Submitted:
28 February 2024
Posted:
28 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Computational Details
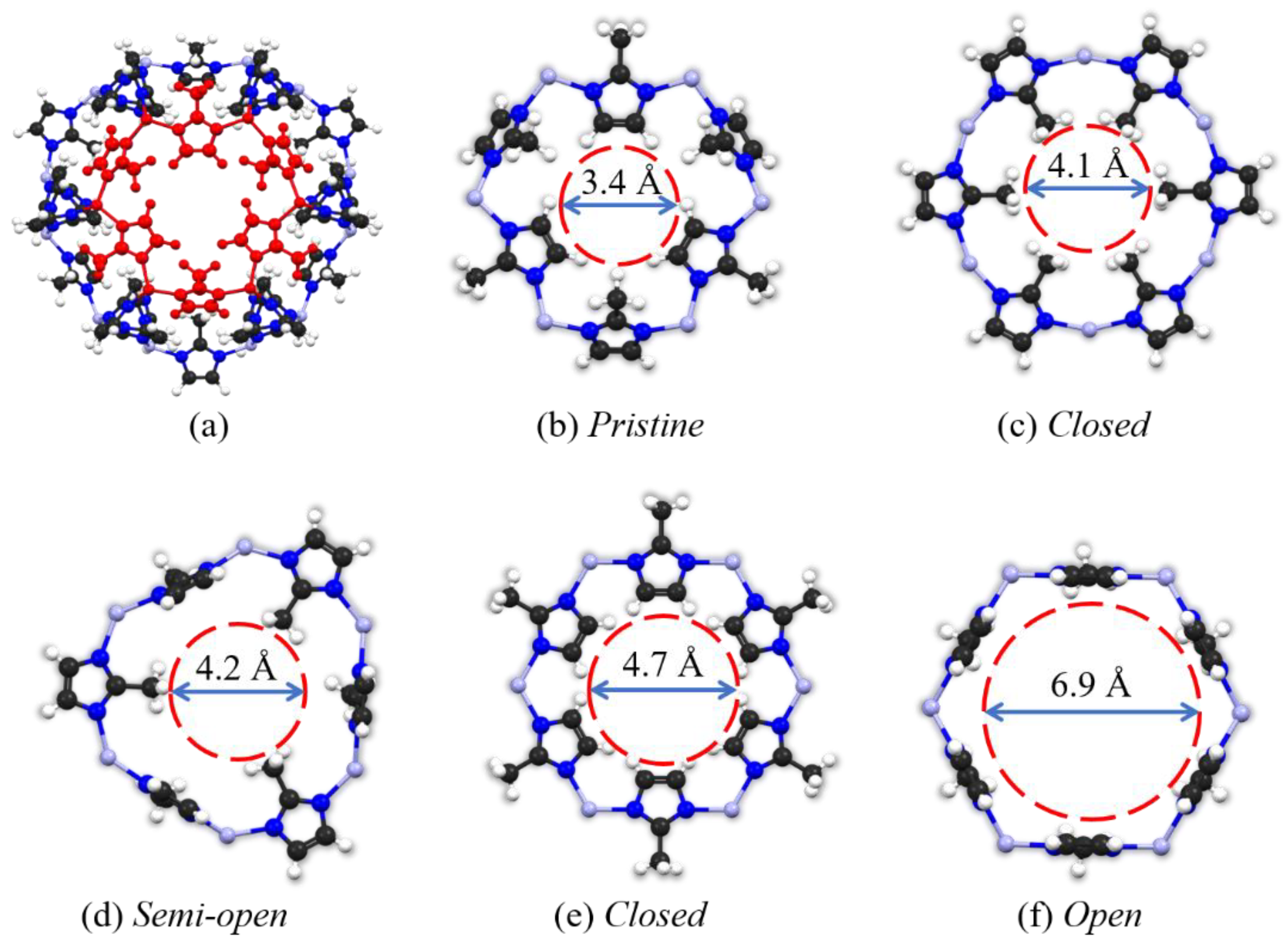
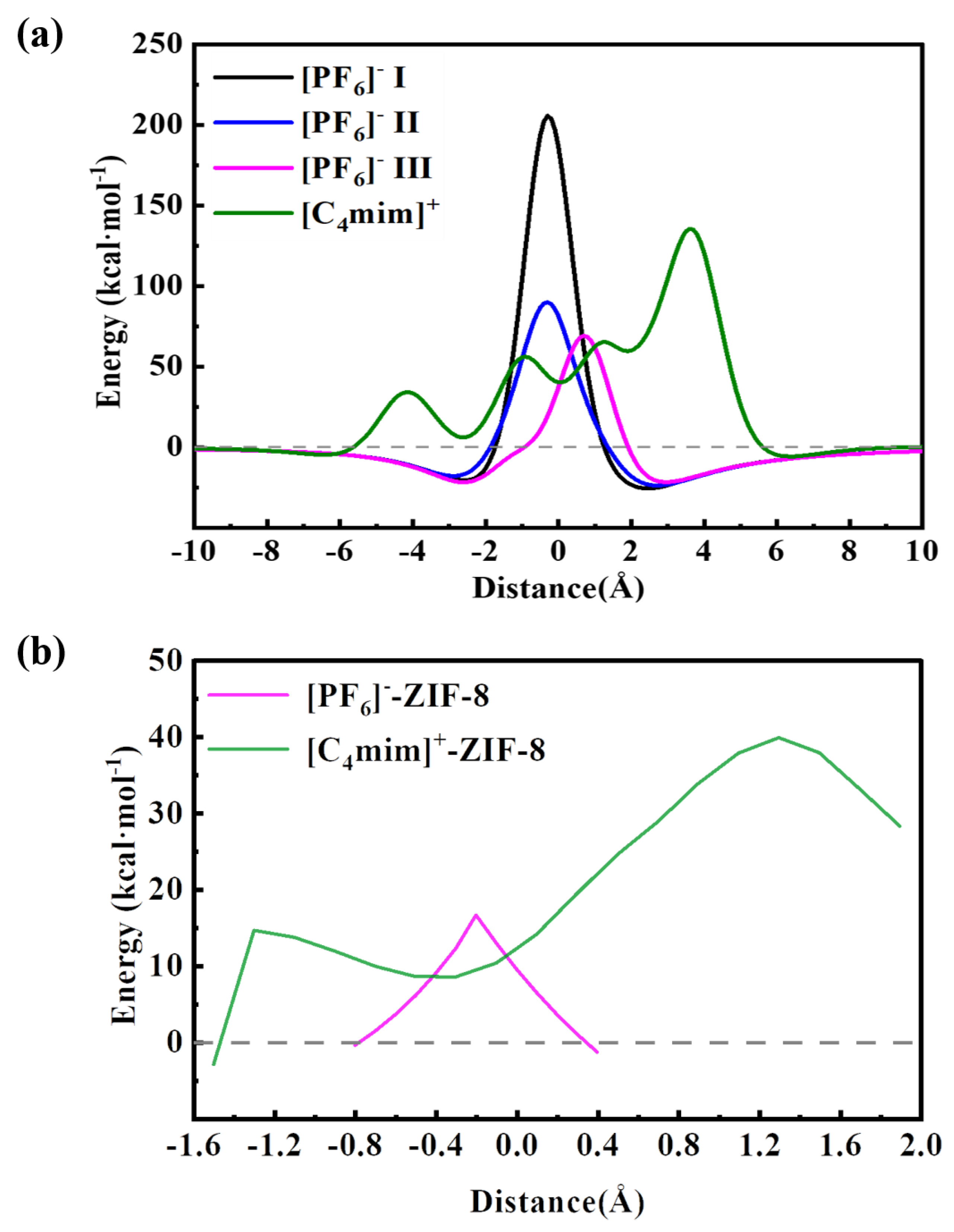
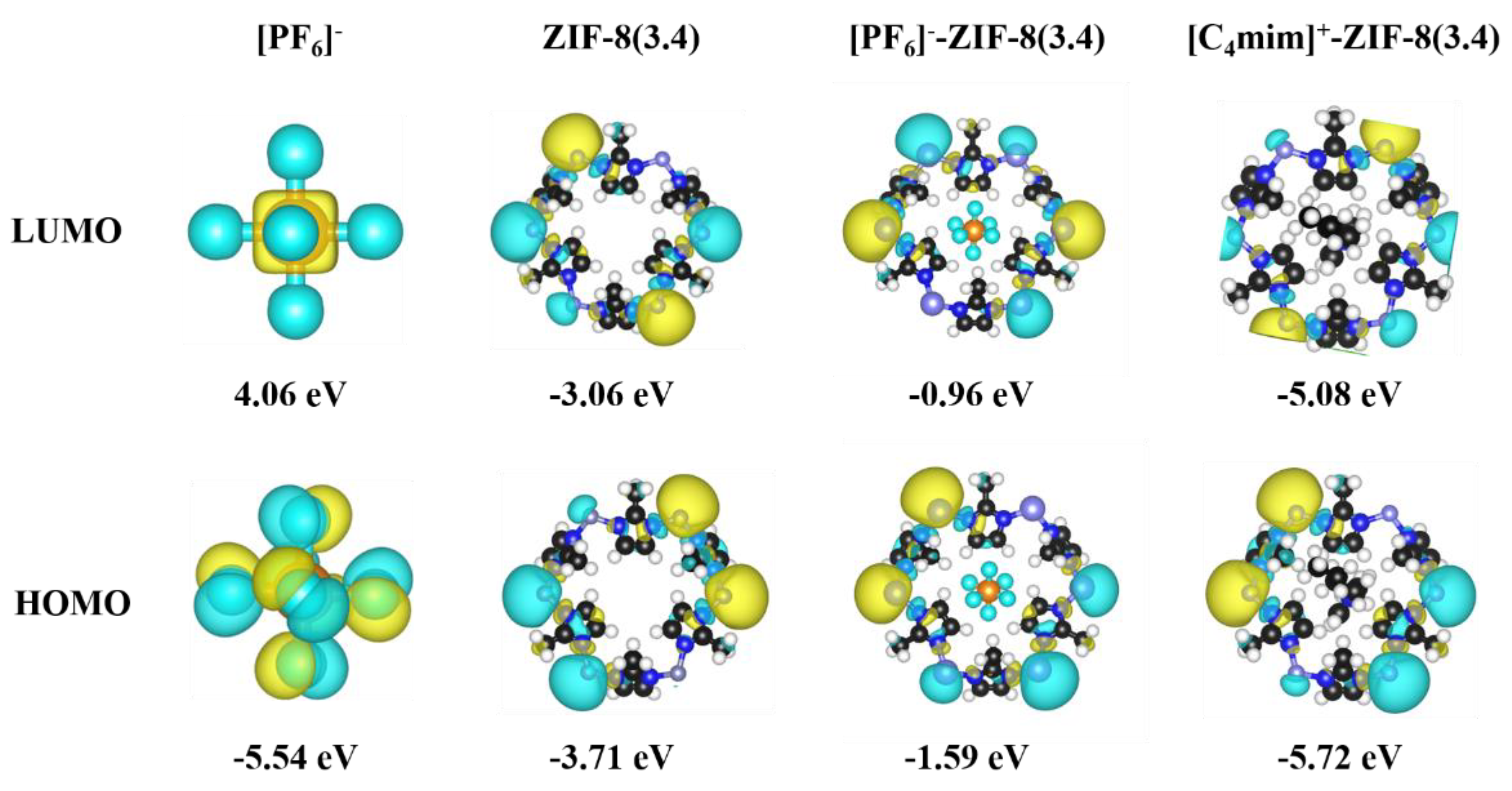
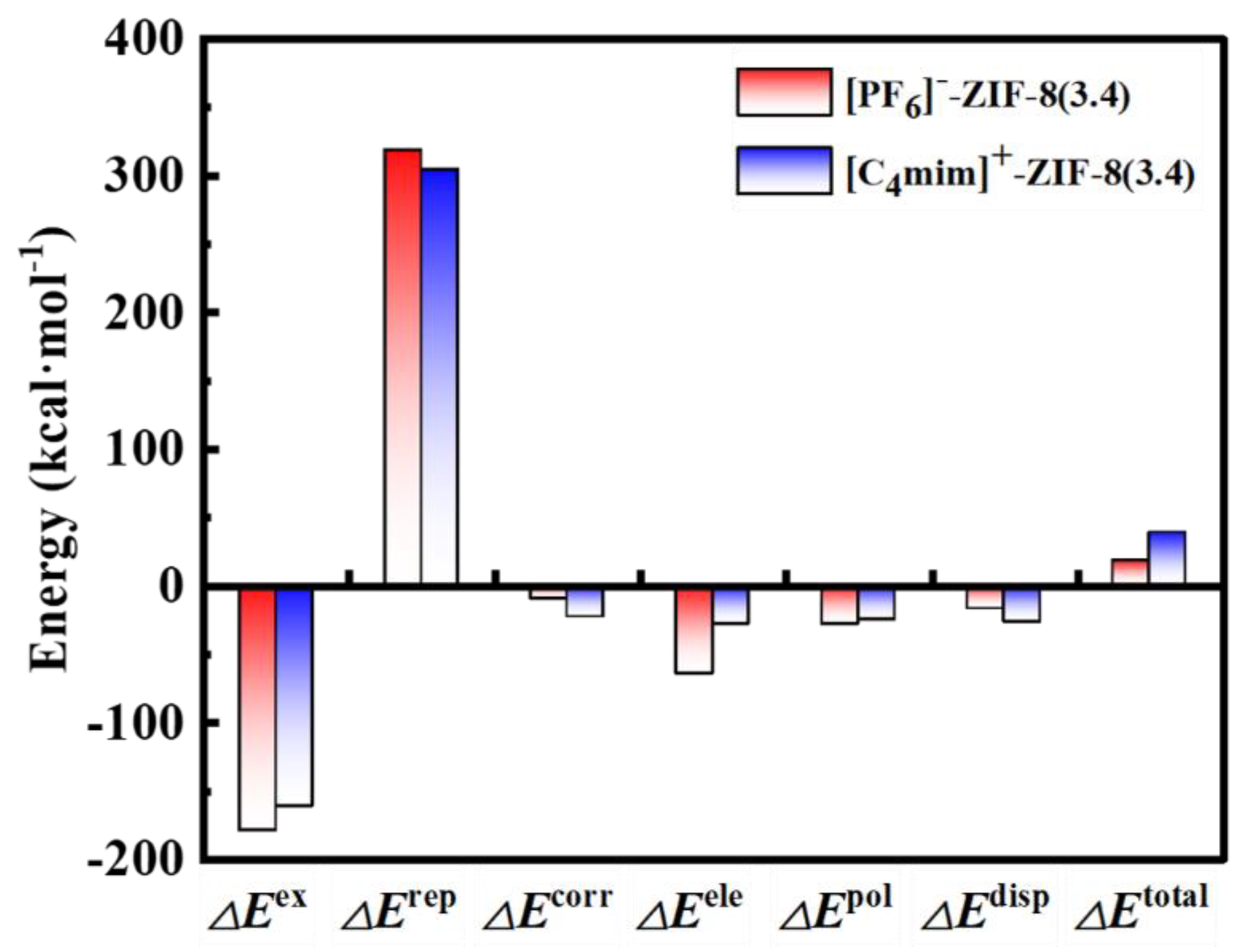
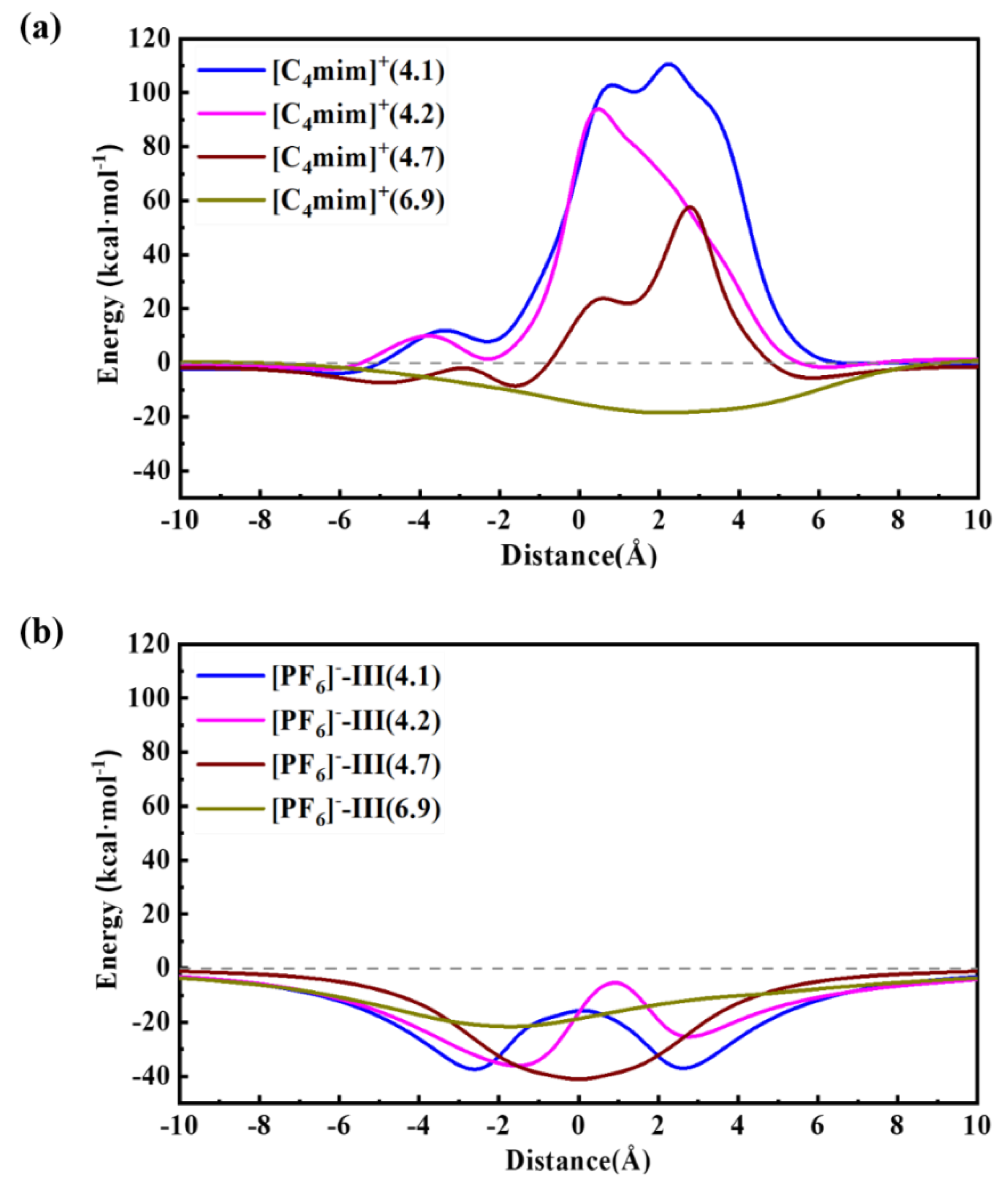
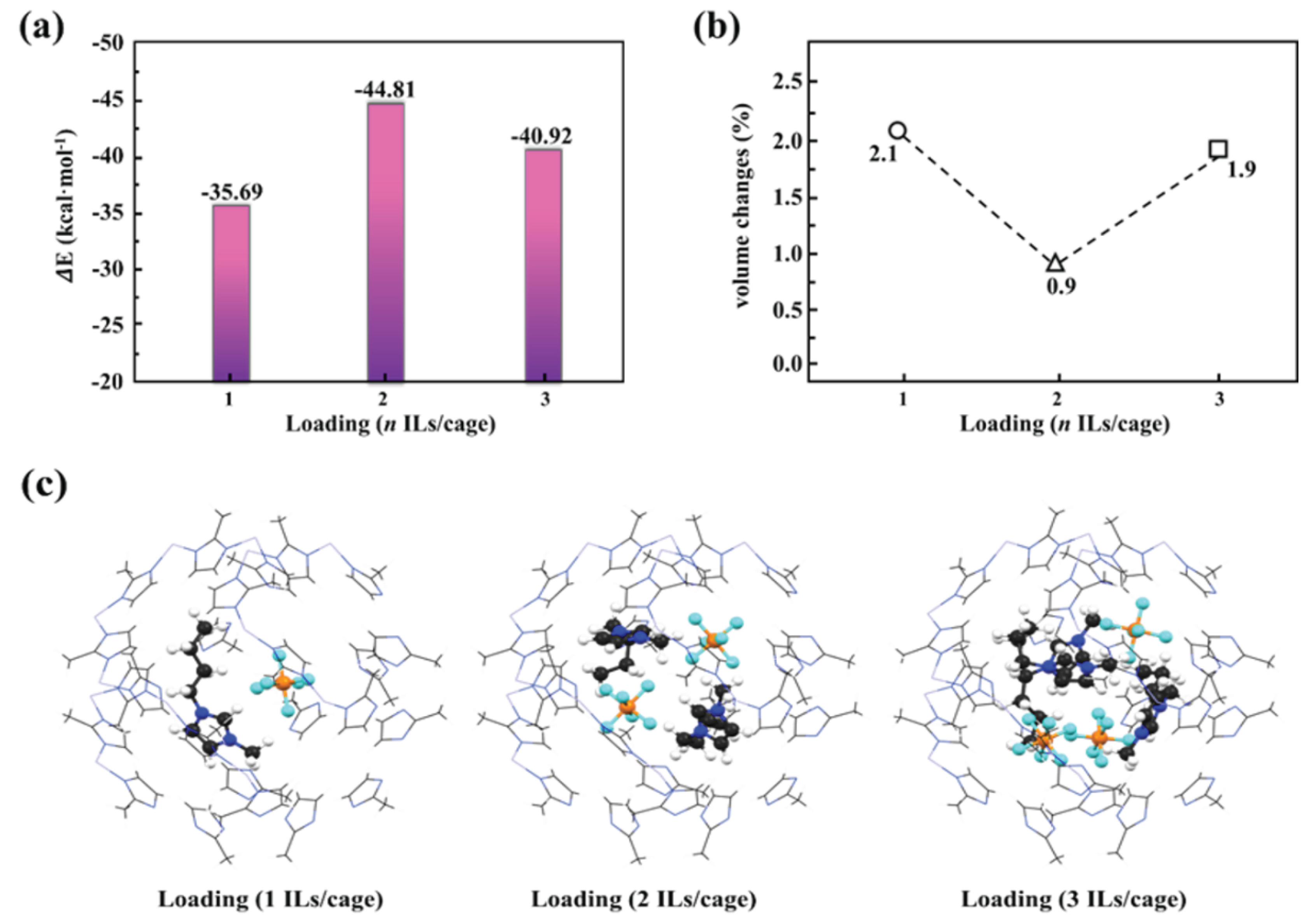
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Li, X.; Chen, K.; Guo, R.; Wei, Z. Ionic Liquids Functionalized MOFs for Adsorption. Chemical Reviews 2023, 123, 10432–10467. [Google Scholar] [CrossRef] [PubMed]
- Durak, O.; Zeeshan, M.; Habib, N.; Gulbalkan, H.C.; Alsuhile, A.A.A.M.; Caglayan, H.P.; Kurtoğlu-Öztulum, S.F.; Zhao, Y.; Haslak, Z.P.; Uzun, A.; Keskin, S. Composites of porous materials with ionic liquids: Synthesis, characterization, applications, and beyond. Microporous and Mesoporous Materials 2022, 332. [Google Scholar] [CrossRef]
- Wu, K.; Miao, X.; Zhao, H.; Liu, S.; Fei, T.; Zhang, T. Selective Encapsulation of Ionic Liquids in UiO-66-NH2 Nanopores for Enhanced Humidity Sensing. ACS Applied Nano Materials 2023, 6, 9050–9058. [Google Scholar] [CrossRef]
- Qian, Y.; Zhang, F.; Pang, H. A Review of MOFs and Their Composites-Based Photocatalysts: Synthesis and Applications. Advanced Functional Materials 2021, 31. [Google Scholar] [CrossRef]
- Friess, K.; Izák, P.; Kárászová, M.; Pasichnyk, M.; Lanč, M.; Nikolaeva, D.; Luis, P.; Jansen, J.C. A Review on Ionic Liquid Gas Separation Membranes. Membranes 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, E.; G. Saiz, P.; Peřinka, N.; Wuttke, S.; Fernández de Luis, R. Printed Capacitive Sensors Based on Ionic Liquid/Metal-Organic Framework Composites for Volatile Organic Compounds Detection. Advanced Functional Materials 2021, 31. [Google Scholar] [CrossRef]
- Tuffnell, J.M.; Morzy, J.K.; Kelly, N.D.; Tan, R.; Song, Q.; Ducati, C.; Bennett, T.D.; Dutton, S.E. Comparison of the ionic conductivity properties of microporous and mesoporous MOFs infiltrated with a Na-ion containing IL mixture. Dalton Transactions 2020, 49, 15914–15924. [Google Scholar] [CrossRef]
- Zeeshan, M.; Nozari, V.; Keskin, S.; Uzun, A. Structural Factors Determining Thermal Stability Limits of Ionic Liquid/MOF Composites: Imidazolium Ionic Liquids Combined with CuBTC and ZIF-8. Industrial & Engineering Chemistry Research 2019, 58, 14124–14138. [Google Scholar]
- Yoshida, Y.; Fujie, K.; Lim, D.W.; Ikeda, R.; Kitagawa, H. Superionic Conduction over a Wide Temperature Range in a Metal–Organic Framework Impregnated with Ionic Liquids. Angewandte Chemie International Edition 2019, 58, 10909–10913. [Google Scholar] [CrossRef]
- Li, H.; Tuo, L.; Yang, K.; Jeong, H.-K.; Dai, Y.; He, G.; Zhao, W. Simultaneous enhancement of mechanical properties and CO2 selectivity of ZIF-8 mixed matrix membranes: Interfacial toughening effect of ionic liquid. Journal of Membrane Science 2016, 511, 130–142. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Z.; Yang, C.; Liu, J.; Shen, H.; Yang, K.; Wang, Z. PIM-based mixed-matrix membranes containing MOF-801/ionic liquid nanocomposites for enhanced CO2 separation performance. Journal of Membrane Science 2021, 636. [Google Scholar] [CrossRef]
- Zeeshan, M.; Keskin, S.; Uzun, A. Enhancing CO2/CH4 and CO2/N2 separation performances of ZIF-8 by post-synthesis modification with [BMIM][SCN]. Polyhedron 2018, 155, 485–492. [Google Scholar] [CrossRef]
- Krokidas, P.; Moncho, S.; Brothers, E.N.; Castier, M.; Economou, I.G. Tailoring the gas separation efficiency of metal organic framework ZIF-8 through metal substitution: a computational study. Physical Chemistry Chemical Physics 2018, 20, 4879–4892. [Google Scholar] [CrossRef] [PubMed]
- Gulbalkan, H.C.; Haslak, Z.P.; Altintas, C.; Uzun, A.; Keskin, S. Assessing CH4/N2 separation potential of MOFs, COFs, IL/MOF, MOF/Polymer, and COF/Polymer composites. Chemical Engineering Journal 2022, 428. [Google Scholar] [CrossRef]
- Ban, Y.; Li, Z.; Li, Y.; Peng, Y.; Jin, H.; Jiao, W.; Guo, A.; Wang, P.; Yang, Q.; Zhong, C.; Yang, W. Confinement of Ionic Liquids in Nanocages: Tailoring the Molecular Sieving Properties of ZIF-8 for Membrane-Based CO2 Capture. Angew Chem Int Ed Engl 2015, 54, 15483–7. [Google Scholar] [CrossRef]
- Kinik, F.P.; Altintas, C.; Balci, V.; Koyuturk, B.; Uzun, A.; Keskin, S. [BMIM][PF6] Incorporation Doubles CO2 Selectivity of ZIF-8: Elucidation of Interactions and Their Consequences on Performance. ACS Applied Materials & Interfaces 2016, 8, 30992–31005. [Google Scholar]
- Koyuturk, B.; Altintas, C.; Kinik, F.P.; Keskin, S.; Uzun, A. Improving Gas Separation Performance of ZIF-8 by [BMIM][BF4] Incorporation: Interactions and Their Consequences on Performance. The Journal of Physical Chemistry C 2017, 121, 10370–10381. [Google Scholar] [CrossRef]
- Ali, S.A.; Khan, A.U.; Mulk, W.U.; Khan, H.; Nasir Shah, S.; Zahid, A.; Habib, K.; Shah, M.U.H.; Othman, M.H.D.; Rahman, S. An Ongoing Futuristic Career of Metal–Organic Frameworks and Ionic Liquids, A Magical Gateway to Capture CO2; A Critical Review. Energy & Fuels 2023, 37, 15394–15428. [Google Scholar] [CrossRef]
- Dong, K.; Liu, X.; Dong, H.; Zhang, X.; Zhang, S. Multiscale Studies on Ionic Liquids. Chemical Reviews 2017, 117, 6636–6695. [Google Scholar] [CrossRef]
- Chen, J.; Dong, K.; Liu, L.; Zhang, X.; Zhang, S. Anti-electrostatic hydrogen bonding between anions of ionic liquids: a density functional theory study. Physical Chemistry Chemical Physics 2021, 23, 7426–7433. [Google Scholar] [CrossRef]
- Kwon, H.T.; Jeong, H.-K.; Lee, A.S.; An, H.S.; Lee, J.S. Heteroepitaxially Grown Zeolitic Imidazolate Framework Membranes with Unprecedented Propylene/Propane Separation Performances. Journal of the American Chemical Society 2015, 137, 12304–12311. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Prakash, M. Tuning the CO2 adsorption by the selection of suitable ionic liquids at ZIF-8 confinement: A DFT study. Applied Surface Science 2019, 491, 633–639. [Google Scholar] [CrossRef]
- Mohamed, A.M.O.; Moncho, S.; Krokidas, P.; Kakosimos, K.; Brothers, E.N.; Economou, I.G. Computational investigation of the performance of ZIF-8 with encapsulated ionic liquids towards CO2 capture. Molecular Physics 2019, 117, 3791–3805. [Google Scholar] [CrossRef]
- Gao, W.; Zheng, W.; Sun, W.; Zhao, L. Understanding the Effective Capture of H2S/CO2 from Natural Gas Using Ionic Liquid@MOF Composites. The Journal of Physical Chemistry C 2022, 126, 19872–19882. [Google Scholar] [CrossRef]
- Thomas, A.; Ahamed, R.; Prakash, M. Selection of a suitable ZIF-8/ionic liquid (IL) based composite for selective CO(2) capture: the role of anions at the interface. RSC Adv 2020, 10, 39160–39170. [Google Scholar] [CrossRef] [PubMed]
- Kavak, S.; Polat, H.M.; Kulak, H.; Keskin, S.; Uzun, A. MIL-53(Al) as a Versatile Platform for Ionic-Liquid/MOF Composites to Enhance CO2 Selectivity over CH4 and N2. Chemistry – An Asian Journal 2019, 14, 3655–3667. [Google Scholar] [CrossRef]
- Zhang, Z.; Jia, X.; Sun, Y.; Guo, X.; Huang, H.; Zhong, C. Pore engineering of ZIF-8 with ionic liquids for membrane-based CO2 separation: bearing functional group effect. Green Chemical Engineering 2021, 2, 104–110. [Google Scholar] [CrossRef]
- Fairen-Jimenez, D.; Moggach, S.A.; Wharmby, M.T.; Wright, P.A.; Parsons, S.; Düren, T. Opening the Gate: Framework Flexibility in ZIF-8 Explored by Experiments and Simulations. Journal of the American Chemical Society 2011, 133, 8900–8902. [Google Scholar] [CrossRef]
- Zhang, C.; Lively, R.P.; Zhang, K.; Johnson, J.R.; Karvan, O.; Koros, W.J. Unexpected Molecular Sieving Properties of Zeolitic Imidazolate Framework-8. The Journal of Physical Chemistry Letters 2012, 3, 2130–2134. [Google Scholar] [CrossRef]
- Zeeshan, M.; Nozari, V.; Yagci, M.B.; Isık, T.; Unal, U.; Ortalan, V.; Keskin, S.; Uzun, A. Core–Shell Type Ionic Liquid/Metal Organic Framework Composite: An Exceptionally High CO2/CH4 Selectivity. Journal of the American Chemical Society 2018, 140, 10113–10116. [Google Scholar] [CrossRef]
- Chang, Y.; Wang, L.; Jiang, Z.; Zhang, R.; Zhu, H.; Zhang, D.; Zhu, J.; Kong, X.; Huang, H. Zwitterionic metal–organic framework with highly dispersed ionic liquid for enhancing CO2 capture. Separation and Purification Technology 2023, 326. [Google Scholar] [CrossRef]
- Yu, T.; Cai, Q.; Lian, G.; Bai, Y.; Zhang, X.; Zhang, X.; Liu, L.; Zhang, S. Mechanisms behind high CO2/CH4 selectivity using ZIF-8 metal organic frameworks with encapsulated ionic liquids: A computational study. Chemical Engineering Journal 2021, 419. [Google Scholar] [CrossRef]
- Yu, T.; Cai, Q.; Lian, G.; Liu, L. Molecular dynamics studies on separation of CO2/CH4 by the ionic liquids encapsulated ZIF-8. Journal of Membrane Science 2022, 644. [Google Scholar] [CrossRef]
- Weinhold, F.; Landis, C.; Glendening, E.J.I. r. i. p. c., What is NBO analysis and how is it useful? 2016, 35, 399–440. [CrossRef]
- Lu, T.; Chen, F. Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 2012, 33, 580–92. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA: a three-dimensional visualization system for electronic and structural analysis. Journal of Applied Crystallography 2008, 41, 653–658. [Google Scholar] [CrossRef]
- Su, P.; Jiang, Z.; Chen, Z.; Wu, W. Energy decomposition scheme based on the generalized Kohn-Sham scheme. J Phys Chem A 2014, 118, 2531–42. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Baldridge, K.K.; Boatz, J.A.; Elbert, S.T.; Gordon, M.S.; Jensen, J.H.; Koseki, S.; Matsunaga, N.; Nguyen, K.A.; Su, S.; Windus, T.L.; Dupuis, M.; Montgomery Jr, J.A. General atomic and molecular electronic structure system. 1993, 14, 1347–1363. [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J.J.T.J. o. p. c., Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. 1994, 98, 11623–11627. [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J Comput Chem 2011, 32, 1456–65. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Kühne, T.D.; Iannuzzi, M.; Del Ben, M.; Rybkin, V.V.; Seewald, P.; Stein, F.; Laino, T.; Khaliullin, R.Z.; Schütt, O.; Schiffmann, F.; Golze, D.; Wilhelm, J.; Chulkov, S.; Bani-Hashemian, M.H.; Weber, V.; Borštnik, U.; Taillefumier, M.; Jakobovits, A.S.; Lazzaro, A.; Pabst, H.; Müller, T.; Schade, R.; Guidon, M.; Andermatt, S.; Holmberg, N.; Schenter, G.K.; Hehn, A.; Bussy, A.; Belleflamme, F.; Tabacchi, G.; Glöß, A.; Lass, M.; Bethune, I.; Mundy, C.J.; Plessl, C.; Watkins, M.; VandeVondele, J.; Krack, M.; Hutter, J. CP2K: An electronic structure and molecular dynamics software package - Quickstep: Efficient and accurate electronic structure calculations. The Journal of Chemical Physics 2020, 152. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zheng, W.; Yan, X.; Dai, Y.; Ruan, X.; Yang, X.; Li, X.; Zhang, N.; He, G. Ionic liquid tuning nanocage size of MOFs through a two-step adsorption/infiltration strategy for enhanced gas screening of mixed-matrix membranes. Journal of Membrane Science 2020, 605. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [PubMed]
- Bühl, M.; Wipff, G. Insights into Uranyl Chemistry from Molecular Dynamics Simulations. ChemPhysChem 2011, 12, 3095–3105. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




