1. Introduction
Pediatric traumatic brain injury (TBI) is a leading cause of disability worldwide [
1,
2], affecting between 47 and 280 children and adolescents per 100,000 every year [
3]. Impairment after TBI may persist for months or even years after the injury, affecting physical [
4], cognitive [
5], emotional [
6], and social functioning [
4]. These impairments significantly impact quality of life, which the World Health Organization has defined as “the individual’s perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards and concerns” [
7]. Health-related quality of life (HRQoL) describes an individual’s subjective physical and mental health status and can classified as generic [
8] or disease-specific HRQoL [
9]. While generic HRQoL encompasses a wide range of parameters related to a variety of health conditions [
8], disease-specific HRQoL focuses on a specific group of patients with specific health conditions and addresses particular disease-specific symptoms [
9]. Thus, disease-specific measures of HRQoL are tailored and particularly sensitive to impairments resulting from a certain health condition and can more sensitively capture HRQoL in a patient population [
10,
11].
The recently developed Quality of Life after Brain Injury – Kid/Adolescent (QOLIBRI-KID/ADO) questionnaire is the first self-reported disease-specific HRQoL instrument for children and adolescents (8–17 years) after TBI [
12]. It was developed from a specifically created item pool based on focus group interviews, national and international Delphi panels, adaptations of items from questionnaires in use in the field of TBI, and cognitive debriefings. It was tested in a German multicenter pilot study in 300 pediatric individuals who had suffered a TBI three months to ten years previously [
12]. The pilot version of the QOLIBRI-KID/ADO consisted of 35 items covering six domains (i.e., Cognition, Self, Daily Life & Autonomy, Social Relationships, Emotions, and Physical Problems). It was found to be reliable and valid for assessing HRQoL in children and adolescents after TBI [
12,
13]. Its psychometric properties were examined using methods referred to as classical test theory (i.e., reliability and validity), supplemented by differential item functioning (DIF) analyses for the age groups. Satisfactory reliability was reported for both the total score (Cronbach’s α = .89) and for the scales (Cronbach’s α = 0.70–0.80) in groups of children and adolescents with and without multiple symptom burden [
12]. Its test-retest reliability was acceptable (intraclass correlation coefficients: 0.42–0.64). Satisfactory construct validity was demonstrated using the Pediatric Quality of Life Inventory (PedsQL) [
14] as a reference measure of generic HRQoL. The results of the psychometric analyses using the item response theory framework indicate that the scales are largely unidimensional, there was no evidence of the assumptions of monotonicity and local independence being violated, and predominantly no DIF occurred in the various patient groups [
13].
Few studies have examined the influence of sociodemographic and clinical variables on HRQoL after pediatric TBI. According to the findings, reduced pediatric HRQoL is associated with older age [
10,
12], being female [
15], suffering from adverse emotional states [
12,
16,
17], a more severe TBI [
18], a lower level of recovery [
12,
19], a higher burden of post-concussion symptoms, and a comparatively recent TBI [
12,
17].
The present study aims to revalidate this 35-item version of the questionnaire, with four items reworded, by replicating the structure and design of the developmental study in a different TBI sample. Several factors associated with impaired HRQoL after pediatric TBI were examined to ensure its construct, discriminant, and convergent validity. We expected lower HRQoL to be associated with older age [
20], being female [
15], higher TBI severity [
18,
21], mental health problems such as anxiety [
22] and depression [
23], and a higher number of post-concussion symptoms [
17]. The final validation of the QOLIBRI-KID/ADO will contribute to assessing the quality of the instrument and its ability to capture the impact of pediatric TBI sequalae on the HRQoL of children and adolescents. By providing a psychometrically robust instrument for measuring TBI-specific HRQoL, we aim to improve diagnosis, treatment, monitoring, and research efforts to reduce the burden of TBI in children and adolescents.
2. Materials and Methods
2.1. Ethical Approval
The study was conducted in accordance with all relevant German and Austrian laws while taking into account the ICH Harmonized Tripartite Guideline for Good Clinical Practice and the World Medical Association Declaration of Helsinki. The study was granted ethical clearance at each of the recruiting centers and informed consent was obtained from all participants in accordance with the German and Austrian laws on data protection. The Ethics Committee of the University Medical Center Goettingen approved the study (application no. 19/4/18).
2.2. Participants
For the statistical analyses, we met the requirements for the statistical power, which suggested a sample size of approximately 140 individuals per age group based on a simulation study [
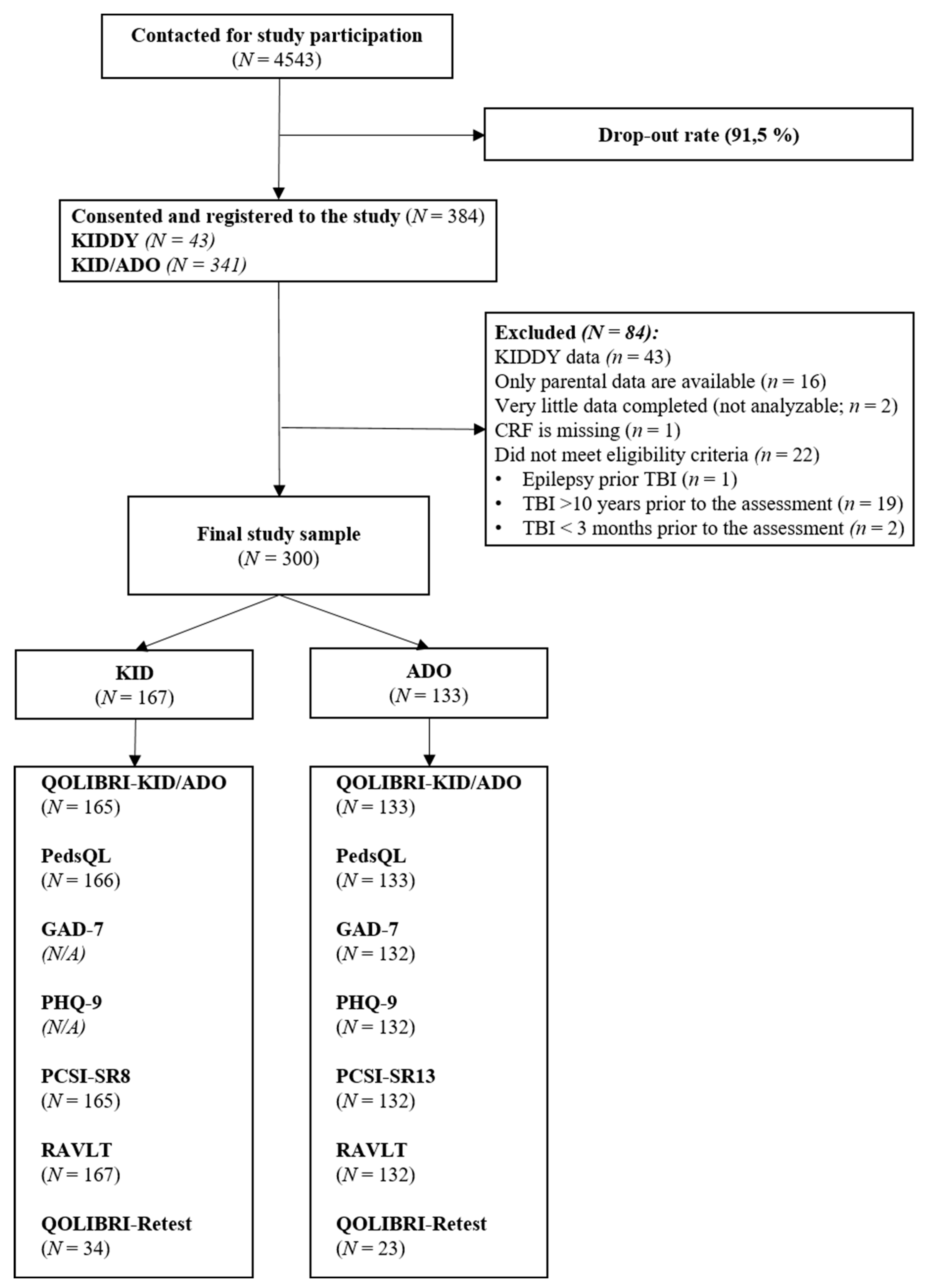
24]. Patients were eligible for participation if they were aged 8 to 17 years at the time of study enrollment (February 2022 until February 2023), had been diagnosed with TBI (at least three months but no more than ten years post injury), were able to understand and answer the questions, and were outpatients (or had recently resumed inpatient treatment). Participants were excluded if the severity of their TBI was not available in their medical records, if they were in a current vegetative state, had a spinal cord injury, had a severe mental illness (e.g., psychosis, autism) or epilepsy prior to the TBI, had diseases leading to death, or very severe polytrauma. All participants and their legal guardians gave written informed consent. The interviews were conducted face-to-face, either online or in person. Parental reports were collected in writing by email, post, or at the medical centers and hospitals. Details of the sample composition are shown in
Figure 1.
2.3. Sociodemographic and Clinical Data
The sociodemographic and clinical information was comparable to that collected in the QOLIBRI adult studies [
25,
26] and the QOLIBRI-KID/ADO pilot study [
12]. Parents provided information about themselves and their participating children, including age and gender, as well as their education and occupation.
Clinical data covering findings from imaging procedures (CT or MRI), cause, date and severity of the TBI, and loss of consciousness were collected from medical records. The TBI severity (mild, moderate, severe) was derived either from the Glasgow Coma Scale (GCS) [
27] or the International Statistical Classification of Diseases and Related Health Problems (ICD-10) diagnosis code (S06.*) [
28] or from the clinical description of the TBI, such as post-traumatic amnesia, need for ventilation and resuscitation, post-traumatic epilepsy, presence of lesions (according to MRI/CT findings), or the need for surgical intervention.
In addition, the time since TBI was obtained from the medical records and subgroups of participants were created using a median split on this time. Additional clinically relevant data were obtained from parental reports. Using a dichotomous scale (“Yes” or “No”), they reported whether the children experienced headaches, other pain, physical impairment, or mental health problems after TBI. To further investigate the presence of persistent symptoms, the mean time since TBI was analyzed and compared between participants with and without these symptoms (parent-reported problems with headaches, other pain, physical impairment, and mental health problems, mild to severe depression or anxiety, and self-reported above-average post-concussion symptoms; for more details see the subsection “Instruments”).
2.4. Instruments
The QOLIBRI-KID/ADO questionnaire tested in the final validation study included 35 items assessing self-reported TBI-specific HRQoL in children and adolescents aged 8–17 years on four “satisfaction” scales (“How satisfied are you with…”) dealing with cognitive abilities, self-concept, perceived autonomy, and social life, and two “bothered” scales (“How bothered are you with…”) describing physical and emotional problems. Responses are provided on a five-point Likert-type scale ranging from “Not at all” to “Very”, supplemented by smileys, using today and the past week as the time frame. As the responses for the two “bothered” scales are worded negatively, they need to be recoded before being aggregated with the satisfaction scales for analysis. The responses for each QOLIBRI-KID/ADO scale are linearly transformed to values ranging from 0 to 100, with higher scores indicating better HRQoL. Scale scores are calculated as the average of the item scores in each scale, and the total score is computed as the average of all scales. Compared with the pilot study, four questions were rephrased and/or misleading or unnecessary examples were removed to improve the comprehensibility of the item content (see
Appendix A,
Table A1).
The Pediatric Quality of Life Inventory (PedsQL™) [
14] is a 23-item generic HRQoL questionnaire covering physical, emotional, social, and school functioning domains. Scale scores can be aggregated to form a total, a physical health, and a psychosocial health score. Participants answer on a five-point Likert-type scale ranging from 0 to 4 (“Never” to “Almost always”). To compute scores, responses are first inverted and then linearly transformed to a scale of 0 to 100, with higher scores indicating better HRQoL.
Anxiety and depression symptoms were rated using the proxy versions of the Generalized Anxiety Disorder 7 (GAD-7) [
29] and the Patient Health Questionnaire 9 (PHQ-9) [
30], respectively, as clinical ratings could not be obtained within the study and self-report questionnaires have not been validated for children younger than twelve years of age [
31,
32]. The GAD-7 captures generalized anxiety disorder as characterized by the DSM-IV [
33]. It assesses the occurrence of seven anxiety symptoms in children or adolescents. Parents rated participants’ symptoms on a four-point Likert-type scale ranging from 0 to 3 (“Not at all” to “Nearly every day”). The sum score of all items, ranging from 0 to 21, represents the severity of the anxiety disorder as assessed by the proxies: minimal (1–4), mild (5–9), moderate (11–14), and severe (15–21) anxiety symptoms [
34]. The PHQ-9 captures the presence and severity of symptoms related to major depression in children and adolescents as characterized by the DSM-IV [
33] criteria. Respondents rate nine depression symptoms on a four-point Likert-type scale from 0 to 3 (“Not at all” to “Nearly every day”). The sum score of the items can range from 0 to 27, with the following severity categories for proxy reports: minimal (1–4), mild (5–10), moderate (11–14), and severe (15–27) depression symptoms [
35].
The German version of the Postconcussion Symptom Inventory (PCSI-SR8 for children and PCSI-SR13 for adolescents) [
36,
37] was used for self-reporting post-concussion symptoms after TBI. For children (aged 8–12 years), 16 items are answered on a three-point Guttman scale ranging from zero to two (“No problem” to “A lot of a problem”). For adolescents, 21 items have to be answered on a seven-point Guttman scale with three anchor categories (0: “Not a problem”, 3: “Moderate problem”, and 6: “Severe problem”). In both age versions, items cover four domains (i.e., physical, emotional, cognitive, and sleep/fatigue), and a total score can be calculated as a sum across all items. Due to the lack of reference values, total scores are categorized as being below, above, or within the average range (M ± 1 SD) of participants who completed the age-appropriate version of the PCSI.
Examiners and clinicians rated functional recovery after pediatric TBI using the King’s Outcome Scale for Closed Head Injury (KOSCHI) [
38] on disability/recovery levels of TBI (3a: “lower severe disability”, 3b: “upper severe disability”, 4a: “lower moderate disability”, 4b: “upper moderate disability”, 5a: “good recovery”, 5b: “intact recovery”).
Verbal learning and memory was assessed using the Rey Auditory Verbal Learning Test (RAVLT) [
39,
40]. Fifteen words are read out loud by the examiners, and these have to be repeated by the children in eight trials. The RAVLT’s learning rate was calculated by subtracting the number of words recalled in Trial I from those in Trial V. They were categorized as being above, below, or within the average range (M ± 1SD) for the respective age group.
2.5. Statistical Analyses
Descriptive statistics at the item, scale, and total score levels included means (
M), standard deviations (
SD), skewness (
SK), and floor and ceiling effects. Skewness was considered absent for values from -0.5 to 0.5; values from ±0.5 to ±1 were considered moderately, and values beyond ±1 heavily skewed, respectively [
41]. Ceiling effects are reported in terms of the percentage of responses in the most satisfied/least bothered category and conversely floor effects in terms of the percentage of responses in the least satisfied/most bothered category [
42].
Using differential item functioning (DIF) analyses, we investigated whether participants’ responses to the final version of the QOLIBRI-KID/ADO were independent of their age. This was analyzed using a logistic ordinal regression of differential item functioning (LORDIF) [
43] based on the age category (children aged 8–12 years, adolescents aged from 13–17 years). For each item as a dependent variable, DIF was evaluated by comparing a LORDIF model including scale scores with a model including scale scores, age category, and an age category-scale score interaction. Whenever the comparison of the models revealed a significant difference (α = 0.01) and the associated effect exceeded a very small effect (McFadden’s pseudo R² > 0.05) [
44], response behavior was assumed to differ between age categories.
Reliability analyses of the final version of the QOLIBRI-KID/ADO took the form of tests for internal consistency and test-retest reliability. Cronbach’s α and McDonald’s ω were calculated for the total and scale scores as indicators of internal consistency. In line with findings from pediatric outcome research, Cronbach’s α ≥ 0.60 [
8] and McDonalds’s ω ≥ 0.70 [
45] were regarded as satisfactory. It has been reasoned in the past that the internal consistency of HRQoL measures may be lower in individuals with cognitive impairment [
12,
46]. We therefore conducted subgroup analyses based on the RAVLT learning rate. To further investigate the reliability of the questionnaire for different TBI-related symptoms, internal consistency was calculated and stratified by severity and symptom groups (for a list and classification, see the subsection “Computational Software, Classification, and Cut-Off Values”). In addition, corrected item-total correlations (CITC) were calculated for individual items and their corresponding scales, with a criterion of CITC > 0.40.
Test-retest reliability was assessed in a subsample of participants who filled in the final version of the QOLIBRI-KID/ADO twice, with an interval of 10 to 20 days between completions. Intraclass correlation coefficients (ICC) were calculated based on a two-way random effects model for the total score and each scale. ICC values > 0.60 [
47] were considered satisfactory. In addition, the standard error of measurement (SEm) [
48] and the minimal detectable change (MDC) were calculated based on the ICC of the total and scale scores. Since there are no clear criteria for acceptable MDC and SEm values, SEm was considered as the total possible uncertainty range of the instrument. A percentage less than 10%, corresponding to a variation of 10 points on the 0 to 100 scale score, was considered satisfactory [
49].
The factorial structure of the final version of the QOLIBRI-KID/ADO was analyzed by means of a confirmatory factor analysis (CFA). A six-factor model representing the six scales of the QOLIBRI-KID/ADO was assumed. Given the ordinal nature of the response categories, diagonally weighted least squares with mean and variance adjustment (WLSMV) was used as the estimation approach for model identification [
50]. As the cut-off values were established for estimating maximum likelihood and not for the estimation method, the following cut-offs for the model fit indices must be interpreted with caution [
51]. The indices used, with their respective desirable cut-offs in parentheses, were: χ2-test statistics (p > 0.01) [
52], χ2/df ratio (≤ 2) [
52], comparative fit index (CFI > 0.95) [
53,
54], Tucker-Lewis index (TLI > 0.95) [
54,
55], root mean square error of approximation and its 90-percent confidence interval (RMSEA < 0.06, CI90%) [
56], as well as the standardized root mean square residual (SRMR < 0.08) [
54].
To examine whether the final version of the QOLIBRI-KID/ADO measures the construct of HRQoL, its convergent validity was investigated using correlation analyses against an established measure of generic HRQoL in children and adolescents, the PedsQL. Pearson correlations were calculated between all the scales, the QOLIBRI-KID/ADO total score, the physical functioning score (equal to the Physical Problems scale), and the psychosocial functioning score (mean of Cognition, Self, Social Relationships, and Emotions scales) with the corresponding PedsQL scale scores.
Discriminant validity was analyzed using correlational analyses with constructs presumed to be less closely related. The association of the construct and relevant sociodemographic and clinical characteristics was investigated using correlational analyses as well as known-groups analyses [
57]. The associations between the QOLIBRI-KID/ADO total and scale scores and the self-reported PCSI-SR8/13 as well as the proxy-reported GAD-7 and PHQ-9 scores were examined using Pearson correlations. The GAD-7, PHQ-9, and PCSI-SR8/13 were assumed to have negative correlations corresponding to a small to medium effect (i.e., r between -0.10 and -0.30) [
58], demonstrating some overlap across the domains and pointing to a possible relationship between lower HRQoL and increased symptom burden. To determine the amount of variance explained, the coefficient of determination (R²) was calculated.
2.6. Missing Values
The occurrence of missing values was not considered a problem if missing values did not exceed five percent of responses to an item. QOLIBRI-KID/ADO scale scores were computed if no more than a third of a scale’s responses were missing and total scores only if all scales yielded a score. Following the manual for the PedsQL [
59], mean scale scores were computed as long as no more than half of a scale’s responses were missing. Similarly, GAD-7 and PHQ-9 mean scores and the PCSI-SR8/13 sum scores were calculated if no more than one third of the items per instrument were missing.
2.7. Computational Software, Classification, and Cut-Off Values
The software R (version 4.3.0) [
60] was used for all the analyses, using the packages psych [
61] for calculating reliability measures, lavaan [
62] for the CFA, and lordif [
63] for DIF analyses.
The following TBI-related symptoms were considered when analyzing the symptom burden: low functional recovery (expressed as a KOSCHI scores below 5), parent-reported post-TBI problems (headache or other aches, physical impairments, or mental health problems: if at least one is reported by a parent), depression and anxiety symptoms (expressed by PHQ-9 and GAD-7 scores above 4, respectively), post-concussion symptoms (expressed as PCSI-SR8/13 scores equal to or greater than 1 SD above the age-adjusted mean), and a low learning rate (expressed as corresponding RAVLT scores equal to or less than 1 SD below the age-adjusted mean). The symptom burden was first examined for the total sample and then in TBI severity groups (i.e., mild vs. moderate/severe).
Cohen’s cut-off criteria were used to assess the strength of associations, indicating a small (0.10), moderate (0.30), or large (0.50) effect [
58]. For the comparisons presented, moderate correlations (r ≥ 0.30) were determined to ascertain the convergent validity; small to medium correlations were expected to indicate sufficient discrimination between the constructs. The mean comparisons for the known-groups validity analyses used one-tailed Student’s t-tests. The correlation analyses used Pearson correlation coefficients.
For statistical comparisons, a p-value less than 0.05 was considered significant for the total score. For the scale comparisons, the p-value was adjusted using the Bonferroni correction (i.e., 0.05/6 = 0.008).
3. Results
3.1. Participants
A total of 300 children and adolescents completed the final version of the QOLIBRI-KID/ADO. Data from 41 participants had to be excluded because only parental data were available, insufficient data was provided, or the inclusion criteria had been violated (one participant was diagnosed with epilepsy prior to TBI, and 22 participants completed the questionnaire outside the allotted time frame of 3 months to 10 years after TBI).
Participants were interviewed face-to-face, either online (60%) or in person (40%). Participants interviewed online exhibited higher QOLIBRI-KID/ADO total scores than those assessed in-person (Monline = 75.67 vs. Min-person = 79.49, t(296) = -2.73, p = .005, d = 0.33). Ninety-four (31%) participants had also taken part in the pilot study, however, prior participation had no effect on the QOLIBRI-KID/ADO total scores in the present study (Mrepeated = 77.78 vs. Mnaïve = 76.95, t(296) = 0.56, p = 0.574, d = 0.07).
Table 1 summarizes the sociodemographic and clinical data. The mean age was 12.6 years (
SD = 2.67,
Min = 8.00,
Max = 17.90) and 54% were male. Mild TBI was the most common type of severity (80%), of which 21 participants (9%) sustained complicated mild TBI [
64] (i.e., mild TBI with brain lesion identified on CT or MRI). Overall, lesions were present in 24% of all participants. The most common onset of TBI was four to ten years prior to study enrollment (67%). The majority of participants displayed favorable functionality (5a/b) based on their KOSCHI score (94%). The parents of half the participants (49%) reported their children as having post-TBI problems. Participants’ self-rated post-concussion symptoms were mostly within the average range for their age group (75%). Most participants showed no or minimal signs of anxiety (71%) or depression (70%). We also examined the symptom characteristics of participants having parent-reported headaches or other pain, physical impairment, and mental health problems, mild to severe anxiety and depression, and above-average post-concussion symptoms in relation to the time since TBI. Only participants with parent-reported problems after TBI differed significantly from those without, with a shorter mean time since injury (
Mproblems = 5.06,
Mno problems = 5.71, t(296) = 2.09,
p = 0.038,
d = 0.24; see
Appendix B,
Table B1).
3.2. Item analyses of the final QOLIBRI-KID/ADO questionnaire: Item Properties, Internal Consistency, and Differential Item Functioning
The item characteristics and distributions are shown in
Table 2. Given the low number of missing values per item (≤ 1%), we assumed these rare cases would not affect the assumption that missingness is completely random. The means of all the items exceeded three on the five-point Likert-type scale, and all items were skewed to the left. Similarly, most responses displayed ceiling effects, a common observation in this kind of HRQoL assessment [
65].
Regarding the contribution of individual items to internal consistency, two items from the Cognition scale (“Decision between two things”, “Orientation”) had corrected item-total correlations below 0.40. The item “Loneliness” from the Emotion scale also led to a slight increase (+0.01) in Cronbach’s α when omitted (see
Table 2).
The DIF analysis did not detect any meaningful differences in the response pattern for individual items between children and adolescents. Although three items (“Planning”, ”Sadness”, “Other injuries”) showed significant differences when comparing models with and without assumed age-group effects, the differences found were not sufficiently large to be considered to have an impact. For details, see the right side of
Table 2.
The internal consistency for the total score of the QOLIBRI-KID/ADO was excellent, with Cronbach’s α = 0.92 and McDonald’s ω = 0.92. The individual QOLIBRI-KID/ADO scales showed good to excellent internal consistency (Cronbach’s α 0.69–0.77; McDonald’s ω 0.76–0.85, see
Table 2 for details) which matches the findings from the pilot study. Subgroup analyses of the internal consistencies for individual scales and subgroups with lower learning rates showed for the most part satisfactory to excellent results (Cronbach’s α 0.65–0.85; McDonald’s ω 0.76–0.89). This was also the case for other subgroups stratified by sociodemographic and clinical characteristics (Cronbach’s α 0.87–0.94; McDonald’s ω 0.89–0.96), except for the group with present post-concussion symptoms on the Cognition scale (Cronbach’s α = 0.52). For details, see
Appendix B,
Table B2.
3.3. Scale analyses of the QOLIBRI-KID/ADO questionnaire: Descriptive Statistics, Test-Retest Reliability, and Factorial Validity
Table 3 summarizes the descriptive statistics for the QOLIBRI-KID/ADO, PedsQL, PHQ-9, GAD-7, and PCSI-SR8/13. The QOLIBRI-KID/ADO mean scale scores were higher than 60, as often observed in HRQoL studies [
65], especially in children and adolescents [
66]. Almost all scales of the QOLIBRI-KID/ADO and the PedsQL were skewed to the left, except for the Cognition and Emotions scales of the QOLIBRI-KID/ADO, which only showed a tendency to be skewed in this direction. On average, the levels of anxiety, depression, and post-concussion symptoms reported were low.
For the test-retest analysis, 57 participants completed the QOLIBRI-KID/ADO questionnaires twice with the specified delay of 10–20 days. The indicitual scale ICCs ranged from 0.57 to 0.74, the total scale ICC was 0.78 and thus almost all ICCs were above the 0.60 criterion, except for the Physical Problems scale (see
Table 4 for details). Expressed as a percentage of the total possible range of the instrument, almost all the SEm values were below the 10% criterion. The MDC of the total score and the scales ranged from 13.35 to 34.91.
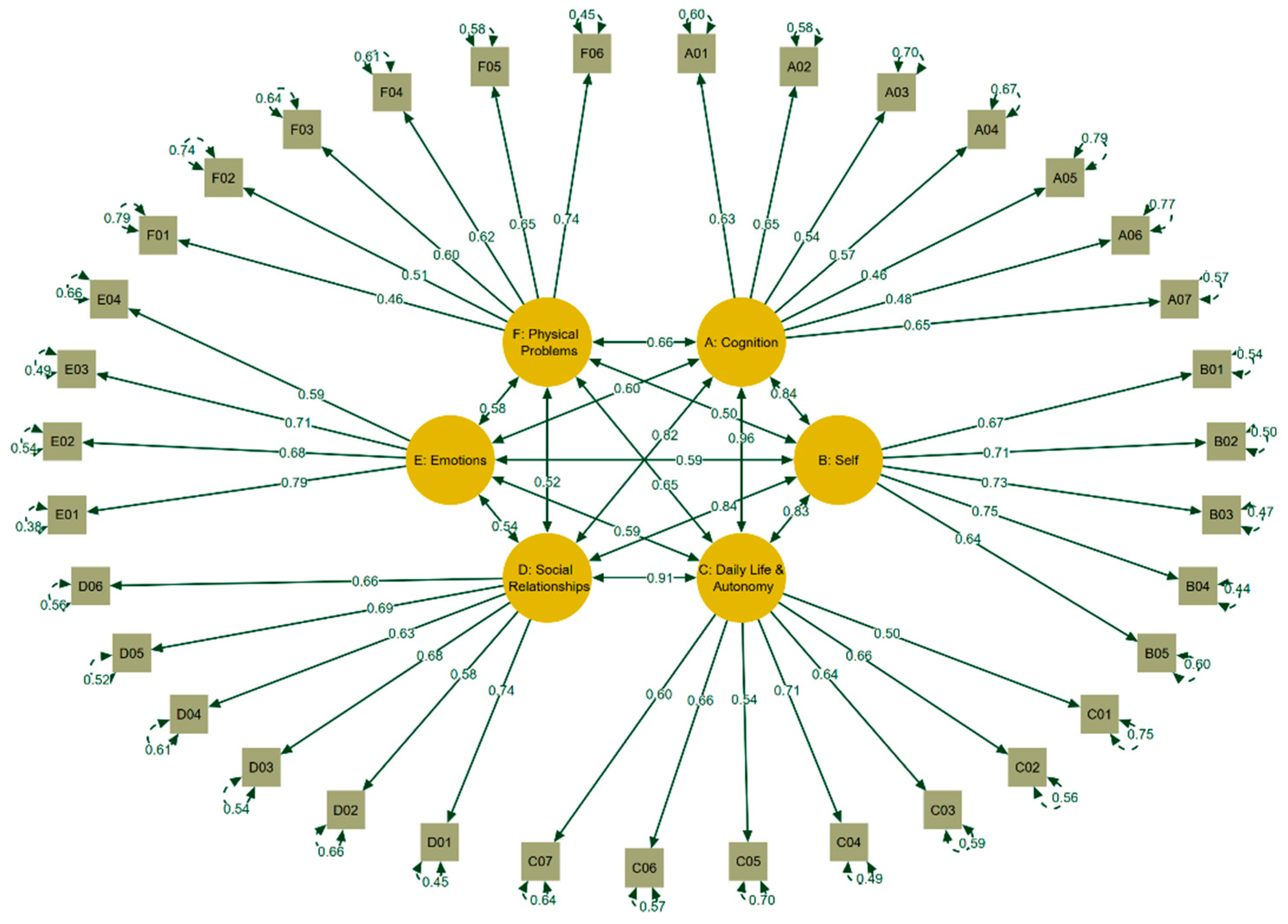
For the factorial validity, an inspection of the response categories revealed that the lowest response categories were seldom chosen by the participants, which made it necessary to collapse the response categories “not at all” and “slightly” for the “satisfaction items” as well as “very” and “quite” for the “bothered scales” for CFA analyses. The six-factor model exhibited fit indices that fulfill the requirements for an excellent fit (CFI = 0.99, TLI = 0.99, RMSEA = 0.03, CI
90% [0.03, 0.04], SRMR = 0.07), with the χ²(545) = 729.20 index being the sole exception (p < 0.01). The significant
p-value can be attributed to the high number of degrees of freedom (545). However, the ratio between χ² and df was 1.34, which did not exceed the acceptable cut-off of 2. These findings match those obtained during the pilot study. The resulting factor loadings are shown in
Figure 2.
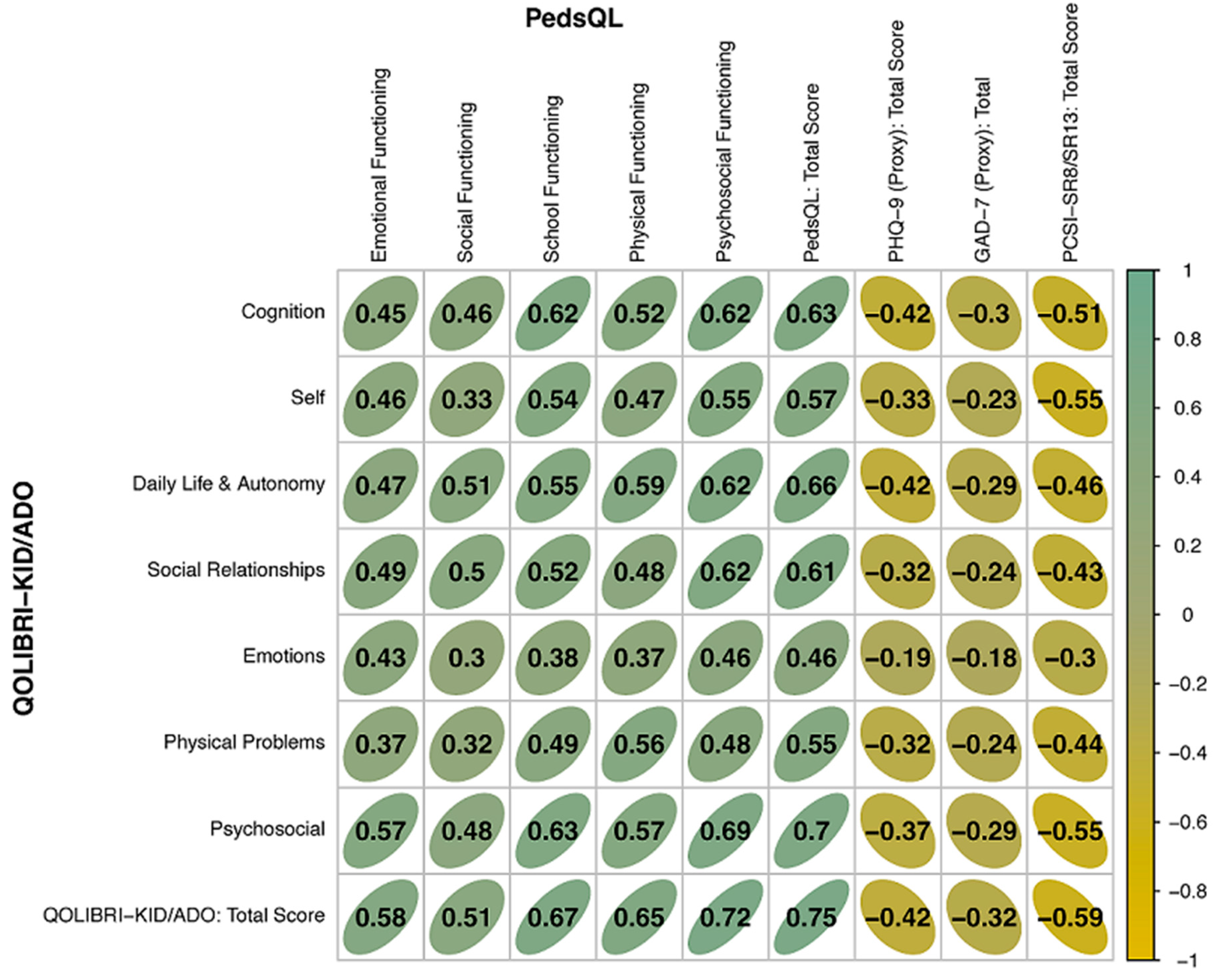
Figure 3 summarizes the Pearson correlation coefficients for the construct validity, and
Appendix B,
Table B3 shows the corresponding explained variance obtained from correlating the QOLIBRI-KID/ADO total and scale scores with the PedsQL total and scale scores, and with the total scores of the GAD-7, the PHQ-9, and the PCSI-SR8/13. The constructs of the QOLIBRI-KID/ADO and PedsQL that were compared included the emotional, social, physical, and psycho-social dimension as well as the total scores. Correlations between the QOLIBRI-KID/ADO and PedsQL scales ranged from
r = 0.43 (Emotions and Emotional Functioning),
r = 0.5 (Social Relationships and Social Functioning),
r = 0.56 (Physical Problems and Physical Functioning),
r = 0.69 (Psychosocial Functioning scale scores) to
r = 0.75 (total scores of QOLIBRI-KID/ADO and PedsQL). The corresponding explained variances were between 18% (emotional), 25% (social), 31% (physical), 48% (psychosocial), and 56% (total), supporting construct validity.
The results of the divergent validity analyses tested against instruments measuring anxiety, depression, and post-concussion symptoms revealed the hypothesized negative correlations. The scales of the QOLIBRI-KID/ADO were in the range of r = -0.42 to r = -0.18 against the GAD-7 and PHQ-9 and in the range of r = -0.3 to r = -0.55 against the PCSI-SR8/13, explaining 3% to 18% of the variability of the scores. The correlations of the total scores were r = -0.42, r = -0.32, and r = -0.59, with an explained variance of 17%, 10%, and 34%, respectively, indicating good discriminant validity of the QOLIBRI-KID/ADO questionnaire compared to the emotional states and post-concussion symptom measures.
Table 5 summarizes the results of known group comparisons that were hypothesized to differ in TBI-related HRQoL, providing further indication of construct validity. Children were found to exhibit significantly higher QOLIBRI-KID/ADO total scores than adolescents. Looking more closely at the scales for which children reported higher HRQoL than adolescents, a significant difference was found for the Self scale (
t(296) = -5.21,
p < .001,
d = -0.61). The total scores of the QOLIBRI-KID/ADO were significantly higher for male participants than the scores of female participants. When examining the individual scales on which males reported higher HRQoL than females, a significant difference was also found on the Self scale (
t(296) = -2.89,
p < .001,
d = -0.34).
Looking at TBI-related characteristics, the QOLIBRI-KID/ADO total and scale scores were unaffected by time since TBI or TBI severity (p > 0.05). Participants without post-concussion symptoms had significantly higher QOLIBRI-KID/ADO total scores (p < 0.05) and scores on all individual scales (padjusted < 0.008) than those with symptoms. Similarly, participants whose parents reported no headaches, other pain, physical impairment, or mental health problems after TBI had significantly higher QOLIBRI-KID/ADO total, Cognition, and Physical Problems scale scores than did participants whose parents reported these problems. Furthermore, participants with a low learning rate did not differ significantly from participants with an average to high learning rate in terms of their QOLIBRI-KID/ADO scores. Regarding emotional states, the absence of depression and anxiety symptoms was associated with higher QOLIBRI-KID/ADO total and all scale scores as compared to the presence of depression and anxiety symptoms, respectively.
4. Discussion
The QOLIBRI-KID/ADO questionnaire was developed as the first disease-specific instrument to assess HRQoL specifically in children and adolescents after TBI. Our results indicate that the final version of the QOLIBRI-KID/ADO is a valid and reliable measure of pediatric TBI-specific HRQoL which can be used in research, diagnostic, and therapeutic contexts. The questionnaire covers six relevant dimensions of life that may play an important role for children and adolescents following TBI [
67]: Cognition, Self, Daily Life and Autonomy, Social Relationships, Emotions, and Physical Problems.
The internal consistency for the total score was high, and satisfactory to good for the six subscales. The internal consistency was also shown to be largely sufficient and satisfactory in different subgroups of children/adolescents with anxiety or depression symptoms or lower learning rates. This underlines the use of the questionnaire in populations with different symptom burdens, which is particularly important for the assessment of HRQoL in diagnostic and therapeutic processes [
68].
Furthermore, the revalidation of the QOLIBRI-KID/ADO questionnaire confirms that it is a reliable, valid, and stable measure over the test-retest interval. The total score displayed very good reliability as indicated by the ICC, and the scales demonstrated a fair to good agreement between test and retest [
47]. The SEm values of less than 10% for the total score and for all scales, except the Emotions and Physical Problems scales, reflect some variability in the data. The MDC values indicate that a change of more than 13 points in the QOLIBRI-KID/ADO total score and a change of 15 points or more in the scale scores can be considered a “true” change [
49] in disease-specific HRQoL after TBI. Longitudinal treatment or therapy studies are recommended to further address the issue of the QOLIBRI-KID/ADO’s sensitivity to change and meaningful clinical change.
The factorial validity analyses replicated the one-level six-factor structure with excellent fit indices, comparable to the findings of both the pilot study [
12] and the adult QOLIBRI assessment [
25]. The one-level six-factor structure supports the notion that the six factors of the multidimensional HRQoL construct should also be considered in research and clinical practice [
69].
There were no meaningful item differences between the two age groups, supporting the use of the same questionnaire from 8 to 17 years of age, allowing aggregated analyses and long-term follow-up using the QOLIBRI-KID/ADO and underscoring the suitability of the questionnaire for comparing children (8–12 years) and adolescents (13–17 years).
The majority of children and adolescents expressed rather high satisfaction with their HRQoL on the Cognition, Self, Daily Life and Autonomy, Social Relationships dimensions and did not report feeling bothered very much by Emotions or Physical problems (QOLIBRI-KID/ADO scores > 62). This observation may be partially explained by the composition of the study sample. Other studies have reported a decrease in HRQoL in individuals after moderate and severe TBI [
18], with chronic health conditions [
70], or with cognitive impairment [
71]. In our sample, anxiety and depression symptoms were reported for more than 30% of the participants, 19% had a low learning rate, and 18% suffered from post-concussion symptoms. Furthermore, 50% of parents reported that their children/adolescents had at least one problem after the TBI: headaches, other pain, physical impairment, or mental health problems.
We observed an unexpected difference in the total score between those tested online and those tested in person. There was a small effect with a significantly lower HRQoL in the online group, which can be attributed to the fact that the online group included more participants who had experienced TBI within the first year (8.4% vs. 0%) and had a higher incidence of moderate/severe TBI (23.4% vs. 14.8%) compared with the group tested face-to-face. Previous study participation (31.3%) had no significant effect on HRQoL.
We replicated all the results of the pilot study regarding the validity of the QOLIBRI-KID/ADO. Evidence of construct validity was obtained by comparing the total score, the physical functioning and the psychosocial functioning scores with the corresponding scales of the generic HRQoL measure, the PedsQL. The correlations of the total scores and both physical and psychosocial functioning scales were fairly high, explaining up to 56% of variance and supporting the construct validity of the new measure. The moderate negative correlations of the total score of the QOLIBRI-KID/ADO with anxiety and depression symptoms are similar to the correlations observed between these concepts in other studies [
22,
23] indicating that higher levels of symptom burden correspond to lower levels of HRQoL and vice versa. However, the construct overlap given by the explained variance is relatively low, which was also hypothesized in terms of the divergent validity. Previous research observed that 11 to 45% of children face the risk of developing mental health issues following pediatric TBI [
72]. In our study, one third of participants exhibited mild to severe symptoms of anxiety and depression, even years after TBI, with a noteworthy decline in HRQoL. Similar mental health outcomes have been documented for children and adolescents coping with chronic health conditions [
16]. Given that the adverse effects of TBI, including mental health problems, may manifest years after injury, early identification of emotional problems in the children and adolescents concerned could improve rehabilitation for the affected children and adolescents and their families.
It is known from reviews of pediatric TBI studies that several factors may influence HRQoL [
18]. As in the developmental pilot study [
12], we found a higher total HRQoL score, and especially a higher score on the Self scale, in the current study in children aged 8–12 years compared with adolescents aged 13–17 years. Other pediatric studies also report increasing health complaints [
73,
74] and lower HRQoL with increasing age [
20,
75,
76,
77,
78]. This may be explained by the fact that adolescence and puberty is a period of major developmental, physical, and social transitions due to the maturation process [
79], and individuals often face challenges in coping with their environment [
80]. It could be also explained by the fact that the younger the children, the less dramatic they experience life changes after TBI as being, because they may have limited memory of their lives before the injury occurred [
23].
Our sample included only slightly more boys (54%), which is relatively more balanced in terms of sex compared with other QOLIBRI studies [
12,
26,
81]. In general, males have a higher incidence of TBI [
82] and report fewer health complaints than females, at least in industrialized countries [
73,
83], resulting in higher HRQoL [
15]. Accordingly and as in the pilot study [
12], boys reported a significantly higher total HRQoL, and the Self scale in particular was rated more positively than among girls. This may be due to a conglomerate of genetic, cultural, and social factors [
83]. Different societal expectations [
76] regarding roles and positions [
84] may explain why girls in general have a less optimistic view of happiness [
78] and are generally more worried [
83]. They tend to internalize problems and negative emotions [
85], which makes them more vulnerable to later mental health problems [
85]. They may therefore benefit from engaging in greater reflection and more communication about their feelings [
78].
As observed in previous research [
18] and in our pilot study [
12], the current study found no association between moderate and severe TBI and lower HRQoL, possibly because TBI severity and HRQoL are not linearly correlated [
86]. In contrast to other studies reporting that longer time since TBI is associated with better HRQoL [
23], we found no significant effect of time since injury on HRQoL. However, when the TBI was more recent we observed a negative effect on HRQoL in those with more post-concussion and depression symptoms. This contrasts with other research suggesting that persistent symptoms and adverse outcomes following early pediatric TBI may not manifest until later in life [
87]. In our study, we found only minimal symptoms reported several years after TBI. This may be due to the predominantly mild severity of the TBI experienced by our study participants. Existing studies have suggested that the persistence of symptoms may be more closely related to TBI severity than to time since TBI or the age of the child [
88]. This issue should be further explored in future studies by additionally controlling for potential confounders [
89] (e.g., other health conditions, comorbidities, or also considering developmental stages leading to depressive symptoms) that may affect adolescents between the time of injury and the assessment of post-concussion, depression symptoms, and HRQoL in the post-acute injury phase.
In line with the pilot study [
12] and other research findings [
23,
90], we found small but significant effects indicating that more post-concussion, anxiety and depression symptoms, as well as parent-reported problems, are associated with lower HRQoL. In general, children who have suffered a TBI are at a higher risk of developing anxiety [
91], depression [
72], and persistent post-concussion symptoms [
17,
92]. It is known from the adult literature [
93] that persistence of symptoms and emotional states compromise HRQoL even years after TBI. Early identification and treatment of emotional challenges in children and adolescents following TBI could enhance and improve the rehabilitation and HRQoL for patients and their families.
Overall, compared with the pilot study, we were able to replicate the robust psychometric structure, the construct validity, and the significant impact of age, sex, anxiety, depression, and post-concussion symptoms on HRQoL assessed using the first disease-specific QOLIBRI-KID/ADO after pediatric TBI. The only effects found in the pilot study but not in the validation study are the impact of low recovery (not analyzable in the present study due to the small sample size) and the very small impact of time since injury on HRQoL. It is important to note that severity did not affect HRQoL in either study, but caution should be exercised in interpreting this finding due to the characteristics of the sample, with children and adolescents predominantly having mild TBI.
4.1. Limitations
This study complements the development and validation of the QOLIBRI-KID/ADO [
12], the first disease-specific questionnaire to assess HRQoL after pediatric TBI. However, some limitations should be mentioned. Concerning the study sample, less than 10% of those originally contacted decided to participate in the study. The reasons for non-participation in pediatric clinical studies are formally unknown, but self-selection bias [
94] may potentially explain the high drop-out rate observed. Other reasons for non-participation may involve parental concerns. Before participating in the study, some parents expressed concern that their children might be too healthy to participate or that they might not remember the accident, while others were concerned that their children might be retraumatized by participating in the study, as is also mentioned in other studies [
95,
96].
Furthermore, like in many observational studies [
18], most participants had experienced a mild TBI and in most cases the injury dated back four to ten years. The sample composition may therefore explain why especially the lower response categories, indicating low satisfaction, were rarely selected by participants. This in turn caused some problems with model estimation, solved by subsequently merging the two lower response categories. This limited variability in the sample could pose challenges in interpreting our results.
A potential limitation may be also the use of parent ratings to assess depression and anxiety in children and adolescents. Discrepancies in agreement between self-reports and parent ratings, particularly in younger children, may limit the validity of parent ratings as proxies for child self-reports [
97,
98].
5. Conclusions
The QOLIBRI-KID/ADO questionnaire is the first validated, disease-specific instrument for measuring disease-specific HRQoL after pediatric TBI. The psychometric properties of this comprehensive 35-item self-report instrument demonstrate the necessary feasibility and measurement characteristics in six dimensions of HRQoL in children and adolescents after TBI. The QOLIBRI-KID/ADO successfully captures the expected theoretical links between the construct of HRQoL and other constructs and aspects, thereby supporting its construct validity. The findings emphasize the importance of implementing prevention strategies that are sensitive to age and sex differences, as well as to anxiety, depression and other symptoms, such as post-concussion or cognitive symptoms, which occur after pediatric TBI. The QOLIBRI-KID/ADO can therefore be applied in research and clinical practice to assess HRQoL after pediatric TBI in order to plan and ameliorate interventions and subsequently outcome after TBI.
Supplementary Materials
There are no supplementary materials.
Author Contributions
Conceptualization, N.v.S., M.Z., K.C.; methodology, N.v.S., M.Z., S.G., K.C.; software, M.Z., S.G.; validation, N.v.S., K.C.; formal analysis, M.Z., S.G.; investigation, N.v.S., U.K., D.T., I.K.K., M.V.B., S.B., M.K., M.H., K.B., M.R., M.L., C.A., A.N., A.K., J.D., U.W., D.P., C.T., A.B., J.S., H.M., K.C.; resources, N.v.S.; data curation, M.Z., S.G.; writing—original draft preparation, N.v.S., M.Z., S.G., K.C.; writing—review and editing, all authors; visualization, N.v.S., M.Z., K.C.; supervision, N.v.S.; project administration, N.v.S., K.C.; funding acquisition, N.v.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Dr. Senckenbergische Stiftung/Clementine Kinderhospital Dr. Christ‘sche Stiftungen (Germany), Deutsche Gesetzliche Unfallversicherung (Germany; grant number FR282), and Uniscientia Stiftung (Switzerland).
Institutional Review Board Statement
The QOLIBRI-KID/ADO study was conducted in accordance with all relevant German laws, including but not limited to the ICH Harmonized Tripartite Guideline for Good Clinical Practice (ICH GCP) and the World Medical Association Declaration of Helsinki (“Ethical Principles for Medical Research Involving Human Subjects”). The study attained ethical clearance at each recruitment center and obtained the informed consent of all participants in accordance with the German law for data protection (General Data Protection Regulation, GDPR). The Ethics Committee of the University Medical Center in Goettingen approved the study (application no. 19/4/18).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors. The data are not publicly available for reasons of data protection.
Acknowledgments
We are immensely grateful to all investigators and our participants for helping us in our efforts to improve care and outcome after pediatric traumatic brain injury. We would also like to thank the additional national and international experts supporting us for the duration of the project, especially during the item generation and reduction process: Anna Adlam, Nada Andelic, Juan Carlos Arango-Lasprilla, Miriam Beauchamp, Jeroen Bekkers, Emily Bennett, Enrico Castelli, Mathilde Chevignard, Gemma Costello, Rita Formisano, Rob Forsyth, Carol Hawley, Fiona Lecky, Marianne Løvstad, Mari Saarinen, Shannon Scratch, Suzanna Watson, Shari Wade, Claudia Armgardt, Monika Bullinger, Katharina Diepold, Christiane Gruß, Ralf Heindorf, Dirk Heinicke, Rainer John, Dorothea Mielke, Ulrike Neirich, Bettina Schulz, Gertrud Wietholt, Angela Woller, and Joy Weber. We would also like to thank all members of the consortium and those who examined our patients: Mattea Ausmeier, Hanna Boenitz, Lea Busch, Johann de Maeyer, Helena Duewel, Anastasia Gorbunova, Louisa Harmsen, Sina Kantelhardt, Maximilian Kluge, Lena Kuschel, Katja Lorenz, Louisa Lohrberg, Isabelle Mueller, Philine Mueller, Anna-Lena Raidl, Maren Roehl, Dorle Schaper, Emma Schmiedekind, Carolin Singelmann, Inga Steppacher, Johanna von Petersdorff, Sophia Mueller, Nico Rodo, Victoria Stefan, Shaghayegh Gorji, Celine Koenig, Carl Jannes Neuse, Alexander Kaiser, Meike Engelbrecht, Agnes Berghuber, Stefan Hillmann, Julius Poppel, Benjamin Nast-Kolb, Hanna Klaeger, Nils Schoenberg, Jonas Pietersteiner, Sophia Hierlmayer, Korbinian Heinrich, Rieke Boeddeker, Elena M. Bonke, Lisa F. Umminger, Leonard B. Jung, Paul S. Raffelhüschen, Lara Pankatz, Paula von Schorlemer, Tim L.T. Wiegand, Nicole Fabri, Clara Lamersdorff, and Philine Rojczyk.
Conflicts of Interest
The authors declare no conflict of interest. The sponsors had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Appendix A
Table A1.
Changes in Wording of the German Items in the Pilot and Final Validation Study translated into English.
Table A1.
Changes in Wording of the German Items in the Pilot and Final Validation Study translated into English.
| |
Wording* |
| Item |
Pilot Study |
Final Validation Study |
| |
How satisfied are you with… |
| Orientation |
… how you find your way around (for example, at home or how you can find the way to school)? |
… how you are able to find your way around (for example, finding your way to school)? |
| Daily Independence |
… being able to do everyday things without assistance (for example, using the toilet, getting dressed)? |
… being able to do things you do every day without help (for example, getting dressed)? |
| Social Activities |
KID: … how you are able to play with your friends (for example, at school, at birthday parties)?
ADO: … how you are able to join in with adolescents (for example, hobbies, parties, sport clubs)?
|
… how you are able to play/ do things with your friends? |
| Family Relationship |
… how you get along with your family (parents, siblings)? |
… how you get along with your family? |
Appendix B
Table B1.
Mean Time in Years Since TBI of Participants with and without Neuropsychiatric Symptoms.
Table B1.
Mean Time in Years Since TBI of Participants with and without Neuropsychiatric Symptoms.
| Symptoms |
Symptoms Present |
|
Symptoms Absent |
|
t-Test Parameters |
|
| |
n |
M (SD) |
|
n |
M (SD) |
|
t |
df |
p |
d |
| Problems after TBI |
147 |
5.06 (2.64) |
|
151 |
5.71 (2.76) |
|
2.09 |
296 |
0.04* |
0.24 |
| Mild to Severe Anxiety |
87 |
5.15 (2.66) |
|
212 |
5.47 (2.74) |
|
0.93 |
297 |
0.35 |
0.12 |
| Mild to Severe Depression |
89 |
4.99 (2.79) |
|
210 |
5.55 (2.67) |
|
1.64 |
297 |
0.10 |
0.21 |
| Post-concussion Symptoms |
47 |
5.13 (2.56) |
|
250 |
5.41 (2.76) |
|
-0.64 |
295 |
0.52 |
-0,10 |
Table B2.
Internal Consistency of the QOLIBRI-KID/ADO Scales for Groups of Participants Stratified by Sociodemographic and Clinical Characteristics.
Table B2.
Internal Consistency of the QOLIBRI-KID/ADO Scales for Groups of Participants Stratified by Sociodemographic and Clinical Characteristics.
| Group |
n |
Cognition |
Self |
Daily Life & Autonomy |
Social Relationships |
Emotions |
Physical Problems |
Total score |
| |
|
α |
ω |
α |
ω |
α |
ω |
α |
ω |
α |
ω |
α |
ω |
α |
ω |
| Moderate/Severe TBI |
60 |
0.68 |
0.76 |
0.71 |
0.82 |
0.76 |
0.84 |
0.80 |
0.86 |
0.64 |
0.84 |
0.69 |
0.81 |
0.91 |
0.93 |
| Mild TBI |
240 |
0.70 |
0.80 |
0.77 |
0.83 |
0.72 |
0.81 |
0.76 |
0.86 |
0.74 |
0.76 |
0.72 |
0.83 |
0.91 |
0.92 |
| Problems Post TBI1
|
147 |
0.68 |
0.73 |
0.74 |
0.80 |
0.70 |
0.78 |
0.76 |
0.85 |
0.69 |
0.82 |
0.70 |
0.80 |
0.90 |
0.92 |
| No Problems Post TBI |
151 |
0.69 |
0.78 |
0.78 |
0.83 |
0.75 |
0.80 |
0.78 |
0.85 |
0.77 |
0.83 |
0.69 |
0.80 |
0.91 |
0.93 |
| Mild to Severe Anxiety |
87 |
0.68 |
0.78 |
0.78 |
0.84 |
0.74 |
0.82 |
0.81 |
0.86 |
0.67 |
0.74 |
0.73 |
0.83 |
0.91 |
0.93 |
| No Anxiety2
|
212 |
0.66 |
0.75 |
0.73 |
0.80 |
0.70 |
0.80 |
0.72 |
0.82 |
0.74 |
0.80 |
0.68 |
0.80 |
0.89 |
0.91 |
| Mild to Severe Depression |
89 |
0.67 |
0.75 |
0.83 |
0.88 |
0.73 |
0.82 |
0.78 |
0.84 |
0.62 |
0.77 |
0.73 |
0.85 |
0.91 |
0.93 |
| No Depression3
|
210 |
0.65 |
0.75 |
0.69 |
0.81 |
0.70 |
0.80 |
0.75 |
0.84 |
0.76 |
0.82 |
0.68 |
0.81 |
0.89 |
0.91 |
| Post-Concussion Symptoms4
|
47 |
0.52 |
0.71 |
0.73 |
0.85 |
0.63 |
0.77 |
0.71 |
0.85 |
0.64 |
0.75 |
0.70 |
0.82 |
0.87 |
0.89 |
| No Post-Concussion Symptoms |
250 |
0.64 |
0.77 |
0.70 |
0.80 |
0.69 |
0.78 |
0.73 |
0.80 |
0.73 |
0.81 |
0.63 |
0.79 |
0.87 |
0.90 |
| Low Learning Rate5
|
57 |
0.75 |
0.85 |
0.82 |
0.89 |
0.79 |
0.84 |
0.78 |
0.84 |
0.65 |
0.76 |
0.75 |
0.87 |
0.93 |
0.95 |
| High Learning Rate |
242 |
0.67 |
0.76 |
0.74 |
0.80 |
0.71 |
0.79 |
0.77 |
0.86 |
0.74 |
0.79 |
0.71 |
0.83 |
0.90 |
0.92 |
Table B3.
Correlations of the QOLIBRI-KID/ADO with the PedsQL, PHQ-9, GAD-7, and PCSI-SR8/SR13: Convergent and Divergent Validity Results (Variance Explained).
Table B3.
Correlations of the QOLIBRI-KID/ADO with the PedsQL, PHQ-9, GAD-7, and PCSI-SR8/SR13: Convergent and Divergent Validity Results (Variance Explained).
| |
|
PedsQL |
PHQ-9 |
GAD-7 |
PCSI-SR8/SR13 |
| |
|
Emotional Functioning |
Social Functioning |
School Functioning |
Physical Functioning |
Psychosocial Functioning |
Total Score |
Total Score |
Total Score |
Total Score |
| QOLIBRI-KID/ADO |
Cognition |
20% |
21% |
39% |
27% |
39% |
40% |
18% |
9% |
26% |
| Self |
21% |
11% |
29% |
22% |
31% |
32% |
11% |
5% |
31% |
| Daily Life & Autonomy |
22% |
26% |
30% |
34% |
39% |
43% |
17% |
8% |
22% |
| Social Relationships |
24% |
25% |
27% |
23% |
38% |
37% |
10% |
6% |
18% |
| Emotions |
18% |
9% |
14% |
14% |
21% |
21% |
4% |
3% |
9% |
| Physical Problems |
14% |
10% |
24% |
31% |
23% |
30% |
10% |
6% |
20% |
| Psychosocial Functioning |
33% |
23% |
39% |
32% |
48% |
49% |
14% |
8% |
30% |
| Total Score |
34% |
26% |
44% |
42% |
52% |
56% |
17% |
10% |
34% |
References
- Araki, T.; Yokota, H.; Morita, A. Pediatric Traumatic Brain Injury: Characteristic Features, Diagnosis, and Management. Neurol. Med. Chir.(Tokyo) 2017, 57, 82–93. [Google Scholar] [CrossRef]
- Gardner, M.T.; O’Meara, A.M.I.; Miller Ferguson, N. Pediatric Traumatic Brain Injury: An Update on Management. Curr Pediatr Rep 2017, 5, 213–219. [Google Scholar] [CrossRef]
- Dewan, M.C.; Mummareddy, N.; Wellons, J.C.; Bonfield, C.M. Epidemiology of Global Pediatric Traumatic Brain Injury: Qualitative Review. World Neurosurgery 2016, 91, 497–509. [Google Scholar] [CrossRef]
- Martinez, A.P.; Scherer, M.J.; Tozser, T. Traumatic Brain Injury (TBI) in School-Based Populations: Common Sequelae and Assistive Technology Interventions. Adv Neurodev Disord 2018, 2, 310–321. [Google Scholar] [CrossRef]
- Ramos-Usuga, D.; Benito-Sánchez, I.; Pérez-Delgadillo, P.; Valdivia-Tangarife, R.; Villaseñor-Cabrera, T.; Olabarrieta-Landa, L.; Arango-Lasprilla, J.C. Trajectories of Neuropsychological Functioning in Mexican Children with Traumatic Brain Injury over the First Year after Injury. NeuroRehabilitation 2019, 45, 295–309. [Google Scholar] [CrossRef]
- Babikian, T.; Merkley, T.; Savage, R.C.; Giza, C.C.; Levin, H. Chronic Aspects of Pediatric Traumatic Brain Injury: Review of the Literature. Journal of Neurotrauma 2015, 32, 1849–1860. [Google Scholar] [CrossRef]
- World Health Organization. Division of Mental Health. WHOQOL-BREF: Introduction, Administration, Scoring and Generic Version of the Assessment: Field Trial Version; World Health Organization: Geneva, 1996; pp. 1–18. [Google Scholar]
- Ravens-Sieberer, U.; Erhart, M.; Wille, N.; Wetzel, R.; Nickel, J.; Bullinger, M. Generic Health-Related Quality-of-Life Assessment in Children and Adolescents. Pharmacoeconomics 2006, 24, 1199–1220. [Google Scholar] [CrossRef] [PubMed]
- Solans, M.; Pane, S.; Estrada, M.-D.; Serra-Sutton, V.; Berra, S.; Herdman, M.; Alonso, J.; Rajmil, L. Health-Related Quality of Life Measurement in Children and Adolescents: A Systematic Review of Generic and Disease-Specific Instruments. Value in Health 2008, 11, 742–764. [Google Scholar] [CrossRef]
- von Steinbuechel, N.; Covic, A.; Polinder, S.; Kohlmann, T.; Cepulyte, U.; Poinstingl, H.; Backhaus, J.; Bakx, W.; Bullinger, M.; Christensen, A.-L.; et al. Assessment of Health-Related Quality of Life after TBI: Comparison of a Disease-Specific (QOLIBRI) with a Generic (SF-36) Instrument. Behavioural Neurology 2016, 2016, 1–14. [Google Scholar] [CrossRef]
- Wiebe, S.; Guyatt, G.; Weaver, B.; Matijevic, S.; Sidwell, C. Comparative Responsiveness of Generic and Specific Quality-of-Life Instruments. J Clin Epidemiol 2003, 56, 52–60. [Google Scholar] [CrossRef]
- Von Steinbuechel, N.; Zeldovich, M.; Greving, S.; Olabarrieta-Landa, L.; Krenz, U.; Timmermann, D.; Koerte, I.K.; Bonfert, M.V.; Berweck, S.; Kieslich, M.; et al. Quality of Life after Brain Injury in Children and Adolescents (QOLIBRI-KID/ADO)—The First Disease-Specific Self-Report Questionnaire after Traumatic Brain Injury. JCM 2023, 12, 4898. [Google Scholar] [CrossRef] [PubMed]
- Zeldovich, M.; Cunitz, K.; Greving, S.; Muehlan, H.; Bockhop, F.; Krenz, U.; Timmermann, D.; Koerte, I.K.; Rojczyk, P.; Roediger, M.; et al. Psychometric Properties of the German Version of the Quality of Life after Brain Injury Scale for Kids and Adolescents (QOLIBRI-KID/ADO) Using Item Response Theory Framework: Results from the Pilot Study. JCM 2023, 12, 3716. [Google Scholar] [CrossRef]
- Varni, J.W.; Seid, M.; Rode, C.A. The PedsQL: Measurement Model for the Pediatric Quality of Life Inventory. Med Care 1999, 37, 126–139. [Google Scholar] [CrossRef]
- Michel, G.; Bisegger, C.; Fuhr, D.C.; Abel, T. ; The KIDSCREEN group Age and Gender Differences in Health-Related Quality of Life of Children and Adolescents in Europe: A Multilevel Analysis. Qual Life Res 2009, 18, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Baumgarten, F.; Cohrdes, C.; Schienkiewitz, A.; Thamm, R.; Meyrose, A.-K.; Ravens-Sieberer, U. Gesundheitsbezogene Lebensqualität und Zusammenhänge mit chronischen Erkrankungen und psychischen Auffälligkeiten bei Kindern und Jugendlichen: Ergebnisse aus KiGGS Welle 2. Bundesgesundheitsbl 2019, 62, 1205–1214. [Google Scholar] [CrossRef]
- Riemann, L.; Voormolen, D.C.; Rauen, K.; Zweckberger, K.; Unterberg, A.; Younsi, A. ; the Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) Investigators and Participants Persistent Postconcussive Symptoms in Children and Adolescents with Mild Traumatic Brain Injury Receiving Initial Head Computed Tomography. Journal of Neurosurgery: Pediatrics 2021, 27, 538–547. [Google Scholar] [CrossRef]
- Di Battista, A.; Soo, C.; Catroppa, C.; Anderson, V. Quality of Life in Children and Adolescents Post-TBI: A Systematic Review and Meta-Analysis. J Neurotrauma 2012, 29, 1717–1727. [Google Scholar] [CrossRef]
- Russell, K.; Selci, E.; Chu, S.; Fineblit, S.; Ritchie, L.; Ellis, M.J. Longitudinal Assessment of Health-Related Quality of Life Following Adolescent Sports-Related Concussion. Journal of Neurotrauma 2017, 34, 2147–2153. [Google Scholar] [CrossRef]
- Fineblit, S.; Selci, E.; Loewen, H.; Ellis, M.; Russell, K. Health-Related Quality of Life after Pediatric Mild Traumatic Brain Injury/Concussion: A Systematic Review. Journal of Neurotrauma 2016, 33, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Anderson, V.; Brown, S.; Newitt, H.; Hoile, H. Long-Term Outcome from Childhood Traumatic Brain Injury: Intellectual Ability, Personality, and Quality of Life. Neuropsychology 2011, 25, 176–184. [Google Scholar] [CrossRef]
- Connolly, E.J.; McCormick, B.F. Mild Traumatic Brain Injury and Psychopathology in Adolescence: Evidence From the Project on Human Development in Chicago Neighborhoods. Journal of Adolescent Health 2019, 65, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Di Battista, A.; Godfrey, C.; Soo, C.; Catroppa, C.; Anderson, V. Depression and Health Related Quality of Life in Adolescent Survivors of a Traumatic Brain Injury: A Pilot Study. PLoS ONE 2014, 9, e101842. [Google Scholar] [CrossRef]
- Mundfrom, D.J.; Shaw, D.G. ; Tian Lu Ke Minimum Sample Size Recommendations for Conducting Factor Analyses. International Journal of Testing 2005, 5, 159–168. [Google Scholar] [CrossRef]
- von Steinbuechel, N.; Wilson, L.; Gibbons, H.; Hawthorne, G.; Höfer, S.; Schmidt, S.; Bullinger, M.; Maas, A.; Neugebauer, E.; Powell, J.; et al. Quality of Life after Brain Injury (QOLIBRI): Scale Development and Metric Properties. Journal of Neurotrauma 2010, 27, 1167–1185. [Google Scholar] [CrossRef]
- von Steinbuechel, N.; Wilson, L.; Gibbons, H.; Hawthorne, G.; Höfer, S.; Schmidt, S.; Bullinger, M.; Maas, A.; Neugebauer, E.; Powell, J.; et al. Quality of Life after Brain Injury (QOLIBRI): Scale Validity and Correlates of Quality of Life. Journal of Neurotrauma 2010, 27, 1157–1165. [Google Scholar] [CrossRef]
- Teasdale, G.; Jennett, B. Assessment of Coma and Impaired Consciousness. A Practical Scale. Lancet 1974, 2, 81–84. [Google Scholar] [CrossRef]
- World Health Organization ICD-10: International Statistical Classification of Diseases and Related Health Problems, 10th Revision, 2nd Ed. 2004.
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.W.; Löwe, B. A Brief Measure for Assessing Generalized Anxiety Disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L. The PHQ-9: A New Depression Diagnostic and Severity Measure. Psychiatric Annals 2002, 32, 509–515. [Google Scholar] [CrossRef]
- Löwe, B.; Decker, O.; Müller, S.; Brähler, E.; Schellberg, D.; Herzog, W.; Herzberg, P.Y. Validation and Standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the General Population. Medical Care 2008, 46, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Allgaier, A.-K.; Pietsch, K.; Frühe, B.; Sigl-Glöckner, J.; Schulte-Körne, G. Screening for Depression in Adolescents: Validity of the Patient Health Questionnaire in Pediatric Care. Depression and Anxiety 2012, 29, 906–913. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, 4th Ed (DSM-IV) 1994.
- Sequeira, S.L.; Morrow, K.E.; Silk, J.S.; Kolko, D.J.; Pilkonis, P.A.; Lindhiem, O. National Norms and Correlates of the PHQ-8 and GAD-7 in Parents of School-Age Children. J Child Fam Stud 2021, 30, 2303–2314. [Google Scholar] [CrossRef] [PubMed]
- Richardson, L.P.; McCauley, E.; Grossman, D.C.; McCarty, C.A.; Richards, J.; Russo, J.E.; Rockhill, C.; Katon, W. Evaluation of the Patient Health Questionnaire (PHQ-9) for Detecting Major Depression among Adolescents. Pediatrics 2010, 126, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Sady, M.D.; Vaughan, C.G.; Gioia, G.A. Psychometric Characteristics of the Postconcussion Symptom Inventory in Children and Adolescents. Archives of Clinical Neuropsychology 2014, 29, 348–363. [Google Scholar] [CrossRef]
- Zeldovich, M.; Krol, L.; Timmermann, D.; Krenz, U.; Arango-Lasprilla, J.C.; Gioia, G.; Brockmann, K.; Koerte, I.K.; Buchheim, A.; Roediger, M.; et al. Psychometric Evaluation and Reference Values for the German Postconcussion Symptom Inventory (PCSI-SR8) in Children Aged 8–12 Years. Frontiers in Neurology 2023, 14. [Google Scholar] [CrossRef]
- Crouchman, M. A Practical Outcome Scale for Paediatric Head Injury. Archives of Disease in Childhood 2001, 84, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Müller, H.; Hasse-Sander, I.; Horn, R.; Helmstaedter, C.; Elger, C.E. Rey Auditory-Verbal Learning Test: Structure of a Modified German Version. Journal of Clinical Psychology 1997, 53, 663–671. [Google Scholar] [CrossRef]
- Strauss, E.; Sherman, E.M.; Spreen, O. Rey Auditory Verbal Learning Test (RAVLT). In A compendium of neuropsychological tests - administration, norms, and commentary; Oxford University Press: New York, NY, 2006; pp. 776–810. [Google Scholar]
- Bulmer, M.G. Principles of Statistics; Dover Publications: New York, 1979; ISBN 978-0-486-63760-0. [Google Scholar]
- Gorbunova, A.; Zeldovich, M.; Voormolen, D.C.; Krenz, U.; Polinder, S.; Haagsma, J.A.; Hagmayer, Y.; Covic, A.; Real, R.G.L.; Asendorf, T.; et al. Reference Values of the QOLIBRI from General Population Samples in the United Kingdom and The Netherlands. JCM 2020, 9, 2100. [Google Scholar] [CrossRef]
- Zumbo, B. A Handbook on the Theory and Methods of Differential Item Functioning (DIF): Logistic Regression Modeling as a Unitary Framework for Binary and Likert-Type (Ordinal) Item Scores; Directorate of Human Resources Research and Evaluation, Department of National Defense: Ottawa, ON, 1999. [Google Scholar]
- Kirk, R.E. Practical Significance: A Concept Whose Time Has Come. Educational and Psychological Measurement 1996, 56, 746–759. [Google Scholar] [CrossRef]
- Hadianfard, H.; Kiani, B.; Azizzadeh Herozi, M.; Mohajelin, F.; Mitchell, J.T. Health-Related Quality of Life in Iranian Adolescents: A Psychometric Evaluation of the Self-Report Form of the PedsQL 4.0 and an Investigation of Gender and Age Differences. Health Qual Life Outcomes 2021, 19, 108. [Google Scholar] [CrossRef] [PubMed]
- von Steinbuechel, N.; Richter, S.; Morawetz, C.; Riemsma, R. Assessment of Subjective Health and Health-Related Quality of Life in Persons with Acquired or Degenerative Brain Injury. Curr. Opin. Neurol. 2005, 18, 681–691. [Google Scholar] [CrossRef]
- Janssens, L.; Gorter, J.W.; Ketelaar, M.; Kramer, W.L.M.; Holtslag, H.R. Health-Related Quality-of-Life Measures for Long-Term Follow-up in Children after Major Trauma. Qual Life Res 2008, 17, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Harvill, L.M. An NCME Instructional Module on. Standard Error of Measurement. Educational Measure: Issues Practice 1991, 10, 33–41. [Google Scholar] [CrossRef]
- van Baalen, B.; Odding, E.; van Woensel, M.P.; van Kessel, M.A.; Roebroeck, M.E.; Stam, H.J. Reliability and Sensitivity to Change of Measurement Instruments Used in a Traumatic Brain Injury Population. Clin Rehabil 2006, 20, 686–700. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Millsap, R.E.; West, S.G.; Tein, J.-Y.; Tanaka, R.; Grimm, K.J. Testing Measurement Invariance in Longitudinal Data with Ordered-Categorical Measures. Psychological Methods 2017, 22, 486–506. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Yang, Y. RMSEA, CFI, and TLI in Structural Equation Modeling with Ordered Categorical Data: The Story They Tell Depends on the Estimation Methods. Behav Res 2019, 51, 409–428. [Google Scholar] [CrossRef]
- Cole, D.A. Utility of Confirmatory Factor Analysis in Test Validation Research. Journal of Consulting and Clinical Psychology 1987, 55, 584–594. [Google Scholar] [CrossRef]
- Bentler, P.M. Comparative Fit Indexes in Structural Models. Psychological bulletin 1990, 107, 238–246. [Google Scholar] [CrossRef]
- Hu, L.; Bentler, P.M. Cutoff Criteria for Fit Indexes in Covariance Structure Analysis: Conventional Criteria versus New Alternatives. Structural Equation Modeling: A Multidisciplinary Journal 1999, 6, 1–55. [Google Scholar] [CrossRef]
- Bentler, P.; Bonett, D. Significance Tests and Goodness-of-Fit in Analysis of Covariance Structures. Psychological Bulletin 1980, 88, 588–606. [Google Scholar] [CrossRef]
- Steiger, J.H. Notes on the Steiger–Lind (1980) Handout. Structural Equation Modeling: A Multidisciplinary Journal 2016, 23, 777–781. [Google Scholar] [CrossRef]
- Davidson, M. Known-Groups Validity. In Encyclopedia of Quality of Life and Well-Being Research; Michalos, A.C., Ed.; Springer: Dordrecht, 2014. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; 2nd ed.; L. Erlbaum Associates: Hillsdale, N.J, 1988; ISBN 978-0-8058-0283-2.
- Varni, J.W. PedsQL TM (Pediatric Quality of Life Inventory TM) Available online:. Available online: https://www.pedsql.org/score.html (accessed on 6 July 2022).
- R Core Team R: A Language and Environment for Statistical Computing 2021.
- Revelle, W. An Introduction to the Psych Package: Part II Scale Construction and Psychometrics. 96.
- Rosseel, Y. Lavaan: An R Package for Structural Equation Modeling. J. Stat. Soft. 2012, 48. [Google Scholar] [CrossRef]
- Choi, S.; Gibbons, L.; Crane, P. Lordif: An R Package for Detecting Differential Item Functioning Using Iterative Hybrid Ordinal Logistic Regression/Item Response Theory and Monte Carlo Simulations. Journal of statistical software 2011, 39, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Lefevre-Dognin, C.; Cogné, M.; Perdrieau, V.; Granger, A.; Heslot, C.; Azouvi, P. Definition and Epidemiology of Mild Traumatic Brain Injury. Neurochirurgie 2021, 67, 218–221. [Google Scholar] [CrossRef] [PubMed]
- von Steinbüchel-Rheinwall, N.; Backhaus, J. Erhebung gesundheitsbezogener Lebensqualität: Gegenwärtiger Stand und Perspektiven. Zeitschrift für Epileptologie 2015, 28, 102–110. [Google Scholar] [CrossRef]
- Varni, J.W.; Limbers, C.A.; Burwinkle, T.M. How Young Can Children Reliably and Validly Self-Report Their Health-Related Quality of Life?: An Analysis of 8,591 Children across Age Subgroups with the PedsQLTM 4.0 Generic Core Scales. Health Qual Life Outcomes 2007, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Krenz, U.; Timmermann, D.; Gorbunova, A.; Lendt, M.; Schmidt, S.; von Steinbuechel, N. Health-Related Quality of Life after Pediatric Traumatic Brain Injury: A Qualitative Comparison between Children’s and Parents’ Perspectives. PLoS ONE 2021, 16, e0246514. [Google Scholar] [CrossRef] [PubMed]
- Holmbeck, G.N.; Thill, A.W.; Bachanas, P.; Garber, J.; Miller, K.B.; Abad, M.; Bruno, E.F.; Carter, J.S.; David-Ferdon, C.; Jandasek, B.; et al. Evidence-Based Assessment in Pediatric Psychology: Measures of Psychosocial Adjustment and Psychopathology. Journal of Pediatric Psychology 2008, 33, 958–980. [Google Scholar] [CrossRef] [PubMed]
- McCauley, S.R.; Wilde, E.A.; Anderson, V.A.; Bedell, G.; Beers, S.R.; Campbell, T.F.; Chapman, S.B.; Ewing-Cobbs, L.; Gerring, J.P.; Gioia, G.A.; et al. Recommendations for the Use of Common Outcome Measures in Pediatric Traumatic Brain Injury Research. J Neurotrauma 2012, 29, 678–705. [Google Scholar] [CrossRef]
- Pinquart, M. Health-Related Quality of Life of Young People With and Without Chronic Conditions. Journal of Pediatric Psychology 2020, 45, 780–792. [Google Scholar] [CrossRef]
- Babikian, T.; Asarnow, R. Neurocognitive Outcomes and Recovery after Pediatric TBI: Meta-Analytic Review of the Literature. Neuropsychology 2009, 23, 283–296. [Google Scholar] [CrossRef]
- Bradbury, K.R.; Williams, C.; Leonard, S.; Holding, E.; Turner, E.; Wagner, A.E.; Piantino, J.; Luther, M.; Hall, T.A. Emotional Aspects of Pediatric Post-Intensive Care Syndrome Following Traumatic Brain Injury. Journ Child Adol Trauma 2021, 14, 177–187. [Google Scholar] [CrossRef]
- Haugland, S.; Wold, B.; Stevenson, J.; Aaroe, L.E.; Woynarowska, B. Subjective Health Complaints in Adolescence. European Journal of Public Health 2001, 11, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Proctor, C.L.; Linley, P.A.; Maltby, J. Youth Life Satisfaction: A Review of the Literature. Journal of Happiness Studies 2008, 10, 583–630. [Google Scholar] [CrossRef]
- Otto, C.; Reiss, F.; Voss, C.; Wüstner, A.; Meyrose, A.-K.; Hölling, H.; Ravens-Sieberer, U. Mental Health and Well-Being from Childhood to Adulthood: Design, Methods and Results of the 11-Year Follow-up of the BELLA Study. Eur Child Adolesc Psychiatry 2021, 30, 1559–1577. [Google Scholar] [CrossRef] [PubMed]
- Meade, T.; Dowswell, E. Health-Related Quality of Life in a Sample of Australian Adolescents: Gender and Age Comparison. Qual Life Res 2015, 24, 2933–2938. [Google Scholar] [CrossRef]
- Freire, T.; Ferreira, G. Health-Related Quality of Life of Adolescents: Relations with Positive and Negative Psychological Dimensions. International Journal of Adolescence and Youth 2018, 23, 11–24. [Google Scholar] [CrossRef]
- Gaspar, T.; de Matos, M.G.; Ribeiro, J.L.P.; Leal, I.; Costa, P.; Erhart, M.; Ravens-Sieberer, U. Quality of Life: Differences Related to Gender, Age, Socio-Economic Status and Health Status in Portugese Teens. Journal of Clinical Child & Adolescent Psychology 2010, 2, 87–104. [Google Scholar]
- Pfeifer, J.H.; Allen, N.B. Puberty Initiates Cascading Relationships Between Neurodevelopmental, Social, and Internalizing Processes Across Adolescence. Biological Psychiatry 2021, 89, 99–108. [Google Scholar] [CrossRef]
- Hampel, P. Brief Report: Coping among Austrian Children and Adolescents. Journal of Adolescence 2007, 30, 885–890. [Google Scholar] [CrossRef]
- Steyerberg, E.W.; Wiegers, E.; Sewalt, C.; Buki, A.; Citerio, G.; De Keyser, V.; Ercole, A.; Kunzmann, K.; Lanyon, L.; Lecky, F.; et al. Case-Mix, Care Pathways, and Outcomes in Patients with Traumatic Brain Injury in CENTER-TBI: A European Prospective, Multicentre, Longitudinal, Cohort Study. The Lancet Neurology 2019, 18, 923–934. [Google Scholar] [CrossRef]
- AWMF. Das Schädel-Hirn-Trauma Im Kindes- Und Jugendalter. Available online: https://www.awmf.org/leitlinien/detail/ll/024-018.html (accessed on 11 March 2022).
- Torsheim, T.; Ravens-Sieberer, U.; Hetland, J.; Välimaa, R.; Danielson, M.; Overpeck, M. Cross-National Variation of Gender Differences in Adolescent Subjective Health in Europe and North America. Social Science & Medicine 2006, 62, 815–827. [Google Scholar] [CrossRef]
- van Wijk, G.; Kolk, A.M.; van den Bosch, W.J.H.M.; van den Hoogen, H.J.M. Male and Female Health Problems in General Practice: The Differential Impact of Social Position and Social Roles. Social Science & Medicine 1995, 40, 597–611. [Google Scholar]
- Gutman, L.M.; Codiroli McMaster, N. Gendered Pathways of Internalizing Problems from Early Childhood to Adolescence and Associated Adolescent Outcomes. J Abnorm Child Psychol 2020, 48, 703–718. [Google Scholar] [CrossRef]
- Ryan, N.P.; Noone, K.; Godfrey, C.; Botchway, E.N.; Catroppa, C.; Anderson, V. Young Adults’ Perspectives on Health-Related Quality of Life after Paediatric Traumatic Brain Injury: A Prospective Cohort Study. Ann Phys Rehabil Med 2019, 62, 342–350. [Google Scholar] [CrossRef]
- Horneman, G.; Folkesson, P.; Sintonen, H.; von Wendt, L.; Emanuelson, I. Health-Related Quality of Life of Adolescents and Young Adults 10 Years after Serious Traumatic Brain Injury. Int J Rehabil Res 2005, 28, 245–249. [Google Scholar] [CrossRef]
- Andruszkow, H.; Deniz, E.; Urner, J.; Probst, C.; Grün, O.; Lohse, R.; Frink, M.; Krettek, C.; Zeckey, C.; Hildebrand, F. Physical and Psychological Long-Term Outcome after Traumatic Brain Injury in Children and Adult Patients. Health and Quality of Life Outcomes 2014, 12, 26. [Google Scholar] [CrossRef]
- Babikian, T. Contextual Considerations for the Increased Risk of Mental Health Problems Following Concussion in Youth. JAMA Netw Open 2022, 5, e221242. [Google Scholar] [CrossRef]
- Pagulayan, K.F.; Hoffman, J.M.; Temkin, N.R.; Machamer, J.E.; Dikmen, S.S. Functional Limitations and Depression After Traumatic Brain Injury: Examination of the Temporal Relationship. Archives of Physical Medicine and Rehabilitation 2008, 89, 1887–1892. [Google Scholar] [CrossRef] [PubMed]
- Albicini, M.; McKinlay, A. A Systematic Review of Anxiety Disorders Following Mild, Moderate and Severe TBI in Children and Adolescents. In A Fresh Look at Anxiety Disorders; Durbano, F., Ed.; InTech, 2015 ISBN 978-953-51-2149-7.
- Fried, E.; Balla, U.; Catalogna, M.; Kozer, E.; Oren-Amit, A.; Hadanny, A.; Efrati, S. Persistent Post-Concussive Syndrome in Children after Mild Traumatic Brain Injury Is Prevalent and Vastly Underdiagnosed. Sci Rep 2022, 12, 4364. [Google Scholar] [CrossRef]
- Pagulayan, K.F.; Temkin, N.R.; Machamer, J.; Dikmen, S.S. A Longitudinal Study of Health-Related Quality of Life After Traumatic Brain Injury. Archives of Physical Medicine and Rehabilitation 2006, 87, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Lieu, J.E.C.; Dewan, K. Assessment of Self-Selection Bias in a Pediatric Unilateral Hearing Loss Study. Otolaryngol Head Neck Surg 2010, 142, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A. Retraumatization and Social Sciences Research Available online:. Available online: https://files.osf.io/v1/resources/rvksp/providers/osfstorage/622b7ba346bf1002635939d5?action=download&direct&version=2 (accessed on 15 February 2024).
- Berry, V. Ethical Considerations in Conducting Family Violence Research. Research Ethics 2009, 5, 91–100. [Google Scholar] [CrossRef]
- Cunitz, K.; Holloway, I.; Harzendorf, A.; Greving, S.; Zeldovich, M.; Krenz, U.; Timmermann, D.; Koerte, I.K.; Bonfert, M.V.; Berweck, S.; et al. Health-Related Quality of Life after Pediatric Traumatic Brain Injury: A Quantitative Comparison between Children’s and Parents’ Perspectives of the QOLIBRI-KID/ADO Questionnaire. Journal of Clinical Medicine. 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Pieper, P.; Garvan, C. Concordance of Child and Parent Reports of Health-Related Quality of Life in Children With Mild Traumatic Brain or Non-Brain Injuries and in Uninjured Children: Longitudinal Evaluation. Journal of Pediatric Health Care 2015, 29, 343–351. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Flow Chart of the Recruitment Process and the Number of Participants per Instrument. Note. TBI = Traumatic brain injury; QOLIBRI-KID/ADO = Quality Of Life after Brain Injury in Children and Adolescents; PedsQL = Pediatric Quality of Life Inventory; GAD-7 = Generalized Anxiety Disorder 7; PHQ-9 = Patient Health Questionnaire 9; PCSI-SR8/13 = Postconcussion Symptom Inventory; RAVLT = Rey Auditory Verbal Learning Test.
Figure 1.
Flow Chart of the Recruitment Process and the Number of Participants per Instrument. Note. TBI = Traumatic brain injury; QOLIBRI-KID/ADO = Quality Of Life after Brain Injury in Children and Adolescents; PedsQL = Pediatric Quality of Life Inventory; GAD-7 = Generalized Anxiety Disorder 7; PHQ-9 = Patient Health Questionnaire 9; PCSI-SR8/13 = Postconcussion Symptom Inventory; RAVLT = Rey Auditory Verbal Learning Test.
Figure 2.
Results of the Confirmatory Factor Analysis of the QOLIBRI-KID/ADO Questionnaire. Note: Standardized coefficients. A1−7: Cognition (6 items: Concentration, Talking to Others, Remembering, Planning, Decision Between Two Things, Orientation, Thinking Speed), B1−5: Self (5 items: Energy, Accomplishment, Appearance, Self-Esteem, Future), C1−7: Daily Life and Autonomy (7 items: Daily Independence, Getting out and About, Manage at School, Social Activities, Decision Making, Support from Others, Ability to Move), D1−6: Social Relationships (6 items: Open up to Others, Family Relationship, Relationship with Friends, Attitudes of Others, Demands from Others), E1−4: Emotions (4 items: Loneliness, Anxiety, Sadness, Anger), F1−6: Physical Problems (6 items: Clumsiness, Other Injuries, Headaches, Pain, Seeing/Hearing, TBI Effects).
Figure 2.
Results of the Confirmatory Factor Analysis of the QOLIBRI-KID/ADO Questionnaire. Note: Standardized coefficients. A1−7: Cognition (6 items: Concentration, Talking to Others, Remembering, Planning, Decision Between Two Things, Orientation, Thinking Speed), B1−5: Self (5 items: Energy, Accomplishment, Appearance, Self-Esteem, Future), C1−7: Daily Life and Autonomy (7 items: Daily Independence, Getting out and About, Manage at School, Social Activities, Decision Making, Support from Others, Ability to Move), D1−6: Social Relationships (6 items: Open up to Others, Family Relationship, Relationship with Friends, Attitudes of Others, Demands from Others), E1−4: Emotions (4 items: Loneliness, Anxiety, Sadness, Anger), F1−6: Physical Problems (6 items: Clumsiness, Other Injuries, Headaches, Pain, Seeing/Hearing, TBI Effects).
Figure 3.
Correlation Results of Convergent and Divergent Validity Analyses. Note: Pearson correlation coefficients.
Figure 3.
Correlation Results of Convergent and Divergent Validity Analyses. Note: Pearson correlation coefficients.
Table 1.
Descriptive Statistics for Demographic and Clinical Characteristics of Participants of the Final Validation Study.
Table 1.
Descriptive Statistics for Demographic and Clinical Characteristics of Participants of the Final Validation Study.
| Variable |
Group |
N (%) |
| Sex |
Female |
137 (46%) |
| Male |
163 (54%) |
| Age |
Children (8–12 years) |
167 (56%) |
| Adolescents (13–17 years) |
133 (44%) |
| Parents’ highest education |
Primary school |
1 (0%) |
| Secondary school |
51 (17%) |
| Vocational school |
54 (18%) |
| College/university |
193 (64%) |
| Unknown or missing |
1 (0%) |
| TBI Severity |
Mild |
240 (80%) |
| Moderate |
30 (10%) |
| Severe |
30 (10%) |
| Cerebral Lesion(s) Found in Neuroimaging |
No |
227 (76%) |
| Yes |
73 (24%) |
| KOSCHI Disability/ Recovery Score |
4b |
15 (5%) |
| 5a |
21 (7%) |
| 5b |
261 (87%) |
| Missing |
3 (1%) |
| Time Since TBI |
<1 Year |
15 (5%) |
| 1–<2 Years |
30 (10%) |
| 2–<4 Years |
55 (18%) |
| 4–10 Years |
200 (67%) |
| Head-/aches, Physical Impairments, or Mental Health Problems after TBI |
No |
151 (50%) |
| Yes |
147 (49%) |
| Missing |
2 (1%) |
| Learning Rate (RAVLT) |
Below average (≤ M – 1SD) |
57 (19%) |
| Average |
177 (59%) |
| Above average (≥ M + 1SD) |
65 (22%) |
| Missing |
1 (0%) |
| GAD-7 |
No anxiety (0) |
46 (15%) |
| Minimal anxiety (1−4) |
166 (55%) |
| Mild to severe anxiety (> 4) |
87 (29%) |
| Missing |
1 (0%) |
| PHQ-9 |
No depression (0) |
34 (11%) |
| Minimal depression (1−4) |
176 (59%) |
| Mild to severe depression (≥ 5) |
89 (30%) |
| Missing |
1 (0%) |
| PCSI-SR8/13 |
Below average (≤ M – 1SD) |
26 (9%) |
| Average |
224 (75%) |
| Above average (≥ M + 1SD) |
47 (16%) |
| Missing |
3 (1%) |
Table 2.
Item and Scale Statistics of the QOLIBRI-KID/ADO questionnaire: Descriptive Statistics, Internal Consistency Parameters, and DIF-Analyses.
Table 2.
Item and Scale Statistics of the QOLIBRI-KID/ADO questionnaire: Descriptive Statistics, Internal Consistency Parameters, and DIF-Analyses.
| |
|
|
Descriptive Statistics |
Internal
Consistency |
CITC |
DIF
Analyses |
| Scale |
Item Abbreviation |
Items |
M |
SD |
%
Missing |
SK |
%
Floor |
%
Ceiling |
α |
Changes
in α if Omitted |
ω |
|
p |
R² |
| Cognition |
|
|
|
|
|
|
|
|
0.69 |
|
0.79 |
|
|
|
| A1 |
Concentration |
3.91 |
0.85 |
1 |
-0.63 |
5 |
72 |
|
-0.07 |
|
0.64 |
0.754 |
|
| A2 |
Talking to Others |
4.45 |
0.69 |
0 |
-0.99 |
1 |
90 |
|
-0.03 |
|
0.46 |
0.153 |
|
| A3 |
Remembering |
3.91 |
0.92 |
1 |
-0.57 |
8 |
70 |
|
-0.03 |
|
0.49 |
0.035 |
|
| A4 |
Planning |
4.06 |
0.96 |
0 |
-0.90 |
7 |
75 |
|
-0.04 |
|
0.51 |
0.003* |
0.015 |
| A5 |
Decision Between Two Things |
3.45 |
1.06 |
0 |
-0.41 |
17 |
51 |
|
-0.01 |
|
0.37 |
0.016 |
|
| A6 |
Orientation |
4.79 |
0.49 |
0 |
-2.70 |
1 |
97 |
|
0.00 |
|
0.27 |
0.212 |
|
| A7 |
Thinking Speed |
3.97 |
0.81 |
0 |
-0.55 |
3 |
74 |
|
-0.08 |
|
0.70 |
0.164 |
|
| Self |
|
|
|
|
|
|
|
|
0.76 |
|
0.81 |
|
|
|
| B1 |
Energy |
3.95 |
0.94 |
0 |
-0.88 |
8 |
74 |
|
-0.03 |
|
0.58 |
0.815 |
|
| B2 |
Accomplishment |
4.07 |
0.69 |
0 |
-0.45 |
2 |
83 |
|
-0.03 |
|
0.58 |
0.820 |
|
| B3 |
Appearance |
4.12 |
0.85 |
1 |
-0.88 |
4 |
78 |
|
-0.08 |
|
0.71 |
0.216 |
|
| B4 |
Self-Esteem |
4.26 |
0.87 |
1 |
-1.16 |
3 |
82 |
|
-0.06 |
|
0.67 |
0.098 |
|
| B5 |
Future |
4.13 |
0.9 |
1 |
-0.94 |
5 |
78 |
|
-0.02 |
|
0.53 |
0.163 |
|
| Daily Life & Autonomy |
|
|
|
|
|
|
|
|
0.73 |
|
0.80 |
|
|
|
| C1 |
Daily Independence |
4.76 |
0.51 |
0 |
-2.17 |
0 |
97 |
|
-0.02 |
|
0.47 |
0.096 |
|
| C2 |
Getting Out and About |
4.64 |
0.62 |
0 |
-1.66 |
1 |
94 |
|
-0.05 |
|
0.62 |
0.028 |
|
| C3 |
Manage at school |
4.22 |
0.82 |
1 |
-1.00 |
3 |
83 |
|
-0.04 |
|
0.55 |
0.503 |
|
| C4 |
Social Activities |
4.56 |
0.73 |
0 |
-1.60 |
2 |
90 |
|
-0.04 |
|
0.56 |
0.485 |
|
| C5 |
Decision Making |
4.12 |
0.85 |
0 |
-0.76 |
4 |
79 |
|
-0.02 |
|
0.50 |
0.739 |
|
| C6 |
Support from Others |
4.32 |
0.78 |
0 |
-0.96 |
3 |
86 |
|
-0.02 |
|
0.47 |
0.560 |
|
| C7 |
Ability to Move |
4.7 |
0.7 |
0 |
-2.87 |
2 |
93 |
|
-0.03 |
|
0.52 |
0.161 |
|
| Social Relationships |
|
|
|
|
|
|
|
|
0.77 |
|
0.85 |
|
|
|
| D1 |
Open up to Others |
4.07 |
0.89 |
0 |
-0.81 |
5 |
77 |
|
-0.05 |
|
0.68 |
0.132 |
|
| D2 |
Family Relationship |
4.42 |
0.75 |
0 |
-1.05 |
1 |
87 |
|
0.00 |
|
0.42 |
0.152 |
|
| D3 |
Relationship with Friends |
4.45 |
0.71 |
0 |
-1.41 |
1 |
91 |
|
-0.05 |
|
0.65 |
0.039 |
|
| D4 |
Friendships |
4.53 |
0.69 |
0 |
-1.44 |
2 |
92 |
|
-0.03 |
|
0.57 |
0.103 |
|
| D5 |
Attitudes of Others |
4.22 |
0.76 |
0 |
-0.75 |
2 |
84 |
|
-0.04 |
|
0.63 |
0.359 |
|
| D6 |
Demands from Others |
4.01 |
0.84 |
0 |
-0.95 |
4 |
78 |
|
-0.04 |
|
0.60 |
0.080 |
|
| Emotions |
|
|
|
|
|
|
|
|
0.73 |
|
0.76 |
|
|
|
| E1 |
Loneliness |
4.01 |
1.13 |
1 |
-0.89 |
13 |
70 |
|
0.01 |
|
0.46 |
0.028 |
|
| E2 |
Anxiety |
3.83 |
1.13 |
0 |
-0.81 |
13 |
67 |
|
-0.07 |
|
0.64 |
0.326 |
|
| E3 |
Sadness |
3.39 |
1.24 |
0 |
-0.44 |
24 |
53 |
|
-0.15 |
|
0.77 |
< 0.001* |
0.016 |
| E4 |
Anger |
3.41 |
1.25 |
0 |
-0.28 |
27 |
51 |
|
-0.04 |
|
0.57 |
0.230 |
|
| Physical Problems |
|
|
|
|
|
|
|
|
0.71 |
|
0.83 |
|
|
|
| F1 |
Clumsiness |
3.55 |
1.21 |
0 |
-0.62 |
21 |
60 |
|
-0.01 |
|
0.44 |
0.028 |
|
| F2 |
Other Injuries |
4.13 |
1.24 |
0 |
-1.24 |
13 |
73 |
|
-0.05 |
|
0.57 |
0.007* |
0.014 |
| F3 |
Headaches |
3.49 |
1.41 |
0 |
-0.58 |
26 |
60 |
|
-0.05 |
|
0.57 |
0.222 |
|
| F4 |
Pain |
3.74 |
1.15 |
0 |
-0.64 |
15 |
62 |
|
-0.05 |
|
0.58 |
0.601 |
|
| F5 |
Seeing/Hearing |
4.25 |
1.11 |
0 |
-1.41 |
11 |
80 |
|
-0.05 |
|
0.57 |
0.019 |
|
| F6 |
TBI Effects |
4.34 |
1.01 |
0 |
-1.57 |
7 |
81 |
|
-0.03 |
|
0.50 |
0.380 |
|
| Total score |
|
|
|
|
|
|
|
|
0.91 |
|
0.92 |
|
|
|
Table 3.
Descriptive Statistics of the QOLIBRI-KID/ADO, PedsQL, PHQ-9, GAD-7, and PCSI-SR8/13 Questionnaires.
Table 3.
Descriptive Statistics of the QOLIBRI-KID/ADO, PedsQL, PHQ-9, GAD-7, and PCSI-SR8/13 Questionnaires.
| Instrument |
Scoring |
Scale |
N |
M |
SD |
Min |
Max |
SK |
| QOLIBRI-KID/ADO |
0−100 |
Cognition |
300 |
76.98 |
12.55 |
42.86 |
100.00 |
-0.47 |
| |
Self |
298 |
77.63 |
15.24 |
15.00 |
100.00 |
-1.03 |
| |
Daily Life & Autonomy |
300 |
86.85 |
11.18 |
42.86 |
100.00 |
-1.30 |
| |
Social Relationships |
300 |
82.09 |
13.27 |
29.17 |
100.00 |
-0.98 |
| |
Emotions |
300 |
66.47 |
22.00 |
6.25 |
100.00 |
-0.40 |
| |
Physical Problems
(= Physical Functioning Score) |
300 |
72.96 |
19.16 |
8.33 |
100.00 |
-0.83 |
| |
Psychosocial Functioning Score |
298 |
75.82 |
12.51 |
26.82 |
100.00 |
-0.63 |
| |
Total Score |
298 |
77.21 |
11.71 |
32.46 |
100.00 |
-0.68 |
| PedsQL |
0−100 |
Emotional Functioning |
300 |
72.13 |
18.21 |
28.13 |
100.00 |
-0.73 |
| |
Social Functioning |
299 |
88.23 |
12.53 |
0.00 |
100.00 |
-1.28 |
| |
School Functioning |
299 |
76.90 |
15.34 |
40.00 |
100.00 |
-0.73 |
| |
Physical Functioning
(= Physical Health Summary Score) |
300 |
84.98 |
12.33 |
25.00 |
100.00 |
-1.39 |
| |
Psychosocial Health Summary Score |
299 |
79.11 |
12.55 |
41.67 |
100.00 |
-0.77 |
| |
Total Scale score |
299 |
81.15 |
11.60 |
36.96 |
100.00 |
-0.98 |
| GAD-7 |
0−21 |
Total Score |
299 |
3.77 |
3.30 |
0.00 |
17.00 |
1.87 |
| PHQ-9 |
0−27 |
Total Score |
299 |
3.39 |
3.19 |
0.00 |
22.00 |
1.44 |
| PCSI-SR8 |
0−32 |
Total Score |
165 |
5.48 |
4.92 |
0.00 |
25.00 |
1.46 |
| PCSI-SR13 |
0−126 |
Total Score |
132 |
20.37 |
18.64 |
0.00 |
82.00 |
1.05 |
Table 4.
Test-Retest Reliability of the QOLIBRI-KID/ADO questionnaire for 57 Participants after 10–20 Days.
Table 4.
Test-Retest Reliability of the QOLIBRI-KID/ADO questionnaire for 57 Participants after 10–20 Days.
| Scale |
ICC(2,1) |
SEm |
MDC |
| Cognition |
0.74 (0.59–0.84) |
6.37 |
17.65 |
| Self |
0.76 (0.62–0.85) |
7.03 |
19.50 |
| Daily Life & Autonomy |
0.62 (0.43–0.76) |
5.68 |
15.74 |
| Social Relationships |
0.62 (0.44–0.76) |
6.95 |
19.26 |
| Emotions |
0.67 (0.45–0.80) |
12.59 |
34.91 |
| Physical Problems |
0.57 (0.33–0.73) |
10.55 |
29.25 |
| Total score |
0.78 (0.60–0.87) |
4.82 |
13.35 |
Table 5.
Results of Known-Group Validity Analyses of the QOLIBRI-KID/ADO total Score Sociodemographic and Clinical Characteristics.
Table 5.
Results of Known-Group Validity Analyses of the QOLIBRI-KID/ADO total Score Sociodemographic and Clinical Characteristics.
| Known Groups |
n |
M |
SD |
t |
df |
p |
d |
| Children |
165 |
78.61 |
10.62 |
-2.32 |
296 |
.01* |
-0.27 |
| Adolescents |
133 |
75.47 |
12.76 |
| Female |
137 |
76.02 |
12.76 |
-2.26 |
296 |
.01* |
-0.14 |
| Male |
161 |
78.61 |
10.96 |
| TBI within the last four years |
100 |
76.19 |
11.89 |
-1.07 |
296 |
.14 |
-0.13 |
| TBI more than four years ago |
200 |
77.72 |
11.61 |
| Moderate-severe TBI |
60 |
76.67 |
12.24 |
-0.40 |
296 |
.35 |
-0.06 |
| Mild TBI |
238 |
77.34 |
11.59 |
|
|
|
|
| Low Functional Recovery1
|
15 |
- |
- |
- |
- |
- |
- |
| High Functional Recovery1
|
280 |
77.61 |
11.34 |
| Post-Concussion Symptoms2
|
47 |
63.98 |
11.81 |
-10.03 |
293 |
<.001* |
-1.60 |
| No Post-Concussion Symptoms2
|
248 |
79.92 |
9.62 |
| Head-/aches, Physical Impairments, or Mental Health Problems3
|
146 |
74.93 |
11.67 |
-3.28 |
294 |
<.001* |
-0.38 |
| No Aches, Impairments, or Problems3
|
150 |
79.33 |
11.41 |
| Low Learning Rate4
|
57 |
77.73 |
12.99 |
0.41 |
295 |
.66 |
0.06 |
| Higher Learning Rate4
|
240 |
77.02 |
11.38 |
| Mild to Severe Anxiety5
|
87 |
72.14 |
13.07 |
-4.96 |
295 |
<.001* |
-0.63 |
| No Anxiety5
|
210 |
79.28 |
10.46 |
| Mild to Severe Depression6
|
88 |
71.46 |
12.74 |
-5.76 |
295 |
<.001* |
-0.73 |
| No Depression6
|
209 |
79.61 |
10.39 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).