Submitted:
26 February 2024
Posted:
26 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hirshberg A, Buchner A. Metastatic tumours to the oral region. An overview. Eur J Cancer B Oral Oncol. novembre 1995;31(6):355–60. [CrossRef]
- Hirshberg A, Berger R, Allon I, Kaplan I. Metastatic Tumors to the Jaws and Mouth. Head Neck Pathol. dicembre 2014;8(4):463–74. [CrossRef]
- McClure SA, Movahed R, Salama A, Ord RA. Maxillofacial Metastases: A Retrospective Review of One Institution’s 15-Year Experience. J Oral Maxillofac Surg. gennaio 2013;71(1):178–88. [CrossRef]
- Shen ML, Kang J, Wen YL, Ying WM, Yi J, Hua CG, et al. Metastatic Tumors to the Oral and Maxillofacial Region: A Retrospective Study of 19 Cases in West China and Review of the Chinese and English Literature. J Oral Maxillofac Surg. aprile 2009;67(4):718–37. [CrossRef]
- Pastremoli, A. [Gingival metastasis, the first clinical sign of a silent kidney carcinoma. A case report]. Minerva Stomatol. dicembre 1991;40(12):825–8.
- Raiss H, Duplomb S, Tartas S, Layachi M, Errihani H. Lingual metastasis as an initial presentation of renal cell carcinoma: a case report. J Med Case Reports. dicembre 2017;11(1):314. [CrossRef]
- Vallalta Morales M, Todolí Parra J, Cervera Miguel JI, Calabuig Alborch JR. Hemiparesia derecha como forma de presentación de carcinoma renal de células claras. An Med Interna [Internet]. luglio 2004;21(7). http://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S0212-71992004000700010&lng=en&nrm=iso&tlng=en.
- Hirshberg A, Leibovich P, Buchner A. Metastatic tumors to the jawbones: analysis of 390 cases. J Oral Pathol Med. settembre 1994;23(8):337–41. [CrossRef]
- Hirshberg A, Leibovich P, Buchner A. Metastases to the oral mucosa: analysis of 157 cases. J Oral Pathol Med. settembre 1993;22(9):385–90. [CrossRef]
- Hirshberg A, Leibovich† P, Horowitz I, Buchner A. Metastatic tumors to postextraction sites. J Oral Maxillofac Surg. dicembre 1993;51(12):1334–7. [CrossRef]
- Ljungberg B, Campbell SC, Cho HY, Jacqmin D, Lee JE, Weikert S, et al. The Epidemiology of Renal Cell Carcinoma. Eur Urol. ottobre 2011;60(4):615–21. [CrossRef]
- Unverzagt S, Moldenhauer I, Nothacker M, Roßmeißl D, Hadjinicolaou AV, Peinemann F, et al. Immunotherapy for metastatic renal cell carcinoma. Cochrane Urology Group, curatore. Cochrane Database Syst Rev [Internet]. 15 maggio 2017 [citato 6 dicembre 2023];2017(5). Disponibile su: http://doi.wiley.com/10.1002/14651858.CD011673.pub2. [CrossRef]
- Zerdes I, Tolia M, Tsoukalas N, Mitsis M, Kardamakis D, Pistevou-Gombaki K, et al. Systemic therapy of metastatic renal cell carcinoma: Review of the current literature. Urol J. febbraio 2019;86(1):3–8. [CrossRef]
- Nazha S, Tanguay S, Kapoor A, Jewett M, Kollmannsberger C, Wood L, et al. Use of Targeted Therapy in Patients with Metastatic Renal Cell Carcinoma: Clinical and Economic Impact in a Canadian Real-Life Setting. Curr Oncol. 1 dicembre 2018;25(6):576–84. [CrossRef]
- Goebell PJ, Staehler M, Müller L, Nusch A, Scheffler M, Sauer A, et al. Changes in Treatment Reality and Survival of Patients With Advanced Clear Cell Renal Cell Carcinoma – Analyses From the German Clinical RCC-Registry. Clin Genitourin Cancer. dicembre 2018;16(6):e1101–15. [CrossRef]
- De Groot S, Redekop WK, Versteegh MM, Sleijfer S, Oosterwijk E, Kiemeney LALM, et al. Health-related quality of life and its determinants in patients with metastatic renal cell carcinoma. Qual Life Res. gennaio 2018;27(1):115–24. [CrossRef]
- Atkins MB, Tannir NM. Current and emerging therapies for first-line treatment of metastatic clear cell renal cell carcinoma. Cancer Treat Rev. novembre 2018;70:127–37. [CrossRef]
- Macleod LC, Hotaling JM, Wright JL, Davenport MT, Gore JL, Harper J, et al. Risk Factors for Renal Cell Carcinoma in the VITAL Study. J Urol. novembre 2013;190(5):1657–61. [CrossRef]
- Cheungpasitporn W, Thongprayoon C, O’Corragain OA, Edmonds PJ, Ungprasert P, Kittanamongkolchai W, et al. The risk of kidney cancer in patients with kidney stones: a systematic review and meta-analysis. QJM. marzo 2015;108(3):205–12. [CrossRef]
- Joh HK, Willett WC, Cho E. Type 2 Diabetes and the Risk of Renal Cell Cancer in Women. Diabetes Care. 1 luglio 2011;34(7):1552–6. [CrossRef]
- Christensson A, Savage C, Sjoberg DD, Cronin AM, Frank O’Brien M, Lowrance W, et al. Association of cancer with moderately impaired renal function at baseline in a large, representative, population-based cohort followed for up to 30 years: Cancer. Int J Cancer. 15 settembre 2013;133(6):1452–8. [CrossRef]
- Choueiri TK, Je Y, Cho E. Analgesic use and the risk of kidney cancer: A meta-analysis of epidemiologic studies. Int J Cancer. 15 gennaio 2014;134(2):384–96. [CrossRef]
- Lambe M, Lindblad P, Wuu J, Remler R, Hsieh C c. Pregnancy and risk of renal cell cancer: a population-based study in Sweden. Br J Cancer. maggio 2002;86(9):1425–9. [CrossRef]
- Kabat GC, Silvera SAN, Miller AB, Rohan TE. A cohort study of reproductive and hormonal factors and renal cell cancer risk in women. Br J Cancer. marzo 2007;96(5):845–9. [CrossRef]
- McKay RR, Kroeger N, Xie W, Lee JL, Knox JJ, Bjarnason GA, et al. Impact of Bone and Liver Metastases on Patients with Renal Cell Carcinoma Treated with Targeted Therapy. Eur Urol. marzo 2014;65(3):577–84. [CrossRef]
- Ðanić P, Ðanić D, Macan D. Tongue metastasis as an initial presentation of renal cell carcinoma. Med Glas Off Publ Med Assoc Zenica-Doboj Cant Bosnia Herzeg. 1 febbraio 2018;15(1):52–8. [CrossRef]
- Makos CP, Psomaderis K. A literature review in renal carcinoma metastasis to the oral mucosa and a new report of an epulis-like metastasis. J Oral Maxillofac Surg Off J Am Assoc Oral Maxillofac Surg. marzo 2009;67(3):653–60. [CrossRef]
- Pires FR, Azevedo RS, Ficarra G, Cardoso AS, Carlos R, Kowalski LP, et al. Metastatic renal cell carcinoma to the oral cavity and clear cell mucoepidermoid carcinoma: comparative clinicopathologic and immunohistochemical study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. aprile 2010;109(4):e22-27. [CrossRef]
- Maiorano E, Altini M, Favia G. Clear cell tumors of the salivary glands, jaws, and oral mucosa. Semin Diagn Pathol. agosto 1997;14(3):203–12.
- Lopez-Beltran A, Carrasco JC, Cheng L, Scarpelli M, Kirkali Z, Montironi R. 2009 update on the classification of renal epithelial tumors in adults. Int J Urol. maggio 2009;16(5):432–43. [CrossRef]
- Eble JN, Sauter G, Epstein LI, Sesterhenn IA. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. ARC Press: Lyon; 2004.
- Gobbo S, Eble JN, Grignon DJ, Martignoni G, MacLennan GT, Shah RB, et al. Clear Cell Papillary Renal Cell Carcinoma: A Distinct Histopathologic and Molecular Genetic Entity. Am J Surg Pathol. agosto 2008;32(8):1239–45. [CrossRef]
- Sangoi AR, Fujiwara M, West RB, Montgomery KD, Bonventre JV, Higgins JP, et al. Immunohistochemical Distinction of Primary Adrenal Cortical Lesions From Metastatic Clear Cell Renal Cell Carcinoma: A Study of 248 Cases. Am J Surg Pathol. maggio 2011;35(5):678–86. [CrossRef]
- Griffin N, Gore ME, Sohaib SA. Imaging in Metastatic Renal Cell Carcinoma. Am J Roentgenol. agosto 2007;189(2):360–70. [CrossRef]
- Corsi A, Guerra F, Grippaudo G, Bosman C. Oral metastasis of renal cell carcinoma. Report of case and critical evaluation of morphologic features for differential diagnosis. Pathologica. dicembre 1994;86(6):665–9.
- Kumamoto H, Yamazaki S, Sato A, Yamaguchi T, Tezuka F, Ooya K. Clear cell odontogenic tumor in the mandible: report of a case with duct-like appearances and dentinoid induction. J Oral Pathol Med. gennaio 2000;29(1):43–7. [CrossRef]
- Nair MK, Burkes EJ, Chai-U-Dom O. Radiographic manifestation of clear cell odontogenic tumor. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontology. febbraio 2000;89(2):250–4. [CrossRef]
- Pastore A, Ciorba A, Soliani M, Di Laora A, Valpiani G, Bianchini C, et al. Secondary malignant tumors of the parotid gland: not a secondary problem! J BUON Off J Balk Union Oncol. 2017;22(2):513–8.
- Franzen A, Buchali A, Lieder A. The rising incidence of parotid metastases: our experience from four decades of parotid gland surgery. Acta Otorhinolaryngol Ital. agosto 2017;37(4):264–9. [CrossRef]
- Majewska H, Skálová A, Radecka K, Stodulski D, Hyrcza M, Stankiewicz C, et al. Renal clear cell carcinoma metastasis to salivary glands – a series of 9 cases: clinico-pathological study. Pol J Pathol. 2016;1:39–45. [CrossRef]
- Ray A, Bhattacharya J, Ganguly S. Renal cell carcinoma presenting with oral tongue metastasis: A rare case presentation. J Cancer Res Ther. 2013;9(1):117. [CrossRef]
- Kalinin Y, Correia-Neto IJ, Do Nascimento SV, De Branco Gonçaves VC, De Andrade BAB, Nonaka CFW, et al. Lingual metastasis as the first presentation of clear cell renal cell carcinoma: Report of a rare case clinically mimicking a benign lesion. Oral Oncol. febbraio 2023;137:106293. [CrossRef]
- Nishii N, Shimamoto H, Ohsako T, Yokokawa M, Sato Y, Ohata Y, et al. Renal cell carcinoma metastasis to the maxillary bone successfully treated with surgery after vascular embolization: a case report. J Med Case Reports. dicembre 2020;14(1):193. [CrossRef]
- Zhang R, Lee CW, Basyuni S, Santhanam V. Mandibular swelling as the initial presentation for renal cell carcinoma: A case report. Int J Surg Case Rep. 2020;70:96–100. [CrossRef]
- Jung SY, Maeng JY, Lee H, Han JJ, Kim SM, Myoung H. Metastasis of Renal Cell Carcinoma to the Mandible. J Craniofac Surg. giugno 2023;34(4):e334–6. [CrossRef]
- Stojanovic M, Krasic D, Trajkovic M, Petrovic V. Rare renal cell carcinoma metastasis to mandibular gingiva: A case report and literature review. Niger J Clin Pract. 2020;23(10):1483. [CrossRef]
- Li L, Friedrich RE, Schmelzle R, Donath K. Metachronous bilateral metastases of renal cell carcinoma to the parotid region. J Oral Maxillofac Surg. aprile 2001;59(4):434–8. [CrossRef]
- Kundu, Eynon-Lewis, Radcliffe. Extensive metastatic renal cell carcinoma presenting as facial nerve palsy. J Laryngol Otol. giugno 2001;115(6):488–90. [CrossRef]
- Park YW, Hlivko TJ. Parotid gland metastasis from renal cell carcinoma. The Laryngoscope. marzo 2002;112(3):453–6. [CrossRef]
- Pritchyk KM, Schiff BA, Newkirk KA, Krowiak E, Deeb ZE. Metastatic Renal Cell Carcinoma to the Head and Neck. The Laryngoscope. settembre 2002;112(9):1598–602. [CrossRef]
- Göğüş Ç, Kiliç Ö, Tulunay Ö, Tulunay Ö, Bedük Y. Solitary metastasis of renal cell carcinoma to the parotid gland 10 years after radical nephrectomy. Int J Urol. ottobre 2004;11(10):894–6. [CrossRef]
- Torres-Carranza E, Garcia-Perla A, Infante-Cossio P, Belmonte-Caro R, Loizaga-Iriondo JM, Gutierrez-Perez JL. Airway obstruction due to metastatic renal cell carcinoma to the tongue. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontology. marzo 2006;101(3):e76–8. [CrossRef]
- Newton JR, O’Donnell M, Samuel PR. A case of renal cell carcinoma metastasizing to the parotid gland. Otolaryngol--Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg. aprile 2007;136(4 Suppl):S65-67. [CrossRef]
- Yoshitomi I, Kawasaki G, Mizuno A, Nishikido M, Hayashi T, Fujita S, et al. Lingual metastasis as an initial presentation of renal cell carcinoma. Med Oncol. dicembre 2011;28(4):1389–94. [CrossRef]
- Morvan JB, Veyrières JB, Mimouni O, Cathelinaud O, Allali L, Verdalle P. Clear-cell renal carcinoma metastasis to the base of the tongue and sphenoid sinus: Two very rare atypical ENT locations. Eur Ann Otorhinolaryngol Head Neck Dis. aprile 2011;128(2):91–4. [CrossRef]
- Balliram S, Goetz L, Ramsoobhag K, Narinesingh D, Medford S, Naraynsingh V. Renal Cell Carcinoma Presenting as a Tongue Lesion. J Oral Maxillofac Surg. luglio 2012;70(7):1605–8. [CrossRef]
- Serouya SM, Dultz LA, Concors SJ, Wang B, Patel KN. Late Solitary Metastasis of Renal Cell Carcinoma to the Submandibular Gland. J Oral Maxillofac Surg. ottobre 2012;70(10):2356–9. [CrossRef]
- Wadasadawala T, Kumar P, Agarwal J, Ghosh-Laskar S. Palliation of dysphagia with radiotherapy for exophytic base tongue metastases in a case of renal cell carcinoma. Indian J Urol. 2011;27(4):550. [CrossRef]
- Deeb R, Zhang Z, Ghanem T. Metastatic Renal Cell Carcinoma to the Parotid Gland in the Setting of Chronic Lymphocytic Leukemia. Case Rep Med. 2012;2012:1–3. [CrossRef]
- Özkiris M, Kubilay U, Sezen O. Cervical lymph node metastasis in renal cell carcinoma. J Oral Maxillofac Pathol. 2011;15(2):211. [CrossRef]
- Ghazali N, Davis C, Barrett AW, Tighe JV. Bilateral Asynchronous Renal Cell Carcinoma with Metastatic Involvement of the Tongue. Case Rep Pathol. 2012;2012:1–4. [CrossRef]
- Lau SYC, Chittleborough TJ, McCracken JA, Wijeratne S. Metastatic clear-cell renal carcinoma to the parotid. ANZ J Surg. ottobre 2012;82(10):760–1. [CrossRef]
- Mazeron R, Fenoll L, Mathieu MC, Dumas I, Haie-Meder C. Brachytherapy for isolated tongue metastasis of renal clear cell carcinoma. Eur Ann Otorhinolaryngol Head Neck Dis. giugno 2013;130(3):149–51. [CrossRef]
- Yanlan C, Liping S, Shaomin C, Zi L. Metastasis to the parotid region as an initial presentation of renal cell carcinoma: A case report. Oncol Lett. marzo 2013;5(3):997–9. [CrossRef]
- Udager AM, Rungta SA. Metastatic renal cell carcinoma, clear cell type, of the parotid gland: A case report, review of literature, and proposed algorithmic approach to salivary gland clear cell neoplasms in fine-needle aspiration biopsies. Diagn Cytopathol. novembre 2014;42(11):974–83. [CrossRef]
- Abbaszadeh-Bidokhty H, Motallebnejad M, Rajabi-Moghaddam M. Metastatic Renal cell Carcinoma Presenting as a clear-cell Tumor in Tongue: A Case Report. Iran J Otorhinolaryngol. luglio 2014;26(76):185–90.
- Kotak A, Merrick G. Presentation of metastatic renal cell carcinoma as a lip lesion. J Surg Case Rep. 9 settembre 2014;2014(9):rju083–rju083. [CrossRef]
- Suojanen J, Färkkilä E, Helkamaa T, Loimu V, Törnwall J, Lindqvist C, et al. Rapidly growing and ulcerating metastatic renal cell carcinoma of the lower lip: A case report and review of the literature. Oncol Lett. novembre 2014;8(5):2175–8. [CrossRef]
- Kudva R, Nayal B, Kantipudi S, Ray S. Metastatic renal cell carcinoma of the buccal mucosa masquerading as a salivary gland neoplasm. J Oral Maxillofac Pathol. 2016;20(3):547. [CrossRef]
- Georgy JT, Mathuram AJ, George AA, Chandramohan J. Renal cell carcinoma presenting as a cutaneous horn and nodules on the gingiva and scalp. BMJ Case Rep. 18 agosto 2017;bcr-2017-220913. [CrossRef]
- Nifosì G, Bressand H, Nifosì AF, Nifosì L, Damseaux P. Epulis-Like Presentation of Gingival Renal Cancer Metastasis. Case Rep Oncol. 10 agosto 2017;10(2):758–63. [CrossRef]
- Vasilyeva D, Peters S, Philipone E, Yoon A. Renal cell carcinoma metastatic to the maxillary gingiva: A case report and review of the literature. J Oral Maxillofac Pathol. 2018;22(4):102. [CrossRef]
- McNattin RF, Dean J, Archie L. Clinical Reports from Memorial Hospital, New York City: A Case of Renal Adenocarcinoma with Unusual Manifestations. The American Journal of Cancer. 1931;1570–6.
- Altinel D, Etit D, Tan A, Bayol Ü, Bulut V, Erdogan IG, et al. Metastatic Renal Cell Carcinoma Initially Presented as a Tongue Mass. Turkish Journal of Pathology. 2010;volume 26(number 3):261–3. [CrossRef]
- Syryło T, Syryło A, Jurkiewicz D, Zieliński H, Piętka T. An upper lip tumour as the presenting symptom of metastatic renal cancer. Otolaryngol Pol. settembre 2010;64(5):318–9. [CrossRef]
- Gil-Julio H, Vázquez-Alonso F, Fernández-Sánchez AJ, Puche-Sanz I, Flores-Martín JF, Cózar JM. Metastasis of Renal Cell Carcinoma to the Buccal Mucosa 19 Years after Radical Nephrectomy. Case Rep Oncol Med. 2012;2012:1–3. [CrossRef]
- Shirazian S, Bahrami N. An oral metastatic carcinoma guiding to discovery of a renal carcinoma: A case report. Journal of Craniomaxillofacial Research. 2016;230–4.
- Schrag AR, Jordan FB. Unusual metastasis from primary hypernephroma. Canadian Medical Association Journal. 1945;53(2):168.
- Carmen BVD, Korbitz BC. ORAL METASTASIS FROM HYPERNEPHROMA*. J Am Geriatr Soc. settembre 1970;18(9):743–6. [CrossRef]
- Friedlander AH, Singer R. Renal adenocarcinoma of the kidney with metastasis to the tongue. J Am Dent Assoc. dicembre 1978;97(6):989–91. [CrossRef]
- Fitzgerald RH, McInnes BK, Manry HC. Renal cell carcinoma involving oral soft tissues. J Oral Maxillofac Surg. settembre 1982;40(9):604–6. [CrossRef]
- Inai T, Kagawa S, Aga Y, Akiyama K. [A renal cell carcinoma with metastasis to the tongue]. Hinyokika Kiyo. agosto 1987;33(8):1240–3. [CrossRef]
- Ishikawa J, Morisue K, Imanishi O, Kamidono S. Renal cell carcinoma metastatic to the tongue: a case report. Hinyokika Kiyo. marzo 1991;37(3):263–5.
- Okabe Y, Ohoka H, Miwa T, Nagayama I, Furukawa M. Renal cell carcinoma metastasis to the tongue. J Laryngol Otol. marzo 1992;106(3):282–4. [CrossRef]
- Shibayama T, Hasegawa S, Nakamura S, Tachibana M, Jitsukawa S, Shiotani A, et al. Disappearance of Metastatic Renal Cell Carcinoma to the Base of the Tongue after Systemic Administration of Interferon-Alpha. Eur Urol. 1993;24(2):297–9. [CrossRef]
- Ziyada WF, Brookes JD, Penman HG. Expectorated tissue leading to diagnosis of renal adenocarcinoma. J Laryngol Otol. dicembre 1994;108(12):1108–10. [CrossRef]
- Airoldi M, Succo G, Valente G, Cavalot A, Gabriele P, Bumma C. Head and Neck Metastases of Renal Cancer after Nephrectomy: A Report of 2 Cases. Tumori J. maggio 1995;81(3):213–4. [CrossRef]
- Aguirre A, Rinaggio J, Diaz-Ordaz E. Lingual metastasis of renal cell carcinoma. J Oral Maxillofac Surg. marzo 1996;54(3):344–7. [CrossRef]
- Konya E, Hara Y, Umekawa T, Uejima S, Sugiyama T, Kurita T. [Two cases of renal cell carcinoma detected by metastasis to another organ]. Hinyokika Kiyo. settembre 1997;43(9):647–50.
- Tomita T, Inouye T, Shinden S, Mukai M. Palliative radiotherapy for lingual metastasis of renal cell carcinoma. Auris Nasus Larynx. maggio 1998;25(2):209–14. [CrossRef]
- Navarro F, Vicente J, Villanueva MJ, Sánchez A, Provencio M, España P. Metastatic Renal Cell Carcinoma to the Head and Neck Area. Tumori J. gennaio 2000;86(1):88–90. [CrossRef]
- Mekni A, Bouraoui S, Touati S, el Ouertani L, el May A. [Linguinal metastasis from clear cell carcinoma of the kidney]. Tunis Med. settembre 2002;80(9):570–3.
- Kyan A, Kato S nosuke. [Renal cell carcinoma metastatic to the base of tongue: a case report]. Hinyokika Kiyo. novembre 2004;50(11):791–3.
- Huang HC, Chang KP, Chen TM, Wu KF, Ueng SH. Renal cell carcinoma metastases in the head and neck. Chang Gung Med J. 2006;29 (4):59–65.
- Cochrane T, Cheng L, Crean J. Renal Cell Carcinoma: A Rare Metastasis to the Tongue – A Case Report. Dent Update. 2 aprile 2006;33(3):186–7. [CrossRef]
- del Rosario Regalado R, Gallana Álvarez S, Creo Martínez T, Herce López J, Pereira Gallardo S. Lingual metastasis from renal carcinoma. Rev Esp Cir Oral y Maxilofac. 2007;29:179–81.
- Longo R, Baldini D, Gasparini G. An atypical tongue metastasis of renal cell carcinoma in a patient with metachronous hepatocellular carcinoma.. Cancer Therapy. 2008;6 (2).
- Kella VKN, Cosgrove JM, Krishnamoorthy V. Synchronous lingual and thyroid metastasis from renal cell carcinoma. Am J Case Rep. 2009.
- Friedmann I, Osborn DA. Metastatic Tumours in the Ear, Nose and Throat Region. J Laryngol Otol. luglio 1965;79(7):576–91. [CrossRef]
- Willis AJTARA. PRIMARY CARCINOMA UNSUSPECTED BY THE CLINICIAN. Med J Aust. agosto 1936;2(7):222–7. [CrossRef]
- Branch C, Norton R. Metastatic hypernephroma of the jaw. N Engl J Med. 1928. [CrossRef]
- Salman I, Langel I. Metastatic tumors of the oral cavity. Oral Surg Oral Med Oral Pathol. novembre 1954;7(11):1141–9. [CrossRef]
- Persson PA, Wallenius K. Metastatic Renal Carcinoma (Hypernephroma) in the Gingiva of the Lower Jaw. Acta Odontol Scand. gennaio 1961;19(2):289–96. [CrossRef]
- Cranin AN, Berman S, Tucker N. Renal-cell carcinoma of the mandibular periodontium. Oral Surg Oral Med Oral Pathol. maggio 1966;21(5):626–31. [CrossRef]
- Buchner A, Begleiter A. Metastatic Renal Cell Carcinoma in the Gingiva Mimicking a Hyperplastic Lesion: Case Report. J Periodontol. luglio 1980;51(7):413–5. [CrossRef]
- Nishimura Y, Yakata H, Kawasaki T, Nakajima T. Metastatic tumours of the mouth and jaws. J Maxillofac Surg. gennaio 1982;10:253–8. [CrossRef]
- Fay JT, Weir GT. Metastatic renal cell carcinoma from a primary tumor removed 14 years previously. J Oral Maxillofac Surg Off J Am Assoc Oral Maxillofac Surg. febbraio 1983;41(2):129–32. [CrossRef]
- Zohar Y, Ben-Tovim R, Gal R, Laurian N. Metastatic carcinoma of oral soft tissue. Head Neck Surg. luglio 1985;7(6):484–6. [CrossRef]
- Tsianos EB, Karentzos C, Papadopoulos NE. Metastatic renal cell carcinoma in the gingiva of the maxilla and mandible: Report of a case. J Oral Maxillofac Surg. novembre 1987;45(11):975–7. [CrossRef]
- Müller-Mattheis V, Hagen M, Frenzel H, Ackermann R. [A rare form of metastasis of renal cell cancer. A case report of intra-oral soft tissue metastasis]. Urol Ausg A. novembre 1989;28(6):355–8.
- Hagen M, Müller-Mattheis V, Frenzel H, Fritzemeier CU. [Intraoral soft tissue metastases of a renal cell carcinoma]. Dtsch Z Mund Kiefer Gesichtschir. 1989;13(2):155–60.
- Salman I, Darlington C. Rare (unusual) malignant tumors of the jaws. Am J Orthod Oral Surg. 1944;30:725. [CrossRef]
- Mallett, SP. A renal-cell metastatic carcinoma involving the mandible and submaxillary gland. Oral Surg Oral Med Oral Pathol. gennaio 1961;14(1):4–7. [CrossRef]
- Meyer I, Shklar G. Malignant tumors metastatic to mouth and jaws. Oral Surg Oral Med Oral Pathol. settembre 1965;20(3):350–62. [CrossRef]
- Godby AF, Sonntag RW, Cosentino BJ. Hypernephroma with metastasis to the mandibular gingiva. Oral Surg Oral Med Oral Pathol. maggio 1967;23(5):696–700. [CrossRef]
- Milobsky SA, Milobsky L, Epstein LI. Metastatic renal adenocarcinoma presenting as periapical pathosis in the maxilla. Oral Surg Oral Med Oral Pathol. gennaio 1975;39(1):30–3. [CrossRef]
- Nagayama M, Oka T. Two cases of clear cell carcinoma found in the jaws. Nagoya J Med Sci. dicembre 1979;42(1–2):1–6.
- Susan LP, Daughtry JD, Stewart BH, Straffon RA. Palatal metastases in renal cell carcinoma. Urology. marzo 1979;13(3):304–5. [CrossRef]
- Matsumoto Y, Yanagihara N. Renal clear cell carcinoma metastatic to the nose and paranasal sinuses. The Laryngoscope. ottobre 1982;92(10 Pt 1):1190–3. [CrossRef]
- Pick JB, Wagner RM, Indresano AT. Initial appearance of renal cell carcinoma as a metastatic mass in the mandible. J Am Dent Assoc. novembre 1986;113(5):759–61. [CrossRef]
- Florine BL, Simonton SC, Sane SM, Stickel FR, Singher LJ, Dehner LP. Clear cell sarcoma of the kidney: Report of a case with mandibular metastasis simulating a benign myxomatous tumor. Oral Surg Oral Med Oral Pathol. maggio 1988;65(5):567–74. [CrossRef]
- Zachariades N, Koumoura F, Vairaktaris E, Mezitis M. Metastatic tumors to the jaws: A report of seven cases. J Oral Maxillofac Surg. settembre 1989;47(9):991–6. [CrossRef]
- Jones GM, Telfer MR, Eveson JW. Metastatic renal clear cell carcinoma of the jaws. Two cases illustrating clinical and pathological diagnostic problems. Br J Oral Maxillofac Surg. giugno 1990;28(3):172–5. [CrossRef]
- Fandella A, Anselmo G, Maccatrozzo L, Frezza D, Marchiori C. Epistaxis in Renal Carcinoma: Case Report. Scand J Urol Nephrol. 1 gennaio 1992;26(1):89–89. [CrossRef]
- G. Lee, S. D. Sharma, K. N. Bullock. An Unusual Case of Renal Cell Carcinoma With Two Rare Metastases. Scand J Urol Nephrol. 1 gennaio 1998;32(3):239–40. [CrossRef]
- Guyot L, Sauvant J, Menasse F, Garcia S, Portier F, Gola R. [Hemorrhagic mandibular metastasis of renal origin: usefulness of therapeutic embolization]. Presse Medicale Paris Fr 1983. 5 giugno 1999;28(20):1066–8.
- Toranzo-Fernandez JM, Falcon-Escobedo R, Sanchez-Hermosillo E, Gonzalez-Mendoza E. Clear cell sarcoma of the kidney metastatic to jaw: case report. J Clin Pediatr Dent. 2000;24(2):137–9.
- Hönig, JF. Inheritance of Hippel-Lindau Disease: A Rare Case of Maxillary Bone Metastasis: J Craniofac Surg. gennaio 2000;11(1):71–2.
- Shetty SC, Gupta S, Nagsubramanium S, Hasan S, Cherry G. Mandibular metastasis from renal cell carcinoma. A case report. Indian J Dent Res Off Publ Indian Soc Dent Res. 2001;12(2):77–80.
- Heinroth S, Bilkenroth U, Eckert AW, Maurer P. Die ossäre Metastase im Oberkiefer als Erstmanifestation eines Nierenzellkarzinoms: Ein Fallbericht. Mund Kiefer Gesichtschir. gennaio 2006;10(1):42–5.
- Madison JF, Frierson HF. Pathologic quiz case 2. Clear cell carcinoma, consistent with metastatic renal cell carcinoma. Arch Otolaryngol Head Neck Surg. maggio 1988;114(5):570–1, 573.
- Kishore M, Chauhan DS, Dogra S. Unusual presentation of renal cell carcinoma: A rare case report. J Lab Physicians. 2018;10(2):241–4. [CrossRef]
- Abro C, Sedhom R, Soni A, Markowski M. Cutaneous finger and tongue metastases in renal cell carcinoma. BMJ Case Rep. 21 giugno 2019;12(6):e230516. [CrossRef]
- Netto R, De Freitas Filho SAJ, Cortezzi W, Merly F, De Andrade VM, Pires FR. Metastasis of Renal Cell Carcinoma Causing Significant Facial Asymmetry. Case Rep Surg. 3 novembre 2019;2019:1–5. [CrossRef]
- Walsh MA, Quinn AJ, Mahesh B. Case report: renal cell carcinoma metastasis to the tongue. J Surg Case Rep. dicembre 2022;2022(12):rjac565. [CrossRef]
- Mrena R, Leivo I, Passador-Santos F, Hagström J, Mäkitie AA. Histopathological findings in parotid gland metastases from renal cell carcinoma. Eur Arch Otorhinolaryngol. settembre 2008;265(9):1005–9. [CrossRef]
- Aljawad M, Alharbi MK, Algahtani SM, Mughallis HM, Almhna SM. Metastasis of Clear Cell Renal Cell Carcinoma to the Parotid Gland: A Case Report. Cureus. agosto 2023;15(8):e43676. [CrossRef]
- Migliorelli A, Caranti A, Manuelli M, Bianchini C, Ciorba A, Pelucchi S. Clear-Cell Renal Cell Carcinoma Metastasis into Pterygomaxillary Fossa - A Case Report. Ann Maxillofac Surg. 2023;13(1):95–7. [CrossRef]
- Maschino F, Guillet J, Curien R, Dolivet G, Bravetti P. Oral metastasis: a report of 23 cases. Int J Oral Maxillofac Surg. febbraio 2013;42(2):164–8. [CrossRef]
- Wallace J, Abelardo E, Ramachandran K, Prabhu V. Renal cell carcinoma uvula metastasis leading to airway compromise: an unusual site. BMJ Case Rep. 8 aprile 2022;15(4):e248098. [CrossRef]
- Ludwig DC, Garcia J, Chang OH, Closmann JJ. Metastatic renal cell carcinoma to the mandible: a case report with clinical and histologic findings. Gen Dent. 2020;68(3):41–4.
- Melnick SJ, Amazon K, Dembrow V. Metastatic renal cell carcinoma presenting as a parotid tumor: a case report with immunohistochemical findings and a review of the literature. Hum Pathol. febbraio 1989;20(2):195–7. [CrossRef]
- Borghi L, Bianchini E, Ballotta MR, Reale D. Metastatic renal cell carcinoma presenting as a parotid tumor: a case report. Pathologica. aprile 1995;87(2):168–70.
- Seijas BP, Franco FL, Sastre RM, García AA, López-Cedrún Cembranos JL. Metastatic renal cell carcinoma presenting as a parotid tumor. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. maggio 2005;99(5):554–7. [CrossRef]
- Goel MC, Williams DW, Evans H, Roberts JG. Lingual metastasis from renal cell carcinoma management and review of the literature. Urol Int. 2003;71(4):418–21. [CrossRef]
- Lenkeit C, Bank J, Shirazi M. Renal Cell Carcinoma in the Head and Neck: Case Presentation of a Patient With a Rare Metastatic Pattern. Cureus. 4 dicembre 2020;12(12):e11894. [CrossRef]
- Ruiz-Oslé S, Prol C, Lardies R, Gaafar A, Barbier L, Arruza A. [Renal Cell Carcinoma metastases in the maxillofacial area: Case series.]. Arch Esp Urol. ottobre 2017;70(8):732–5.
- Doykos, JD. Wilms’ tumor metastiatic to mandible and oral mucosa. Report of a case. Oral Surg Oral Med Oral Pathol. febbraio 1969;27(2):220–4. [CrossRef]
- Schwab B, Lee WT. Bilateral renal cell carcinoma metastasis in the oral cavity. Am J Otolaryngol. 2012;33(1):154–5. [CrossRef]
- Erkilic S, Keskinruzgar A, Bozdag Z, Gunhan O. Metastasis of a Renal Collecting Duct Adenocarcinoma to the Oral Cavity After Tooth Extraction. J Craniofac Surg. giugno 2017;28(4):e398–9. [CrossRef]
- Lee YH, Lee JI. Metastatic carcinoma of the oral region: An analysis of 21 cases. Med Oral Patol Oral Cirugia Bucal. 1 maggio 2017;22(3):e359–65. [CrossRef]
- Guimarães DM, Pontes FSC, Miyahara LAN, Guerreiro MYR, de Almeida MCL, Pontes HAR, et al. Metastatic Renal Cell Carcinoma to the Oral Cavity. J Craniofac Surg. settembre 2016;27(6):e533-534. [CrossRef]
- Owosho AA, Xu B, Kadempour A, Yom SK, Randazzo J, Ghossein RA, et al. Metastatic solid tumors to the jaw and oral soft tissue: A retrospective clinical analysis of 44 patients from a single institution. J Cranio-Maxillo-fac Surg Off Publ Eur Assoc Cranio-Maxillo-fac Surg. agosto 2016;44(8):1047–53. [CrossRef]
- Nisi M, Izzetti R, Graziani F, Gabriele M. Renal Cell Carcinoma Metastases to the Oral Cavity: Report of 2 Cases and Review of Literature. J Oral Maxillofac Surg Off J Am Assoc Oral Maxillofac Surg. settembre 2020;78(9):1557–71. [CrossRef]
- Lang EE, Patil N, Walsh RM, Leader M, Walsh MA. A case of renal cell carcinoma metastatic to the nose and tongue. Ear Nose Throat J. maggio 2003;82(5):382–3. [CrossRef]
- Bućin E, Andréasson L, Björlin G. Metastases in the oral cavity. Case reports. Int J Oral Surg. ottobre 1982;11(5):321–5. [CrossRef]
- Marioni G, Gaio E, Poletti A, Derosas F, Staffieri A. Uncommon metastatic site of renal adenocarcinoma: the oral tongue. Acta Otolaryngol (Stockh). marzo 2004;124(2):197–201. [CrossRef]
- van der Waal RIF, Buter J, van der Waal I. Oral metastases: report of 24 cases. Br J Oral Maxillofac Surg. febbraio 2003;41(1):3–6. [CrossRef]
- Fukuda M, Miyata M, Okabe K, Sakashita H. A case series of 9 tumors metastatic to the oral and maxillofacial region. J Oral Maxillofac Surg Off J Am Assoc Oral Maxillofac Surg. agosto 2002;60(8):942–4. [CrossRef]
- Dehner, LP. Tumors of the mandible and maxilla in children. II. A study of 14 primary and secondary malignant tumors. Cancer. luglio 1973;32(1):112–20.
- Morii, T. A case report of metastatic clear cell carcinoma of the oral cavity. Japanese Journal of Oral and Maxillofacial Surgery. 1975;21 (2):213–6. [CrossRef]
- Sidhu SS, Parkash H, Chopra P. Renal metastatic carcinoma of the mandible. J Dent. giugno 1982;10(2):103–6. [CrossRef]
- Sánchez Aniceto G, García Peñín A, de la Mata Pages R, Montalvo Moreno JJ. Tumors metastatic to the mandible: analysis of nine cases and review of the literature. J Oral Maxillofac Surg Off J Am Assoc Oral Maxillofac Surg. marzo 1990;48(3):246–51. [CrossRef]
- Maestre-Rodríguez O, González-García R, Mateo-Arias J, Moreno-García C, Serrano-Gil H, Villanueva-Alcojol L, et al. Metastasis of renal clear-cell carcinoma to the oral mucosa, an atypical location. Med Oral Patol Oral Cirugia Bucal. 1 novembre 2009;14(11):e601-604. [CrossRef]
- Will TA, Agarwal N, Petruzzelli GJ. Oral cavity metastasis of renal cell carcinoma: a case report. J Med Case Reports. 29 settembre 2008;2:313. [CrossRef]
- Nesbitt AL, Lim ZLT, Chan KJ, Zardawi I, Pridgeon SW. Metastatic renal cell carcinoma presenting with both acute stroke and an oral lesion. Urol Case Rep. marzo 2019;23:75–7. [CrossRef]
- Patel S, Barros J, Nwizu NN, Ogbureke KUE. Metastatic renal cell carcinoma to the oral cavity as first sign of disease: A case report. Clin Case Rep. agosto 2020;8(8):1517–21. [CrossRef]
- Narea-Matamala G, Fernández-Toro M de los A, Villalabeitía-Ugarte E, Landaeta-Mendoza M, Rojas-Alcayaga G. Oral metastasis of renal cell carcinoma, presentation of a case. Med Oral Patol Oral Cirugia Bucal. 1 novembre 2008;13(11):E742-744.
- Massaccesi M, Morganti AG, Serafini G, Di Lallo A, Deodato F, Picardi V, et al. Late tonsil metastases from renal cell cancer: a case report. Tumori. 2009;95(4):521–4. [CrossRef]
- Shinozaki Y, Ito H, Nakayama R, Noguchi T, Jinbu Y, Kusama M, et al. Metastatic Clear Cell Carcinoma of the Mandible in a Patient with Renal Cancer undergoing Haemodialysis. Asian Journal of Oral and Maxillofacial Surgery. 2009;21(1–2):43–7. [CrossRef]
- Ohmura S, Kitagawa T, Kida Y, Fujita K, Masuda M, Ohtani T. Renal cell carcinoma metastatic to the mandibular angle. Japanese Journal of Oral and Maxillofacial Surgery,. 1981;27(5):662–7. [CrossRef]
- Nakano H, Naito K, Suzuki S, Naito K, Kubota T, Takizawa S. Metastatic renal cell carcinoma in the cheek: report of a case. Journal of Oral and Maxillofacial Surgery, Medicine, and Pathology. 2013;25(3):291–3. [CrossRef]
- Ficarra G, Pierleoni L, Panzoni E. Metastatic renal cell carcinoma involving Wharton’s duct. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontology. maggio 1996;81(5):580–3. [CrossRef]
- Tunio MA, AlAsiri M, Ahmad S, Fareed M, Bayoumi Y. Tongue metastasis as an initial manifestation of metastasis in renal cell carcinoma: A case report. J Solid Tumors. 31 marzo 2012;2(2):p39. [CrossRef]
- Milner P, Janas A, Grzesiak-Janas G. Clear cell renal carcinoma metastasis in the oral cavity – Case report. J Pre-Clin Clin Res. 12 gennaio 2015;8(2):127–9. [CrossRef]
- Santana LN, Ribeiro JT, Domingues M, De Oliveira MG, Rivero LF, Carrard VC, et al. A rare case of oral metastasis of renal clear cell carcinoma: case report and review of literature. Journal of Oral Diagnosis. 2000.
- Kizaekka A, Chengot P, Mannion C. Recurrent oral metastatic lesion of renal cell carcinoma - A case report. Int J Oral Craniofacial Sci. 13 settembre 2019;5(2):024–6. [CrossRef]
- Paraskevopoulos K, Vahtsevanos K, Ntomouchtsis, A, Kalaitsidou I, Patrikidou A, Andreadis C, et al. Metastatic tumors to the oral cavity - A retrospective analysis. Interna- tional Rsearch Journal of Otolaryn- gology. 2021;4(10).
- Morita Y, Iwagami T, Kawakita C, Kusuyama Y, Niki-Yonekawa A, Morita N. Oral metastasis of renal cell carcinoma mimicking recurrence of excised malignant myoepithelioma: A case report. Mol Clin Oncol [Internet]. 18 maggio 2018 [citato 6 dicembre 2023]; Disponibile su: http://www.spandidos-publications.com/10.3892/mco.2018.1630. [CrossRef]
- Prol C, Ruiz-Oslé S, Malaxetxebarría S, Dolado A, Del Hoyo OM, Barbier L. Oral and Maxillary Metastases: Retrospective Clinical Analysis of 21 Cases. Rev Esp Cir Oral Maxilofac [Internet]. 2019 [citato 6 dicembre 2023];41. Disponibile su: http://gestorrecom.inspiranetwork.com/fichaArticulo.aspx?iarf=220689767-747237414271. [CrossRef]
- Shimono H, Hirai H, Oikawa Y, Mochizuki Y, Kuroshima T, Tomioka H, et al. Metastatic tumors in the oral region: a retrospective chart review of clinical characteristics and prognosis. Oral Surg Oral Med Oral Pathol Oral Radiol. dicembre 2021;132(6):648–52. [CrossRef]
- Ali RAE, Mohamed KEH. Metastatic Clear Cell Renal Cell Carcinoma Presenting with a Gingival Metastasis. Clin Pract. 10 giugno 2016;6(2):847. [CrossRef]
- Selvi F, Faquin WC, Michaelson MD, August M. Three Synchronous Atypical Metastases of Clear Cell Renal Carcinoma to the Maxillary Gingiva, Scalp and the Distal Phalanx of the Fifth Digit: A Case Report. J Oral Maxillofac Surg Off J Am Assoc Oral Maxillofac Surg. giugno 2016;74(6):1286.e1-9. [CrossRef]
- Jatti D, Puri G, Aravinda K, Dheer DS. An atypical metastasis of renal clear cell carcinoma to the upper lip: a case report. J Oral Maxillofac Surg Off J Am Assoc Oral Maxillofac Surg. febbraio 2015;73(2):371.e1-6. [CrossRef]
- Sikka S, Sikka P, Kaur G, Shetty DC. A review of histopathological and immunohistochemical parameters in diagnosis of metastatic renal cell carcinoma with a case of gingival metastasis. J Cancer Res Ther. 2013;9(1):105–7. [CrossRef]
- Ganini C, Lasagna A, Ferraris E, Gatti P, Paglino C, Imarisio I, et al. Lingual metastasis from renal cell carcinoma: a case report and literature review. Rare Tumors. 26 giugno 2012;4(3):e41. [CrossRef]
- Lutcavage GJ, Branham GB, Winterholler BW, Wood DA. Renal cell carcinoma metastasis to the hard palate. J Oral Maxillofac Surg Off J Am Assoc Oral Maxillofac Surg. luglio 1984;42(7):469–74. [CrossRef]
- Azam F, Abubakerr M, Gollins S. Tongue metastasis as an initial presentation of renal cell carcinoma: a case report and literature review. J Med Case Reports. 25 luglio 2008;2:249. [CrossRef]
- Basely M, Bonnel S, Maszelin P, Verdalle P, Bussy E, de Jaureguiberry JP. A rare presentation of metastatic renal clear cell carcinoma to the tongue seen on FDG PET. Clin Nucl Med. settembre 2009;34(9):566–9. [CrossRef]
- Mansourian E, Ahmadnia H, Amirmajdi N. Renal cell carcinoma presenting as mandibular metastasis. Saudi J Kidney Dis Transplant. 2013;24(4):789. [CrossRef]
- Ord RA, Malins T, Ward-Booth PR. Vascular metastatic renal carcinoma of the maxilla. Report of two cases. Int J Oral Maxillofac Surg. aprile 1990;19(2):106–9. [CrossRef]
- Capodiferro S, Limongelli L, Mastropasqua MG, Favia G, Lajolo C, Colella G, et al. Metastatic Tumors of the Oro-Facial Tissues: Clear Cell Renal Cell Carcinoma. A Clinico-Pathological and Immunohistochemical Study of Seven Cases. J Clin Med. 17 aprile 2020;9(4):1151. [CrossRef]
- Andabak Rogulj A, Tomasovic Loncaric C, Muller D, Blivajs I, Andabak M, Vucicevic Boras V, et al. Solid malignant metastases in the jaw bones. Br J Oral Maxillofac Surg. ottobre 2018;56(8):705–8. [CrossRef]
- Derakhshan S, Rahrotaban S, Mahdavi N, Mirjalili F. Metastatic renal cell carcinoma presenting as maxillary lesion: Report of two rare cases. J Oral Maxillofac Pathol JOMFP. gennaio 2018;22(Suppl 1):S39–43. [CrossRef]
- Altuntaş O, Petekkaya İ, Süslü N, Güllü İ. Renal cell carcinoma metastatic to the tongue: a case report and review of the literature. J Oral Maxillofac Surg Off J Am Assoc Oral Maxillofac Surg. giugno 2015;73(6):1227–30. [CrossRef]
- Amiruddin S, Yunus MRM. Tongue mass in post nephrectomy patient. Egypt J Ear Nose Throat Allied Sci. luglio 2013;14(2):147–9. [CrossRef]
- Lieder A, Guenzel T, Lebentrau S, Schneider C, Franzen A. Diagnostic relevance of metastatic renal cell carcinoma in the head and neck: An evaluation of 22 cases in 671 patients. Int Braz J Urol. aprile 2017;43(2):202–8. [CrossRef]
- Kase AM, George DJ, Ramalingam S. Clear Cell Renal Cell Carcinoma: From Biology to Treatment. Cancers (Basel). 2023 Jan 21;15(3):665. [CrossRef]
- Schütz V, Lin H, Kaczorowski A, Zschäbitz S, Jäger D, Stenzinger A, Duensing A, Debus J, Hohenfellner M, Duensing S. Long-Term Survival of Patients with Stage T1N0M1 Renal Cell Carcinoma. Cancers (Basel). 2023 Dec 6;15(24):5715. [CrossRef]
- Heng, D.Y.; Xie, W.; Regan, M.M.; Harshman, L.C.; Bjarnason, G.A.; Vaishampayan, U.N.; Mackenzie, M.; Wood, L.; Donskov, F.; Tan, M.H.; et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: A population-based study. Lancet Oncol. 2013, 14, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Brierley DJ, Crane H, Hunter KD. Lumps and Bumps of the Gingiva: A Pathological Miscellany. Head Neck Pathol. 2019 Mar;13(1):103-113. [CrossRef]
- Ide F, Obara K, Mishima K, Saito I, Horie N, Shimoyama T, et al. Peripheral odontogenic tumor: a clinicopathologic study of 30 cases: general features and hamartomatous lesions. J Oral Pathol Med. 2005;34(9):552–557. [CrossRef]
- Wulfrank D, Speelman T, Pauwels C, Roels H, De Schryver A. Extranodal non-Hodgkin’s lymphoma of the head and neck. Radiother Oncol. 1987;8(3):199–207. [CrossRef]
- Epstein JB, Epstein JD, Le ND, Gorsky M. Characteristics of oral and paraoral malignant lymphoma: a population-based review of 361 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontics. 2001;96:519–525. [CrossRef]
- Wu J, Fantasia JE, Kaplan R. Oral manifestations of acute myelomonocytic leukemia: a case report and review of the classification of leukemias. J Periodontol. 2002;73(6):664–668. [CrossRef]
- Irani, S. Metastasis to the Jawbones: A review of 453 cases. J Int Soc Prev Community Dent. 2017 Mar-Apr;7(2):71-81. [CrossRef]
- Franzen A, Buchali A, Lieder A. The rising incidence of parotid metastases: our experience from four decades of parotid gland surgery. Acta Otorhinolaryngol Ital. 2017 Aug;37(4):264-269. [CrossRef]
- Azam, F. , Abubakerr, M. & Gollins, S. Tongue metastasis as an initial presentation of renal cell carcinoma: a case report and literature review. J Med Case Reports 2, 249 (2008). [CrossRef]
| AUTHORS | Years | Site | Histotype | Gender | Age | First sign of Disease |
|---|---|---|---|---|---|---|
| Ray et al (41) | 2013 | Tongue | RCC | M | 65 | Yes |
| Kalinin et al (42) | 2023 | Tongue | ccRCC | F | 58 | yes |
| Nishii et al (43) | 2020 | Maxillary bone | ccRCC | M | 89 | No |
| Zhang et al (44) | 2020 | Mandibular bone | RCC | F | 56 | Yes |
| Jung et al (45) | 2023 | Mandibular bone | RCC | F | 22 | Yes |
| Stojanovic et al (46) | 2020 | Gingiva | RCC | M | 53 | Yes |
| Li et al (47) | 2001 | Parotid | RCC | M | 63 | No |
| Kundu et al (48) | 2001 | Parotid | ccRCC | M | 61 | Yes |
| Park and Hlivko (49) | 2002 | Parotid | ccRCC | F | 83 | No |
| Pritchyk et al (50) | 2002 | Lip Maxillary bone Tongue |
RCC RCC RCC |
M F M |
70 53 60 |
Yes |
| Göğüş et al (51) | 2004 | Parotid | ccRCC | F | 59 | No |
| Torres-Carranza et al (52) | 2006 | Tongue | ccRCC | F | 49 | No |
| Newton et al (53) | 2007 | Parotid | ccRCC | F | 74 | No |
| Yoshitomi et al (54) | 2011 | Tongue | ccRCC | M | 47 | Yes |
| Morvan et al (55) | 2011 | Tongue | ccRCC | F | 48 | No |
| Balliram et al (56) | 2012 | Tongue | pRCC | M | 72 | Yes |
| Serouya et al (57) | 2012 | Submandibular gland | ccRCC | M | 60 | No |
| Wadasadawala et al (58) | 2011 | Tongue | RCC | M | 48 | No |
| Deeb et al (59) | 2012 | Parotid | RCC | M | 82 | No |
| Özkiriş et al (60) | 2011 | Cervical lymph nodes | ccRCC | F | 56 | No |
| Ghazali et al (61) | 2012 | Tongue | ccRCC | F | 64 | No |
| Lau et al (62) | 2012 | Parotid | ccRCC | F | 79 | No |
| Mazeron et al (63) | 2013 | Tongue | ccRCC | M | 66 | Yes |
| Yanlan et al (64) | 2013 | Parotid | ccRCC | F | 44 | Yes |
| Udager and Rungta (65) | 2014 | Parotid | ccRCC | M | 64 | No |
| Abbaszadeh-Bidokhty et al (66) | 2014 | Tongue | ccRCC | M | 80 | No |
| Kotak and Merrick (67) | 2014 | Lip | ccRCC | M | 64 | No |
| Suojanen et al (68) | 2014 | Lip | ccRCC | M | 71 | No |
| Kudva et al (69) | 2016 | Buccal mucosa | ccRCC | F | 36 | Yes |
| Georgy et al (70) | 2017 | Gingiva | ccRCC | M | 63 | Yes |
| Nifosì et al (71) | 2017 | Gingiva | ccRCC | M | 58 | No |
| Raiss et al (6) | 2017 | Tongue | RCC | M | 55 | Yes |
| Vasilyeva et al (72) | 2018 | Gingiva | RCC | F | 78 | Yes |
| McNattin and Dean (73) | 1931 | Tongue | Tubular Adenocarcinoma | M | 58 | Yes |
| Altinel et al (74) | 2010 | Tongue | ccRCC | M | 67 | Yes |
| Syryło et al (75) | 2010 | Lip | ccRCC | M | 59 | Yes |
| Gil-Julio et al (76) | 2012 | Buccal mucosa | ccRCC | M | 65 | No |
| Shirazian and Bahrami (77) | 2016 | Gingiva | ccRCC | M | 45 | Yes |
| Schrag and Jordan (78) | 1945 | Tongue | RCC | M | 34 | No |
| Carmen and Korbitz (79) | 1970 | Tongue | ccRCC | M | 77 | No |
| Friedlander et al (80) | 1978 | Tongue | RCC | M | 84 | No |
| Fitzgerald et al (81) | 1982 | Gingiva and Tongue | RCC | M | 63 | No |
| Inai et al (82) | 1987 | Tongue | RCC | M | 42 | No |
| Ishikawa et al (83) | 1991 | Tongue | RCC | F | 58 | No |
| Okabe et al (84) | 1992 | Tongue | ccRCC | M | 58 | No |
| Shibayama et al (85) | 1993 | Tongue | RCC | M | 41 | No |
| Ziyada et al (86) | 1994 | Tongue | ccRCC | M | 59 | Yes |
| Airoldi et al (87) | 1995 | Tongue | RCC | M | 51 | No |
| Aguirre et al (88) | 1996 | Tongue | ccRCC | F | 82 | Yes |
| Konya et al (89) | 1997 | Tongue | RCC | M | 59 | Yes |
| Tomita et al (90) | 1998 | Tongue | ccRCC | M | 50 | No |
| Navarro et al (91) | 2000 | Tongue | ccRCC | M | 62 | No |
| Mekni et al (92) | 2002 | Tongue | ccRCC | M | 63 | No |
| Kyan and Kato (93) | 2004 | Tongue | ccRCC | M | 66 | No |
| Huang et al (94) | 2006 | Tongue Parotid |
RCC ccRCC |
F F |
76 56 |
No No |
| Cochrane et al (95) | 2006 | Tongue | RCC | M | 41 | No |
| Del Rosario Regalado et al (96) | 2007 | Tongue | RCC | M | 81 | No |
| Longo et al (97) | 2008 | Tongue | RCC | M | 68 | No |
| Kella et al (98) | 2009 | Tongue | ccRCC | F | 67 | Yes |
| Friedmann and Osborn (99) | 1965 | Maxillary bone | RCC | M | 63 | No |
| Trinca and Willis (100) | 1936 | Tongue | RCC | M | 57 | Yes |
| Branch and Norton (101) | 1928 | Gingiva | ccRCC | F | 64 | Yes |
| Salman and Langel (102) | 1954 | Gingiva | RCC | F | 62 | No |
| Persson and Wallenius (103) | 1961 | Gingiva | ccRCC | F | 60 | No |
| Cranin et al (104) | 1966 | Gingiva | RCC | M | 72 | No |
| Buchner and Begleiter (105) | 1980 | Gingiva | ccRCC | M | 46 | No |
| Nishimura et al (106) | 1982 | Mandibular bone Gingiva Mandibular bone Mandibular bone |
RCC RCC Transitionalcell cR RCC |
F M M F |
61 72 61 36 |
yes yes no yes |
| Fay and Weir (107) | 1983 | Gingiva | ccRCC | F | 18 | No |
| Zohar et al (108) | 1985 | Gingiva | ccRCC | F | 54 | Yes |
| Tsianos et al (109) | 1987 | Gingiva | RCC | M | 78 | No |
| Müller-Mattheis et al (110) | 1989 | Gingiva | RCC | F | 47 | No |
| Hagen et al (111) | 1989 | Gingiva | RCC | F | 46 | No |
| Corsi et al (35) | 1994 | Lip | ccRCC | M | 44 | No |
| Salman and Darlington (112) | 1944 | Hard palate | ccRCC | F | 54 | No |
| Mallet (113) | 1961 | Mandibular bone | ccRCC | F | 72 | Yes |
| Meyer and Shklar (114) | 1965 | Parotid Maxillary bone Mandibular bone Mandibular bone |
RCC RCC Reticulumcell sarc. RCC |
M F M M |
48 73 43 57 |
No No No No |
| Godby et al (115) | 1967 | Gingiva | ccRCC | M | 45 | No |
| Milobsky et al (116) | 1975 | Maxillary bone | RCC | F | 66 | Yes |
| Nagayama and Oka (117) | 1979 | Mandibular bone Hard palate |
ccRCC ccRCC |
F F |
61 43 |
yes |
| Susan et al (118) | 1979 | Hard palate Hard palate |
ccRCC ccRCC |
M M |
53 62 |
yes yes |
| Matsumoto and Yanagihara (119) | 1982 | Maxillary bone Maxillary bone |
ccRCC ccRCC |
M M |
73 48 |
yes yes |
| Pick et al (120) | 1986 | Mandibular bone | ccRCC | M | 71 | Yes |
| Florine et al (121) | 1988 | Mandibular bone | Clear cell sarcoma | M | 15 m. | No |
| Zachariades et al (122) | 1989 | Mandibular bone | RCC | M | 78 | No |
| Jones and al (123) | 1990 | Mandibular bone Mandibular bone |
ccRCC ccRCC |
F F |
62 52 |
yes yes |
| Fandella et al (124) | 1992 | Maxillary bone | ccRCC | M | 62 | Yes |
| Lee et al (125) | 1998 | Maxillary bone | RCC | M | 76 | Yes |
| Guyot et al (126) | 1999 | Mandibular bone | RCC | M | 83 | No |
| Toranzo-Fernandez et al (127) | 2000 | Mandibular bone | Clear cell sarcoma | M | 8 | Yes |
| Honig (128) | 2000 | Maxillary bone | RCC | M | 46 | No |
| Shetty et al (129) | 2001 | Mandibulr bone | RCC | M | 62 | Yes |
| Heinroth et al (130) | 2006 | Maxillary bone | ccRCC | F | 53 | yes |
| Ðanić et al (26) | 2018 | Tongue | RCC | M | 51 | yes |
| Madison and Frierson (131) | 1988 | Tongue Tongue |
ccRCC ccRCC |
M M |
29 63 |
No No |
| Kishore et al (132) | 2018 | Lip | ccRCC | M | 54 | No |
| Abro et al (133) | 2019 | Tongue | RCC | M | 54 | No |
| Netto et al (134) | 2019 | Gingiva | RCC | M | 68 | Yes |
| Walsh et al (135) | 2022 | Tongue | ccRCC | M | 63 | No |
| Mrena et al (136) | 2008 | Parotid Parotid Parotid |
ccRCC RCC RCC |
F F F |
58 76 62 |
Yes No No |
| Aljawad et al (137) | 2023 | Parotid | ccRCC | M | 65 | No |
| Migliorelli et al (138) | 2023 | Maxillary bone | ccRCC | F | 54 | Yes |
| Maschino et al (139) | 2013 | Maxillary bone Maxillary bone Parotid Tongue |
ccRCC ccRCC ccRCC RCC |
M F M M |
73 84 78 66 |
No No No No |
| Wallace et al (140) | 2022 | Soft palate | ccRCC | M | 50 | No |
| Ludwig et al (141) | 2020 | Mandibular bone | ccRCC | M | 78 | Yes |
| Melnick et al (142) | 1989 | Parotid | ccRCC | M | 72 | Yes |
| Borghi et al (143) | 1995 | Parotid | ccRCC | M | 68 | No |
| Seijas et al (144) | 2005 | Parotid | ccRCC | M | 67 | Yes |
| Goel et al (145) | 2003 | Tongue | ccRCC | M | 62 | Yes |
| Lenkeit et al (146) | 2020 | Tongue | RCC | M | 71 | No |
| Ruiz-Oslé et al (147) | 2017 | Parotid Mandibular bone Gingiva Masticatory space |
RCC RCC RCC RCC |
M M M F |
72 55 62 52 |
yes yes yes no |
| Doykos (148) | 1969 | Mandibular bone | Wilm’s Tumor | F | 9 | Yes |
| Schwab and Lee (149) | 2012 | Maxillary bone | ccRCC | M | 63 | No |
| Erkilic et al (150) | 2017 | Gingiva | Collecting duct adenocarcinoma | F | 54 | Yes |
| Lee and Lee (151) | 2017 | Mandibular bone | RCC | M | 62 | No |
| Guimarães et al (152) | 2016 | Gingiva | ccRCC | F | 31 | No |
| Owosho et al (153) | 2016 | Mandibular bone Mandibular bone Gingiva Buccal mucosa Buccal mucosa Gingiva Buccal mucosa |
RCCRCCRCCRCCRCCRCCRCC | F F F M M M M |
61 63 18 75 70 59 66 |
No No No No No No No |
| Nisi et al (154) | 2020 | Tongue Buccal mucosa |
ccRCC ccRCC |
M M |
61 71 |
yes yes |
| Lang et al (155) | 2003 | Tongue | ccRCC | M | 45 | No |
| Bucín et al (156) | 1982 | Gingiva | RCC | M | 65 | No |
| Marioni et al (157) | 2004 | Tongue | ccRCC | F | 87 | No |
| Van der Wall et al (158) | 2003 | Soft palate Maxillary bone Mandibular bone Buccal mucosa |
ccRCC ccRCC ccRCC ccRCC |
F F M M |
62 64 48 67 |
No No No No |
| Fukuda et al (159) | 2002 | Mandibular bone | RCC | M | 76 | No |
| Makos and Psomaderis (27) | 2009 | Gingiva | ccRCC | M | 63 | No |
| Dehner (160) | 1973 | Mandibular bone | Wilm’s Tumor | F | 6 | No |
| Morii (161) | 1975 | Buccal mucosa | ccRCC | M | 63 | No |
| Sidhu (162) | 1982 | Mandibular bone | RCC | F | 32 | Yes |
| Sánchez Aniceto et al (163) | 1990 | Mandibular bone | RCC | M | 54 | Yes |
| Maestre-Rodríguez et al (164) | 2009 | Gingiva | ccRCC | M | 52 | Yes |
| Will et al (165) | 2008 | Floor of mouth | ccRCC | M | 63 | no |
| Nesbitt et al (166) | 2019 | Gingiva | Sarcomatoid RCC | M | 59 | Yes |
| Patel et al (167) | 2020 | Gingiva | ccRCC | F | 59 | yes |
| Narea-Matamala et al (168) | 2008 | Gingiva | RCC | M | 74 | yes |
| Massaccesi et al (169) | 2009 | Tonsil | ccRCC | M | 76 | yes |
| Shinozaki et al (170) | 2009 | Mandibular bone | ccRCC | F | 76 | No |
| Ohmura et al (171) | 1981 | Mandibular bone | ccRCC | M | 53 | No |
| Nakano et al (172) | 2013 | Gingiva | ccRCC | M | 72 | No |
| Ficarra et al (173) | 1996 | Wharton’s duct | ccRCC | M | 73 | No |
| Tunio et al (174) | 2012 | Tongue | ccRCC | M | 35 | No |
| Milner et al (175) | 2014 | Hard palate | ccRCC | M | 67 | Yes |
| Santana et al (176) | 2000 | Gingiva | ccRCC | M | 63 | Yes |
| Kizaekka et al (177) | 2019 | Tongue | ccRCC | M | 77 | No |
| Paraskevopoulos et al (178) | 2021 | Mandibular bone | ccRCC | M | 72 | Yes |
| Morita et al (179) | 2018 | Buccal mucosa | ccRCC | M | 75 | No |
| Prol et al (180) | 2019 | Mandibular bone Gingiva Gingiva Mandibular bone Masticatory space |
ccRCC ccRCC ccRCC chRCC ccRCC |
M M F M M |
55 62 52 56 65 |
No No No No No |
| Shimono et al (181) | 2021 | Mandibular bone Maxillary bone Tongue |
RCCRCCRCC | M M M |
62 89 63 |
Yes No no |
| Ali and Mohamed (182) | 2016 | Gingiva | ccRCC | M | 60 | Yes |
| Selvi et al (183) | 2016 | Gingiva | ccRCC | M | 51 | No |
| Jatti et al (184) | 2015 | Lip | ccRCC | M | 60 | No |
| Sikka et al (185) | 2013 | Gingiva | ccRCC | M | 73 | Yes |
| Ganini et al (186) | 2012 | Tongue | ccRCC | M | 70 | No |
| Lutcavage et al (187) | 1984 | Hard palate | RCC | M | 55 | No |
| Azam et al (188) | 2008 | Tongue | ccRCC | M | 78 | Yes |
| Basely et al (189) | 2009 | Tongue | ccRCC | F | 46 | No |
| Ahmadnia et al (190) | 2013 | Mandibular bone | ccRCC | M | 57 | Yes |
| Ord et al (191) | 1990 | Maxillary bone Maxillary bone |
RCC RCC |
M M |
58 73 |
yes |
| Capodiferro et al (192) | 2020 | Gingiva Tongue Mandibular bone Mandibular bone Parotid Parotid Mandibular bone |
ccRCC ccRCC ccRCC ccRCC ccRCC ccRCC ccRCC |
F M M M M F M |
69 56 45 63 55 55 60 |
No No No No No No No |
| Andabak Rogulj et al (193) | 2018 | Maxillary bone Maxillary bone Mandibular bone Maxillary bone Mandibular bone |
ccRCC ccRCC RCC RCC RCC |
M M F M F |
65 58 64 61 68 |
No No No No No |
| Derakhshan et al (194) | 2018 | Maxillary boneMaxillary bone | ccRCCccRCC | M M |
54 51 |
yes yes |
| Altuntaş et al (195) | 2014 | Tongue | pRCC | M | 70 | No |
| Amiruddin and Yunus (196) | 2013 | Tongue | ccRCC | M | 66 | No |
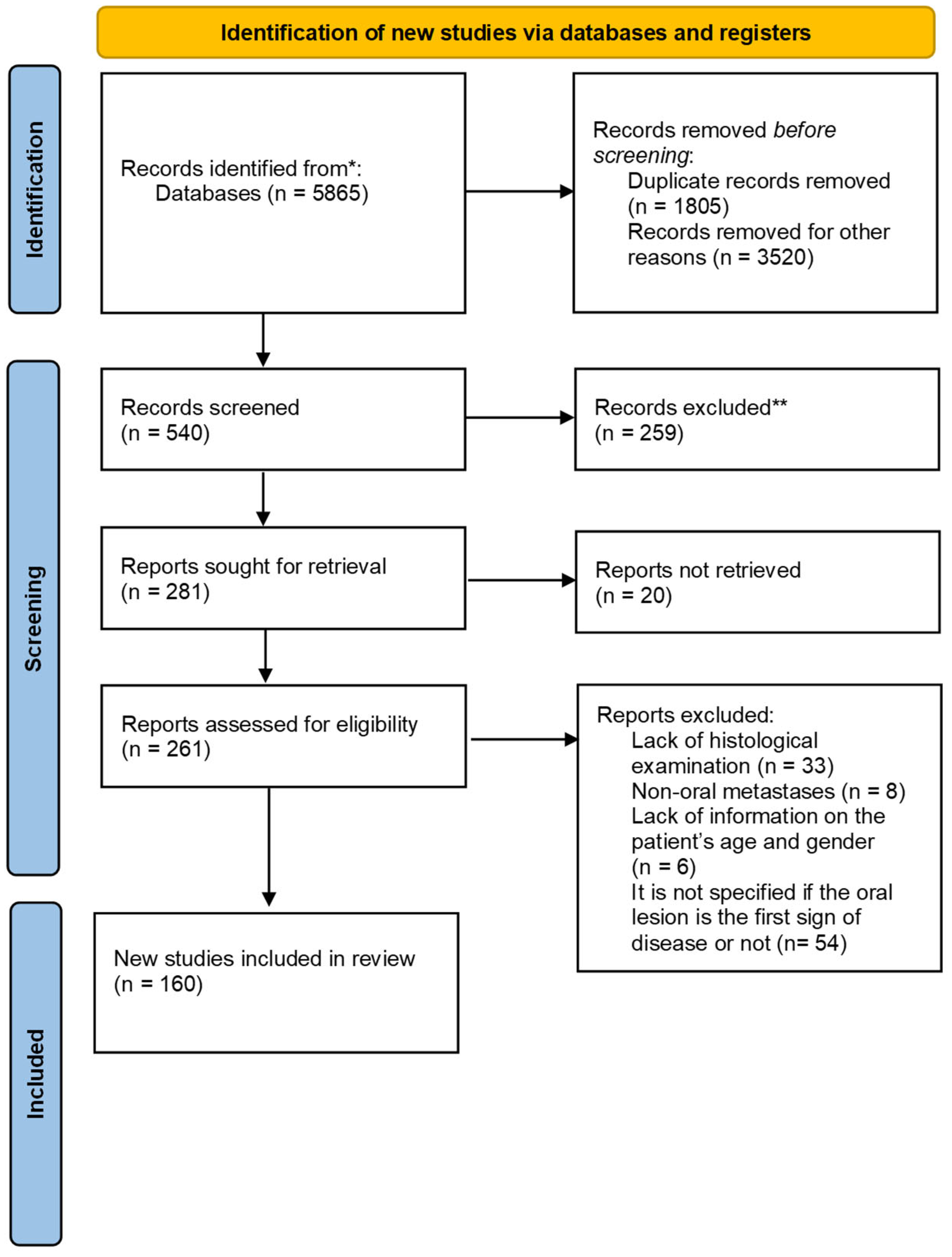
| SITE | CASES | |
|---|---|---|
| tongue | 55 | 26% |
| mandibular bone | 40 | 18.9% |
| gingiva | 39 | 18.4% |
| maxillary bone | 23 | 10.9% |
| parotid gland | 22 | 10.4% |
| buccal mucosa | 11 | 5.2% |
| lips | 7 | 3.3% |
| hard palate | 6 | 2.8% |
| soft palate | 2 | 0.9% |
| masticatory space | 2 | 0.9% |
| submandibular gland | 2 | 0.9% |
| lymph nodes | 1 | 0.4% |
| tonsil | 1 | 0.4% |
| oral floor | 1 | 0.4% |
| GENDER | CASES | |
| male | 148 | 70.1% |
| female | 63 | 29.85% |
| Authors | Site | Gender | Age | First sign of Disease | Clinical presentation | Radiological aspect |
|---|---|---|---|---|---|---|
| Kalinin et al (42) | Tongue | F | 58 | yes | Painless nodule | - |
| Nishii et al (43) | Maxillary bone | M | 89 | No | Swelling of the left maxillary ginguva | Osteolytic area |
| Kundu et al (48) | Parotid | M | 61 | Yes | Facial weakness and post-auricular pain | - |
| Park and Hlivko (49) | Parotid | F | 83 | No | infra-auricular swelling | - |
| Göğüş et al (51) | Parotid | F | 59 | No | pre-auricular swelling | - |
| Torres-Carranza et al (52) | Tongue | F | 49 | No | Pedunculated painless mass | - |
| Newton et al (53) | Parotid | F | 74 | No | Pre- auricular swelling | - |
| Yoshitomi et al (54) | Tongue | M | 47 | Yes | mass | - |
| Morvan et al (55) | Tongue | F | 48 | No | Painful mass | - |
| Serouya et al (57) | Submandibular gland | M | 60 | No | Submandibular mass | - |
| Özkiriş et al (60) | Cervical lymph nodes | F | 56 | No | Multiple mass in neck region | - |
| Ghazali et al (61) | Tongue | F | 64 | No | Painless mass | - |
| Lau et al (62) | Parotid | F | 79 | No | Parotid mass | - |
| Mazeron et al (63) | Tongue | M | 66 | Yes | Exophytic mass | - |
| Yanlan et al (64) | Parotid | F | 44 | Yes | Painless mass in parotid region | - |
| Udager and Rungta (65) | Parotid | M | 64 | No | Painless mass in parotid region | - |
| Abbaszadeh-Bidokhty et al (66) | Tongue | M | 80 | No | Swelling | - |
| Kotak and Merrick (67) | Lip | M | 64 | No | Asymptomatic swelling | - |
| Suojanen et al (68) | Lip | M | 71 | No | Spontaneously bleeding mass | - |
| Kudva et al (69) | Buccal mucosa | F | 36 | Yes | Painful ulcer | Bone erosion |
| Georgy et al (70) | Gingiva | M | 63 | Yes | Gingival nodule | - |
| Nifosì et al (71) | Gingiva | M | 58 | No | small painful reddish indurated swelling | - |
| Altinel et al (74) | Tongue | M | 67 | Yes | Tongue mass | - |
| Syryło et al (75) | Lip | M | 59 | Yes | Upper lip nodule | - |
| Gil-Julio et al (76) | Buccal mucosa | M | 65 | No | Discomfort in left cheek | - |
| Shirazian and Bahrami (77) | Gingiva | M | 45 | Yes | red-purple rubbery, sessile exophytic lesion with smooth surface | Saucer shape resorption of the crestal bone |
| Carmen and Korbitz (79) | Tongue | M | 77 | No | Painful mass | - |
| Okabe et al (84) | Tongue | M | 58 | No | Painless mass | - |
| Ziyada et al (86) | Tongue | M | 59 | Yes | Tongue mass | - |
| Aguirre et al (88) | Tongue | F | 82 | Yes | swelling | - |
| Tomita et al (90) | Tongue | M | 50 | No | Hemorragic mass | - |
| Navarro et al (91) | Tongue | M | 62 | No | Exophytic lesion | - |
| Mekni et al (92) | Tongue | M | 63 | No | NA | - |
| Kyan and Kato (93) | Tongue | M | 66 | No | Tongue mass | - |
| Huang et al (94) | Parotid | F | 56 | No | Bilateral enlarging mass in parotid region | - |
| Kella et al (98) | Tongue | F | 67 | Yes | NA | - |
| Branch and Norton (101) | Gingiva | F | 64 | Yes | Epulis-like mass | - |
| Persson and Wallenius (103) | Gingiva | F | 60 | No | Rapidly growing swelling | - |
| Buchner and Begleiter (105) | Gingiva | M | 46 | No | Rapidly growing mass | - |
| Fay and Weir (107) | Gingiva | F | 18 | No | Soft, fluctuant mass | Demarcated radiolucency |
| Zohar et al (108) | Gingiva | F | 54 | Yes | Soft, friable red mass | - |
| Corsi et al (35) | Lip | M | 44 | No | NA | - |
| Salman and Darlington (112) | Hard palate | F | 54 | No | Ulcerated nodule | NA |
| Mallet (113) | Mandibular bone | F | 72 | Yes | Pain and swelling | Osteolytic area |
| Godby et al (115) | Gingiva | M | 45 | No | Gingival mass | Bone resorption |
| Nagayama and Oka (117) | Mandibular bone Hard palate |
F F |
61 43 |
yes |
Swelling Palate’s perforation |
Osteolytic area NA |
| Susan et al (118) | Hard palate Hard palate |
M M |
53 62 |
yes yes |
Swelling Pedunculated lesion |
NA NA |
| Matsumoto and Yanagihara (119) | Maxillary bone Maxillary bone |
M M |
73 48 |
yes yes |
Cheek’s swelling epistaxis |
Osteolytic area NA |
| Pick et al (120) | Mandibular bone | M | 71 | Yes | Swelling | mixed radiolucent and radiopaque lesion |
| Jones and al (123) | Mandibular bone Mandibular bone |
F F |
62 52 |
yes yes |
Swelling Swelling |
osteolytic area osteolytic area |
| Fandella et al (124) | Maxillary bone | M | 62 | Yes | epistaxis | NA |
| Heinroth et al (130) | Maxillary bone | F | 53 | yes | Painful swelling | opacity in the maxillary sinus |
| Madison and Frierson (131) | Tongue Tongue |
M M |
29 63 |
No No |
NA NA |
- - |
| Kishore et al (132) | Lip | M | 54 | No | swelling | - |
| Walsh et al (135) | Tongue | M | 63 | No | Pedunculated lesion | - |
| Mrena et al (136) | Parotid |
F |
58 |
Yes |
Non-tender nodule | - |
| Aljawad et al (137) | Parotid | M | 65 | No | Non-tender mass | - |
| Migliorelli et al (138) | Maxillary bone | F | 54 | Yes | Facial pain | Bone erosion |
| Maschino et al (139) | Maxillary bone Maxillary bone Parotid |
M F M |
73 84 78 |
No No No |
Exophytic mass Pain, discomfort Rapid growth mass |
Osteolytic lesion NA |
| Wallace et al (140) | Soft palate | M | 50 | No | Globular lesion | - |
| Ludwig et al (141) | Mandibular bone | M | 78 | Yes | Painful swelling and paresthesia | NA |
| Melnick et al (142) | Parotid | M | 72 | Yes | Parotid mass | - |
| Borghi et al (143) | Parotid | M | 68 | No | Painless swelling | - |
| Seijas et al (144) | Parotid | M | 67 | Yes | Painless mass | - |
| Goel et al (145) | Tongue | M | 62 | Yes | Swelling | - |
| Schwab and Lee (149) | Maxillary bone | M | 63 | No | Bilateral, friable masses with a foul odor | NA |
| Guimarães et al (152) | Gingiva | F | 31 | No | Painful growth | Enlargement of the periodontal ligament |
| Nisi et al (154) | Tongue Buccal mucosa |
M M |
61 71 |
yes yes |
Swelling Large mass |
- - |
| Lang et al (155) | Tongue | M | 45 | No | Pedunculated mass | - |
| Marioni et al (157) | Tongue | F | 87 | No | Exophytic, ulcerated mass | - |
| Van der Wall et al (158) | Soft palate Maxillary bone Mandibular bone Buccal mucosa |
F F M M |
62 64 48 67 |
No No No No |
NA NA NA NA |
- - - - |
| Makos and Psomaderis (27) | Gingiva | M | 63 | No | Epulis-like mass | - |
| Morii (161) | Buccal mucosa | M | 63 | No | NA | - |
| Maestre-Rodríguez et al (164) | Gingiva | M | 52 | Yes | Granulomatous gingival lesion | - |
| Will et al (165) | Floor of mouth | M | 63 | no | Indurated mass | - |
| Patel et al (167) | Gingiva | F | 59 | yes | pink-red, oval, ulcerated lesion with a white pseudomembranous surface | - |
| Massaccesi et al (169) | Tonsil | M | 76 | yes | dysphagia | - |
| Shinozaki et al (170) | Mandibular bone | F | 76 | No | swelling | Multilocular bone destruction |
| Ohmura et al (171) | Mandibular bone | M | 53 | No | NA | NA |
| Nakano et al (172) | Gingiva | M | 72 | No | swelling | - |
| Ficarra et al (173) | Wharton’s duct | M | 73 | No | Movable mass in the floor of the mouth | - |
| Tunio et al (174) | Tongue | M | 35 | No | Painless swelling | - |
| Milner et al (175) | Hard palate | M | 67 | Yes | Irregularly shaped lump | none |
| Santana et al (176) | Gingiva | M | 63 | Yes | Double lobe nodule | Radiolucent lesion |
| Kizaekka et al (177) | Tongue | M | 77 | No | Pedunculated lesion | - |
| Paraskevopoulos et al (178) | Mandibular bone | M | 72 | Yes | NA | - |
| Morita et al (179) | Buccal mucosa | M | 75 | No | Swelling and facial asymmetry | - |
| Prol et al (180) | Mandibular bone Gingiva Gingiva Masticatory space |
M M F M |
55 62 52 65 |
No No No No |
Mass Mass NA Mass |
NA - - NA |
| Ali and Mohamed (182) | Gingiva | M | 60 | Yes | Gingival mass | Erosive bone changes |
| Selvi et al (183) | Gingiva | M | 51 | No | Rapidly progressive, painless exophytic lesion | Destruction of the alveolar bone |
| Jatti et al (184) | Lip | M | 60 | No | Ulcerated nodule | - |
| Sikka et al (185) | Gingiva | M | 73 | Yes | Multiple painless swelling | - |
| Ganini et al (186) | Tongue | M | 70 | No | Ulcerated lesion | - |
| Azam et al (188) | Tongue | M | 78 | Yes | Pedunculated lesion, difficulty in swallowing solid | - |
| Basely et al (189) | Tongue | F | 46 | No | Swelling on the left side of the neck | - |
| Ahmadnia et al (190) | Mandibular bone | M | 57 | Yes | Swelling, trismus | Radiolucent lesion |
| Capodiferro et al (192) | Gingiva Tongue Mandibular bone Mandibular bone Parotid Parotid Mandibular bone |
F M M M M F M |
69 56 45 63 55 55 60 |
No No No No No No No |
Large fungating mass Large fungating mass - - growing mass growing mass - |
Bone rarefaction - Osteolytic area Osteolytic area - - Osteolytic area |
| Andabak Rogulj et al (193) | Maxillary bone Maxillary bone |
M M |
65 58 |
No No |
Mobility of tooth Exophytic lesion |
NA NA |
| Derakhshan et al (194) | Maxillary boneMaxillary bone | M M |
54 51 |
yes yes |
Pain and swelling Polypoid mass |
intraosseous radiolucency |
| Amiruddin and Yunus (196) | Tongue | M | 66 | No | Painless mass | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





