Submitted:
23 February 2024
Posted:
26 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Epidemiology of HF in T1D
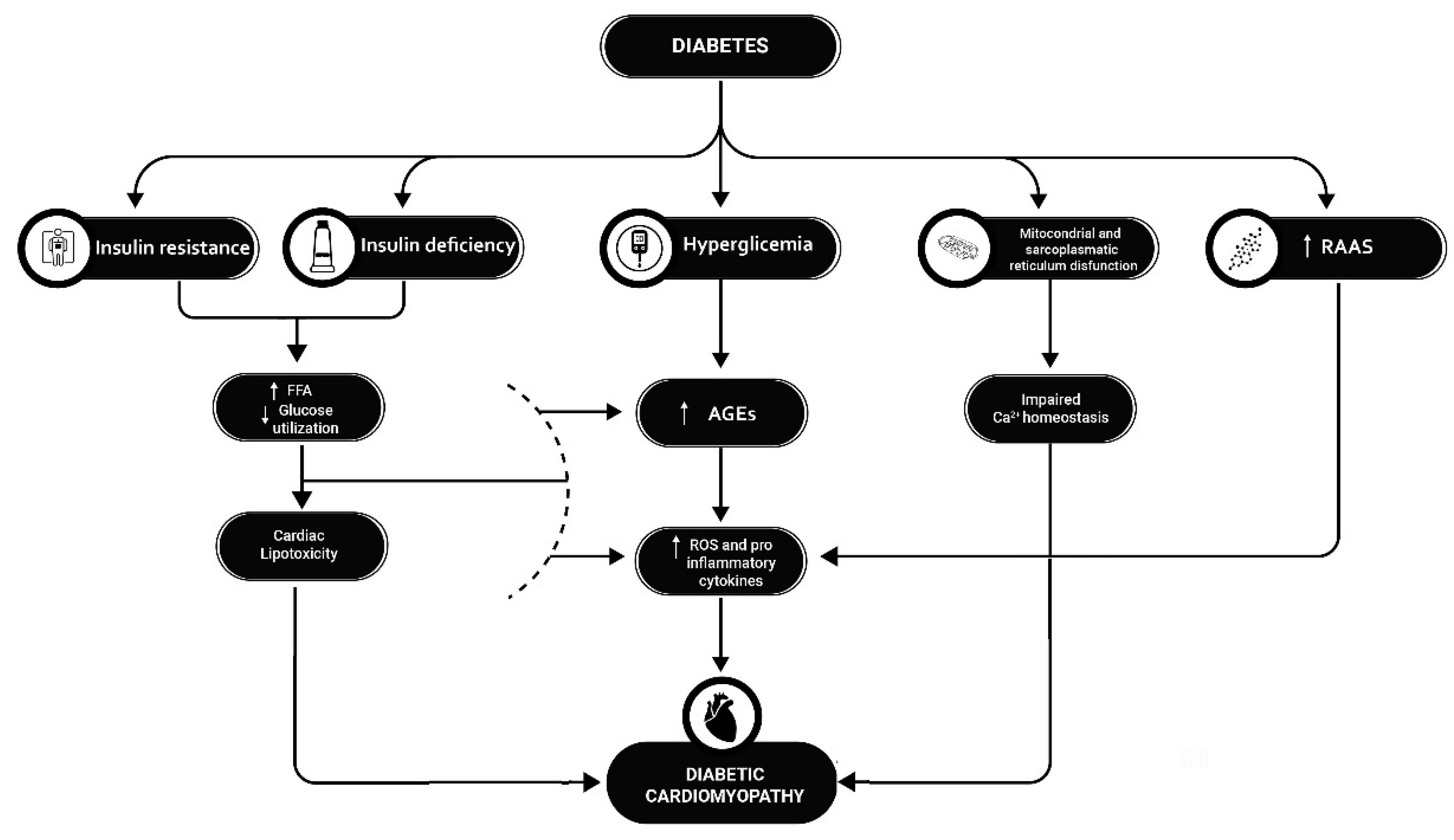
3. Pathophysiology
3.1. Abnormal insulin metabolic signaling
3.2. Hyperglycemia and AGEs
3.3. Cardiac lipotoxicity
3.4. Autonomic neuropathy
3.5. Insulin Deficiency
3.6. Mitochondrial and myocardial sarcoplasmic reticulum dysfunction
3.7. Inappropriate activation of RAAS system
4. Diagnosis
5. Prognosis
6. SGLT2-i in subjects with type 1 diabetes and heart failure
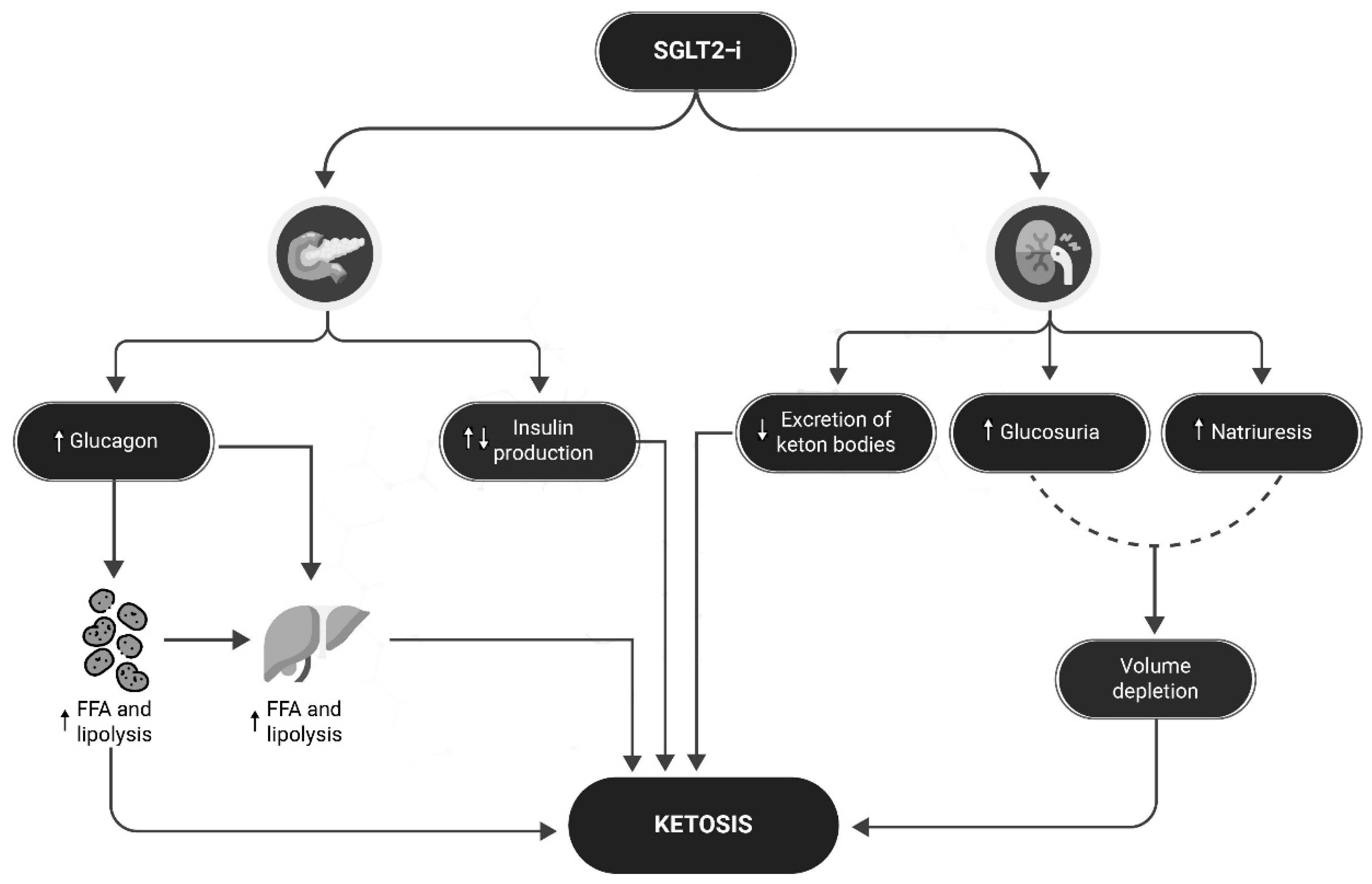
7. Ketoacidosis: could be this problem be overcome?
8. Sensors for continuous monitoring of ketonemia
8.1. Continuous Ketone Monitoring: A New Paradigm for Physiologic Monitoring
8.2. Utility of ketone measurement in the prevention, diagnosis and management of DKA
9. Limitations
10. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McHugh, K.; et al. Heart Failure with Preserved Ejection Fraction and Diabetes: JACC State-of-the-Art Review. J Am Coll Cardiol 2019, 73, 602–611. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. European Heart Journal 2021, 42, 3599–3726. [Google Scholar] [PubMed]
- Ohkuma, T.; et al. Diabetes as a risk factor for heart failure in women and men: a systematic review and meta-analysis of 47 cohorts including 12 million individuals. Diabetologia 2019, 62, 1550–1560. [Google Scholar] [CrossRef] [PubMed]
- Lehrke, M.; Marx, N. Diabetes Mellitus and Heart Failure. Am J Med 2017, 130, S40–s50. [Google Scholar] [CrossRef] [PubMed]
- McAllister, D.A.; et al. Incidence of Hospitalization for Heart Failure and Case-Fatality Among 3.25 Million People With and Without Diabetes Mellitus. Circulation 2018, 138, 2774–2786. [Google Scholar] [CrossRef]
- Kenny, H.C.; Abel, E.D. Heart Failure in Type 2 Diabetes Mellitus. Circ Res 2019, 124, 121–141. [Google Scholar] [CrossRef]
- Seferović, P.M.; et al. Type 2 diabetes mellitus and heart failure: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2018, 20, 853–872. [Google Scholar] [CrossRef]
- Packer, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. New England Journal of Medicine 2020, 383, 1413–1424. [Google Scholar]
- McMurray, J.J.V.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Wiviott, S.D.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 2019, 380, 347–357. [Google Scholar] [CrossRef]
- Zinman, B.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; et al. Angiotensin–Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. New England Journal of Medicine 2019, 381, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet 2003, 362, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Federation, I.D. Type 1 Diabetes. I.D. Federation 2020.
- Avogaro, A.; et al. Incidence of heart failure in patients with type 1 diabetes: a systematic review of observational studies. J Endocrinol Invest 2021, 44, 745–753. [Google Scholar] [CrossRef]
- Kluger, A.Y.; et al. Cardiorenal Outcomes in the CANVAS, DECLARE-TIMI 58, and EMPA-REG OUTCOME Trials: A Systematic Review. Rev Cardiovasc Med 2018, 19, 41–49. [Google Scholar]
- Petrie, D.; et al. Recent trends in life expectancy for people with type 1 diabetes in Sweden. Diabetologia 2016, 59, 1167–1176. [Google Scholar] [CrossRef]
- Group, D.E.S.R. Mortality in Type 1 Diabetes in the DCCT/EDIC Versus the General Population. Diabetes Care 2016, 39, 1378–1383. [Google Scholar]
- Lee, Y.B.; et al. Risk of early mortality and cardiovascular disease in type 1 diabetes: a comparison with type 2 diabetes, a nationwide study. Cardiovasc Diabetol 2019, 18, 157. [Google Scholar] [CrossRef]
- MacDonald, M.R.; et al. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: An analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. European Heart Journal 2008, 29, 1377–1385. [Google Scholar] [CrossRef]
- Gerber, Y.; et al. A Contemporary Appraisal of the Heart Failure Epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Internal Medicine 2015, 175, 996. [Google Scholar] [PubMed]
- Tsao, C.W.; et al. Temporal Trends in the Incidence of and Mortality Associated With Heart Failure With Preserved and Reduced Ejection Fraction. JACC Heart Fail 2018, 6, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Bode, D.; et al. Dual SGLT-1 and SGLT-2 inhibition improves left atrial dysfunction in HFpEF. Cardiovascular Diabetology 2021, 20, 7. [Google Scholar] [CrossRef] [PubMed]
- Dharam, J.; Kumbhani, M. SM, FACC Breakthrough results for Jardiance® (empagliflozin) confirm EMPEROR-Preserved as first and only successful trial for heart failure with preserved ejection fraction. 2021.
- AIFA. Nota Informativa Importante su FORXIGA (dapagliflozin). 2021; Available from: https://www.aifa.gov.it/-/nota-informativa-importante-su-forxiga-dapagliflozin-.
- Agency, U.M.a.H.p.R. Dapagliflozin (Forxiga): no longer authorised for treatment of type 1 diabetes mellitus. 2021.
- Lind, M.; et al. Glycaemic control and incidence of heart failure in 20,985 patients with type 1 diabetes: an observational study. Lancet 2011, 378, 140–146. [Google Scholar] [CrossRef]
- Wilhelmsen, L.; et al. Heart failure in the general population of men--morbidity, risk factors and prognosis. J Intern Med 2001, 249, 253–261. [Google Scholar] [PubMed]
- Chadalavada, S.; et al. Women With Diabetes Are at Increased Relative Risk of Heart Failure Compared to Men: Insights From UK Biobank. Front Cardiovasc Med 2021, 8, 658726. [Google Scholar] [CrossRef]
- Rawshani, A.; et al. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register-based cohort study. Lancet 2018, 392, 477–486. [Google Scholar] [CrossRef]
- Jia, G.; Demarco, G.; Sowers, J.R. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nature Reviews Endocrinology 2016, 12, 144–153. [Google Scholar] [CrossRef]
- Dhalla, N.S.; et al. Pathogenesis of cardiac dysfunction in diabetes mellitus. Can J Cardiol 1985, 1, 263–281. [Google Scholar]
- Belke, D.D.; et al. Altered metabolism causes cardiac dysfunction in perfused hearts from diabetic (db/db) mice. American Journal of Physiology-Endocrinology and Metabolism 2000, 279, E1104–E1113. [Google Scholar] [CrossRef]
- Wallace, A.S.; et al. Obesity and Chronic Kidney Disease in US Adults With Type 1 and Type 2 Diabetes Mellitus. The Journal of Clinical Endocrinology & Metabolism 2022, 107, 1247–1256. [Google Scholar]
- Erqou, S.; et al. Association between glycated haemoglobin and the risk of congestive heart failure in diabetes mellitus: systematic review and meta-analysis. European Journal of Heart Failure 2013, 15, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.B.; et al. Acute Hyperglycemia Attenuates Endothelium-Dependent Vasodilation in Humans In Vivo. Circulation 1998, 97, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Way, K.J.; Katai, N.; King, G.L. Protein kinase C and the development of diabetic vascular complications. Diabetic Medicine 2001, 18, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Genuth, S.; et al. Glycation and carboxymethyllysine levels in skin collagen predict the risk of future 10-year progression of diabetic retinopathy and nephropathy in the diabetes control and complications trial and epidemiology of diabetes interventions and complications participants with type 1 diabetes. Diabetes 2005, 54, 3103–3111. [Google Scholar] [PubMed]
- Xanthis, A.; et al. Receptor of Advanced Glycation End Products (RAGE) Positively Regulates CD36 Expression and Reactive Oxygen Species Production in Human Monocytes in Diabetes. Angiology 2009, 60, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Soro-Paavonen, A.; et al. Advanced glycation end-products induce vascular dysfunction via resistance to nitric oxide and suppression of endothelial nitric oxide synthase. J Hypertens 2010, 28, 780–788. [Google Scholar] [CrossRef]
- Lee, T.-W.; et al. PPARs modulate cardiac metabolism and mitochondrial function in diabetes. Journal of Biomedical Science 2017, 24. [Google Scholar] [CrossRef]
- Balcıoğlu, A.S. Diabetes and cardiac autonomic neuropathy: Clinical manifestations, cardiovascular consequences, diagnosis and treatment. World Journal of Diabetes 2015, 6, 80. [Google Scholar] [CrossRef]
- Raev, D.C. Which left ventricular function is impaired earlier in the evolution of diabetic cardiomyopathy? An echocardiographic study of young type I diabetic patients. Diabetes Care 1994, 17, 633–639. [Google Scholar] [CrossRef]
- Fein, F.S.; et al. Reversibility of diabetic cardiomyopathy with insulin in rats. Circulation Research 1981, 49, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Penpargkul, S.; et al. The effect of diabetes on performance and metabolism of rat hearts. Circulation Research 1980, 47, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Herrero, P.; et al. Increased myocardial fatty acid metabolism in patients with type 1 diabetes mellitus. J Am Coll Cardiol 2006, 47, 598–604. [Google Scholar] [CrossRef]
- Dillmann, W.H. Diabetic Cardiomyopathy. Circ Res 2019, 124, 1160–1162. [Google Scholar] [CrossRef] [PubMed]
- Hölscher, M.E.; Bode, C.; Bugger, H. Diabetic Cardiomyopathy: Does the Type of Diabetes Matter? Int J Mol Sci 2016, 17. [Google Scholar]
- Lin, Y., Y. Tang, and F. Wang, The Protective Effect of HIF-1α in T Lymphocytes on Cardiac Damage in Diabetic Mice. Ann Clin Lab Sci 2016, 46, 32–43. [Google Scholar]
- Liu, Z.W.; et al. Protein kinase RNA-like endoplasmic reticulum kinase (PERK) signaling pathway plays a major role in reactive oxygen species (ROS)-mediated endoplasmic reticulum stress-induced apoptosis in diabetic cardiomyopathy. Cardiovasc Diabetol 2013, 12, 158. [Google Scholar] [CrossRef]
- Zhao, X.-Y.; et al. Decreased cardiac sarcoplasmic reticulum Ca2+-ATPase activity contributes to cardiac dysfunction in streptozotocin-induced diabetic rats. Journal of Physiology and Biochemistry 2006, 62, 1. [Google Scholar] [CrossRef]
- Marciniak, S.J.; Ron, D. Endoplasmic Reticulum Stress Signaling in Disease. Physiological Reviews 2006, 86, 1133–1149. [Google Scholar] [CrossRef]
- Yi, C.H., H.; Vakifahmetoglu-Norberg, H.; Yuan, J. Integration of apoptosis and metabolism. Cold Spring Harb Symp Quant Biol 2011, 76, 375–387. [Google Scholar] [CrossRef]
- Singh, V.P.; et al. Intracellular angiotensin II production in diabetic rats is correlated with cardiomyocyte apoptosis, oxidative stress, and cardiac fibrosis. Diabetes 2008, 57, 3297–3306. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.A. Impact of hyperglycemia on the renin angiotensin system in early human type 1 diabetes mellitus. J Am Soc Nephrol 1999, 10, 1778–1785. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.E.; et al. Differential Clinical Profiles, Exercise Responses, and Outcomes Associated With Existing HFpEF Definitions. Circulation 2019, 140, 353–365. [Google Scholar] [CrossRef]
- Reddy, Y.N.V.; et al. A Simple, Evidence-Based Approach to Help Guide Diagnosis of Heart Failure With Preserved Ejection Fraction. Circulation 2018, 138, 861–870. [Google Scholar] [CrossRef]
- Schilling, J.D.; Mann, D.L. Diabetic Cardiomyopathy. Heart Failure Clinics 2012, 8, 619–631. [Google Scholar] [CrossRef]
- Tofte, N.; et al. Comparison of Natriuretic Peptides as Risk Markers for All-Cause Mortality and Cardiovascular and Renal Complications in Individuals With Type 1 Diabetes. Diabetes Care 2021, 44, 595–603. [Google Scholar]
- Gohar, A.; et al. Mid-regional pro-atrial natriuretic peptide for the early detection of non-acute heart failure. Eur J Heart Fail 2019, 21, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Costacou, T., A.; Saenger, A.K.; Orchard, T.J. High-Sensitivity Cardiac Troponin-T and N-Terminal Prohormone of B-Type Natriuretic Peptide in Relation to Cardiovascular Outcomes in Type 1 Diabetes. Diabetes Care 2020, 43, 2199–2207. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, M.; et al. Frequency of Reduced Left Ventricular Contractile Efficiency and Discoordinated Myocardial Relaxation in Patients Aged 16 to 21 Years With Type 1 Diabetes Mellitus (from the Emerald Study). Am J Cardiol 2020, 128, 45–53. [Google Scholar] [CrossRef]
- Gustafsson, I.; et al. Influence of diabetes and diabetes-gender interaction on the risk of death in patients hospitalized with congestive heart failure. Journal of the American College of Cardiology 2004, 43, 771–777. [Google Scholar] [CrossRef]
- Association, A.D. Introduction: Standards of Medical Care in Diabetes—2022, Diabetes Care 2021, 45(Supplement_1), S1-S2.
- Wing, R.R.; et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013, 369, 145–154. [Google Scholar] [PubMed]
- Gregg, E.W.; et al. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol 2016, 4, 913–921. [Google Scholar] [PubMed]
- Liu, C.; et al. Efficacy and safety of metformin for patients with type 1 diabetes mellitus: a meta-analysis. Diabetes Technol Ther 2015, 17, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Neal, B.; Perkovic, V.; Matthews, D.R. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med 2017, 377, 2099. [Google Scholar] [CrossRef]
- Bhatt, D.L.; et al. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N Engl J Med 2021, 384, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; et al. Sotagliflozin in Patients with Diabetes and Chronic Kidney Disease. New England Journal of Medicine 2020, 384, 129–139. [Google Scholar] [CrossRef]
- Filippatos, G.; et al. Empagliflozin for Heart Failure With Preserved Left Ventricular Ejection Fraction With and Without Diabetes. Circulation 2022, 146, 676–686. [Google Scholar] [CrossRef]
- Modi, A.; Agrawal, A.; Morgan, F. Euglycemic Diabetic Ketoacidosis: A Review. Curr Diabetes Rev 2017, 13, 315–321. [Google Scholar] [CrossRef]
- Abu-Zaid, A.; et al. A systematic review and dose-response meta-analysis on the efficacy of dapagliflozin in patients with type 1 diabetes mellitus. Pharmacol Res 2021, 165, 105456. [Google Scholar] [CrossRef]
- Musso, G.; et al. Efficacy and safety of dual SGLT 1/2 inhibitor sotagliflozin in type 1 diabetes: meta-analysis of randomised controlled trials. Bmj 2019, 365, l1328. [Google Scholar] [CrossRef]
- Rosenstock, J.; et al. Empagliflozin as Adjunctive to Insulin Therapy in Type 1 Diabetes: The EASE Trials. Diabetes Care 2018, 41, 2560–2569. [Google Scholar] [CrossRef]
- Kim, Y.J., S.; Hwang, S.D.; Lim, S. Effects of Sodium-Glucose Cotransporter Inhibitor/Glucagon-Like Peptide-1 Receptor Agonist Add-On to Insulin Therapy on Glucose Homeostasis and Body Weight in Patients With Type 1 Diabetes: A Network Meta-Analysis. Front Endocrinol (Lausanne) 2020, 11, 553. [Google Scholar] [CrossRef]
- Merovci, A.; et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest 2014, 124, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.I.; Blau, J.E.; Rother, K.I. SGLT2 Inhibitors May Predispose to Ketoacidosis. J Clin Endocrinol Metab 2015, 100, 2849–2852. [Google Scholar] [CrossRef] [PubMed]
- Hanas, R.; Lindgren, F.; Lindblad, B. A 2-yr national population study of pediatric ketoacidosis in Sweden: predisposing conditions and insulin pump use. Pediatr Diabetes 2009, 10, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Realsen, J., H.; Goettle, H.; Chase, H.P. Morbidity and mortality of diabetic ketoacidosis with and without insulin pump care. Diabetes Technol Ther 2012, 14, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
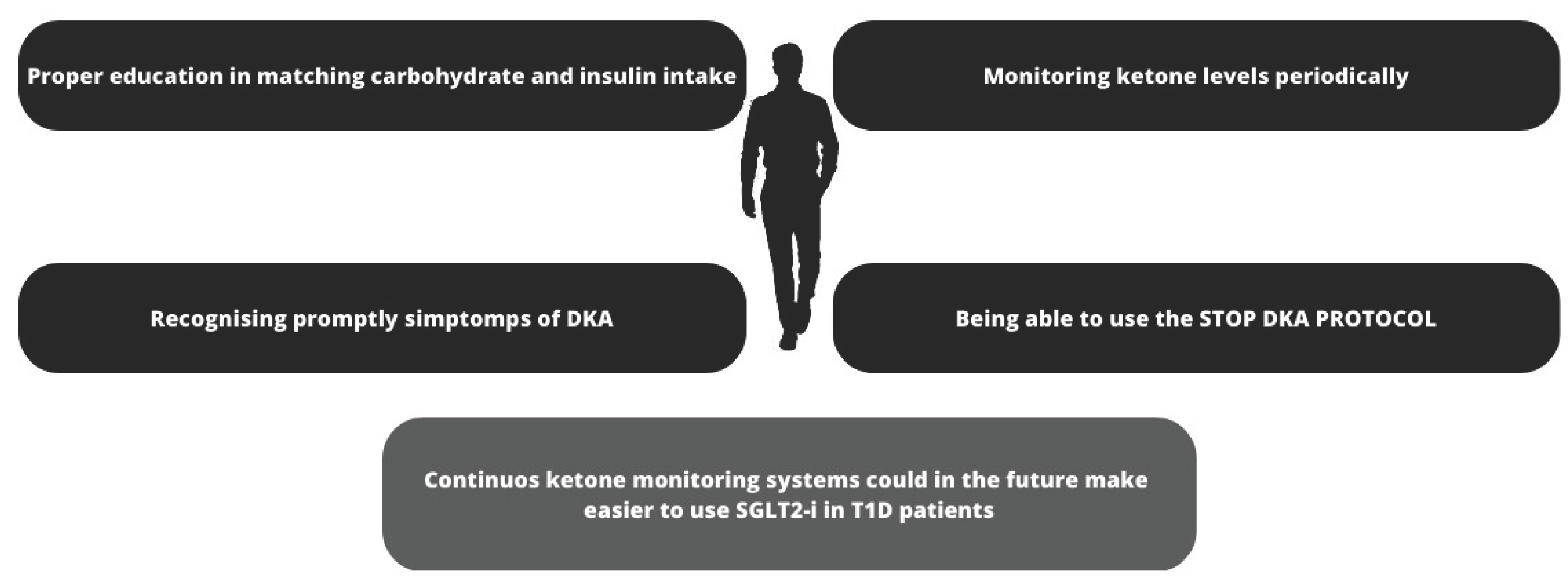
- Danne, T.; et al. International Consensus on Risk Management of Diabetic Ketoacidosis in Patients With Type 1 Diabetes Treated With Sodium–Glucose Cotransporter (SGLT) Inhibitors. Diabetes Care 2019, 42, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, F.; et al. An integrated wearable microneedle array for the continuous monitoring of multiple biomarkers in interstitial fluid. Nature Biomedical Engineering.
- Goldenberg, R.M.; et al. Sodium-glucose co-transporter inhibitors, their role in type 1 diabetes treatment and a risk mitigation strategy for preventing diabetic ketoacidosis: The STOP DKA Protocol. Diabetes, Obesity and Metabolism 2019, 21, 2192–2202. [Google Scholar] [CrossRef]
- Garg SK, P.A.; Buse, J.B.; Danne, T. Strategy for Mitigating DKA Risk in Patients with Type 1 Diabetes on Adjunctive Treatment with SGLT Inhibitors: A STICH Protocol. Diabetes Technology & Therapeutics 2018, 20, 571–575. [Google Scholar]
- Zhang, J.Y.; et al. Continuous Ketone Monitoring: A New Paradigm for Physiologic Monitoring. Journal of Diabetes Science and Technology 2021, 15. [Google Scholar]
- Abbott's Biowearable: One Sensor for Glucose, Ketones. Abbott, 2022.
- Misra, S.; Oliver, N.S. Utility of ketone measurement in the prevention, diagnosis and management of diabetic ketoacidosis. Diabetic Medicine 2015.
- Jefferies, C.A.; et al. Preventing Diabetic Ketoacidosis. Pediatr Clin North Am 2015, 62, 857–871. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




