Submitted:
25 February 2024
Posted:
26 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
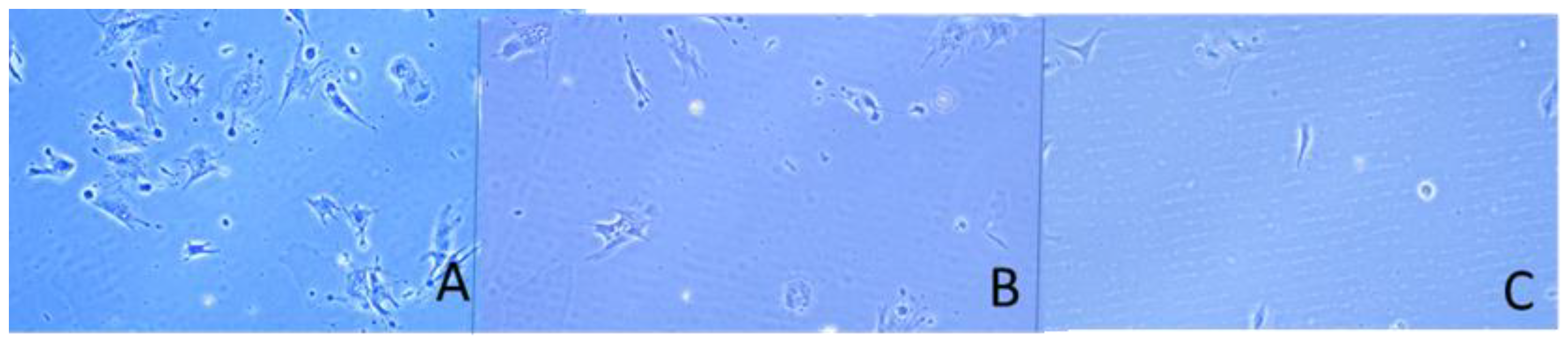
2.1. Morphological observations
2.2. Enzymatic remodelling of glycated collagen by the adhering cells
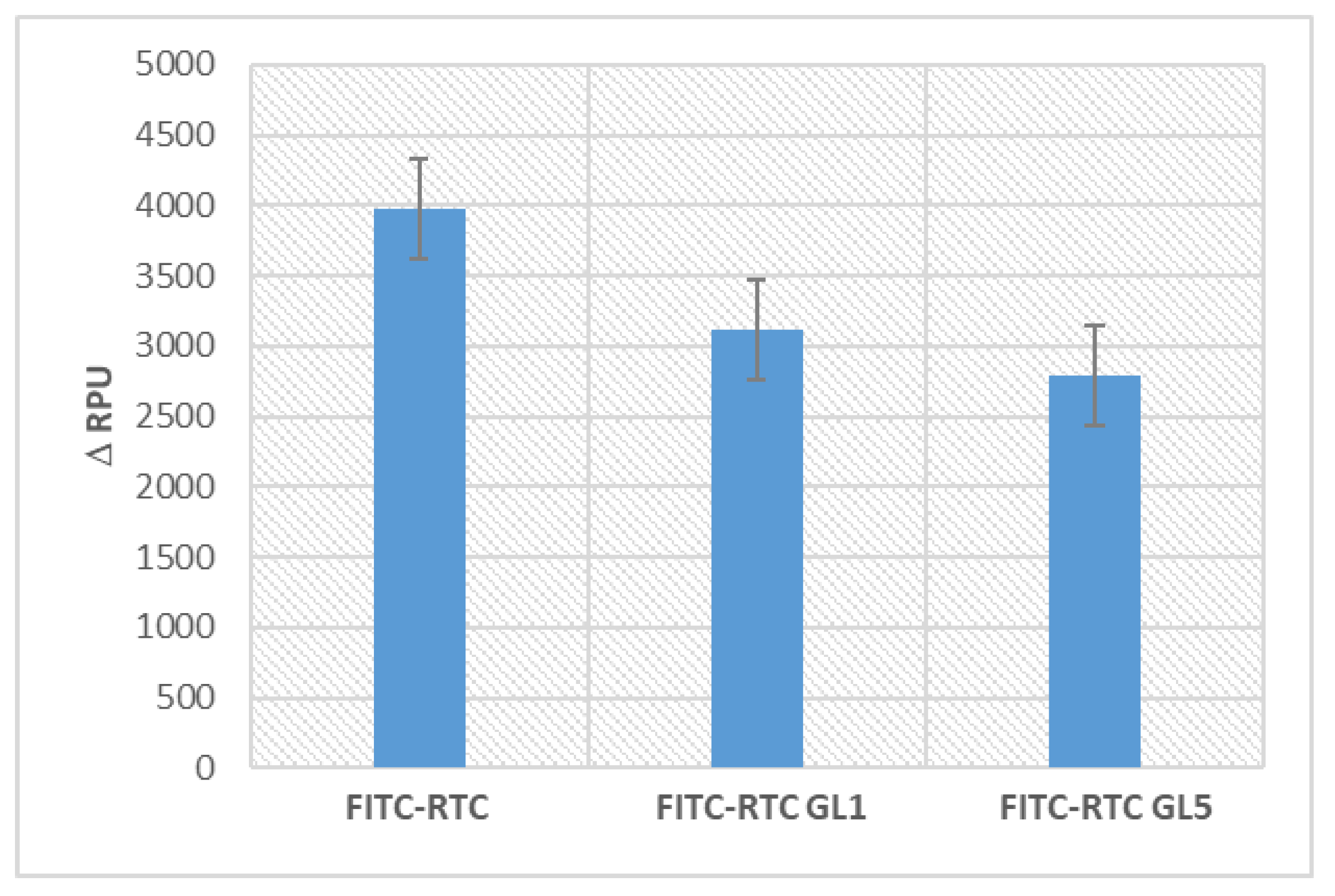
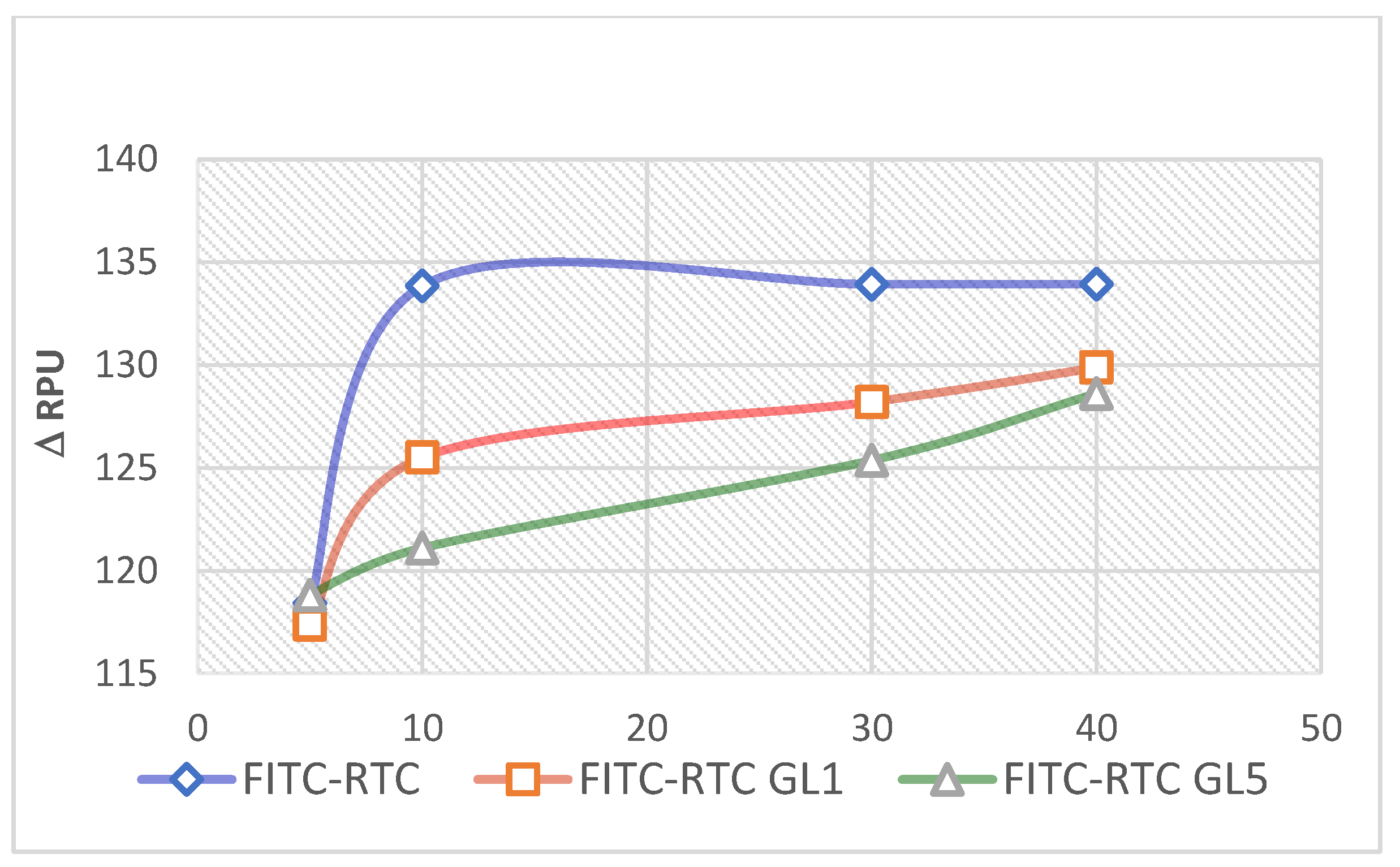
2.3. Enzymatic degradation of glycated FITC Collagen in cells-free system
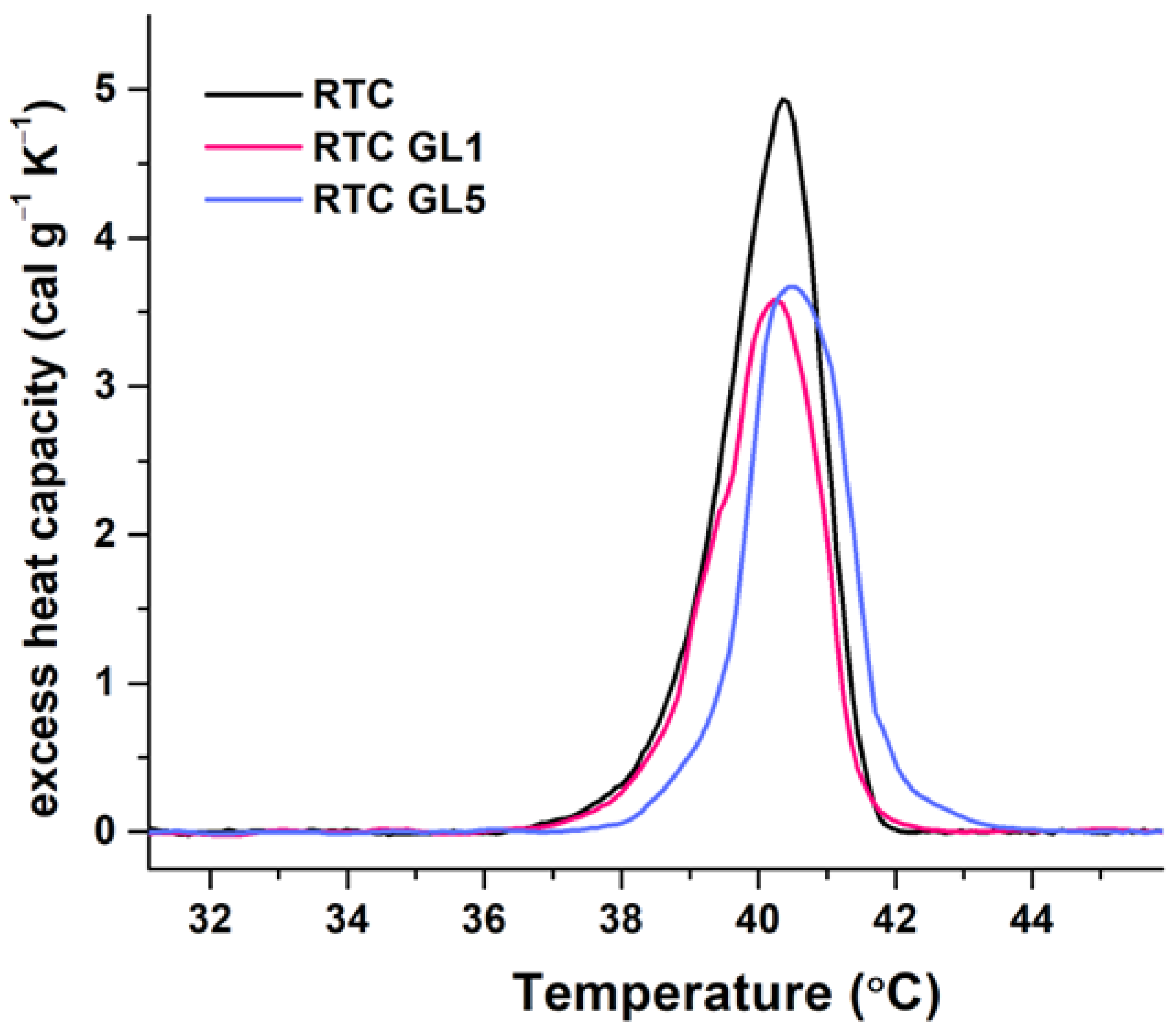
2.4. Differential Scanning Calorimetry (DSC) Measurements
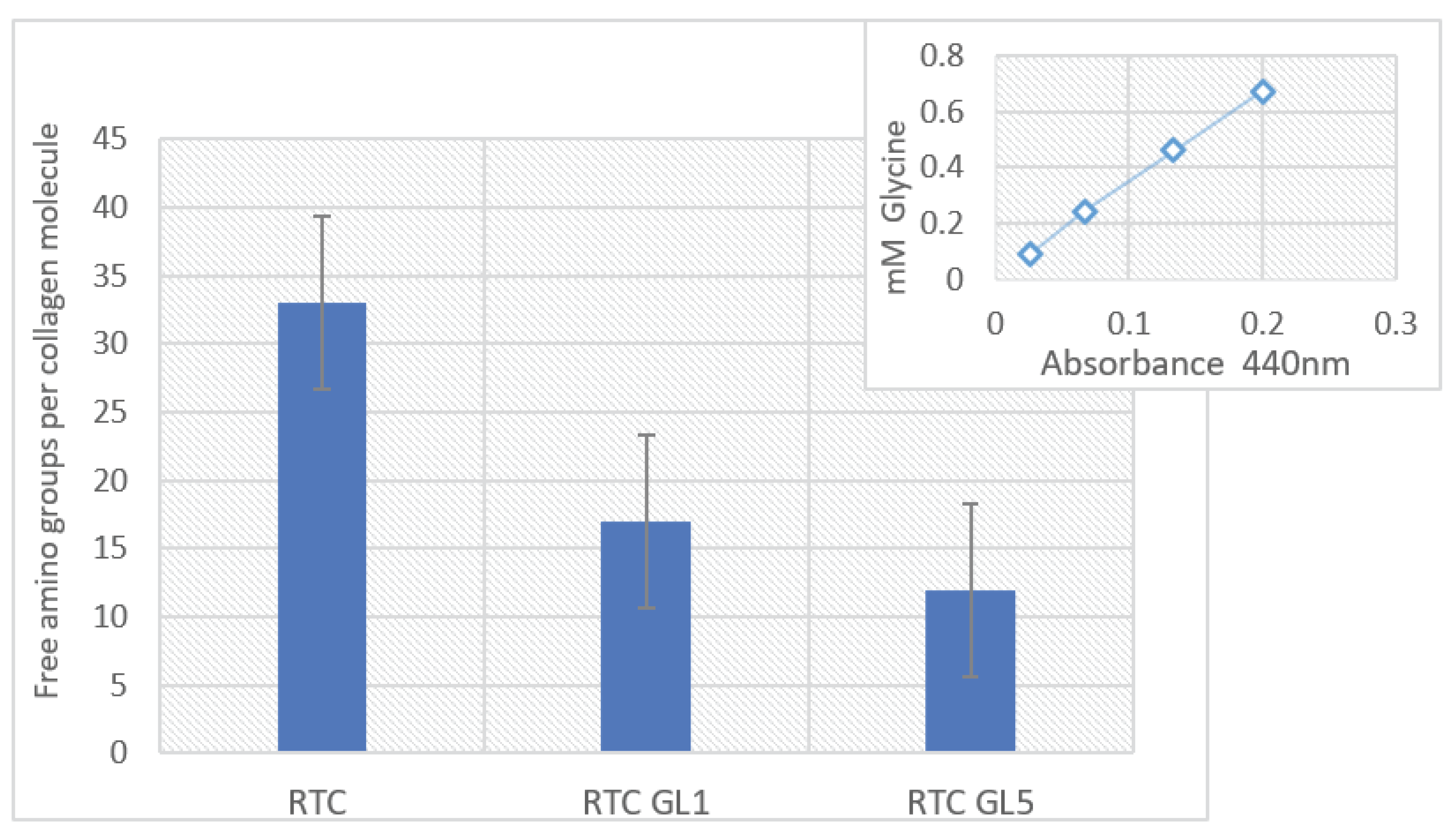
2.5. Measurements on the extent of glycation
3. Discussion
4. Materials and Methods
4.1. Collagen procedures
4.1.1. Collagen Preparation
4.1.2. Fluorescent Labelling of Collagen
4.1.3. Preparation of glycated collagen
4.2. TNBS method for quantifying free amino groups
4.3. Cells
4.3.1. Morphological Studies
4.3.2. Overall cells morphology and focal adhesion formation
4.3.4. Quantitative Morphometry Analysis of Raw Format Images by ImageJ
4.3.5. Measurement of Collagen Degradation by ADMSC
4.4. FITC-Collagen Degradation in Cell-Free System
4.5. DSC Measurements
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bächinger, H. P.; Mizuno, K.; Vranka; J. A., & Boudko; S. P. (2010). Collagen formation and structure. In Comprehensive Natural Products II: Chemistry and Biology (Vol. 5, pp. 469-530). Elsevier Ltd.
- Rennert, R. C. ;M. Sorkin; R. K Garg, and G. C Gurtner Stem cell recruitment after injury: lessons for regenerative medicine, Regen Med. 2012 Nov; 7(6): 833–850. [CrossRef]
- Rashid, U.; Yousaf, A.; Yaqoob, M. et al. Characterization and differentiation potential of mesenchymalstem cells isolated from multiple canine adipose tissue sources. BMC Vet Res (2021), 17, 388. [CrossRef] [PubMed]
- Jiang, N.; Tian, X.; Wang, Q. et al. Regulation Mechanisms and Maintenance Strategies of Stemness in Mesenchymal Stem Cells. Stem Cell Rev and Rep (2023). [CrossRef]
- Baglio, S.R.; Pegtel, D.M.; Baldini, N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front Physiol. 2012 Sep 6;3:359. [CrossRef]
- Kolf, C.M.; Cho, E. & Tuan, R.S. Mesenchymal stromal cells: Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther 9, 204 (2007). [CrossRef]
- K, C.-H.; Johnson, P. H.; Batten, P.; Sarathchandra, P.; Chambers, R.C.; Taylor P., M.; Yacoub, M.H.; Chester, A.H. Collagen synthesis by mesenchymal stem cells and aortic valve interstitial cells in response to mechanical stretch, Cardiovascular Research, Volume 71, Issue 3, 06, Pages 548–556. 20 August. [CrossRef]
- Rogers, J.D.; Yeganegi, A.; Richardson W. J. Mechano-Regulation of Fibrillar Collagen Turnover by Fibroblasts. In Mechanobiology Handbook, Second Edition, Ed.2nd, 2018,ImprintCRC Press, Pages20, eBook ISBN9780429444982.
- Alt, E.; Yan, Y.; Gehmert, S.; Song, Y.H.; Altman, A.; Gehmert, S.; Vykoukal, D.; Bai, X. Fibroblasts share mesenchymal phenotypes with stem cells, but lack their differentiation and colony-forming potential. Biol Cell. 2011 Apr;103(4):197-208. Erratum in: Biol Cell. 2011 Aug;103(8):403. PMID: 21332447. [CrossRef]
- Lee, C. H.; Moioli, E. K. , & Mao, J. J. (2006). Fibroblastic Differentiation of Human Mesenchymal Stem Cells using Connective Tissue Growth Factor. Conference Proceedings :... Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual Conference, 1, 775. [CrossRef]
- Burk, J.; Sassmann, A.; Kasper, C.; Nimptsch, A.; Schubert, S. Extracellular Matrix Synthesis and Remodeling by Mesenchymal Stromal Cells Is Context-Sensitive Int J Mol Sci. 2022 Feb; 23(3): 1758. [CrossRef]
- Capobianco, E.; White, V. ; Sosa, M et al. Regulation of Matrix Metalloproteinases 2 and 9 Activities by Peroxynitrites in Term Placentas From Type 2 Diabetic Patients. Reproductive Sciences. 2012;19(8):814-822. [CrossRef]
- Almalki, S.G.; Agrawal, D.K. Effects of matrix metalloproteinases on the fate of mesenchymal stem cells. Stem Cell Res Ther 7, 129 (2016). [CrossRef]
- Wu, M.; Cronin, K.; Crane, J.S. Biochemistry, Collagen Synthesis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan. Available from: https://www.ncbi.nlm.nih. 5077. [Google Scholar]
- Avery, N. C. & Bailey, A. J. Enzymic and non-enzymic cross-linking mechanisms in relation to turnover of collagen: relevance to aging and exercise. Scand. J. Med. Sci. Sports. (2005) 15, 231–40.
- Yamauchi, M.; Sricholpech, M. Lysine post-translational modifications of collagen. Essays Biochem. 2012;52:113-33. [CrossRef]
- Hennet, T. Collagen Glycosylation. 1: Current Opinion in Structural Biology 2019, 56:131–138.
- Baumann, S. , & Hennet, T. Collagen Accumulation in Osteosarcoma Cells lacking GLT25D1 Collagen Galactosyltransferase. 2016. Journal of Biological Chemistry, 291(35), 18514-18524. [CrossRef]
- Rabbani, N. , & Thornalley, P. J. (2021).Protein glycation – biomarkers of metabolic dysfunction and early-stage decline in health in the era of precision medicine. Redox Biology, 42,101920. [CrossRef]
- Perrone, A.; Giovino, A.; Benny, J.; Martinelli, F. Advanced Glycation End Products (AGEs): Biochemistry, Signaling, Analytical Methods, and Epigenetic Effects. Oxid Med Cell Longev. 2020 Mar 18;2020:3818196. [CrossRef]
- R, Li; Rajan, R.; Wong, WCV.; Reid, D.G.; Duer, M.J.; Somovilla, V.J.; Martinez-Saez, N.; Bernardes, G.J.L.; Hayward, R.; Shanahan, C.M. In situ characterization of advanced glycation end products (AGEs) in collagen and model extracellular matrix by solid state NMR. – Chem Commun (Camb) (2017) 53, 13316. [CrossRef]
- Revell, C. K.; Jensen, O. E.; Shearer, T.; Lu, Y.; Holmes, D. F. , & Kadler, K. E. (2021). Collagen fibril assembly: New approaches to unanswered questions. Matrix Biology Plus, 12, 100079. [CrossRef]
- Komsa Penkova, R.; Stavreva, G.; Belemezova, K.; Kyurkchiev, S.; Todinova, S.; Altankov, G. Mesenchymal Stem-Cell Remodeling of Adsorbed Type-I Collagen-The Effect of Collagen Oxidation. Int. J. Mol. Sci. 2022, 23, 3058. [Google Scholar] [CrossRef] [PubMed]
- Magin, C.M.; Alge, D.L.; Anseth, K.S. Bio-inspired 3D microenvironments: a new dimension in tissue engineering. Biomed Mater. 2016 Mar 4;11(2):022001. [CrossRef]
- Komsa-Penkova, R.; Stoycheva, S.; Tonchev, P.; Stavreva, G.; Todinova, S.; Georgieva, G.; Yordanova, A.; Kyurkchiev, S.; Altankov, G. Morphological and Quantitative Evidence for Altered Mesenchymal Stem Cell Remodeling of Collagen in an Oxidative Environment—Peculiar Effect of Epigallocatechin-3-Gallate. Polymers. 2022, 14, 3957. [Google Scholar] [CrossRef] [PubMed]
- de Almeida., L.G.N; Thode, H.; Eslambolchi, Y.; Chopra, S.; Young, D.; Gill, S.; Devel, L.; Dufour, A. de Almeida. L.G.N; Thode, H.; Eslambolchi, Y.; Chopra, S.; Young, D.; Gill, S.; Devel, L.; Dufour, A. Matrix Metalloproteinases: From Molecular Mechanisms to Physiology, Pathophysiology, and Pharmacology. Pharmacol Rev. 2022 Jul;74(3):712-768.
- Mikulikova, K.; Eckhardt, A.; Pataridis, S.; Miksik, I. Study of posttranslational non-enzymatic modifications of collagen using capillary electrophoresis/mass spectrometry and high performance liquid chromatography/mass spectrometry. JChromatogr A 1155: 125-133, 2007.
- Fedintsev, A.; Moskalev, A. Stochastic non-enzymatic modification of long-lived macromolecules - A missing hallmark of aging. Ageing Res Rev. 2020 Sep;62:101097. [CrossRef] [PubMed]
- Avery, N.C.; Bailey, A.J. The effects of the Maillard reaction on the physical properties and cell interactions of collagen. Pathol Biol (Paris). 2006 Sep;54(7):387-95. [CrossRef] [PubMed]
- Evens, L.; Beliën, H.; Deluyker, D.; Bronckaers, A.; Gervois, P.; Hendrikx, M.; Bito, V. The Impact of Advanced Glycation End-Products (AGEs) on Proliferation and Apoptosis of Primary Stem Cells: A Systematic Review. Stem Cells Int. 2020 Nov 14;2020:8886612. [CrossRef]
- Sharma, S.D.; Pandey, B.N.; Mishra, K.P.; Sivakami, S. Amadori product and age formation during nonenzymatic glycosylation of bovine serum albumin in vitro. J Biochem Mol Biol Biophys. 2002 Aug;6(4):233-42. [CrossRef] [PubMed]
- Robins, S.P.; Bailey, A.J. (1972). Age-related changes in collagen: the identification of reducible lysine-carbohydrate condensation products. Biochem. Biophys.Res.Commun. 48, 76–84. [CrossRef]
- Cohen, M. P., & Ziyadeh, F. N. (1994). Amadori glucose adducts modulate mesangial cell growth and collagen gene expression. Kidney International, 45(2), 475-484. [CrossRef]
- Singh, R.; Barden, a, Mori, T. & Beilin, L. Advanced glycation end-products: a review. Diabetologia. 44, 129–46 (2001).
- Yan, S. F.; Ramasamy, R.; Naka, Y. & Schmidt, A. M. Glycation, Inflammation, and RAGE A Scaffold for the Macrovascular Complications of Diabetes and Beyond, 1159–1169 (2003).
- Ahmed, N. Advanced glycation endproducts — role in pathology of diabetic complications. Diabetes Res. Clin. Pract. 67, 3–21 (2005).
- Dariya, B., & Nagaraju, G. P. Advanced glycation end products in diabetes, cancer and phytochemical therapy. Drug Discovery Today, 25(9), 1614-1623. [CrossRef]
- Singh, R. ; Barden, a, Mori, T. & Beilin, L. Advanced glycation end-products: a review. Diabetologia. 44, 129–46 (2001).
- Yan, S. F.; Ramasamy, R.; Naka, Y. & Schmidt, A. M. Glycation, Inflammation, and RAGE A Scaffold for the Macrovascular Complications of Diabetes and Beyond, 1159–1169 (2003).
- Ahmed, N. Advanced glycation endproducts — role in pathology of diabetic complications. Diabetes Res. Clin. Pract. 67, 3–21 (2005).
- Dariya, B., & Nagaraju, G. P. (2020). Advanced glycation end products in diabetes, cancer, and phytochemical therapy. Drug Discovery Today, 25(9), 1614-1623. [CrossRef]
- Kawano, E. , Takahashi, S.; Sakano, Y.; Fujimoto D. Nonenzymatic glycation alters properties of collagen as a substratum for cells. Matrix. 1990 Oct;10(5):300-5. [CrossRef] [PubMed]
- Verzijl, N.; DeGroot, J.; Ben, Z.C. Brau-Benjamin O, Maroudas A, Bank RA, Mizrahi J, Schalkwijk CG, Thorpe SR, Baynes JW, Bijlsma JW, Lafeber FP, TeKoppele JM. Crosslinking by advanced glycation end products increases the stiffness of the collagen network in human articular cartilage: a possible mechanism through which age is a risk factor for osteoarthritis. Arthritis Rheum. 2002; 46: 114–123. .
- Said, G.; Guilbert, M.; Millerot-Serrurot, E.; Van Gulick, L.; Terryn, C.; Garnotel, R.; Jeannesson, P. Impact of carbamylation and glycation of collagen type I on migration of HT1080 human fibrosarcoma cells. Int J Oncol. 2012 Jun;40(6):1797-804. [CrossRef] [PubMed]
- Chen, S.V.;Brodsky, D.M.; Goligorsky, D.J.; Hampel, H. Li, S.S. Gross, et al., Glycated collagen I induces premature senescence-like phenotypic changes in endothelial cells, Circ. Res. 90 (2002) 1290–1298. [CrossRef]
- Jost, T.; Zipprich, A.; Glomb, M.A. Analysis of Advanced Glycation Endproducts in Rat Tail Collagen and Correlation to Tendon Stiffening. J Agric Food Chem. 2018 Apr 18;66(15):3957-3965. [CrossRef] [PubMed]
- Fu, H.L.; Valiathan, R.R.; Arkwright, R.; Sohail, A.; Mihai, C.; Kumarasiri, M. et al. Discoidin domain receptors: unique receptor tyrosine kinases in collagen-mediated signaling. J Biol Chem. (2013) 288:7430–7. [CrossRef]
- Nørregaard, K.S.; Krigslund, O.; Behrendt, N.; Engelholm, L.H.; Jürgensen, H.J. The collagen receptor uPARAP/Endo180 regulates collectins through unique structural elements in its FNII domain. J Biol Chem. 2020 Jul 3;295(27):9157-9170. [CrossRef]
- Olivares-Navarrete,R.; S. E. Rodil; S.L. Hyzy; G.R. Dunn; A.Almaguer-Flores; Z.Schwartz; and B.D. Boyan. Role of Integrin Subunits in Mesenchymal Stem Cell Differentiation and Osteoblast Maturation on Graphitic Carbon-coated Microstructured Surfaces Biomaterials. 2015 May; 51: 69–79.
- Heino, J. The collagen family members as cell adhesion proteins. Bioessays. 2007 Oct;29(10):1001-10. [CrossRef] [PubMed]
- Zhang WM, Kapyla, J.; Puranen J.S.; Knight C.G.; Tiger CF, et al.. a2b1 integrin recognizes the GFOGER sequence in interstitial collagens. 2003, J Biol Chem 278:7270–7277.
- Ruoslahti, E, and M. D. Pierschbacher. “New Perspectives in Cell Adhesion: RGD and Integrins.” Science, vol. 238, no. 4826, 1987, pp. 491–97. JSTOR. https://www.jstor.org/stable/1700530. Accessed 3 Jan. 2024.
- Davidenko N, Hamaia S, Bax DV, Malcor JD, Schuster CF, Gullberg D, Farndale RW, Best SM, Cameron RE. Selecting the correct cellular model for assessing of the biological response of collagen-based biomaterials. Acta Biomater. 2018 Jan;65:88-101. [CrossRef]
- Bansode, S. , Bashtanova, U., Li, R., Clark, J., Müller, K. H., Puszkarska, A., Goldberga, I., Chetwood, H. H., Reid, D. G., Colwell, L. J., Skepper, J. N., Shanahan, C. M., Schitter, G., Mesquida, P., & Duer, M. J. Glycation changes molecular organization and charge distribution in type I collagen fibrils. Scientific Reports, 2020.10(1), 1-13. [CrossRef]
- Rodrigues, M. , Griffith, L.G. & Wells, A. Growth factor regulation of proliferation and survival of multipotential stromal cells. Stem Cell Res Ther 1, 32 (2010). [CrossRef]
- Hidalgo A, Sanz-Rodriguez F, Rodriguez-Fernandez JL, et al. Chemokine stromal cell-derived factor-1α modulates VLA-4 integrin-dependent adhesion to fibronectin and VCAM-1 on bone marrow hematopoietic progenitor cells. Exp Hematol. 2001;29(3):345–355.
- Liesveld, J. L. , Sharma, N., & Aljitawi, O. S.. Stem cell homing: From physiology to therapeutics. STEM CELLS, 2020, 38(10), 1241-1253. [CrossRef]
- Komsa Penkova, R.; Spirova, R.; Bechev, B. Modification of Lowry’s method for collagen concentration measurement. J. Biochem.Biophys. Methods 1996, 32, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, N.; Leikin, S. Does the triple helical domain of type I collagen encode molecular recognition and fiber assembly while telopeptides serve as catalytic domains? Effect of proteolytic cleavage on fibrillogenesis and on collagen-collagen interaction in fibers. J. Biol. Chem. 1999, 274, 36083–36088. [Google Scholar] [CrossRef] [PubMed]
- Doyle, A.D. Fluorescent Labeling of Rat-tail Collagen for 3D Fluorescence Imaging. Bio-Protoc. 2018, 8, e2919. Available online: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/documents/222/544/f7250pis.pdf (accessed on 20 December 2023). [CrossRef]
- Dandia H., K. Makkad, P. Tayalia. Glycated collagen – a 3D matrix system to study pathological cell behavior.Biomaterials Science, 2019, № 8, p. 3480-3488. [CrossRef]
- Bubnis, W. and Ofner, C. . The determination of epsilon-amino groups in soluble and poorly soluble proteinaceous materials by a spectrophotometric method using trinitrobenzenesulfonic acid. Anal Biochem. 1992, 297, 129–33. [Google Scholar]
- Boudaoud, A.; Burian, A.; Borowska-Wykret, D.; Uyttewaal, M.; Wrzalik, R.; Kwiatkowska, D.; Hamant, O. FibrilTool, an ImageJplug-in to quantify fibrillar structures in raw microscopy images. Nat. Protoc. 2014, 9, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Jedeszko, C.; Sameni, M.; Olive, M.B.; Moin, K.; Sloane, B.F. Visualizing Protease Activity in Living Cells: From Two Dimensions to Four Dimensions. Curr. Protoc. Cell Biol. 2008, 39, 4–20. [Google Scholar] [CrossRef] [PubMed]
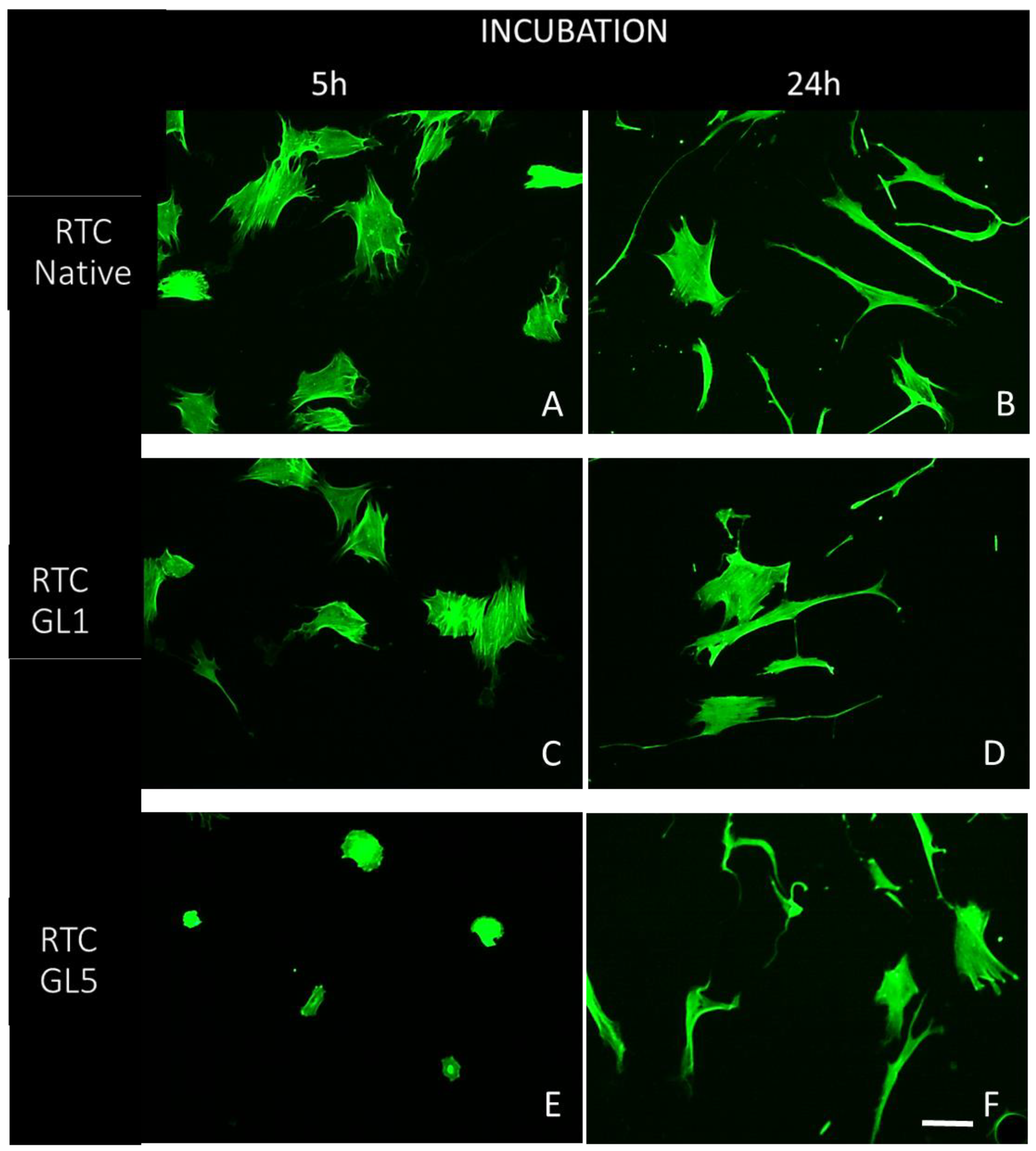
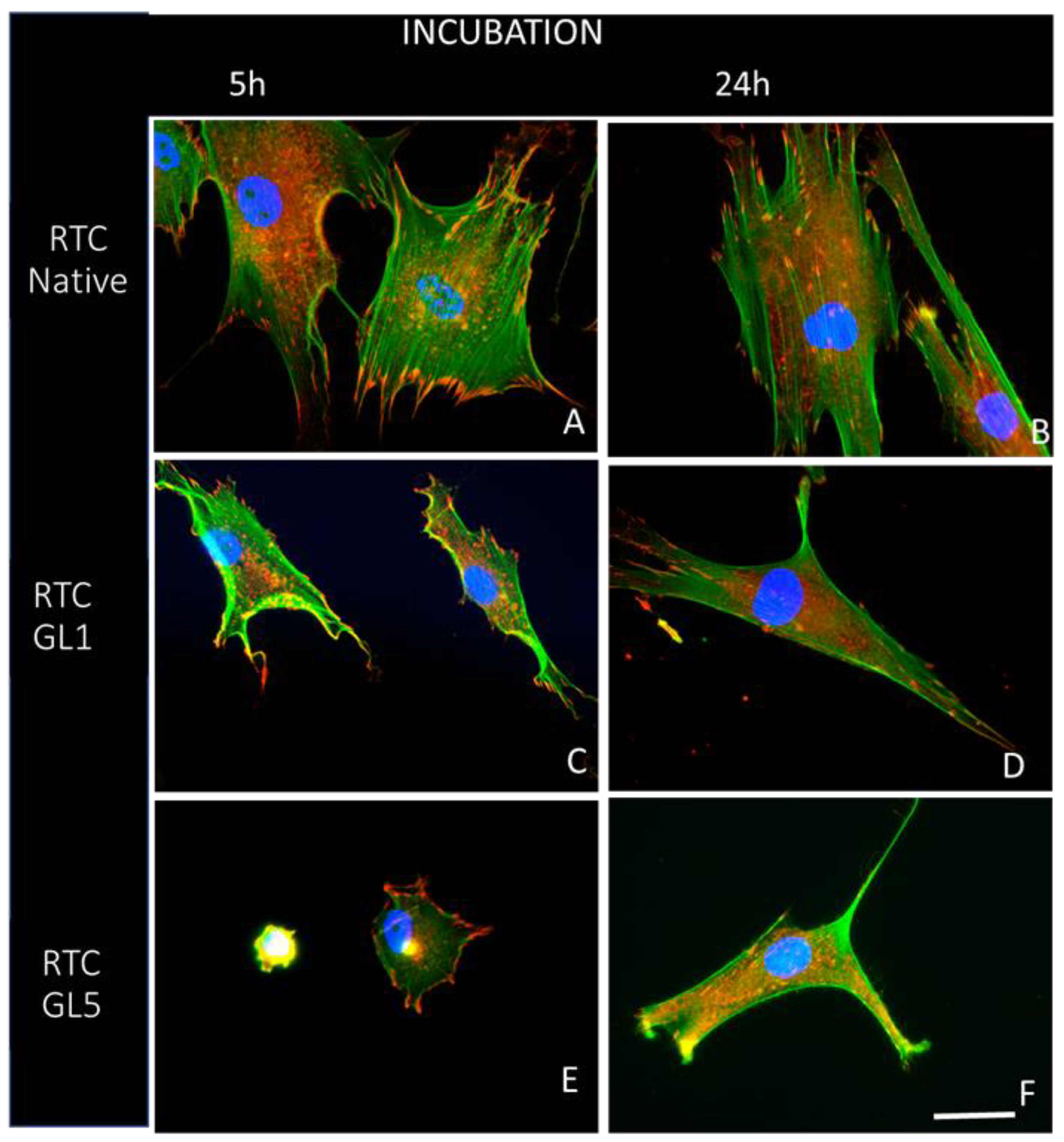

| Conditions | 5 h | 24 h | ||||
|---|---|---|---|---|---|---|
| Sample | RTC | RTC GL1 | RTC GL5 | RTC | RTC GL1 | RTC GL5 |
| CSA (μm2) | 246,8 ±143 | 216,8 ± 146 | 163,7 ± 109 | 249,1 ± 150 | 210,7 ± 171 | 226,4 ± 123 |
| Perimeter (μm) | 112,9 ± 56,0 | 95,1 ± 39 | 86,2 ± 42 | 130,4 ± 53 | 117,6 ± 66 | 164,5 ± 76,7 |
| AR | 1,5 ± 0,4 | 1,9 ± 0,9 | 1,7 ± 0,6 | 3,4 ± 2,3 | 3,6 ± 2,5 | 3,9 ± 2,7 |
| CSI | 0,30 ± 0,21 | 0,34 ± 0,19 | 0,31 ± 0,14 | 0,21 ± 0,12 | 0,23 ± 0,17 | 0,14 ± 0,11 |
| Sample | Tm (°C) |
∆Hcal (cal g−1) |
cPex (cal.g−1K−1) |
Tm ½ (°C) |
|---|---|---|---|---|
| RTC native | 40.4 | 8.76 | 4.93 | 1.57 |
| RTC GL1 (glycated for 24) | 40.2 | 6.82 | 3.58 | 1.72 |
| RTC GL5(glycated for 120h) | 40.5 | 6.98 | 3.67 | 1.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





