Submitted:
28 February 2024
Posted:
29 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
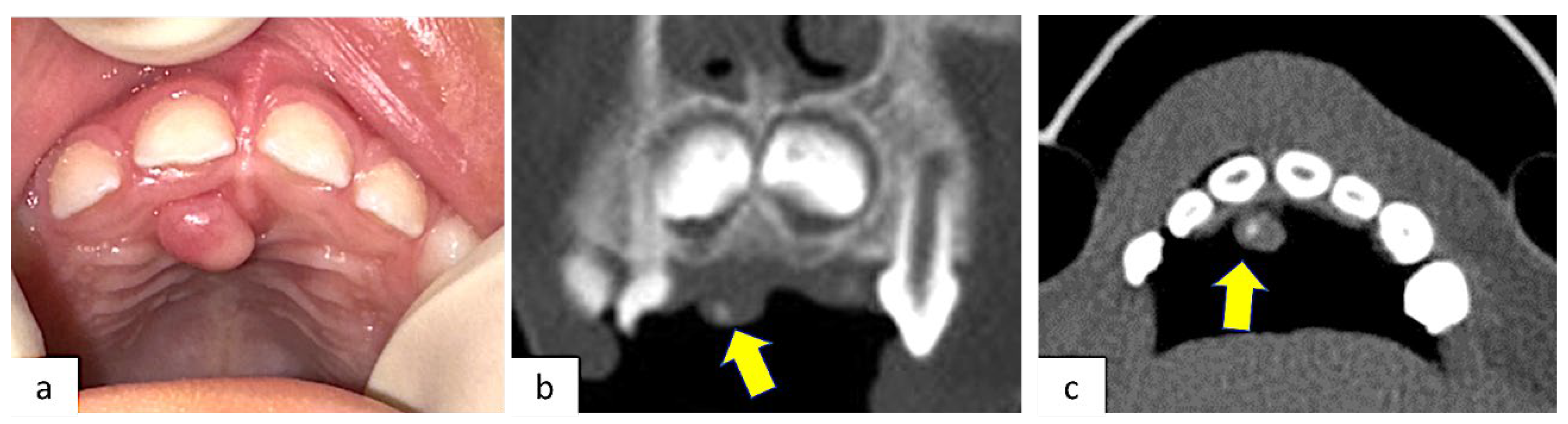
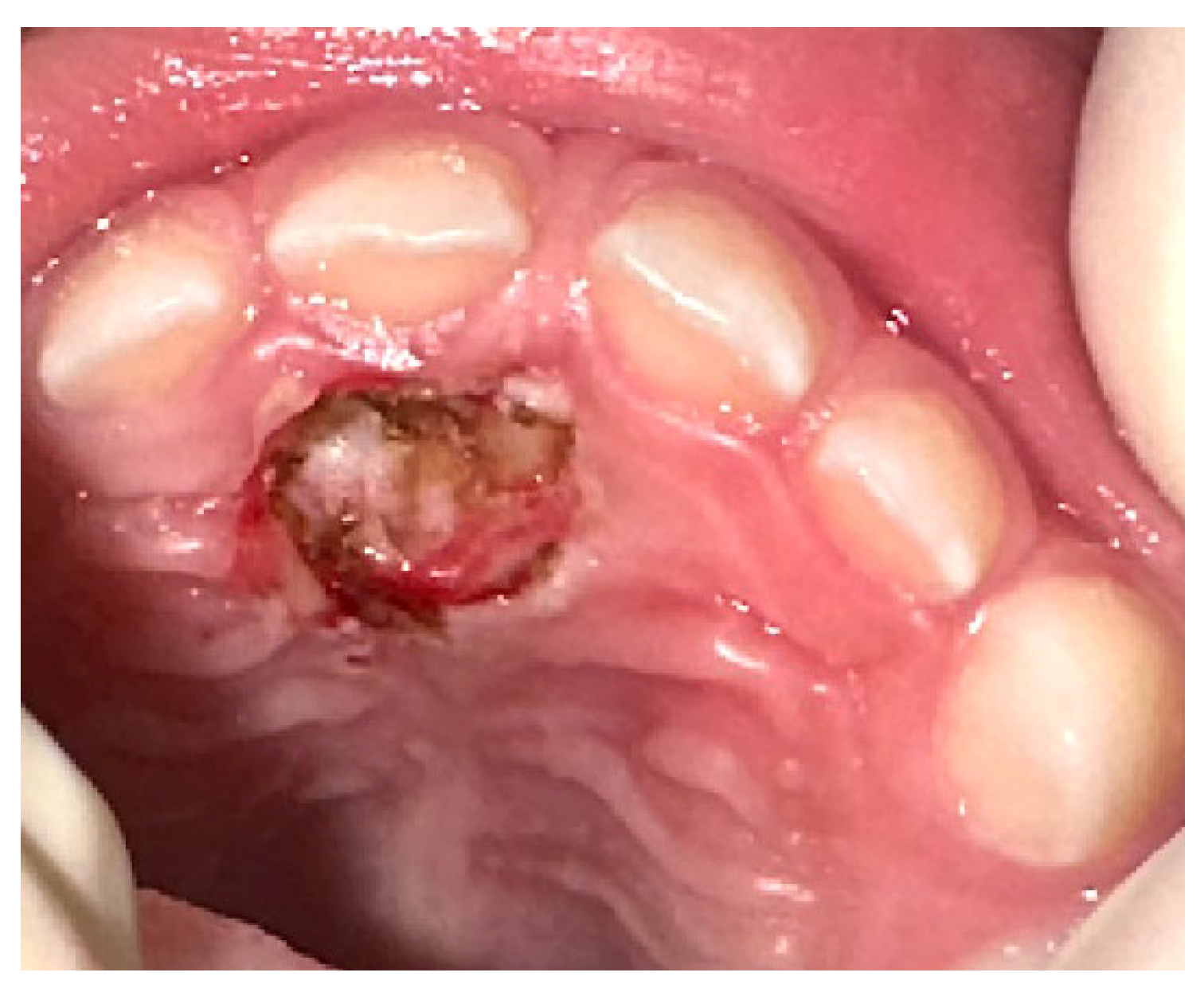
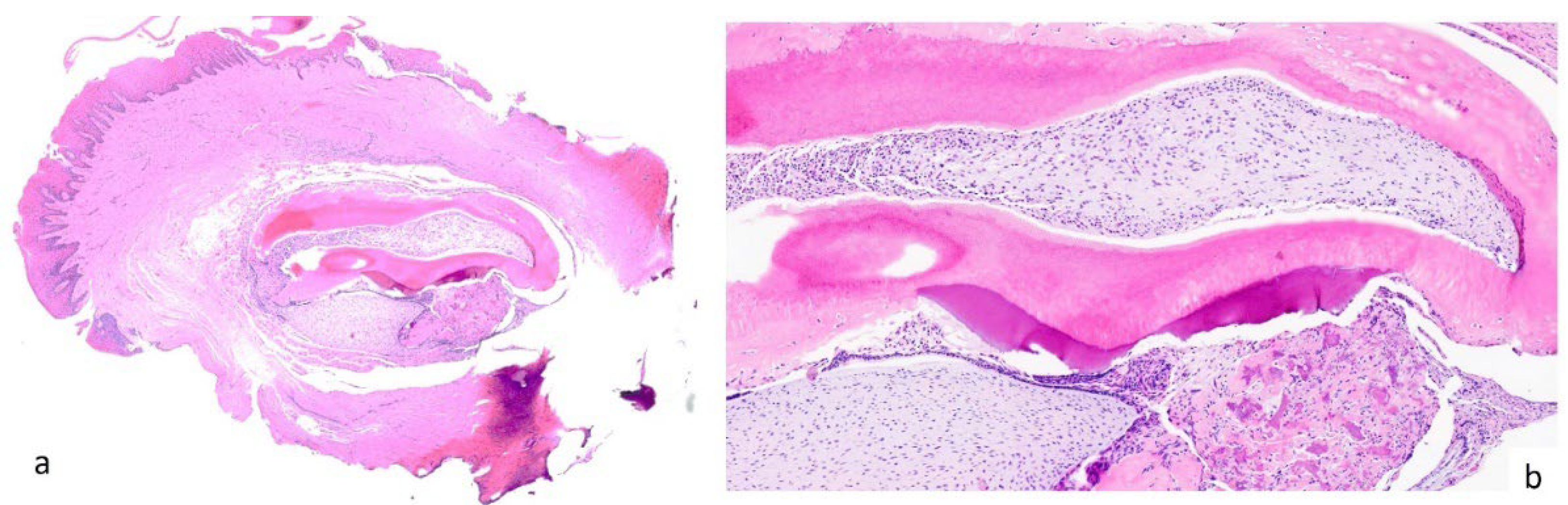
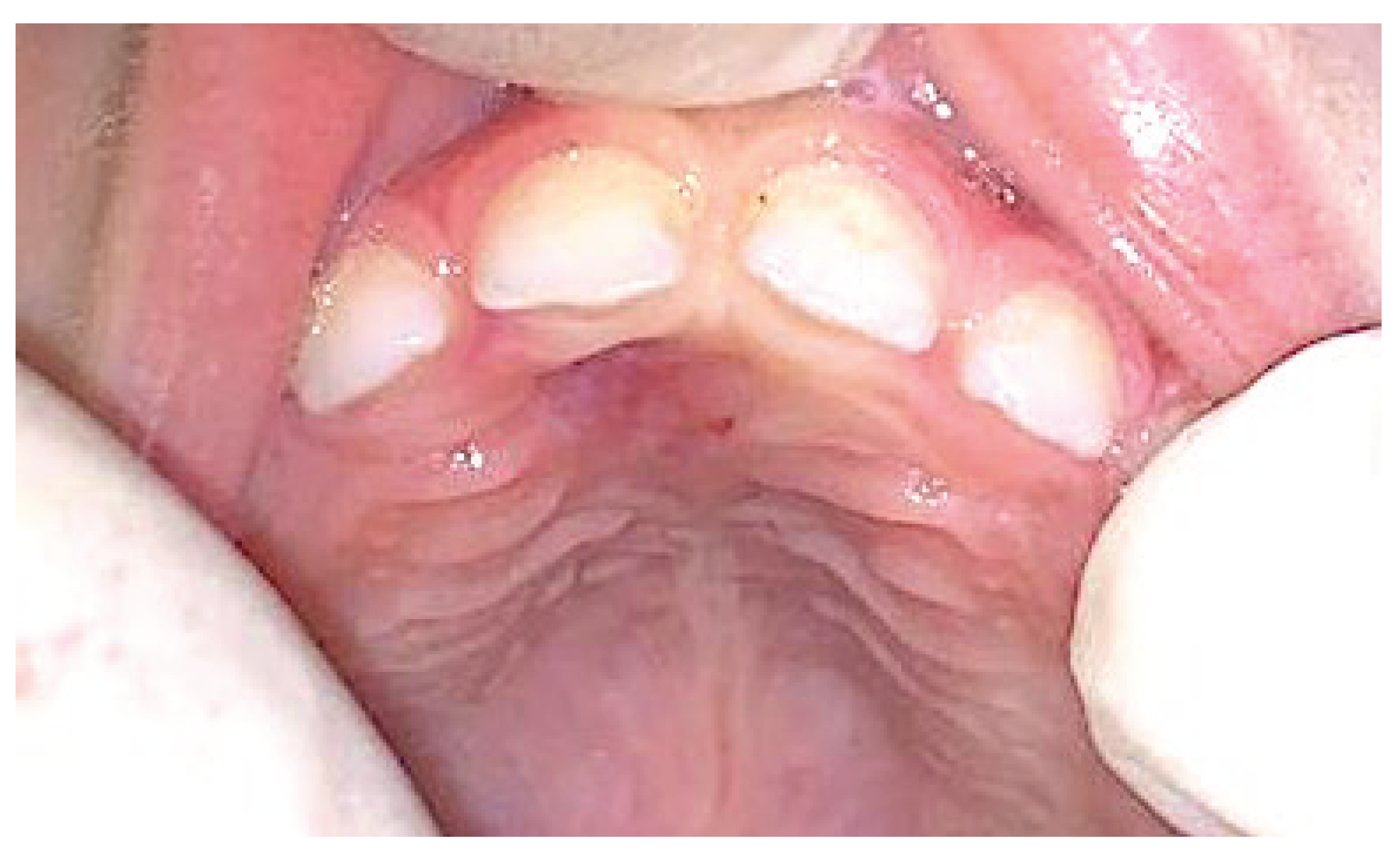
2. Case Presentation
3. Results
4. Literature Review
5. Discussion
5.1. Epidemiology
5.2. Etiopathological Theories
5.3. Clinico-Radiological Features and Differential Diagnosis
5.4. POF in Pediatric Patients: Analysis of the Recent Literature
5.5. Therapeutic Strategies
5.6. Limitations and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Eversole, L.R.; Rovin, S. Reactive Lesions of the Gingiva. J. Oral Pathol. 1972, 1, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Nilesh, K.; Punde, P.; Patil, N.S.; Gautam, A. Central Ossifying Fibroma of Mandible. BMJ Case Rep. 2020, 13, e239286. [Google Scholar] [CrossRef]
- Bawazir, M.; Islam, M.N.; Cohen, D.M.; Fitzpatrick, S.; Bhattacharyya, I. Gingival Fibroma: An Emerging Distinct Gingival Lesion with Well-Defined Histopathology. Head. Neck Pathol. 2021, 15, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Lázare, H.; Peteiro, A.; Pérez Sayáns, M.; Gándara-Vila, P.; Caneiro, J.; García-García, A.; Antón, I.; Gándara-Rey, J.M.; Suárez-Peñaranda, J.M. Clinicopathological Features of Peripheral Ossifying Fibroma in a Series of 41 Patients. Br. J. Oral Maxillofac. Surg. 2019, 57, 1081–1085. [Google Scholar] [CrossRef]
- Shahrabi-Farahani, S.; Pencarinha, D.M.; Anderson, M. SATB2 Immunoexpression in Peripheral Ossifying Fibroma and Peripheral Odontogenic Fibroma. Head. Neck Pathol. 2022, 16, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.-R.; Li, X.-F.; Zhang, R.; Chen, Y.; Li, T.-J. GNAS Mutational Analysis in Differentiating Fibrous Dysplasia and Ossifying Fibroma of the Jaw. Mod. Pathol. 2013, 26, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Dutra, K.L.; Longo, L.; Grando, L.J.; Rivero, E.R.C. Incidence of Reactive Hyperplastic Lesions in the Oral Cavity: A 10 Year Retrospective Study in Santa Catarina, Brazil. Braz. J. Otorhinolaryngol. 2019, 85, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Rallan, M.; Pathivada, L.; Rallan, N.S.; Grover, N. Peripheral Ossifying Fibroma. Case Reports 2013, 2013, bcr2013009010–bcr2013009010. [Google Scholar] [CrossRef]
- Mortazavi, H.; Safi, Y.; Baharvand, M.; Rahmani, S.; Jafari, S. Peripheral Exophytic Oral Lesions: A Clinical Decision Tree. Int. J. Dent. 2017, 2017, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Anuradha, B.R.; Penumarty, S.; Charan, C.R.; Swati, M. Application of 810-Nm Diode Laser in the Management of Peripheral Ossifying Fibroma. J. Indian. Soc. Periodontol. 2015, 19, 224–226. [Google Scholar] [CrossRef]
- Yu, J.L.; Kapur, R.P.; Susarla, S.M. Recurrent Gingival Lesions in a Pediatric Patient. Plast. Reconstr. Surg. - Glob. Open 2022, 10, e4382. [Google Scholar] [CrossRef] [PubMed]
- Maymone, M.B.C.; Greer, R.O.; Burdine, L.K.; Dao-Cheng, A.; Venkatesh, S.; Sahitya, P.C.; Maymone, A.C.; Kesecker, J.; Vashi, N.A. Benign Oral Mucosal Lesions: Clinical and Pathological Findings. J. Am. Acad. Dermatol. 2019, 81, 43–56. [Google Scholar] [CrossRef] [PubMed]
- S, S.; Gujjari, S.K.; Sreeshyla, H.S. Peripheral Ossifying Fibroma in Pregnancy: A Multifactorial Consequence. IJMDS 2014, 518–523. [Google Scholar] [CrossRef]
- Franco-Barrera, M.J.; Zavala-Cerna, M.G.; Fernández-Tamayo, R.; Vivanco-Pérez, I.; Fernández-Tamayo, N.M.; Torres-Bugarín, O. An Update on Peripheral Ossifying Fibroma: Case Report and Literature Review. Oral Maxillofac. Surg. 2016, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hsien-Yen Hung; Chia-Chuan Chang; Julia Yu-Fong Chang; Chuan-Hang Yu; Yi-Ping Wang; Bu-Yuan Liu; Chun-Pin Chiang Peripheral Ossifying Fibroma: A Clinicopathological Study of 27 Cases. J. Dent. Sci. 2007, 2. [CrossRef]
- Ojo, M.A.; Omoregie, O.F.; Altini, M.; Coleman, H. A Clinico-Pathologic Review of 56 Cases of Ossifying Fibroma of the Jaws with Emphasis on the Histomorphologic Variations. Niger. J. Clin. Pract. 2014, 17, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Childers, E.L.B.; Morton, I.; Fryer, C.E.; Shokrani, B. Giant Peripheral Ossifying Fibroma: A Case Report and Clinicopathologic Review of 10 Cases from the Literature. Head. Neck Pathol. 2013, 7, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Godinho, G.V.; Silva, C.A.; Noronha, B.R.; Silva, E.J.; Volpato, L.E. Peripheral Ossifying Fibroma Evolved From Pyogenic Granuloma. Cureus 2022, 14, e20904. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Chandra, S.; Gupta, S.; Srivastava, S. Heterogeneous Conceptualization of Etiopathogenesis: Oral Pyogenic Granuloma. Natl. J. Maxillofac. Surg. 2019, 10, 3. [Google Scholar] [CrossRef]
- Lima, M.D.M.; Teixeira, R.G.; Bonecker, M.; De Camargo Moraes, P.; Mantesso, A. Recurrent Multicentric Peripheral Ossifying Fibroma-like Lesion in a Child: A Case Report. BMC Res. Notes 2014, 7, 673. [Google Scholar] [CrossRef]
- Agarwal, P.; Chug, A.; Kumar, S.; Jain, K. Palatal Peripheral Ossifying Fibroma: A Rare Occurrence. Int. J. Health Sci. (Qassim) 2019, 13, 63–66. [Google Scholar] [PubMed]
- Popli, H.; Singh, H.; Gupta, A.; Kamboj, M. Peripheral Ossifying Fibroma Veiling a Nasopalatine Duct Cyst: An Unusual Concurrence. Indian. J. Otolaryngol. Head. Neck Surg. 2022, 74, 1459–1461. [Google Scholar] [CrossRef] [PubMed]
- Moon, W.-J.; Choi, S.Y.; Chung, E.C.; Kwon, K.H.; Chae, S.W. Peripheral Ossifying Fibroma in the Oral Cavity: CT and MR Findings. Dentomaxillofac Radiol. 2007, 36, 180–182. [Google Scholar] [CrossRef] [PubMed]
- Buchner, A.; Shnaiderman, A.; Vared, M. Pediatric Localized Reactive Gingival Lesions: A Retrospective Study from Israel. Pediatr. Dent. 2010, 32, 486–492. [Google Scholar] [PubMed]
- Ferraresso, L.F.O.T.; Fagundes, F.A.U.; Padovese, M.; Singi, P.; Paiva, M.F.; Inagaki-Nomura, L.T.; Dezan-Garbelini, C.C.; Boer, F.A.C. Peripheral Odontogenic Fibroma in a Child with Ellis-van Creveld Syndrome: Case Report. Spec. Care Dent. 2023, scd.12855. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Gupta, S.; Hussain, I.; Augustine, J.; Ghosh, S.; Gupta, S. A Rare Case of Peripheral Ossifying Fibroma in an Infant. Contemp. Clin. Dent. 2021, 12, 81. [Google Scholar] [CrossRef] [PubMed]
- Tavares, T.S.; Da Costa, A.A.S.; Freire-Maia, F.B.; Souza, L.N.; Zarzar, P.M.; Martins-Júnior, P.A.; Aguiar, M.C.F.; Mesquita, R.A.; Caldeira, P.C. Unusual Exophytic Gingival Lesion in a Newborn Treated with Diode Laser. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 130, e74–e79. [Google Scholar] [CrossRef] [PubMed]
- Choubey, S.; Banda, N.R.; Banda, V.R.; Vyawahare, S. Peripheral Cementifying Fibroma: A Clinical Diagnostic Dilemma. Case Reports 2013, 2013, bcr2013009472–bcr2013009472. [Google Scholar] [CrossRef] [PubMed]
- Hasanuddin, S.; Malleshwar, Y. Idiopathic Peripheral Ossifying Fibroma in a Young Adolescent Girl: A Very Rare Clinical Presentation. J. Indian. Soc. Periodontol. 2017, 21, 329–332. [Google Scholar] [CrossRef]
- Nair, K.K.; Nausheen, E.; Chaudhuri, K.; Hariharan, M.; Ramesh, S. Laser-Assisted Management of a Rare Presentation of Peripheral Ossifying Fibroma in an Infant. Cureus 2021, 13, e20417. [Google Scholar] [CrossRef]
- Tewari, N.; Mathur, V.P.; Mridha, A.; Bansal, K.; Sardana, D. 940 Nm Diode Laser Assisted Excision of Peripheral Ossifying Fibroma in a Neonate. Laser Ther. 2017, 26, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Delbem, A.C.B.; Cunha, R.F.; Silva, J.Z.; Soubhia, A.M.P. Peripheral Cemento-Ossifying Fibroma in Child. A Follow-up of 4 Years. Report of a Case. Eur. J. Dent. 2008, 2, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Rm.; Bavle, R.; Umashankar, D.; Sharma, R. Congenital Epulis of the Newborn. J. Oral Maxillofac. Pathol. 2015, 19, 407. [Google Scholar] [CrossRef]
- Crusoé-Rebello, I.; Torres, M.; Burgos, V.; Oliveira, C.; Santos, J. dos; Azevedo, R.; Campos, P. Hybrid Lesion: Central Giant Cell Granuloma and Benign Fibro-Osseous Lesion. Dentomaxillofacial Radiol. 2009, 38, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Missias, E.M.; Nascimento, E.; Pontual, M.; Pontual, A.A.; Freitas, D.Q.; Perez, D.; Ramos-Perez, F. Prevalence of Soft Tissue Calcifications in the Maxillofacial Region Detected by Cone Beam CT. Oral Dis. 2018, 24, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Galeotti, A.; Garret Bernardin, A.; D’Antò, V.; Ferrazzano, G.F.; Gentile, T.; Viarani, V.; Cassabgi, G.; Cantile, T. Inhalation Conscious Sedation with Nitrous Oxide and Oxygen as Alternative to General Anesthesia in Precooperative, Fearful, and Disabled Pediatric Dental Patients: A Large Survey on 688 Working Sessions. Biomed. Res. Int. 2016, 2016, 7289310. [Google Scholar] [CrossRef] [PubMed]
- Katanec, T.; Budak, L.; Brajdić, D.; Gabrić, D. Atypical Peripheral Ossifying Fibroma of the Mandible. Dent. J. (Basel) 2022, 10, 9. [Google Scholar] [CrossRef]
- Cavalcante, I.-L.; Barros, C.-C.; Cruz, V.-M.; Cunha, J.-L.; Leão, L.-C.; Ribeiro, R.-R.; Turatti, E.; Andrade, B.-A.; Cavalcante, R.-B. Peripheral Ossifying Fibroma: A 20-Year Retrospective Study with Focus on Clinical and Morphological Features. Med. Oral Patol. Oral Cir. Bucal 2022, 27, e460–e467. [Google Scholar] [CrossRef] [PubMed]
- Verma, E.; Chakki, A.B.; Nagaral, S.C.; Ganji, K.K. Peripheral Cemento-Ossifying Fibroma: Case Series Literature Review. Case Rep. Dent. 2013, 2013, 930870. [Google Scholar] [CrossRef]
- Ortega-Concepción, D.; Cano-Durán, J.A.; Peña-Cardelles, J.-F.; Paredes-Rodríguez, V.-M.; González-Serrano, J.; López-Quiles, J. The Application of Diode Laser in the Treatment of Oral Soft Tissues Lesions. A Literature Review. J. Clin. Exp. Dent. 2017, 9, e925–e928. [Google Scholar] [CrossRef]
- Capodiferro, S.; Loiudice, A.M.; Pilolli, G.; Lajolo, C.; Giuliani, M.; Maiorano, E.; Favia, G. Diode Laser Excision of Chondroid Lipoma of the Tongue with Microscopic (Conventional and Confocal Laser Scanning) Analysis. Photomed. Laser Surg. 2009, 27, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Capodiferro, S.; Kazakova, R. Laser-Assisted Gingivectomy to Treat Gummy Smile. Dent. Clin. North. Am. 2022, 66, 399–417. [Google Scholar] [CrossRef] [PubMed]
- Limongelli, L.; Capodiferro, S.; Tempesta, A.; Sportelli, P.; Dell’Olio, F.; Angelelli, G.; Maiorano, E.; Favia, G. Early Tongue Carcinomas (Clinical Stage I and II): Echo-Guided Three-Dimensional Diode Laser Mini-Invasive Surgery with Evaluation of Histological Prognostic Parameters. A Study of 85 Cases with Prolonged Follow-Up. Lasers Med. Sci. 2020, 35, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Capodiferro, S.; Limongelli, L.; Tempesta, A.; Maiorano, E.; Favia, G. Diode Laser Treatment of Venous Lake of the Lip. Clin. Case Rep. 2018, 6, 1923–1924. [Google Scholar] [CrossRef] [PubMed]
- Tenore, G.; Mohsen, A.; Nuvoli, A.; Palaia, G.; Rocchetti, F.; Di Gioia, C.R.T.; Cicconetti, A.; Romeo, U.; Del Vecchio, A. The Impact of Laser Thermal Effect on Histological Evaluation of Oral Soft Tissue Biopsy: Systematic Review. Dent. J. 2023, 11, 28. [Google Scholar] [CrossRef]
- AAPD. Policy on use of Lasers for pediatric dental patients. AAPD Reference Manual 2013, 36, 75–77.
- American Academy of Pediatric Dentistry. Policy on the use of lasers for pediatric dental patients. The Reference Manual of Pediatric Dentistry. Chicago, Ill.: American Academy of Pediatric Dentistry; 2022, 131–134.
| AUTHOR | YEAR | PATIENTS | AGE | SEX | LOCALIZATION | DIMENSIONS | CLINICAL PRESENTATION | RADIOLOGICAL EXAMS |
ANESTHESIOLOGICAL REGIMEN | DIAGNOSIS | RECURRENCE |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Deus Moura Lima [20] |
2014 | 1 | 9 | M | Generalized | Diffuse | Extensive exophytic, sessile, firm lesion with areas of ulcerated surface and a pink-to-red coloration that covered almost all the upper and lower teeth | OPT Periapical radiogram |
Local anesthesia | POF | 30 times |
|
Botazzo Delbem [32] |
2008 | 1 | 5 | F | Anterior maxilla Alveolar ridge |
1,5 cm | “lump” in the right upper alveolar margin between teeth 52 and 53 | Periapical radiogram | Local anesthesia | PCOF | No |
|
Rallan [8] |
2013 | 1 | 12 | M | Anterior maxilla Retroincisal (palate) |
2 × 2 cm | well-circumscribed, sessile, and erythematous upper retroincisal swelling, firm on palpation | Periapical radiogram | Local anesthesia | POF | No |
|
Choubey [28] |
2013 | 1 | 13 | F | Anterior mandibula | 3 × 1.5 cm | Large gingival overgrowth | Periapical radiogram | Local anesthesia | PCF | No |
|
Singh [26] |
2020 | 1 | 5 months | M | Anterior mandibula | 1.5 × 1 cm | Two distinct, firm, soft pedunculated growths; normal-appearing overlying mucosa | Periapical radiogram | Local anesthesia | POF | No |
|
Franco-Barrera [14] |
2015 | 1 | 11 | F | Anterior maxilla Right canine region |
4 × 3 cm | sessile exophytic lesion without changes in color, indurations, or ulceration. | OPT CT |
General anesthesia | POF | No |
|
Yu [11] |
2022 | 1 | 13 | F | Generalized | Diffuse | Diffuse hyperplastic gingival tissue across the maxillary and mandibular alveolar segments | OPT | Local anesthesia | POF | 1 time |
|
Hasanuddin [29] |
2017 | 1 | 15 | F | Anterior maxilla Vestibular middle-line |
2.4 × 2 cm | Elevated, oval-shaped mass with a smooth, shiny surface without bleeding or ulceration. | OPT | Local anesthesia | POF | No |
|
Tomáz Ferraresso [25] |
2022 | 1 | 6 | M | Anterior mandibula | 0.5 × 1 cm | Sessile, firm, whitish nodule located on the alveolar ridge | OPT | Local anesthesia | POF | No |
|
Soares Tavares [27] |
2020 | 1 | 2 months | F | Anterior mandibula | 1 x 0.5 cm | Nodular, firm, pedunculated lesion and covered by pink, flat, smooth mucosa | Occlusal radiogram | Local anesthesia | POF | No |
|
Nair [30] |
2021 | 1 | 3 months | F | Anterior mandibula | 0.5 x 1 cm | Solitary pedunculated nodular swelling | No radiograph | Local anesthesia | POF | No |
|
Tewari [31] |
2017 | 1 | 2 months | M | Anterior mandibula | 2.5 x 1 cm | Pink, nodular, pedunculated growth with smooth, intact surface | No radiograph | Local anesthesia | POF | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





