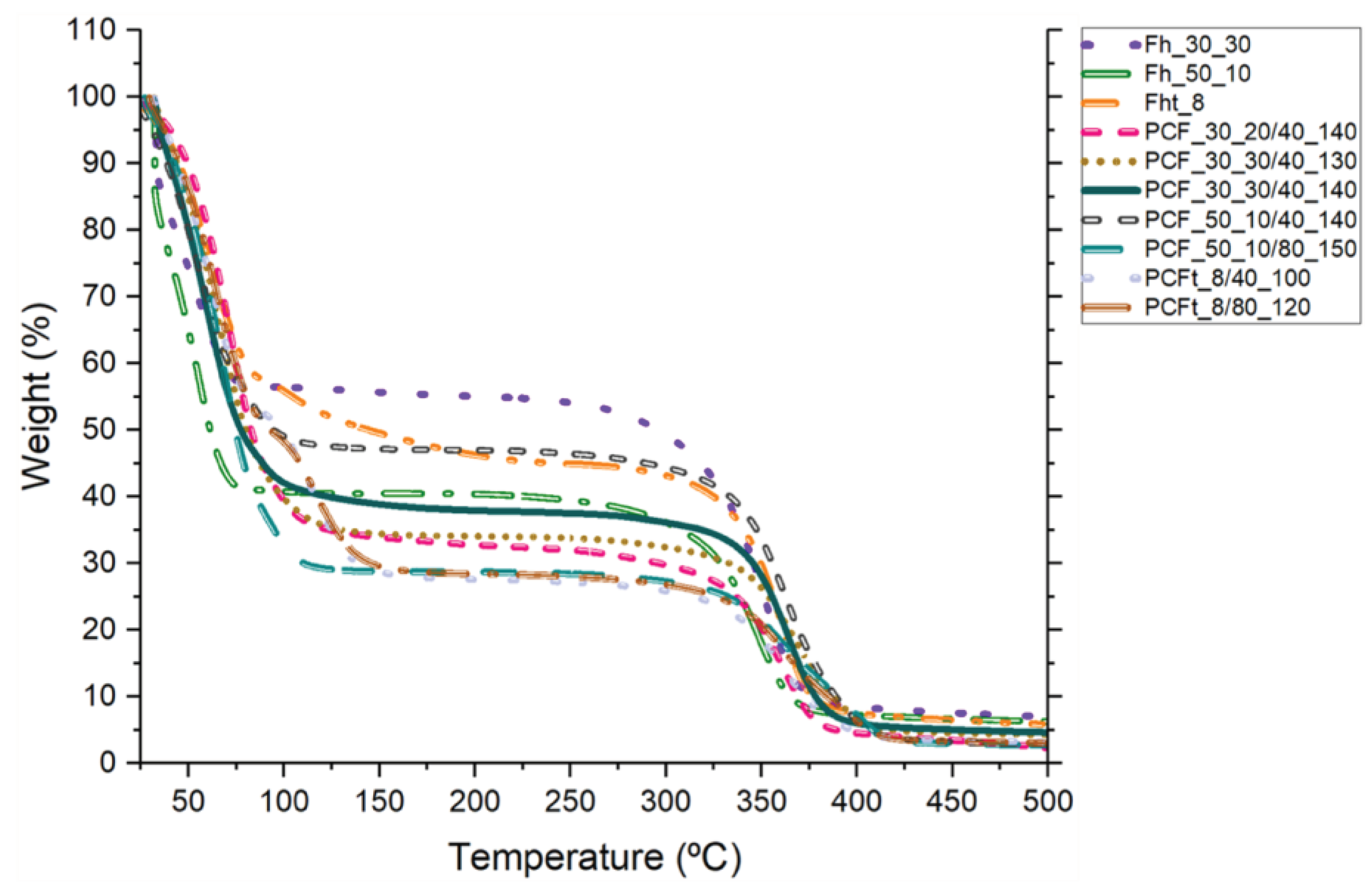
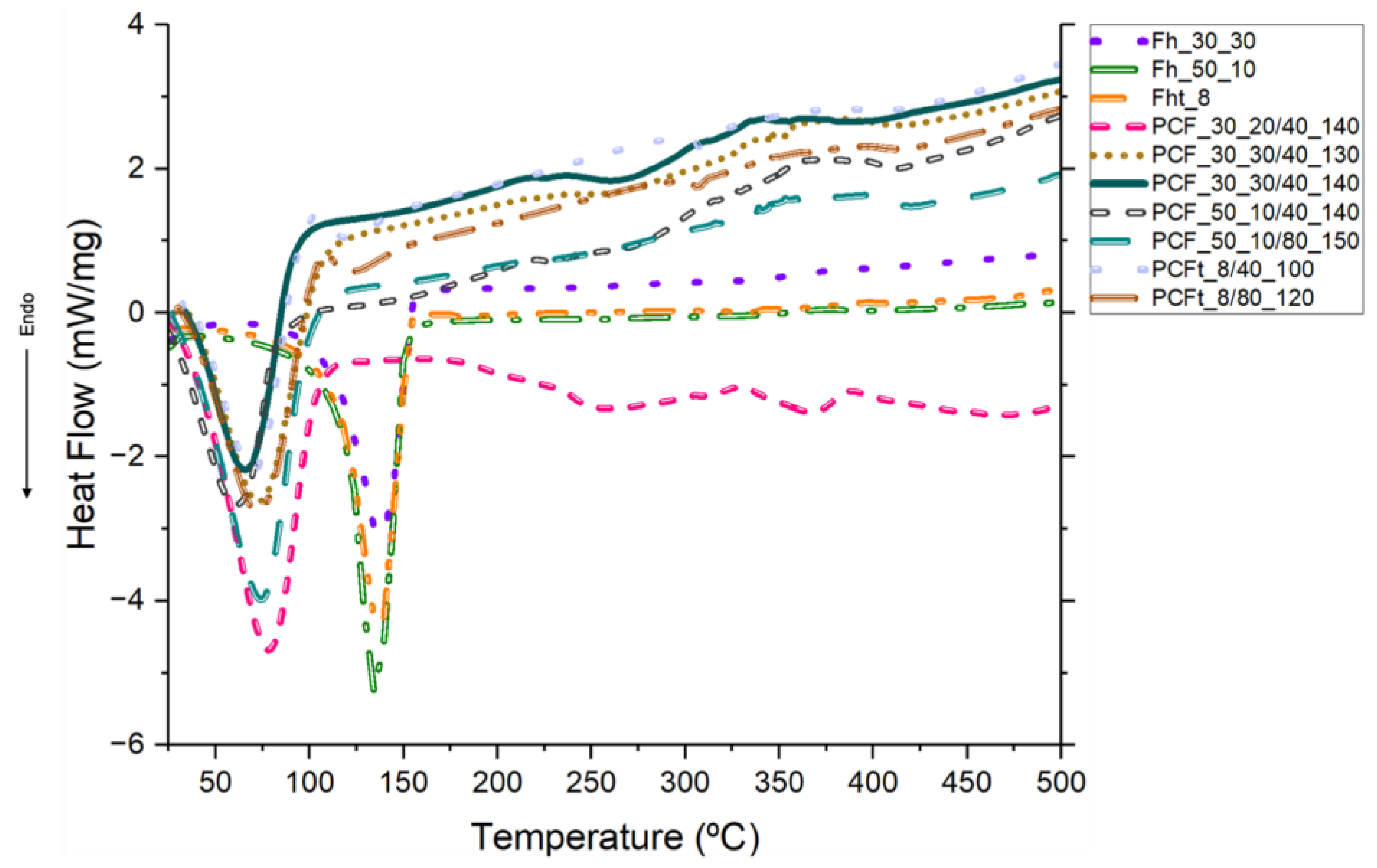
| Samples | %GA | DS |
|---|---|---|
| CAt | 42.70 | 2.83 |
| CAt1 | 42.74 | 2.83 |
| CAt2 | 42.96 | 2.84 |
| Average | 42.80 ± 0.141 | 2.83 ± 0.011 |
| Samples | Fibre Type | Mn CA | wt. % CA/CAt |
wt. % PEG |
Ejection Velocity PEG (mL/min) |
|---|---|---|---|---|---|
| Fu_a_b | Uniaxial | 30,000 and 50,000 |
10, 20 and 30 | - | - |
| Fut_b | Uniaxial | - | 8 | - | - |
| Fh_a_b | Hollow | 30,000 and 50,000 |
10, 20 and 30 | - | - |
| Fht_b | Hollow | - | 8 | - | - |
| PCF_a_b/x_y | Phase Change Fibre | 30,000 and 50,000 |
10, 20 and 30 | 40,60 and 80 | 0.130, 0.140 and 0.150 |
| PCFt_b/x_y | Phase Change Fibre | - | 8 | 40 and 80 | 0.100 and 0.120 |
| Samples | Peak Temperature (°C) | Melting Point (J/g) |
|---|---|---|
| Fh_30_30 | 139.84 | 111.78 |
| Fh_50_10 | 134.42 | 140.46 |
| Fht_8 | 137.52 | 134.39 |
| PCF_30_20/40_140 | 78.02 | 108.43 |
| PCF_30_30/40_130 | 72.26 | 56.38 |
| PCF_30_30/40_140 | 64.87 | 50.51 |
| PCF_50_10/40_140 | 61.61 | 70.91 |
| PCF_50_10/80_150 | 74.96 | 106.40 |
| PCFt_8/40_100 | 69.83 | 44.97 |
| PCFt_8/80_120 | 72.84 | 41.52 |
| Samples | Maximum elongations at break (%) | Breaking strength (kPa) |
|---|---|---|
| Fh_30_30 | 28.86 ± 1.59 | 133.18 ± 6.57 |
| PCF_30_10/60_150 | 1.59 ± 0.14 | 105.54 ± 14.73 |
| PCF_30_10/80_150 | 2.46 ± 0.30 | 212.83 ± 52.80 |
| PCF_30_20/40_140 | 14.32 ± 0.22 | 1023.64 ± 52.56 |
| PCF_30_30/40_130 | 28.46 ± 1.08 | 1094.60 ± 150.04 |
| PCF_30_30/40_140 | 22.42 ± 0.84 | 1122.49 ± 145.56 |
| PCF_30_30/40_150 | 13.07 ± 0.93 | 920.01 ± 104.02 |
| PCF_30_30/80_150 | 25.40 ± 0.63 | 264.68 ± 22.18 |
| Fh_50_10 | 16.50 ± 0.95 | 117.28 ± 30.03 |
| PCF_50_10/40_130 | 11.71 ± 0.60 | 60.28 ± 5.86 |
| PCF_50_10/40_140 | 16.20 ± 0.99 | 100.63 ± 13.22 |
| PCF_50_10/80_140 | 12.98 ± 0.58 | 75.03 ± 17.68 |
| PCF_50_10/80_150 | 21.62 ± 0.42 | 105.69 ± 15.68 |
| Fht_8 | 4.00 ± 0.71 | 61.36 ± 18.46 |
| PCFt_8/40_100 | 7.18 ± 0.62 | 28.06 ± 14.20 |
| PCFt_8/80_120 | 9.02 ± 0.66 | 39.75 ± 9.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





