Introduction
The bones are one of the most common site for secondary localization of various tumours, including lung, breast, prostatic, and renal cancer. The management of symptomatic bone metastases of the scapula remains a significant clinical challenge, mostly due to its complex anatomy and its proximity to critical neurovascular structures [
1]. Most patients have their quality of life largely limited by the impairment of their shoulder mobility and function and tumor-related pain [
2]. Conventional therapies including surgical resection, chemotherapy, and radiation are effective, but they are often accompanied by severe side effects. Most of the patients who benefit from locoregional treatments have also already undergone chemo-radiotherapy with insufficient results [
1,
2,
3].
Arterial embolization has emerged as a promising minimally invasive technique in the palliative treatment of painful bone metastases [
4,
5,
6,
7]. Arterial embolization reduces blood supply to the tumor, resulting in necrosis, and size reduction, and in some cases offers a certain degree of ossification of the lesion. Embolic therapy has grown in application to various anatomical sites - the spine, pelvis, and long bones, with encouraging results [
6,
8,
9,
10]. However, limited literature is available on the outcomes of arterial embolization for pain reduction and disease control in metastases of the scapula.
We report our experience of arterial embolizations for scapular metastases during a seven-year period (2016 to 2023). Demographic data have been analyzed along with the recurrence of symptoms and the Visual Analogue Scale (VAS) scores at several intervals after the procedure. Arterial embolization may play a significant role in pain palliation and disease control in patients with metastases of the scapula.
Materials and Methods
A review of the digital records of patients who underwent palliative arterial embolization for scapular metastases from 2016 to 2023 was performed. All patients including men and women 18 years of age or older with a proven diagnosis of metastatic disease to the scapula with significant pain that could not be controlled adequately with systemic therapy or conservative management were included. All patients that received arterial embolization as a preoperative treatment, or that underwent surgery in the next 3 moths were excluded.
All embolization procedures in this series were done using a mixture of Glubran and Lipiodol in a 1:2 ratio. A total of 17 embolization procedures were performed on 15 patients. Symptoms recurred in two patients who subsequently received a second embolization at the same site. Age and sex were taken as demographic data. The primary outcome was the variation in pain, assessed by the Visual Analogue Scale (VAS). The VAS score was reported at baseline before the procedure, then after 1, 3, 6, and 12 months from embolization. The second outcome was decreased size. The size of the tumor was measured by two senior musculoskeletal radiologists using volumetric segmentation to calculate the total tumor volume expressed in cm3. Symptom recurrence and the need for repeated procedures were also noted. All complications were classified using the CIRSE classification system for complications [
11].
Mean and standard deviation were used in measuring continuous variables (age, VAS scores), while frequencies and ratios were used for categorical data (sex, recurrence). A comparison of pre-and post-embolization VAS scores was done with the use of a paired t-test; p<0.05 was set as the cutoff for statistical significance.
Statistical Analysis
Descriptive statistics on the sample are reported as mean (SD), as far as continuous variables are concerned, and as fraction/total for the nominal variables.
Distribution of VAS values and tumor diameter were compared through the Wilcoxon Signed-Rank Test. Statistical significance threshold was set to be p<0.05.
Embolization Procedure
Vascular mapping was performed to identify the pathologic vessels of the metastatic lesions and evaluate if arterial embolization was appropriate and safe. Therefore digital subtraction angiography using contrast media: Iomeprol 300mg/ml (Iomeron; Bracco, Milan, Italy) and iohexol 350 mg/mL (Omnipaque; GE Healthcare, Milan, Italy), angiographic equipment: Siemens Nexaris Angio-CT system (Siemens Healthcare, Forchheim, Germany) and Philips Integris V3000 Cesar-SCP-Visub (Philips Medical Systems, Eindhoven, the Netherlands) was performed before proceeding to each embolization.
The angiographic and embolization procedures were performed via femoral artery catheterization using the Seldinger technique. Local anesthesia was administered in the site of access. The first step in every procedure the diagnostic arteriogram of the subclavian artery, and then a selective and super-selective catheterization of the pathologic feeding vessels of the tumor was performed with a Vertebra catheter (Terumo, Tokyo, Japan). After evaluating the tumor vascularity and arterial anatomy, the major tumor-feeding arteries were selected and embolization was performed through a coaxial 2.7- to 2.9-French microcatheter (Progreat
®, Terumo, Tokyo, Japan). The main approach used was selective catheterization of the circumflex scapular artery, the dorsal scapular artery and the thoracic-acromial trunk. The embolizing agent was NBCA (1 mL) mixed with 33% lipiodol (2 mL). The mixture was then aspirated in 1 mL syringes and depending on the entity of the flow of the pathologic vessels, 0.1-0.2 mL of the embolizing mixture was injected under fluoroscopic guidance and washed out using 2 mL of 5% glucose solution. The success of the embolization was evaluated with a second arteriography at the same site. If the occlusion was not complete, a second embolization was performed using the same technique. On average, 0.5-2 mL of NBCA was used for each embolization. The final result was documented by an angiogram performed at the end of the procedure (
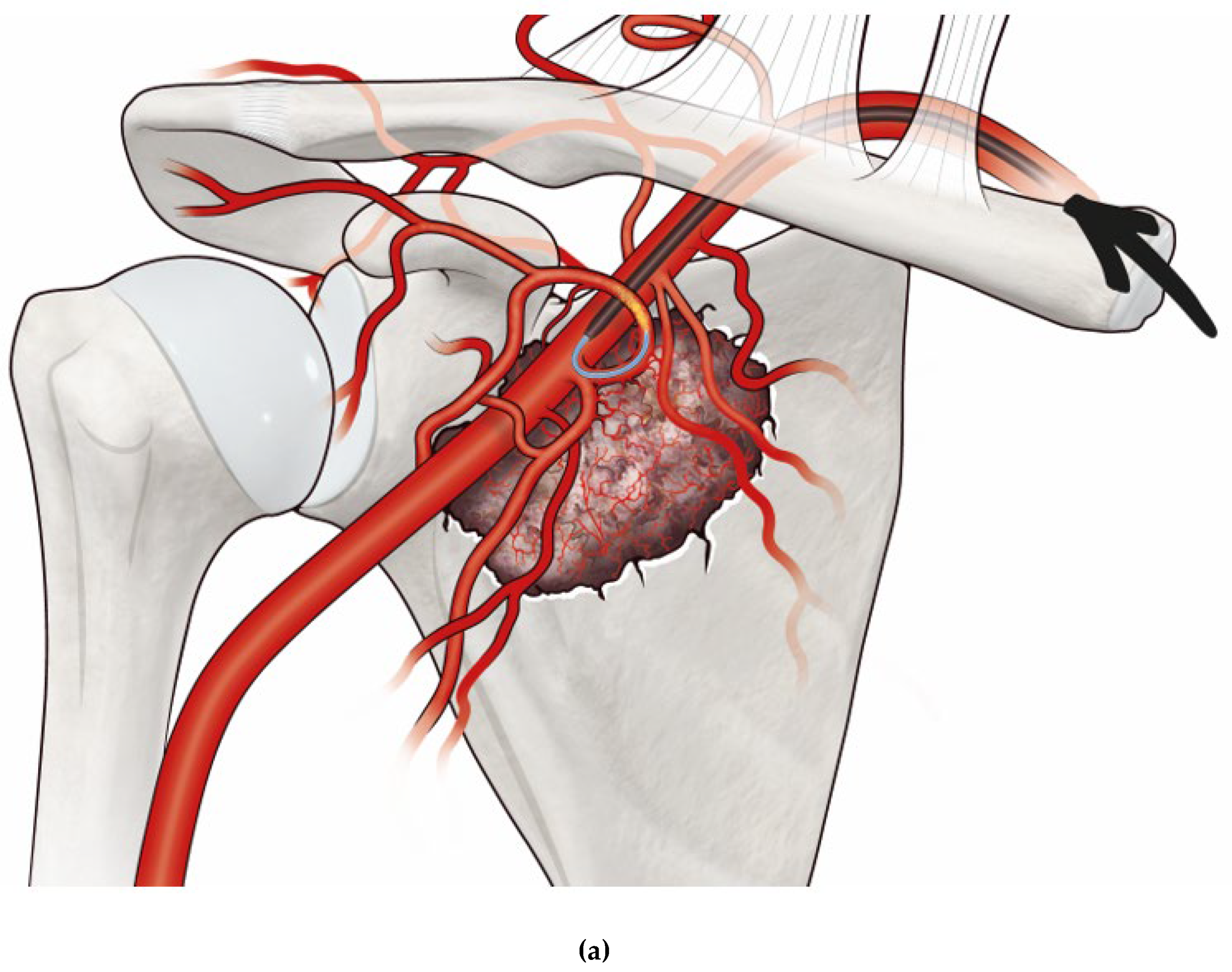
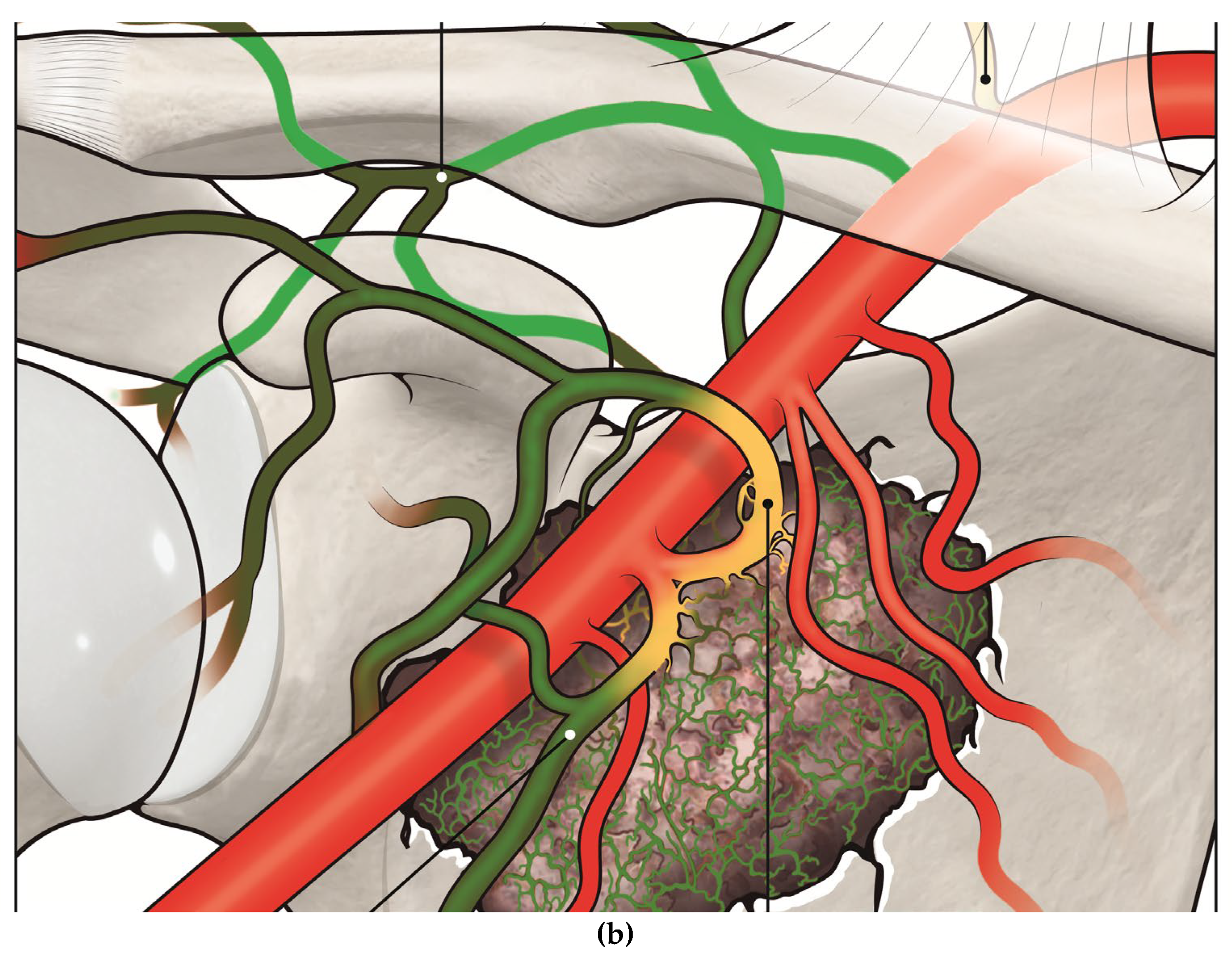
Figure 1).
Figure 1.
a. Illustration of a metastatic lesion in the scapula with its feeding vessels with a catheter and microcatheter system (arrow) positioned in the circumflex scapular artery, injecting embolizing material (illustrated as yellow), and b. post-embolization occlusion of the tumor’s feeding vessels (occluded vessels are painted in green).
Figure 1.
a. Illustration of a metastatic lesion in the scapula with its feeding vessels with a catheter and microcatheter system (arrow) positioned in the circumflex scapular artery, injecting embolizing material (illustrated as yellow), and b. post-embolization occlusion of the tumor’s feeding vessels (occluded vessels are painted in green).
Variations in pain were measured using the VAS score, before the procedure and again at 1, 3, 6, and 12 months afterward. Patients experiencing recurrence of symptoms were considered for repeated embolization.
Comprehensive information regarding the procedure, its potential risks and benefits, and other treatment alternatives was provided to all patients, who gave their informed consent before proceeding.
Results
The study cohort included 14 patients (9 males, 5 females) with a mean age of 62 years (SD 10.2) who underwent palliative arterial embolization for scapular metastases. The primary arteries embolized were the circumflex scapular artery, the dorsal scapular artery and the thoracic-acromial trunk. A mixture of Glubran and Lipiodol in a 1:2 ratio was used for embolization, with an average of 1.1 mL (Range 0.5-1.5 mL) used per procedure (
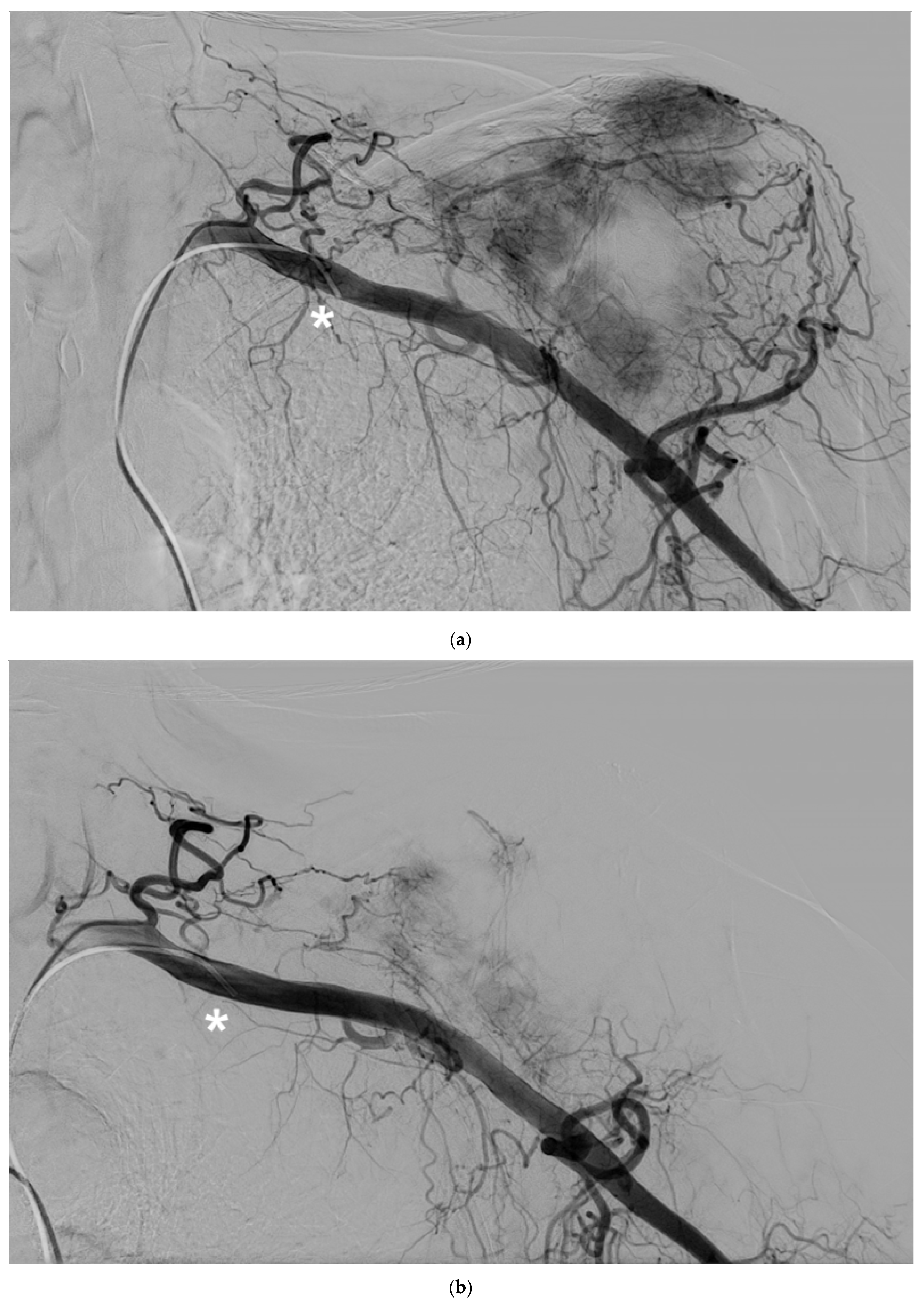
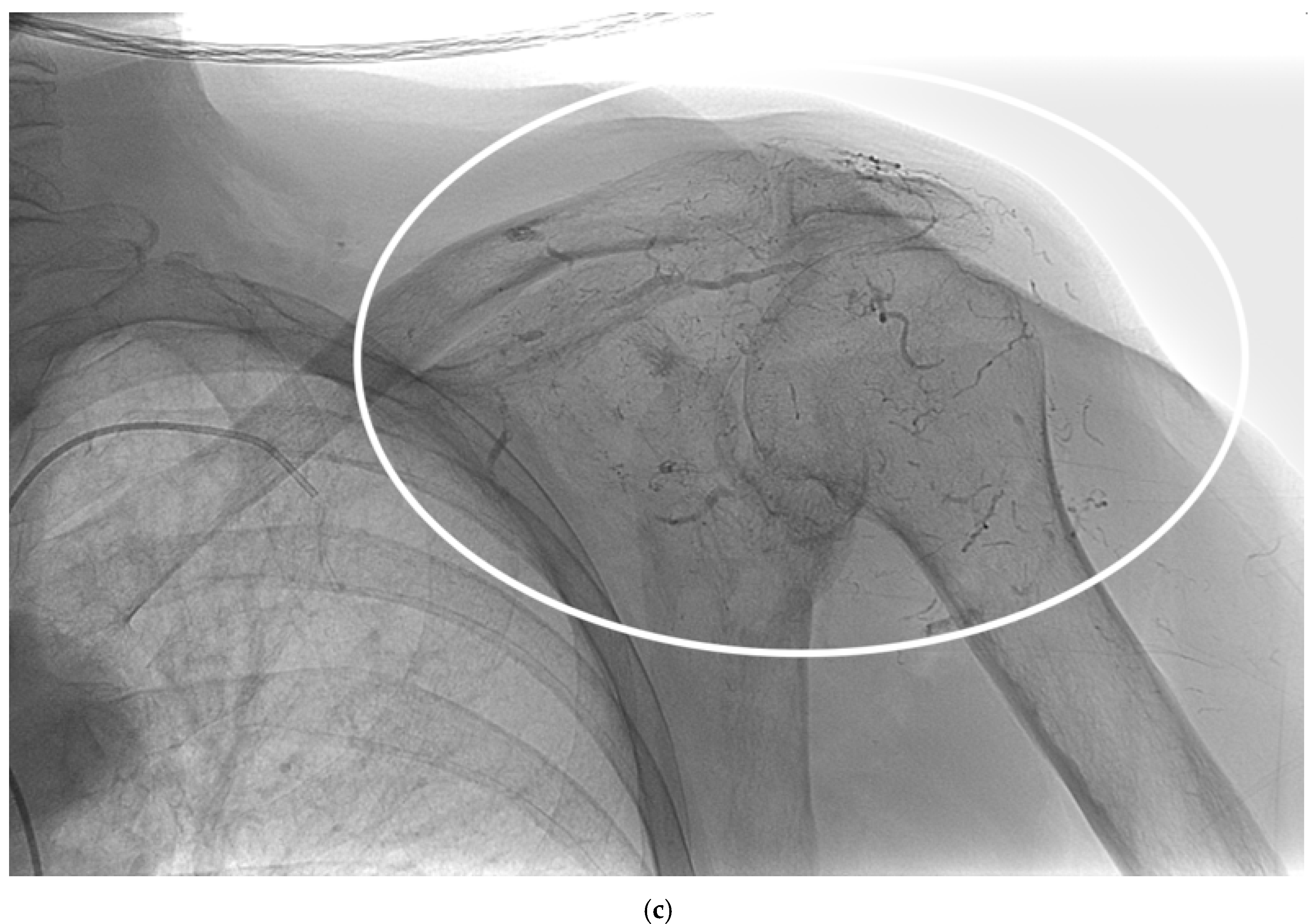
Figure 2).
Figure 2.
A 62-year-old man with a single metastasis from lung cancer in the body of the scapula; a) arteriography performed with a 5F vertebral catheter positioned in the subclavian artery shows pathologic vascularization of the tumor and b) post-embolization arteriography performed from the same injection point, demonstrating near complete devascularization of the scapular lesion. C) Non-subtracted digital fluoroscopy demonstrating extensive presence of lipiodol/Glubran mixture in the embolized vessels (circle).
Figure 2.
A 62-year-old man with a single metastasis from lung cancer in the body of the scapula; a) arteriography performed with a 5F vertebral catheter positioned in the subclavian artery shows pathologic vascularization of the tumor and b) post-embolization arteriography performed from the same injection point, demonstrating near complete devascularization of the scapular lesion. C) Non-subtracted digital fluoroscopy demonstrating extensive presence of lipiodol/Glubran mixture in the embolized vessels (circle).
A total of 17 embolization procedures were conducted, with two patients requiring repeat embolization due to symptom recurrence.
Lung cancer was the most common primary tumor, found in 6 patients (42.9%). This was followed by breast cancer and renal cell carcinoma, each accounting for 4 patients (28.6%). The remaining patient had a primary source of hepatocellular carcinoma (7.1%).
The Visual Analogue Scale (VAS) was used to measure pain relief, with scores recorded at baseline, and at 1, 3, 6, and 12 months post-embolization. Baseline VAS score was a mean of 7.2 (SD 1.5). At one month following the procedure, there was a significant decrease in VAS scores to a mean of 2.8 (SD 0.9). This trend of pain reduction continued over the follow-up period, with the mean VAS score decreasing to 1.6 (SD 0.6) at 12 months post-procedure.
Metastatic tumor size was reduced from a mean of 192.2 cm
3 (range 38.7 to 480.1 cm
3) pre-embolization to a mean of 177.3 cm
3 (range 37.2 to 455.5 cm
3) at the 12-month follow-up (p<0.05) (
Figure 3). None of the patients experienced embolization-related complications.
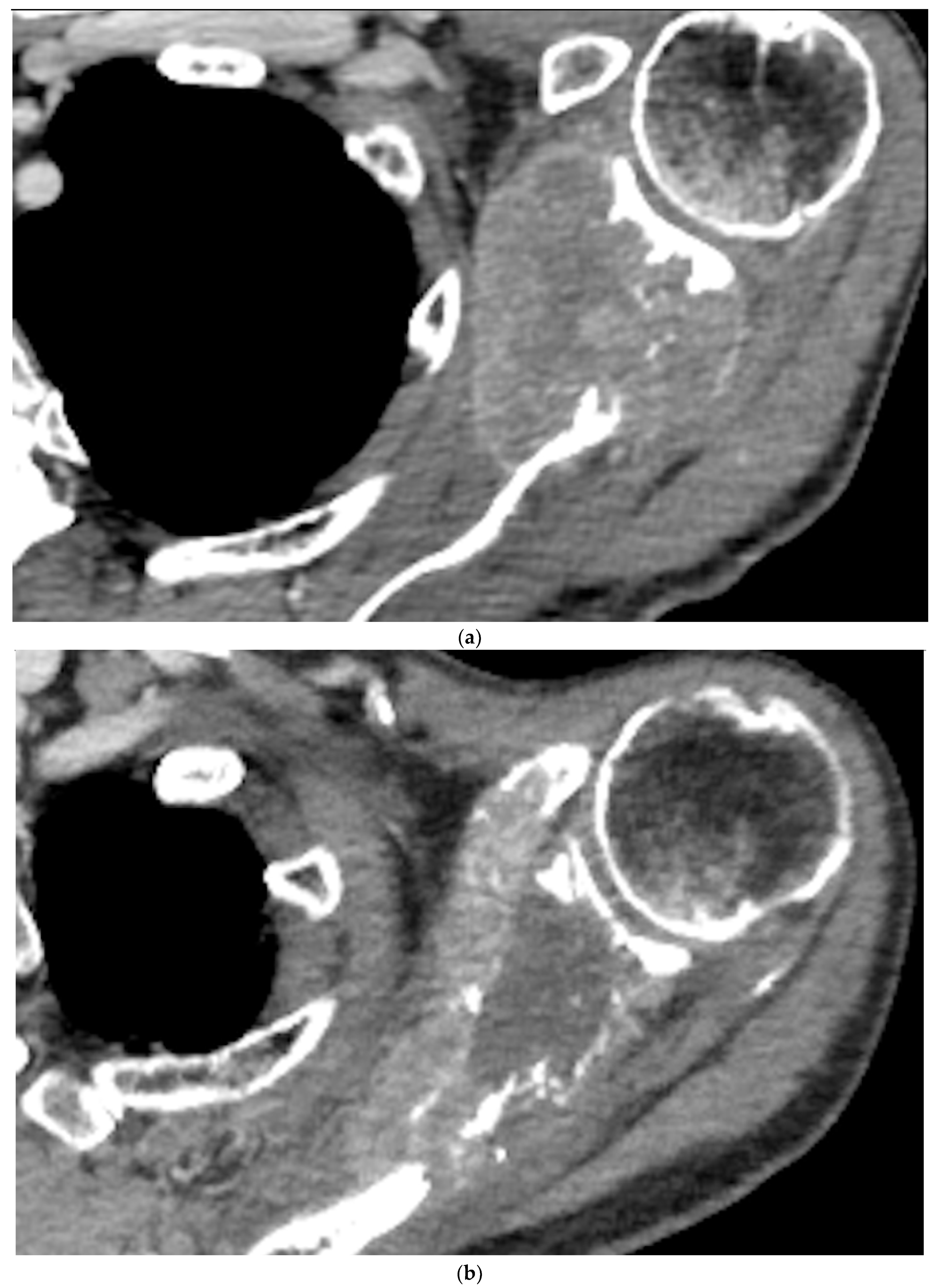
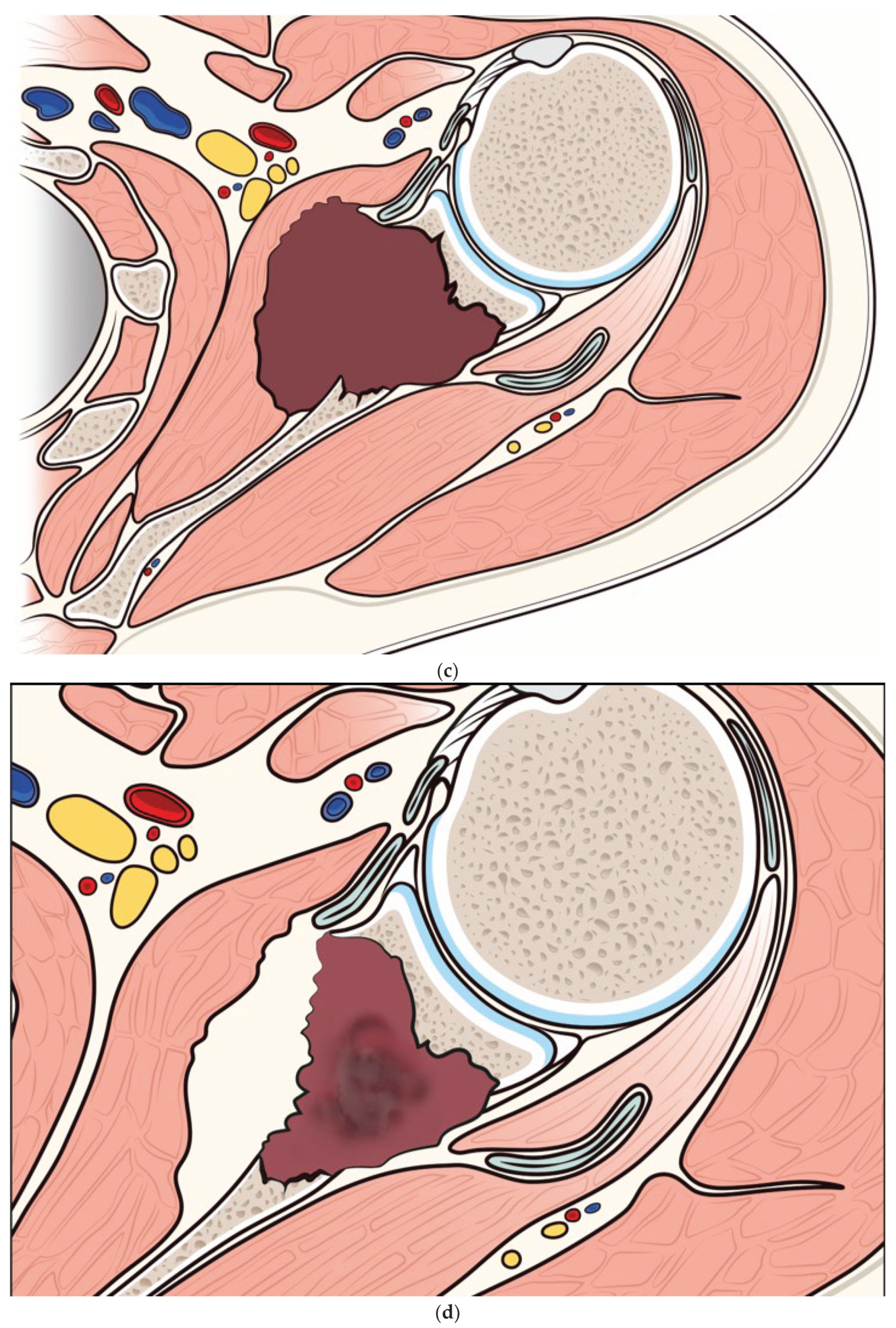
Figure 3.
a) Pre-embolization CT showing a metastatic lesion from renal cancer in the body of the scapula and b) 12-month post-embolization CT showing increased ossification of the lytic lesion of the scapula, with significant reduction of extraskeletal pathologic tissue. c) and d) illustration of the evolution of a metastatic lesion of the scapula before and after arterial embolization.
Figure 3.
a) Pre-embolization CT showing a metastatic lesion from renal cancer in the body of the scapula and b) 12-month post-embolization CT showing increased ossification of the lytic lesion of the scapula, with significant reduction of extraskeletal pathologic tissue. c) and d) illustration of the evolution of a metastatic lesion of the scapula before and after arterial embolization.
Discussion
The management of metastatic bone disease has been the subject of many advances in medical and surgical treatments over the past few decades, however, the treatment of scapular lesions poses a serious challenge, due to the complex skeletal and neurovascular structures involved [
3,
12,
13,
14]. In our series, all patients were not candidate for surgical intervention due to limited life expectancy and the high risk for damage to neurovascular structures. Besides arterial embolization, other minimally invasive treatments are suitable for pain reduction and local disease control of bone metastases, some examples are cryoablation or other thermal ablations such as microwave of RFA [
15,
16,
17], but in the reported cases, arterial embolization was deemed more appropriate due to the size of the lesions, the lytic nature, and the close proximity to neurovascular structures [
18,
19]. Our findings align with those from previous studies demonstrating the efficacy of arterial embolization in pain relief for bone metastases in different anatomical locations [
4,
8,
9,
10]. In the study cohort, a significant pain reduction was achieved for a prolonged time, and even in case of recurrence, repeating embolization was feasible and led to pain reduction even in the two refractory cases, this highlights the repeatability of the procedure, or the planning of multiple treatments in case of very large lesions [
20].
This study has several major limitations to be considered, mainly its retrospective nature and the small sample size.
In conclusion, arterial embolization should be considered for pain palliation and local disease control for patients with metastases of the scapula. The technique is feasible, safe, and repeatable in case of recurrence of symptoms.
References
- Houdek MT, Wilke BK, Barlow JD. Management of Scapular Tumors. Orthop Clin North Am. 2023;54:101–8.
- Hargiss JB, Labott JR, Broida SE, Rose PS, Barlow JD, Houdek MT. Outcome of Scapular Ewing Sarcoma. Anticancer Res. 2022;42:3869–72.
- Klein A, Birkenmaier C, Baur-Melnyk A, Roeder F, Jansson V, Dürr HR. Functional results after oncological scapula resections. J Shoulder Elbow Surg. 2022;31:333–40.
- Rossi G, Mavrogenis AF, Rimondi E, Braccaioli L, Calabrò T, Ruggieri P. Selective embolization with N-butyl cyanoacrylate for metastatic bone disease. J Vasc Interv Radiol. 2011;22:462–70.
- Papalexis N, Parmeggiani A, Peta G, Spinnato P, Miceli M, Facchini G. Minimally Invasive Interventional Procedures for Metastatic Bone Disease: A Comprehensive Review. Curr Oncol. 2022;29:4155–77.
- Facchini G, Di Tullio P, Battaglia M, Bartalena T, Tetta C, Errani C, et al. Palliative embolization for metastases of the spine. Eur J Orthop Surg Traumatol. 2016;26:247–52.
- Facchini G, Parmeggiani A, Peta G, Martella C, Gasbarrini A, Evangelisti G, et al. The role of percutaneous transarterial embolization in the management of spinal bone tumors: a literature review. Eur Spine J. 2021;30:2839–51.
- Forauer AR, Kent E, Cwikiel W, Esper P, Redman B. Selective palliative transcatheter embolization of bony metastases from renal cell carcinoma. Acta Oncol. 2007;46:1012–8.
- Koike Y, Takizawa K, Ogawa Y, Muto A, Yoshimatsu M, Yagihashi K, et al. Transcatheter arterial chemoembolization (TACE) or embolization (TAE) for symptomatic bone metastases as a palliative treatment. Cardiovasc Intervent Radiol. 2011;34:793–801.
- Papalexis N, Peta G, Vara G, Spinnato P, Errani C, Martella C, et al. Palliative Arterial Embolization for Metastases of the Sternum. Cardiovasc Intervent Radiol. 2023;46:794–8.
- Filippiadis DK, Binkert C, Pellerin O, Hoffmann RT, Krajina A, Pereira PL. Cirse Quality Assurance Document and Standards for Classification of Complications: The Cirse Classification System. Cardiovasc Intervent Radiol. 2017;40:1141–6.
- Shashaa MN, Alkarrash MS, Kitaz MN, Hawash S, Otaqy MB, Tarabishi J, et al. Ewing’s sarcoma in scapula, epidemiology, clinical manifestation, diagnosis and treatment: A literature review. Ann Med Surg (Lond). 2022;77:103617.
- Song Y, Liu J, Cao L, Yu B-H, Sun T, Shi L, et al. Clinical and Imaging Features of Tumors in the Scapula. Curr Med Imaging Rev. 2022;18:674–83.
- Errani C, Bazzocchi A, Spinnato P, Facchini G, Campanacci L, Rossi G, et al. What’s new in management of bone metastases? Eur J Orthop Surg Traumatol. 2019;29:1367–75.
- Kurup AN, Callstrom MR. Ablation of musculoskeletal metastases: pain palliation, fracture risk reduction, and oligometastatic disease. Tech Vasc Interv Radiol. 2013;16:253–61.
- Gardner CS, Ensor JE, Ahrar K, Huang SY, Sabir SH, Tannir NM, et al. Cryoablation of Bone Metastases from Renal Cell Carcinoma for Local Tumor Control. J Bone Joint Surg Am. 2017;99:1916–26.
- Papalexis N, Savarese LG, Peta G, Errani C, Tuzzato G, Spinnato P, et al. The New Ice Age of Musculoskeletal Intervention: Role of Percutaneous Cryoablation in Bone and Soft Tissue Tumors. Curr Oncol. 2023;30:6744–70.
- Ma Y, Wallace AN, Waqar SN, Morgensztern D, Madaelil TP, Tomasian A, et al. Percutaneous Image-Guided Ablation in the Treatment of Osseous Metastases from Non-small Cell Lung Cancer. Cardiovasc Intervent Radiol. 2018;41:726–33.
- Kujak JL, Liu PT, Johnson GB, Callstrom MR. Early experience with percutaneous cryoablation of extra-abdominal desmoid tumors. Skeletal Radiol. 2010;39:175–82.
- Chiras J, Adem C, Vallée J-N, Spelle L, Cormier E, Rose M. Selective intra-arterial chemoembolization of pelvic and spine bone metastases. Eur Radiol. 2004;14:1774–80.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).