1. Introduction
Severe asthma (SA) is a debilitating chronic disorder that affects approximately 10% of asthma patients worldwide [
1,
2,
3]. Due to its heterogeneous nature, distinct phenotypes and endotypes have been identified and prompted the sub-classification of the disease according to clinical characteristics, and functional and inflammatory parameters [
4]. Severe eosinophilic asthma (SEA) is one of the predominant subtypes of SA [
5,
6]; its pathophysiology is defined by an extensive type 2 (T2) inflammatory process mainly driven by the proliferation and activation of eosinophils. Accordingly, eosinophil number is increased in blood and sputum of SEA patients; other key characteristics are a scarce respiratory function that further deteriorates over time, and recurrent and/or life-threatening exacerbations [
7,
8,
9]. Given the high burden of its manifestations and the poor prognosis, SEA has a devastating impact on patients’ quality of life (QoL), which can be even worsened by the presence of comorbidities, among which chronic rhinosinusitis with nasal polyposis (CRSwNP) is one of the most frequently observed [
10,
11].
The recommended background therapies, which include inhaled corticosteroids (ICS) and a second controller (usually a long-acting b2-agonist [LABA]) [
12] are not always effective in managing SEA symptoms. Based on their potent anti-inflammatory action, oral corticosteroids (OCS) have been traditionally added to background medications in cases of inadequate asthma control, to prevent exacerbations. However, given the cumulative risk of significant adverse effects and mortality associated with their usage, even moderate dosages of OCS should be avoided [
12,
13,
14,
15,
16].
The development of several biological therapies has represented a giant step forward in the treatment of T2-high SA. Six biologics have thus far received approval (omalizumab, mepolizumab, reslizumab, benralizumab, dupilumab, tezepelumab); by targeting distinct pathways involved in the pathophysiology of the disease, they ensure superior efficacy and safety than OCS [
17]. Based on their different mechanism of action (MoA), each one of these pharmacological agents is expected to be more successful in patients whose asthma is predominantly sustained by the corresponding inflammatory endotype. However, SA patients frequently show overlapping T2-high features [
18]; as a result, a precise pheno-endotypization is required to identify the driving pathway of the disease and anticipate the most effective biologic treatment.
Overall, the great clinical outcomes displayed by SA patients treated with biologic therapies have highlighted the potential of reaching a status of remission from the disease. To clarify this concept, a consensus on the criteria that define clinical remission (CR), both complete (cCR) and partial (pCR), was recently reached by a panel of experts from the Severe Asthma Network Italy (SANI) study group, allowing for standardized assessment of patients regardless of the biologic treatment received [
19,
20].
Benralizumab is a monoclonal antibody (mAb) approved for the treatment of SEA [
21]. It is a humanized afucosilated immunoglobulin (Ig) Gk1 mAb that binds both interleukin 5 receptor alpha (IL-5Rα) and Fc gamma receptor IIIa (FcγRIIIa), expressed abundantly by eosinophils and natural killer (NK) cells, respectively. The simultaneous recognition of the two receptors allows benralizumab to activate antibody-dependent cell-mediated cytotoxicity (ADCC), a process through which NK cells induce the apoptosis of eosinophils [
22,
23]. The consequent nearly complete depletion of eosinophils differentiates benralizumab from the anti IL-5 mepolizumab and reslizumab [
24], and determines the well-established efficacy of benralizumab in SEA patients [
25]. In a recent study, benralizumab has been shown to have profound immunological effects that are not limited to eosinophil apoptosis but include an increase in NK cell proliferation, maturation and cytotoxic activity, and modulation of T cell subsets. Intriguingly, the number of circulating CD3+T cells and activated NK cells significantly correlated with improvement in lung function parameters in benralizumab-treated SEA patients [
26]. These results deepen our understanding of benralizumab MoA, which appears to be more complex than what was traditionally thought. These data suggest that the improvement in respiratory outcomes mediated by benralizumab may be due to a profound immunological modulation that takes place even in the absence of eosinophils.
To date, the 5-year-long MELTEMI trial represents the longest study evaluating the effects of benralizumab on SEA patients [
27]; in addition, few real-life studies have investigated benralizumab effectiveness over 20-month to 4-year-long periods [
25,
28,
29,
30,
31,
32,
33]. A marked and long-lasting reduction in exacerbations has been consistently shown across all studies, with impressive results obtained from the Italian ANANKE study, showing a reduction of 94.9% and 96.9% in all and severe annual exacerbation rate (AER) respectively, after 96 weeks of treatment [
25]. Positive outcomes have also been demonstrated in lung function and OCS reduction; however, the extent and the durability of benralizumab effectiveness on respiratory outcomes for periods longer than 2 years is still uncertain [
31,
33].
This Italian multicentric retrospective study includes a cohort of 108 SEA patients treated with benralizumab for up to 3 years. The data show changes in multiple clinical outcomes and provide a comprehensive analysis, as well as novel evidence, of benralizumab long-term effectiveness.
2. Materials and Methods
This is an observational retrospective study; data were collected from 9 Italian SANI centres specialized in the treatment of SA (Brescia, Catania, Modena, Montebelluna, Padova, Varese, Verona, Siena, Roma).
SA was diagnosed according to the European Respiratory Society (ERS) and the American Thoracic Society (ATS) guidelines [
1]. Benralizumab was prescribed to adult patients as per Italian clinical practice, according to the eligibility criteria established by the Italian regulatory drug agency (Agenzia Italiana del Farmaco, AIFA). To be eligible to benralizumab treatment, patients must have had blood eosinophil count (BEC) ≥300 cells/µL in absence of OCS treatment, and must meet one of the two following conditions: (1) at least two exacerbations in the previous 12 months despite maximum inhalation therapy (step 4-5 of the GINA document) treated with systemic steroid or requiring hospitalization; (2) continuous OCS treatment received during the previous year in addition to maximal inhaled therapy [
34]. Benralizumab was given subcutaneously at a dose of 30 mg; after the first three doses, which were administered every four weeks, benralizumab was administered every eight weeks.
A total of 108 patients were enrolled between January 2018 and February 2021; follow-up visits took place at 6, 12, 24,36 months after benralizumab initiation. Personal information including sociodemographic, clinical, functional and laboratory data were recorded as per clinical practice at baseline and during follow-up visits. Data were retrospectively collected from medical charts between April and June More in detail, the baseline characteristics included: age, gender, body mass index (BMI), smoking habit, age at asthma diagnosis, age at benralizumab initiation, comorbidities), use and dose of asthma therapies (background inhaled medications, OCS, biologics received prior to benralizumab use), laboratory tests (BEC, IgE, fractioned exhaled nitric oxide (FeNO)), exacerbations, as determined by treating physicians (expressed as AER), and pre- and post-bronchodilator (BD) lung function parameters including forced expiratory volume in 1 second (FEV1), and forced vital capacity (FVC). All respiratory measurements are pre-BD, unless otherwise specified.
Various patient reported outcomes (PROs) were also used to assess patients’ asthma control (asthma control test, ACT, asthma control questionnaire-6, ACQ), and QoL (asthma quality of life questionnaire, AQLQ). Sinonasal symptoms severity was investigated specifically in comorbid patients with CRSwNP using visual analogue scale (VAS) and sinonasal outcome test 22 (SNOT 22). The absolute differences in AER between 0 and 12 months and between 0 and 36 months were computed. Changes in laboratory parameters, AER, lung function parameters, PROs, background therapies and OCS were evaluated over time. The number and percentages of patients reaching either pCR or cCR (defined according to the criteria detailed by Canonica et al. [
19]) were determined at 12, 24 and 36 months; CR was calculated for each time point either from the corresponding previous time point (i.e., baseline to 12 months; 12 months to 24 months; 24 months to 36 months) or from baseline. pCR was defined by three criteria: 1) no use of OCS, accompanied by two of the following: either 1) good asthma control defined by ACT score≥20, and/or 2) elimination of exacerbations and/or 3) pulmonary stability. cCR was achieved by patients who met all the four criteria of: 1) no use of OCS, 2) good asthma control defined by ACT score≥20, 3) elimination of exacerbations, and 4) pulmonary stability [
19].
Informed consent was obtained from all patients involved in the study. This was a SANI study; the study was conducted in conformity with the Declaration of Helsinki and was approved by the Central Ethics Committee for the SANI Network.
Statistical analyses
For descriptive analyses, continuous variables were given as mean with standard deviations (SD) or median with range or interquartile range (IQR) and categorical variables were expressed as number of subjects (n) and percentage values.
Linear, logistic and negative binomial mixed-effect regression models were performed on the continuous, dichotomy and count values, respectively, to evaluate changes in AER, lung function, PROs scores, OCS and use of asthma medication over time. Regression coefficients, odds ratios (ORs) and exponential regression coefficients associated with each outcome were calculated with 95% confidence intervals (CI) for each factor.
The centre and subject variability were considered as random effects in all mixed-effect regression models. The likelihood ratio test was used as a test of statistical significance and p-values were adjusted for multiple comparisons by using the Holm correction method.
Differences, with a p-value less than 0.05, were selected as significant. Data were acquired and analysed in in R v4.3.1 software environment [
35].
3. Results
3.1. Patients’ characteristics at baseline
The demographics, biochemical and clinical characteristics of the study participants at baseline are summarised in table Briefly, a total of 108 patients (59.26% females, mean age 55.96 years) took part in this study; the population was highly comorbid, with 94 patients (87.04%) with at least one comorbidity. Chronic rhinosinusitis (CRS), and specifically CRSwNP, was the most common comorbidity, being present in 65 patients (60.19%). A total of 28 out of 104 patients (26.96%) had bronchiectasis and 4 out of 96 patients (4.71%) had eosinophilic granulomatosis with polyangiitis (EGPA). The mean age at asthma diagnosis was 35.68 years (15.95). All patients used ICS and LABA as background therapies, the mean ICS dose used by patients was high (1006.93 mcg/day (402.56)) and the majority of patients required additional asthma medications, including long-acting muscarinic antagonists (LAMA) (79 out of 107 patients, 73.15%), anti leukotrienes (anti LT) (52 out of 107 patients, 48.15%), theophylline (6 out of 106 patients, 5.66%). OCS were taken by 67 patients (62.04%) at a mean dose of 7.95 mg/day (7.42); a total of 41 patients used OCS dose higher than 5 mg/day (61.19%). Of note, benralizumab was the first biologic therapy used in 89 patients (82.41%), while 19 patients were switched to benralizumab after being treated with other biologics (either omalizumab or mepolizumab).
Biochemical analyses revealed that patients had a median BEC of 600 cells/mm3 (28-3350), a median total IgE level of 209 IU/ml (4.9-1940) (n=91) and a mean FeNO of 61.95 ppb (55.66) (n=55). The frequency of asthma exacerbations approached 4 events/year, with a mean AER of 3.84 (3.18) (n=107); the phenotype of exacerbations was assessed in 45 patients, among which 14 (31.11%) had exacerbations of non-infectious nature, whereas the rest of patients experienced either viral (15.56%) or bacterial induced (53.33%) exacerbations. Patients were also characterized by suboptimal lung function, showing a mean FEV1/FVC ratio of 54.11 (24.87) (n=91), mean FEV1 of 1.94 L (0.83) (n=101) and mean FEV1 predicted of 74.5% (20.68) (n=86).
Overall, asthma was uncontrolled despite the use of multiple medications and patients’ QoL was poor, as demonstrated by the low scores achieved in various PROs, including ACT (n=104), ACQ (n=39) and AQLQ (n=31) (mean values of 14.41, 2.33 and 3.76, respectively). VAS (n=60) and SNOT 22 (n=57) were administered to comorbid patients with CRSwNP and showed a mean score of 5.14 (3.13) and 47.21 (20.67) respectively, indicative of moderate sinonasal symptom severity [
36].
Table 1.
Demographic, social and clinical characteristics of patient population at baseline. Data refer to n=108 patients, unless otherwise specified, and are expressed as number of subjects (percentage), mean (SD), or median (range) as appropriate.
Table 1.
Demographic, social and clinical characteristics of patient population at baseline. Data refer to n=108 patients, unless otherwise specified, and are expressed as number of subjects (percentage), mean (SD), or median (range) as appropriate.
3.2. Benralizumab reduced exacerbations and inflammatory markers
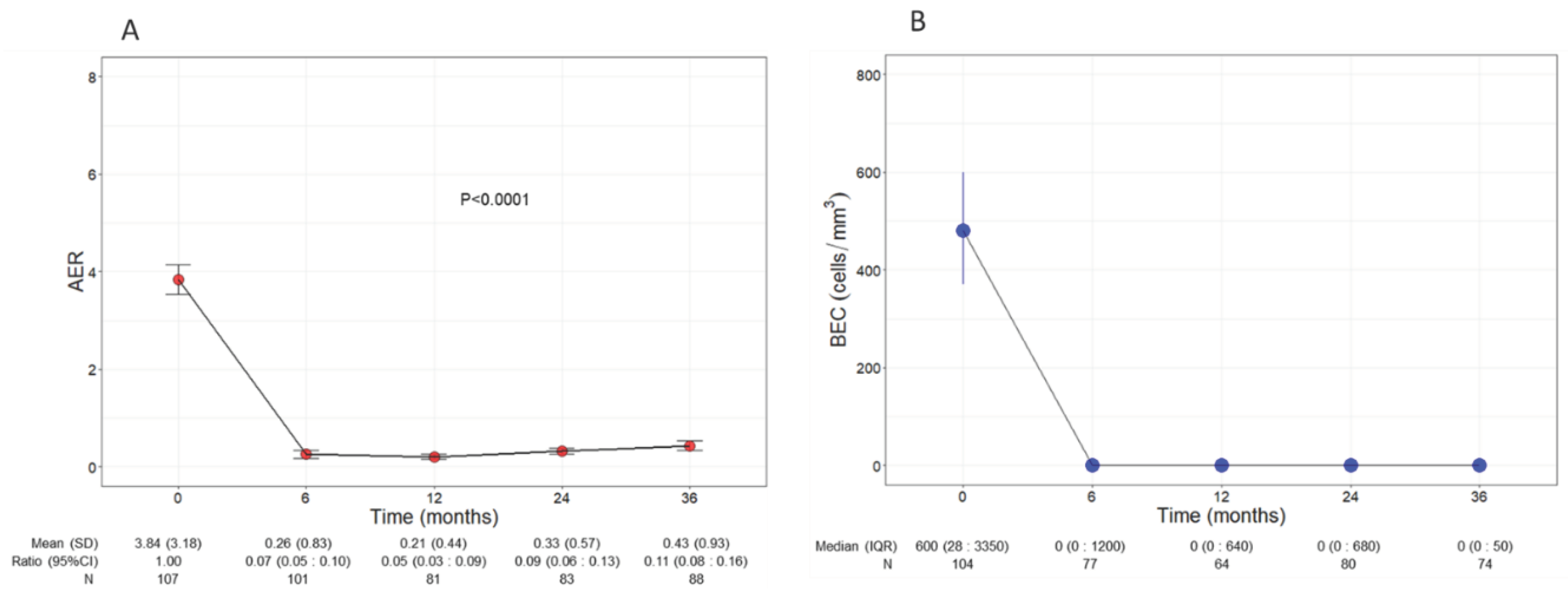
Patients experienced a significant and remarkable reduction of AER throughout the three years of treatment with benralizumab (p<0.0001,
Figure 1A); baseline AER declined from a mean of 3.84 (3.18) to 0.26 (0.83) (0.07, CI 0.05:0.01) already after 6 months of treatment and remained low at all time points, reaching 0.43 (0.93) (0.11, CI 0.08:0.16) at 36 months. Compared with baseline, the decline of AER amounted to 93%, 95%, 91% and 89% at 6, 12, 24 and 36 months, respectively.
Consistent with its anti-eosinophilic effect, benralizumab treatment induced an almost complete depletion of BEC that was sustained over time, with a median BEC of 0 (0 : 50) displayed at all time points (
Figure 1B). The drop in BEC was accompanied by a persistent reduction in FeNO, which decreased from a mean of 61.95 ppb (55.66) at baseline to 42.27 ppb (32.31) at 36 months (p=0.001,
Table S1).
3.3. Benralizumab improved lung function
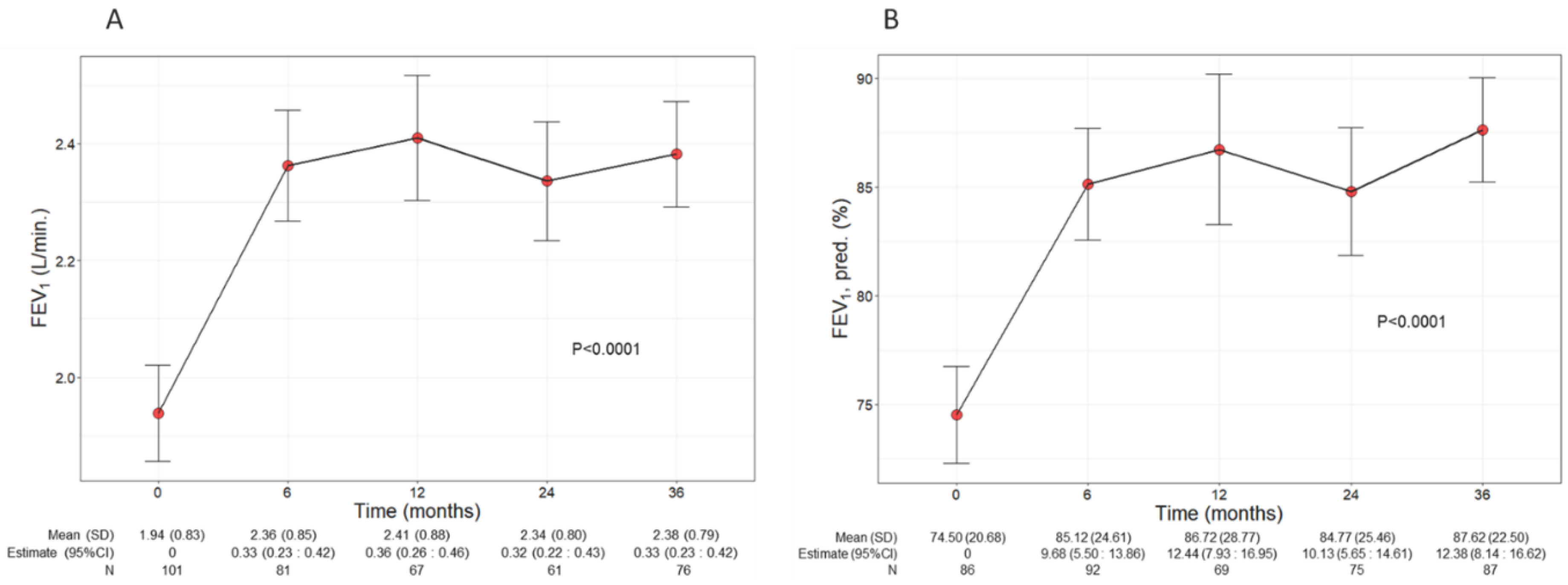
Patients treated with benralizumab ameliorated their lung function throughout the 36-month period, with a significant increment observed in FEV
1 (p<0.0001) (
Figure 2). The mean volume of FEV
1 increased from 1.94 L (0.83) at baseline to 2.38 L (0.79) at 36 months (+440 mL), with the maximal value of 2.41 L (0.88) recorded at 12 months (
Figure 2A), while percentage of predicted FEV
1 peaked at 36 months (87.62%, with a mean increase of 13.12% from baseline) (12.38, CI 8.14:16.62) (
Figure 2B). Variations in other respiratory measurements are reported in
Table S1.
3.4. Benralizumab increased asthma control and QoL
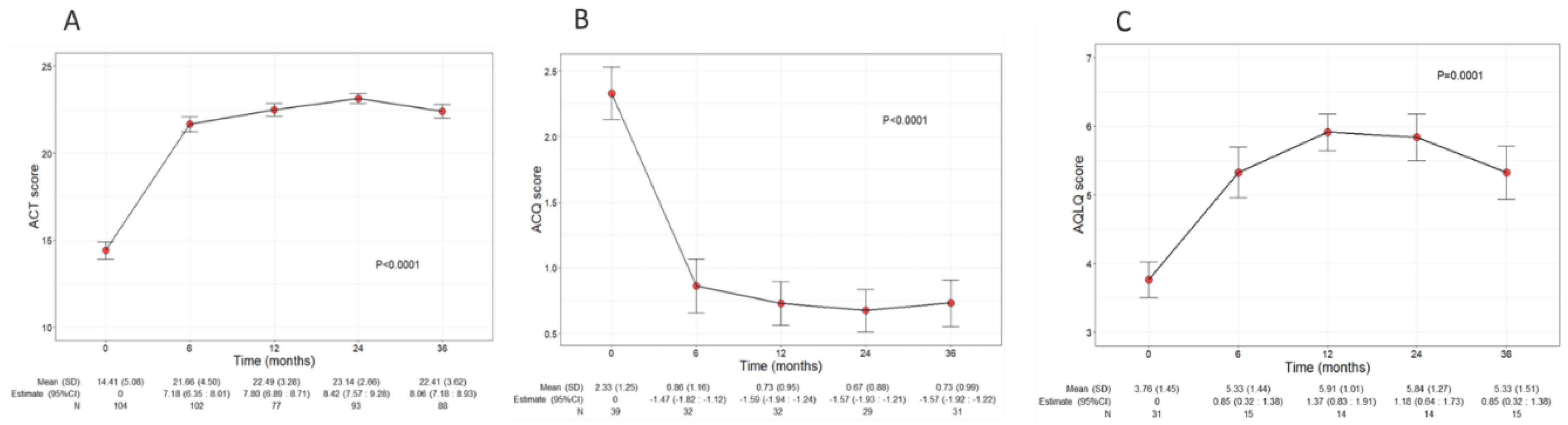
Asthma control and QoL significantly ameliorated during benralizumab treatment, with net improvements observed already at 6 months after the start of the treatment (
Figure 3A). In detail, the mean ACT score was 14.41 (5.08) at baseline and increased to 22 points (4.5) at 6 months (7.80, CI 6.89:8.71); ACT score remained either stable or further increased throughout the treatment period, indicating a durable good control of asthma. In addition, benralizumab enhanced patients’ QoL as determined by the significant changes of both ACQ and AQLQ scores over time (mean ACQ: from 2.33 to 0.73, p<0.001; mean AQLQ: from 3.76 to 5.33, p=0.001) (
Figure 3B and 3C).
3.5. Benralizumab alleviated sinonasal symptoms in comorbid patients
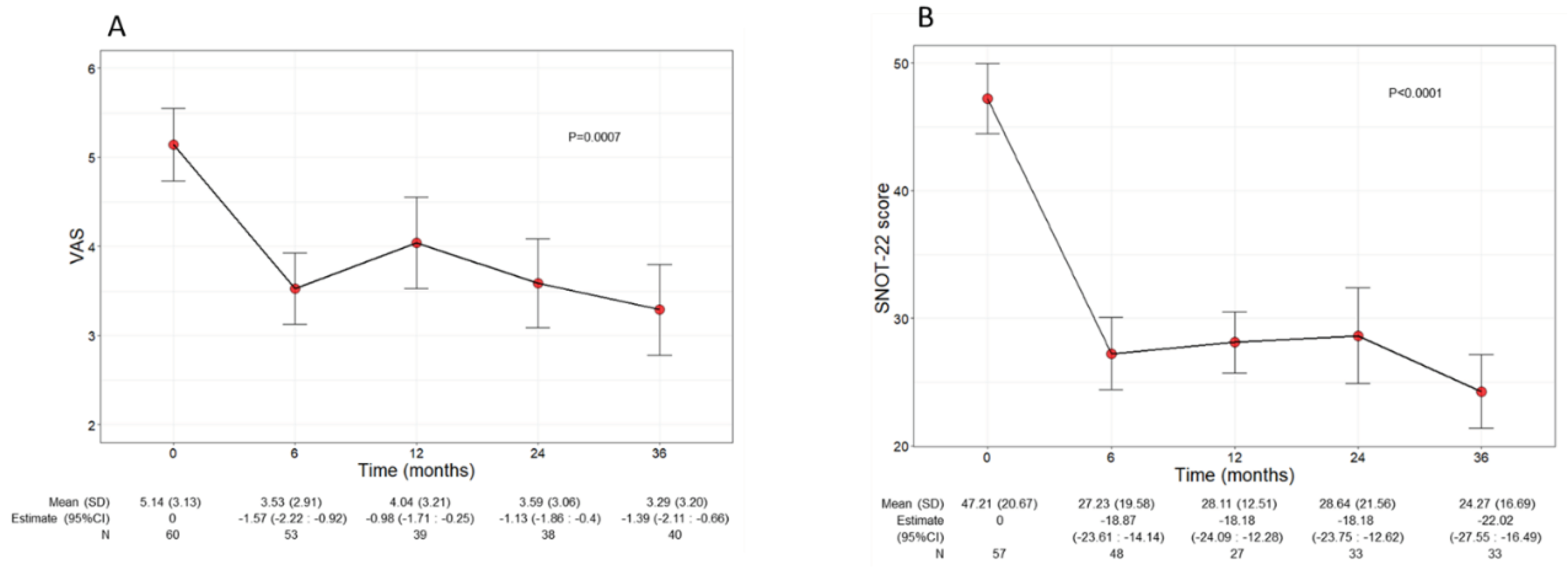
Beyond the asthma control improvement, benralizumab also decreased the severity of sinonasal symptoms in the subset of comorbid patients with CRSwNP. The mean VAS score significantly declined over the treatment period (p=0.0010), decreasing from 5.14 (3.13) at baseline to 3.29 (3.20) at 36 months (-1.45, CI -2.18:-0.71) (
Figure 4A). In parallel, the mean SNOT 22 score also significantly dropped (p<0.0001) from 47.21 (20.67) at baseline to 24.27 (16.69) at 36 months (-22.02, CI -27.55:-16.49) (
Figure 4B).
3.6. Benralizumab decreased the use of OCS
As shown in
Figure 5, a significant reduction in OCS use was observed over time (p<0.0001). The mean dose of OCS decreased to 2.1 mg/day (5.50) at 6 months (-5.52, CI -6.91:-4.41) and continued to decline, reaching a mean dose of 1.20 mg/day (4.80) at 36 months (-6.37, CI -7.88:-4.85). A total of 39 out of 64 (60.94%) eliminated the use of OCS already after 6 months from the start of benralizumab, and 38 out of 45 patients (84.44%) remained free from OCS at 36 months. Only 3 patients (6.67%) maintained (or increased) the baseline OCS dose, showing no reduction at 36 months (
Table 2).
The change in OCS use was also investigated in patients grouped according to their baseline OCS dose (≤5 mg/day or >5 mg/day) (
Figure 5 and
Table S2). The overall population and the subgroup of patients with baseline OCS dose >5 mg/day showed a similar OCS reduction pattern, with mean OCS doses of 1.20 (4.80) and 1.90 (5.60) at 36 month, respectively (
Figure 5). An almost identical percentage of patients discontinued OCS at 36 months regardless of baseline OCS dose (85.71% patients with OCS ≤5 mg/day and 83.33% patients with OCS >5 mg/day) (
Table S2).
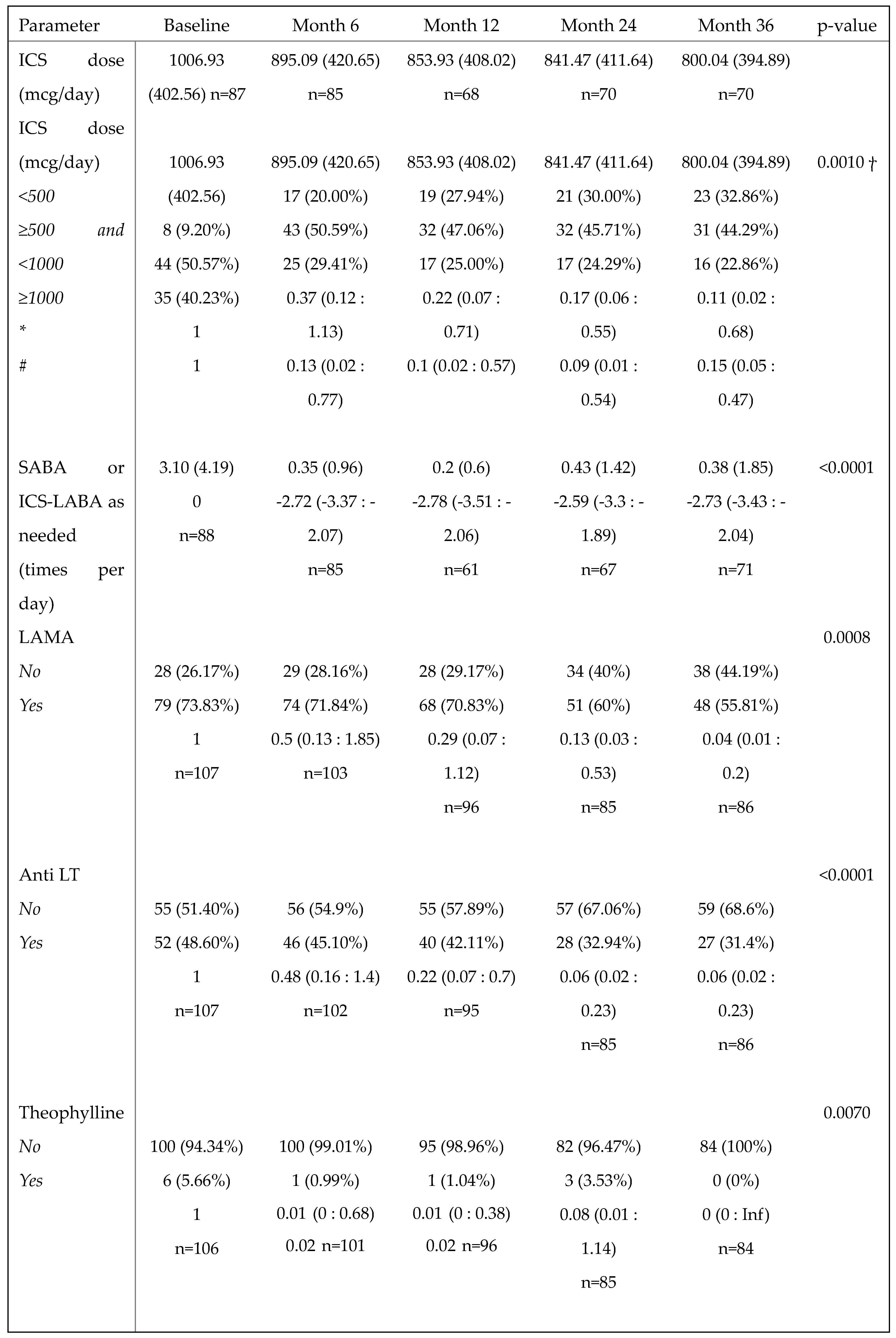
3.7. Benralizumab reduced the need for asthma background medication
The daily ICS dose used by patients decreased progressively over time, from a mean of 1006.93 (402.56) at baseline (n=87) to a mean of 800.04 (394.89) at 36 months (n=70), with an overall reduction of 20.55%. Accordingly, the percentage of patients taking a low dose of ICS (<500 mcg/day) increased from 9.20% at baseline to 32.86% at 36 months, and a significant reduction in the required dose of ICS was observed over time (p=0.0010,
Table 3). In particular, the chances of patients requiring a medium ICS dose (≥500 and <1000 mcg/day) at 12, 24 and 36 months were reduced by 78%, 83% and 89% compared with baseline (OR (95%CI): 0.22 (0.07:0.71), 0.17 (0.06: 0.55) and 0.11 (0.02:0.68), respectively), and the chances of requiring a high ICS dose (≥1000 mcg/day) at 6, 12, 24 and 36 months were reduced by 87%, 90%, 91% and 85% compared with baseline (OR (95%CI) =0.13 (0.02:0.77), 0.10 (0.02:0.57), 0.09 (0.01:0.54) and 0.15 (0.05:0.47), respectively) (
Table 3). Benralizumab treatment not only lowered the ICS dose but also decreased the need for other asthma medications, such as the use of as-needed relievers SABA or ICS/LABA (p<0.0001), LAMA (p=0.0008), anti LT (p<0.0001) and theophylline (p=0.0102) (
Table 3).
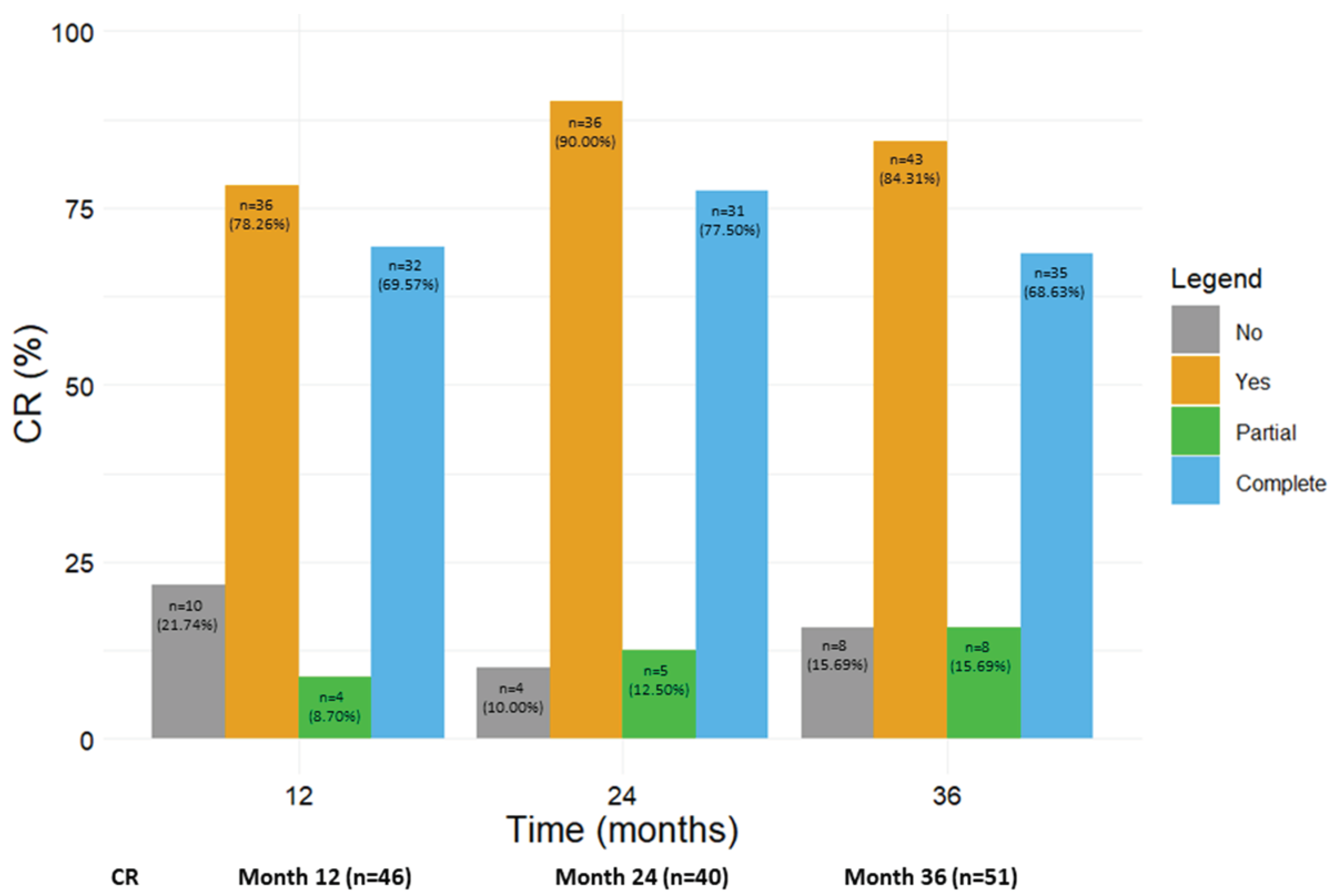
3.8. Benralizumab promoted the achievement of CR
Figure 6 shows the number and percentage of patients who achieved any CR, including pCR and cCR, at 12, 24 and 36 months (from the corresponding previous time point). A total of 36 out of 46 patients (78.26%) reached CR (either pCR, 8.70% or cCR, 69.57%) after 12 months; the rate of CR further increased at the following time points, with 90.00% and 84.31% of patients in CR at 24 and 36 months, respectively. When pCR and cCR were considered separately, the percentage of patients in pCR steadily increased from 8.70% at 12 months to 15.69% at 36 months, while most patients were in cCR from 12 months onwards (69.57% at 12 months, 77.50% at 24 months and 68.63% at 36 months). The percentage of patients who did not achieve any kind of CR dropped from 21.74% at 12 months to 10.00% and 15.69% at 24 and 36 months respectively (
Figure 6). We also considered the percentage of patients who reached CR from baseline to each time point; as shown in
Figure S1, similar results were obtained (at 36 months, 85.71% of patients achieved any CR, with 12.50% patients in pCR and 73.21% patients in cCR).
4. Discussion
This study provides a comprehensive analysis of benralizumab effectiveness by retrospectively evaluating a total of 108 Italian patients with SEA treated for up to 36 months. Even though a high number of real-life studies have thus far evaluated the effectiveness of benralizumab on SEA patients, to our knowledge this is the first study conducted on more than 100 SEA patients treated over a period longer than 2 years. The longest real-life study (up to 48 months) was published by Numata and colleagues however, only 23 SEA patients were initially included and fewer were followed for the entire period [
33]. Similarly, Caminati and colleagues evaluated asthma outcomes in 68 mepolizumab-switched patients treated with benralizumab for a median period of 31 months [
30]. In a more recent work, Fyles et al. considered a population of 81 SEA patients treated with either mepolizumab or benralizumab for up to 36 months; however, the majority of data were presented for the overall population, thus it is not possible to extrapolate the clinical improvements experienced by benralizumab-treated patients for each single outcome [
31].
The baseline clinical characteristics reveal a severely compromised SEA patient population. The presence of circulating eosinophils (600 cells/mm3), the high AER (3.84), the suboptimal FEV1/FVC and FEV1 (predicted: 74.5%) and the low scores of various PROs (with a mean ACT score: 14.41) confirm the poor control of the disease. In addition, the high percentages of patients taking OCS and experiencing comorbidities, among which CRSwNP in 60.19% patients, bronchiectasis in almost 27% patients and EGPA in 4.71% patients, further corroborate the high disease burden in our benralizumab-treated population.
The results from this long-term study reinforce the remarkable effectiveness of benralizumab in minimizing the number of exacerbations while maintaining minimal BEC throughout the study period. Benralizumab rapidly decreased the frequency of exacerbations, with a reduction of AER that persisted throughout the 36-month period (AER reduction ranging from 89% at 36 months to 95% at 12 months). Although the phenotype of exacerbations was determined in a small subgroup of patients at baseline only, we may speculate that benralizumab reduced all types of exacerbations, both infectious and non-infectious. This hypothesis is supported by: 1) the extensive effect seen throughout the treatment period; 2) the high prevalence of bacterial-mediated exacerbations (more than 50%) observed at baseline; and 3) benralizumab novel MoA, which implies a broad modulation of the immune system, including increased activation of NK cells [
26]. Considering the antiviral and antibacterial role exerted by NK cells, it is plausible that their increased function contributed to prevent infections and infectious-related exacerbations in patients during benralizumab treatment.
These data demonstrate that the effectiveness of benralizumab in preventing exacerbations is truly long-lasting, and the prominent extent of AER reduction is consistent with previous real-life studies [
25,
28,
29,
30].
Although benralizumab was anticipated to have a positive impact on AER over time, variable results have been published regarding its ability to enhance lung function over the long term. In our study, benralizumab significantly increased FEV
1 over time, with a remarkable +440 mL volume gain and predicted levels reaching normal values up to 36 months. This is the first time that benralizumab has been shown to induce a durable increase in respiratory function in real-life up to 3 years; these results are in line with the recently published 96-week data from the ANANKE study, in which both pre-BD FEV
1 and FVC peaked after 96 weeks of treatment [
25], and complement the data obtained from randomized clinical trials (RCT), where the initial improvement in lung function was stabilized over a two-year period [
37]. As already mentioned, the novel MoA of benralizumab postulated by Bergantini et al., which involves the modulation of circulating CD3+T subsets and increased activation of NK cells even in absence of eosinophils, may play a key role in benralizumab-mediated long-term improvement in lung function [
26]. On the other hand, Numata and colleagues reported a decline in FEV
1 levels registered after 24 months [
33]. In light of the data obtained from our and other studies, it is possible that this result is biased, due to the low number of patients considered in the study. The authors also speculated that the observed FEV
1 reduction may be caused by an airway obstruction mechanism induced by the long-term administration of a single biologic, or a decrease adherence to inhaled therapies, or a physiological decline in pulmonary function [
33]. Regardless of the reason justifying the different results, more studies with a greater number of patients are needed to ascertain benralizumab long-term effectiveness on lung function.
As measured by ACT questionnaire, asthma control was significantly improved, with a mean ACT score greater than 21 at all timepoints. The significant results obtained in ACQ and AQLQ reinforced the achievement of good asthma control and demonstrated an overall improvement of QoL.
Benralizumab not only induced profound beneficial effects in term of asthma symptoms but also improved sinonasal symptoms in comorbid patients with CRSwNP, as demonstrated by the significant and progressive changes recorded in VAS and SNOT Although benralizumab currently lacks the indication for the treatment of CRSwNP, growing evidence indicates that the anti IL-5R has a positive impact on nasal symptoms in comorbid patients [
38,
39,
40,
41]. Notably, a recent work by Santomasi and colleagues showed that benralizumab was not only effective in decreasing SNOT 22 score, but it also significantly reduced nasal polyps score (NPS), determined by nasal endoscopy, and the number of nasal eosinophils and neutrophils, assessed via nasal cytology, in SEA patients with CRSwNP [
41]. Collectively, these data corroborate the theory of “united airway disease” [
42], implying that SEA and CRSwNP share the same eosinophilic-driven pathophysiology in comorbid patients, and benralizumab could indeed represent the optimal therapeutic strategy to tackle both pathologies simultaneously.
The marked OCS-sparing effect of benralizumab is well-recognized. In the pivotal PONENTE study, almost 63% of patients completely eliminated the use of OCS and more than 80% of patients either eliminated OCS or maintained a minimum dose due to adrenal insufficiency [
43]. Similarly, OCS dose has been either reduced or zeroed in real-life studies where patients were treated for periods longer than one year [
25,
29,
30,
33]. Our data indicate that benralizumab induced a durable OCS reduction and this effect further increased over time. Indeed, the minimal mean dose of OCS was registered at 36 months; at this time point, almost the totality of patients (93.33%) successfully decreased their OCS dose by any extent, and 84.44% patients permanently discontinued OCS therapy. To our knowledge, these percentages are the highest ever recorded in the literature. The sub-analysis conducted on patients requiring a daily OCS dose of either ≤ or >5 mg substantiated the findings from the PONENTE trial, which showed that benralizumab OCS-sparing effect was independent of the baseline dose [
43].
Beyond the high rate of OCS reduction and elimination, benralizumab treatment was associated with a net and significant decrease in the dose of maintenance ICS dose, with an overall reduction of 20.5% and a decreased probability of requiring medium and high ICS doses over time. Consistently, there were more than 20% patients transitioning from medium or high ICS doses to low ICS doses (<500 mcg/day) at 36 months. We also observed a progressive decline in the use of all other asthma therapies, including LAMA, anti LT, theophylline, and as needed SABA and/or ICS/LABA. Notably, patients could reduce all these medications while maintaining good asthma control, as demonstrated by ACT and ACQ scores. To date, the effect of benralizumab on asthma medication other than OCS has not been extensively investigated. In the RCT SHAMAL, up to 92% of patients successfully reduced their ICS dose and 96% maintained such reduction up to 48 weeks [
44]. In real-life, maintenance medications (ICS dose reduction and/or LABA, LAMA montelukast interruption) were decreased in 66.3% patients over a mean treatment period of 19.7 months. Similarly to our data, they also observe a 25% reduction in mean ICS dose [
29].
Since the advent of biologic therapies for asthma and the compelling amelioration of patients’ symptoms, the achievement of CR in SA patients has become possible. However, the criteria defining CR used in the various studies published so far have been somehow arbitrary. Recently, a Delphi consensus reached by members of the SANI study group agreed on the criteria to identify patients in pCR and cCR [
19]. Based on these criteria, our results show that CR, and specifically cCR, was achieved by more than the half of the patients at all time points considered (up to 90.00% patients in CR at 24 months, of which 77.50% patients were in cCR). These data mean that benralizumab could permanently eradicate the disease in the vast majority of patients during the three-year study duration. CR was evaluated in previous studies, both RCT and in real life; the results show that benralizumab induced CR in percentages of SEA patients ranging from 14.5% (in the SIROCCO and CALIMA RCTs) [
45] to 43% (in the real-world XALOC-1 study [
46]). The percentage of patients achieving CR in our study seems to exceed the results previously reported, however attention should be paid to the criteria employed to define CR, as they vary across the studies, and they differ from the criteria used here. For instance, in the XALOC-1 study the percentage of patients reaching CR (43%) was calculated without including any respiratory parameters and considering an ACT score ≥16 points [
46] . Importantly, Campisi et al. found that SEA patients achieved CR more frequently in absence of bronchiectasis [
47], suggesting a negative impact of this comorbidity on benralizumab effectiveness [
47]. The inclusion of a variable proportion of patients with this comorbidity, accompanied by the methodological differences used across the studies, may justify the variable results obtained so far. Nevertheless, the rate of CR that emerges from real-world investigations seems to be consistently higher than that of RCTs [
33,
45,
46,
47]. In our study, CR was evaluated in all patients, including those with bronchiectasis (which affected approximately a quarter of our patient population). The strict criteria employed in our study to define cCR and the inclusion of patients with bronchiectasis add further value to the rates of cCR reported here, which are unprecedented, but justified by the striking effect of benralizumab observed in all the single outcomes (exacerbations, lung function, asthma control and OCS use).
The retrospective design of this study represents its main limitation, as it is associated with considerable loss of data during the 36-month treatment period. As already mentioned above, additional studies will be needed to validate our results over even longer treatment periods and by considering greater numbers of patients. Ideally, future studies will be conducted with a prospective design. A thorough evaluation of safety would also be valuable to confirm the long-term safety of benralizumab already observed in the MELTEMI study [
27].
In conclusion, this study offers a comprehensive assessment of benralizumab long-term effectiveness on SEA by examining a meaningful sample population at various time points, up to 36 months of treatment. These impressive data not only comprehensively illustrate the long-lasting response to benralizumab in all the considered asthma clinical outcomes, but also reveal the simultaneous positive effects on CRSwNP symptoms. The large percentage of patients who reached either pCR or cCR is indicative of the long-term well-being induced by benralizumab and support its role as a disease-modifying anti-asthmatic therapy for the management of SEA.
Supplementary Materials
Table S1: Descriptive statistics with a summary output of mixed-model on FeNO and lung function parameters recorded at baseline and during the treatment with benralizumab (n=108 unless otherwise specified). Mean (SD) and n values or n (percentage) and beta regression coefficients with 95% CI are reported for each time point. Table S2: Extent of OCS dose reduction during treatment with benralizumab in patients grouped according to OCS dose at baseline (≤5 mg/day or >5 mg/day) Figure S1: Number and percentage of patients who achieved and did not achieve CR (either pCR or cCR) at 12, 24 and 36 months during the treatment with benralizumab (values calculated from baseline). .
Author Contributions
Conceptualization, Laura Pini; Investigation, Laura Pini, Diego Bagnasco, Bianca Beghè, Fulvio Braido, Paolo Cameli, Marco Caminati, Cristiano Caruso, Claudia Crimi, Gabriella Guarnieri, Manuela Latorre, Francesco Menzella, Claudio Micheletto, Andrea Vianello, Dina Visca, Benedetta Bondi, Yehia Elmasri, Jordan Giordani, Andrea Mastrototaro, Matteo Maule, Alessandro Pini, Stefano Piras, Martina Zappa, Gianenrico Senna, Antonio Spanevello, Pierluigi Paggiaro, Francesco Blasi and Giorgio Canonica; Resources, Laura Pini, Diego Bagnasco, Bianca Beghè, Fulvio Braido, Paolo Cameli, Marco Caminati, Cristiano Caruso, Claudia Crimi, Gabriella Guarnieri, Manuela Latorre, Francesco Menzella, Claudio Micheletto, Andrea Vianello, Dina Visca, Benedetta Bondi, Yehia Elmasri, Jordan Giordani, , Andrea Mastrototaro, Matteo Maule, Alessandro Pini, Stefano Piras, Martina Zappa, Gianenrico Senna, Antonio Spanevello, Pierluigi Paggiaro, Francesco Blasi and Giorgio Canonica; Writing – original draft, Laura Pini; Writing – review & editing, Laura Pini, Diego Bagnasco, Bianca Beghè, Fulvio Braido, Paolo Cameli, Marco Caminati, Cristiano Caruso, Claudia Crimi, Gabriella Guarnieri, Manuela Latorre, Francesco Menzella, Claudio Micheletto, Andrea Vianello, Dina Visca, Benedetta Bondi, Yehia Elmasri, Jordan Giordani, , Andrea Mastrototaro, Matteo Maule, Alessandro Pini, Stefano Piras, Martina Zappa, Gianenrico Senna, Antonio Spanevello, Pierluigi Paggiaro, Francesco Blasi and Giorgio Canonica. All authors have read and agreed to the submitted version of the manuscript.
Funding
This project was supported by AstraZeneca.
Institutional Review Board Statement
The SANI registry was constructed according to the Declarations of Helsinki and Oviedo. The study was approved by the Central Ethics Committee for the SANI Network (Comitato Etico Area Vasta Nord-Ovest Toscana; protocol number: study number 1245/2016, protocol ID: 73714).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author (Prof. Pini,
laura.pini@unibs.it).
Acknowledgments
The authors would like to thank all the participants who took part in the study and their families. Statistical analyses were conducted by Fabio Gallo, Ph.D.; writing and editorial assistance were provided by Alessandra Rossi, Ph.D., on behalf of EDRA S.p.A.
Conflicts of Interest
To be communicated upon request.
References
- Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–. [CrossRef]
- Hekking P-PW, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135:896–. [CrossRef]
- Bagnasco D, Paggiaro P, Latorre M, Folli C, Testino E, Bassi A, et al. Severe asthma: One disease and multiple definitions. World Allergy Organization Journal. 2021;14:. [CrossRef]
- Buhl R, Humbert M, Bjermer L, Chanez P, Heaney LG, Pavord I, et al. Severe eosinophilic asthma: a roadmap to consensus. Eur Respir J. 2017;49:. [CrossRef]
- Perez-de-Llano L, Tran TN, Al-ahmad M, Alacqua M, Bulathsinhala L, Busby J, et al. Characterization of Eosinophilic and Non-Eosinophilic Severe Asthma Phenotypes and Proportion of Patients with These Phenotypes in the International Severe Asthma Registry (ISAR). C21 ADVANCES IN ADULT AND PEDIATRIC ASTHMA PHENOTYPING AND ENDOTYPING. American Thoracic Society; p. A4525–A.
- Maio S, Baldacci S, Bresciani M, Simoni M, Latorre M, Murgia N, et al. RItA: The Italian severe/uncontrolled asthma registry. Allergy. 2018;73:683–. [CrossRef]
- Bakakos A, Loukides S, Bakakos P. Severe Eosinophilic Asthma. J Clin Med. 2019;
- de Groot JC, Ten Brinke A, Bel EHD. Management of the patient with eosinophilic asthma: a new era begins. ERJ Open Res. 2015;1:00024–.
- Heaney LG, Perez de Llano L, Al-Ahmad M, Backer V, Busby J, Canonica GW, et al. Eosinophilic and Noneosinophilic Asthma: An Expert Consensus Framework to Characterize Phenotypes in a Global Real-Life Severe Asthma Cohort. Chest. 2021;160:814–.
- Laidlaw TM, Mullol J, Woessner KM, Amin N, Mannent LP. Chronic Rhinosinusitis with Nasal Polyps and Asthma. The Journal of Allergy and Clinical Immunology: In Practice. 2021;9:1133–.
- Massoth L, Anderson C, McKinney KA. Asthma and Chronic Rhinosinusitis: Diagnosis and Medical Management. Medical Sciences. 2019;7:. [CrossRef]
- 2023 GINA MAIN REPORT. Available from: https://ginasthma.org/wp-content/uploads/2023/05/GINA-2023-Full-Report-2023-WMS.pdf.
- Price DB, Trudo F, Voorham J, Xu X, Kerkhof M, Ling Zhi Jie J, et al. Adverse outcomes from initiation of systemic corticosteroids for asthma: long-term observational study. J Asthma Allergy. 2018;11:193–. [CrossRef]
- Lee H, Ryu J, Nam E, Chung SJ, Yeo Y, Park DW, et al. Increased mortality in patients with corticosteroid-dependent asthma: a nationwide population-based study. Eur Respir J. 2019;54:. [CrossRef]
- Bourdin A, Molinari N, Vachier I, Pahus L, Suehs C, Chanez P. Mortality: a neglected outcome in OCS-treated severe asthma. Eur Respir J. 2017;50:. [CrossRef]
- Lommatzsch M, Brusselle GG, Levy ML, Canonica GW, Pavord ID, Schatz M, et al. A2BCD: a concise guide for asthma management. The Lancet Respiratory Medicine. 2023;11:573–. [CrossRef]
- Chen W, Tran TN, Sadatsafavi M, Murray R, Wong NCB, Ali N, et al. Impact of Initiating Biologics in Patients With Severe Asthma on Long-Term Oral Corticosteroids or Frequent Rescue Steroids (GLITTER): Data From the International Severe Asthma Registry. The Journal of Allergy and Clinical Immunology: In Practice. 2023;11:2732–. [CrossRef]
- Chen M, Shepard K 2nd, Yang M, Raut P, Pazwash H, Holweg CTJ, et al. Overlap of allergic, eosinophilic and type 2 inflammatory subtypes in moderate-to-severe asthma. Clin Exp Allergy. 2021;51:546–.
- Canonica GW, Blasi F, Carpagnano GE, Guida G, Heffler E, Paggiaro P, et al. Severe Asthma Network Italy Definition of Clinical Remission in Severe Asthma: A Delphi Consensus. The Journal of Allergy and Clinical Immunology: In Practice. 2023;S. [CrossRef]
- Lommatzsch M, Brusselle GG, Canonica GW, Jackson DJ, Nair P, Buhl R, et al. Disease-modifying anti-asthmatic drugs. The Lancet. 2022;399:1664–. [CrossRef]
- fasenra-epar-medicine-overview_en.pdf.
- Dagher R, Kumar V, Copenhaver AM, Gallagher S, Ghaedi M, Boyd J, et al. Novel mechanisms of action contributing to benralizumab’s potent anti-eosinophilic activity. Eur Respir J. 2022; [CrossRef]
- Caminati M, Bagnasco D, Vaia R, Senna G. New horizons for the treatment of severe, eosinophilic asthma: benralizumab, a novel precision biologic. Biologics. 2019;13:89–. [CrossRef]
- Menzella F, Biava M, Bagnasco D, Galeone C, Simonazzi A, Ruggiero P, et al. Efficacy and steroid-sparing effect of benralizumab: has it an advantage over its competitors? DIC. 2019;8:1–.
- Vultaggio A, Aliani M, Altieri E, Bracciale P, Brussino L, Caiaffa MF, et al. Long-term effectiveness of benralizumab in severe eosinophilic asthma patients treated for 96-weeks: data from the ANANKE study. Respir Res. 2023;24:. [CrossRef]
- Bergantini L, d’Alessandro M, Pianigiani T, Cekorja B, Bargagli E, Cameli P. Benralizumab affects NK cell maturation and proliferation in severe asthmatic patients. Clinical Immunology. 2023;253:. [CrossRef]
- Korn S, Bourdin A, Chupp G, Cosio BG, Arbetter D, Shah M, et al. Integrated Safety and Efficacy Among Patients Receiving Benralizumab for Up to 5 Years. J Allergy Clin Immunol Pract. 2021;9:4381-4392.e. [CrossRef]
- Vitale C, Maglio A, Pelaia C, D’Amato M, Ciampo L, Pelaia G, et al. Effectiveness of Benralizumab in OCS-Dependent Severe Asthma: The Impact of 2 Years of Therapy in a Real-Life Setting. JCM. 2023;12:. [CrossRef]
- Sposato B, Scalese M, Camiciottoli G, Carpagnano GE, Pelaia C, Santus P, et al. Severe asthma and long-term Benralizumab effectiveness in real-life. Eur Rev Med Pharmacol Sci. 2022;26:7461–. [CrossRef]
- Caminati M, Marcon A, Guarnieri G, Miotti J, Bagnasco D, Carpagnano GE, et al. Benralizumab Efficacy in Late Non-Responders to Mepolizumab and Variables Associated with Occurrence of Switching: A Real-Word Perspective. JCM. 2023;12:. [CrossRef]
- Fyles F, Nuttall A, Joplin H, Burhan H. Long-Term Real-World Outcomes of Mepolizumab and Benralizumab Among Biologic-Naive Patients With Severe Eosinophilic Asthma: Experience of 3 Years’ Therapy. The Journal of Allergy and Clinical Immunology: In Practice. 2023;11:2715–. [CrossRef]
- Risco M, Sotomayor J, Alvarez-Sala P, Piorno I, Diaz-Campos R, Moya B, et al. LONG-TERM EFFECTIVENESS AND SAFETY OF BENRALIZUMAB FOR UNCONTROLLED EOSINOPHILIC ASTHMA IN REAL-WORD PRACTICE. Journal of Allergy and Clinical Immunology. 2022;149:AB. [CrossRef]
- Numata T, Araya J, Okuda K, Miyagawa H, Minagawa S, Ishikawa T, et al. Long-Term Efficacy and Clinical Remission After Benralizumab Treatment in Patients with Severe Eosinophilic Asthma: A Retrospective Study. JAA. 2022;Volume 15:1731–. [CrossRef]
- Piano terapeutico AIFA per la prescrizione SSN di Fasenra (benralizumab) nell’asma grave eosinofilico refrattario [Internet]. Gazzetta Ufficiale della Repubblica Italiana; Available from: https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=2019-02-12&atto.codiceRedazionale=19A00829&elenco30giorni=false.
- https://www.r-project.org/.
- Toma S, Hopkins C. Stratification of SNOT-22 scores into mild, moderate or severe and relationship with other subjective instruments. Rhin. 2016;54:129–.
- FitzGerald JM, Bleecker ER, Bourdin A, Busse WW, Ferguson GT, Brooks L, et al. Two-Year Integrated Efficacy And Safety Analysis Of Benralizumab In Severe Asthma. J Asthma Allergy. 2019;12:401–. [CrossRef]
- Nolasco S, Crimi C, Pelaia C, Benfante A, Caiaffa MF, Calabrese C, et al. Benralizumab Effectiveness in Severe Eosinophilic Asthma with and without Chronic Rhinosinusitis with Nasal Polyps: A Real-World Multicenter Study. J Allergy Clin Immunol Pract. 2021;9:4371-4380.e. [CrossRef]
- Lombardo N, Pelaia C, Ciriolo M, Della Corte M, Piazzetta G, Lobello N, et al. Real-life effects of benralizumab on allergic chronic rhinosinusitis and nasal polyposis associated with severe asthma. Int J Immunopathol Pharmacol. 2020;34:. [CrossRef]
- Bagnasco D, Brussino L, Bonavia M, Calzolari E, Caminati M, Caruso C, et al. Efficacy of Benralizumab in severe asthma in real life and focus on nasal polyposis. Respiratory Medicine. 2020;171:. [CrossRef]
- Santomasi C, Buonamico E, Dragonieri S, Iannuzzi L, Portacci A, Quaranta N, et al. Effects of benralizumab in a population of patients affected by severe eosinophilic asthma and chronic rhinosinusitis with nasal polyps: a real life study. Acta Biomedica Atenei Parmensis. 2023;94:e. [CrossRef]
- Kanda A, Kobayashi Y, Asako M, Tomoda K, Kawauchi H, Iwai H. Regulation of Interaction between the Upper and Lower Airways in United Airway Disease. Medical Sciences. 2019;7:. [CrossRef]
- Menzies-Gow A, Gurnell M, Heaney LG, Corren J, Bel EH, Maspero J, et al. Oral corticosteroid elimination via a personalised reduction algorithm in adults with severe, eosinophilic asthma treated with benralizumab (PONENTE): a multicentre, open-label, single-arm study. Lancet Respir Med. 2022;10:47–. [CrossRef]
- Jackson DJ, Heaney LG, Humbert M, Kent BD, Hiljemark L, Olinger L, et al. Late Breaking Abstract - SHAMAL: reduction of maintenance inhaled corticosteroids in patients with severe eosinophilic asthma treated with benralizumab: a randomised phase 4 study. Airway pharmacology and treatment [Internet]. European Respiratory Society; 2023 [cited 2023 Nov 12]. p. RCTAvailable from: http://erj.ersjournals.com/lookup/doi/10.1183/13993003.congress-2023.RCT798.
- Menzies-Gow A, Hoyte FL, Price DB, Cohen D, Barker P, Kreindler J, et al. Clinical Remission in Severe Asthma: A Pooled Post Hoc Analysis of the Patient Journey with Benralizumab. Adv Ther. 2022;39:2065–.
- Jackson D, Pelaia G, Padilla-Galo A, Watt M, Kayaniyil S, Boarino S, et al. Asthma Clinical Remission with Benralizumab in an Integrated Analysis of the Real-World XALOC-1 Study. Journal of Allergy and Clinical Immunology. 2023;151:AB. [CrossRef]
- Campisi R, Nolasco S, Pelaia C, Impellizzeri P, D’Amato M, Portacci A, et al. Benralizumab Effectiveness in Severe Eosinophilic Asthma with Co-Presence of Bronchiectasis: A Real-World Multicentre Observational Study. JCM. 2023;12:3953. [CrossRef]
Figure 1.
Change in AER (A) and BEC (B) during the treatment with benralizumab. Data were recorded at baseline and at 6, 12, 24 and 36 months. Mean (SD), n values and exponential beta regression coefficients (i.e.: ratio) with 95% CI, or median (IQR) and n values are reported for each time point. .
Figure 1.
Change in AER (A) and BEC (B) during the treatment with benralizumab. Data were recorded at baseline and at 6, 12, 24 and 36 months. Mean (SD), n values and exponential beta regression coefficients (i.e.: ratio) with 95% CI, or median (IQR) and n values are reported for each time point. .
Figure 2.
Change in FEV1 volume (A) and percentage of predicted (B) during the treatment with benralizumab. Data were recorded at baseline and at 6, 12, 24 and 36 months. Mean (SD), n values and regression coefficients (i.e., estimate) with 95% CI are reported for each time point. .
Figure 2.
Change in FEV1 volume (A) and percentage of predicted (B) during the treatment with benralizumab. Data were recorded at baseline and at 6, 12, 24 and 36 months. Mean (SD), n values and regression coefficients (i.e., estimate) with 95% CI are reported for each time point. .
Figure 3.
Change in ACT (A), ACQ (B) and AQLQ (C) scores during the treatment with benralizumab. Data were recorded at baseline and at 6, 12, 24 and 36 months. Mean (SD), n values and regression coefficients (i.e.: estimate) with 95% CI are reported for each time point. .
Figure 3.
Change in ACT (A), ACQ (B) and AQLQ (C) scores during the treatment with benralizumab. Data were recorded at baseline and at 6, 12, 24 and 36 months. Mean (SD), n values and regression coefficients (i.e.: estimate) with 95% CI are reported for each time point. .
Figure 4.
Change in VAS (A) and SNOT 22 (B) score during the treatment with benralizumab. Data were recorded at baseline and at 6, 12, 24 and 36 months. Mean values (SD), n and regression coefficients (i.e.: Estimate) with 95% CI are reported for each time point. .
Figure 4.
Change in VAS (A) and SNOT 22 (B) score during the treatment with benralizumab. Data were recorded at baseline and at 6, 12, 24 and 36 months. Mean values (SD), n and regression coefficients (i.e.: Estimate) with 95% CI are reported for each time point. .
Figure 5.
Change in OCS dose in the overall patient population and in patients with baseline OCS dose >5 mg/day during the treatment with benralizumab. Data were recorded at baseline and at 6, 12, 24 and 36 months. Mean (SD), n values and regression coefficients (i.e.: Estimate) with 95% CI are reported for each time point. .
Figure 5.
Change in OCS dose in the overall patient population and in patients with baseline OCS dose >5 mg/day during the treatment with benralizumab. Data were recorded at baseline and at 6, 12, 24 and 36 months. Mean (SD), n values and regression coefficients (i.e.: Estimate) with 95% CI are reported for each time point. .
Figure 6.
Number and percentage of patients who achieved and did not achieve CR (either pCR or cCR) at 12, 24 and 36 months during the treatment with benralizumab (from previous time point). .
Figure 6.
Number and percentage of patients who achieved and did not achieve CR (either pCR or cCR) at 12, 24 and 36 months during the treatment with benralizumab (from previous time point). .
Table 2.
Extent of OCS dose reduction achieved by patients during the treatment with benralizumab. Data are expressed as n (%).
Table 2.
Extent of OCS dose reduction achieved by patients during the treatment with benralizumab. Data are expressed as n (%).
| Extent of OCS reduction |
Month 6 (n=64) |
Month 12 (n=47) |
Month 24 (n=43) |
Month 36 (n=45) |
| Any reduction |
52 (81.25%) |
41 (87.23%) |
39 (90.7%) |
42 (93.33%) |
| ≥90 % |
39 (60.94%) |
34 (72.34%) |
34 (79.07%) |
38 (84.44%) |
| ≥75 % |
42 (65.62%) |
38 (80.85%) |
37 (86.05%) |
39 (86.67%) |
| ≥50 % |
50 (78.12%) |
41 (87.23%) |
38 (88.37%) |
42 (93.33%) |
| ≥25 % |
51 (79.69%) |
41 (87.23%) |
39 (90.7%) |
42 (93.33%) |
| No reduction |
12 (18.75%) |
6 (12.77%) |
4 (9.3%) |
3 (6.67%) |
| Elimination |
39 (60.94%) |
34 (72.34%) |
33 (76.74%) |
38 (84.44%) |
| Extent of OCS reduction |
Month 6 (n=64) |
Month 12 (n=47) |
Month 24 (n=43) |
Month 36 (n=45) |
| Any reduction |
52 (81.25%) |
41 (87.23%) |
39 (90.7%) |
42 (93.33%) |
| ≥90 % |
39 (60.94%) |
34 (72.34%) |
34 (79.07%) |
38 (84.44%) |
| ≥75 % |
42 (65.62%) |
38 (80.85%) |
37 (86.05%) |
39 (86.67%) |
| ≥50 % |
50 (78.12%) |
41 (87.23%) |
38 (88.37%) |
42 (93.33%) |
| ≥25 % |
51 (79.69%) |
41 (87.23%) |
39 (90.7%) |
42 (93.33%) |
| No reduction |
12 (18.75%) |
6 (12.77%) |
4 (9.3%) |
3 (6.67%) |
| Elimination |
39 (60.94%) |
34 (72.34%) |
33 (76.74%) |
38 (84.44%) |
Table 3.
Change in asthma medication use during the treatment with benralizumab. Descriptive statistics with a summary output of mixed-model on asthma medications other than OCS (ICS, SABA or ICS-LABA as needed, LAMA, anti LT and theophylline) recorded at baseline and during the treatment with benralizumab. Mean (SD) and n values or n (percentage) and the beta regression coefficient (or OR where appropriate) with 95% CI are reported for each time point.
Table 3.
Change in asthma medication use during the treatment with benralizumab. Descriptive statistics with a summary output of mixed-model on asthma medications other than OCS (ICS, SABA or ICS-LABA as needed, LAMA, anti LT and theophylline) recorded at baseline and during the treatment with benralizumab. Mean (SD) and n values or n (percentage) and the beta regression coefficient (or OR where appropriate) with 95% CI are reported for each time point.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).