Submitted:
24 February 2024
Posted:
26 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
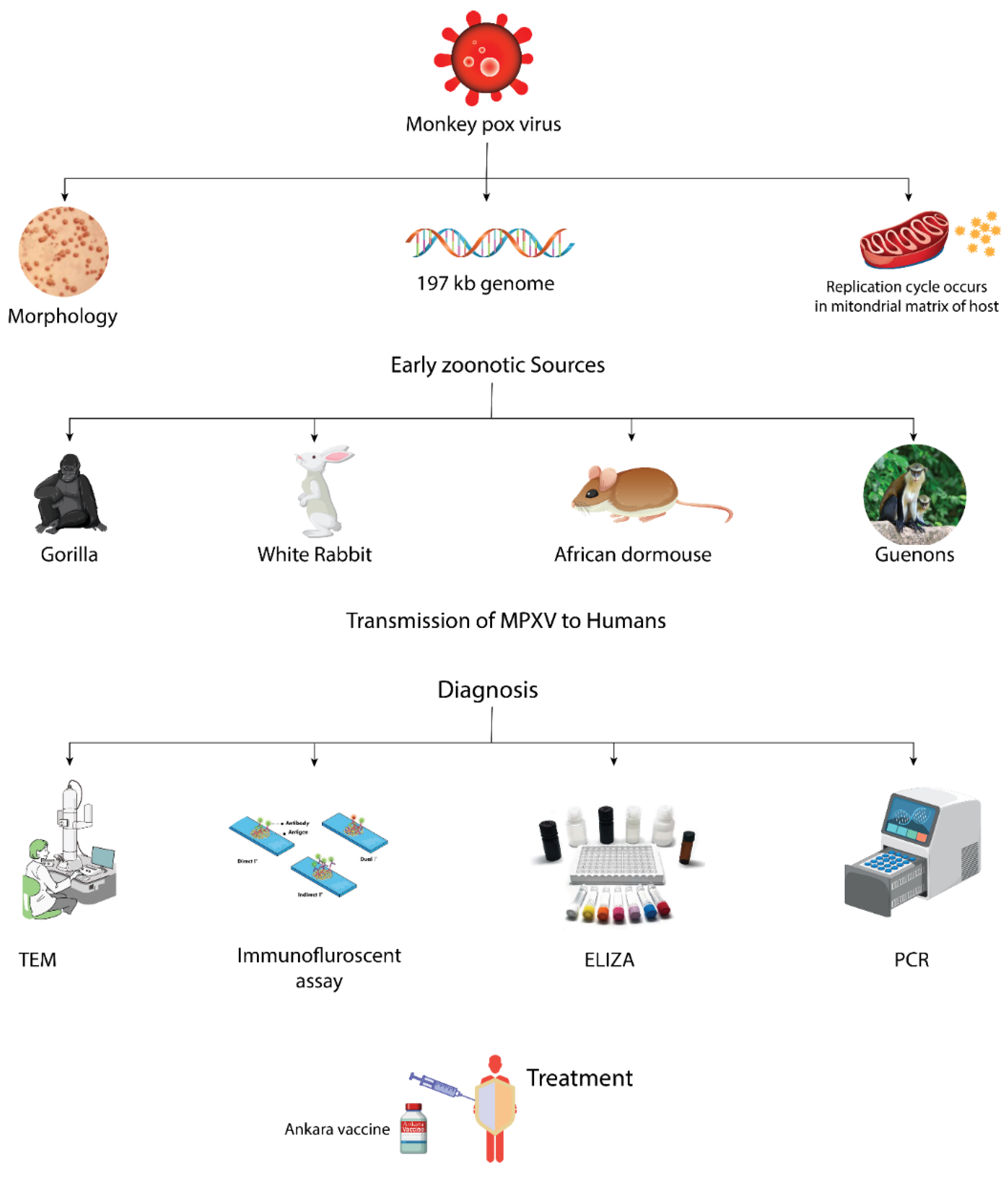
2. Molecular Biology
2.1. Morphology
2.2. Genome
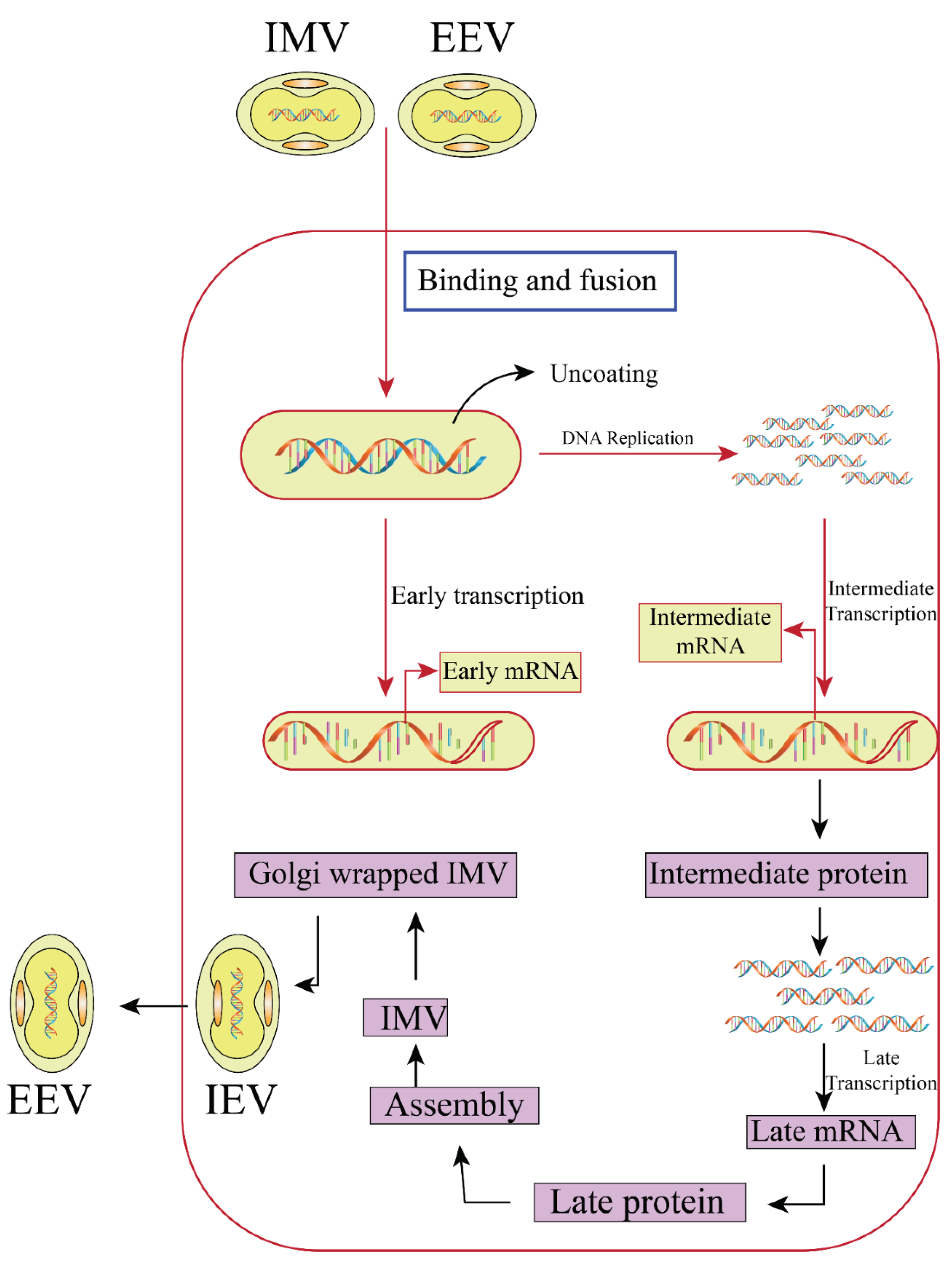
2.3. Replication Cycle
2. Early Insights into Zoonotic Origins of Monkeypox
| Species Name | Family | Commonly Called | Determination Techniques | Remarks | References |
|---|---|---|---|---|---|
| Graphiurus spp. | Gliridae | African dormouse | Outbreak in pocket pets; Infection investigations in the Lab | After infection, in vivo imaging experiments were carried out. | [49,50] |
| Cynomys ludovicanus | Sciuridae | Black-tailed priarie dog | Outbreak in pocket pets; Infection investigations in the Lab | After infection, in vivo imaging experiments were carried out. | [49,51] |
| Cercopithecus spp. | Ceropithicediae | Guenons | Field examinations | no virus or antigen DNA identified, OPX antibody positive | [52] |
| Gorilla sp. | Hominidae | Gorilla | Outbreak, zoological park | Morbidity reported | [53] |
| Cricetomys spp. | Nesomyidae | Giant pouched rat | Outbreak in pocket pets; Infection investigations in the Lab | After infection, in vivo imaging experiments were carried out. | [49,54] |
| Mus musculus | Muridae | Laboratory mouse, domestic mouse | Infection studies in laboratory | Adult immunological competent mice are often resistant to wild-derived castaneus strains, which have been found to be sensitive in laboratory trials. | [55,56] |
| Myrmecophaga tridactyla | Myrmecophagidae | New World giant anteater | Outbreak, zoological park | Morbidity reported | [53] |
| Petrodromus tetradactylus | Macroscelididae | elephant shrews | Field examinations | no virus or antigen DNA identified, OPX antibody positive | [57,58] |
| Oryctolagus cuniculus | Leporidae | White rabbit | Infection investigations in laboratory | Adult animals are normally not vulnerable; however, outcome varies depending on the inoculation route and the animal's genetic background. | [59] |
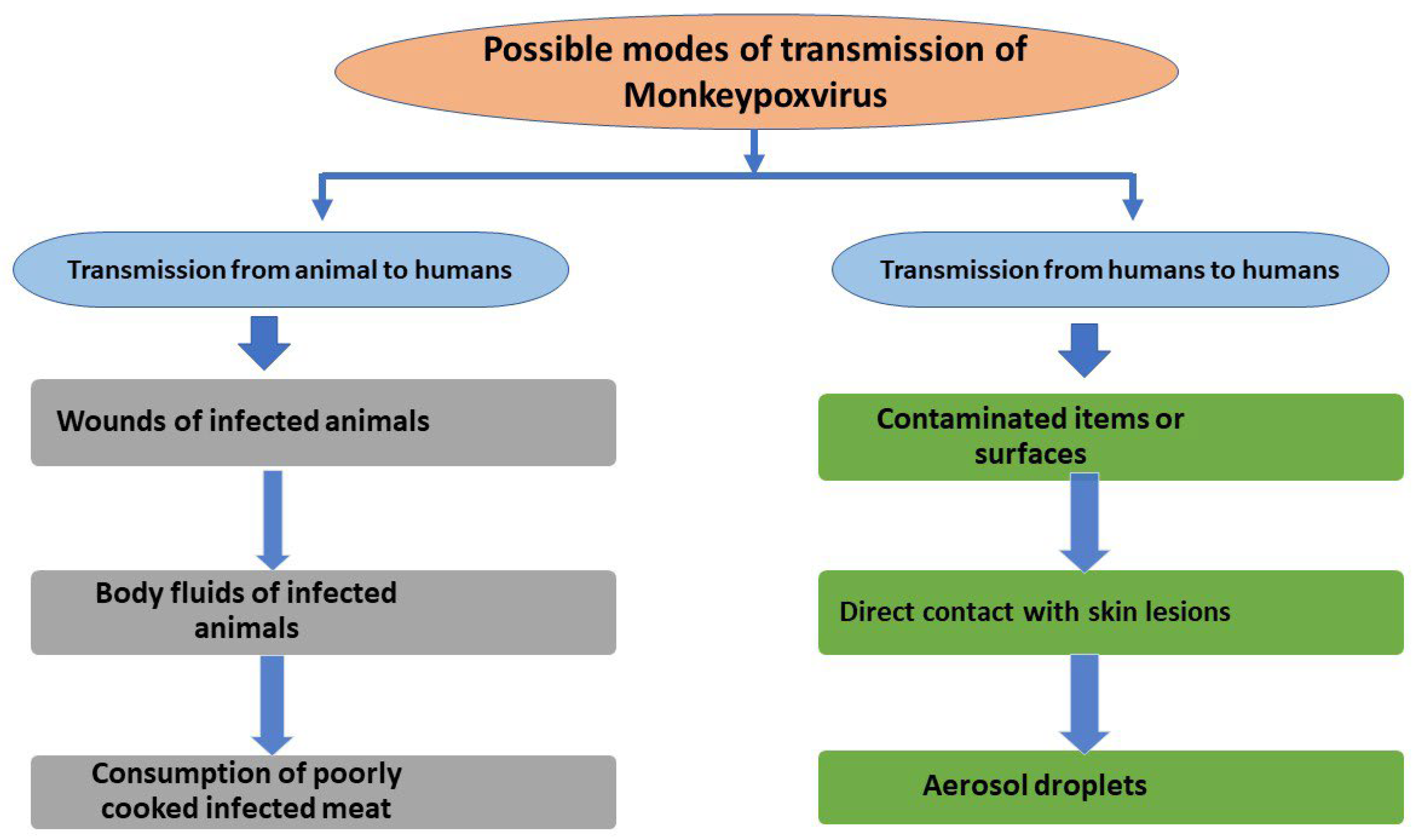
3. Transmission of MPXV to Humans
4. The Evolving Epidemiology of Human Monkeypox
5. Clinical Presentation of Monkeypox Virus
6. Diagnosis
7. Treatment and Management
8. Preventive Strategies
9. Discussion
10. Conclusions and Future Perspective
References
- Howard, C.R.; Fletcher, N.F. Emerging virus diseases: Can we ever expect the unexpected? Emerg Microbes Infect. 2012;1(12):e46. [CrossRef]
- Kabuga, A.I.; El Zowalaty, M.E. A review of the monkeypox virus and a recent outbreak of skin rash disease in Nigeria. J. Med Virol. 2019, 91, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Nathanson, N. (2016). The Human Toll of Viral Diseases: Past Plagues and Pending Pandemics. In Viral Pathogenesis (pp. 3-16). Academic Press. [CrossRef]
- Vaughan, A.; Aarons, E.; Astbury, J.; Brooks, T.; Chand, M.; Flegg, P.; Hardman, A.; Harper, N.; Jarvis, R.; Mawdsley, S.; et al. Human-to-Human Transmission of Monkeypox Virus, United Kingdom, October 2018. Emerg. Infect. Dis. 2020, 26, 782–785. [Google Scholar] [CrossRef] [PubMed]
- Ladnyj ID, Ziegler P, Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull World Health Organ 1972; 46:593–7.
- Alakunle, E.; Moens, U.; Nchinda, G.; Okeke, M.I. Monkeypox Virus in Nigeria: Infection Biology, Epidemiology, and Evolution. Viruses 2020, 12, 1257. [Google Scholar] [CrossRef] [PubMed]
- Kugelman JR, Johnston SC, Mulembakani PM, Kisalu N, Lee MS, Koroleva G, McCarthy SE, Gestole MC, Wolfe ND, Fair JN, Schneider BS, Wright LL, Huggins J, Whitehouse CA, Wemakoy EO, Muyembe-Tamfum JJ, Hensley LE, Palacios GF, Rimoin AW. Genomic variability of monkeypox virus among humans, Democratic Republic of the Congo. Emerg Infect Dis. 2014 Feb;20(2):232-9. [CrossRef]
- Walsh, D. Poxviruses: Slipping and sliding through transcription and translation. PLoS Pathog. 2017 Nov;13(11):e1006634. [CrossRef]
- Nguyen PY, Ajisegiri WS, Costantino V, Chughtai AA, MacIntyre CR. Reemergence of Human Monkeypox and Declining Population Immunity in the Context of Urbanization, Nigeria, 2017-2020. Emerg Infect Dis. 2021 Apr;27(4). [CrossRef]
- Sklenovská N, Van Ranst M. Emergence of Monkeypox as the Most Important Orthopoxvirus Infection in Humans. Front Public Health. 2018; 6:241. [CrossRef]
- Durski KN, McCollum AM, Nakazawa Y; et al. Emergence of monkeypox – west and Central Africa, 1970–2017. MMWR. 2018; 67:306–310.
- U.K. Health Security Agency. Monkeypox cases confirmed in England -latest updates. 2022. [Online]. Available from: https://www.gov.uk/ government/news/monkeypox-cases-confirmed-in-england-latest-updates. [Accessed on 25 May 2022].
- World Health Organization. Multi-country monkeypox outbreak in non- endemic countries. 2022. [Online]. Available from: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON385. [Accessed on 25 May 2022]. Back to cited text no. 9.
- Sklenovská N, Van Ranst M. Emergence of monkeypox as the most important orthopoxvirus infection in humans. Front Public Health 2018; 6: 241. [CrossRef]
- World Health Organization. Monkeypox-United Kingdom of Great Britain and Northern Ireland. 2022. [Online]. Available from: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON381.[Accessed on 25 May 2022].
- Shanmugaraj B, Malla A, Khorattanakulchai N, Phoolcharoen W. SARS- CoV-2 omicron variant: Could it be another threat? J Med Virol 2022; 94(4): 1284-1288. [CrossRef]
- Shanmugaraj, B.; Phoolcharoen, W.; Khorattanakulchai, N. Emergence of monkeypox: Another concern amidst COVID-19 crisis. Asian Pac. J. Trop. Med. 2022, 15, 193–195. [Google Scholar] [CrossRef]
- Ferreira Barreto-Vieira D, Monika Barth O (2015) Negative and positive staining in transmission electron microscopy for virus diagnosis. In: Shah MM (ed) Microbiology in agriculture and human health. InTech, Croatia. [CrossRef]
- Jahrling PB, Huggins JW, Ibrahim MS, Lawler JV, Martin JW (2007) Smallpox and related orthopoxviruses. In: Dembek ZF, Borden I (eds) Medical aspects of biological warfare. Borden Institute, Walter Reed Army Medical Center; Office of the Surgeon General, United States Army; United States Army Medical Dept. Center and School, Washington, DC.
- Moss B, Damon I (2013) Chapter: Poxviridae. In: Knipe DM, Howley PM (eds) Fields virology, 6th edn. Wolters Kluwer Health/Lippincott Williams & Wilkins, Philadelphia.
- Ladnyi ID, Jezek Z, Fenner F, Henderson DA, Arita I (1988) Chapter: Human monkeypox and other poxvirus infections of man. In: Smallpox and its eradication. WHO.
- Resch W, Hixson KK, Moore RJ, Lipton MS, Moss B (2007) Protein composition of the vaccinia virus mature virion. Virology 358(1):233–247. [CrossRef]
- Sklenovská, N. (2020). Monkeypox Virus. In: Malik, Y.S., Singh, R.K., Dhama, K. (eds) Animal-Origin Viral Zoonoses. Livestock Diseases and Management. Springer, Singapore. [CrossRef]
- Shchelkunov SN, Totmenin AV, Safronov PF, Mikheev MV, Gutorov VV, Ryazankina OI, Moss B (2002) Analysis of the monkeypox virus genome. Virology 297(2):172–194. [CrossRef]
- Wittek R, Moss B (1980) Tandem repeats within the inverted terminal repetition of vaccinia virus DNA. Cell 21(1):277–284.
- Baroudy BM, Venkatesan S, Moss B (1982) Incompletely base-paired flip-flop terminal loops link the two DNA strands of the vaccinia virus genome into one uninterrupted polynucleotide chain. Cell 28(2):315–324.
- Seet BT, Johnston JB, Brunetti CR, Barrett JW, Everett H, Cameron C, McFadden G (2003) Poxviruses and immune evasion. Annu Rev Immunol 21:377423. [CrossRef]
- Shchelkunov SN, Totmenin AV, Babkin IV, Safronov PF, Ryazankina OI, Petrov NA, Sandakhchiev LS (2001) Human monkeypox and smallpox viruses: Genomic comparison. FEBS Lett 509(1):66–70. [CrossRef]
- Likos AM, Sammons SA, Olson VA, Frace AM, Li Y, Olsen-Rasmussen M, Damon IK (2005) A tale of two clades: Monkeypox viruses. J Gen Virol 86(Pt 10):2661–2672. [CrossRef]
- Afonso CL, Tulman ER, Lu Z, Zsak L, Sandybaev NT, Kerembekova UZ, Rock DL (2002) The genome of camelpox virus. Virology 295(1):1–9. [CrossRef]
- Chen N, Li G, Liszewski MK, Atkinson JP, Jahrling PB, Feng Z, Buller RM (2005b) Virulence differences between monkeypox virus isolates from West Africa and the Congo basin. Virol J 340(1):46–63. [CrossRef]
- Tulman ER, Delhon G, Afonso CL, Lu Z, Zsak L, Sandybaev NT, Rock DL (2006) Genome of horsepox virus. J Virol 80(18):9244–9258. [CrossRef]
- Barry M, Wasilenko ST, Stewart TL, Taylor JM (2004) Apoptosis regulator genes encoded by poxviruses. Prog Mol Subcell Biol 36:19–37. [CrossRef]
- Buller RM, Palumbo GJ (1991) Poxvirus pathogenesis. Microbiol Rev 55(1):80–122. [CrossRef]
- Moss B (2016) Membrane fusion during poxvirus entry. Semin Cell Dev Biol 60:89–96. [CrossRef]
- Bray M, Buller M (2004) Looking back at smallpox. Clin Infect Dis 38(6):882–889. [CrossRef]
- Roberts KL, Smith GL (2008) Vaccinia virus morphogenesis and dissemination. Trends Microbiol 16(10):472–479. [CrossRef]
- Arita I, Henderson DA. Monkeypox and whitepox viruses in west and Central Africa. Bull World Health Organ. 1976;53:347–353.
- Gispen R, Brand-Saathof BB, Hekker AC. Monkeypox-specific antibodies in human and simian sera from the Ivory Coast and Nigeria. Bull World Health Organ. 1976;53:355–360.
- Khodakevich, L., Ježek, Z., & Kinzanzka, K. (1986). Isolation of monkeypox virus from wild squirrel infected in nature. Isolation of monkeypox virus from wild squirrel infected in nature., (Jan. 11), 98-99. [CrossRef]
- Arita I, Jezek Z, Khodakevich L; et al. Human monkeypox: A newly emerged orthopoxvirus zoonosis in the tropical rain forests of Africa. Am J Trop Med Hyg. 1985;34:781–789. [CrossRef]
- Khodakevich L, Szczeniowski M, Nambu MD; et al. Monkeypox virus in relation to the ecological features surrounding human settlements in Bumba zone, Zaire. Trop Geogr Med. 1987;39:56–63.
- Khodakevich L, Jezek Z, Messinger D. Monkeypox virus: Ecology and public health significance. Bull World Health Organ. 1988;66:747–752.
- Khodakevich L, Szczeniowski M, Manbu MD; et al. The role of squirrels in sustaining monkeypox virus transmission. Trop Geogr Med. 1987;39:115–122.
- Doshi RH, Guagliardo SAJ, Dzabatou-Babeaux A; et al. Strengthening of surveillance during monkeypox outbreak, republic of the Congo, 2017. Emerg Infect Dis. 2018;24:1158–1160. [CrossRef]
- Hutin YJ, Williams RJ, Malfait P; et al. Outbreak of human monkeypox, democratic republic of Congo, 1996 to 1997. Emerg Infect Dis. 2001;7:434–438. [CrossRef]
- Radonic A, Metzger S, Dabrowski PW; et al. Fatal monkeypox in wild-living sooty mangabey, Cote d’Ivoire, 2012. Emerg Infect Dis. 2014;20:1009–1011. [CrossRef]
- Doty JB, Malekani JM, Kalemba LN; et al. Assessing monkeypox virus prevalence in small mammals at the human-animal interface in the democratic republic of the Congo. Viruses. 2017;9:283. [CrossRef]
- Hutson CL, Lee KN, Abel J; et al. Monkeypox zoonotic associations: Insights from laboratory evaluation of animals associated with the multi-state US outbreak. Am J Trop Med Hyg. 2007;76:757–768. [CrossRef]
- Earl PL, Americo JL, Cotter CA; et al. Comparative live bioluminescence imaging of monkeypox virus dissemination in a wild-derived inbred mouse (Mus musculus castaneus) and outbred African dormouse (Graphiurus kelleni). Virology. 2015;475:150–158. [CrossRef]
- Falendysz EA, Londono-Navas AM, Meteyer CU; et al. Evaluation of monkeypox virus infection of black-tailed prairie dogs (Cynomys ludovicianus) using in vivo bioluminescent imaging. J Wildl Dis. 2014;50:524–536.
- Breman JG, Nakano JH, Coffi E; et al. Human poxvirus disease after smallpox eradication. Am J Trop Med Hyg. 1977;26:273–281. [CrossRef]
- Arita I, Henderson DA. Smallpox and monkeypox in non-human primates. Bull World Health Organ. 1968;39:277–283.
- Falendysz EA, Lopera JG, Lorenzsonn F; et al. Further assessment of monkeypox virus infection in gambian pouched rats (cricetomys gambianus) using in vivo bioluminescent imaging. PLoS Negl Trop Dis. 2015;9:e0004130. [CrossRef]
- Americo JL, Moss B, Earl PL. Identification of wild-derived inbred mouse strains highly susceptible to monkeypox virus infection for use as small animal models. J Virol. 2010;84:8172–8180. [CrossRef]
- Earl PL, Americo JL, Moss B. Genetic studies of the susceptibility of classical and wild-derived inbred mouse strains to monkeypox virus. Virology. 2015;481:161–165. [CrossRef]
- Hutin YJ, Williams RJ, Malfait P; et al. Outbreak of human monkeypox, democratic republic of Congo, 1996 to 1997. Emerg Infect Dis. 2001;7:434–438. [CrossRef]
- Doty JB, Malekani JM, Kalemba LN; et al. Assessing monkeypox virus prevalence in small mammals at the human-animal interface in the democratic republic of the Congo. Viruses. 2017;9:283. [CrossRef]
- Marennikova SS, Seluhina EM. Susceptibility of some rodent species to monkeypox virus, and course of the infection. Bull World Health Organ. 1976;53:13–20.
- Prier JE, Sauer RM. A pox disease of monkeys. Ann N Y Acad Sci 1960;85:951-9. [CrossRef]
- Wenner HA, Macasaet D, Kamitsuka PS, Kidd P. MonkeypoxI. Clinical, virologic and immunologic studies. Am J Epidemiol 1968;87:551-66. [CrossRef]
- Nakoune E, Lampaert E, Ndjapou SG et al. A Nosocomial Outbreak of Human Monkeypox in the Central African Republic. Open Forum Infect Dis. 2017;4:ofx168. [CrossRef]
- Durski KN, McCollum AM, Nakazawa Y et al. Emergence of Monkeypox - West and Central Africa, 1970-2017. MMWR Morb Mortal Wkly Rep. 2018;67:306-10. [CrossRef]
- Sklenovská N, Van Ranst M. Emergence of Monkeypox as the Most Important Orthopoxvirus Infection in Humans. Front Publ Hlth. 2018;6:241. [CrossRef]
- Quiner CA, Moses C, Monroe BP et al. Presumptive risk factors for monkeypox in rural communities in the Democratic Republic of the Congo. PLoS ONE. 2017;12:e0168664. [CrossRef]
- Marennikova SS, Seluhina EM, Malceva NN, Cimiskjan KL, Macevic GR. Isolation and properties of the causal agent of a new variola-like disease (monkeypox) in man. Bull World Hlth Organ 1972;46:599–611.
- World Health Organization. Monkeypox. 2019. https://www.who.int/newsroom/factsheets/detail/monkeypox. Accessed 9 December 2019.
- Rimoin AW, Mulembakani PM, Johnston SC; et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc Natl Acad Sci U S A 2010; 107:16262–7. [CrossRef]
- Centers for Disease Control and Prevention. Human monkeypox— Kasai Oriental, Zaire, 1996–1997. MMWR Morb Mortal Wkly Rep 1997; 4614:304–7.
- Jezek Z, Grab B, Szczeniowski MV, Paluku KM, Mutombo M. Human monkeypox: Secondary attack rates. Bull World Health Organ 1988; 66:465–70.
- Hussain AN, Hussain F, Alam M, Cleri DJ. Smallpox treatment and management. 2019. https://emedicine.medscape.com/article/237229-treatment. Accessed 08 March 2021.
- World Population Review. Median age 2021. http://worldpopulationreview.com/countries/median-age. Accessed 22 January 2020.
- Levine RS, Peterson AT, Yorita KL, Carroll D, Damon IK, Reynolds MG. Ecological niche and geographic distribution of human monkeypox in Africa. PLoS ONE 2007; 2:e176. [CrossRef]
- Hutin YJ, Williams RJ, Malfait P; et al. Outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg Infect Dis 2001; 7:434–8. [CrossRef]
- Reynolds MG, Doty JB, McCollum AM, Olson VA, Nakazawa Y. Monkeypox re-emergence in Africa: A call to expand the concept and practice of One Health. Expert Rev Anti Infect Ther. 2019; 17:129–39. [CrossRef]
- Yinka-Ogunleye A, Aruna O, Ogoina D; et al. Reemergence of human monkeypox in Nigeria, 2017. Emerg Infect Dis 2018; 24:1149–51. [CrossRef]
- Guarner J, Johnson BJ, Paddock CD; et al.; Veterinary Monkeypox Virus Working Group. Monkeypox transmission and pathogenesis in prairie dogs. Emerg Infect Dis 2004; 10:426–31. [CrossRef]
- Bartlett, J. Monkeypox review. Medscape 2003. https://wwwnc.cdc.gov/eid/article/26/4/19%E2%80%901164_article. Accessed 08 March 2021.
- Simpson, K. , Heymann, D., Brown, C. S., Edmunds, W. J., Elsgaard, J., Fine, P.,... & Wapling, A. (2020). Human monkeypox–After 40 years, an unintended consequence of smallpox eradication. Vaccine, 38(33), 5077-5081. [CrossRef]
- Public Health England. Monkeypox diagnosed in England—First case of onward transmission. 2019. https://www.who.int/csr/don/01-october-2020-monkeypox-drc/en/#:~:text=WHO%20risk%20assessment,group%20of%20viruses%20as%20smallpox.
- Likos AM, Simmons SA; et al. A tale of two clades: Monkeypox viruses. J Gen Virol 2005; 86:1661–2672. [CrossRef]
- Whitehouse, E. R. , Bonwitt, J., Hughes, C. M., Lushima, R. S., Likafi, T., Nguete, B.,... & Reynolds, M. G. (2021). Clinical and epidemiologic findings from enhanced monkeypox surveillance in Tshuapa Province, Democratic Republic of the Congo during 2011–2015.
- World Health Organization. Monkeypox—Democratic Republic of the Congo. 2020. https://www.who.int/csr/don/01-october-2020-monkeypox-drc/en/. Accessed 08 February 2021.
- Lederman ER, Reynolds MG, Karem K, et al. Prevalence of antibodies against orthopoxviruses among residents of Likouala region, Republic of Congo: Evidence for monkeypox virus exposure. Am J Trop Med Hyg 2007; 77:1150–6. [CrossRef]
- Doshi RH, Guagliardo SAJ, Doty JB; et al. Epidemiologic and ecologic investigations of monkeypox, Likouala department, Republic of the Congo, 2017. Emerg Infect Dis 2019; 25:273–81. [CrossRef]
- Davi SD, Kissenkötter J, Faye M; et al. Recombinase polymerase amplification assay for rapid detection of monkeypox virus. Diagn Microbiol Infect Dis 2019; 95:41–5. [CrossRef]
- Li D, Wilkins K, McCollum AM; et al. Evaluation of the GeneXpert for human monkeypox diagnosis. Am J Trop Med Hyg 2017; 96:405–10. [CrossRef]
- World Health Organization. Outbreaks and emergencies bulletin. https://www.afro.who.int/healthtopics/disease-outbreaks/outbreaksand-other-emergencies-updates. Accessed 08 March 2021.
- Petersen BW, Kabamba J, McCollum AM; et al. Vaccinating against monkeypox in the Democratic Republic of the Congo. Antiviral Res 2019; 162:171–7. [CrossRef]
- Reynolds MG, McCollum AM, Nguete B, Shongo Lushima R, Petersen BW. Improving the care and treatment of monkeypox patients in low-resource settings: Applying evidence from contemporary biomedical and smallpox biodefense research. Viruses 2017; 9:380. [CrossRef]
- Damon, I. K. (2011). Status of human monkeypox: Clinical disease, epidemiology and research. Vaccine, 29, D54-D59. [CrossRef]
- McCollum, A. M., & Damon, I. K. (2014). Human monkeypox. Clinical infectious diseases, 58(2), 260-267.
- Yinka-Ogunleye, A., Aruna, O., Ogoina, D., Aworabhi, N., Eteng, W., Badaru, S., ... & Ihekweazu, C. (2018). Reemergence of human monkeypox in Nigeria, 2017. Emerging infectious diseases, 24(6), 1149. [CrossRef] [PubMed]
- Kalthan, E., Tenguere, J., Ndjapou, S. G., Koyazengbe, T. A., Mbomba, J., Marada, R. M.,... & Nakoune, E. R. (2018). Investigation of an outbreak of monkeypox in an area occupied by armed groups, Central African Republic. Medecine et Maladies Infectieuses, 48(4), 263-268. [CrossRef]
- Falcinelli, S. D., Chertow, D. S., & Kindrachuk, J. (2016). Integration of global analyses of host molecular responses with clinical data to evaluate pathogenesis and advance therapies for emerging and re-emerging viral infections. ACS infectious diseases, 2(11), 787-799. [CrossRef]
- Chen, N., Li, G., Liszewski, M. K., Atkinson, J. P., Jahrling, P. B., Feng, Z., ... & Buller, R. M. L. (2005). Virulence differences between monkeypox virus isolates from West Africa and the Congo basin. Virology, 340(1), 46-63. [CrossRef]
- Damon, IK. Status of human monkeypox: Clinical disease, epidemiology, and research. Vaccine. 2011;29:D54-D59. [CrossRef]
- Reynolds MG, Carroll DS, Karem KL. Factors affecting the likelihood of monkeypox’s emergence and spread in the post-smallpox era. Curr Opin Virol. 2012;2(3):335-43. [CrossRef]
- Oladoye, M. J. (2021). Monkeypox: A Neglected Viral Zoonotic Disease. Electronic Journal of Medical and Educational Technologies, 14(2), em2108. [CrossRef]
- McCollum AM, Damon IK. Human monkeypox. Clin Infect Dis. 2014 Jan;58(2):260-7. [PubMed] [Reference list].
- Petersen BW, Kabamba J, McCollum AM, Lushima RS, Wemakoy EO, Muyembe Tamfum JJ, Nguete B, Hughes CM, Monroe BP, Reynolds MG. Vaccinating against monkeypox in the Democratic Republic of the Congo. Antiviral Res. 2019 Feb;162:171-177. [CrossRef]
- Sklenovská N, Van Ranst M. Emergence of Monkeypox as the Most Important Orthopoxvirus Infection in Humans. Front Public Health. 2018;6:241. [CrossRef]
- Reynolds MG, McCollum AM, Nguete B, Shongo Lushima R, Petersen BW. Improving the Care and Treatment of Monkeypox Patients in Low-Resource Settings: Applying Evidence from Contemporary Biomedical and Smallpox Biodefense Research. Viruses. 2017 Dec 12;9(12). [CrossRef]
- Quiner CA, Moses C, Monroe BP; et al. Presumptive risk factors for monkeypox in rural communities in the Democratic Republic of the Congo. Yang Y, ed. PLoS ONE. 2017; 12(2): e0168664. [CrossRef]
- Petersen, E., Kantele, A., Koopmans, M., Asogun, D., Yinka-Ogunleye, A., Ihekweazu, C., & Zumla, A. (2019). Human monkeypox: Epidemiologic and clinical characteristics, diagnosis, and prevention. Infectious Disease Clinics, 33(4), 1027-1043. [CrossRef]
- Bass J, Tack DM, McCollum AM; et al. Enhancing health care worker ability to detect and care for patients with monkeypox in the Democratic Republic of the Congo. Int Health 2013;5:237–43. [CrossRef]
- Reynolds MG, McCollum AM, Nguete B, Shongo Lushima R, Petersen BW. Improving the care and treatment of monkeypox patients in low-resource settings: Applying evidence from contemporary biomedical and smallpox biodefense research. Viruses 2017;9:E380. [CrossRef]
- World Health Organization. R&D blueprint: List of blueprint priority diseases. Geneva, Switzerland: World Health Organization; 2018.
- Shiferaw ML, Doty JB, Maghlakelidze G; et al. Frameworks for preventing, detecting, and controlling zoonotic diseases. Emerg Infect Dis 2017;23. [CrossRef]
- Durski, K. N., McCollum, A. M., Nakazawa, Y., Petersen, B. W., Reynolds, M. G., Briand, S.,... & Khalakdina, A. (2018). Emergence of monkeypox—West and central Africa, 1970–2017. Morbidity and mortality weekly report, 67(10), 306. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




