Submitted:
25 February 2024
Posted:
26 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
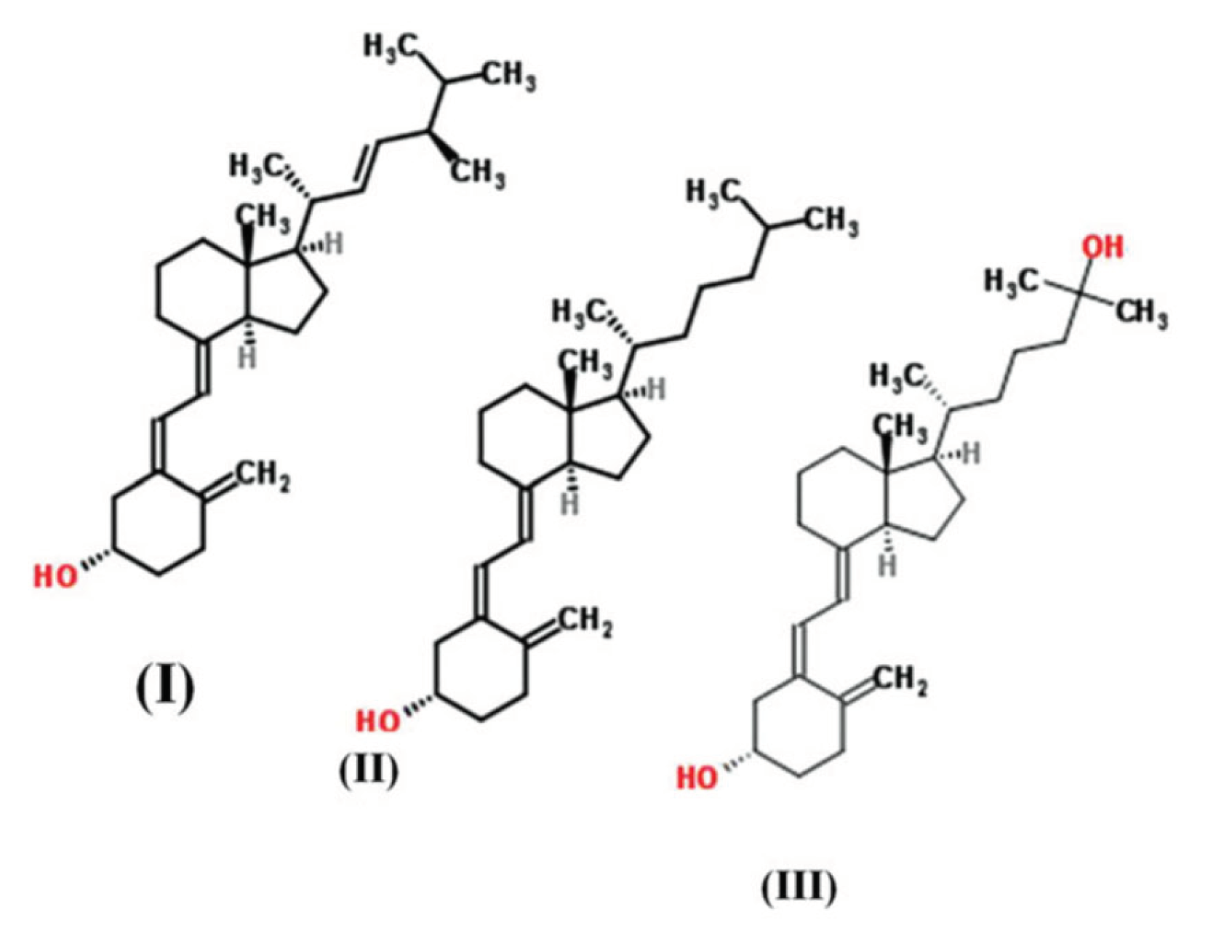
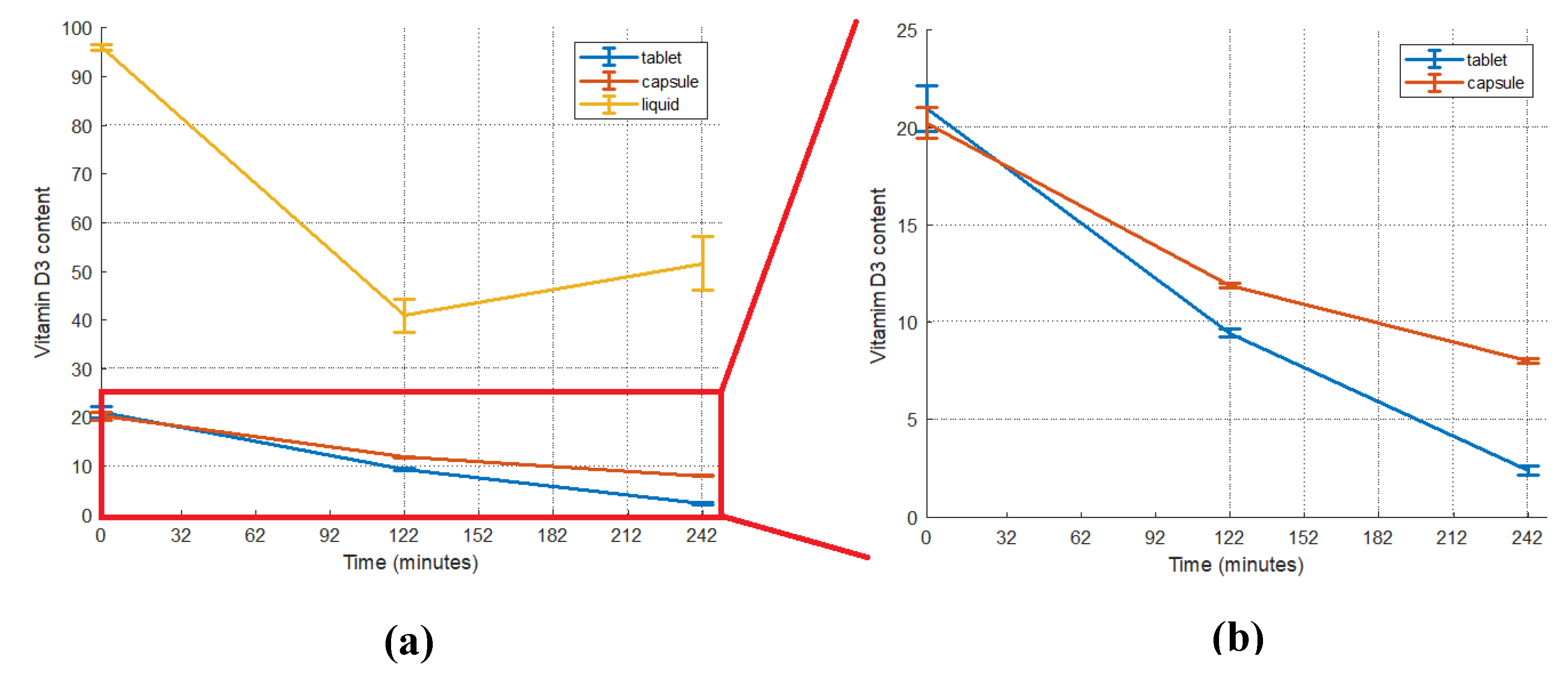
2.1. Vitamin D3 content of foods and supplements
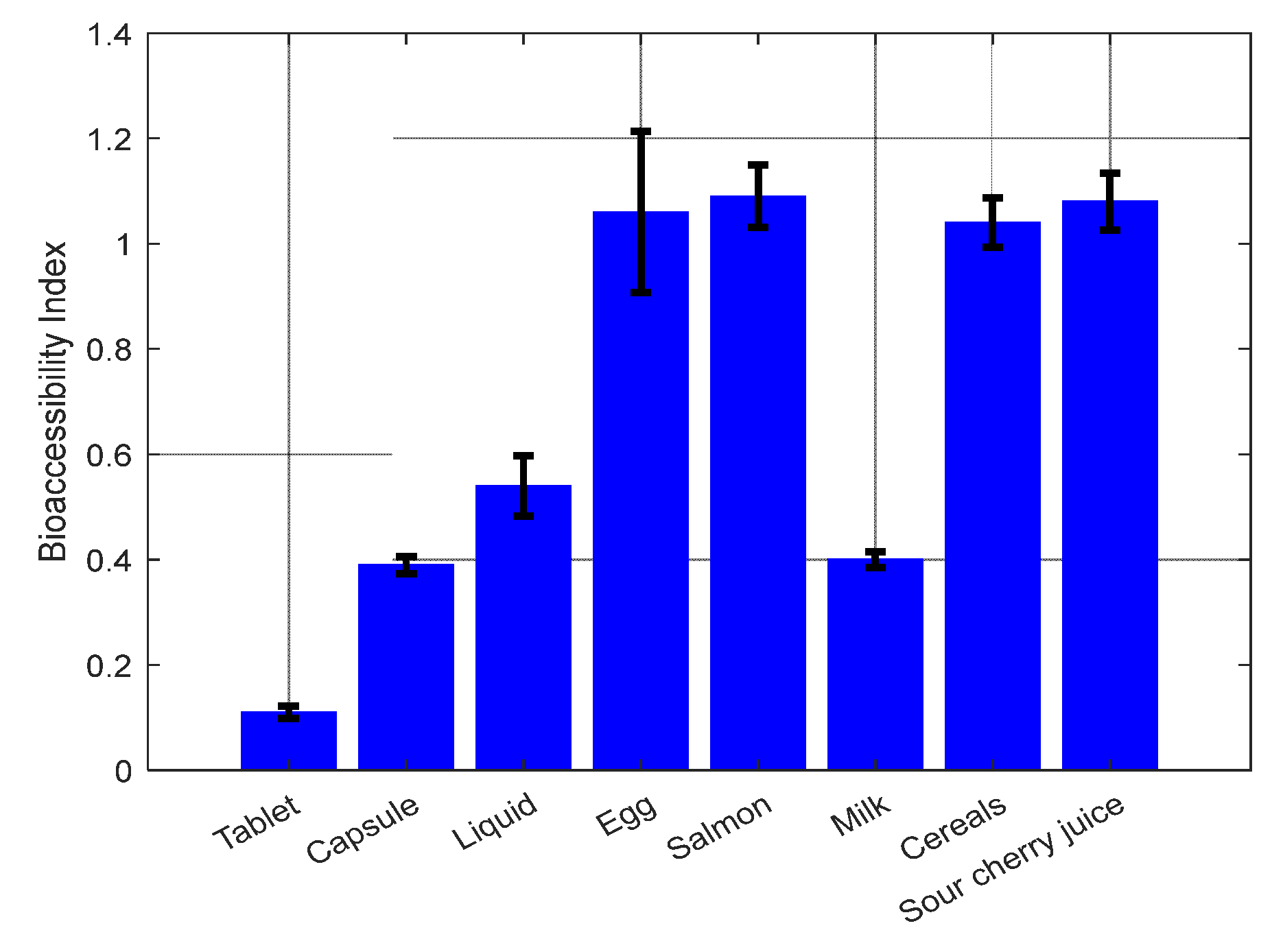
2.2. Vitamin D3 bioaccessibility
2.2.1. Vitamin D3 Bioaccessibility from Supplements
2.2.2. Vitamin D3 Bioaccessibility from Foods
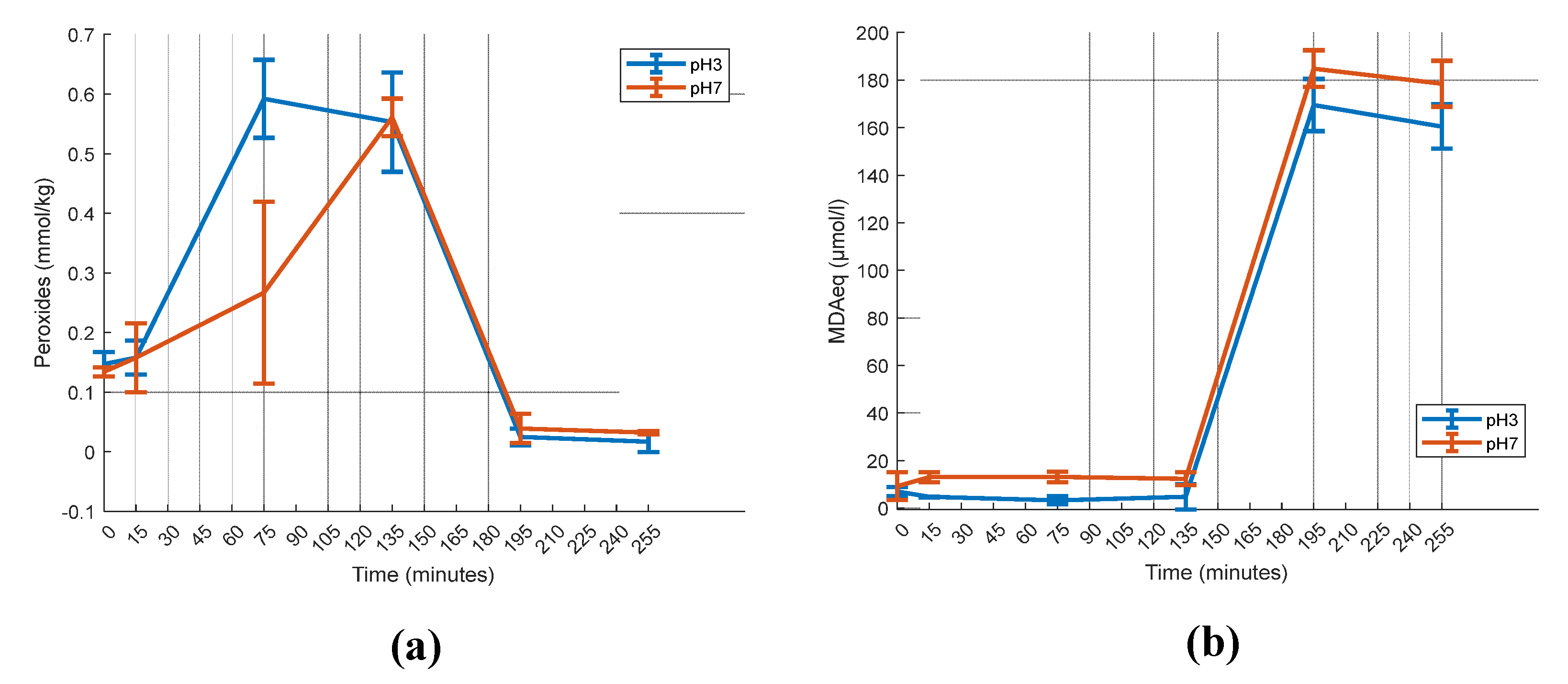
2.3. Gastric pH effect on vitamin D3 Bioaccessibility
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2 Digestion Procedure
4.2.1. Oral Phase
4.2.2. Gastric Phase
4.2.3. Intestinal Phase
4.3. Vitamin D3 Isolation
4.3.1. Samples with Saponification
4.3.2. Samples without Saponification
4.4. High Performance Liquid Chromatography (HPLC)
4.5. Bioaccessibility Index
4.6. Oxidation Measurement
4.6.1. Peroxide Value
4.6.2. Thiobarbituric Acid Method (TBARS)
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Combs, G. F.; McClung, J. P. Sources of the Vitamins. In The Vitamins; Elsevier, 2017; pp. 501–530. [Google Scholar]
- Janoušek, J.; Pilařová, V.; Macáková, K.; Nomura, A.; Veiga-Matos, J.; Silva, D. D. da; Remião, F.; Saso, L.; Malá-Ládová, K.; Malý, J.; Nováková, L.; Mladěnka, P. Vitamin D: Sources, Physiological Role, Biokinetics, Deficiency, Therapeutic Use, Toxicity, and Overview of Analytical Methods for Detection of Vitamin D and Its Metabolites. Crit Rev Clin Lab Sci 2022, 59, 517–554. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J. Cellular and Molecular Effects of Vitamin D on Carcinogenesis. Arch Biochem Biophys 2012, 523, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Khazai, N.; Judd, S. E.; Tangpricha, V. Calcium and Vitamin D: Skeletal and Extraskeletal Health. Curr Rheumatol Rep 2008, 10, 110. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Li, X.; Liu, W.; Xiao, Y.; Tang, H.; Wu, Y.; Xiong, Y.; Gao, S. The Role of Vitamin D in the Prevention and Treatment of SARS-CoV-2 Infection: A Meta-Analysis of Randomized Controlled Trials. Clinical Nutrition 2023, 42, 2198–2206. [Google Scholar] [CrossRef] [PubMed]
- Bendik, I.; Friedel, A.; Roos, F. F.; Weber, P.; Eggersdorfer, M. Vitamin D: A Critical and Essential Micronutrient for Human Health. In Frontiers in Physiology; Frontiers Research Foundation; p. 2014.
- Cui, A.; Zhang, T.; Xiao, P.; Fan, Z.; Wang, H.; Zhuang, Y. Global and Regional Prevalence of Vitamin D Deficiency in Population-Based Studies from 2000 to 2022: A Pooled Analysis of 7.9 Million Participants. Front Nutr 2023, 10, 1070808. [Google Scholar] [CrossRef] [PubMed]
- Borel, P.; Caillaud, D.; Cano, N. J. Vitamin D Bioavailability: State of the Art. Crit Rev Food Sci Nutr 2015, 55, 1193–1205. [Google Scholar] [CrossRef] [PubMed]
- Traub, M. L.; Finnell, J. S.; Bhandiwad, A.; Oberg, E.; Suhaila, L.; Bradley, R. Impact of Vitamin D3 Dietary Supplement Matrix on Clinical Response. J Clin Endocrinol Metab 2014, 99, 2720–2728. [Google Scholar] [CrossRef]
- Fox, C. B.; Kim, J.; Le, L. V.; Nemeth, C. L.; Chirra, H. D.; Desai, T. A. Micro/Nanofabricated Platforms for Oral Drug Delivery. Journal of Controlled Release 2015, 431–444. [Google Scholar] [CrossRef]
- Joye, I. J.; Davidov-Pardo, G.; McClements, D. J. Nanotechnology for Increased Micronutrient Bioavailability. Trends Food Sci Technol 2014, 40, 168–182. [Google Scholar] [CrossRef]
- Šimoliūnas, E.; Rinkūnaitė, I.; Bukelskienė, Ž.; Bukelskienė, V. Bioavailability of Different Vitamin D Oral Supplements in Laboratory Animal Model. Medicina (Lithuania) 2019, 55. [Google Scholar] [CrossRef]
- Grossmann, R. E.; Tangpricha, V. Evaluation of Vehicle Substances on Vitamin D Bioavailability: A Systematic Review. Molecular Nutrition and Food Research. 2010, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Helde Frankling, M.; Norlin, A. C.; Hansen, S.; Wahren Borgström, E.; Bergman, P.; Björkhem-Bergman, L. Are Vitamin D3 Tablets and Oil Drops Equally Effective in Raising S-25-Hydroxyvitamin D Concentrations? A Post-Hoc Analysis of an Observational Study on Immunodeficient Patients. Nutrients 2020, 12. [Google Scholar] [CrossRef]
- Maurya, V. K.; Aggarwal, M. Factors Influencing the Absorption of Vitamin D in GIT: An Overview. Journal of Food Science and Technology Springer India November 1. 2017, 54, 3753–3765. [Google Scholar] [CrossRef] [PubMed]
- Natri, A.-M.; Salo, P.; Vikstedt, T.; Palssa, A.; Huttunen, M.; Kärkkäinen, M. U. M.; Salovaara, H.; Piironen, V.; Jakobsen, J.; Lamberg-Allardt, C. J. Bread Fortified with Cholecalciferol Increases the Serum 25-Hydroxyvitamin D Concentration in Women as Effectively as a Cholecalciferol Supplement. J Nutr 2006, 136, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Biancuzzo, R. M.; Young, A.; Bibuld, D.; Cai, M. H.; Winter, M. R.; Klein, E. K.; Ameri, A.; Reitz, R.; Salameh, W.; Chen, T. C.; Holick, M. F. Fortification of Orange Juice with Vitamin D2 or Vitamin D 3 Is as Effective as an Oral Supplement in Maintaining Vitamin D Status in Adults. American Journal of Clinical Nutrition 2010, 91, 1621–1626. [Google Scholar] [CrossRef] [PubMed]
- Reboul, E.; Goncalves, A.; Comera, C.; Bott, R.; Nowicki, M.; Landrier, J. F.; Jourdheuil-Rahmani, D.; Dufour, C.; Collet, X.; Borel, P. Vitamin D Intestinal Absorption Is Not a Simple Passive Diffusion: Evidences for Involvement of Cholesterol Transporters. Mol Nutr Food Res 2011, 55, 691–702. [Google Scholar] [CrossRef]
- Hornbuckle, W. E.; Simpson, K. W.; Tennant, B. C. Gastrointestinal Function. Clinical Biochemistry of Domestic Animals 2008, 413–457. [Google Scholar]
- Reboul, E. Intestinal Absorption of Vitamin D: From the Meal to the Enterocyte. Food and Function. Royal Society of Chemistry 2015, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, B.; Argin, S.; Ozilgen, M.; McClements, D. J. Nanoemulsion Delivery Systems for Oil-Soluble Vitamins: Influence of Carrier Oil Type on Lipid Digestion and Vitamin D3 Bioaccessibility. Food Chem 2015, 187, 499–506. [Google Scholar] [CrossRef]
- Goncalves, A.; Gleize, B.; Roi, S.; Nowicki, M.; Dhaussy, A.; Huertas, A.; Amiot, M. J.; Reboul, E. Fatty Acids Affect Micellar Properties and Modulate Vitamin D Uptake and Basolateral Efflux in Caco-2 Cells. Journal of Nutritional Biochemistry 2013, 24, 1751–1757. [Google Scholar] [CrossRef]
- Temova Rakuša, Ž.; Pišlar, M.; Kristl, A.; Roškar, R. Comprehensive Stability Study of Vitamin D3 in Aqueous Solutions and Liquid Commercial Products. Pharmaceutics 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Yang, X.; Yang, L.; Liu, Z. L.; Zhang, F. Autoxidation of Isotachysterol. Tetrahedron 2004, 60, 2881–2888. [Google Scholar] [CrossRef]
- Esmaeili, M.; Yekta, R.; Abedi, A. S.; Ghanati, K.; Derav, R. Z.; Houshyarrad, A.; Dehkordi, Z. S.; Ajami, M.; Mahmoudzadeh, M. Encapsulating Vitamin D: A Feasible and Promising Approach to Combat Its Deficiency. Pharmaceutical Sciences Tabriz University of Medical Sciences April 1. 2022, 194–207. [Google Scholar] [CrossRef]
- Diarrassouba, F.; Remondetto, G.; Liang, L.; Garrait, G.; Beyssac, E.; Subirade, M. Effects of Gastrointestinal PH Conditions on the Stability of the β-Lactoglobulin/Vitamin D3 Complex and on the Solubility of Vitamin D3. Food Research International 2013, 52, 515–521. [Google Scholar] [CrossRef]
- Xiang, C.; Gao, J.; Ye, H.; Ren, G.; Ma, X.; Xie, H.; Fang, S.; Lei, Q.; Fang, W. Development of Ovalbumin-Pectin Nanocomplexes for Vitamin D3 Encapsulation: Enhanced Storage Stability and Sustained Release in Simulated Gastrointestinal Digestion. Food Hydrocoll 2020, 106, 105926. [Google Scholar] [CrossRef]
- Sharifi, F.; Jahangiri, M. Investigation of the Stability of Vitamin D in Emulsion-Based Delivery Systems. Chemical Industry & Chemical Engineering Quarterly 2017, 24, 157–167. [Google Scholar]
- Sams, L.; Paume, J.; Giallo, J.; Carrière, F. Relevant PH and Lipase for in Vitro Models of Gastric Digestion. Food Funct 2016, 7, 30–45. [Google Scholar] [CrossRef]
- Wang, X.; Ye, A.; Lin, Q.; Han, J.; Singh, H. Gastric Digestion of Milk Protein Ingredients: Study Using an in Vitro Dynamic Model. J Dairy Sci 2018, 101, 6842–6852. [Google Scholar] [CrossRef] [PubMed]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; Clemente, A.; Corredig, M.; Dupont, D.; Dufour, C.; Edwards, C.; Golding, M.; Karakaya, S.; Kirkhus, B.; Le Feunteun, S.; Lesmes, U.; Macierzanka, A.; Mackie, A. R.; Martins, C.; Marze, S.; McClements, D. J.; Ménard, O.; Minekus, M.; Portmann, R.; Santos, C. N.; Souchon, I.; Singh, R. P.; Vegarud, G. E.; Wickham, M. S. J.; Weitschies, W.; Recio, I. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat Protoc 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Food Code 2022 | FDA. Available online: https://www.fda.gov/food/fda-food-code/food-code-2022 (accessed 2023-07-25).
- Schmid, A.; Walther, B. Natural Vitamin D Content in Animal Products. Advances in Nutrition 2013, 4, 453–462. [Google Scholar] [CrossRef]
- Temova, Ž.; Roškar, R. Stability-Indicating HPLC–UV Method for Vitamin D3 Determination in Solutions, Nutritional Supplements and Pharmaceuticals. J Chromatogr Sci 2016, 54, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, E.; Cunningham, J.; Sherriff, J.; Lucas, R.; Greenfield, H.; Arcot, J.; Strobel, N.; Black, L. Vitamin D3 and 25-Hydroxyvitamin D3 Content of Retail White Fish and Eggs in Australia. Nutrients 2017, 9, 647. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, J.; Smith, C.; Bysted, A.; Cashman, K. D. Vitamin D in Wild and Farmed Atlantic Salmon (Salmo Salar)—What Do We Know? Nutrients 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Flores-Aldana, M.; Rivera-Pasquel, M.; García-Guerra, A.; Pérez-Cortés, J. G.; Bárcena-Echegollén, J. E. Effect of Vitamin D Supplementation on (25(OH)D) Status in Children 12–30 Months of Age: A Randomized Clinical Trial. Nutrients 2023, 15, 2756. [Google Scholar] [CrossRef] [PubMed]
- Villamor, E.; Oliveros, H.; Marín, C.; López-Arana, S.; Agudelo-Cañas, S. Increased Serum Total and Free 25-Hydroxyvitamin D with Daily Intake of Cholecalciferol-Fortified Skim Milk: A Randomized Controlled Trial in Colombian Adolescents. Journal of Nutrition 2023, 153, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
- Sollano-mendieta, X. C.; Meza-márquez, O. G.; Osorio-revilla, G.; Téllez-medina, D. I. Effect of In Vitro Digestion on the Antioxidant Compounds and Antioxidant Capacity of 12 Plum (Spondias Purpurea L.) Ecotypes. Foods 2021, 10. [Google Scholar] [CrossRef]
- Hemery, Y. M.; Fontan, L.; Moench-Pfanner, R.; Laillou, A.; Berger, J.; Renaud, C.; Avallone, S. Influence of Light Exposure and Oxidative Status on the Stability of Vitamins A and D3 during the Storage of Fortified Soybean Oil. Food Chem 2015, 184, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Floros, S.; Toskas, A.; Pasidi, E.; Vareltzis, P. Bioaccessibility and Oxidative Stability of Omega-3 Fatty Acids in Supplements, Sardines and Enriched Eggs Studied Using a Static In Vitro Gastrointestinal Model. Molecules 2022, Vol. 27, Page 415 2022, 27, 415. [Google Scholar] [CrossRef]
- Jakobsen, J.; Knuthsen, P. Stability of Vitamin D in Foodstuffs during Cooking. Food Chem 2014, 148, 170–175. [Google Scholar] [CrossRef]
- Szlinder-Richert, J.; Malesa-Ciećwierz, M. Effect of Household Cooking Methods on Nutritional Value of Cod and Salmon-Twin Fillet Approach. Carpathian Journal of Food Science and Technology 2018, 10, 142–157. [Google Scholar]
- Mahmoodani, F.; Perera, C. O.; Fedrizzi, B.; Abernethy, G.; Chen, H. Degradation Studies of Cholecalciferol (Vitamin D3) Using HPLC-DAD, UHPLC-MS/MS and Chemical Derivatization. Food Chem 2017, 219, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Lee, H. J.; Shin, C.; Chun, Y. S.; Kim, J.; Jung, H.; Choung, J.; Shim, S. M. Physicochemical Properties and Bioavailability of Naturally Formulated Fat-Soluble Vitamins Extracted from Agricultural Products for Complementary Use for Natural Vitamin Supplements. Food Sci Nutr 2020, 8, 5660–5672. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.; Gonçalves, R. F. S.; Pinheiro, A. C.; Manrique, Y. A.; Barreiro, M. F.; Lopes, J. C. B.; Dias, M. M. In Vitro Digestion and Bioaccessibility Studies of Vitamin E-Loaded Nanohydroxyapatite Pickering Emulsions and Derived Fortified Foods. LWT 2022, 154, 112706. [Google Scholar] [CrossRef]
- Mahmoodani, F.; Perera, C. O.; Abernethy, G.; Fedrizzi, B.; Chen, H. Lipid Oxidation and Vitamin D3 Degradation in Simulated Whole Milk Powder as Influenced by Processing and Storage. Food Chem 2018, 261, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodani, F.; Perera, C. O.; Abernethy, G.; Fedrizzi, B.; Greenwood, D.; Chen, H. Identification of Vitamin D3 Oxidation Products Using High-Resolution and Tandem Mass Spectrometry. J Am Soc Mass Spectrom 2018, 29, 1442–1455. [Google Scholar] [CrossRef] [PubMed]
- Bochkov, V. N.; Oskolkova, O. V.; Birukov, K. G.; Levonen, A. L.; Binder, C. J.; Stöckl, J. Generation and Biological Activities of Oxidized Phospholipids. Antioxid Redox Signal 2010, 12, 1009. [Google Scholar] [CrossRef] [PubMed]
- Vélez-Alavez, M.; Méndez-Rodriguez, L. C.; De Anda Montañez, J. A.; Mejía, C. H.; Galván-Magaña, F.; Zenteno-Savín, T. Vitamins C and E Concentrations in Muscle of Elasmobranch and Teleost Fishes. Comp Biochem Physiol A Mol Integr Physiol 2014, 170, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Sun, J.; Liu, Y.; Zeng, H.; Su, Y.; Yang, Y. ACE Inhibitory Peptides and Antioxidant Peptides Derived from in Vitro Digestion Hydrolysate of Hen Egg White Lysozyme. Food Chem 2012, 135, 1245–1252. [Google Scholar] [CrossRef]
- Young, D.; Nau, F.; Pasco, M.; Mine, Y. Identification of Hen Egg Yolk-Derived Phosvitin Phosphopeptides and Their Effects on Gene Expression Profiling against Oxidative Stress-Induced Caco-2 Cells. J Agric Food Chem 2011, 59, 9207–9218. [Google Scholar] [CrossRef]
- Remanan, M. K.; Wu, J. Antioxidant Activity in Cooked and Simulated Digested Eggs. Food Funct 2014, 5, 1464–1474. [Google Scholar] [CrossRef]
- Lipkie, T. E.; Ferruzzi, M. G.; Weaver, C. M. Low Bioaccessibility of Vitamin D2 from Yeast-Fortified Bread Compared to Crystalline D2 Bread and D3 from Fluid Milks. Food Funct 2016, 7, 4589–4596. [Google Scholar] [CrossRef]
- Hernández-Olivas, E.; Muñoz-Pina, S.; Sánchez-García, J.; Andrés, A.; Heredia, A. Understanding the Role of Food Matrix on the Digestibility of Dairy Products under Elderly Gastrointestinal Conditions. Food Research International 2020, 137, 109454. [Google Scholar] [CrossRef]
- Zhou, H.; Zheng, B.; Zhang, Z.; Zhang, R.; He, L.; McClements, D. J. Fortification of Plant-Based Milk with Calcium May Reduce Vitamin D Bioaccessibility: An in Vitro Digestion Study. J Agric Food Chem 2021, 69, 4223–4233. [Google Scholar] [CrossRef]
- Dima, C.; Dima, S. Bioaccessibility Study of Calcium and Vitamin D3 Co-Microencapsulated in Water-in-Oil-in-Water Double Emulsions. Food Chem 2020, 303, 125416. [Google Scholar] [CrossRef]
- Forrest, S. A.; Yada, R. Y.; Rousseau, D. Interactions of Vitamin D3 with Bovine β-Lactoglobulin A and β-Casein. J Agric Food Chem 2005, 53, 8003–8009. [Google Scholar] [CrossRef]
- Antoine, T.; Icard-Vernière, C.; Scorrano, G.; Salhi, A.; Halimi, C.; Georgé, S.; Carrière, F.; Mouquet-Rivier, C.; Reboul, E. Evaluation of Vitamin D Bioaccessibility and Mineral Solubility from Test Meals Containing Meat and/or Cereals and/or Pulses Using in Vitro Digestion. Food Chem 2021, 347. [Google Scholar] [CrossRef]
- Li, Y.; Ma, D.; Sun, D.; Wang, C.; Zhang, J.; Xie, Y.; Guo, T. Total Phenolic, Flavonoid Content, and Antioxidant Activity of Flour, Noodles, and Steamed Bread Made from Different Colored Wheat Grains by Three Milling Methods. Crop J 2015, 3, 328–334. [Google Scholar] [CrossRef]
- Siyuan, S.; Tong, L.; Liu, R. H. Corn Phytochemicals and Their Health Benefits. Food Science and Human Wellness 2018, 7, 185–195. [Google Scholar] [CrossRef]
- Aguillón-Osma, J.; Luzardo-Ocampo, I.; Cuellar-Nuñez, M. L.; Maldonado-Celis, M. E.; Loango-Chamorro, N.; Campos-Vega, R. Impact of in Vitro Gastrointestinal Digestion on the Bioaccessibility and Antioxidant Capacity of Bioactive Compounds from Passion Fruit (Passiflora Edulis) Leaves and Juice Extracts. J Food Biochem 2019, 43, e12879. [Google Scholar] [CrossRef]
- Goebel, S.; Avallone, S.; Detchewa, P.; Prasajak, P.; Sriwichai, W. Natural and Synthetic Antioxidants Prevent the Degradation of Vitamin D3fortification in Canola Oil during Baking and in Vitro Digestion. Applied Science and Engineering Progress 2021, 14. [Google Scholar] [CrossRef]
- Blanco, A.; Blanco, G. Digestion - Absorption. Med Biochem 2017, 251–273. [Google Scholar]
- Wang, W.; Cui, C.; Wang, Q.; Sun, C.; Jiang, L.; Hou, J. Effect of PH on Physicochemical Properties of Oil Bodies from Different Oil Crops. J Food Sci Technol 2019, 56, 49. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D. Nonclassic Actions of Vitamin D. J Clin Endocrinol Metab 2009, 94, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Yanhai, Z.; Yan, J.; Qun, X.; Rohrer, J. Simultaneous Determination of Vitamins A, E, and D3 in Milk-Based Nutritionals by On-Line Two-Dimensional HPLC.
- Zhu, Y.; Yang, S.; Huang, Y.; Huang, J.; Li, Y. Effect of in Vitro Gastrointestinal Digestion on Phenolic Compounds and Antioxidant Properties of Soluble and Insoluble Dietary Fibers Derived from Hulless Barley. J Food Sci 2021, 86, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Richards, M. P.; Hultin, H. O. Effect of PH on Lipid Oxidation Using Trout Hemolysate as a Catalyst: A Possible Role for Deoxyhemoglobin. J Agric Food Chem 2000, 48, 3141–3147. [Google Scholar] [CrossRef] [PubMed]
- Peroxide Value Method. 2023. Available online: https://www.protocols.io/view/Peroxide-Value-Method-4rm7vz12lx1w/v1 (accessed 2023-08-01).
- Christie, W. W.; Han, X. Isolation of Fatty Acids and Identification by Spectroscopic and Related Techniques. Lipid Analysis 2012, 181–211. [Google Scholar]
- Shantha, N. C.; Decker, E. A. Rapid, Sensitive, Iron-Based Spectrophotometric Methods for Determination of Peroxide Values of Food Lipids. J AOAC Int 1994, 77, 421–424. [Google Scholar] [CrossRef]
- Lemon, D.W. An improved TBA test for rancidity. New Series Circular No. 51, 1975. [Google Scholar]
| Food Sample | Vitamin D3 detected content (μg/g1) | Bioaccessibility Index (BI) | ||||
|---|---|---|---|---|---|---|
| Initial | Thermally processed | Stomach | Intestine | |||
| Natural | Egg | 0.06±0.004 b | 0.03±0.005 c | 0.08±0.007 a | 0.06±0.008 b | 1.06±0.153 |
| Salmon | 0.50±0.021 c | 0.38±0.020 d | 0.74±0.015 a | 0.55±0.019 b | 1.10±0.060 | |
| Fortified | Milk | 1.53±0.056 a | N/A | 0.62±0.007 b | 0.61±0.004 b | 0.40±0.015 |
| Cereals | 0.89±0.040 a, b | N/A | 0.84±0.005 b | 0.92±0.006 a | 1.04±0.046 | |
| Sour cherry juice | 1.15±0.005 c | N/A | 1.20±0.008 b | 1.24±0.003 a | 1.08±0.054 | |
| Gastric pH Value | Vitamin D3 Detected Content (μg/ml) | BI | ||
|---|---|---|---|---|
| Initial | Stomach | Intestine | ||
| 1 | 95.93±0.64 a | 39.87±8.97 b, B | 70.86±4.58 c, A | 0.74±0.05 |
| 3 | 40.95±2.69 b, B | 51.71±5.46 c, B | 0.54±0.06 | |
| 5 | 47.14±3.71b, A B | 51.62±2.08 b, B | 0.54±0.02 | |
| 7 | 53.65±6.55 b, A | 41.28±2.89 c, C | 0.43±0.03 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





