Submitted:
28 February 2024
Posted:
29 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Altamirano, M.J.; De La Rosa, J.; Martínez, F.J. Arribazones de la especie exótica Rugulopteryx okamurae (E.Y. Dawson) I.K. Hwang, W.J. Lee and H.S. Kim (Dictyotales, Orchrophyta) en el Estrecho de Gibraltar: Primera cita para el Atlántico y España. Algas, 2016; 52, 20. [Google Scholar]
- García-Gómez, J.C.; Sempere-Valverde, J.; González, A.R.; Martínez-Chacón, M.; Olaya-Ponzone, L.; Sánchez-Moyano, E.; Ostalé-Valriberas, E.; Megina, C. From exotic to invasive in record time: The extreme impact of Rugulopteryx okamurae (Dictyotales, Ochrophyta) in the Strait of Gibraltar. Sci. Total Environ. 2020, 704, 135408. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Ibáñez, A.; Chebaane, S.; Sempere-Valverde, J.; Faria, J.; Ramalhosa, P.; Kaufmann, M.; Florido, M.; Albert-Fonseca, A.; Canning-Clode, J.; Gestoso, I.; Cacabelos, E. A worrying arrival: the first record of brown macroalga Rugulopteryx okamurae in Madeira Island and its invasive risk. BioInvasions Rec. 2022, 11(4), 912–924. [Google Scholar] [CrossRef]
- García-Gómez, J.C.; Florido, M.; Olaya-Ponzone, L.; Sempere-Valverde, J.; Megina, C. The invasive macroalgae Rugulopteryx okamurae: substrata plasticity and spatial colonization pressure on resident macroalgae. Front. Ecol. Environ. 2021, 9, 631754. [Google Scholar] [CrossRef]
- Patón, D.; García-Gómez, J.C.; Loring, J.; Torres, A. Composting the invasive toxic seaweed Rugulopteryx okamurae using five invertebrate species, and a mini-review on composting macroalgae. Waste Biomass Valorization 2022, 14, 167–184. [Google Scholar] [CrossRef]
- Bouchon, D.; Zimmer, M.; Dittmer, J. The terrestrial isopod microbiome: An all-in-one toolbox for animal-microbe interactions of ecological relevance. Front. Microbiol. 2016, 7, 1472. [Google Scholar] [CrossRef] [PubMed]
- Hornung, E. Evolutionary adaptation of oniscidean isopods to terrestrial life: Structure, physiology and behavior. Terr. Arthropod Rev. 2011, 4, 95–130. [Google Scholar] [CrossRef]
- Ghemarr, C.; Bouslama, M.F.; Ayari, A.; Nasri-Ammar, K. Population structure & dynamics of Porcellio laevis (latreille, 1804) in northern Tunisia. Vie et Milieu 2016, 66(2), 209–218. [Google Scholar] [CrossRef]
- Lardies, M.A.; Bozinovic, F. Genetic variation for plasticity in physiological and life-history traits among populations of an invasive species, the terrestrial isopod Porcellio laevis. Evol. Ecol. Res. 2008, 10, 747–762. [Google Scholar]
- García, Ll. Orden Isopoda: Suborden Oniscidea. Revista IDE@ - SEA, 2015, 78, 1–12. [Google Scholar]
- García, Ll. Nuevos registros de Isópodos terrestres (Crustacea: Oniscidea) en España meridional (Andalucía y Murcia). Rev. Soc. Gad. Hist. Nat. 2019, 13, 27–32. [Google Scholar]
- Tóth, Z.; Hornung, E.; Szlavecz, K. Urban effects on saprophagous macroarthropods are mainly driven by climate: A global meta-analysis. Sci. Total. Environ. 2021, 797, 149182. [Google Scholar] [CrossRef] [PubMed]
- Sfenthourakis, S.; Hornung, E. Isopod distribution and climate change. ZooKeys 2018, 801, 25–61. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, N.G.C.; Cardoso, D.N.; Morgado, R.; Soares, A.M.V.; Loureiro, S. Long-term exposure of the isopod Porcellionides pruinosus to nickel: Costs in the energy budget and detoxification enzymes. Chemosphere 2015, 135, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, M. Nutrition in terrestrial isopods (Isopoda: Oniscidea): an evolutionary-ecological approach. Biol. Rev. Camb. Philos. Soc. 2002, 77(4), 455–93. [Google Scholar] [CrossRef]
- Hassall, M.; Tuck, J.M. Sheltering behavior of terrestrial isopods in grasslands. Invertebr. Biol. 2007, 126(1), 46–56. [Google Scholar] [CrossRef]
- Vittori, M.; Vodnik, K.; Blejec, A. Changes in cuticle structure during growth in two terrestrial isopods (Crustacea: Isopoda: Oniscidea). Nauplius 2020, 28, e2020041. [Google Scholar] [CrossRef]
- Csonka, D.; Halasy, K.; Buczkó, K.; Hornung, E. Morphological traits - desiccation resistance – habitat characteristics: a possible key for distribution in woodlice (Isopoda, Oniscidea). In Isopods in a Changing World; Hornung, E., Taiti, S., Szlavecz, K., Eds.; ZooKeys 2018; Volume 801, pp. 481-499.
- Khemaissia, H.; Raimond, M.; Ayari, A.; Jelassi, R.; Souty-Grosset, C.; Nasri-Ammar, K. Cuticular differences of the exoskeleton relative to habitat preferences among three terrestrial isopods. Biol. 2018, 73, 477–483. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, Z.; Yang, L.; Li, H.; Ge, B. Body size and weight of pill bugs (Armadillidium vulgare) vary between urban green space habitats. Anim. 2023, 13, 857. [Google Scholar] [CrossRef]
- Broly, P.; Deneubourg, J.L.; Devigne, C. Benefits of aggregation in woodlice: a factor in the terrestrialization process? . Insect. Soc. 2013, 60, 419–435. [Google Scholar] [CrossRef]
- Hassall, M.; Edwards, D.P.; Carmenta, R.; Derhé, M.A.; Moss, A. Predicting the effect of climate change on aggregation behaviour in four species of terrestrial isopods. Behav. 2010, 147(2), 151–164. [Google Scholar] [CrossRef]
- Brigić, A.; Antonović, I.; Alegro, A.; Segota, V.; Bujan, J. Terrestrial isopods (Isopoda: Oniscidea) as unexpected inhabitants of extreme habitats. Eur. J. Soil Biol. 2017, 88, 66–71. [Google Scholar] [CrossRef]
- Quinlan, M.C.; Hadley, N.F. Water relations of the terrestrial isopods Porcellio laevis and Porcellionides pruinosus (Crustacea, Oniscoidea). J. Comp. Physiol. 1983, 151, 155–161. [Google Scholar] [CrossRef]
- Zimmer, M.; Brauckmann, H.J. Geographical and annual variations in the phenology of some terrestrial isopods (Isopoda, Oniscidea). Biol. Bratislava 1997, 52(2), 281–289. [Google Scholar]
- Magura, T.; Hornung, E.; Tóthmérész, B. Abundance patterns of terrestrial isopods along an urbanization gradient. Community Ecol. 2008, 9(1), 115–120. [Google Scholar] [CrossRef]
- Folguera, G.; Bastías, D.A.; Caers, J.; Rojas, J.M.; Piulachs, M.D.; Bellés, X.; Bozinovic, F. An experimental test of the role of environmental temperature variability on ectotherm molecular, physiological and life-history traits: Implications for global warming. Comp. Biochem. Physiol. A, 2011, 159, 242–246. [Google Scholar] [CrossRef]
- Wagler, R. Effective techniques for the care, reproduction and utilization of the terrestrial isopod Porcellio scaber in your science classroom. Am. Biol. Teach. 2020, 82(4), 266–268. [Google Scholar] [CrossRef]
- Broly, P.; Mullier, R.; Deneubourg, J.L.; Devigne, C. Aggregation in woodlice: Social interaction and density effects. In Advances in Terrestrial Isopod Biology; Štrus, J., Taiti, S., Sfenthourakis, S., Eds.; ZooKeys 2012; Volume 176, pp. 133–144.
- Kight, S.L. Reproductive ecology of terrestrial isopods (Crustacea: Oniscidea). Terr. Arthropod Rev. 2008, 1, 95–110. [Google Scholar] [CrossRef]
- Kight, S.L.; Nevo, M. Female terrestrial isopods, Porcellio laevis Latreille (Isopoda: Oniscidea) reduce brooding duration and fecundity in response to physical stress. J. Kansas Entomol. Soc. 2004, 77(3), 285–287. [Google Scholar] [CrossRef]
- Quadros, A.F.; Araujo, P.B. An assemblage of terrestrial isopods (Crustacea) in southern Brazil and its contribution to leaf litter processing. Revista Brasileira de Zoologia 2008, 25(1), 58–66. [Google Scholar] [CrossRef]
- Lardies, M.A.; Carter, M.J.; Bozinovic, F. Dietary effects on life history traits in a terrestrial isopod: The importance of evaluating maternal effects and trade-offs. Oecol. 2004, 138(3), 387–395. [Google Scholar] [CrossRef] [PubMed]
- Ŝpaldon̂ová, A.; Frouz, J. The role of Armadillidium vulgare (Isopoda: Oniscidea) in litter decomposition and soil organic matter stabilization. Appl. Soil Ecol. 2014, 83, 186–192. [Google Scholar] [CrossRef]
- Zimmer, M.; Brune, A. Physiological properties of the gut lumen of terrestrial isopods (Isopoda: Oniscidea): adaptive to digesting lignocellulose? . J. Comp. Physiol. B 2005, 175, 275–283. [Google Scholar] [CrossRef]
- Zimmer, M.; Kautz, G.; Topp, W. Do woodlice and earthworms interact synergistically in leaf litter decomposition? Funct. Ecol. 2005, 19, 7–16. [Google Scholar] [CrossRef]
- Sade, Y.B.; De Moraes, D.T.; Beltrão, P.J.; Vicentim, M.P. Cellulases from invertebrate animals. In Bioethanol and Beyond: Advances in Production Process and Future Directions; Brenzo, M., Ed.; Nova Science Pub. Inc., USA, 2018; pp. 261-284.
- Domínguez-Santos, R.; Pérez-Cobas, A.E.; Cuti, P.; Pérez-Brocal, V.; García-Ferris, C.; Moya, A.; Latorre, A.; Gil, R. Inter-kingdom gut microbiome and resistome of the cockroach Blattella germanica. MSystems 2021, 6, e01213-20. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Fonck, M.; Van Hal, J.; Cornelissen, J.H.C.; Berg, M.P. Diversity of macro-detritivores in dead wood is influenced by tree species, decay stage and environment. Soil Biol. Biochem. 2014, 78, 288–297. [Google Scholar] [CrossRef]
- Koubová, A.; Lorenc, F.; Horváthová, T.; Chroňáková, A.; Šustr, V. Millipede gut-derived microbes as a potential source of cellulolytic enzymes. World J. Microbiol. Biotechnol. 2023, 39, 169. [Google Scholar] [CrossRef] [PubMed]
- König, H.; Varma, A. Intestinal microorganisms of termites and other invertebrates. Springer Berlin, Heidelberg, Germany, 2006; 473 pp.
- Udovic, M.; Drobne, D.; Lestan, D. An in vivo invertebrate bioassay of Pb, Zn and Cd stabilization in contaminated soil. Chemosphere 2013, 92, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Odendaal, J.P.; Reinecke, A.J. Quantifying histopathological alterations in the hepatopancreas of the woodlouse Porcellio laevis (Isopoda) as a biomarker of cadmium exposure. Ecotoxicol. Environ. Saf. 2003, 56, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, M.G.; Hassall, M. Woodlice (Isopoda: Oniscidea): their potential for assessing sustainability and use as bioindicators. Agric. Ecosyst. Environ. 1999, 74, 157–165. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9(7), 671–675. [Google Scholar] [CrossRef]
- Bauer, D.F. Constructing confidence sets using rank statistics. J. Am. Stat. Assoc. 1972, 67, 687–690. [Google Scholar] [CrossRef]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.r-project.org/ (accessed on 30 January 2024).
- Bordiean, A.; Krzyżaniak, M.; Stolarski, M.J.; Peni, D. Growth potential of yellow mealworm reared on industrial residues. Agric. 2020, 10, 599. [Google Scholar] [CrossRef]
- Yasmin, N.; Jamuda, M.; Panda, A.K.; Samal, K.; Nayak, J.K. Emission of greenhouse gases (GHGs) during composting and vermicomposting: Measurement, mitigation, and perspectives. Energy Nexus 2022, 7, 100092. [Google Scholar] [CrossRef]
- Patón, D.; García-Gómez, J.C. Blatticomposting of food waste, production estimates, chemical composition and CO2 emissions savings: A case study. Waste Biomass Valorization 2023, 14, 3811–3826. [Google Scholar] [CrossRef]
- Casal-Porras, I.; Zubía, E.; Brun, F.G. Dilkamural: A novel chemical weapon involved in the invasive capacity of the alga Rugulopteryx okamurae in the Strait of Gibraltar, Estuarine. Coast. Shelf Sci. 2021, 257, 107398. [Google Scholar] [CrossRef]
- Škarková, P.; Kos, M.; Drobne, D.; Vávrová, M.; Jemec, A. Effects of food salinization on terrestrial crustaceans Porcellio scaber. Appl. Soil Ecol. 2016, 100, 1–7. [Google Scholar] [CrossRef]
- Quadros, A.F.; Zimmer, M.; Araujo, P.B.; Kray, J.G. Litter traits and palatability to detritivores: a case study across bio-geographical boundaries. Nauplius 2014, 22(2), 103–111. [Google Scholar] [CrossRef]

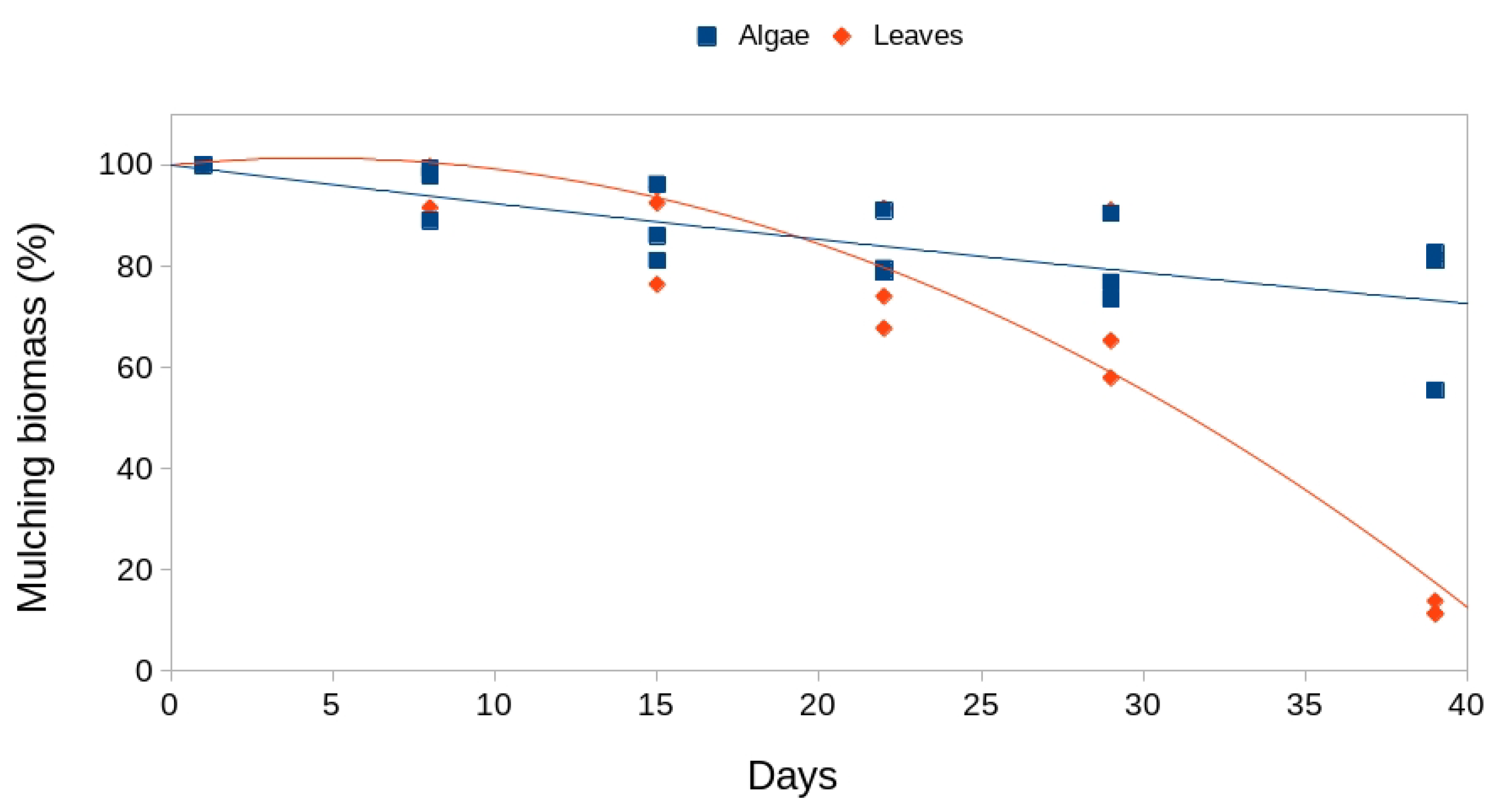
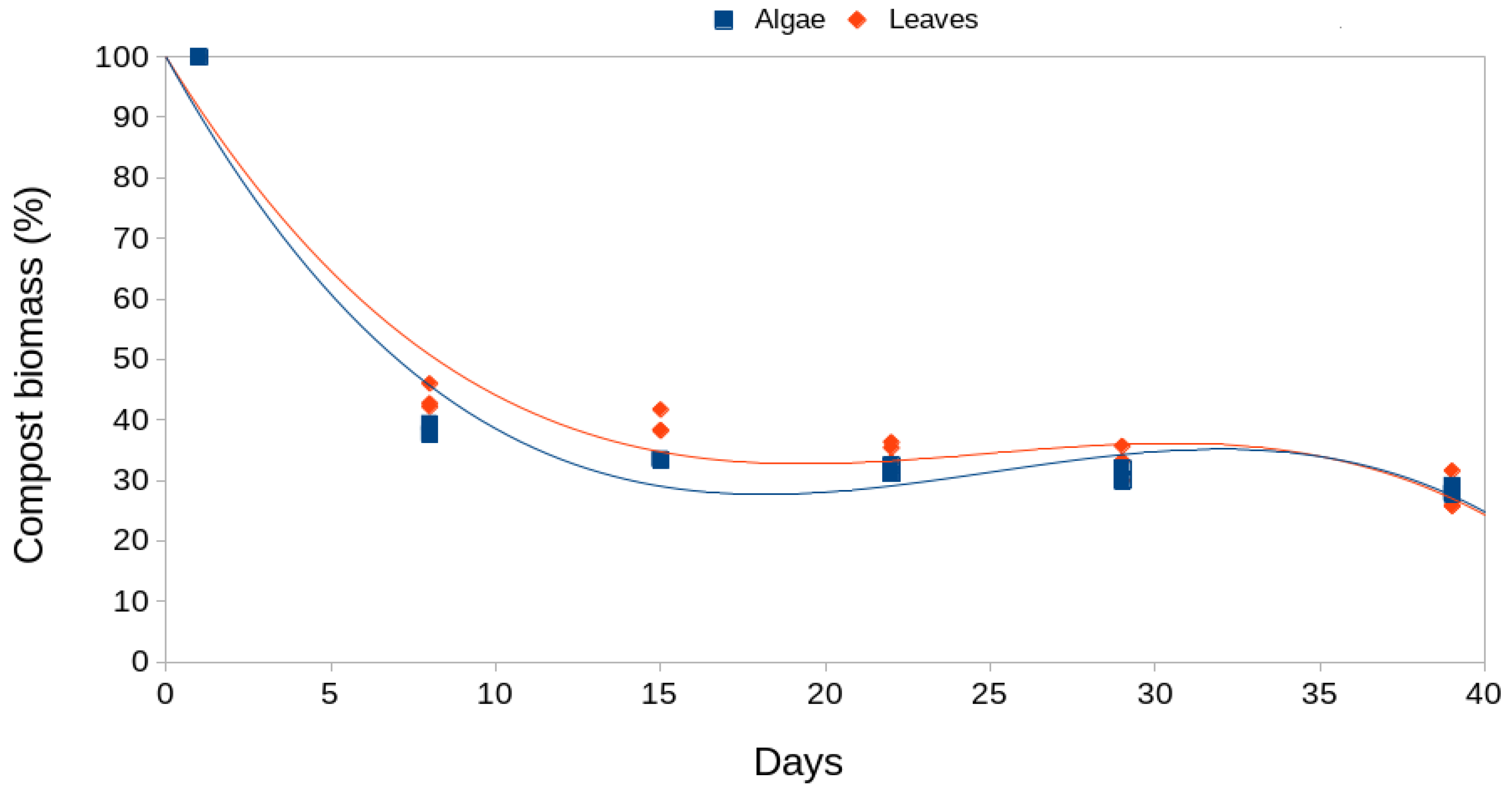
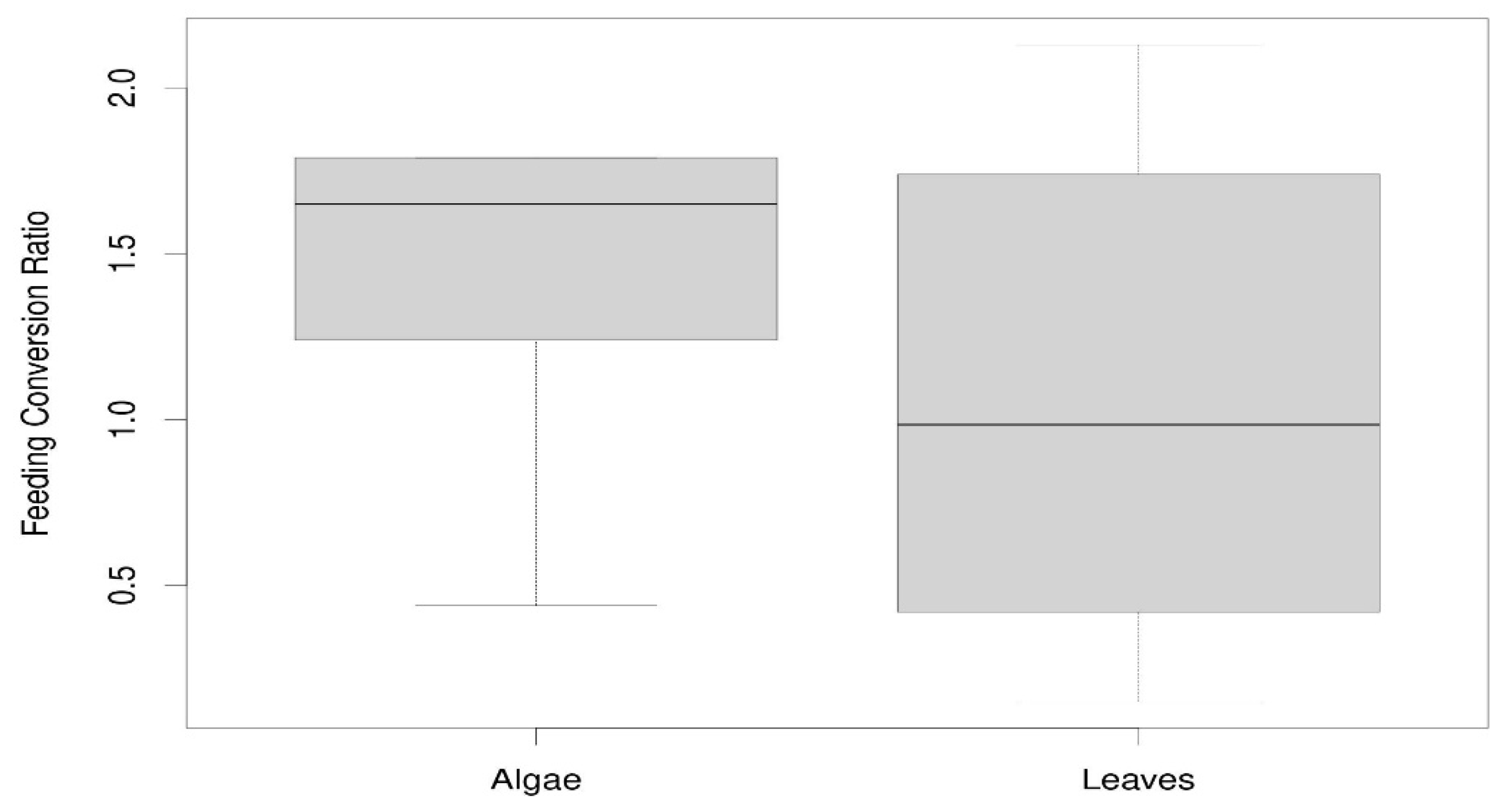
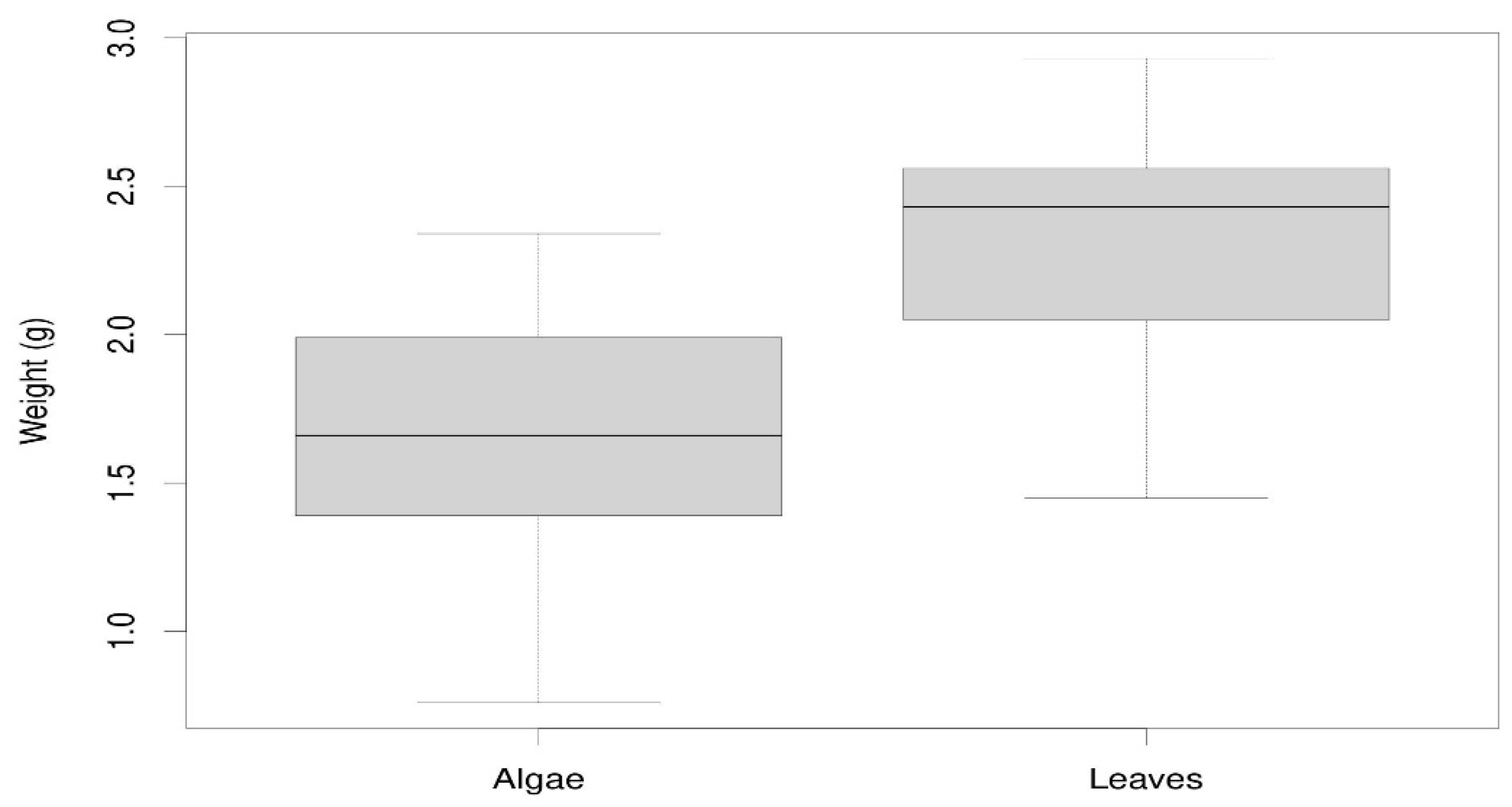
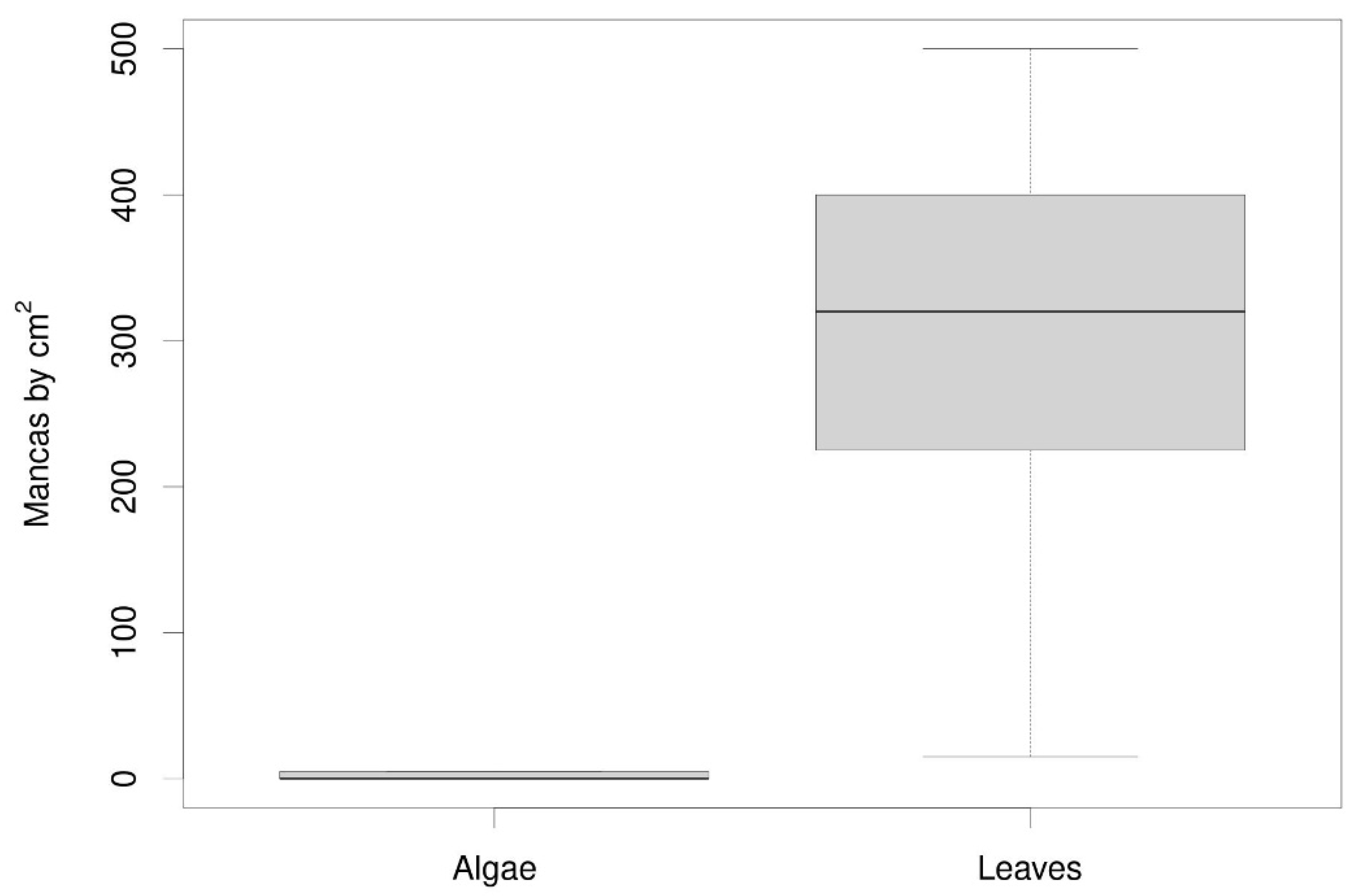


| Parameter | Algae | Leaves |
|---|---|---|
| Model | Biomass(%) = 86.61 – 22.04*Days +1.73*Days² | Biomass(%) = 74.43 – 65.06*Days – 28.24*Days² |
| Fitted R² | 0.986 | 0.933 |
| F-statistic | 179.7 *** | 35.84 ** |
| Significant terms | Intercept, X | All |
| Parameter | Algae | Leaves |
|---|---|---|
| Model | Biomass(%) = 43.71 - 77.11*Days + 59.66*Days² - 40.93*Days³ | Biomass(%) = 46.44 – 80.55*Days + 51.21*Days² - 36.21*Days³ |
| Fitted R² | 0.958 | 0.954 |
| F-statistic | 130.20 *** | 119.00 ** |
| Significant terms | All | All |
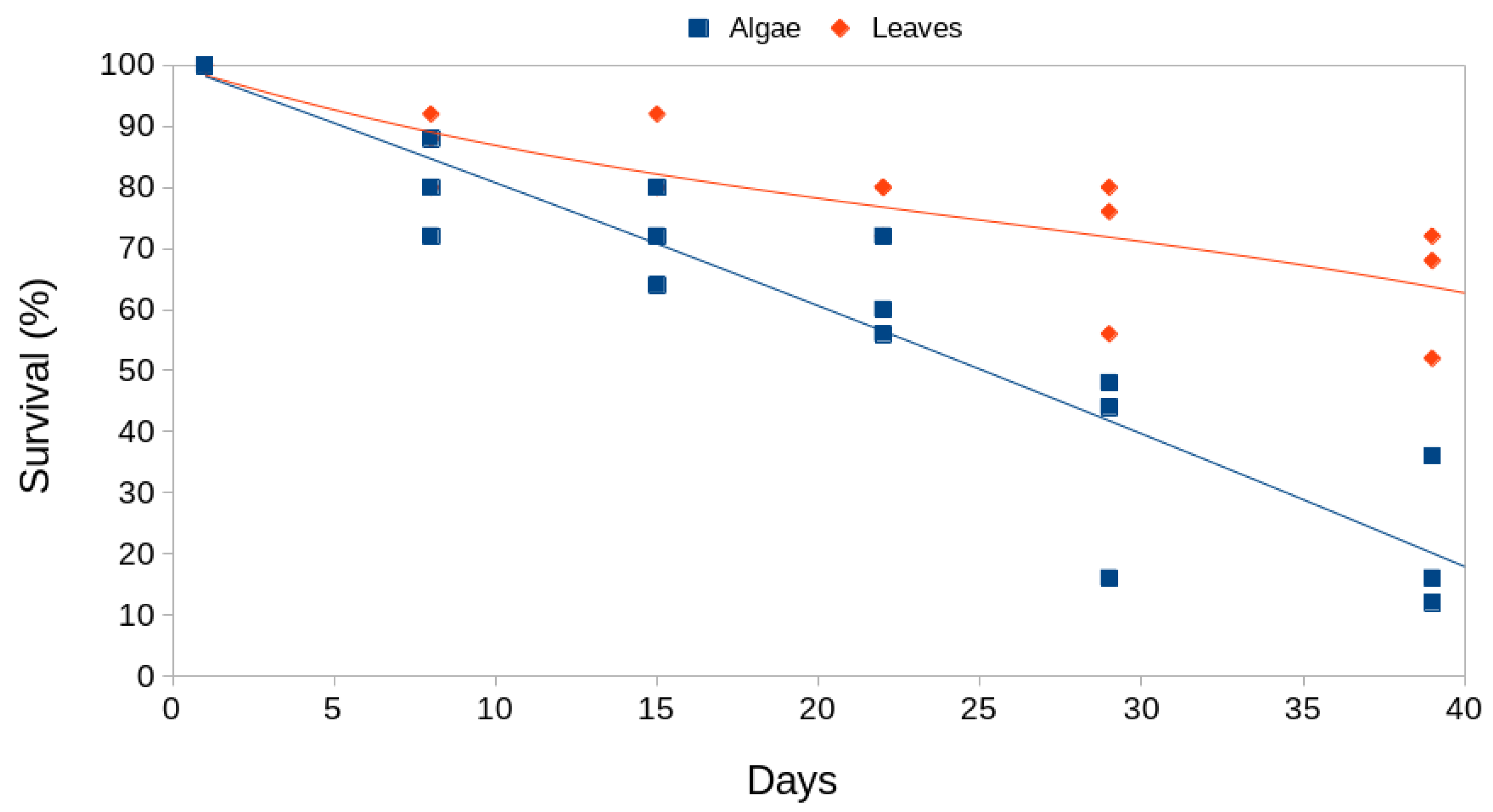
| Parameter | Algae | Leaves |
|---|---|---|
| Model | Survival (%) = 101.05 – 2.06*Days | Survival (%) = 97.50 – 0.90*Days |
| Fitted R² | 0.875 | 0.717 |
| F-statistic | 119.9 *** | 44.03 *** |
| Significant terms | All | All |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




