Submitted:
27 February 2024
Posted:
28 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study design and population
2.2. Sampling
2.3. Serological analysis
2.4. Statistical analysis
3. Results
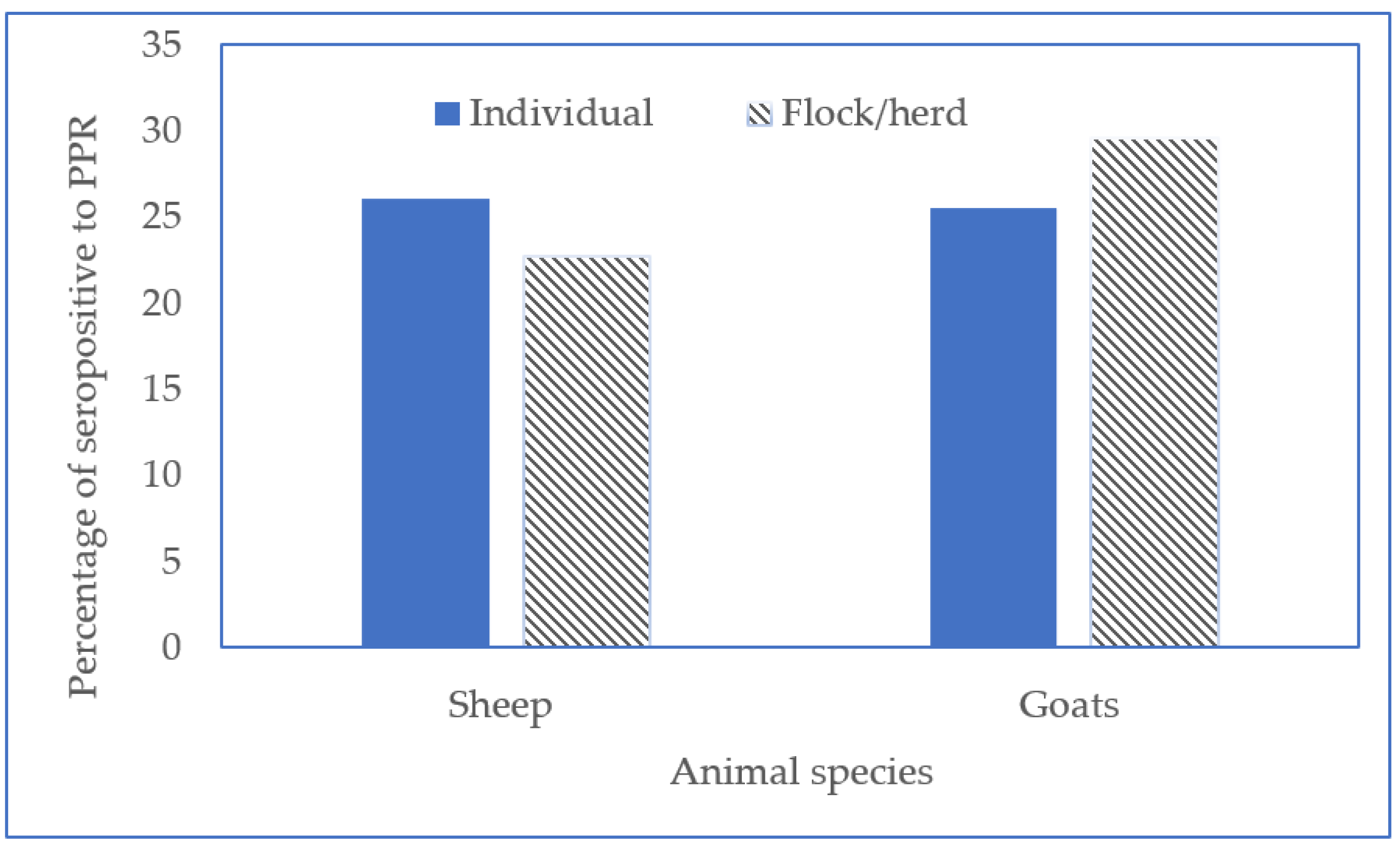
3.1. Prevalence of PPR
3.2. Risk factors analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OIE. Peste des Petits Ruminants (Infection with Peste des Petits Ruminants) in OIE Terrestrial Mannual 2019. 2020. p. 1–16.
- Benfield, C.T.O.; Legnardi, M.; Mayen, F.; Almajali, A.; Cinardi, G.; Wisser, D.; Chaka, H.; Njeumi, F. Peste des Petits Ruminants in the Middle East: epidemiological situation and status of control and eradication activities after the first phase of the PPR Global Eradication Program (2017–2021). Animals 2023, 13, 1196. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.A.; Rich, K.M.; Mariner, J.C.; Anderson, J.; Jeggo, M.; Thevasagayam, S.; Cai, Y.; Peters, A.R.; Roeder, P. The economic impact of eradicating Peste des Petits Ruminants: A benefit-cost analysis. PLoS ONE 2016, 11, e0149982. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, M., Jamal, S. M., Hussain, M., Ali, Q. Incidence of Peste des Petits Ruminants (PPR) virus in sheep and goat as detected by immuno–capture ELISA (Ic ELISA). Small Rumin. Res. 2008, 75, 256–259. [CrossRef]
- Gibbs, E. P., Taylor, W. P., Lawman, M. J., Bryant, J. Classification of Peste des Petits Ruminants virus as the fourth member of the genus Morbillivirus. Intervirology, 1979, 11, 268–274. [CrossRef]
- Amarasinghe, G. K., Ayllón, M. A., Bào, Y., Basler, C. F., Bavari, S., Blasdell, K. R., Briese, T., Brown, P. A., Bukreyev, A., Balkema-Buschmann, A., et al. Taxonomy of the order Mononegavirales: update 2019. Archives of virology, 2019, 164, 1967–1980. [CrossRef]
- Dahar, P., Sreenivasa, B. P., Barrett, T., Corteyn, M., Singh, R. P., Bandyopadhyay, S. K. Recent epidemiology of Peste des Petits Ruminants virus (PPRV). Veterinary Microbiology, 2002, 88, 153–159. [CrossRef]
- Parida, S., Selvaraj, M., Gubbins, S., Pope, R., Banyard, A., Mahapatra, M. Quantifying levels of Peste des Petits Ruminants (PPR) virus in excretions from experimentally infected goats and its importance for nascent PPR Eradication Programme. Viruses, 2019, 11, 249. [CrossRef]
- Fine, A.E.; Pruvot, M.; Benfield, C.T.O.; Caron, A.; Cattoli, G.; Chardonnet, P.; Dioli, M.; Dulu, T.; Gilbert, M.; Kock, R.; et al. Eradication of Peste des Petits Ruminants virus and the wildlife-livestock interface. Front. Vet. Sci. 2020, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Dundon, W.G.; Diallo, A.; Cattoli, G. Peste des Petits Ruminants in Africa: a review of currently available molecular epidemiological data, 2020. Arch. Virol. 2020, 165, 2147–2163. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, M.; Pachauri, R.; Subramaniam, S.; Banyard, A.C.; ChandraSekar, S.; Ramakrishnan, M.A.; Njeumi, F.; Muthuchelvan, D.; Parida, S. Ongoing assessment of the molecular evolution of Peste des Petits Ruminants virus continues to question viral origins. Viruses 2021, 13, 2144. [Google Scholar] [CrossRef] [PubMed]
- Benfield, C.T.O.; Hill, S.; Shatar, M.; Shiilegdamba, E.; Damdinjav, B.; Fine, A.; Willett, B.; Kock, R.; Bataille, A. Molecular Epidemiology of Peste des Petits Ruminants virus emergence in critically endangered Mongolian saiga antelope and other wild ungulates. Virus Evol. 2021, 7, veab062. [Google Scholar] [CrossRef] [PubMed]
- Gargadennec, L.; Lalanne, A. La Peste Des Petits Ruminants. Bull. Serv. Zoo. Epizoot. AOF 1942, 5, 16–21. [Google Scholar]
- Al-Majali, A.M., Hussain, N.O., Amarin, N.M., Majok, A.A. Seroprevalence of, and risk factors for, peste des petits ruminants in sheep and goats in Northern Jordan. Prev Vet Med. 2008, 85(1-2), 1-8. [CrossRef]
- Boshra, H., Truong, T., Babiuk, S., & Hemida, M. G. Seroprevalence of Sheep and Goat Pox, Peste Des Petits Ruminants and Rift Valley Fever in Saudi Arabia. PloS One, 2015, 10, e0140328. [CrossRef]
- Mariner, J. C., Jones, B. A., Rich, K. M., Thevasagayam, S., Anderson, J., Jeggo, M., Cai, Y., Peters, A. R., Roeder, P. L. The Opportunity to Eradicate Peste des Petits Ruminants. J. Immunol. 2016, 196, 3499–3506. [CrossRef]
- WOAH WAHIS. Available online: https://wahis.woah.org/ (accessed on 22 March 2023).
- Libeau, G., Préhaud, C., Lancelot, R., Colas, F., Guerre, L., Bishop, D. H., Diallo, A. Development of a competitive ELISA for detecting antibodies to the Peste des Petits Ruminants virus using a recombinant nucleoprotein. Res. Vet. Sci. 1995, 58, 50-5. [CrossRef]
- Ozkul, A., Akca, Y., Alkan, F., Barrett, T., Karaoglu, T., Dagalp, S. B., Anderson, J., Yesilbag, K., Cokcaliskan, C., Gencay, A., Burgu, I. Prevalence, distribution, and host range of Peste des petits ruminants virus, Turkey. Emerg. Infect. Dis. 2002, 8, 708–712. [CrossRef]
- Zahur, A. B., Ullah, A., Hussain, M., Irshad, H., Hameed, A., Jahangir, M., Farooq, M. S. Sero-epidemiology of peste des petits ruminants (PPR) in Pakistan. Prev. Vet. Med. 2011, 102, 87–92. [CrossRef]
- Sharawi, S.S.; Yousef, M.R.; Al-Hofufy, A.N.; Al-Blowi, M.H. Isolation, Serological and Real time PCR diagnosis of Peste des Petites Ruminants virus in naturally exposed Arabian Gazelle in Saudi Arabia. Vet. World 2010, 3, 489–494. [CrossRef]
- Fathelrahman, E.M.; Reeves, A.; Mohamed, M.S.; Ali, Y.M.E.; El Awad, A.I.; Bensalah, O.K.; Abdalla, A.A. Epidemiology and cost of Peste des Petits Ruminants (PPR) eradication in small ruminants in the United Arab Emirates - disease spread and control strategies simulations. Animals 2021, 11, 2649. [Google Scholar] [CrossRef] [PubMed]
- Hussain, K.J. Sero-prevalence with risk factor for Peste des Petits Ruminants (PPR) in sheep and goats using competitive ELISA in Mosul, Iraq. Basrah J. Vet. Res. 2021, 20, 160–171. [Google Scholar] [CrossRef]
- Al-Dubaib M. A. Peste des Petits Ruminants morbillivirus infection in lambs and young goats at Qassim region, Saudi Arabia. Trop. Anim. Health Prod. 2009, 41, 217–220. [CrossRef] [PubMed]
- Abdellatif, M., Mahmoud, A. Z., Shazali, L. Prevalence of PPR-virus antibodies in sheep, goats and camels in Hail, Saudi Arabia. Brit. J. Virol. 2016, 3, 86-89. [CrossRef]
- Taylor, W. The global eradication of Peste des Petits Ruminants (PPR) within 15 years – is this a pipe dream? Trop. Anim. Health Prod. 2016, 48, 559–567. [Google Scholar] [CrossRef]
- Singh, R. P., Bandyopadhyay, S. K., Sreenivasa, B. P., Dhar, P. Production and characterization of monoclonal antibodies to Peste des Petits Ruminants (PPR) virus. Vet. Res. Commun. 2004, 28, 623–639. [CrossRef]
- Acharya, N., Poudel, S. P., Acharya, K. P. Cross-sectional sero-prevalence study of Peste des Petits Ruminants (PPR) in goats of Syangja and Kaski districts of Nepal. Virus Dis. 2018, 29, 173–179. [CrossRef]
- Torsson, E., Berg, M., Misinzo, G., Herbe, I., Kgotlele, T., Päärni, M., Roos, L., Blomström, A. Ståhl, K., Wensman, J. J. Seroprevalence and risk factors for Peste des Petits Ruminants and selected differential diagnosis in sheep and goats in Tanzania. Infect. Ecol. Epidemiol. 2017, 7, 1-12. [CrossRef]
- Mahamat, O., Doungous, T., Kebkiba, B., Oumar, H. A., Oussiguéré, A., Yacoub, A. H., Goudja, A., Guindé, M., & Moussa, A. H. Seroprevalence, geographical distribution, and risk factors of Peste des Petits Ruminants in the Republic of Chad. J. Adv. Vet. Anim. Res. 2018, 5, 420–425. [CrossRef]
- Moumin, G., Moussa, C., Teshale, S., Gezahegne, M. Seroprevalence and risk factors for Peste des Petits Ruminants in sheep and goats in Djibouti. OIE Revue Scientifique et Technique, 2018, 37, 961–969. [CrossRef]
- Kgotlele. T., Torsson, E., Kasanga, C. J. Seroprevalence of Peste des Petits Ruminants virus from samples collected in different regions of Tanzania in 2013 and 2015. J. Vet. Sci. Technol. 2016, 7, 1–5. [CrossRef]
| Variable | No. Negative (%) | No. Positive (%) | Odd ratio | |
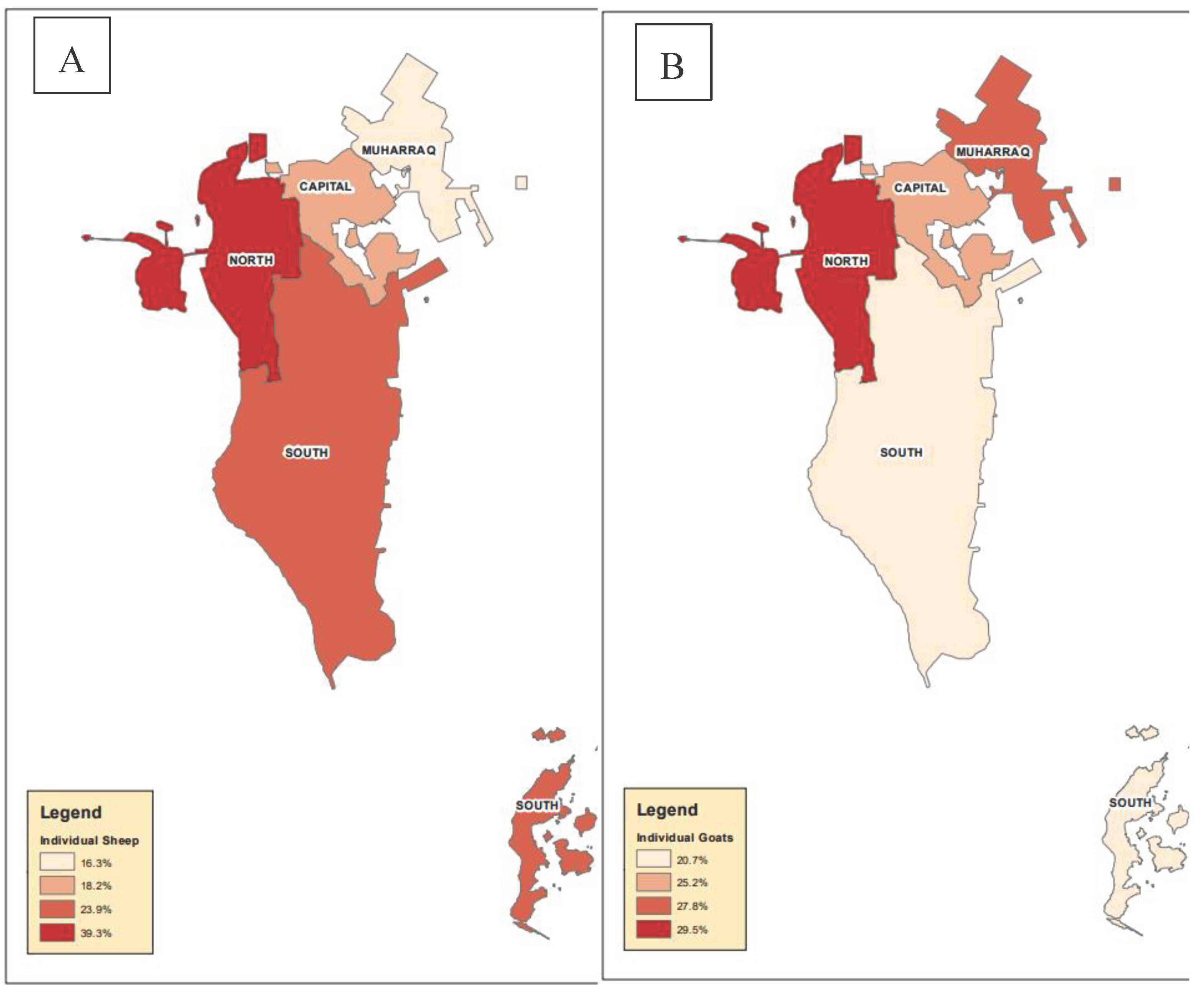
| Governorate | Capital | 279 (81.8) | 62 (18.2) | 3.3 |
| Muharraq | 113 (83.7) | 22 (16.3) | ||
| Northern | 230 (60.7) | 149 (39.3)* | ||
| Southern | 295 (76.6) | 90 (23.9) | ||
| Age | < 12 months | 210 (76.9) | 63 (23.1) | ---- |
| 12 – 36 months | 234 (74.5) | 80 (25.5) | ||
| > 36 months | 473 (72.4) | 180 (27.6) | ||
| Sex | Male | 140 (69.7) | 61 (30.3)* | 1.4 |
| Female | 765 (76.3) | 238 (23.7) | ||
| Health status | Excellent | 288 (83.7) | 56 (16.3) | 1.9 |
| good | 257 (77.2) | 76 (22.8) | ||
| Weak | 117 (64.3) | 65 (35.7)* | ||
| Poor | 40 (81.6) | 9 (18.4) | ||
| Variable | No. Negative (%) | No. Positive (%) | Odd ratio | |
|---|---|---|---|---|
| Governorate | Capital | 243 (74.8) | 82 (25.2) | 1.2 |
| Muharraq | 114 (72.2) | 44 (27.8) | ||
| Northern | 263 (70.5) | 110 (29.5)* | ||
| Southern | 292 (79.3) | 76 (20.7) | ||
| Age | < 12 months | 172 (66.9) | 85 (33.1)* | 1.3 |
| 12 – 36 months | 228 (73.1) | 84 (26.9) | ||
| > 36 months | 512 (78.2) | 143 (21.8) | ||
| Sex | Male | 148 (63) | 87 (37)* | 2.0 |
| Female | 742 (77.5) | 215 (22.5) | ||
| Health status | Excellent | 229 (75.3) | 75 (24.7) | 1.2 |
| good | 267 (73.6) | 96 (26.4) | ||
| Weak | 106 (66.3) | 54 (33.8)* | ||
| Poor | 32 (71.1) | 13 (28.9) | ||
| Variable | No. Negative (%) | No. Positive (%) | Odd ratio | |
|---|---|---|---|---|
| Governorate | Capital | 35 (83.3) | 7 (16.7) | 3.5 |
| Muharraq | 25 (83.3) | 5 (16.7) | ||
| Northern | 4 (44.4) | 5 (55.6)* | ||
| Southern | 28 (73.7) | 10 (26.3) | ||
| Herd size | < 50 | 74 (84.1) | 14 (15.9) | 1.6 |
| 50 – 200 | 13 (61.9) | 8 (38.1) | ||
| > 200 | 5 (50) | 5 (50)* | ||
| Farming | Traditional | 90 (77.6) | 26 (22.4) | --- |
| Semi-intensive | 2 (66.7) | 1 (33.3) | ||
| Purpose | Trading and meat | 0 (0.0) | 1 (100) | --- |
| Breeding | 92 (78) | 26 (22) | ||
| Source of water | Ground water | 22 (73.3) | 8 (26.7) | --- |
| Municipality water | 70 (79.5) | 18 (20.5) | ||
| Presence of dogs | Yes | 7 (70) | 3 (30) | --- |
| No | 85 (78) | 24 (22) | ||
| Use of disinfectants | Yes | 4 (100) | 0 (0.0) | --- |
| No | 88 (76.5) | 27 (23.5) | ||
| Disposal of dead | Call municipality | 77 (81.1) | 18 (18.9) | --- |
| Incineration | 2 (50) | 2 (50) | ||
| Burial | 13 (65) | 7 (35) | ||
| Presence of abortion | Yes | 33 (78.6) | 9 (21.4) | --- |
| No | 59 (76.6) | 18 (23.4) | ||
| Variable | No. Negative (%) | No. Positive (%) | Odd ratio | |
|---|---|---|---|---|
| Governorate | Capital | 30 (71.4) | 12 (28.6) | --- |
| Muharraq | 23 (76.7) | 7 (23.3) | ||
| Northern | 31 (72.1) | 12 (27.9) | ||
| Southern | 4 (40) | 6 (60) | ||
| Herd size | < 50 | 72 (72) | 28 (28) | --- |
| 50 – 100 | 10 (71.4) | 4 (28.6) | ||
| > 100 | 6 (54.5) | 5 (45.5) | ||
| Farming | Traditional | 84 (73) | 31 (27) | 4.0 |
| Semi-intensive | 4 (40) | 6 (60)* | ||
| Purpose | Trading and meat | 0 (0.0) | 4 (100) | --- |
| Breeding | 88 (72.7) | 33 (27.3) | ||
| Source of water | Ground water | 23 (65.7) | 12 (34.3) | --- |
| Municipality water | 65 (73) | 24 (27) | ||
| Presence of dogs | Yes | 9 (75) | 3 (25) | --- |
| No | 79 (69.9) | 34 (30.1) | ||
| Use of disinfectants | Yes | 5 (71.4) | 2 (28.6) | --- |
| No | 79 (73.1) | 29 (26.9) | ||
| Disposal of dead | Call municipality | 68 (67.3) | 33 (32.7) | --- |
| Incineration | 5 (83.1) | 1 (16.9) | ||
| Burial | 15 (83.3) | 3 (16.7) | ||
| Presence of abortion | Yes | 28 (70) | 12 (30) | --- |
| No | 60 (70.6) | 25 (29.4) | ||
| Animal Species | Variable | B | S.E. | Sig. | Odd ratio |
|---|---|---|---|---|---|
| Sheep | Constant | 19.010 | 0.402 | 0.000 | |
| Southern | -0.055 | 0.429 | 0.898 | ||
| Northern | 1.121 | 0.349 | 0.001 | 3.07 | |
| Goats | Constant | -1.939 | 0.482 | 0.000 | |
| Southern | 0.590 | 0.320 | 3.398 | ||
| Northern | 0.705 | 0.341 | 4.264 | 2.02 | |
| < 12 months | 0.724 | 0.237 | 9.371 | 2.06 | |
| > 36 months | 0.476 | 0.235 | 4.092 | ||
| Male | 0.789 | 0.191 | 17.059 | 2.20 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





