1. Introduction
The need to improve fuel efficiency and safety has led to a high and growing demand for high-strength steels in the automotive industry. The potential of weight reduction directly depends on mechanical properties improvement, which are in turn controlled by the microstructural features. In that context, the precipitation of amorphous Si3N4 into α-Fe has received a growing attention over the last ten years [1–5]. There are several reasons for this interest. On the one hand, the nanometric size and high density of these particles significantly increase the strength of the alloy [6,7]. On the other hand, the amorphous Si3N4 has a density half that of iron, imparting a substantial weight saving potential to Si3N4-containing steel sheets for automotive applications [6] .
From a more fundamental point of view, the precipitation of Si3N4 in α−Fe has very singular characters. (i) The amorphous nature of the precipitates is unusual for nitrides and carbides in steels and, more generally, for precipitates formed at low temperature in a metal matrix [1, 4]. (ii) These amorphous precipitates are stable over remarkably long treatment durations and, under certain conditions can transform into a crystalline phase of the same composition [5]. (iii) The observed precipitates display a cuboidal shape, which is remarkable since it is usually explained by crystallographic orientation relationships that do not exist here since Si3N4 is amorphous [1,4,5]. Indeed, considering solely the interfacial energy would lead to expect a sphere. (iv) The development of uniquely octapod-shaped nanosized amorphous silicon nitride precipitates in a ferrite matrix was observed at 650°C (not at 570°C) [7]. It was explained (but not proved) by the anisotropic growth induced by anisotropic stress field around the developing precipitates after nucleation. We will come back to this point later.
Morphological instabilities are not only fascinating from a scientific point of view, they also play an important role in the evolution of mechanical properties. For example, the transformation of a spherical particle into a cubic particle negatively affects the ductility of the material due to the increase in plastic strain concentration in the particle sharp corners [8,9]. In superalloys, it is also recognised that changes in precipitate morphology during exposure to typical service temperature influence the mechanical properties [10,11]. It is therefore important to gain a better understanding of the phenomena behind morphological instabilities. To this end, a number of studies have been carried out in Ni-based, Ti-Al based, Al-based alloys during ageing [12−21]. For instance, in Ni-based superalloys, sphere-to-cube and cube-to-plate shape transitions were reported together with the so-called “reverse coarsening” corresponding to the splitting of a single cuboidal precipitate into a pair of plates. Another well-known example is the evolution of Al3Sc precipitates from spheres to cuboids and then to petals [19−20]. Even if it is difficult to summarise all the conclusions of these studies, given their scale, most of the morphological instabilities can be explained on the basis of thermodynamic arguments, taking into account the contributions of interfacial and elastic energies as well as their anisotropies [22,23]. However, kinetic instability phenomena can play an important role. Indeed, as suggested very recently in the case of precipitation of iron-rich precipitates in Cu-Fe-Co alloys, the morphological instability which turns the flat facets into concave ones cannot be understood by means of thermodynamic arguments only [24]. The kinetic instability phenomena linked to diffusional processes necessarily play a significant role in the observed changes.
In this work, we highlight a size-dependent morphological instability that occurs during the pure growth of Si3N4 in α-ferrite at 570°C. The precipitates nucleate in the form of spheres and gradually transform into cubes and further, into octapods. To clarify the mechanisms involved, the microstructural evolutions was investigated using Transmission Electron Microscopy (TEM) and 3D modelling able to describe the time-evolution of both chemical and mechanical fields and their interactions. In particular, this work clarifies for the first time the interplay between diffusional processes and local mechanical fields in the morphological instability of amorphous Si3N4 precipitates in ferrite during nitriding, an important point for controlling the mechanical properties of nitrogen steels.
4. Results and Discussions
Intense precipitation was revealed on replica bright field TEM images (
Figure 7). The precipitates observed were between 15 and 50 nm in size in the Fe-1.5wt% alloy nitrided for 8h. They form homogeneously and heterogeneously on the ferritic grain boundaries. Regions in the proximity of some grain boundaries are found to be free of precipitates. These precipitate free zones (PFZ’s) are usually explained by two possible effects. First, precipitates may nucleate first at the grain boundaries (GBs), which are prime nucleation sites, thereby depleting the solute from the adjacent matrix and drastically reducing the driving force for precipitation in this zone. Second, a grain boundary is a sink for vacancies so that regions adjacent to the boundary are unable to nucleate the precipitates, even though the matrix may be supersaturated with solute.
On a larger scale, the majority of precipitates are cuboid in shape. It is worth noting that the smallest particles adopt a more spheroidal shape (see arrows 1 and 2 in
Figure 8a). This point will be detailed later in the paper. The diffraction pattern from an isolated extracted particle and the high-resolution image from a thin foil confirm the amorphous nature of the precipitates (
Figure 8b and c). This result is consistent with earlier observations [1,2,4,5]. The composition of these precipitates has also been investigated through various approaches both direct and indirect [1,4,5]. EPMA measurements showed that the average ratio of Si/N was 0.76 ± 0.06 to be compared with the expected 0.75 of stoichiometric Si
3N
4 [1]. Direct electron energy loss spectroscopy (EELS) analyses of the precipitates in the TEM gave a composition of Si
3N
3.98±0.16 [4]. These techniques do, however, raise the question of the precise measurement of nitrogen concentration. Recently, in the same alloys than studied here (Fe-3.5 wt%Si), and using 3D Atom Probe Tomography (3D ATP) we have shown that Si content in silicon nitrides is ~42 at% and N concentration is ~55.5 at%, yielding a Si/N ratio of 0.757 [71]. This ratio is in excellent agreement with the expected 0.75 stoichiometric ratio for Si
3N
4, and suggests that the precipitates deviate little from the Si
3N
4 stoichiometry. All experimental data converge to a Si
3N
4 stoichiometric precipitate in a large range of experimental conditions, which suggests a low tolerance of the phase for deviation from stoichiometry, as is expected for a covalent compound [71]. Based on these experimental pieces of evidence, it will be assumed in the following that the amorphous precipitates are stoichiometric Si
3N
4 silicon nitride.
By positioning the
directions of ferrite on a brightfield TEM image taken along the
zone axis, it was found that all intragranular precipitates having a cuboid shape were systematically oriented with respect to the ferrite matrix (
Figure 9) [6]. As it can be seen in
Figure 9, the faces of the cuboidal shapes are always parallel to {100} α-Fe planes. Upon closer examination using Scanning Transmission Electron Microscopy (STEM) method (bright field mode), it can be noticed that the shape of the precipitates evolves with their size (
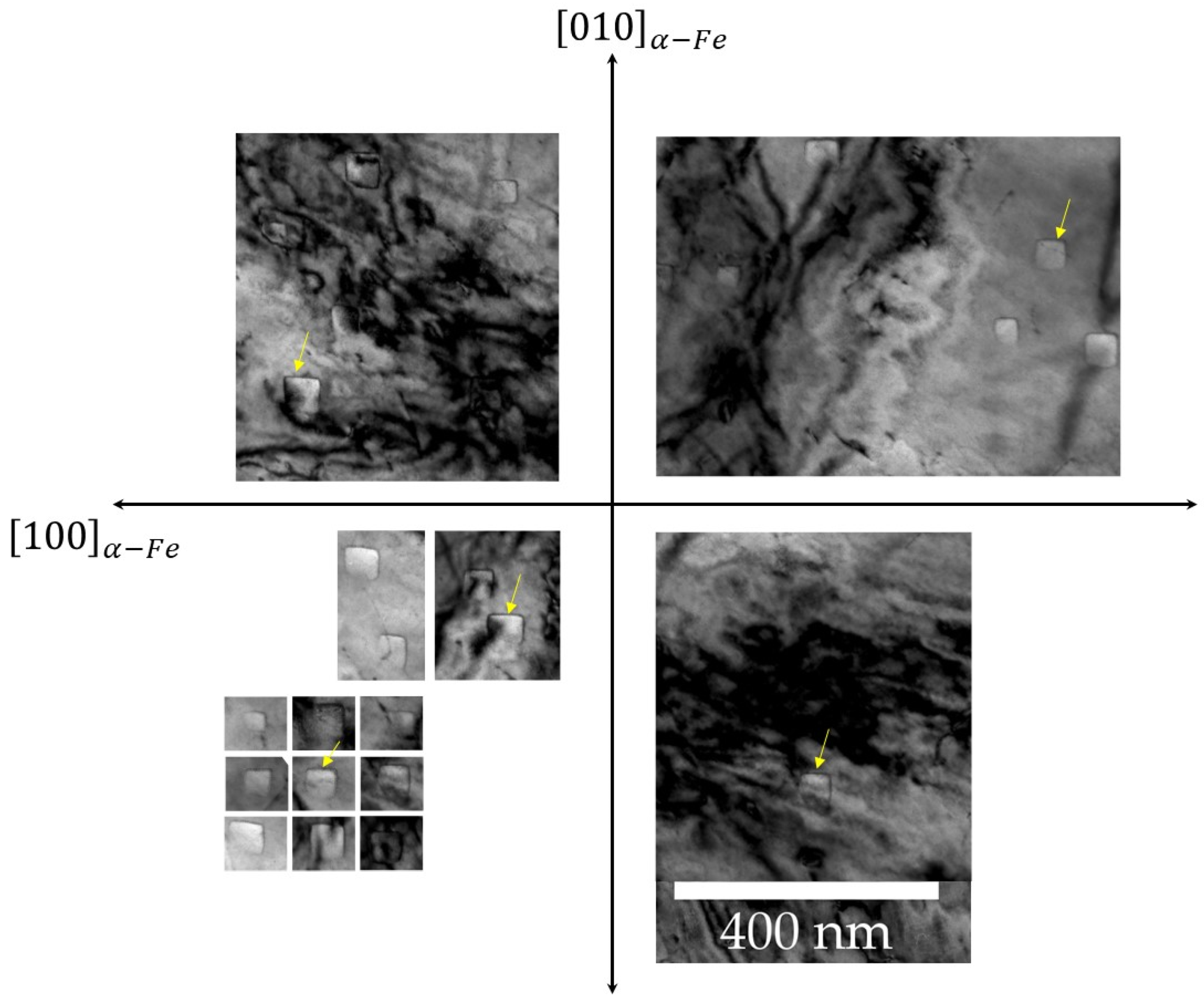
Figure 10). At small sizes, the precipitates have a shape that is close to a sphere and as they grow, they turn into cubes with bulging faces. As they grow even further, the faces become concave and the corners of the cuboid develop into lobes, resulting in an ear (or octapod)-like morphology.
We were particularly interested in the morphological instability sequence. Working exclusively on extractive replicas (see an example in the top left insert of
Figure 11), the relationship between equivalent size and morphology was studied for the Fe-1.5wt%Si alloy nitrided for 8h. Several fields covering about 1.5 µm² were analysed and we proceeded step by step. Firstly, the different morphologies observed were classified according to three types: spheroid, cuboid and octapod-like morphology. Next, the average characteristic size of particles with the same morphology was measured; the characteristic size being taken as the diameter for spheroids and the corner to corner distance for cuboids and octapod-like particles. Although subject to inaccuracies in shape and mean size, this method nevertheless allows a semi-quantitative study to be carried out. The morphological instability of precipitates can be further characterised by the non-dimensional length parameter
yielding the largest length of precipitate along the <111> direction and the smallest length along the <001> direction of the matrix. In the case of spherical particle,
, for cube,
, and
corresponds to the concave cuboid shape preceding the octapod-like shape of precipitates. This shows that there is a one-to-one link between particle size and particle morphology (
Figure 11). For an average characteristic size of less than 25 nm, we found that amorphous Si3N4 particles are spheroid-shaped, for sizes between 25 and 45 nm they are cuboid-shaped and for sizes greater than 45 nm they have an are octapod-like morphology characterised by the formation of lobes at the corners of the particles which can be described as a branching phenomenon (see the sequence shown schematically on the left of
Figure 11). It is interesting to note that (i) the cuboid shape observed is characterised by a parameter
, which means here that the corners of the cube are smooth. (ii) for
we observe the formation of a concave surface on the (100) faces.
To analyse the observations made, it should be noted that, on the one hand, the anisotropy of the interfacial energy between amorphous Si
3N
4 and ferrite is expected to be small, analogous to liquid solid interfaces [72], and consequently, equilibrium shape obtained from the Wulff construction would be close to sphere. On the other, the formation of amorphous Si
3N
4 in the ferrite is accompanied by internal stresses because the two phases have different molar volumes. Consequently, we must consider that the equilibrium shape of precipitates from minimising the sum of the interfacial and the elastic energies at constant volume. The relative contributions of elastic and interfacial energy to the total energy of the system can be evaluated through the dimensionless parameter
defined as follow [73,74]:
where
is the misfit strain,
is an elastic constant of the matrix,
l is the characteristic size of the precipitates and
is the surface energy. It is worth noting that the quantity
scales the elastic energy density.
Although this approach can only be applied rigorously under specific conditions (for example same elastic constants for the matrix and the precipitates), it nevertheless provides a simple explanation of our observations on the morphological transition between spheroid and cuboid shapes. For small
(i. e. small
l and/or small elastic energy density), interfacial energy is the dominant factor in setting the equilibrium shape. In this case, the spheroid shape is expected to be stabilised. This corresponds perfectly to our observations, as most Si
3N
4 amorphous particles with a characteristic size of less than 25 nm are spheroid-shaped. Since
scales linearly with
l, it is clear that as the particle increases in size, the effects of the elastic stress on the equilibrium shape become more important. In that case, the spherical particle is no longer in equilibrium. The development of a fourfold anisotropy in the particle shape is then expected, and depends on the elastically hard and soft directions of the matrix [74]. In our case, it is well known that the Young’s modulus of the α-Fe matrix phase in the <100> directions are lower than in both the <110> and <111> directions; the <100> directions corresponding to the elastically soft directions [75]. It can be shown in 2D that the high interfacial concentration near the <100> direction results in the flow of mass to the region of the interface near the <110> direction which, is low in concentration [74]. This is expected to produce an increase in curvature along the elastically hard <110> directions and decrease in curvature along the elastically soft <100> directions of the matrix. The resulting morphology would be a cuboid shape that never develops the sharp corners of flat sides characteristic of a cube and whose faces would be always parallel to {100} α-Fe planes. This is in perfect agreement with our observations, as we have shown that all cuboid-shaped Si
3N
4 particles have faces parallel to {100} α-Fe planes (see
Figure 9). This work also provides experimental evidences that misfitting particles with nearly isotropic interfacial energy in an elastically anisotropic medium can exhibit some degree of fourfold anisotropy and that the magnitude of the fourfold anisotropy will increase with particle size. The origin of such a phenomenon is mainly linked to the elastic strain energy. However, large
does not necessarily mean that the misfit must be large, since
scales linearly with the particle size
l. Thus, even particles with small misfits will eventually exhibit the effects of elastic stress for sufficiently large particle sizes.
To better understand the whole sequence of morphological instability, it is now interesting to focus on the transition from cuboid to octapod-like morphology. Observations show that this transition takes place via a transformation of quasi flat-facets into concave ones (see
Figure 10 and
Figure 11). However, neither the elastic strain energy nor the interfacial energy favour the formation of a concave interface. It is therefore reasonable to suspect that the morphological instability results from kinetic effects. To verify this hypothesis, we propose to evaluate the effects of diffusion fields alone, in the presence and absence of internal stresses, using the 3D model developed. The model system chosen corresponds to an
A-
B binary composed by two phases
α and
β. The initial state corresponds to a single particle
β of cubic shape placed in a matrix
α. The initial volume fraction of
β is
. The elastic constants used for the simulation correspond to those of ferrite for the
α phase and Si
3N
4 for the
β phase. These data are given in
Table 2.
Figure 12 gives the evolution of both the volume fraction
and the morphology of a particle
β, initially cubic in shape, as a function of the dimensionless time
.Firstly, as expected, the growth kinetics is lowered when the misfit between particle and the matrix increases. This is also clearly exhibited on the 3D morphologies obtained at the same dimensionless time
for which the volume fraction of
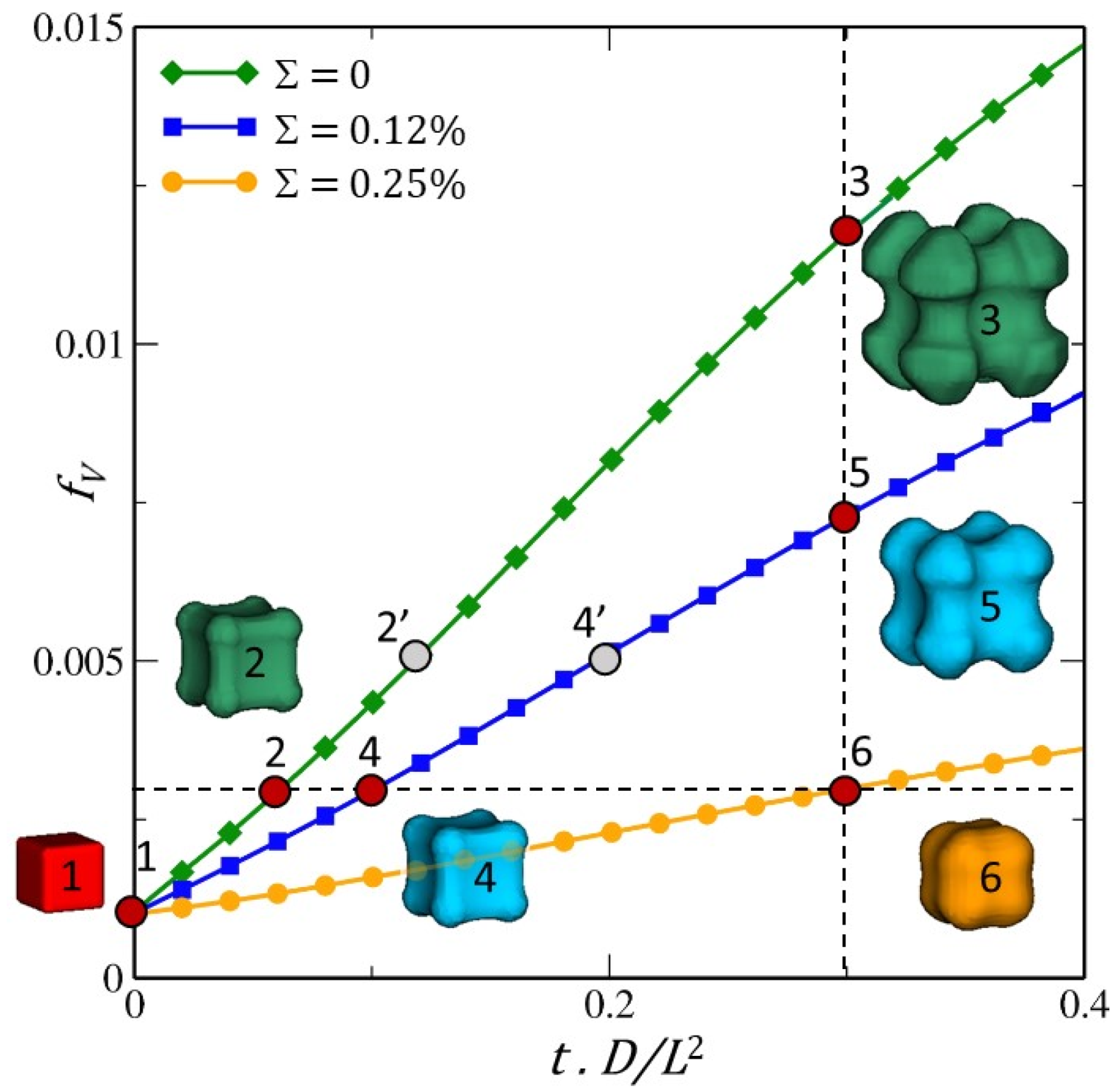
β are higher as the misfit decreases (see points 3, 5, 6 in
Figure 12). Secondly, it is clear that the resulting morphology depends on the misfit and therefore on internal stresses. The formation of the octapod-like morphology seems to be favoured in the absence of internal stresses.
It can be seen that when the driving force for growth is relatively high (3 and 5 in
Figure 12), the lobes of the cube appear facetted and, compared with observations, much less pointed in the < 111> directions. This can be explained mainly by the fact that the interfacial and anisotropic effects of diffusion were neglected to better highlight and to decouple the effects of stress and diffusion fields on morphological evolution.
To go further, we consider two different misfit values,
, and a volume fraction of
β of
(points 2′ and 4′ in
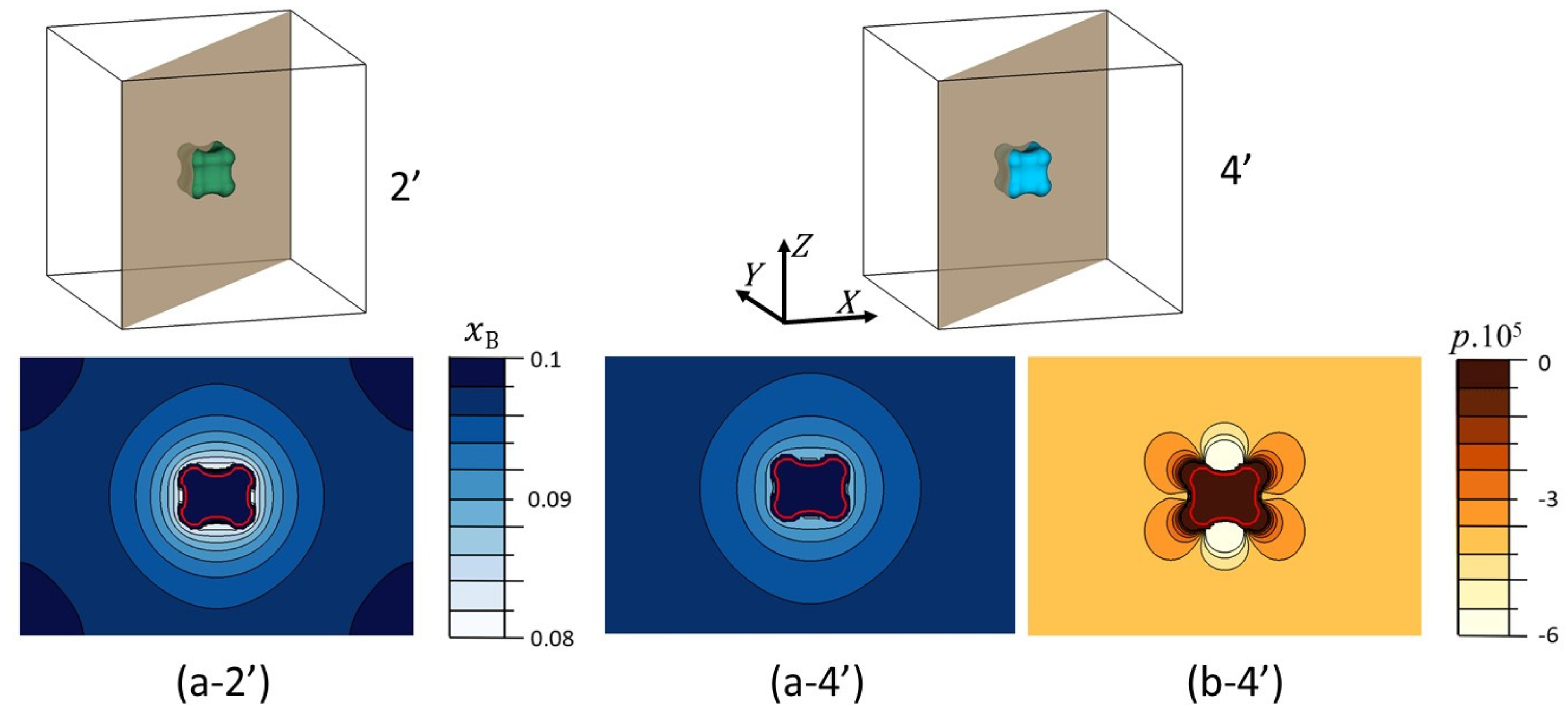
Figure 12). The analysis of the composition and stress fields in a (110) plane of the matrix α shows
Figure 13 that : (i) the composition gradient around the particle is less marked in the presence of internal stresses (comparison between
Figure 13a-2′ and
Figure 13a-4′). This is perfectly consistent with the fact that internal stresses slow down the growth kinetics of β. (ii) Both composition and stress fields are anisotropic (see
Figure 13a-4′ and
Figure 13b-4′). (iii) in both cases,
and
, we observe the formation of lobes in the corners of the cube, i. e. along the <111> directions of the matrix. The latter can appear well before the diffusion fields overlap (clearly visible in
Figure 13a-2′). It is therefore not mainly linked to the interaction between the concentration fields around the particle and the boundaries of the system.
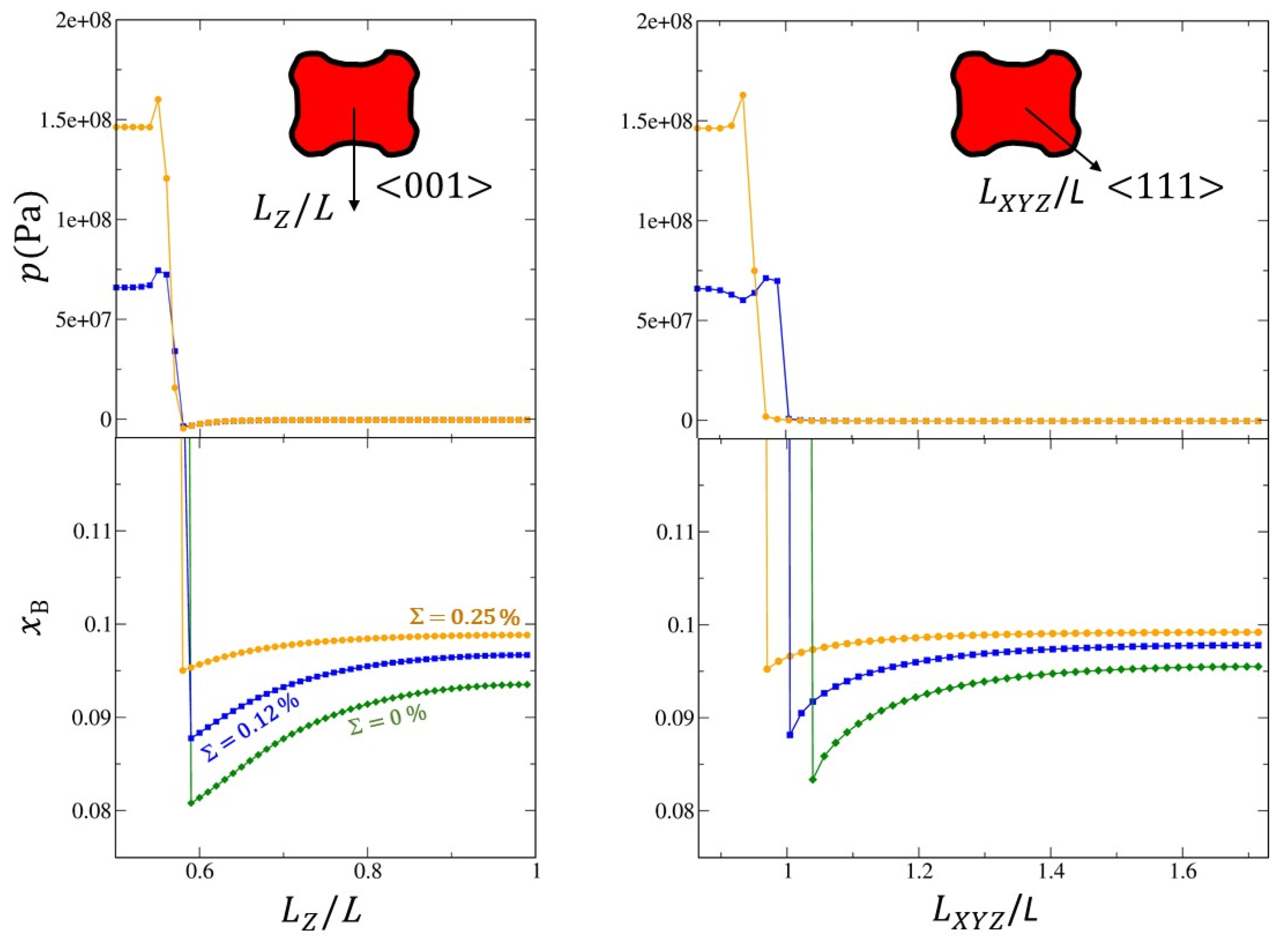
These general observations provide an overview of the dynamics of the morphological instability. To refine them, both the concentration and stress profiles were plotted in the <001> and <111> directions of the matrix as a function of the misfit (
Figure 14).
The concentration gradients at the
interface are a marker of the interface mobility and therefore of the growth rate of β into α. When there are internal stresses (for
and
), particle β is in compression and matrix α in tension. The greater the misfit, the greater the level of stress in the particle
β. We clearly show
Figure 14 that the concentration gradient in the matrix at the
interface is steeper in the <111> direction than in the <001> direction. This explains why the corners of the cuboid grow faster than the faces.
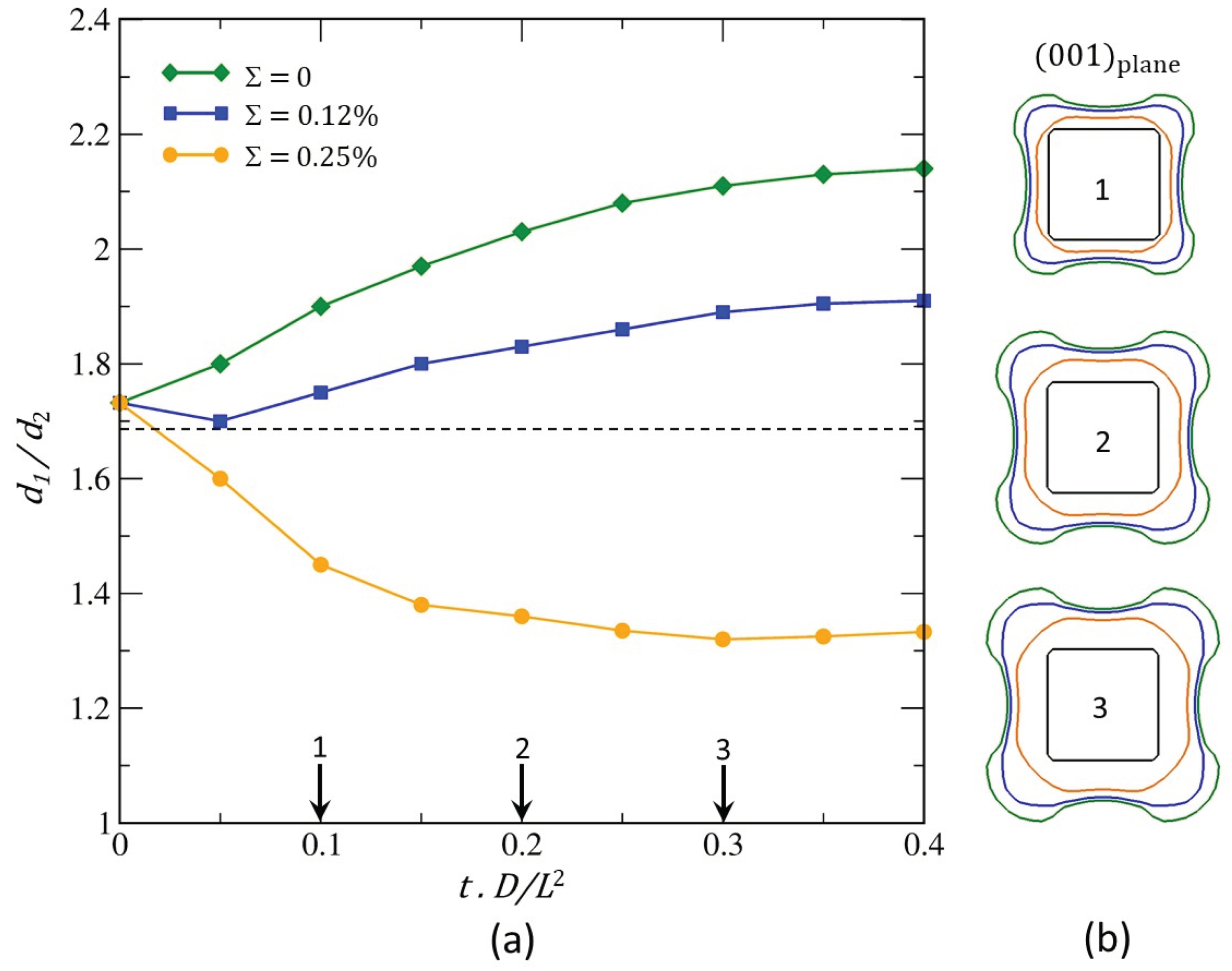
This process has two consequences. Firstly, accelerated growth of the β phase in the <111> directions at the expense of growth in the <001> directions. Secondly, the formation of a concave surface on the faces of the cube. Consequently, the cuboid/octapod-like morphology instability is indeed the result of a kinetic effect induced by the diffusion fields. However, internal stresses play an important role in this instability and, then on morphological evolution. Indeed, we can suspect that the relative growth of β in the <111> and <001> directions depends on the misfit. To confirm this, the evolution of the dimensionless parameter
yielding the largest length of precipitate along the <111> direction and the smallest length along the <100> direction was plotted as a function of
and dimensionless time (
Figure 15a). The morphological evolution in the (001) plane of the matrix are given for three dimensionless time 0.1, 0.2 and 0.3 (
Figure 15b). Remember that
and
correspond to the spherical and cubic shapes respectively, it is clear that diffusion field alone destabilises the cube to octapod-like shape (green curve in
Figure 15a and green outline in
Figure 15b) because the
ratio increases as the
β particle grows. When the internal stresses increase (i.e., the misfit) we can have two different situations. One in which the
ratio increases as the particle grows for a misfit of 0.12% (blue curve in
Figure 15a). In this case, lobes in the corners of the cube and concavity of the faces of the cube will develop leading to the formation of an octapod-like morphology (blue outline in
Figure 15b). However, this process will be kinetically less pronounced than in the absence of internal stresses. The other in which the
ratio decreases as the particle grows for a misfit of 0.25% (yellow curve in
Figure 15a). In that case, the octapod-like morphology is no longer observed. The internal stresses tend to stabilise the cuboid or even to destabilise cuboid to spheroid shape (yellow outline in
Figure 15b). The origin of such a phenomenon can be explained from the mechano-chemical potential gradient, which tends to cause the B atoms to diffuse from the most stressed areas (i. e. the regions located in the immediate vicinity of the cuboid corners in the matrix. See
Figure 13b-4′) towards the least stressed ones (close to the faces (001). See white zones in Figure
Figure 13b-4′). These results were recently confirmed by observations and analysis relating to the morphological transition from cube to petal of the iron-rich particle in the Cu-Fe-Co system formation [
24]. Furthermore, it was shown that one of the conditions for branching instability to occur is that elastic driving force should be smaller than the chemical driving to permit growth of the particle corners [76]. This is perfectly consistent with our work.
As a consequence, the morphological instability from cuboid to octapod-like morphology observed is linked to the interaction between the diffusion fields and the stress fields and result of two antagonistic effects. On the one hand, a stabilising effect linked to the internal stresses and, on the other, a destabilising effect linked to the anisotropy of the diffusion fields.
It is interesting to note that the co-formation of crystalline Si3N4 and octapod-shaped nanosized amorphous Si3N4 precipitates in a ferrite matrix was observed upon nitriding of Fe–4.5 at.%Si alloy for 48h at 650°C [3]. The octapod morphology was attributed to the highly anisotropic stress field around the developing precipitates, which would favour the development of lobes in the <111> directions of the matrix. Our findings show instead that the elastic stress field favours the growth of a cuboid precipitate to the detriment of an octapod one. However, this raises questions regarding the presence of an octapod-like morphology in a system where high internal stresses are expected. In this work it was established that the octapod-like morphology formation is the result of a balance between chemical driving force and elastic driving force. In our experiments, this is observed when the characteristic size of amorphous Si3N4 is around 45 nm. This would mean that for particle sizes greater than 45nm, the elastic driving force should be smaller than the chemical driving force. In the case of nitriding, this hypothesis is very realistic for two main reasons. Firstly, the system can be considered as open due to the steady supply of nitrogen atoms from the atmosphere, which enables a large chemical driving force throughout the process [77]. Secondly, in these systems many mechanisms of stress relaxation operate. For example, a local redistribution of nitrogen in specific sites that accommodate stress is possible. This mechanism has already been observed in Fe–N thin films [78] and in Fe–N–Si sputtered films [79]. Interestingly, it was suggested that the presence of the amorphous Si3N4 precipitates requires such a stress relaxation mechanism to be operating. Indeed, experimentally, it has been shown that amorphous Si3N4 can be destabilized in favour of crystalline α-Si3N4 [5] in a low nitrogen activity atmosphere.
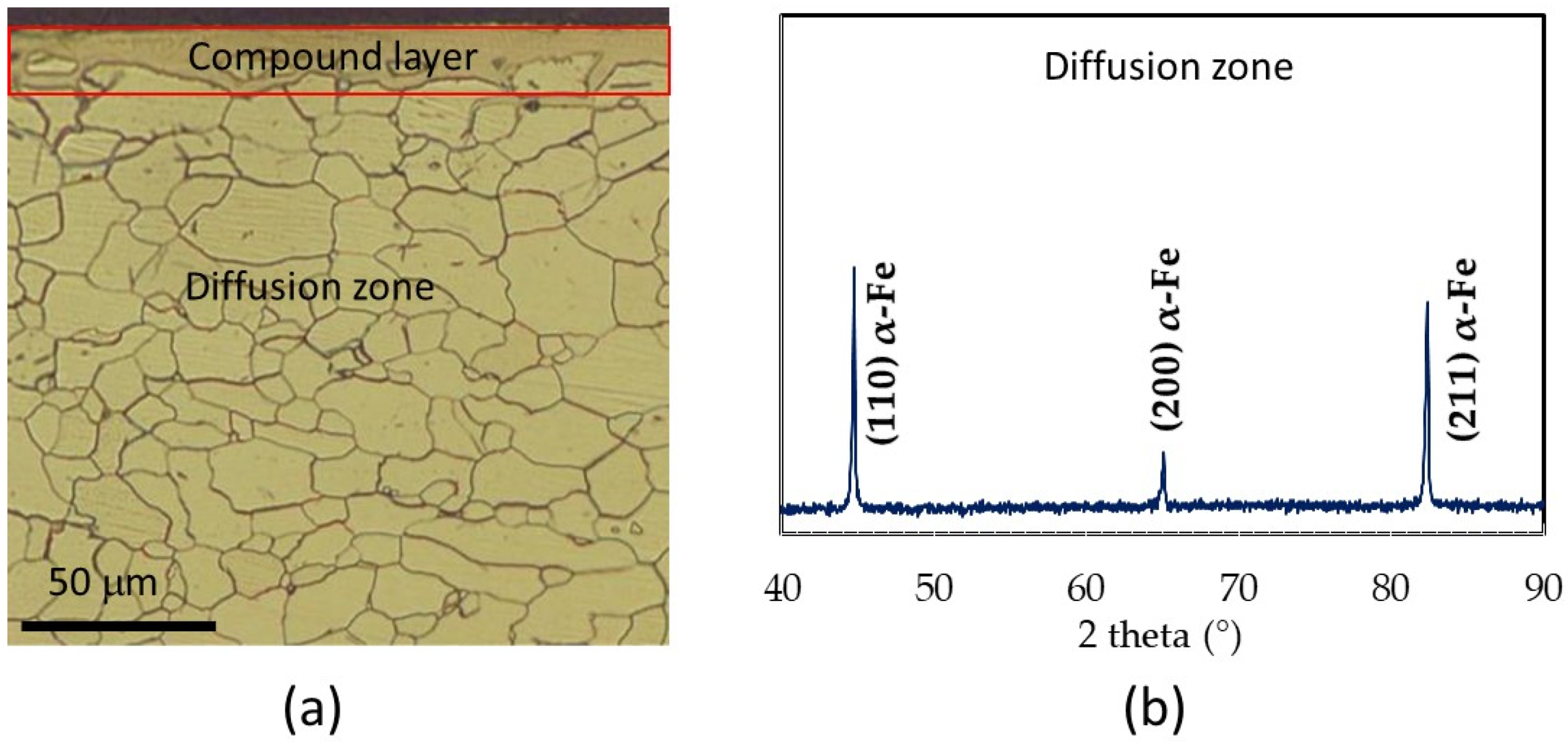
Figure 1.
(a) Micrograph obtained after nitriding of the Fe-1.5wt%Si sample at 570 °C for 8 h; (b) Diffraction pattern associated with the diffusion zone.
Figure 1.
(a) Micrograph obtained after nitriding of the Fe-1.5wt%Si sample at 570 °C for 8 h; (b) Diffraction pattern associated with the diffusion zone.
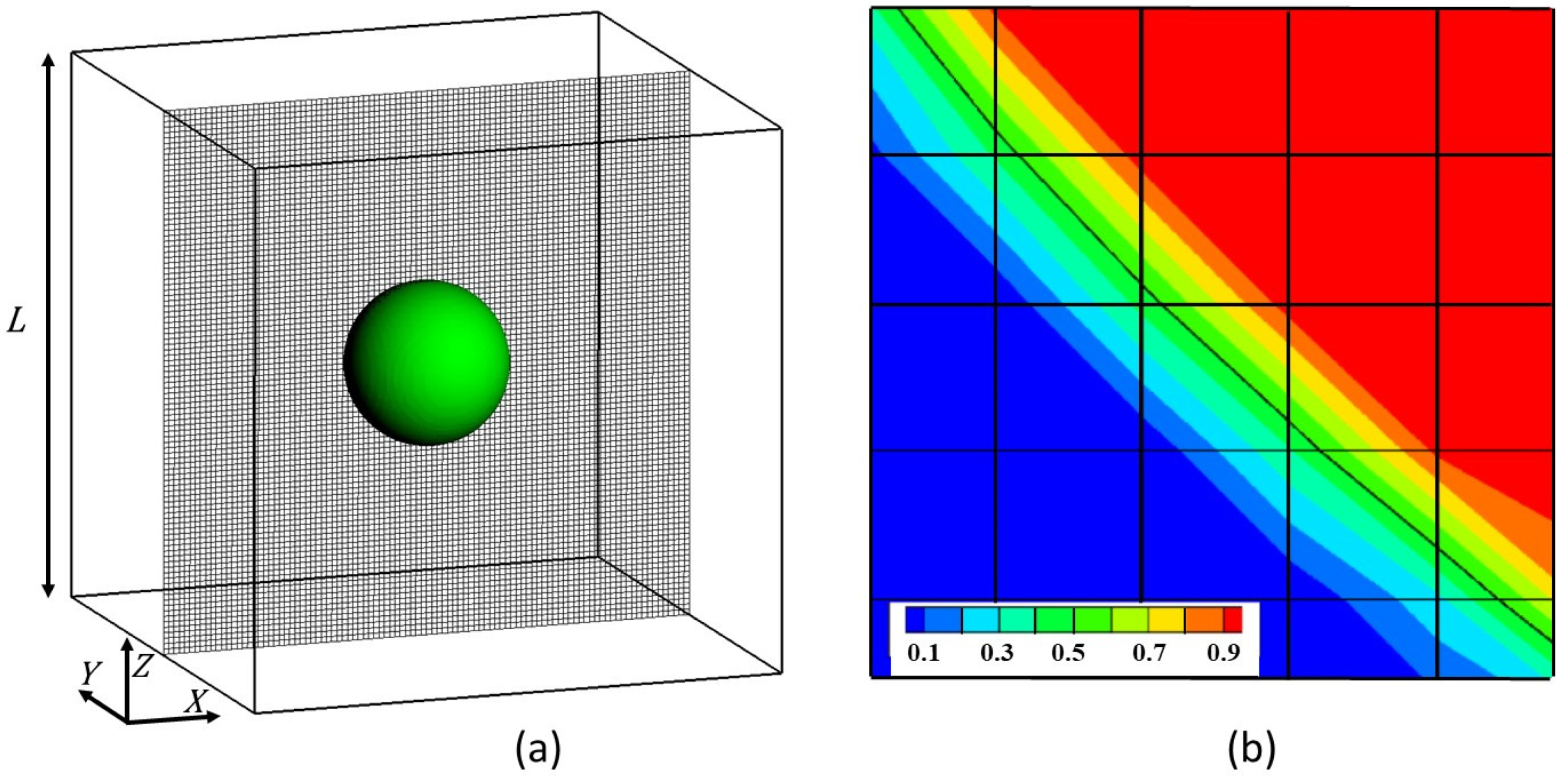
Figure 2.
(a) 3D representation of a particle/matrix system into the simulation box of length L and 2D visualization of the mesh grid in the (100)-plane; (b) 2D visualization of the phase function ϕ discriminating the matrix (blue color) from the particle (red color). At the interface, the ϕ-function varies continuously. The location of the interface is defined for with the full line in black color.
Figure 2.
(a) 3D representation of a particle/matrix system into the simulation box of length L and 2D visualization of the mesh grid in the (100)-plane; (b) 2D visualization of the phase function ϕ discriminating the matrix (blue color) from the particle (red color). At the interface, the ϕ-function varies continuously. The location of the interface is defined for with the full line in black color.
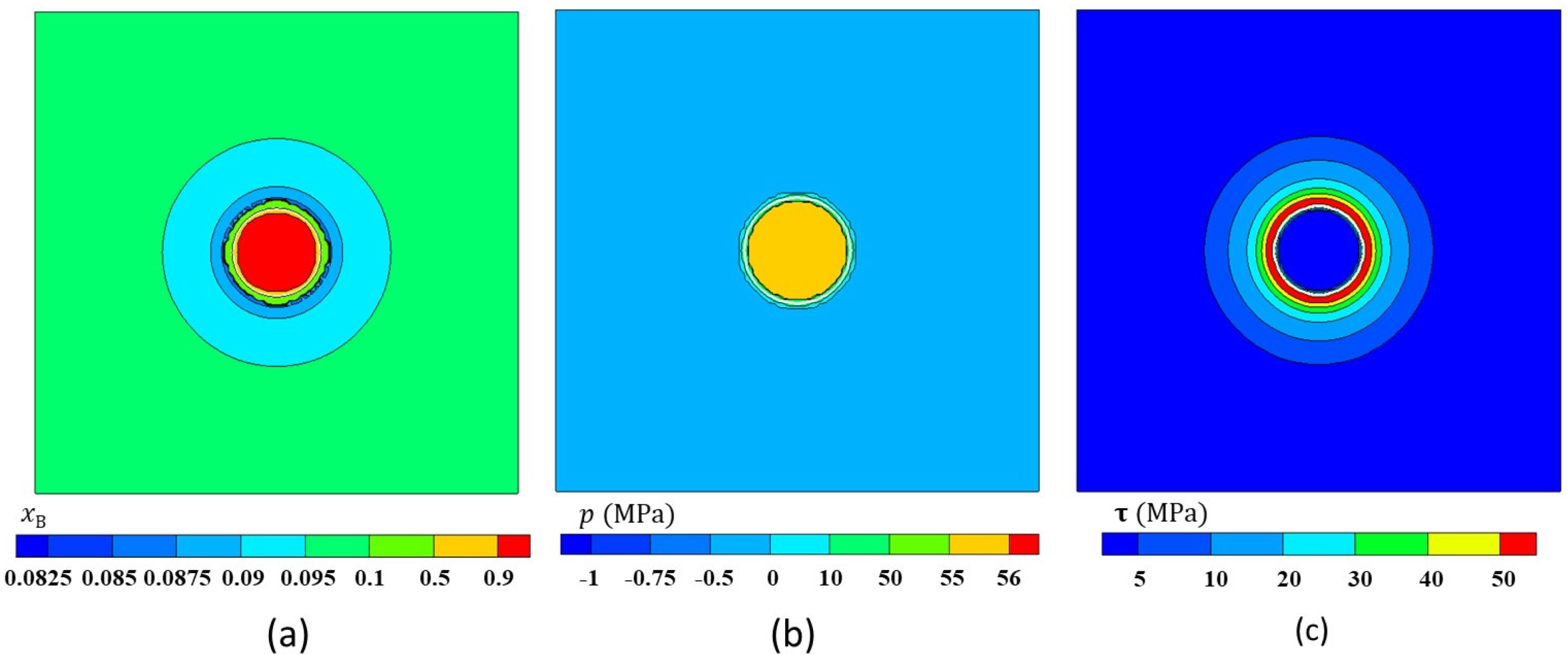
Figure 3.
Maps within the three equivalent middle planes , , and at dimensionless time of: (a) Molar fraction of B-atoms; (b) Pressure. Note that the positive value of pressure corresponds to compressive pressure, whereas negative value corresponds to tensile pressure; (c) Normalized shear stress.
Figure 3.
Maps within the three equivalent middle planes , , and at dimensionless time of: (a) Molar fraction of B-atoms; (b) Pressure. Note that the positive value of pressure corresponds to compressive pressure, whereas negative value corresponds to tensile pressure; (c) Normalized shear stress.
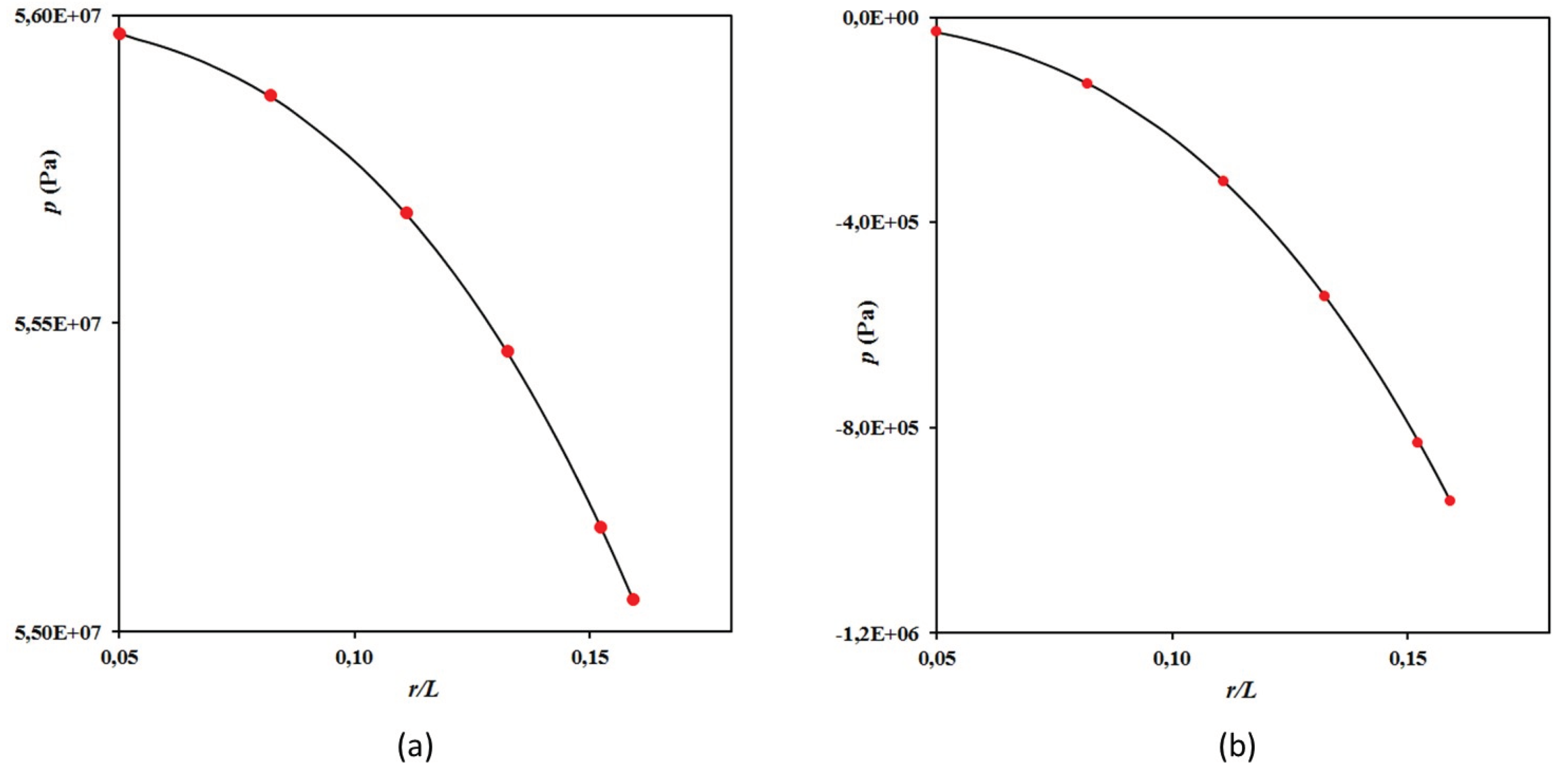
Figure 4.
Evolution of the pressure characteristics in the system as a function of the dimensionless particle radius r/L at: (a) The -point located in the β-precipitate; (b) The -point located in the α-matrix. This evolution is compared for some volume fractions (red dots) to that obtained analytically from the Scherer’s model [68].
Figure 4.
Evolution of the pressure characteristics in the system as a function of the dimensionless particle radius r/L at: (a) The -point located in the β-precipitate; (b) The -point located in the α-matrix. This evolution is compared for some volume fractions (red dots) to that obtained analytically from the Scherer’s model [68].
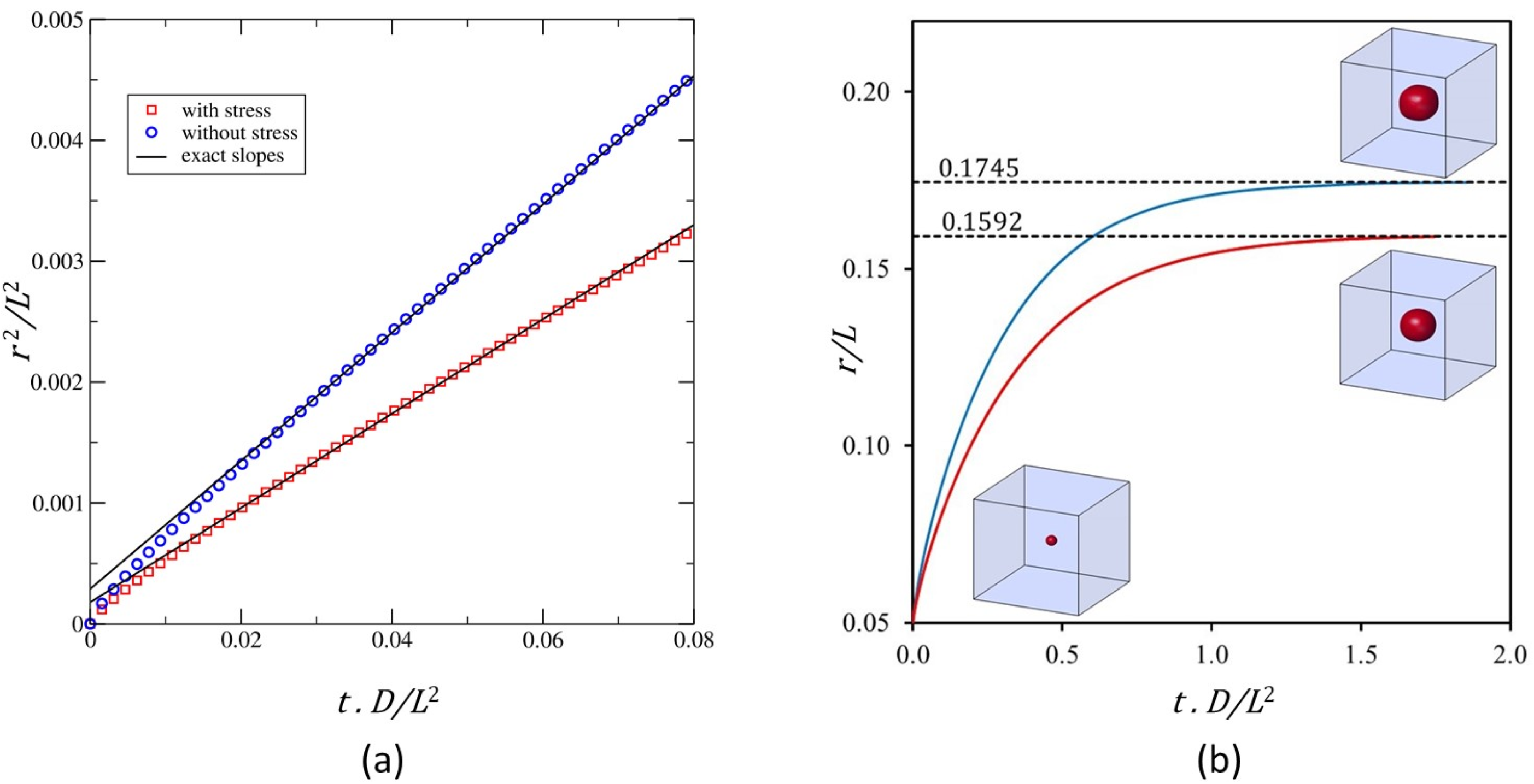
Figure 5.
Comparison of the particle growth kinetics. The blue curve and the red curve correspond respectively to the unstressed and stressed cases: (a) Evolution of the square of the dimensionless particle radius as a function of dimensionless time; (b) Evolution of the dimensionless particle radius.The dashed line corresponds to the final equilibrium values.
Figure 5.
Comparison of the particle growth kinetics. The blue curve and the red curve correspond respectively to the unstressed and stressed cases: (a) Evolution of the square of the dimensionless particle radius as a function of dimensionless time; (b) Evolution of the dimensionless particle radius.The dashed line corresponds to the final equilibrium values.
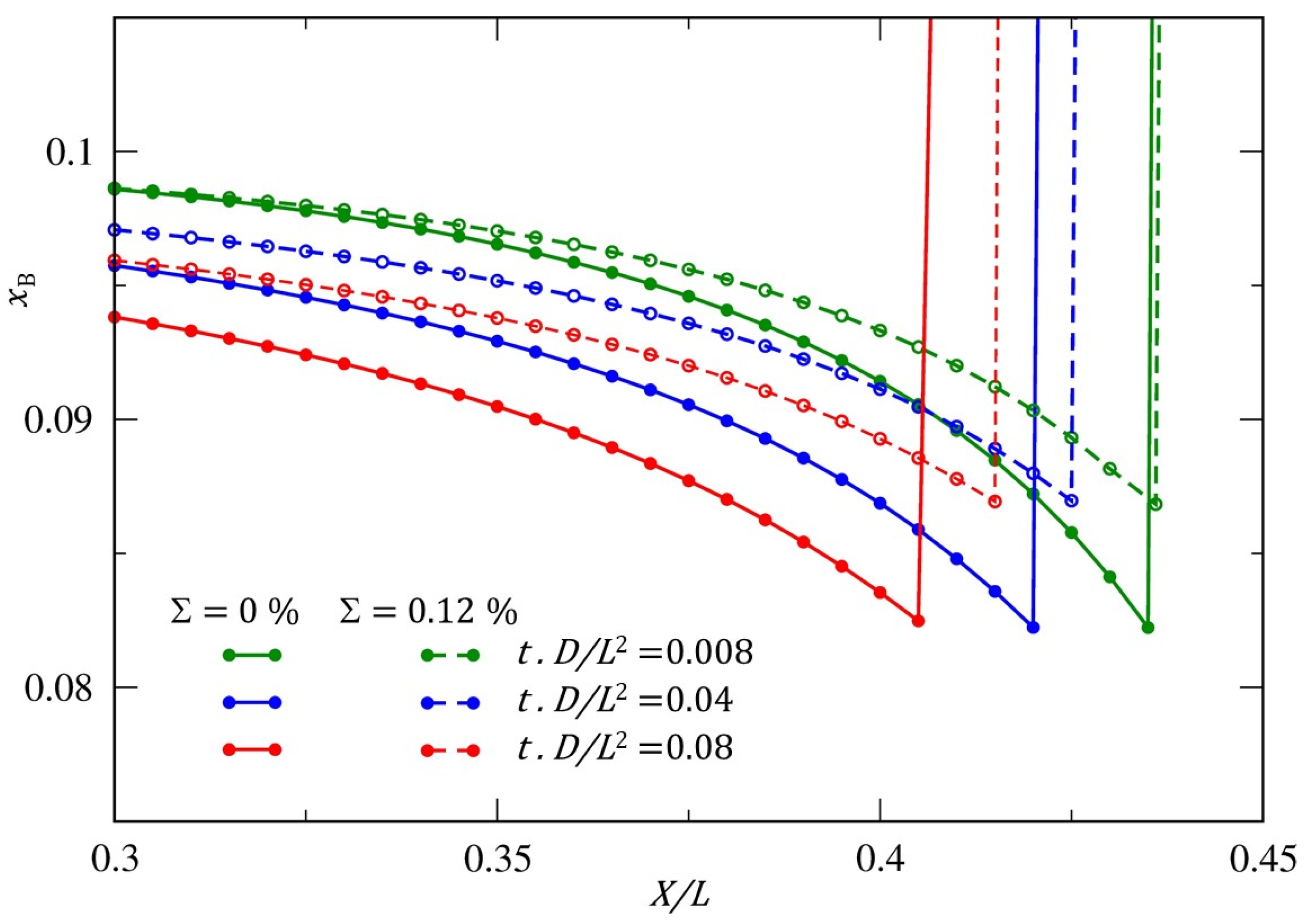
Figure 6.
Comparison of the atom fraction profiles of B-atoms within the α-phase along the three equivalent middle lines, (X/L, Y/L = 0.5, Z/L = 0.5)-line, (X/L = 0.5, Y/L, Z/L = 0.5)-line and the (X/L=0.5, Y/L=0.5, z/L)-line (x/L, y/L = 0.5, z/L = 0.5)-line during the particle growth. The solid lines and the dashed lines correspond to the unstressed and stressed case, respectively.
Figure 6.
Comparison of the atom fraction profiles of B-atoms within the α-phase along the three equivalent middle lines, (X/L, Y/L = 0.5, Z/L = 0.5)-line, (X/L = 0.5, Y/L, Z/L = 0.5)-line and the (X/L=0.5, Y/L=0.5, z/L)-line (x/L, y/L = 0.5, z/L = 0.5)-line during the particle growth. The solid lines and the dashed lines correspond to the unstressed and stressed case, respectively.
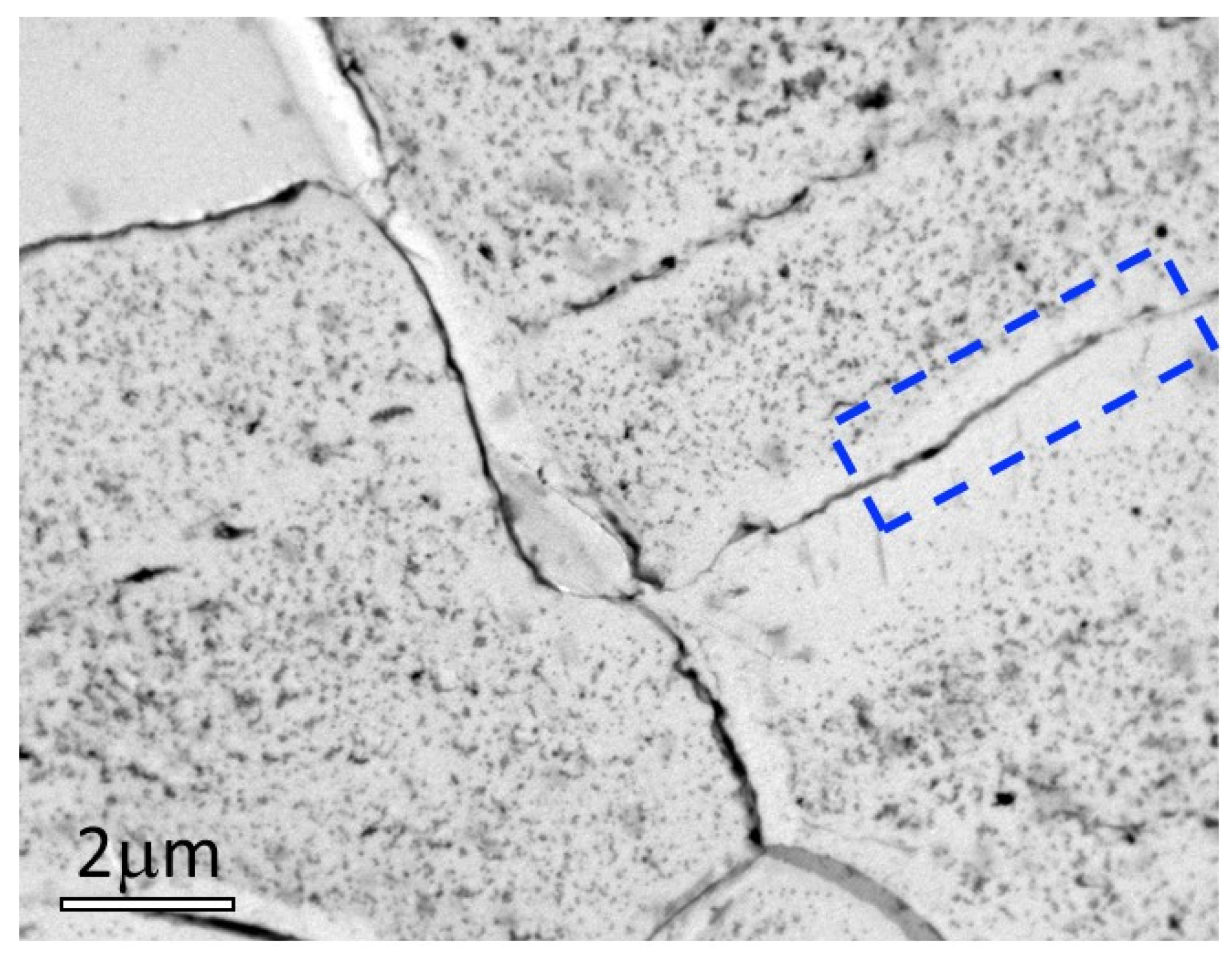
Figure 7.
Bright field TEM images of a replica extracted around 50 μm below the surface of the Fe-1.5wt%Si sample nitrided for 8 h. The micrographs show the intense precipitation of silicon nitride particles. It is of interest to note the thin depleted area along the grain boundary as shown in the dotted area.
Figure 7.
Bright field TEM images of a replica extracted around 50 μm below the surface of the Fe-1.5wt%Si sample nitrided for 8 h. The micrographs show the intense precipitation of silicon nitride particles. It is of interest to note the thin depleted area along the grain boundary as shown in the dotted area.
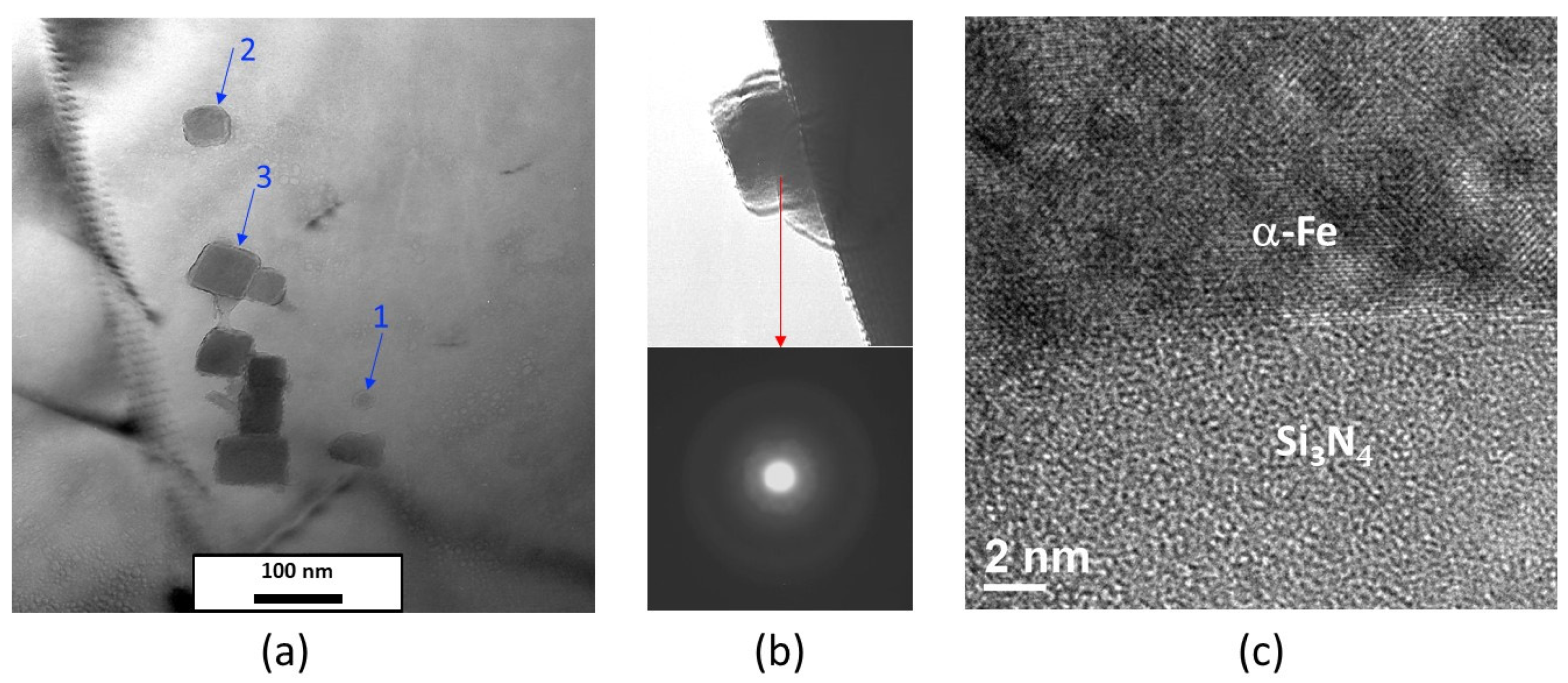
Figure 8.
(a) Bright field TEM image at 200kV of a sample of Fe-1.5wt% Si nitrided for 2h at 570°C. The arrows numbered 1, 2 and 3 show that the particles can have a quasi-spherical and/or cuboid morphology; b) Electron diffraction pattern on isolated particle extracted; (c) the contrast observed using high-resolution TEM support the amorphous nature of Si3N4.
Figure 8.
(a) Bright field TEM image at 200kV of a sample of Fe-1.5wt% Si nitrided for 2h at 570°C. The arrows numbered 1, 2 and 3 show that the particles can have a quasi-spherical and/or cuboid morphology; b) Electron diffraction pattern on isolated particle extracted; (c) the contrast observed using high-resolution TEM support the amorphous nature of Si3N4.
Figure 9.
Bright field TEM images of a sample of Fe–1.5wt% Si nitrided for 4 h at 570°C in the zone axis. The images were rotated in such a way that the of the ferrite are represented in an orthogonal reference frame. The faces of these cuboids are all systematically parallel to {100} planes of the matrix as indicated by the arrows.
Figure 9.
Bright field TEM images of a sample of Fe–1.5wt% Si nitrided for 4 h at 570°C in the zone axis. The images were rotated in such a way that the of the ferrite are represented in an orthogonal reference frame. The faces of these cuboids are all systematically parallel to {100} planes of the matrix as indicated by the arrows.
Figure 10.
Bright field image of a thin foil prepared using FIB on Fe-3.3wt%Si nitrided for 4h at 570°C. There is a correlation between particle size and morphology. The smallest particles are spheroid-shaped, those of intermediate size are cuboid-shaped, while the largest have concave cuboid-shape with lobes formation at the corners.
Figure 10.
Bright field image of a thin foil prepared using FIB on Fe-3.3wt%Si nitrided for 4h at 570°C. There is a correlation between particle size and morphology. The smallest particles are spheroid-shaped, those of intermediate size are cuboid-shaped, while the largest have concave cuboid-shape with lobes formation at the corners.
Figure 11.
– Relationship between the measured characteristic sizes of amorphous Si3N4 precipitates, the dimensionless parameter and their observed morphologies in the Fe-1.5wt% nitride for 8 h. for a sphere, for a cube and corresponds to the concave cuboid shape preceding the formation of lobes at the corners of the particles. The inserts at the top-left and in the figure correspond to TEM images obtained on replicas. Morphological instabilities and the corresponding critical sizes are shown schematically on the right of the figure.
Figure 11.
– Relationship between the measured characteristic sizes of amorphous Si3N4 precipitates, the dimensionless parameter and their observed morphologies in the Fe-1.5wt% nitride for 8 h. for a sphere, for a cube and corresponds to the concave cuboid shape preceding the formation of lobes at the corners of the particles. The inserts at the top-left and in the figure correspond to TEM images obtained on replicas. Morphological instabilities and the corresponding critical sizes are shown schematically on the right of the figure.
Figure 12.
Effect of misfit on the growth kinetics and morphological evolution of the β particle as a function of dimensionless time. Three misfit values, Σ, were considered: 0.25%, 0.12% and 0%. Points 2, 4, 6 and 3, 5, 6 are located on volume iso-fraction and iso-dimensionless time , respectively. Points 2′ and 4′ correspond to a volume fraction of 0.5% and a misfit of 0.12 % and 0.25 % respectively.
Figure 12.
Effect of misfit on the growth kinetics and morphological evolution of the β particle as a function of dimensionless time. Three misfit values, Σ, were considered: 0.25%, 0.12% and 0%. Points 2, 4, 6 and 3, 5, 6 are located on volume iso-fraction and iso-dimensionless time , respectively. Points 2′ and 4′ correspond to a volume fraction of 0.5% and a misfit of 0.12 % and 0.25 % respectively.
Figure 13.
Distribution of both the composition and stress fields in the (110) plane of the matrix
α for the two points 2′ and 4′ shown in
Figure 12 for
. The particle
β is located inside the red outline. (a-2′) Concentration map of
B atoms in the absence of internal stresses (
0); (a-4′) Concentration map of
B atoms for the misfit
; (b-4′) Stress field for
.
Figure 13.
Distribution of both the composition and stress fields in the (110) plane of the matrix
α for the two points 2′ and 4′ shown in
Figure 12 for
. The particle
β is located inside the red outline. (a-2′) Concentration map of
B atoms in the absence of internal stresses (
0); (a-4′) Concentration map of
B atoms for the misfit
; (b-4′) Stress field for
.
Figure 14.
Evolution of the concentration and stress profiles along the <001> and <111> directions of the matrix as a function of misfit (, and ). Obviously, the stress profile for was not plotted because there are no internal stresses in this case.
Figure 14.
Evolution of the concentration and stress profiles along the <001> and <111> directions of the matrix as a function of misfit (, and ). Obviously, the stress profile for was not plotted because there are no internal stresses in this case.
Figure 15.
Evolution of: (a) The dimensionless parameter yielding the largest length of precipitate along the <111> direction and the smallest length along the <001> direction as a function of misfit and dimensionless time; (b) The morphology of precipitate β as a function of misfit and at three given dimensionless time 0.1, 0.2 and 0.3 in the (001)-plane.
Figure 15.
Evolution of: (a) The dimensionless parameter yielding the largest length of precipitate along the <111> direction and the smallest length along the <001> direction as a function of misfit and dimensionless time; (b) The morphology of precipitate β as a function of misfit and at three given dimensionless time 0.1, 0.2 and 0.3 in the (001)-plane.
Table 1.
Parameters for the calculation of the kinetic coefficients κ for unstressed and stressed configurations.
Table 1.
Parameters for the calculation of the kinetic coefficients κ for unstressed and stressed configurations.
| |
|
|
|
(s-1) |
ω |
κ |
| Without stress |
1.0 |
0.08 |
0.01 |
1.0 x10-6
|
0.021739 |
0.1151359 |
| With stress |
1.0 |
0.08 |
0.01 |
1.0 x10-6
|
0.01663 |
0.0994093 |
Table 2.
Data used for modelling.
Table 2.
Data used for modelling.
| |
Symbol |
α-phase |
β-phase |
| Initial molar fraction |
|
0.1 |
1 |
| Equilibrium molar fraction |
|
0.08 |
1 |
| Young’s modulus (GPa) |
EY |
178 |
320 |
| Poisson coefficient |
ν |
0.30 |
0.26 |