Submitted:
26 February 2024
Posted:
26 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals – Whey Cheese Extract
2.2. Silver Nanoparticle Synthesis
2.3. Characterisation Techniques
2.4. Statistical Analysis, Experimental Design, and Pareto Analysis
2.5. Total Protein Assay
3. Results
3.1. UV-Vis Spectra Analysis
3.2. AgNP Characterization
3.3. Bradford Assay
3.4. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
| 1 | B. O. D.: Biochemical Oxygen Demand |
| 2 | UV – Vis spectra without the addition of either of the reactants did not show any absorption feature in the measured range. |
References
- Kinsella JE, Whitehead DM. Proteins in Whey: Chemical, Physical, and Functional Properties, 1989, p. 343–438. [CrossRef]
- Elafros G. Novel process for cleaning waste from cheese industry 2014.
- Ha E, Zemel MB. Functional properties of whey, whey components, and essential amino acids: Mechanisms underlying health benefits for active people (Review). Journal of Nutritional Biochemistry 2003;14:251–8. [CrossRef]
- Chen H. Functional Properties and Applications of Edible Films Made of Milk Proteins. J Dairy Sci 1995;78:2563–83. [CrossRef]
- Gounga ME, Xu S-Y, Wang Z. Whey protein isolate-based edible films as affected by protein concentration, glycerol ratio and pullulan addition in film formation. J Food Eng 2007;83:521–30. [CrossRef]
- Gunasekaran S, Ko S, Xiao L. Use of whey proteins for encapsulation and controlled delivery applications. J Food Eng 2007;83:31–40. [CrossRef]
- Asefian S, Ghavam M. Green and environmentally friendly synthesis of silver nanoparticles with antibacterial properties from some medicinal plants. BMC Biotechnol 2024;24. [CrossRef]
- Youssef AM, El-Sayed SamahM. Bionanocomposites materials for food packaging applications: Concepts and future outlook. Carbohydr Polym 2018;193:19–27. [CrossRef]
- Dipankar C, Murugan S. The green synthesis, characterization and evaluation of the biological activities of silver nanoparticles synthesized from Iresine herbstii leaf aqueous extracts. Colloids Surf B Biointerfaces 2012;98:112–9. [CrossRef]
- Shankar S, Rhim JW. Amino acid mediated synthesis of silver nanoparticles and preparation of antimicrobial agar/silver nanoparticles composite films. Carbohydr Polym 2015;130:353–63. [CrossRef]
- Srisod S, Motina K, Inprasit T, Pisitsak P. A green and facile approach to durable antimicrobial coating of cotton with silver nanoparticles, whey protein, and natural tannin. Prog Org Coat 2018;120:123–31. [CrossRef]
- Ghodake G, Shinde S, Saratale RG, Kadam A, Saratale GD, Patel R, et al. Whey peptide-encapsulated silver nanoparticles as a colorimetric and spectrophotometric probe for palladium(II). Microchimica Acta 2019;186:763. [CrossRef]
- Zeng A, Wang B, Zhang C, Yang R, Yu S, Zhao W. Physicochemical properties and antibacterial application of silver nanoparticles stabilized by whey protein isolate. Food Biosci 2022;46. [CrossRef]
- Viorica RP, Pawel P, Boguslaw B. Use of Lactobacillus paracasei isolated from whey for silver nanocomposite synthesis: Antiradical and antimicrobial properties against selected pathogens. J Dairy Sci 2021;104:2480–98. [CrossRef]
- Lai YR, Lai JT, Wang SSS, Kuo YC, Lin TH. Silver nanoparticle-deposited whey protein isolate amyloid fibrils as catalysts for the reduction of methylene blue. Int J Biol Macromol 2022;213:1098–114. [CrossRef]
- Rigopoulos N, Thomou E, Kouloumpis A, Lamprou ER, Petropoulea V, Gournis D, et al. Optimization of Silver Nanoparticle Synthesis by Banana Peel Extract Using Statistical Experimental Design, and Testing of their Antibacterial and Antioxidant Properties. Curr Pharm Biotechnol 2019;20:858–73. [CrossRef]
- Rigopoulos N, Gkaliouri CM, Sakavitsi V, Gournis D. Full Factorial Design Synthesis of Silver Nanoparticles Using Origanum vulgare 2023. [CrossRef]
- El-Naggar NEA, Abdelwahed NAM. Application of statistical experimental design for optimization of silver nanoparticles biosynthesis by a nanofactory Streptomyces viridochromogenes. Journal of Microbiology 2014;52:53–63. [CrossRef]
- Biswas S, Mulaba-Bafubiandi AF. Optimization of process variables for the biosynthesis of silver nanoparticles by Aspergillus wentii using statistical experimental design. Advances in Natural Sciences: Nanoscience and Nanotechnology 2016;7:045005. [CrossRef]
- Montgomery DC. Design and Analysis of Experiments Eighth Edition. 1984.
- Asadzadeh F, Maleki-Kaklar M, Soiltanalinejad N, Shabani F. Central composite design optimization of zinc removal from contaminated soil, using citric acid as biodegradable chelant. Sci Rep 2018;8. [CrossRef]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–54. [CrossRef]
- Grintzalis K, Georgiou CD, Schneider Y-J. An accurate and sensitive Coomassie Brilliant Blue G-250-based assay for protein determination. Anal Biochem 2015;480:28–30. [CrossRef]
- Dada AO, Adekola FA, Adeyemi OS, Bello OM, Oluwaseun AC, Awakan OJ, et al. Exploring the Effect of Operational Factors and Characterization Imperative to the Synthesis of Silver Nanoparticles. Silver Nanoparticles - Fabrication, Characterization and Applications, InTech; 2018. [CrossRef]
- Quinten M. Optical Properties of Nanoparticle Systems. Wiley - Vch Verlag GmbH & Co. KGaA; 2011. [CrossRef]
- Khan MAM, Kumar S, Ahamed M, Alrokayan SA, AlSalhi MS. Structural and thermal studies of silver nanoparticles and electrical transport study of their thin films. Nanoscale Res Lett 2011;6:1–8. [CrossRef]
- Molleman B, Hiemstra T. Surface Structure of Silver Nanoparticles as a Model for Understanding the Oxidative Dissolution of Silver Ions. Langmuir 2015;31:13361–72. [CrossRef]
- Valenti LE, Giacomelli CE. Stability of silver nanoparticles: agglomeration and oxidation in biological relevant conditions. Journal of Nanoparticle Research 2017;19. [CrossRef]
- Manikandan V, Velmurugan P, Park J-H, Chang W-S, Park Y-J, Jayanthi P, et al. Green synthesis of silver oxide nanoparticles and its antibacterial activity against dental pathogens. 3 Biotech 2017;7:72. [CrossRef]
- Gallardo OAD, Moiraghi R, Macchione MA, Godoy JA, Pérez MA, Coronado EA, et al. Silver oxide particles/silver nanoparticles interconversion: susceptibility of forward/backward reactions to the chemical environment at room temperature. RSC Adv 2012;2:2923. [CrossRef]
- Ahmed S, Ahmad M, Swami BL, Ikram S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J Adv Res 2016;7:17–28. [CrossRef]
- Nelson DL, Cox MM. Lehninger Principles of Biochemistry 7th. vol. 2. Macmillan International Higher Education; 2017.
- Deshmukh AR, Gupta A, Kim BS. Ultrasound Assisted Green Synthesis of Silver and Iron Oxide Nanoparticles Using Fenugreek Seed Extract and Their Enhanced Antibacterial and Antioxidant Activities. Biomed Res Int 2019;2019:1–14. [CrossRef]
- Trivedi MK, Dahryn Trivedi AB, Khemraj Bairwa HS. Fourier Transform Infrared and Ultraviolet-Visible Spectroscopic Characterization of Biofield Treated Salicylic Acid and Sparfloxacin. Nat Prod Chem Res 2015;03. [CrossRef]
- Alavi M, Karimi N. Characterization, antibacterial, total antioxidant, scavenging, reducing power and ion chelating activities of green synthesized silver, copper and titanium dioxide nanoparticles using Artemisia haussknechtii leaf extract. Artif Cells Nanomed Biotechnol 2018;46:2066–81. [CrossRef]
- Ruíz-Baltazar Á de J, Reyes-López SY, Mondragón-Sánchez M de L, Estevez M, Hernández-Martinez AR, Pérez R. Biosynthesis of Ag nanoparticles using Cynara cardunculus leaf extract: Evaluation of their antibacterial and electrochemical activity. Results Phys 2018;11:1142–9. [CrossRef]
- Gharibshahi L, Saion E, Gharibshahi E, Shaari A, Matori K. Structural and Optical Properties of Ag Nanoparticles Synthesized by Thermal Treatment Method. Materials 2017;10:402. [CrossRef]
- Gbassi G, Yolou F, Sarr S, Atheba P, Amin C, Ake M. Whey proteins analysis in aqueous medium and in artificial gastric and intestinal fluids. Int J Biol Chem Sci 2012;6. [CrossRef]
- Kora AJ, Arunachalam J. Green Fabrication of Silver Nanoparticles by Gum Tragacanth ( Astragalus gummifer ): A Dual Functional Reductant and Stabilizer. J Nanomater 2012;2012:1–8. [CrossRef]
- Kaushik R, Saran S, Isar J, Saxena RK. Statistical optimization of medium components and growth conditions by response surface methodology to enhance lipase production by Aspergillus carneus. J Mol Catal B Enzym 2006;40:121–6. [CrossRef]
- Boumba VA, Voutsinas LP, Philippopoulos CD. Composition and nutritional value of commercial dried whey products from feta cheese manufacture. Int J Dairy Technol 2001;54:141–5. [CrossRef]
- Mohebrad B, Rezaee A, Sohrabi B. Effect of isoelectric point on cheese whey wastewater treatment using a microbial electrochemical system. Bioelectrochemistry 2019;130. [CrossRef]
- Tran QH, Nguyen VQ, Le AT. Corrigendum: Silver nanoparticles: Synthesis, properties, toxicology, applications and perspectives (Advances in Natural Sciences: Nanoscience and Nanotechnology 4 (033001) DOI: 10.1088/2043-6262/4/3/033001). Advances in Natural Sciences: Nanoscience and Nanotechnology 2018;9. [CrossRef]
- Trieu QA, Le CTB, Pham CM, Bui TH. Photocatalytic degradation of methylene blue and antibacterial activity of silver nanoparticles synthesized from Camellia sinensis leaf extract. J Exp Nanosci 2023;18. [CrossRef]
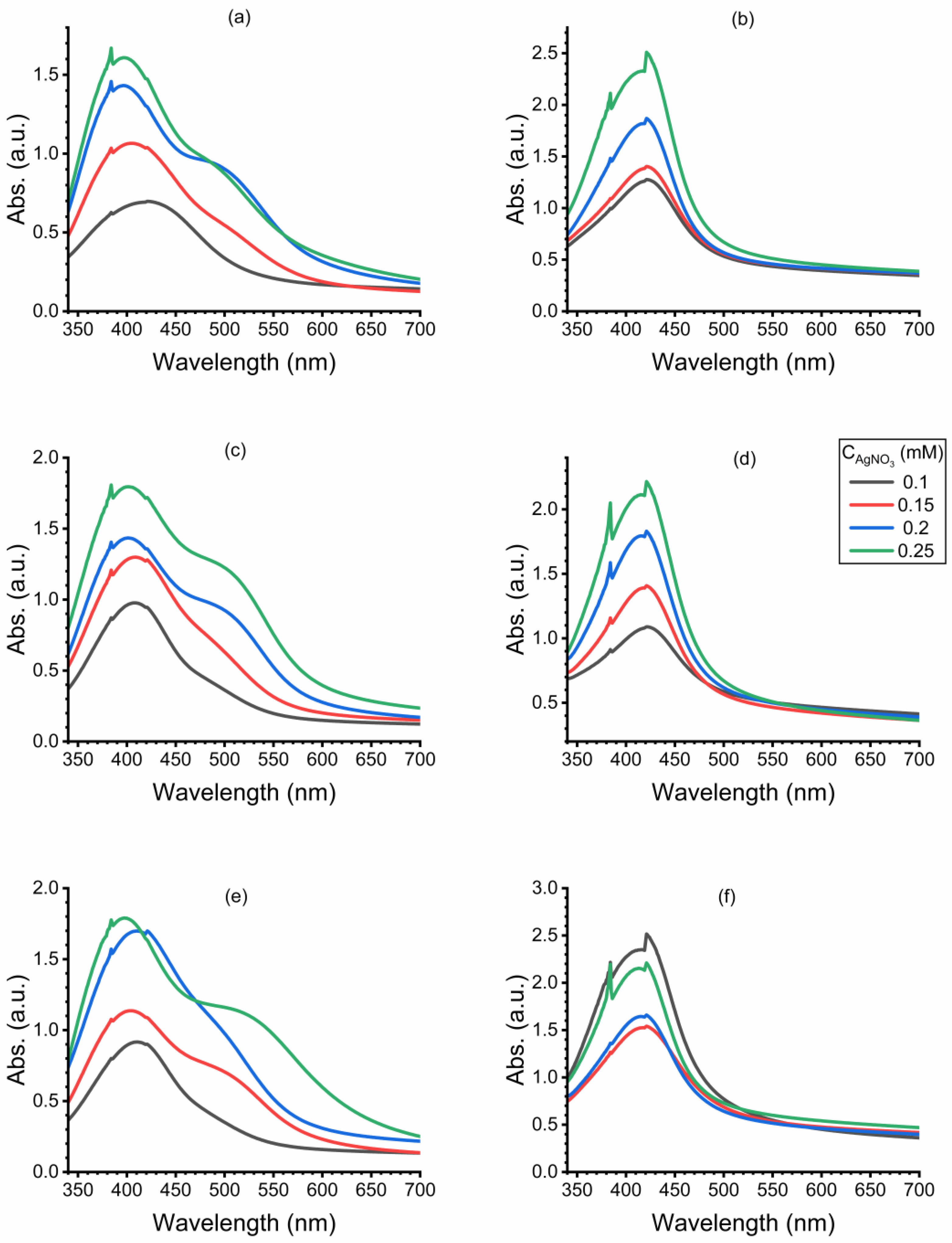
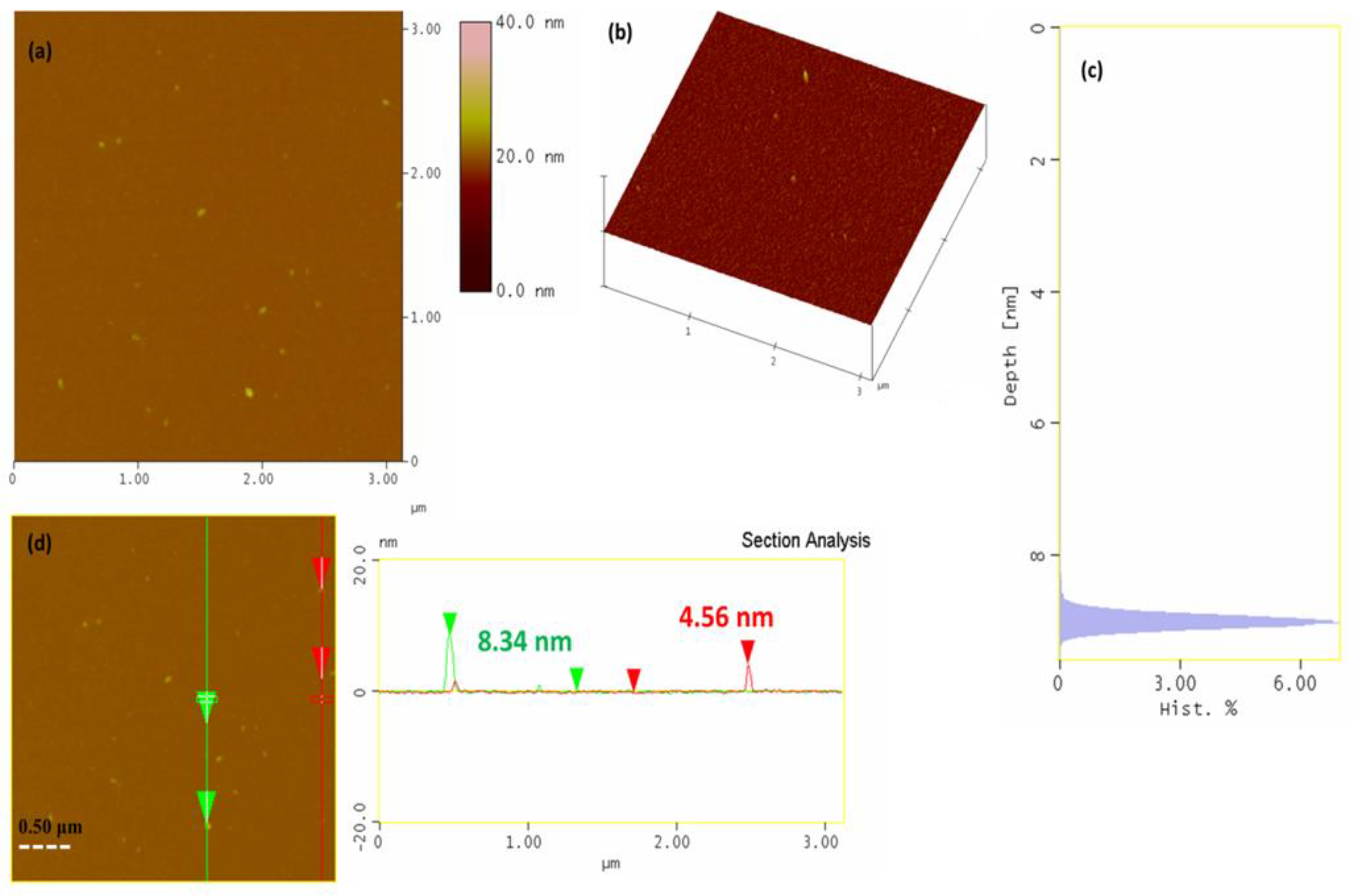
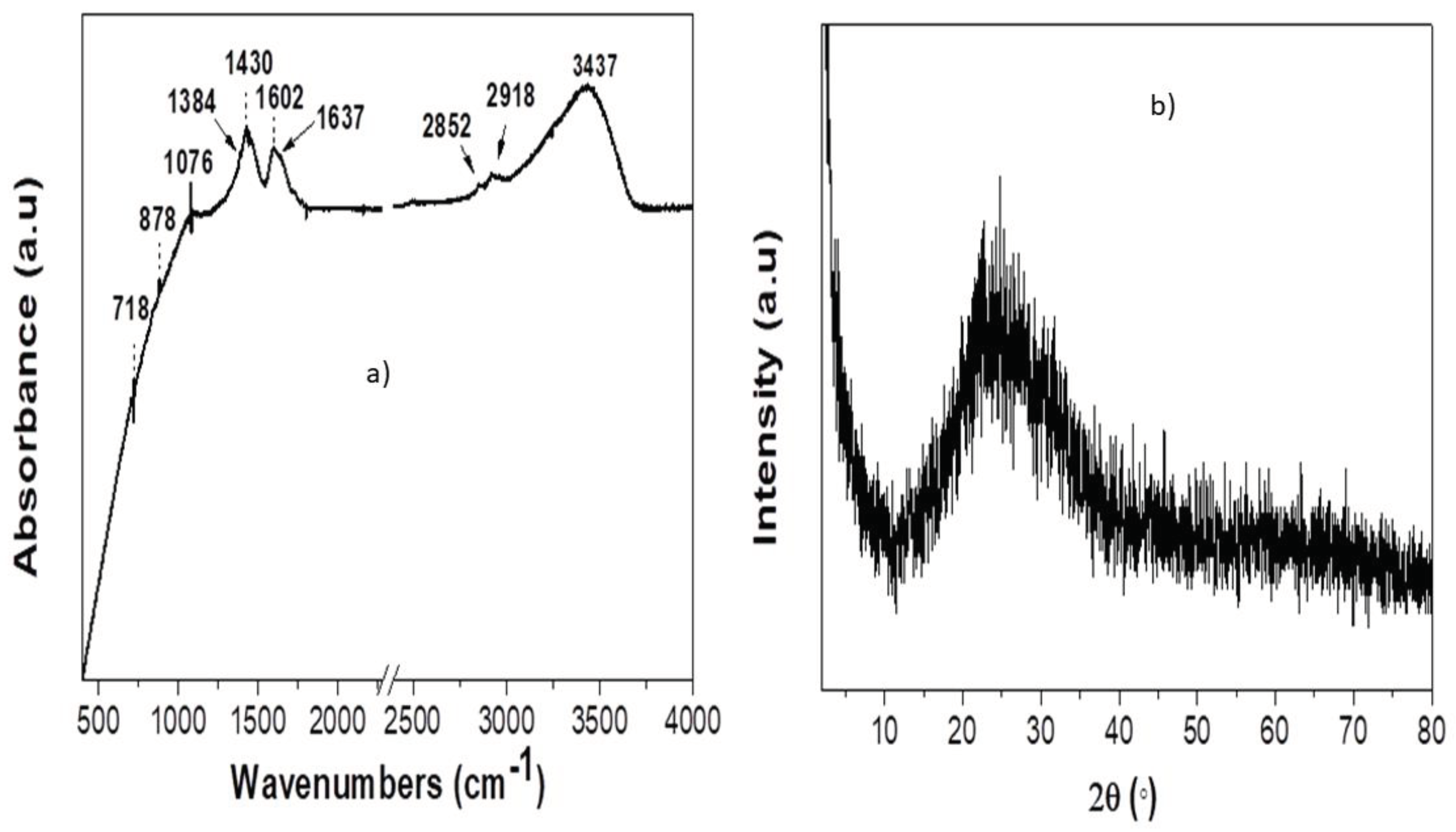
| Run | X1 (Cnitric mM) |
X2 (CWhey % v/v) |
X3 (CNaOH mM) | X4 (Time min) |
Responses | ||
| λ0 (nm) | A (a.u.a) |
FWHM (nm) | |||||
| 1 | 0 (0.3) | 0 (0.8) | -2(10) | 0 (40) | 411 | 250 | 101 |
| 2 | -1 (0.15) | -1 (0.4) | -1 (20) | 1 (50) | 413 | 191 | 106 |
| 3 | -1 (0.15) | 1 (1.2) | -1 (20) | 1 (50) | 415 | 202 | 97 |
| 4 | -1 (0.15) | -1 (0.4) | -1 (20 | -1 (30) | 410 | 190 | 104 |
| 5 | -1 (0.15) | 1 (1.2) | -1 (20) | -1 (30) | 423 | 171 | 111 |
| 6 | 1 (0.45) | -1 (0.4) | -1 (20) | 1 (50) | 403 | 421 | 126 |
| 7 | 1 (0.45) | 1 (1.2) | -1 (20) | -1 (30) | 405 | 282 | 132 |
| 8 | 1 (0.45) | -1 (0.4) | -1 (20) | -1 (30) | 398 | 490 | 139 |
| 9 | 1 (0.45) | 1 (1.2) | -1 (20) | 1 (50) | 399 | 346 | 112 |
| 10 | -2 (0) | 0 (0.8) | 0 (30) | 0 (40) | n/ab | n/ab | n/ab |
| 11 | 0 (0.3) | -2 (0) | 0 (30) | 0 (40) | 487 | 62 | 335 |
| 12 | 0 (0.3) | 0 (0.8) | 0 (30) | 0 (40) | 407 | 274 | 105 |
| 13 | 0 (0.3) | 0 (0.8) | 0 (30) | -2 (20) | 410 | 322 | 105 |
| 14 | 0 (0.3) | 0 (0.8) | 0 (30) | 2 (60) | 409 | 312 | 131 |
| 15 | 0 (0.3) | 2 (1.6) | 0 (30) | 0 (40) | 402 | 311 | 95 |
| 16 | 2 (0.6) | 0 (0.8) | 0 (30) | 0 (40) | 406 | 724 | 170 |
| 17 | -1 (0.15) | 1 (1.2) | 1 (40) | -1 (30) | 422 | 221 | 107 |
| 18 | -1 (0.15) | -1 (0.4) | 1 (40) | 1 (50) | 412 | 175 | 111 |
| 19 | -1 (0.15) | 1 (1.2) | 1 (40) | 1 (50) | 418 | 229 | 108 |
| 20 | -1 (0.15) | -1 (0.4) | 1 (40) | -1 (30) | 410 | 150 | 107 |
| 21 | 1 (0.45) | -1 (0.4) | 1 (40) | -1 (30) | 401 | 284 | 128 |
| 22 | 1 (0.45) | -1 (0.4) | 1 (40) | 1 (50) | 401 | 215 | 109 |
| 23 | 1 (0.45) | 1 (1.2) | 1 (40) | 1 (50) | 401 | 315 | 124 |
| 24 | 1 (0.45) | 1 (1.2) | 1 (40) | -1 (30) | 400 | 382 | 117 |
| 25 | 0 (0.3) | 0 (0.8) | 2 (50) | 0 (40) | 405 | 306 | 110 |
| A595nm (a.u.) | BSA-eq (μg/ml) | BSA per μg of Whey (μg/μL whey) |
| 0.647 | 16.93 | 4.84 |
| 0.640 | 16.69 | 4.77 |
| 0.636 | 16.55 | 4.73 |
| Variables | Coefficient | Coefficient SE | t- test | p-value |
| Intercept | 408.69 | 0.862 | 474.12 | 0.000 |
| Cnitric (X1) | 17.40 | 1.36 | 12.77 | 0.000 |
| CWhey (X2) | 10.067 | 0.528 | 19.07 | 0.000 |
| CNaOH (X3) | 0.393 | 0.528 | 0.74 | 0.478 |
| Time (X4) | -0.373 | 0.305 | -1.22 | 0.256 |
| X1X1 | -9.373 | 0.681 | -13.75 | 0.000 |
| X2X2 | 8.908 | 0.341 | 26.14 | 0.000 |
| X3X3 | -0.011 | 0.341 | -0.03 | 0.974 |
| X1X2 | -1.798 | 0.373 | -4.82 | 0.001 |
| X2X3 | 0.015 | 0.373 | 0.04 | 0.968 |
| X2X4 | -1.689 | 0.373 | -4.53 | 0.002 |
| X3X4 | 0.297 | 0.373 | 0.80 | 0.449 |
| X2X2X2 | -7.869 | 0.215 | -36.52 | 0.000 |
| X3X3X3 | -0.497 | 0.215 | -2.31 | 0.050 |
| X1X2X2 | -24.55 | 1.41 | -17.37 | 0.000 |
| X2X3X4 | 0.990 | 0.373 | 2.65 | 0.029 |
| Variables | Coefficient | Coefficient SE | t- test | p-value |
| Intercept | 302.8 | 18.5 | 16.38 | 0.000 |
| Cnitric (X1) | 29.8 | 14.5 | 2.06 | 0.073 |
| CWhey (X2) | -18.1 | 11.3 | -1.60 | 0.148 |
| CNaOH (X3) | -31.3 | 11.3 | -2.77 | 0.024 |
| Time (X4) | -3.97 | 6.54 | -0.61 | 0.560 |
| X1X1 | -1.1 | 14.6 | -0.07 | 0.943 |
| X2X2 | -29.10 | 7.31 | -3.98 | 0.004 |
| X3X3 | -6.16 | 7.31 | -0.84 | 0.424 |
| X1X2 | -12.72 | 8.00 | -1.59 | 0.151 |
| X1X3 | -22.78 | 8.00 | -2.85 | 0.022 |
| X1X4 | -12.84 | 8.00 | -1.60 | 0.147 |
| X2X3 | 38.41 | 8.00 | 4.80 | 0.001 |
| X1X1X1 | 45.8 | 10.1 | 4.53 | 0.002 |
| X2X2X2 | 20.12 | 4.62 | 4.35 | 0.002 |
| X3X3X3 | 11.32 | 4.62 | 2.54 | 0.040 |
| X1X2X3 | 21.80 | 8.00 | 2.72 | 0.026 |
| Variables | Coefficient | Coefficient SE | t- test | p-value |
| Intercept | 105.63 | 2.84 | 37.22 | 0.000 |
| Cnitric (X1) | -13.18 | 2.22 | -5.94 | 0.002 |
| CWhey (X2) | 18.31 | 1.74 | 10.54 | 0.000 |
| CNaOH (X3) | 0.18 | 1.00 | 0.18 | 0.867 |
| Time (X4) | -6.28 | 1.74 | -3.61 | 0.015 |
| X1X1 | -20.91 | 2.24 | -9.32 | 0.000 |
| X2X2 | 27.29 | 1.12 | 24.33 | 0.000 |
| X4X4 | 2.98 | 1.12 | 2.66 | 0.045 |
| X1X2 | -0.71 | 1.23 | -0.58 | 0.586 |
| X1X3 | -2.98 | 1.23 | -2.42 | 0.060 |
| X1X4 | -2.32 | 1.23 | -1.89 | 0.117 |
| X2X3 | 1.57 | 1.23 | 1.28 | 0.257 |
| X2X4 | -0.09 | 1.23 | -0.07 | 0.944 |
| X3X4 | 2.38 | 1.23 | 1.94 | 0.111 |
| X1X1X1 | 21.79 | 1.55 | 14.05 | 0.000 |
| X2X2X2 | -19.587 | 0.709 | -27.61 | 0.000 |
| X4X4X4 | 3.197 | 0.709 | 4.51 | 0.006 |
| X1X2X4 | 2.37 | 1.23 | 1.93 | 0.111 |
| X2X3X4 | 2.77 | 1.23 | 2.25 | 0.074 |
| Peak wavelength λ0 | Peak Area A | FWHM | |||
| Term | Per. Efa (%) | Term | Per. Ef (%) | Term | Per. Ef (%) |
| 24.35 | 14.24 | 6.76 | |||
| 8.15 | 8.73 | 13.05 | |||
| 7.07 | 24.81 | 17.02 | |||
| 6.38 | 35.27 | 29.00 | |||
| 0.26 | 6.81 | 0.35 | |||
| 0.23 | 2.15 | 18.49 | |||
| 4.98 | 7.99 | 14.94 | |||
| 0.02 | 0.4 | ||||
| 48.48 | |||||
| 0.08 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





