1. Introduction
With the enhancement of aesthetic requirements and the development of novel dental materials, tooth-colored materials like composite resins (CRs) have been widely used in dentistry. Due to their strong mechanical properties and good aesthetic characteristics, CRs have gradually become the most commonly used filling and adhesive materials in clinic, surpassing the use of traditional silver amalgam and the glass ionomer cement (GIC) [
1,
2,
3]. However, the occurrence of secondary caries at the edge of the CR restorations has become the main cause of secondary filling treatments [
4]. Furthermore, the secondary filling operations often produce more damage to the hard tissues of teeth, as well as more thermal or chemical stimulus to the dental pulps, which may also lead to the filling failures [
5]. It was reported that the possibility of secondary caries occurring around the CRs was even greater than that using other filling materials such as the GIC or the amalgam [
6,
7]. Thus, secondary caries has become an urgent problem in dental clinic. The replacement of CRs has undoubtedly become a heavy burden on the expenditure on health.
Moreover, the polymerization shrinkage occurring in the curing procedure also makes the application of CRs in posterior teeth more stressful, especially in repairing the extensive dental defects [
8]. In order to improve the defects of CRs, studies have been conducted to reduce the polymerization shrinkage by improving the filling technique, using the bonding agents in combination [
9,
10,
11]. However, the complexity of filling procedure may not only increase the operating time but also improve the technical sensitivity [
12,
13]. Hitherto, there are still no new materials or new technologies that can avoid the microleakage occurring at the edge of the CRs completely. On the other hand, as far as we know that bacterial infection is considered the main cause of secondary caries after restoration, yet the specific cariogenic bacteria and the pathological process are not clear. Thus, adjusting the compositions to develop novel CRs with antibacterial properties has also become one of the research hotspots currently [
14].
Back to the early 20
th century, the application of fluoride agents had become an important way to maintain oral health [
15]. The anti-caries property of fluoride agents is mainly achieved by maintaining a certain concentration of fluoride ions in saliva locally, which forms a mineralization system with calcium ions and phosphate ions together. Calcium fluoride and fluorapatite generated can inhibit the demineralization and promote remineralization process. Furthermore, fluoride can also act on the acid-producing bacteria such as mutans streptococci (S. mutans) directly. While blocking the functions of enzymes related to glycolysis and cellular oxidation, fluoride also inhibits the intake of glucose by the bacteria. Fluoride has been proved to play an excellent anti-caries property in the traditional GIC, and has also been tried to be used in the development of novel antibacterial CRs nowadays [
16]. Since the 1980s, fluoride-containing CRs have been used in the orthodontic adhesives and the fissure sealants, while there were few studies on them as direct filling materials [
17]. But it is worth noting that the release of fluoride ions may result in the porous structure of the material, which will weaken the mechanical properties and wear resistance [
18].
In recent years, nano-zirconia powders have been applied as inorganic fillers to enhance the mechanical properties of CRs. On the one hand, as one of the commonly used reinforcing materials in biomedicine, zirconia presents excellent strength and biocompatibility. On the other hand, addition of nanoparticles is conducive to increase the aesthetic performance and wear resistance of materials. Furthermore, it was suggested that nanoparticles possess stronger prevention in bacterial adhesion and biofilm formation due to their large surface-volume ratio [
19]. Some commercial CRs products contained nano-zirconia fillers have been used in clinic so far, yet few studies focused on their antibacterial property. Thus, we proposed a hypothesis that combination fluoride with nano-zirconia through a certain way in the fillers of a novel CR would do help to maintain the clinical performance while exerting good fluoride-releasing property. In the early stage of our research, ammonium zirconium hexafluoride was added into zirconium salt as the source of fluoride ions. A kind of high-purity fluoride-doped nano-zirconia particles were synthesized through chemical precipitation and the calcination procedure. A series of experiments were carried out to prove that both the nanoparticles and the novel CR loaded with fluoride-doped nano-zirconia fillers possessed definite release of fluoride ions, and the released ions could inhibit the growth of S. mutans effectively [
20].
In the present work, the composition of fillers was optimized furtherly according to the preliminary results. Fluoride release from the experimental CR into media with different pH values were measured to simulated the caries processes. The action mode of antibacterial effect was explored here. Furthermore, thermocycling test were used to simulate the intraoral aging process of the experimental CR. The mechanical performance and cytotoxicity were also evaluated to verify its clinical application prospects.
2. Materials and Methods
Fluoride-doped nano-zirconia particles were coated with the silane coupling agent KH-570 to improve the interfacial combination between the fillers and resin matrix. The resin matrix consisted of a 70:30 (w/w) bisphenol-A glycidyl dimethacrylate (Bis-GMA) and tri-ethylene glycol dimethacrylate (TEGDMA). The initiator system consisted of 0.5 wt% camphorquinone (CQ) and 1 wt% ethoxylated bisphenol A dimethacrylate (DMAEMA). The experimental CRs were formulated with the resin matrix and varying concentrations of the silaned fillers (25, 50 wt%) through in situ dispersion method. The mixtures were filled to Teflon molds and light cured for 40 seconds on both sides (1200mW/cm2, Elipar S10, 3M Espe, Seefeld, Germany). For comparison, specimens of the pure resin matrix without fillers were also prepared in the same way.
One specimen of each group of the experimental CRs selected and coated with electrically-conductive material. The surface morphology and the dispersion of nanoparticles in CRs were investigated by Field emission scanning electron microscopy (FESEM, MERLIN, ZEISS, Gemany).
Mechanical Properties
Specimens of size 25 mm × 2 mm × 2 mm were prepared in each group (N=5). Before the test, every specimen was polished sequentially using #800, #1000, #2000, and #3000 abrasive papers and then stored in the saline solution at 37 ℃ for 24 hours. After drying thoroughly, the specimens were placed on the universal mechanical testing machine (Instron 5566, Instron, UK) for flexure strength test at a span length of 20 mm and a speed of 0.5 mm/min. At the same time, the fragmentation load was recorded and the flexure strength was calculated according to the formula: FS = 3Fl/2bh2. (Where FS means the flexure strength (unit: MPa), F means the fragmentation load (unit: N), l means the span length, b and h mean the width and height of specimen (unit: mm)).
Cylindrical specimens with a diameter of 10 mm and a height of 5 mm were prepared (N=3) and polished sequentially. After stored in the saline solution at 37 ℃ for 24 hours and dried, each specimen was fixed on the mechanical reciprocating friction and wear device (MWF-02, sdbaohang machinery manufacturing co., ltd, China). A Co-Cr stainless steel ball with a diameter of 5 mm was used as the grinding part, and the abradant was prepared by mixing 4:1 (w/w) fluorite powder and water. A 30 N loading force was applied to the specimens during 6000 friction cycles, and the speed of the reciprocating friction movement was 300 rpm/min. The digital micrometer was used to measure the height of specimens before and after the test. Moreover, one specimen was selected randomly to observe the microscopic morphology of the wear surface under SEM.
Fluoride Release
Fluoride release of the experimental CRs into various storage media with different pH values during 28 days was determined using a pH meter (a-AB41PH ZH, OHAUS, China) with fluoride ion selective electrode (F, STISE22, OHAUS, China). Three specimens of each group were eluted in either 2 mL distilled water, 2mL acidic buffer (20 mM KCl, 154 mM NaCl, 3.6 mM NaH2PO4·H2O, pH 4.2) or 2 mL neutral buffer (1.9 mM CaCl2, 30mM KCl, HEPES, pH 7.0). All samples were stored in a shaker at 37 ℃. The extraction media were collected in the 1st, 3rd, 7th ,14th and 28th days and exchanged by fresh storage media at the time of each measurement. The fluoride ions concentration in the extraction media was measured. Before determination of the samples, the selective electrode was fully activated and calibrated with a series of fluoride standards ranging from 0.001 ppm to 100 ppm.
Antibacterial Property
Streptococcus mutans (S.mutans UA159, Guangdong Microbial Culture Collection Center, China), which was considered as the primary cariogenic bacteria, was used as the experimental strain. The frozen strain was revived and incubated in sterile Brain Heart Infusion broth-agar (BHI-agar, BD, USA) media under anaerobic condition at 37 ℃. A single bacteria colony was selected and transferred into fresh BHI media before the experiment. The microplate reader (Elx800, BioTek, USA) was used to regulate and control the absorbance of S.mutans strain at the wavelength of 600 nm.
Specimens with 8mm in diameter and 2 mm thick of each group were prepared (N=3) and sterilized by ultraviolet light for 2 hours before the antibacterial test. The specimens of Resin without the experimental fillers were prepared and taken as control group. All specimens were placed in a sterile 48-well plate with 500 uL fresh BHI media and 50 uL of bacterial suspension (1 × 106 CFU/mL). After incubated for 24 hours at 37 ℃, the bacteria grow and form biofilms on the surface of specimens. Then, the specimens were transferred to another 48-well plate and washed gently with PBS to get rid of the planktonic bacteria. The biofilm formed on the surface of each specimen was collected by shaking and washing with 500 uL fresh BHI media. And the planktonic bacteria in the original 48-well plate were also collected and mixed well. All experimental bacteria suspensions were diluted to 10-5 times and inoculated on BHI-agar plates to count the bacteria colonies.
The metabolic activities of both planktonic bacteria and biofilm were estimated by the Cell Counting Kit-8 assay (CCK-8, Dojindo, Japan). After co-culturing with the experimental CRs for 24 hours, the planktonic bacteria and biofilm were collected and transferred to a new 48-well plate by the method as above-mentioned in 2.7.1. 50 uL of CCK-8 liquid was added into each well and incubated for another 2 hours in the incubator at 37 ℃. Finally, the absorbance at 450 nm was determined by the microplate reader (Elx800, BioTek, USA).
Aging Test
Specimens with 8mm in diameter and 2 mm thick of each group were prepared (N=3) for thermal aging test. No thermal aging status was denoted as time T0. Then the specimens were subjected to 10000 cycles of thermocycling between 5 ℃ and 55 ℃ with a transfer time of 30 s, which was applied to simulate the thermal aging in 1 year. The time at which the 10000 cycles ended was denoted as T1.
Color values of the specimens were measured at T
0 and T
1, respectively, using a tooth color comparator (VITA Easyshade V, VITA Zahnfabrik H. Rauter GmbH & Co.KG, Germany) in 3-point measurement mode. The color comparator was recalibrated before each measurement. After all measurements, average of the CIELab values (L*a*b*) was calculated for each specimen. And the ΔE00 value was calculated with the online color calculator (CIEDE2000 color system,
www.colormine.org) to show the color change from before to after the thermo aging procedure.
The microhardness values of all specimens were measured using a microhardness (Vickers) testing device (Micro Hardness Tester HMV-G-FA, SHIMADZU, Japan) at T0 and T1. A load of HV0.1 (980.7mN) was applied to the surface of each specimen in 3 points selected randomly for 10 seconds. And averages of the microhardness values before and after thermal aging procedure were calculated.
Cytotoxicity
Specimens with 8mm in diameter and 2 mm thick of each group were prepared (N=5) and sterilized with 75 % ethanol for three times. Each specimen was immersed into Dulbecco’s modified Eagle’s medium (DMEM) high glucose medium (HyClone, USA), which contained 10 % fetal bovine serum (Gibco, USA) and 1 % pecicillin-streptomycin (EveryGreen, China). Then, the eluates were collected after the incubation at 37 ℃ for 3 days. A total of human dental pulp cells (HDPs) was seeded per well in a 96-well plate and cultured with DMEM media. After incubated at 37 ℃ for 24 hours, the media was replaced with the eluates of each group. Cells incubated in the pure media without eluates were used as the negative control. The alamar blue kit (Invitrogen, USA) was used for cytotoxicity test according to the manufacturer’s instruction. Fluorescence intensities of HDPs at 1, 3, 5 and 7 days in response to the eluates of experimental CRs were measured using the microplate reader.
Statistical Analysis
The data obtained were analyzed with SPSS Statistical software (version 25.0, IBM, Armonk, USA). One-way analysis of variance (ANOVA) and Post hoc analysis for multiple comparisons were performed. P < 0.05 was considered as statistically significant.
4. Discussion
Dental composite resins (CRs) are the most widely used direct restorative materials in clinic because of their high mechanical strength and aesthetic superiority. However, as the traditional CRs has little resistance to bacterial infection, which was considered as the major cause of dental caries, secondary caries might occur adjacent to the CRs restoration margins and shorten their lifespans ultimately. Thus, the development of antibacterial CRs is one of the most important investigations regarding novel dental materials.
The antibacterial action of fluoride agents against cariogenic bacteria has been widely appreciated by previous studies. Fluoride ions in saliva can diffuse into bacterial cells in the form of HF, where they decompose into both hydrogen ions and fluoride ions. This process can not only inhibit the action of enzymes directly but also stimulate more HF to diffuse into the cells [
21]. In addition, the increase of hydrogen ions within the cells can reduce bacterial acid production[
22]. Given the definite antibacterial effect of fluoride agents, various fluoride-releasing restorative materials have emerged and were considered as fluoride reservoirs, which might increase the fluoride level locally [
23,
24]. To date, addition of fluoride-containing inorganic fillers was considered as the major method to develop novel fluoride-releasing CRs. Both the soluble salts such as calcium fluoride (CaF
2) and the slightly soluble salts like ytterbium fluoride (YbF
2) and fluoro-alimino-silicate glass (FAG) had been applied in previous studies. However, it was reported that most of them showed a “burst release” of fluoride ions and the dissolution of fluoride agents had an adverse effect on the mechanical properties [
18]. It is not hard to spot that those inorganic fluoride agents were added by mixing with other fillers and there were only physical compatibilities among all particles, without chemical combination. Thus, the release of fluoride might damage the dense structure of the materials and furtherly declined the strength. It is necessary to improve the chemical structure of inorganic fillers to replace the way of adding fluoride agents directly. Cheng et al. produced a kind of core-shell nanofibers containing sodium fluoride (NaF) and used as fillers of CRs [
25]. Results showed that fluoride releasing with minor burst release could be achieved, which was quite superior to the case of adding NaF nanocrystals directly. Similarly, a novel LiAl-F layered double hydroxide (LDH) was also developed by Su et al. and was supposed to be a fluoride reservoir filler for CRs [
24]. However, this kind of researches were still in the early stage of exploration and were expected to be carried on furtherly.
Although the best way of developing fluoride-releasing CRs has not yet been concluded, the previous studies suggested that it was of necessity to develop a novel CRs with efficient fluoride-releasing effect and proper mechanical properties. Recently, nano-zirconia particles have been used as the reinforcing fillers in dental CRs [
26]. Zirconium salts, which were a kind of raw compositions for the synthesis of zirconia particles, has been proven to exhibit strong chelate formation characteristics. It could form coordination bonds with multiple fluoride ions, forming a highly efficient “fluoride ions receptors” [
27]. For this reason, zirconium salts were frequently used for fluoride removal in drinking water [
28]. Burgess et al. introduced a novel monomer with zirconium fluoride chelate and confirmed it could also be applied to dental resin-based materials [
29]. Furthermore, in the process of plasma fluorination of yttrium stabilized zirconia, Wolter et al. observed that the content of oxygen atoms gradually decreased with the increase of fluorine atoms in X-ray photoelectron spectroscopy [
30]. The result indicated that fluorine atoms might replace oxygen atoms to occupy the spatial position in zirconia cells and form chemical bond with zirconia, without destroying the microstructure of zirconia cells. In the preliminary study, a kind of novel CR loaded with fluoride-doped nano-zirconia (F-zirconia) particles was prepared and proved to show proper fluoride-releasing and good antibacterial property. Furtherly, the effect of pH values on fluoride release and the action mode of antibacterial activities were explored here. In addition, the mechanical performance and aging properties, as well as the cytotoxicity of the experimental CR were also evaluated [
20].
Previous studies revealed that addition of nano-zirconia fillers could improve the mechanical strength of CRs, but might also reduce the transparency. When the content of nano-zirconia fillers reached 55 wt%, the CRs could not be cured completely through light-curing [
26]. Thus, we prepared two groups of experimental CRs loaded with 25 wt% or 50 wt% F-zirconia fillers, respectively.
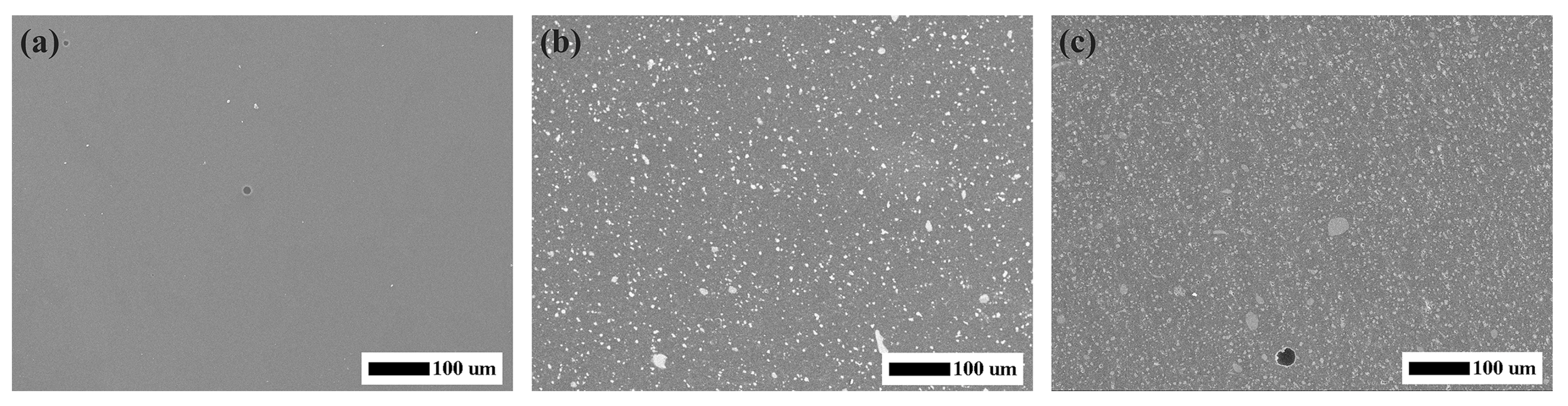
Figure 1 showed the SEM micrographs of the experimental CRs, and it could be seen that nano-fillers distributed well over the whole surface of resin matrix. Good dispersibility of fillers was attributed to the mechanical strength of CRs [
31]. As presented in
Table 1, the three-point bending test showed that the addition of F-zirconia fillers could maintain the flexure strength of experimental CRs. The flexure strengths of all three groups of CRs were > 80 MPa, which could meet to the ISO 4049 standard [
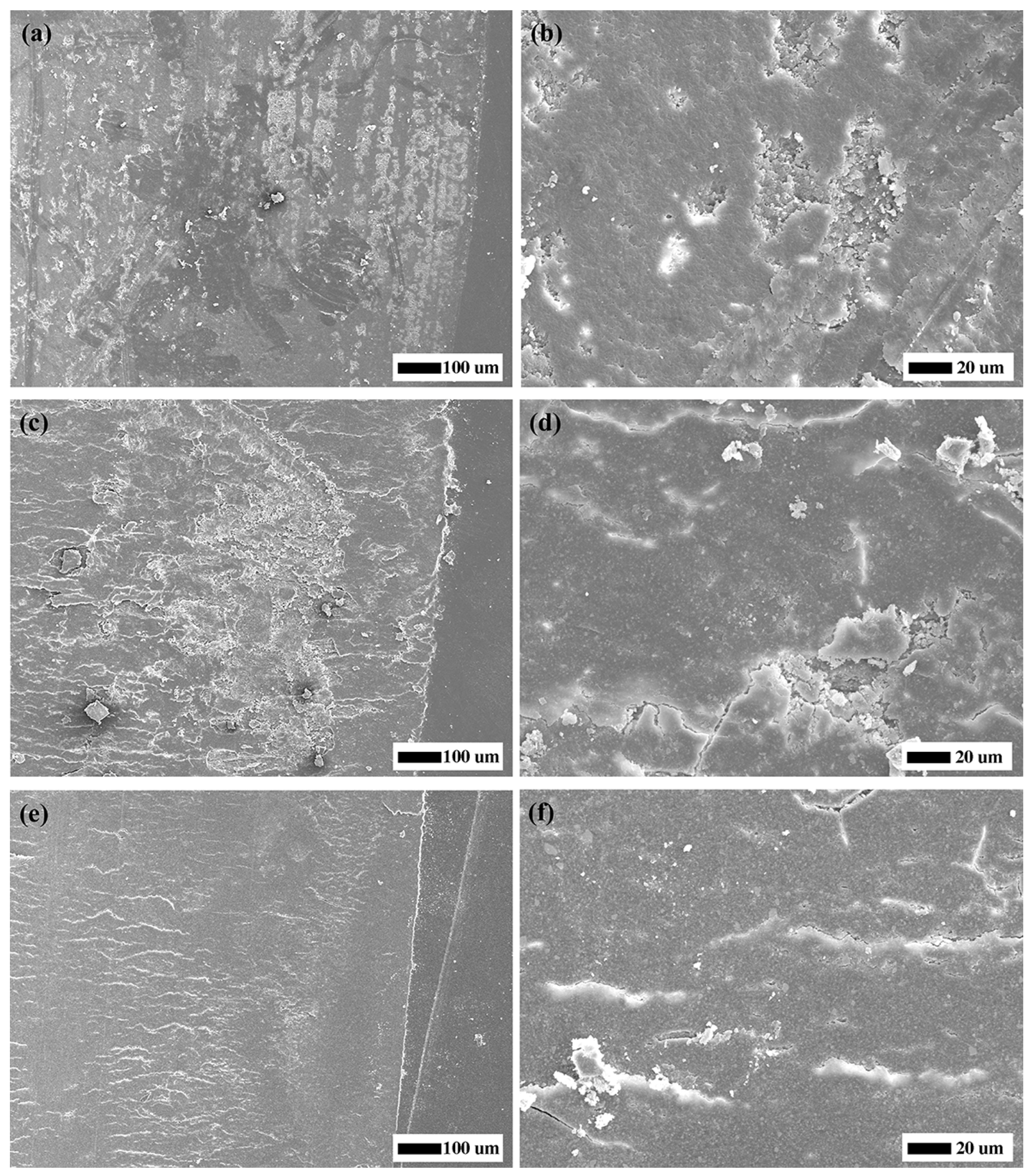
32]. Furtherly, compared to the control group, addition of F-zirconia fillers could significantly improve the wear resistance of experimental CRs. With the increase of F-zirconia fillers, height loss in the surface wear zones decreased significantly and the wear zones were relatively flat (
Table 1 and
Figure 2).
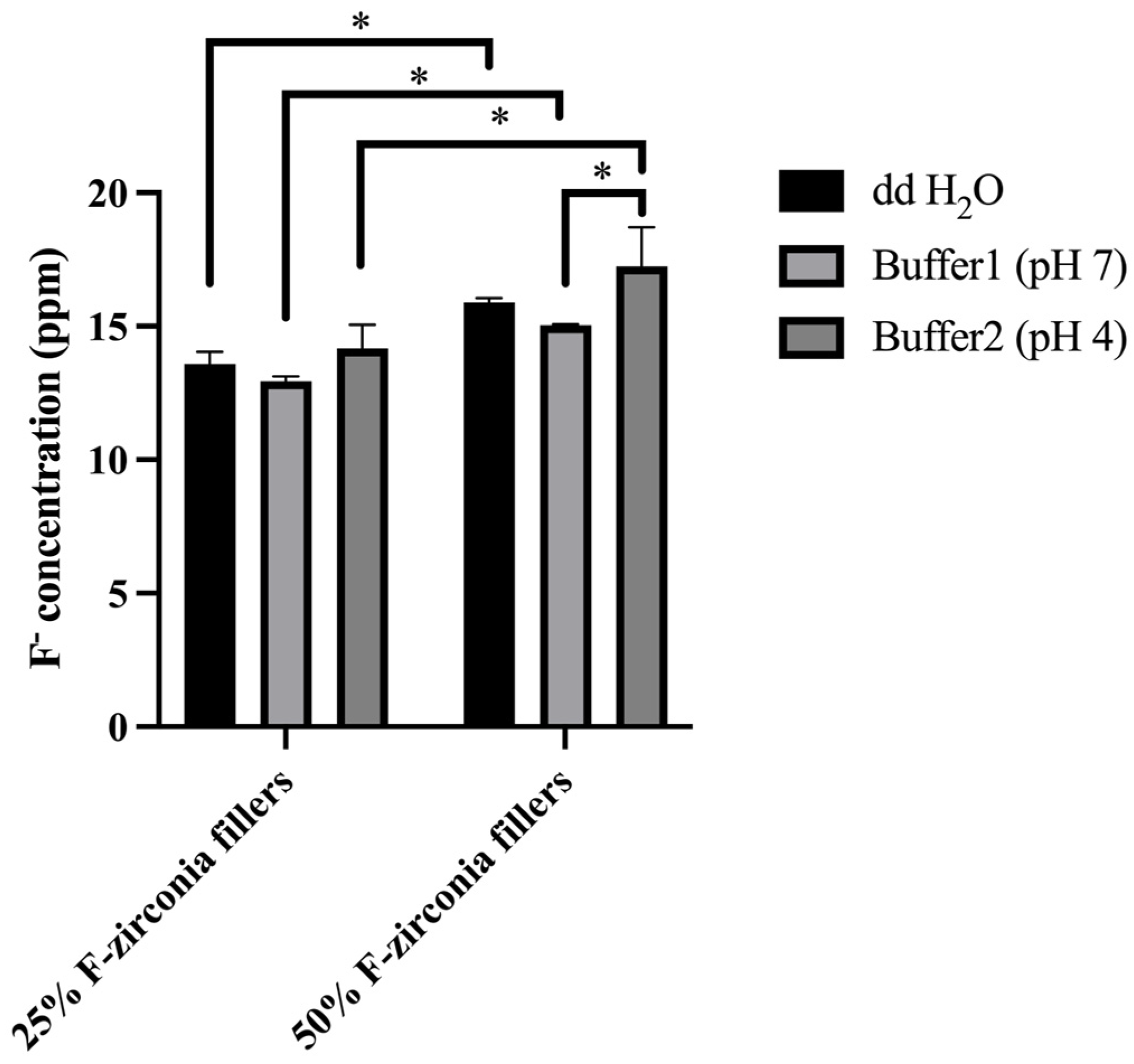
The daily and cumulative amounts of fluoride release from the experimental CRs were presented in
Table 2 and
Figure 3. In general, the two groups of experimental CRs showed continuous fluoride release within 28 days, and the fluoride release was positively correlated with the content of F-zirconia fillers. The significantly higher amounts of fluoride release were observed during the first 7 days. And the daily amounts of fluoride release decreased in day 14 and 28. It was considered that the fluoride ions were dissolved from the surface of specimens in the early stage. Subsequently, a longer time was required for the fluoride ions to diffuse from the inner part of specimens. The trend of fluoride release here was consistent with that in other similar studies [
33,
34]. Compared with the traditional GICs, which contained a large amount of aluminum fluosilicate fillers, the experimental CRs showed lower fluoride release, relatively. But it was reported that only 0.03 ppm to 0.07 ppm fluoride ions could be contributed to transform teeth demineralization to the remineralization phase [
35]. Moreover, Marquis R.E. suggested that fluoride ions could inhibit oral bacteria when the concentration reached 0.1mM [
36]. Thus, all observations during 28 days were within the expected range.
Meanwhile, the influence of various pH values on the release of fluoride ions from the experimental CRs was also investigated here. It was discussed that fluoride might interfere with the dynamics of dental caries process
in vivo, so
in vitro models that could simulated the caries process were recommended to test the effects of fluoride-releasing materials [
37,
38]. One of the most important causes of dental caries is the dissolution of acids produced by cariogenic bacteria [
39]. And it was reported that the incidence of caries increased significantly when pH value dropped to 4.0 ~ 5.5 [
40]. Thus, it is of necessity to verify the fluoride release property of the experimental CRs in acidic media. As shown in
Table 2 and
Figure 3, it was interesting to find that the amount of fluoride release in the deionized water (ddH
2O) was higher than that in the neutral buffer (pH 7.0). Like in previous studies, ddH
2O was used as an accurate model of fluoride release from dental materials, and a reduced amount of fluoride release could be found when using neutral buffer or artificial saliva [
41,
42]. The reason for this was likely to be the multi-ionic environment accelerated the equilibration of fluoride ions. It could also be found that the amount of fluoride release increased in the acidic buffer (pH 4.2) in this study, but the change was not significant. Therefore, the result revealed that intraoral fluoride release from the experimental CRs might be enhanced on restorations or caries surfaces that were eroded by plaque-associated acids.
Among various of bacterial species in the oral environment, S. mutans was proven to play a major role in the formation of plaque biofilms and the development of caries [
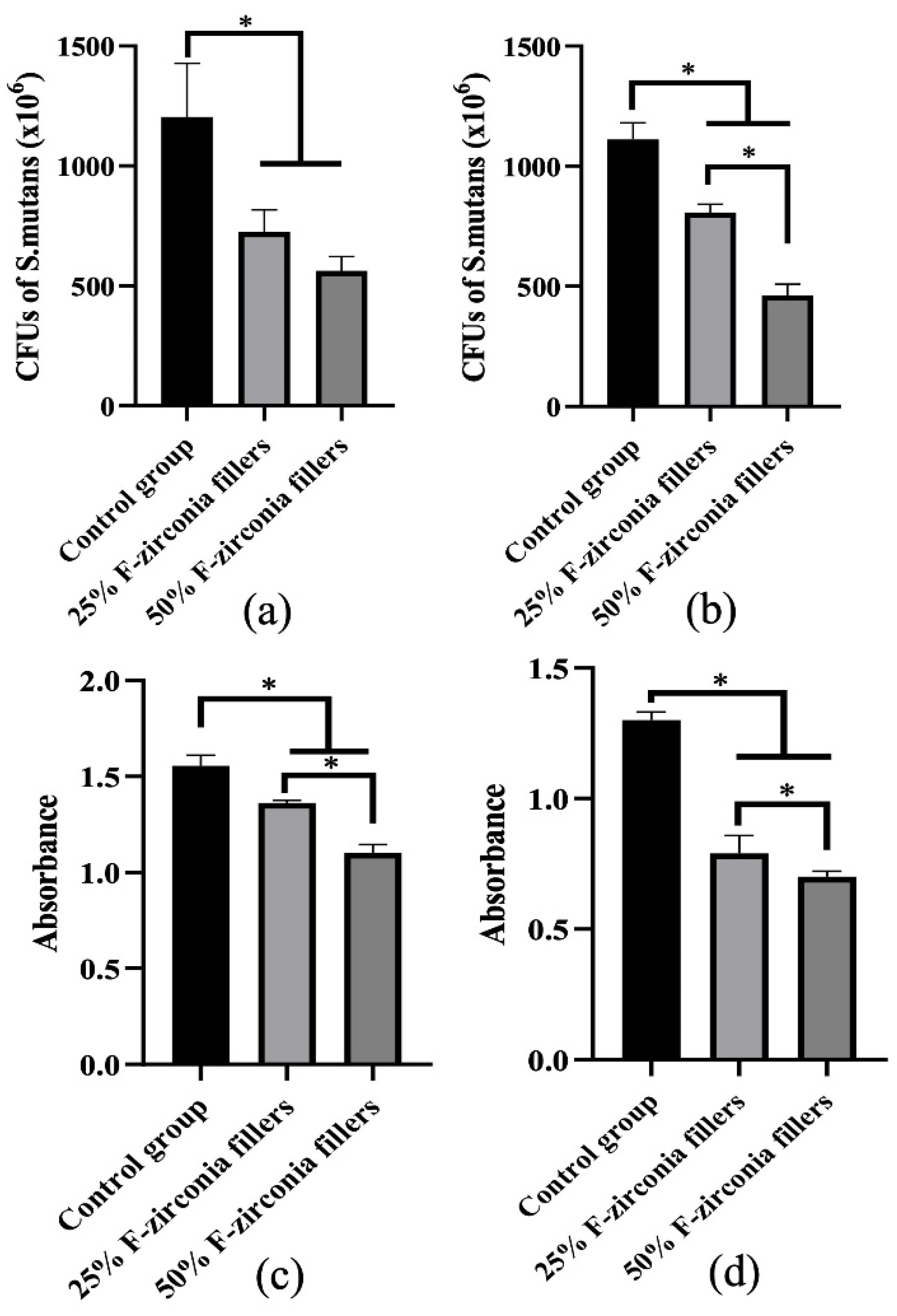
43]. Therefore, antibacterial effects of the experimental CRs were investigated against S. mutans biofilms on the surfaces, as well as the planktonic bacteria. The results of antibacterial effect were shown in
Figure 4. Compared with the control group, all two experimental groups showed obvious decrease in S. mutans colonies when against whether the biofilms or the planktonic bacteria. Furtherly, the result of CCK-8 assay confirmed that the experimental CRs effectively inhibited the metabolic activities of S. mutans, and the antibacterial effect was enhanced with the increase of fluoride-doped nano-zirconia fillers content. In fact, a previous research suggested that the antibacterial effect of fluoride agents occurred by the diffusion of fluoride ions [
44]. Nonetheless, the result here indicated that the experimental CRs could inhibit the growth of S. mutans not only by releasing of the fluoride ions in the media but also through direct contact with the biofilms on the surfaces. The large surface-to-volume ratio of nanoparticles on the surfaces of specimens could help to inhibit the bacterial adhesion and biofilm formation [
4]. Moreover, as the CRs wearing out slowly during the chewing movement, more fluoride-doped nano-zirconia fillers shall be exposed to the oral environment and show antibacterial effect continuously.
Thermal cycling process was applied to simulate the aging of dental materials during temperature fluctuations that occur in the mouth. It was carried out at temperatures equivalent to the intraoral temperature, ranging from 5 ℃ to 55 ℃, and 10000 cycles might be equivalent to 1 year [
45]. Color change (ΔE00 value) and microhardness of the experimental CRs affected by aging were presented in
Table 3 and
Table 4. The color stability of restoration materials was important to meet the esthetic demands in dentistry. It was reported that a ΔE00 values between 0.8 and 1.8 were considered acceptable for color changes in clinic that could be detected by the human eyes [
46]. Based on the results in this study, the control group and the experimental groups had the average ΔE00 values of 1.69, 1.20 and 0.78, respectively, which were all within the acceptable range. The control group showed the highest ΔE00 value. The resin matrix, especially the TEGDMA monomer, might be the major reason for color change. It was indicated that TEGDMA exhibited high hydrophilicity and interfere with the color stability [
47]. Addition of the fluoride-doped nano-zirconia fillers could reduce the color change of experimental CRs. Moreover, it was observed that the microhardness values of all specimens decreased slightly after the thermal aging progress. This might be the result of cracks in the cross-linking of resin structure and the weak bonding between the matrix and fillers. But the variation of microhardness values among three groups was not statistically different.
As we all know, the restorative procedure was usually associated with loss of significant amounts of hard tissues due to caries attack. Application of composite resin following caries removal would contribute to potential adverse effects on the pulp tissue. Jiang et al. [
48]studied the impact of various dentin thicknesses on the cytotoxicity of three commercial restorative materials in vitro and showed that one of the materials reached cytotoxic levels when the thickness was at 1 mm. It is of significance to evaluate the cytotoxicity with cells in pulp tissue. As such we chose human dental pulp cells (HDPs) in cell culture here like previous studies [
49,
50]. Statistical results as showed in
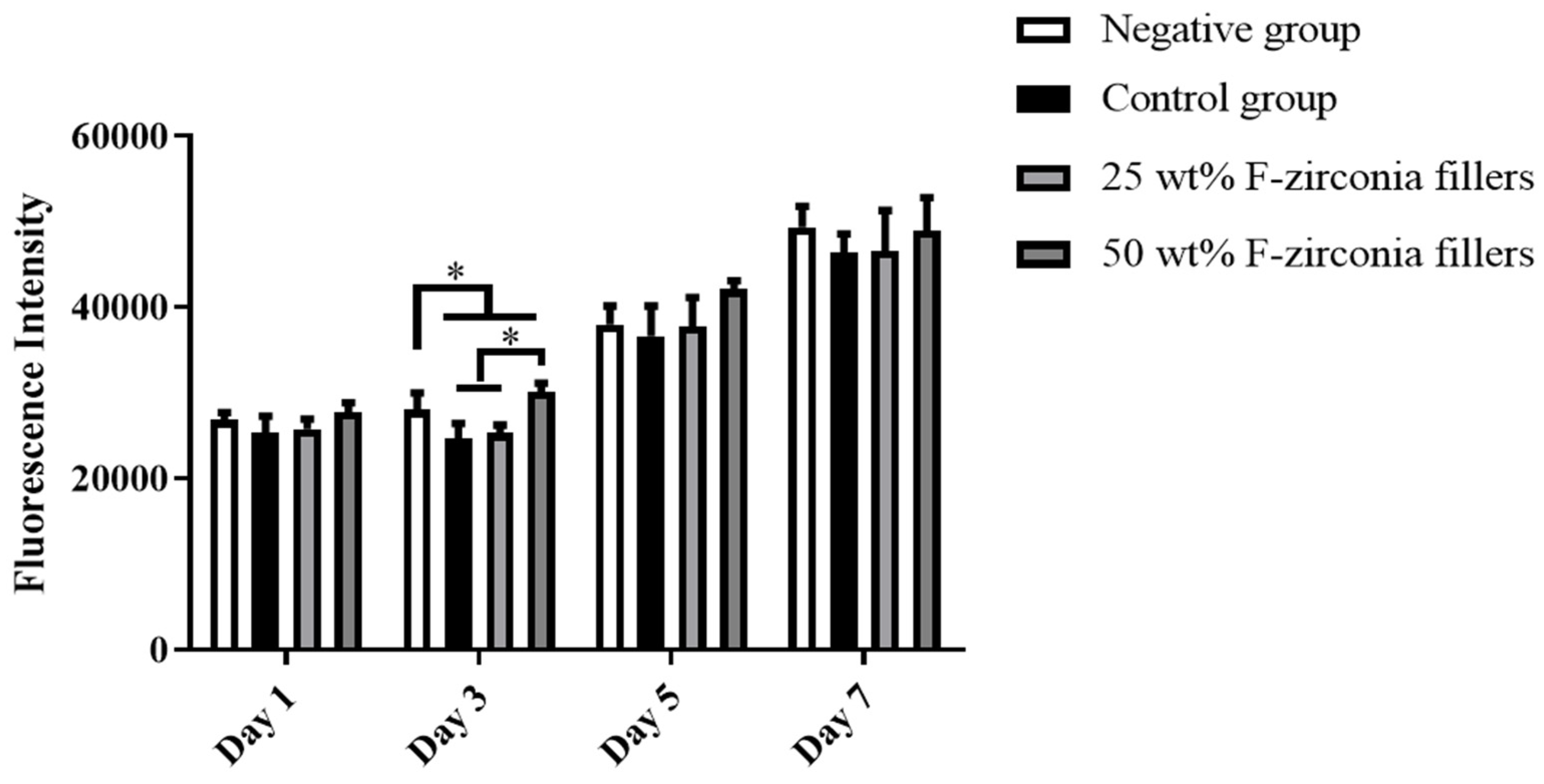
Figure 5 revealed that the viabilities of HDPs exposed to eluates of the experimental CRs were not significantly different from that of the negative group. Therefore, the experimental CRs developed here showed no significant cytotoxicity and was qualified for application in clinic.
There are still some limitations in this study. Given the anti-caries effects of fluoride ions include both inhibition of the cariogenic bacteria and remineralization of dentin or enamel, additional investigations are indeed required to clarify the remineralization of the novel CRs. In addition, the experimental conditions can not fully simulate the oral environments, which are also affected by the washing process of saliva and the effect of enzymes. Thus, further in vivo tests are needed before clinic application.