1. Introduction
Left ventricle free wall rupture (LVFWR) is a life-threatening mechanical complication of acute myocardial infraction (AMI). Although nowadays rare, due to early and effective reperfusion therapy, it may represent significant diagnostic and therapeutic dilemma and is associated with high morbidity and mortality. Early identification and effective surgical treatment may save the patient’s life. We present our case report of LVFWR leading to double false aneurism of left ventricular inferior wall to discuss the management of this uncommon but important entity.
2. Case
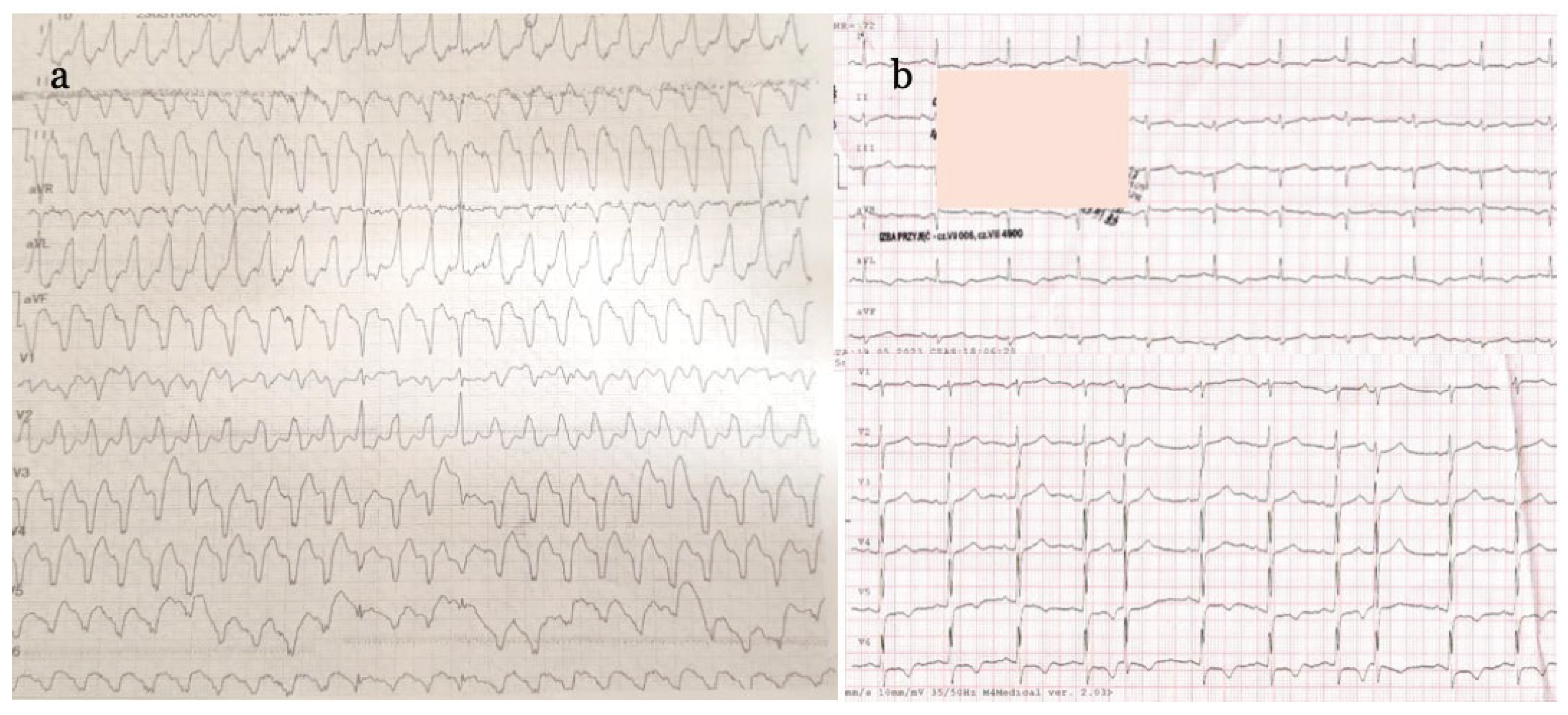
55-year-old male patient, without previous cardiovascular history but with typical atherosclerosis risk factors (obesity, dyslipidemia, smoking), was admitted May last year to emergency department (ED) of our hospital after an episode of syncope with dyspnea, lightheadedness, and palpitations. He reported chest pain of two weeks duration and progressive weakness. On physical examination the patient was hypotensive, had irregular and weak arterial pulse, tachypnea and orthopnea, pulmonary rales, and desaturation. On electrocardiogram (ECG) wide-QRS complex tachycardia was evident (
Figure 1a). He received oxygen face mask and diuretics and, while preparing for electrical cardioversion, was administered amiodarone 600 mg iv bolus which successfully stopped ventricular tachycardia (VT). However, atrial fibrillation appeared with pathological Q waves in inferior leads and additional signs of antero-lateral ischemia - inverted T waves in I, aVL, and V5-V6 precordial leads (
Figure 1b).
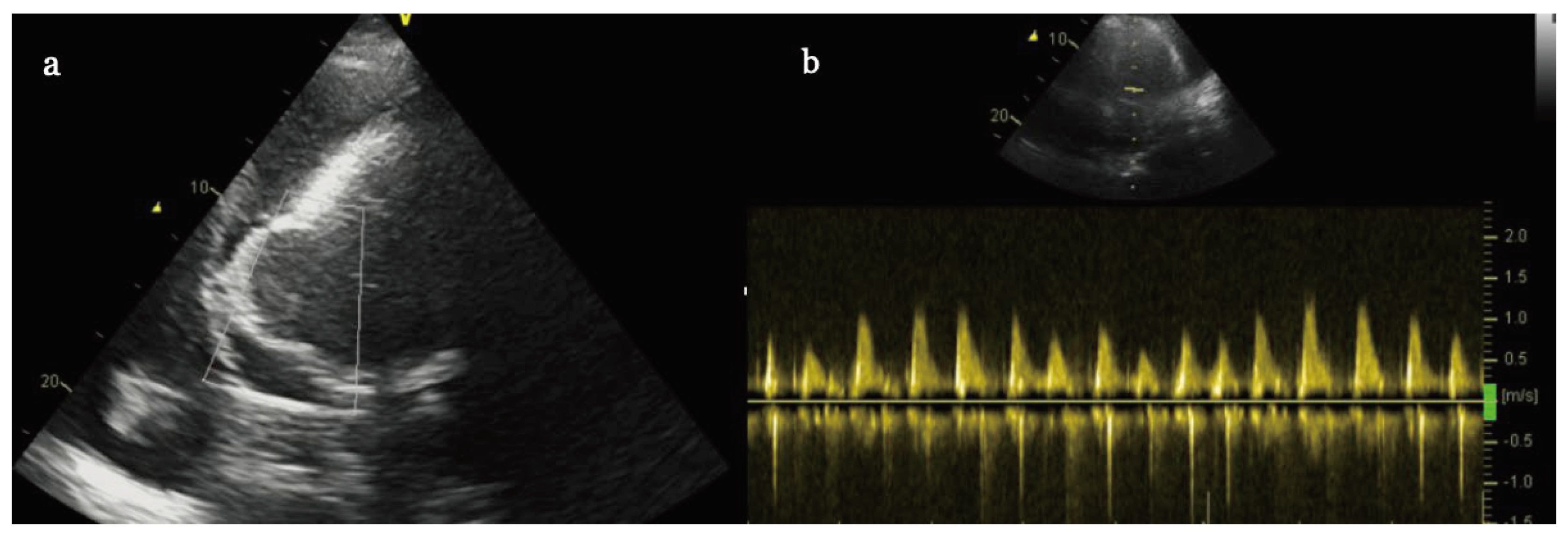
Laboratory samples were positive for high sensitivity troponins and D-dimers. On echocardiographic (ECHO) examination, significant volume (4,5 cm) of pericardial effusion with signs of cardiac tamponade was observed with global left ventricle ejection fraction (EF) of only 25%. In addition, we observed thin walled, partially hyperechoic and dyskinetic aneurism in inferior location (
Figure 2 a-b). It is worth to mention that because of acute dyspnea and orthopnea the patient was unable to recumbent which made ECHO examination very difficult with poor acustic window and only subcostal projections were useful to recognize the aneurysm.
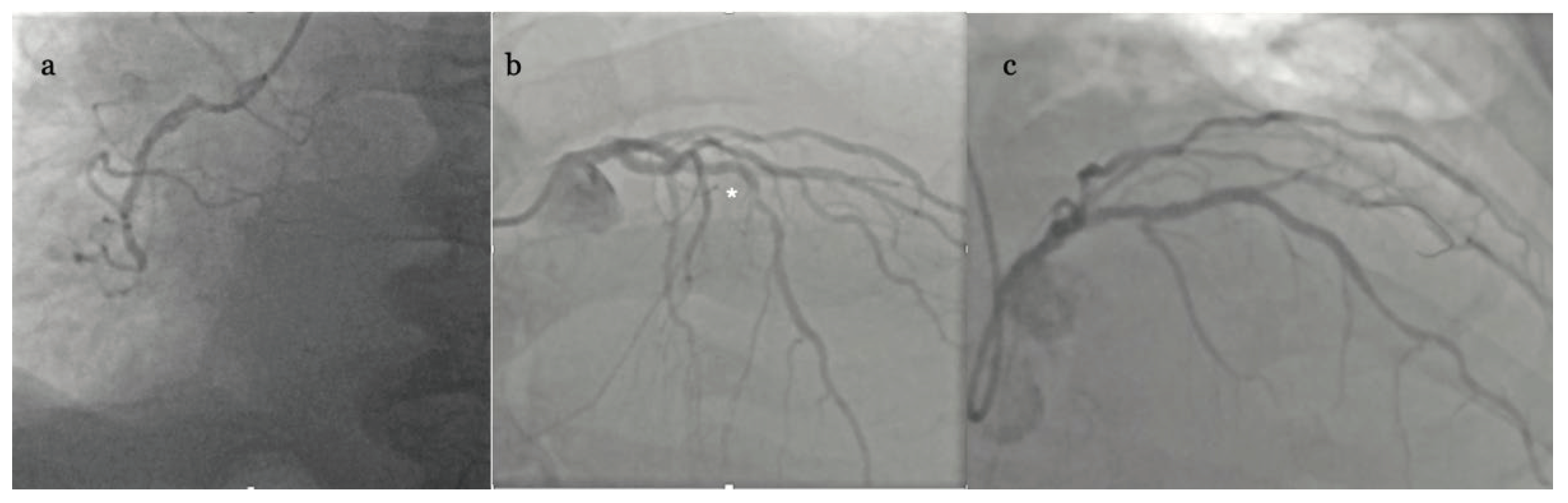
The patient was admitted to our invasive cardiology ward and sent for urgent coronary angiography (CAG) which revealed occlusion of right coronary artery (RCA) in 2
nd segment without collateral circulation and significant stenosis in middle (7
th) segment of left anterior descending artery (LAD) (
Figure 3 a-b).
At this point the whole clinical picture indicated recent but sustained transmural myocardial infarction complicated by subacute left ventricular inferior free wall rupture (LVFWR) with subsequent formation of false aneurysm. No loading dose of P2Y12 inhibitors were given and there was no attempt at reopening of RCA (signs of necrosis on ECG and ECHO, no residual chest pain), instead urgent cardiosurgical consultation was requested. The decision was made to send the patient to reference cardiovascular surgery center (120 km apart) with the idea of definite repair (aneurism excision, left internal mammary bypass graft to LAD). The transport was uneventful and done by helicopter emergency medical service (HEMS). But the surgical team on-duty perceived the risk of open-heart surgery too high and instead performed only drainage of blood from pericardial sac (1300 cc) using substernal approach.
After intervention the patient was sent back to our institution, was hemodynamically stable and soon weaned from catecholamines but received antibiotics due to Staphylococcus aureus surgical-site infection. On 10
th postoperative day cardiac computer tomography (CT) was done which showed residual 7 mm of fluid and inferior wall aneurysm of 54 x 82 x 29 mm with only 2,5 mm wall thickness. We asked for Heart Team Consultation pointing again for the need of aneurismectomy, but the decision was made to recommend percutaneous coronary intervention (PCI) of LAD and observe the clinical status of the patient. Accordingly, PCI was performed (18 days from acute presentation) with one drug eluting stent (DES) implantation to mid LAD under intravascular ultrasound (IVUS) guidance with optimal result (
Figure 3c). The patient was discharged home in stable condition on clopidogrel and apixaban, guideline directed heart failure medical therapy as well as on amiodarone treatment due to persisting runs of non-sustained VT (nsVT) on ECG (
Figure 4).
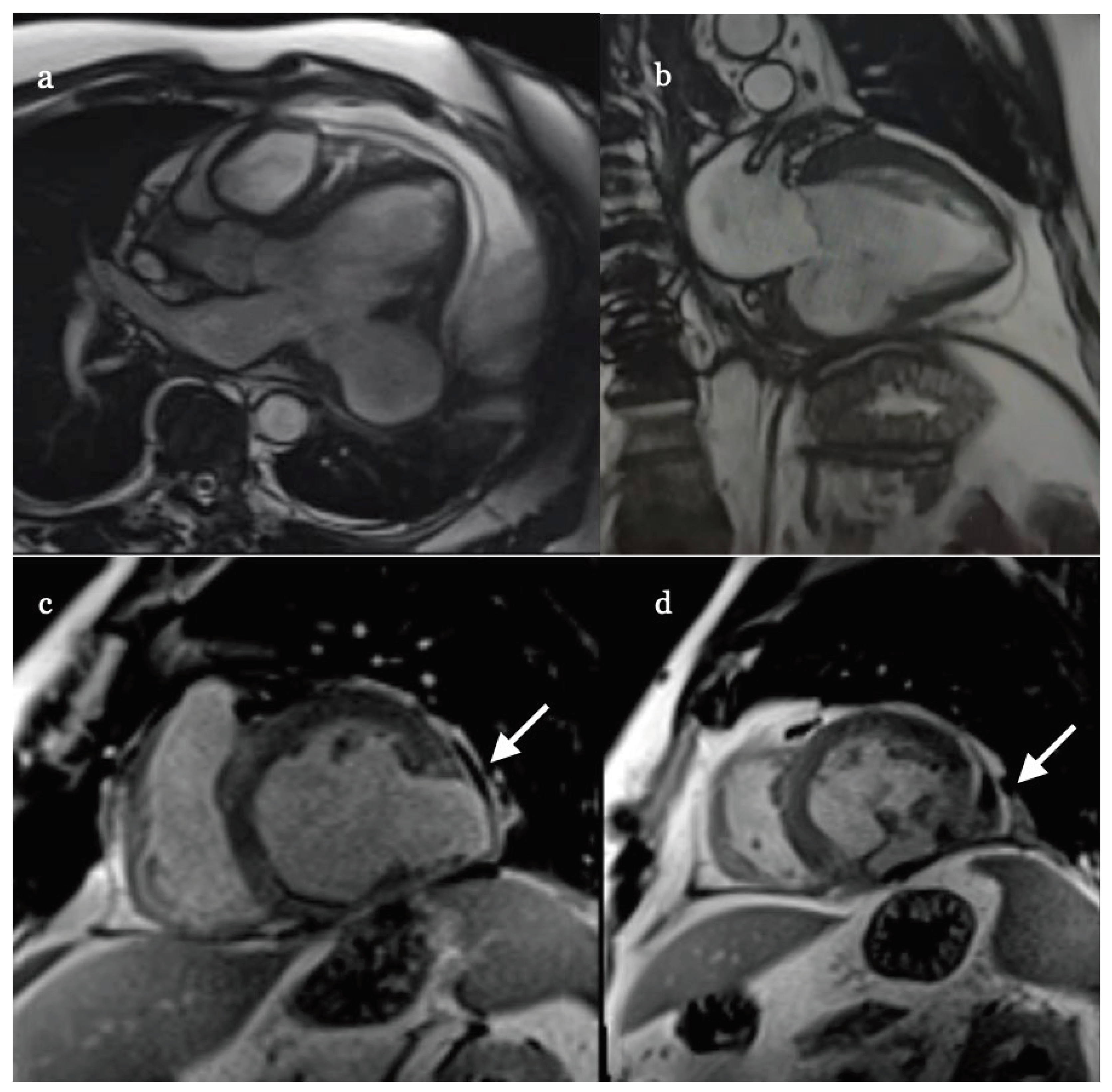
A month later the patient was electively re-admitted for cardiac magnetic resonance imaging (cMRI) which showed large (60 x 50 x 27 mm) and well-organized aneurysm but with further thinning (1 mm) of its wall (
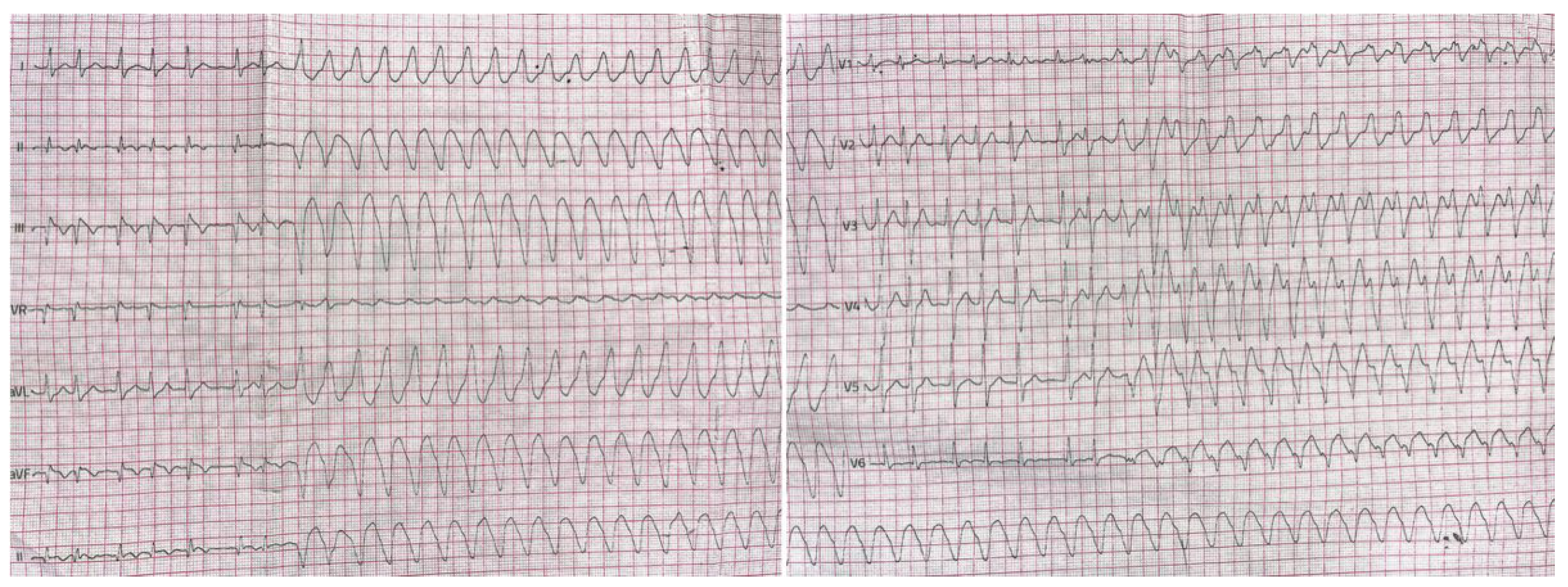
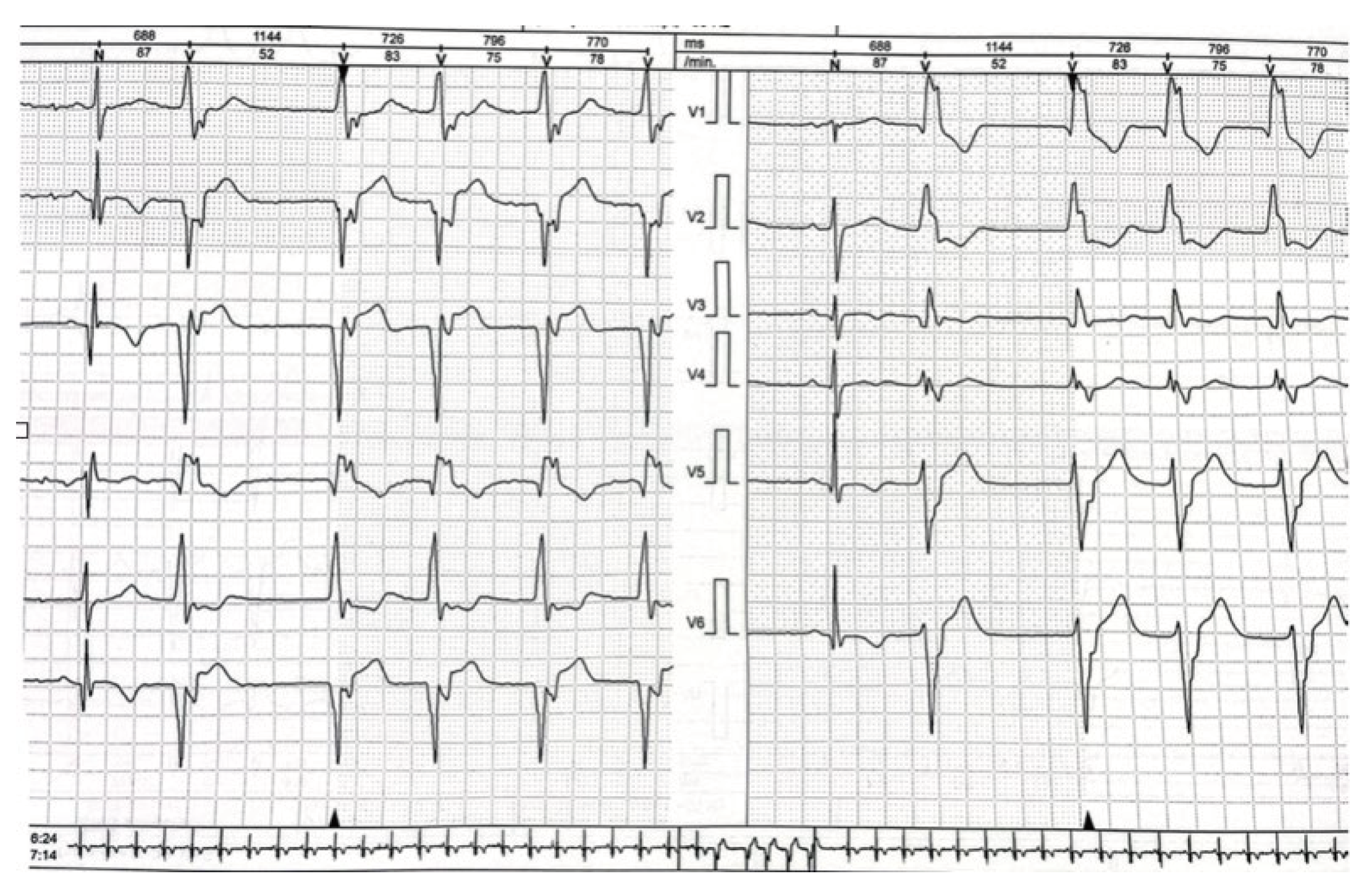
Figure 5 a-d). Because of continuing runs of ventricular rhythm on ECG Holter despite amiodarone oral therapy (
Figure 6), signs, and symptom of heart failure in New York Heart Association (NYHA) II class, and no significant improvement of EF implantable cardioverter-defibrillator and dual chamber stimulator (ICD-DR) was implanted on that occasion as well.
During hospitalization due to progressive thinning of the aneurysm (as evidenced by cMRI) the case was also reconsulted by cardiac surgeon, and this time qualified for open-heart surgery because of perceived very high risk of fatal re-rupture.
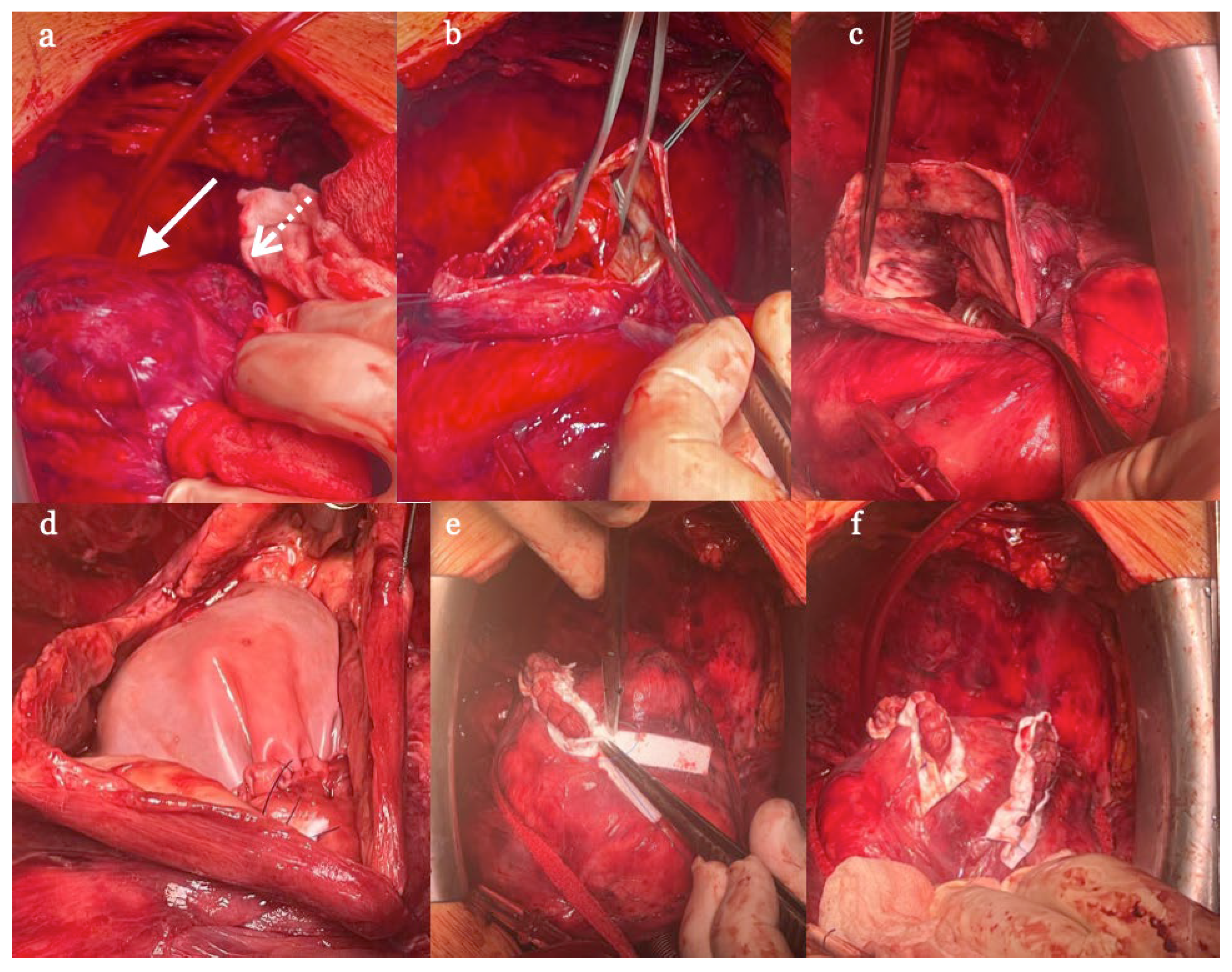
The reconstructive surgery was done electively in September, i.e. 4 months after acute presentation and 3 months after PCI upon temporary withdrawal of Clopidogrel. Intraoperatively rather unexpectedly two aneurysms were found. The larger one was cut open and thrombus material was removed. A bovine pericardial patch was used to close the defect preserving the left ventricular volume and shape. The smaller one was directly closed with double layer suture line. (
Figure 7 a-f). Patient was discharged home on 8
th postoperative day. On follow-up visit one month after the surgery (October last year) ICD-DR interrogation found no recurrence of VT, sinus rhythm, and 25% of atrial pacing (probably the effect of combined therapy of Metoprolol and Amiodarone). On ECHO exam done in December (3 months after the operation) EF improved to 38%, and patient was now well and free from heart failure symptoms.
3. Discussion
Left ventricle free wall rupture (LVFWR) is a life-threatening mechanical complication of acute myocardial infraction (AMI). Historically, the first free wall rupture after AMI was described by William Harvey in 1647, and first series of 11 postmortem cases was reported in 1769 by Morgagni, who ironically died himself of ventricular rupture [
1]. Although nowadays extremely rare, thanks to effective and early reperfusion therapy, it may still happen with current literature reporting an incidence between 0.01% and 0.5% of AMI cases [
2]. Unfortunately, it represents significant diagnostic and therapeutic dilemma. First, the patient is usually hemodynamically unstable, hence the cardiac computed tomography (CT) and in particular cardiac magnetic resonance imaging (cMRI) may be impossible to perform and the acustic window of echo examination may be insufficient to make an indisputable diagnosis. Therefore the high level of suspicion is needed. The presence of reduced myocardial wall thickness, hemopericardium or pericardial clots and cardiac tamponade especially in an unstable patient with late presentation AMI, are the most relevant findings. Additionally, color doppler may reveal discontinuity of cardiac wall by showing bi-directional flow between left ventricle and extracardiac echo-free space. For that reason, the echo examination should be the standard of care, otherwise the correct diagnosis might easily be missed, and anticoagulant, dual antiplatelet therapy initiated, and PCI procedure performed which may worsen the situation or even lead to death. In suitable patient cMRI owing to its ability to visualize heart in great detail can complement the diagnosis by identifying contained ventricular rupture, as was documented in our patient.
Still, even when correctly diagnosed, the outcome of patients who develop LVFWR is usually fatal [
3], although some patients with acute or subacute rupture present a window of opportunity for intervention. Prompt surgery though challenging and associated with high mortality remains the only the treatment of choice. Before surgery hemodynamic stabilization by catecholamine infusion, insertion of intraaortic balloon pump (IABP) and pericardial tamponade drainage may be needed (but percutaneous approach can be ineffective because of organized blood clots). In case of cardiac arrest extracorporeal membrane oxygenation (ECMO) may be the only chance for survival.
There exist controversies about whether to perform coronary angiography before surgery. Some advocate definite surgical treatment without any delay to “save time”. However, knowing coronary anatomy beforehand can be very helpful, first because it may help to decide where and how to put sutures during surgery and second in case of presence of significant stenoses in non-infarct related arteries coronary bypass grafting (CABG) can be done on the same occasion which might exert positive impact on survival [
4].
Surgical techniques depend on the kind of the rupture i.e. blowout or oozing type. The first is characterized by abrupt and acute course and is associated with active bleeding and macroscopic tear in the infarcted myocardium. On the other hand, oozing LVFWR is considered an incomplete rupture and have subacute presentation characterized by epicardial extravasation or slow bleeding which may be temporarily sealed by clot or pericardial adhesion. Broadly two different methods of repair exist: sutureless (STL) and sutured (ST). STL technique is accomplished using collagen sponge or pericardium patch fixed on epicardium with surgical adhesive. ST denotes repair with stiches to close the myocardial tear or fix the patch on the myocardium. Initially sutured approached was the only possibility and comprise of linear closure, infarctectomy and closure, and finally patch covering. In linear closure technique the ventricular tear is closed with horizontal Prolene® mattress sutures with two supporting Teflon® felts. It is important not to place the stiches along ischemic areas, because it usually generates myocardial tear and increase bleeding. Infarctectomy and closure comprise excision of necrotic myocardial tissue followed by replacement using a prosthetic patch fashioned to fit the space and sutured with pledgeted interrupted sutures. Finally, in patch covering methode the ventricular rupture and surrounding infarcted myocardium are covered with a patch grafted to the healthy epicardium by Prolene® running sutures with meticulous attention not to involve coronary arteries. More recently invention of surgical glues has allowed broader application of sutureless techniques, even without cardiopulmonary bypass (CBP), but whenever there is an active bleeding there remain concerns whether the glued patch can withstand the intraventricular pressure and prevent pseudoaneurysm formation. In summary, the chosen method depends on type of rupture as was discussed, but the principle is always to relief the tamponade, close the tear anchoring its rims to the healthy tissue preventing re-rupture, and finally save the geometry of the heart [
1].
Because of rarity of LVFWR little is known about clinical results of repair and most published work consist of case reports or single-center experience [
5,
6,
7]. The only multicenter study we found was retrospective and evaluated the early outcome and prognostic factors of operative mortality of 140 patients who underwent cardiac surgery for LVFWR [
7]. The mean age of patients was 69,4 years. The authors identified two types of rupture – blowout (43,6%) and oozing (56,4%). The surgical techniques were ST in 61,4% and STL in the remaining cases. The mean interval from AMI to LVFWR was 50,6±84,7 hours and from LVFWR to surgery was 4,7±6 hours. Overall operative mortality was 36,4% with low cardiac output syndrome as a main cause of perioperative death. Myocardial rerupture after surgery occurred in 10 patients (7.1%). Multivariable analysis revealed that preoperative left ventricular ejection fraction (P < .001), cardiac arrest at presentation (P=.011), female sex (P= .044), and the need for preoperative extracorporeal life support (P= .003) were independent predictors for operative mortality [
8].
In another interesting study, the authors were able to gather retrospectively 35 cases of LVFWR operated in a single cardiac surgical center at a time span of 29 years (1990-2019). The mean age of patients was 68,9 years, 65,7% were male. The oozing type was encountered in 29 individuals, and blowout in 6 subjects. Sutured repaired was performed in 77,1%. In-hospital mortality rate was 28.6% with low cardiac output syndrome as a main cause of death. The multivariable analysis identified age >75 years, preoperative cardiac arrest and concurrent ventricular septal rupture (VSR) as independent predictors of in-hospital death [
7].
In case of subacute presentation of post AMI left ventricular aneurysm with concomitant ventricular septal rupture (VSD) there is increasingly used strategy of delayed surgery with the use of mechanical support devices. Temporary veno-arterial extracorporeal membrane oxygenation (VA-ECMO) may significantly contribute to good therapeutic results by stabilizing the patient, maintaining tissue oxygenation, obtaining time for myocardial remodeling, and elimination of the effect of antiplatelet therapy. Additionally, in VSD even without shock, ECMO help unload the ventricle and prevent deterioration [
5].
Our case is unique for two reasons. The first is that the surgery was very late (4
th months after AMI), which is rather exceptional, and one may even argue that it was only a play of chance that the patient had not died suddenly before. While there are reports of LVFWR treated conservatively [
9] delay or even withhold from surgery could not be universally recommended, because overall survival of this approach is poor – only 10% as evidenced in the retrospective cohort study of 107 patients [
10]. Indeed, despite our patient was clinically stable, we observed progressive thinning of the wall indicating the imminent risk of cardiac rupture despite our recommendation to completely refrain from strenuous activities. The second issue was ventricular arrythmia originating from aneurysmal tissue which was resistant to pharmacologic treatment necessitating ICD implantation, but which disappeared after the operation. The third problem was the thromboembolic risk associated with thrombus formation inside the cavity of aneurysm. Our patient received non-vitamin K anticoagulant apixaban, because of concomitant atrial fibrillation which, although debatable, was successful in prevention of the embolic events. Finally, the large aneurysm worsened patient’s left ventricular function aggravating heart failure symptoms as evidenced by improvement of EF and his clinal status after the surgery.
The other peculiarity is that in fact two false aneurysms were discovered during the surgery, although it was not detected in radiologic examinations. One larger and closer to apex of left ventricle which was repaired with the pericardial patch and the smaller one nearer to the interventricular septum and the basis of the heart directly closed with double layer suture line. The possible explanation of this finding is that more than one rapture of ventricular free wall might had occurred on two separate occasions indicating even higher risk of death for that patient.
4. Conclusions
In conclusion, we presented unusual case of double left ventricular false aneurysm complicating late presentation AMI successfully treated successfully with the combination of urgent surgical drainage of tamponade, staged PCI of non-culprit lesion, preventive implantation of ICD-DR, guideline directed heart failure medical therapy and delayed but effective reconstructive operation.
Funding
This research received no external funding.
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- M. Matteucci et al., “Treatment strategies for post-infarction left ventricular free-wall rupture,” Eur. Hear. J. Acute Cardiovasc. Care, vol. 8, no. 4, pp. 379–387, 2019. [CrossRef]
- Elbadawi et al., “Temporal Trends and Outcomes of Mechanical Complications in Patients With Acute Myocardial Infarction,” JACC Cardiovasc. Interv., vol. 12, no. 18, pp. 1825–1836, 2019. [CrossRef]
- J. K. French et al., “Mechanical Complications After Percutaneous Coronary Intervention in ST-Elevation Myocardial Infarction (from APEX-AMI),” Am. J. Cardiol., vol. 105, no. 1, pp. 59–63, 2010. [CrossRef]
- Leva C, Bruno PG, Gallorini C, et al. Complete myocardial revascularization and sutureless technique for left ventricu- lar free wall rupture: Clinical and echocardiographic results. Interact Cardiovasc Thorac Surg 2006; 5: 408–412. [CrossRef]
- K. Wróbel, K. Zbikowska, R. Wojdyga, E. Pirsztuk, M. Zygier, and K. Kurnicka, “The role of temporary mechanical circulatory support in an effective surgical treatment of a left ventricular aneurysm and a ventricular septal defect in a patient after anterior wall myocardial infarction,” Kardiol. Pol., vol. 79, no. 6, pp. 718–719, 2021. [CrossRef]
- S. Gill, D. J. Rakhit, S. K. Ohri, and S. P. Harden, “Left ventricular true and false aneurysms identified by cardiovascular magnetic resonance,” Br. J. Radiol., vol. 84, no. 998, pp. 308–309, 2011. [CrossRef]
- Matteo Matteucci Sandro Ferrarese Vittorio Mantovani et al., “Surgical repair of left ventricular free-wall rupture complicating acute myocardial infarction: a single-center 30 years of experience,” Front. Cardiovasc. Med., vol. Volume 10, 2023. [CrossRef]
- M. Matteucci et al., “Surgical Treatment of Post-Infarction Left Ventricular Free-Wall Rupture: A Multicenter Study,” Ann. Thorac. Surg., vol. 112, no. 4, pp. 1186–1192, 2021. [CrossRef]
- L. Yan, H. Wang, B. Su, J. Fan, M. Wang, and X. Zhao, “Survival after left ventricular free wall rupture following acute myocardial infarction by conservative treatment,” Am. J. Emerg. Med., vol. 39, pp. 21–23, 2021. [CrossRef]
- Blinc A, Noc M, Pohar B, et al. Subacute rupture of the left ventricular free wall after acute myocardial infarction. Three cases of long-term survival without emergency surgical repair. Chest 1996; 109: 565–567. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).