Submitted:
22 February 2024
Posted:
22 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
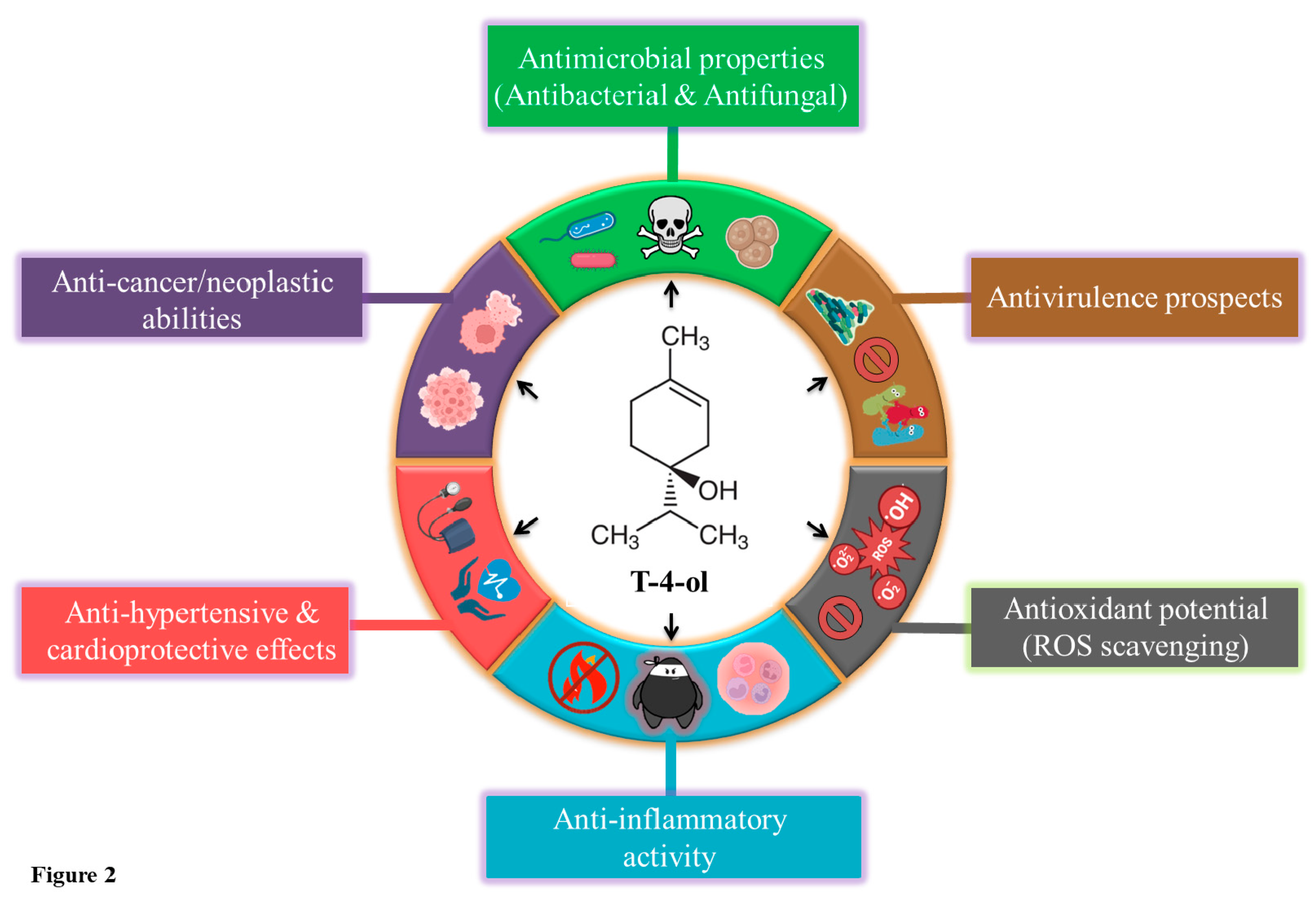
2. Biological Properties of T-4-ol: A Comprehensive Overview
2.1. Antibacterial Potential of T-4-ol: Prospecting Alternatives to Antibiotics
2.2. Anti-Fungal Prospects of T-4-ol: Beyond the Antibacterial Spectrum
2.3. Quorum Quenching and Antivirulent Potential of T-4-ol: A Recent Insight
2.4. Antioxidant Activity of T-4-ol: On the Hunt for Radical Scavenging
2.5. Anti-Inflammatory Activity of T-4-ol: The Conquest Against Swelling
2.6. Anti-Hypertensive and Cardioprotective Effects of T-4-ol: Heart of Gold
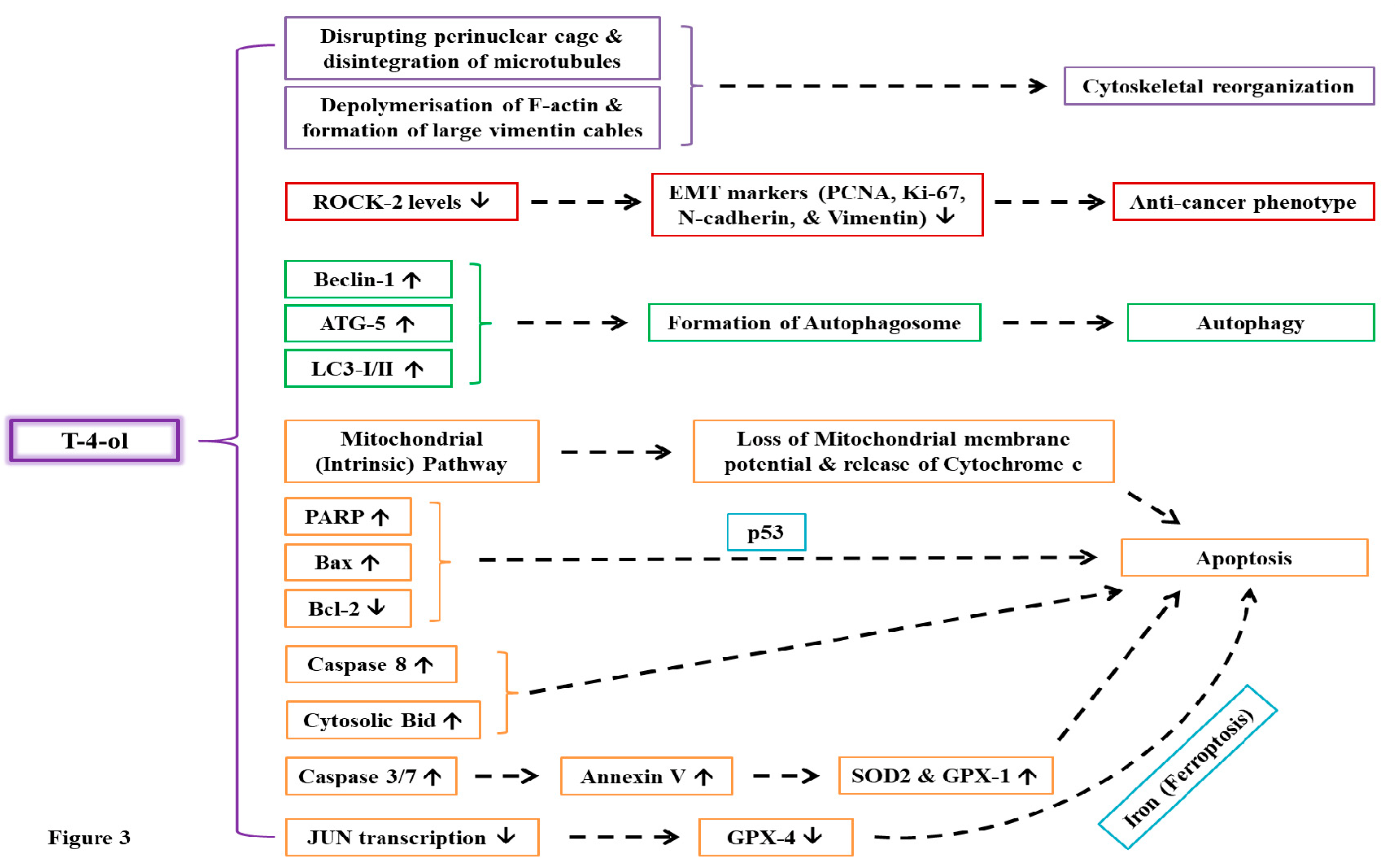
2.7. Anti-Cancer Activity of T-4-ol: Combating a Devastating Disease
3. Conclusion
Author Contributions
Data Availability Statement
Acknowledgements
Conflicts of Interest
Abbreviations
References
- Chadha, J.; Khullar, L. Subinhibitory concentrations of nalidixic acid alter bacterial physiology and induce anthropogenic resistance in a commensal strain of Escherichia coli in vitro. Lett. Appl. Microbiol. 2021, 73, 623–633. [Google Scholar] [CrossRef]
- WHO. WHO publishes list of bacteria for which new antibiotics are urgently needed (2024). https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed. (Accessed 22 February 2024).
- D’andrea, M.M.; Fraziano, M.; Thaller, M.C.; Rossolini, G.M. The Urgent Need for Novel Antimicrobial Agents and Strategies to Fight Antibiotic Resistance. Antibiotics 2019, 8, 254. [Google Scholar] [CrossRef] [PubMed]
- Chadha, J.; Harjai, K.; Chhibber, S. Repurposing phytochemicals as anti-virulent agents to attenuate quorum sensing-regulated virulence factors and biofilm formation in Pseudomonas aeruginosa. Microb. Biotechnol. 2021, 15, 1695–1718. [Google Scholar] [CrossRef] [PubMed]
- Chadha, J.; Gupta, M.; Nagpal, N.; Sharma, M.; Adarsh, T.; Joshi, V.; Tiku, V.; Mittal, T.; Nain, V.K.; Singh, A.; et al. Antibacterial potential of indigenous plant extracts against multidrug-resistant bacterial strains isolated from New Delhi region. GSC Biol. Pharm. Sci. 2021, 14, 185–196. [Google Scholar] [CrossRef]
- B. Ali, N.A. Al-Wabel, S. Shams, A. Ahamad, S.A. Khan, F. Anwar, Essential oils used in aromatherapy: A systemic review, Asian Pacific Journal of Tropical Biomedicine 5(8) (2015) 601-611.
- Mohamed, A.A.; Alotaibi, B.M. Essential oils of some medicinal plants and their biological activities: a mini review. J. Umm Al-Qura Univ. Appl. Sci. 2022, 9, 40–49. [Google Scholar] [CrossRef]
- Alamgir, A.N.M. Phytoconstituents—Active and Inert Constituents, Metabolic Pathways, Chemistry and Application of Phytoconstituents, Primary Metabolic Products, and Bioactive Compounds of Primary Metabolic Origin, 74 (2018) 25-164.
- Kawatra, S. Gupta, R. Dhankhar, P. Singh, P. Gulati, Application of Phytochemicals in Therapeutic, Food, Flavor, and Cosmetic Industries, (2022) 85-108. [CrossRef]
- Chandrasekara, A.; Shahidi, F. Herbal beverages: Bioactive compounds and their role in disease risk reduction - A review. J. Tradit. Complement. Med. 2018, 8, 451–458. [Google Scholar] [CrossRef]
- Khaleel, C.; Tabanca, N.; Buchbauer, G. α-Terpineol, a natural monoterpene: A review of its biological properties. Open Chem. 2018, 16, 349–361. [Google Scholar] [CrossRef]
- Hart, P.; Brand, C.; Carson, C.; Riley, T.; Prager, R.; Finlay-Jones, J. Terpinen-4-ol, the main component of the essential oil of Melaleuca alternifolia (tea tree oil), suppresses inflammatory mediator production by activated human monocytes. Inflamm. Res. 2000, 49, 619–626. [Google Scholar] [CrossRef]
- Fatma, G.; Mouna, B.F.; Mondher, M.; Ahmed, L. In-vitro assessment of antioxidant and antimicrobial activities of methanol extracts and essential oil of Thymus hirtus sp. algeriensis. Lipids Heal. Dis. 2014, 13, 114–114. [Google Scholar] [CrossRef] [PubMed]
- Drug Bank. Terpinen-4-ol (2022). https://go.drugbank.com/drugs/DB12816. (Accessed on 22 February 2024).
- PubChem. 4-Terpineol (2024). https://pubchem.ncbi.nlm.nih.gov/compound/4-Terpineol. (Accessed on 22 February 2024).
- Le, M.T.; Nguyen, N.M.; Le, X.T. Enriching terpinen-4-ol from tea tree (Melaleuca alternifolia) oil using vacuum fractional distillation: Effect of column and packings on the separation. IOP Conf. Series: Earth Environ. Sci. 2021, 947. [Google Scholar] [CrossRef]
- Davies, N.W.; Larkman, T.; Marriott, P.J.; Khan, I.A. Determination of Enantiomeric Distribution of Terpenes for Quality Assessment of Australian Tea Tree Oil. J. Agric. Food Chem. 2016, 64, 4817–4819. [Google Scholar] [CrossRef]
- Foreverest. Terpinen-4-ol (2023). https://foreverest.net/products/extractives-synthetic/terpinen-4-ol.html. (Accessed on 22 February 2024).
- pkCSM-pharmacokinetics. pkCSM: predicting small-molecule pharmacokinetic properties using graph-based signatures (2024). https://biosig.lab.uq.edu.au/pkcsm/. (Accessed on 22 February 2024).
- Su, C.-W.; Tighe, S.; Sheha, H.; Cheng, A.M.S.; Tseng, S.C.G. Safety and efficacy of 4-terpineol against microorganisms associated with blepharitis and common ocular diseases. BMJ Open Ophthalmol. 2018, 3, e000094. [Google Scholar] [CrossRef]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (Tea Tree) Oil: a Review of Antimicrobial and Other Medicinal Properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef]
- Cha, J.; Jeong, M.; Jeong, S.; Moon, S.; Kil, B.; Yun, S.; Lee, K.; Song, Y. Chemical composition and antimicrobial activity of the essential oil of Cryptomeria japonica. Phytotherapy Res. 2007, 21, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Mondello, F.; Girolamo, A.; Scaturro, M.; Ricci, M.L. Determination of Legionella pneumophila susceptibility to Melaleuca alternifolia Cheel (tea tree) oil by an improved broth micro-dilution method under vapour controlled conditions. J. Microbiol. Methods 2009, 77, 243–248. [Google Scholar] [CrossRef]
- Pankey, G.A.; Sabath, L.D. Clinical Relevance of Bacteriostatic versus Bactericidal Mechanisms of Action in the Treatment of Gram-Positive Bacterial Infections. Clin. Infect. Dis. 2004, 38, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, L.; Figueiredo, P.; Souza, H.; Sousa, A.; Andrade-Júnior, F.; Medeiros, D.; Nóbrega, J.; Silva, D.; Martins, E.; Barbosa-Filho, J.; et al. Terpinen-4-ol as an Antibacterial and Antibiofilm Agent against Staphylococcus aureus. Int. J. Mol. Sci. 2020, 21, 4531. [Google Scholar] [CrossRef]
- Cheng, F.; Mo, Y.; Chen, K.; Shang, X.; Yang, Z.; Hao, B.; Shang, R.; Liang, J.; Liu, Y. Integration of metabolomics and transcriptomics indicates changes in MRSA exposed to terpinen-4-ol. BMC Microbiol. 2021, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Johansen, B.; Duval, R.E.; Sergere, J.-C. First Evidence of a Combination of Terpinen-4-ol and α-Terpineol as a Promising Tool against ESKAPE Pathogens. Molecules 2022, 27, 7472. [Google Scholar] [CrossRef]
- Chadha, J.; Moudgil, G.; Harjai, K. Synergism Between α-Terpineol and Terpinen-4-ol Potentiates Antivirulence Response Against Pseudomonas aeruginosa. Indian J. Microbiol. 2024, 1–5. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, R.; Li, L.; Zhou, X.; Li, Z.; Jia, R.; Song, X.; Zou, Y.; Yin, L.; He, C.; et al. The Antibacterial Mechanism of Terpinen-4-ol Against Streptococcus agalactiae. Curr. Microbiol. 2018, 75, 1214–1220. [Google Scholar] [CrossRef]
- F. Mondello, S. F. Mondello, S. Fontana, M. Scaturro, A. Girolamo, M. Colone, A. Stringaro, M.D. Vito, M.L. Ricci, Terpinen-4-ol, the Main Bioactive Component of Tea Tree Oil, as an Innovative Antimicrobial Agent against Legionella pneumophila, Pathogens 11(6) (2022) 682. [CrossRef]
- Bucci, A.R.; Marcelino, L.; Mendes, R.K.; Etchegaray, A. The antimicrobial and antiadhesion activities of micellar solutions of surfactin, CTAB and CPCl with terpinen-4-ol: applications to control oral pathogens. World J. Microbiol. Biotechnol. 2018, 34, 86. [Google Scholar] [CrossRef] [PubMed]
- Bordini, E.A.F.; Tonon, C.C.; Francisconi, R.S.; Magalhães, F.A.C.; Huacho, P.M.M.; Bedran, T.L.; Pratavieira, S.; Spolidorio, L.C.; Spolidorio, D.P. Antimicrobial effects of terpinen-4-ol against oral pathogens and its capacity for the modulation of gene expression. Biofouling 2018, 34, 815–825. [Google Scholar] [CrossRef]
- Huacho, P.M.M.; Herrero, E.R.; Verspecht, T.; Pauwels, M.; Marcantonio, E.; Spolidorio, D.M.P.; Teughels, W. Terpinen-4-ol and carvacrol affect multi-species biofilm composition. Biofouling 2019, 35, 561–572. [Google Scholar] [CrossRef]
- Mondello, F.; De Bernardis, F.; Girolamo, A.; Cassone, A.; Salvatore, G. In vivo activity of terpinen-4-ol, the main bioactive component of Melaleuca alternifolia Cheel (tea tree) oil against azole-susceptible and -resistant human pathogenic Candida species. BMC Infect. Dis. 2006, 6, 158–158. [Google Scholar] [CrossRef] [PubMed]
- Francisconi, R.S.; Huacho, P.M.M.; Tonon, C.C.; Bordini, E.A.F.; Correia, M.F.; Sardi, J.D.C.O.; Spolidorio, D.M.P. Antibiofilm efficacy of tea tree oil and of its main component terpinen-4-ol against Candida albicans. Braz. Oral Res. 2020, 34, e050. [Google Scholar] [CrossRef] [PubMed]
- Francisconi, R.S.; Maquera-Huacho, P.M.; Tonon, C.C.; Calixto, G.M.F.; Sardi, J.d.C.O.; Chorilli, M.; Spolidorio, D.M.P. Terpinen-4-ol and nystatin co-loaded precursor of liquid crystalline system for topical treatment of oral candidiasis. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Wang, J.; Shao, X.; Xu, F.; Wang, H. Antifungal modes of action of tea tree oil and its two characteristic components against Botrytis cinerea. J. Appl. Microbiol. 2015, 119, 1253–1262. [Google Scholar] [CrossRef]
- Barra, V. Coroneo, S. Dessi, P. Cabras, A. Angioni, Characterization of the Volatile Constituents in the Essential Oil of Pistacia lentiscus L. from Different Origins and Its Antifungal and Antioxidant Activity, Journal of Agricultural and Food Chemistry 55(17) (2007) 7093-7098. [CrossRef]
- R.S.N. Brilhante, É.P. Caetano, R.A.C.d. Lima, F.J.d.F. Marques, D.d.S.C.M. Castelo-Branco, C.V.S.d. Melo, G.M.d.M. Guedes, J.S.d. Oliveira, Z.P.d. Camargo, J.L.B. Moreira, A.J. Monteiro, T.d.J.P.G. Bandeira, R.d.A. Cordeiro, M.F.G. Rocha, J.J.C. Sidrim, Terpinen-4-ol, tyrosol, and β-lapachone as potential antifungals against dimorphic fungi, Brazilian Journal of Microbiology 47(4) (2016) 917-924.
- Morcia, C.; Malnati, M.; Terzi, V. In vitroantifungal activity of terpinen-4-ol, eugenol, carvone, 1,8-cineole (eucalyptol) and thymol against mycotoxigenic plant pathogens. Food Addit. Contam. Part A 2011, 29, 1–8. [Google Scholar] [CrossRef]
- Kerekes, E.-B.; Deák. ; Takó, M.; Tserennadmid, R.; Petkovits, T.; Vágvölgyi, C.; Krisch, J. Anti-biofilm forming and anti-quorum sensing activity of selected essential oils and their main components on food-related micro-organisms. J. Appl. Microbiol. 2013, 115, 933–942. [Google Scholar] [CrossRef]
- Bose, S.K.; Chauhan, M.; Dhingra, N.; Chhibber, S.; Harjai, K. Terpinen-4-ol attenuates quorum sensing regulated virulence factors and biofilm formation in Pseudomonas aeruginosa. Futur. Microbiol. 2020, 15, 127–142. [Google Scholar] [CrossRef]
- Noumi, E.; Merghni, A.; Alreshidi, M.M.; Haddad, O.; Akmadar, G.; De Martino, L.; Mastouri, M.; Ceylan, O.; Snoussi, M.; Al-Sieni, A.; et al. Chromobacterium violaceum and Pseudomonas aeruginosa PAO1: Models for Evaluating Anti-Quorum Sensing Activity of Melaleuca alternifolia Essential Oil and Its Main Component Terpinen-4-ol. Molecules 2018, 23, 2672. [Google Scholar] [CrossRef]
- Merghni, N. Haddaji, N. Bouali, K.F. Alabbosh, M. Adnan, M. Snoussi, E. Noumi, Comparative Study of Antibacterial, Antibiofilm, Antiswarming and Antiquorum Sensing Activities of Origanum vulgare Essential Oil and Terpinene-4-ol against Pathogenic Bacteria, Life 12(10) (2022) 1616. [CrossRef]
- Zhao, L.; Duan, F.; Gong, M.; Tian, X.; Guo, Y.; Jia, L.; Deng, S. (+)-Terpinen-4-ol Inhibits Bacillus cereus Biofilm Formation by Upregulating the Interspecies Quorum Sensing Signals Diketopiperazines and Diffusing Signaling Factors. J. Agric. Food Chem. 2021, 69, 3496–3510. [Google Scholar] [CrossRef]
- Kim, H.-J.; Chen, F.; Wu, C.; Wang, X.; Chung, H.Y.; Jin, Z. Evaluation of Antioxidant Activity of Australian Tea Tree (Melaleuca alternifolia) Oil and Its Components. J. Agric. Food Chem. 2004, 52, 2849–2854. [Google Scholar] [CrossRef] [PubMed]
- S. Aslam, W. Younis, M.N.H. Malik, S. Jahan, Alamgeer, A.M. Uttra, M.U. Munir, M. Roman, Pharmacological evaluation of anti-arthritic potential of terpinen-4-ol using in vitro and in vivo assays, Inflammopharmacology 30(3) (2022) 945-959. [CrossRef]
- Badr, M.M.; Taktak, N.E.M.; Badawy, M.E.I. Comparison of the antimicrobial and antioxidant activities of tea tree (Melaleuca alternifolia) oil and its main component terpinen-4-ol with their nanoemulsions. Egypt. J. Chem. 2022, 66, 111–120. [Google Scholar] [CrossRef]
- Koh, K.; Pearce, A.; Marshman, G.; Finlay-Jones, J.; Hart, P. Tea tree oil reduces histamine-induced skin inflammation. Br. J. Dermatol. 2002, 147, 1212–1217. [Google Scholar] [CrossRef]
- Brand, C.; Ferrante, A.; Prager, R.; Riley, T.; Carson, C.; Finlay-Jones, J.; Hart, P. The water-soluble components of the essential oil of Melaleuca alternifolia (tea tree oil) suppress the production of superoxide by human monocytes, but not neutrophils, activated in vitro. Inflamm. Res. 2001, 50, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Pongprayoon, U.; Soontornsaratune, P.; Jarikasem, S.; Sematong, T.; Wasuwat, S.; Claeson, P. Topical antiinflammatory activity of the major lipophilic constituents of the rhizome of Zingiber cassumunar. Part I: The essential oil. Phytomedicine 1997, 3, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, K.; Hayama, K.; Ishijima, S.A.; Maruyama, N.; Irie, H.; Kurihara, J.; Abe, S. Suppression of Inflammatory Reactions by Terpinen-4-ol, a Main Constituent of Tea Tree Oil, in a Murine Model of Oral Candidiasis and Its Suppressive Activity to Cytokine Production of Macrophages in Vitro. Biol. Pharm. Bull. 2013, 36, 838–844. [Google Scholar] [CrossRef]
- M.N.M. Nogueira, S.G. Aquino, C. Rossa Junior, D.M.P. Spolidorio, Terpinen-4-ol and alpha-terpineol (tea tree oil components) inhibit the production of IL-1β, IL-6 and IL-10 on human macrophages, Inflammation Research 63(9) (2014) 769-778. [CrossRef]
- Kim, T.; Song, B.; Cho, K.S.; Lee, I.-S. Therapeutic Potential of Volatile Terpenes and Terpenoids from Forests for Inflammatory Diseases. Int. J. Mol. Sci. 2020, 21, 2187. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, P.; Lu, X.; Li, Y.; Liu, J.; Liu, B.; Fu, Y.; Cao, Y.; Zhang, N. In Vivo and In Vitro Study on the Efficacy of Terpinen-4-ol in Dextran Sulfate Sodium-Induced Mice Experimental Colitis. Front. Immunol. 2017, 8, 558. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Xu, L.; Zhao, Q.; Zhang, Y.-Y.; Shen, C.-Q. The Protective Effects of Terpinen-4-ol on LPS-Induced Acute Lung Injury via Activating PPAR-γ. Inflammation 2018, 41, 2012–2017. [Google Scholar] [CrossRef] [PubMed]
- Taga, I.; Lan, C.Q.; Altosaar, I. Plant Essential Oils and Mastitis Disease: Their Potential Inhibitory Effects on Pro-inflammatory Cytokine Production in Response to Bacteria Related Inflammation. Nat. Prod. Commun. 2012, 7, 675–82. [Google Scholar] [CrossRef] [PubMed]
- Y. Higashi, Y. Kihara, K. Noma, Endothelial dysfunction and hypertension in aging, Hypertension Research 35(11) (2012) 1039-1047. [CrossRef]
- Cunha, G.; Fechine, F.; Bezerra, F.F.; Silveira, E.; Canuto, K.; Moraes, M. Comparative study of the antihypertensive effects of hexane, chloroform and methanol fractions of essential oil of Alpinia zerumbet in rats Wistar. Rev. Bras. de Plantas Med. 2016, 18, 113–124. [Google Scholar] [CrossRef]
- S. Lahlou, L.F.L. Interaminense, J.H. Leal-Cardoso, G.P. Duarte, Antihypertensive effects of the essential oil of Alpinia zerumbet and its main constituent, terpinen-4-ol, in DOCA-salt hypertensive conscious rats, Fundamental & Clinical Pharmacology 17(3) (2003) 323-330. [CrossRef]
- S. Lahlou, C.A. Galindo, J.H. Leal-Cardoso, M.C. Fonteles, G.P. Duarte, Cardiovascular Effects of the Essential Oil of Alpinia zerumbet Leaves and its Main Constituent, Terpinen-4-ol, in Rats: Role of the Autonomic Nervous System, Planta Medica 68(12) (2002) 1097-1102. [CrossRef]
- Nascimento, N.R.F.; Leal-Cardoso, J.H.; A Lessa, L.M.; Roriz-Filho, J.S.; A Cunha, K.M.; Fonteles, M.C. Terpinen-4-ol: mechanisms of relaxation on rabbit duodenum. J. Pharm. Pharmacol. 2005, 57, 467–474. [Google Scholar] [CrossRef]
- Maia-Joca, R.P.M.; Joca, H.C.; Ribeiro, F.J.P.; Nascimento, R.V.D.; Silva-Alves, K.S.; Cruz, J.S.; Coelho-De-Souza, A.N.; Leal-Cardoso, J.H. Investigation of terpinen-4-ol effects on vascular smooth muscle relaxation. Life Sci. 2014, 115, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, L.; Tu, M.; Huang, M.; Chen, Y.; Pan, D.; Peng, J.; Shen, X. The ameliorative effect of terpinen-4-ol on ER stress-induced vascular calcification depends on SIRT1-mediated regulation of PERK acetylation. Pharmacol. Res. 2021, 170, 105629. [Google Scholar] [CrossRef] [PubMed]
- Gondim, A.N.S.; Lara, A.; Santos-Miranda, A.; Roman-Campos, D.; Lauton-Santos, S.; Menezes-Filho, J.E.R.; de Vasconcelos, C.M.L.; Conde-Garcia, E.A.; Guatimosim, S.; Cruz, J.S. (-)-Terpinen-4-ol changes intracellular Ca2+ handling and induces pacing disturbance in rat hearts. Eur. J. Pharmacol. 2017, 807, 56–63. [Google Scholar] [CrossRef]
- Elansary, H.O.; Abdelgaleil, S.A.M.; Mahmoud, E.A.; Yessoufou, K.; Elhindi, K.; El-Hendawy, S. Effective antioxidant, antimicrobial and anticancer activities of essential oils of horticultural aromatic crops in northern Egypt. BMC Complement. Altern. Med. 2018, 18, 214. [Google Scholar] [CrossRef]
- Calcabrini, A.; Stringaro, A.; Toccacieli, L.; Meschini, S.; Marra, M.; Colone, M.; Arancia, G.; Molinari, A.; Salvatore, G.; Mondello, F. Terpinen-4-ol, The Main Component of Melaleuca Alternifolia (Tea Tree) Oil Inhibits the In Vitro Growth of Human Melanoma Cells. J. Investig. Dermatol. 2004, 122, 349–360. [Google Scholar] [CrossRef]
- Di Martile, M.; Garzoli, S.; Sabatino, M.; Valentini, E.; D’aguanno, S.; Ragno, R.; Del Bufalo, D. Antitumor effect of Melaleuca alternifolia essential oil and its main component terpinen-4-ol in combination with target therapy in melanoma models. Cell Death Discov. 2021, 7, 1–13. [Google Scholar] [CrossRef]
- Bozzuto, G.; Mariano, F.; Costa, I.; Calcabrini, A.; Molinari, A. Tea Tree Oil and Terpinen-4-Ol Induce Cytoskeletal Reorganization of Human Melanoma Cells. Planta Medica Int. Open 2022, 9, e34–e53. [Google Scholar] [CrossRef]
- Greay, S.J.; Ireland, D.J.; Kissick, H.T.; Levy, A.; Beilharz, M.W.; Riley, T.V.; Carson, C.F. Induction of necrosis and cell cycle arrest in murine cancer cell lines by Melaleuca alternifolia (tea tree) oil and terpinen-4-ol. Cancer Chemother. Pharmacol. 2009, 65, 877–888. [Google Scholar] [CrossRef]
- Hayes, A.J.; Leach, D.N.; Markham, J.L.; Markovic, B. In vitro Cytotoxicity of Australian Tea Tree Oil using Human Cell Lines. J. Essent. Oil Res. 1997, 9, 575–582. [Google Scholar] [CrossRef]
- N. Casalle and C.R. de Andrade, Cytotoxic and mutagenic capacity of TTO and terpinen-4-ol in oral squamous cell carcinoma, bioRxiv (2020). [CrossRef]
- Wu, C.-S.; Chen, Y.-J.; Chen, J.J.W.; Shieh, J.-J.; Huang, C.-H.; Lin, P.-S.; Chang, G.-C.; Chang, J.-T.; Lin, C.-C. Terpinen-4-ol Induces Apoptosis in Human Nonsmall Cell Lung Cancer In Vitro and In Vivo. Evidence-Based Complement. Altern. Med. 2012, 2012, 1–13. [Google Scholar] [CrossRef]
- Khaw-On, P.; Banjerdpongchai, R. Induction of Intrinsic and Extrinsic Apoptosis Pathways in the Human Leukemic MOLT-4 Cell Line by Terpinen-4-ol. Asian Pac. J. Cancer Prev. 2012, 13, 3073–3076. [Google Scholar] [CrossRef] [PubMed]
- Banjerdpongchai, R.; Khaw-On, P. Terpinen-4-ol Induces Autophagic and Apoptotic Cell Death in Human Leukemic HL-60 Cells. Asian Pac. J. Cancer Prev. 2013, 14, 7537–7542. [Google Scholar] [CrossRef] [PubMed]
- Shapira, S.; Pleban, S.; Kazanov, D.; Tirosh, P.; Arber, N. Terpinen-4-ol: A Novel and Promising Therapeutic Agent for Human Gastrointestinal Cancers. PLOS ONE 2016, 11, e0156540. [Google Scholar] [CrossRef]
- Nakayama, K.; Murata, S.; Ito, H.; Iwasaki, K.; Villareal, M.O.; Zheng, Y.-W.; Matsui, H.; Isoda, H.; Ohkohchi, N. Terpinen-4-ol inhibits colorectal cancer growth via reactive oxygen species. Oncol. Lett. 2017, 14, 2015–2024. [Google Scholar] [CrossRef]
- Cao, W.; Tian, R.; Pan, R.; Sun, B.; Xiao, C.; Chen, Y.; Zeng, Z.; Lei, S. Terpinen-4-ol inhibits the proliferation and mobility of pancreatic cancer cells by downregulating Rho-associated coiled-coil containing protein kinase Bioengineered 2022, 13, 8643–8656. [CrossRef]
- Cao, W.; Li, Y.; Zeng, Z.; Lei, S. Terpinen-4-ol Induces Ferroptosis of Glioma Cells via Downregulating JUN Proto-Oncogene. Molecules 2023, 28, 4643. [Google Scholar] [CrossRef]
- Kong, J.-O.; Park, I.-K.; Choi, K.-S.; Shin, S.-C.; Ahn, Y.-J. Nematicidal and Propagation Activities of Thyme Red and White Oil Compounds toward Bursaphelenchus xylophilus (Nematoda: Parasitaphelenchidae). . 2007, 39, 237–42. [Google Scholar]
- Walton, S.F.; McKinnon, M.; Pizzutto, S.; Dougall, A.; Williams, E.; Currie, B.J. Acaricidal Activity of Melaleuca alternifolia (Tea Tree) Oil. Arch. Dermatol. 2004, 140, 563–566. [Google Scholar] [CrossRef]
- D.P.d. Sousa, F.F.F. Nóbrega, L.C.S.L.d. Morais, R.N.d. Almeida, Evaluation of the Anticonvulsant Activity of Terpinen-4-ol, Zeitschrift für Naturforschung C 64(1-2) (2009) 1-5.
- Nóbrega, F.F.F.; Salvadori, M.G.S.S.; Masson, C.J.; Mello, C.F.; Nascimento, T.S.; Leal-Cardoso, J.H.; de Sousa, D.P.; Almeida, R.N. Monoterpenoid Terpinen-4-ol Exhibits Anticonvulsant Activity in Behavioural and Electrophysiological Studies. Oxidative Med. Cell. Longev. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Tighe, S.; Gao, Y.-Y.; Tseng, S.C.G. Terpinen-4-ol is the Most Active Ingredient of Tea Tree Oil to KillDemodexMites. Transl. Vis. Sci. Technol. 2013, 2, 2. [Google Scholar] [CrossRef]
- Y. Yong, B. Fang, Y. Huang, J. Li, T. Yu, L. Wu, C. Hu, X. Liu, Z. Yu, X. Ma, R. Gooneratne, S. Li, A.M. Abd El-Aty, X. Ju, Tea Tree Oil Terpinen-4-ol Protects Gut Barrier Integrity by Upregulation of Tight Junction Proteins via the ERK1/2-Signaling Pathway, Frontiers in Nutrition 8 (2022). [CrossRef]
- Maior, L.d.F.S.; Maciel, P.P.; Ferreira, V.Y.N.; Dantas, C.d.L.G.; de Lima, J.M.; Castellano, L.R.C.; Batista, A.U.D.; Bonan, P.R.F. Antifungal activity and Shore A hardness of a tissue conditioner incorporated with terpinen-4-ol and cinnamaldehyde. Clin. Oral Investig. 2019, 23, 2837–2848. [Google Scholar] [CrossRef]
- Astani, A.; Reichling, J.; Schnitzler, P. Comparative study on the antiviral activity of selected monoterpenes derived from essential oils. Phytotherapy Res. 2009, 24, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Pérez-López, A.J.; Saura, D.; Lorente, J.; Carbonell-Barrachina, A. Limonene, linalool, α-terpineol, and terpinen-4-ol as quality control parameters in mandarin juice processing. Eur. Food Res. Technol. 2005, 222, 281–285. [Google Scholar] [CrossRef]
- Souza, C.F.; Baldissera, M.D.; Silva, L.d.L.; Geihs, M.A.; Baldisserotto, B. Is monoterpene terpinen-4-ol the compound responsible for the anesthetic and antioxidant activity of Melaleuca alternifolia essential oil (tea tree oil) in silver catfish? Aquaculture 2017, 486, 217–223. [Google Scholar] [CrossRef]
- Li, Z.; Wang, N.; Wei, Y.; Zou, X.; Jiang, S.; Xu, F.; Wang, H.; Shao, X. Terpinen-4-ol Enhances Disease Resistance of Postharvest Strawberry Fruit More Effectively than Tea Tree Oil by Activating the Phenylpropanoid Metabolism Pathway. J. Agric. Food Chem. 2020, 68, 6739–6747. [Google Scholar] [CrossRef] [PubMed]
- Baldissera, M.D.; Grando, T.H.; Souza, C.F.; Gressler, L.T.; Stefani, L.M.; da Silva, A.S.; Monteiro, S.G. In vitro and in vivo action of terpinen-4-ol, γ-terpinene, and α-terpinene against Trypanosoma evansi. Exp. Parasitol. 2016, 162, 43–48. [Google Scholar] [CrossRef]
- Kumar, A.; Mills, S.; Bazaka, K.; Bajema, N.; Atkinson, I.; Jacob, M.V. Biodegradable optically transparent terpinen-4-ol thin films for marine antifouling applications. Surf. Coatings Technol. 2018, 349, 426–433. [Google Scholar] [CrossRef]
- Grant, D.S.; Ahmed, J.; Whittle, J.D.; Michelmore, A.; Vasilev, K.; Bazaka, K.; Jacob, M.V. Comparative Study of Natural Terpenoid Precursors in Reactive Plasmas for Thin Film Deposition. Molecules 2021, 26, 4762. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Al-Jumaili, A.; Prasad, K.; Bazaka, K.; Mulvey, P.; Warner, J.; Jacob, M.V. Pulse Plasma Deposition of Terpinen-4-ol: An Insight into Polymerization Mechanism and Enhanced Antibacterial Response of Developed Thin Films. Plasma Chem. Plasma Process. 2019, 40, 339–355. [Google Scholar] [CrossRef]
- Kumar, A.; Al-Jumaili, A.; Bazaka, K.; Mulvey, P.; Warner, J.; Jacob, M.V. In-Situ Surface Modification of Terpinen-4-ol Plasma Polymers for Increased Antibacterial Activity. Materials 2020, 13, 586. [Google Scholar] [CrossRef]
- Chiavacci, L.; Manaia, E.B.; Kaminski, R.C.K.; de Oliveira, A.G.; Corrêa, M.A. Multifunction hexagonal liquid-crystal containing modified surface TiO2 nanoparticles and terpinen-4-ol for controlled release. Int. J. Nanomed. 2015, 10, 811–819. [Google Scholar] [CrossRef]
- Z. Yang, Z. Xiao, H. Ji, Solid inclusion complex of terpinen-4-ol/β-cyclodextrin: kinetic release, mechanism and its antibacterial activity, Flavour and Fragrance Journal 30(2) (2014) 179-187. [CrossRef]
- Ge, Y.; Tang, J.; Fu, H.; Fu, Y. Terpinen-4-ol liposomes-incorporated chitosan/polyethylene oxide electrospun nanofibrous film ameliorates the external microenvironment of healing cutaneous wounds. J. Appl. Polym. Sci. 2020, 138, 49670. [Google Scholar] [CrossRef]
- Marini, V.G.; Martelli, S.M.; Zornio, C.F.; Caon, T.; Simões, C.M.O.; Micke, G.A.; de Oliveira, M.A.L.; Machado, V.G.; Soldi, V. Biodegradable Nanoparticles Obtained from Zein as a Drug Delivery System for Terpinen‐4‐Ol. Quimica Nova 2014. [Google Scholar] [CrossRef]
- da Cunha, J.A.; Junior, G.B.; da Silva, E.G.; Scheeren, C.d. .; Fausto, V.P.; Salbego, J.; Vaucher, R.d.A.; de Vargas, A.C.; Baldisserotto, B. The survival and hepatic and muscle glucose and lactate levels of Rhamdia quelen inoculated with Aeromonas hydrophila and treated with terpinen-4-ol, carvacrol or thymol. Microb. Pathog. 2018, 127, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.-M.; Zhang, C.-L.; Li, P. Characterization, Antibiofilm, and Mechanism of Action of Novel PEG-Stabilized Lipid Nanoparticles Loaded with Terpinen-4-ol. J. Agric. Food Chem. 2012, 60, 6150–6156. [Google Scholar] [CrossRef]
- Messaoud, R.; El Fekih, L.; Mahmoud, A.; Ben Amor, H.; Bannour, R.; Doan, S.; Khairallah, M. Improvement in ocular symptoms and signs in patients with Demodex anterior blepharitis using a novel terpinen-4-ol (2.5%) and hyaluronic acid (0.2%) cleansing wipe. Clin. Ophthalmol. 2019, ume 13, 1043–1054. [CrossRef]
- Arici, C.; Mergen, B.; Yildiz-Tas, A.; Bahar-Tokman, H.; Tokuc, E.; Ozturk-Bakar, Y.; Kutlubay, Z.; Sahin, A. Randomized double-blind trial of wipes containing terpinen-4-ol and hyaluronate versus baby shampoo in seborrheic blepharitis patients. Eye 2021, 36, 869–876. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Demodex Blepharitis Treatment Study (DBTS) (2017). https://classic.clinicaltrials.gov/ct2/show/NCT01647217. (Accessed 22 February 2024). 22 February.
- Epstein, I.J.; Rosenberg, E.D.; Stuber, R.B.; Choi, M.B.; Donnenfeld, E.D.; Perry, H.D. Double-Masked and Unmasked Prospective Study of Terpinen-4-ol Lid Scrubs With Microblepharoexfoliation for the Treatment of Demodex Blepharitis. Cornea 2020, 39, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Evren Kemer, E.E. Karaca, D. Özek, Efficacy of cyclic therapy with terpinen-4-ol in Demodex blepharitis, European Journal of Ophthalmology 31(3) (2020) 1361-1366.
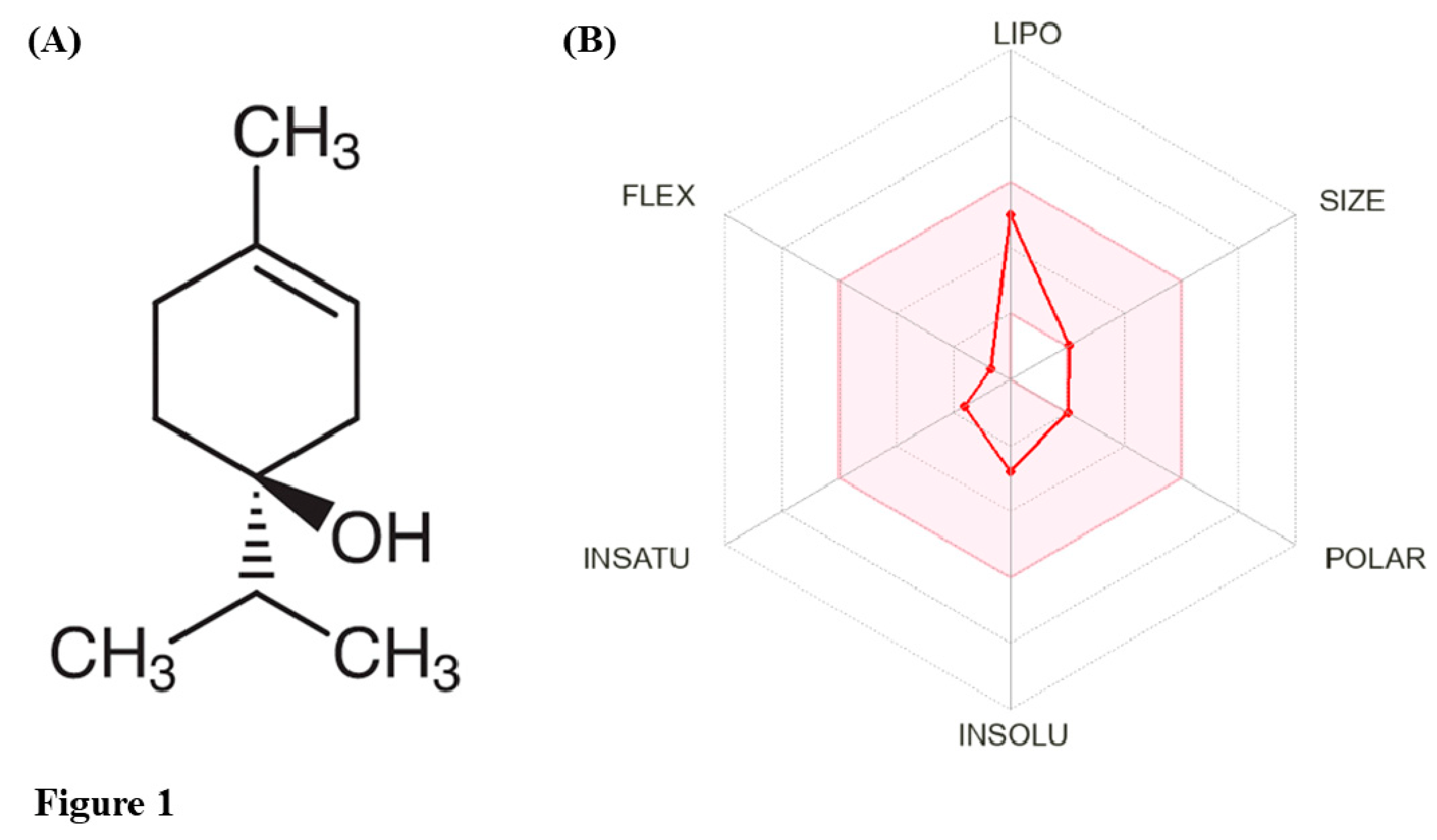
| S. No. | Descriptor | T-4-ol |
|---|---|---|
| 1 | Molecular weight | 154.253 g/mol |
| 2 | LogP | 2.5037 |
| 3 | # Rotatable bonds | 1 |
| 4 | # Acceptors | 1 |
| 5 | # Donors | 1 |
| 6 | Surface area | 69.123 |
| Pharmacokinetic property | Model name | Predicted value | Measurement units |
|---|---|---|---|
| Absorption | Water solubility | -2.386 | Numeric (log mol/L) |
| Caco2 permeability | 1.353 | Numeric (log Papp in 10-6 cm/s) | |
| Intestinal absorption (human) | 96.364 | Numeric (% Absorbed) | |
| Skin Permeability | -2.012 | Numeric (log Kp) | |
| P-glycoprotein substrate | Yes | Categorical (Yes/No) | |
| P-glycoprotein I inhibitor | No | Categorical (Yes/No) | |
| P-glycoprotein II inhibitor | No | Categorical (Yes/No) | |
| Distribution | VDss (human) | 0.331 | Numeric (log L/kg) |
| Fraction unbound (human) | 0.458 | Numeric (Fu) | |
| BBB permeability | 0.359 | Numeric (log BB) | |
| CNS permeability | -2.099 | Numeric (log PS) | |
| Metabolism | CYP2D6 substrate | No | Categorical (Yes/No) |
| CYP3A4 substrate | No | Categorical (Yes/No) | |
| CYP1A2 inhibitor | Yes | Categorical (Yes/No) | |
| CYP2C19 inhibitor | No | Categorical (Yes/No) | |
| CYP2C9 inhibitor | No | Categorical (Yes/No) | |
| CYP2D6 inhibitor | No | Categorical (Yes/No) | |
| CYP3A4 inhibitor | No | Categorical (Yes/No) | |
| Excretion | Total Clearance | 1.056 | Numeric (log mL/min/kg) |
| Renal OCT2 substrate | No | Categorical (Yes/No) | |
| Toxicity | AMES toxicity | No | Categorical (Yes/No) |
| Maximum tolerated dose (human) | 1.273 | Numeric (log mg/kg/day) | |
| hERG I inhibitor | No | Categorical (Yes/No) | |
| hERG II inhibitor | No | Categorical (Yes/No) | |
| Oral Rat Acute Toxicity (LD50) | 2.131 | Numeric (mol/kg) | |
| Oral Rat Chronic Toxicity (LOAEL) | 1.872 | Numeric (log mg/kg-bw/day) | |
| Hepatotoxicity | No | Categorical (Yes/No) | |
| Skin Sensitization | Yes | Categorical (Yes/No) | |
| T. Pyriformis toxicity | -0.009 | Numeric (log μg/L) | |
| Minnow toxicity | 1.417 | Numeric (log mM) |
| S.No. | Pharmacological property reported | Effects described | Reference |
|---|---|---|---|
| 1 | Nematicidal activity | Moderate activity against adult worms of Bursaphelenchus xylophilus with LD50 2.61 mg/mL | [80] |
| 2 | Acaricidal/Pesticidal activity | T-4-ol (2.1%) killed 85% of Sarcoptes scabiei (scabies mites) within 1 h and lowered worm survival rates significantly | [81] |
| 3 | Anticonvulsant activity | Administering T-4-ol (200 mg/kg body weight) decreased spontaneous motor activity within 30 min of dosing and lowered pentylenetetrazole- and picrotoxin-induced convulsions in Swiss mice | [82] |
| 4 | Anticonvulsant activity | Intraperitoneal injection of T-4-ol (0.1 and 1.0 mM) inhibited pentylenetetrazole-induced convulsions in Wistar rats and Swiss mice | [83] |
| 5 | Anti-parasitic activity | T-4-ol (1%) effectively killed Demodex mites within 88 min of exposure in vitro. Demodex-infested patient receiving Cliradex lid cleanser (containing T-4-ol) for 8 weeks resulted in worm clearance and clearer eyelashes | [84] |
| 6 | Protection against inflammatory bowel disease | Treatment with T-4-ol attenuated LPS-induced damage in intestinal porcine epithelial cell lines (IPEC-J2) in vitro and lowered DSS-stimulated colitis in C57BL6/J mice in vivo by preventing LPS-mediated phosphorylation of ERK | [85] |
| 7 | Improved biomaterial quality and softness | Incorporation of cinnamaldehyde and T-4-ol to tissue conditioner (SoftoneTM) lowers Shore A hardness of prosthodontic dentures, thereby reducing the amount of plasticizers and imparting anti-fungal potential against C. albicans | [86] |
| 8 | Anti-viral activity | Exposure with T-4-ol at 75 and 100 μg/mL resulted in 68.9% and 99.6% killing of herpes simplex virus-1 | [87] |
| 9 | Quality indicator | Poor quality/stability of Spanish mandarin juices was assessed by the formation of ill-flavouring constituents (T-4-ol and α-terpineol) and decomposition of linalool and D-limonene | [88] |
| 10 | Anesthetic activity | T-4-ol (300-1000 μL/L) induced anesthesia in Rhamdia quelen (silver catfish) with induction times ranging between 103-630 s and recovery period of 134-673 s | [89] |
| 11 | Induction of disease resistance | T-4-ol treatment of post-harvest strawberry inoculated with Botrytis cinerea prevented disease incidence of by 44.4% after 48 h through upregulation of genes involved in phenylpropanoid biosynthesis and flavonoid metabolism pathways | [90] |
| 12 | Anti-trypanosomal activity | T-4-ol treatment (1% and 2%) resulted in 100% killing of Trypanosoma evansi within 3 h in vitro but failed to protect mice against trypanosomal infection (mastigote form) | [91] |
| S. No. | Formulation reported | Effects described in the study | Reference |
|---|---|---|---|
| 1 | Biodegradable antifouling coating for marine applications | Deposition of T-4-ol on cover slips using a two-stage technique involving plasma-enhanced chemical vapor deposition increased surface smoothness, hydrophobicity, and transmission efficiency. T-4-ol-based coatings were also successfully field-tested for their antifouling properties in Curralea Lake, Australia | [92] |
| 2 | Plasma-polymerized thin films | T-4-ol-derived plasma polymerized films demonstrated antimicrobial and antifouling activity against S. aureus. Additionally, the coatings exhibited biocompatibility towards human fibroblast cells and BALB/c mouse macrophages | [93] |
| 3 | Pulse plasma-assisted thin films | Thin films deposited on borosilicate glass formed stable coatings that retained the monomeric structure of T-4-ol, increasing surface wettability (water contact angle), surface energy, and imparting antibacterial potential against P. aeruginosa | [94] |
| 4 | Plasma-assisted thin films | Zinc oxide nanoparticle-modified T-4-ol plasma polymers were developed and biophysically characterized. The functionalized films demonstrated improved hydrophobicity with a marked increase in the water contact angle, UV absorption, and enhanced antibacterial properties (~ 3 folds) against E. coli | [95] |
| 5 | Liquid-crystalline preparations for photoprotection | Biophysically characterized liquid-crystalline formulations of T-4-ol-functionalized titanium dioxide nanoparticles showcased hexagonal phase structures that permitted consistent release of T-4-ol by obeying zero-order kinetics. Controlled release of titanium dioxide nanoparticles and T-4-ol from the formulation showed photoprotective properties, indicating their possible application as a transparent inorganic sunscreen | [96] |
| 6 | Solid inclusion preparation | Novel formulation composed of solid T-4-ol/β-cyclodextrin inclusion complex prepared using freeze-drying with enhanced stability, sustained drug release, and antibacterial activity (1.25-5.0 mg/mL) against E. coli, P. aeruginosa, and S. aureus | [97] |
| 7 | Nanofibrous film for wound healing | Incorporating liposomal preparation of T-4-ol (6%) improved the structure and morphology of chitosan/polyethylene oxide nanofibers. The nanofibrous dressing effectively absorbed simulated tissue fluid, maintaining optimal moisture content, and mimicking human skin properties. It harbored pro-coagulant property, in vitro biodegradability along with antibacterial activity against C. albicans, E. coli, and S. aureus | [98] |
| 8 | Biodegradable nanoparticles for drug delivery | T-4-ol-loaded zein (corn protein) nanoparticles were formulated with an encapsulation efficiency of 91% for prolonged and sustained release of T-4-ol for its possible application in the treatment of melanoma | [99] |
| 9 | Nanoencapsulation for improved drug delivery | Rhamdia quelen (silver catfish) treated with nanoformulations of T-4-ol (5-25 mg/L for 6 days) prevented Aeromonas hydrophila infection and improved survival rates of silver catfish | [100] |
| 10 | Liposomal nanoparticles for antifungal prospects | Polyethylene glycol-stabilized lipid nanoparticles-containing T-4-ol displayed remarkable stability, high-capacity drug loading, sustained release of the phytochemical accompanied with antifungal and antifouling properties against C. albicans (MIC ~ 5 μg/mL and MBEC ~ 10 μg/mL). Moreover, the liposomal formulation delivered T-4-ol into the yeast mitochondria, thereby disrupting enzyme-dependent cellular respiration and impairing biofilm formation | [101] |
| S.No. | Target disease | Study design | Number of Participants | Findings/Results | Trial number | Reference |
|---|---|---|---|---|---|---|
| 1 | Seborrheic blepharitis | Randomized, open, two-parallel group comparative investigation | 48 | Cleansing wipes impregnated with T-4-ol (2.5%) and hyaluronic acid (0.2%), termed ‘Blephademodex®’, substantially lowered ocular discomfort associated with Demodex (eyelash mite) blepharitis on day 8 and 29 in both the groups with no signs of allergy. Total cylindrical dandruff was reduced by 30.4% in Group 1 (once daily) and 43.5% in Group 2 patients (twice daily) | Not available | [102] |
| 2 | Seborrheic blepharitis | Randomized, double-blind, comparative clinical trial | 48 | Lid wipes containing hyaluronate and T-4-ol (Hy-ter®) was more efficacious over baby shampoo in reducing blepharitis symptoms at 8th and 12th weeks. Although both the treatments resulted in lowered Demodex count, non-invasive tear breakup time was higher for lid wipes (4 weeks) as compared to baby shampoo (8 weeks) | NCT04441528 | [103] |
| 3 | Chronic blepharitis | Randomized and parallel clinical trial | 17 | Lid scrubs loaded with T-4-ol were parallelly assessed along placebo group in treating ocular demodicosis. Changes in Demodox mite count (primary objective) and changes in bulbar conjunctival hyperemia and lid margin redness (secondary objective) were monitored. Phase I trials completed but data not released in public domain | NCT01647217 | [104] |
| 4 | Demodex blepharitis | Randomized and double-masked investigation | 46 | Demodex folliculorum infestation levels were significantly lowered with both terpinen-4-ol lid scrubs (Cliradex) and sham lid scrubs following on-site microblepharoexfoliation. However, clinical significance could not be elucidated | Not available | [105] |
| 5 | Demodex blepharitis | Retrospective observational case series | 30 | Cyclic treatment with T-4-ol-soaked wipes for 2 weeks (twice a day) was successful in improving lid margin, tear breakup time, and ocular surface disease index. Following second cycle after 7-10 gap, disease symptoms were lowered along with significantly improved tear function tests | Not available | [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





