Submitted:
29 February 2024
Posted:
29 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Sample and group formation
2.2. Electrocardiographic data
2.3. Patient follow-up
2.4. Statistical analysis
3. Results
3.1. Sample analysis
3.2. ECG data
3.2.1. QRS complex width measurement
3.2.2. ST-segment elevation
3.3. Follow-up data
3.3.1. 6-month follow-up
3.3.2. 6-year follow-up
3.4. ECG data in MACE prediction
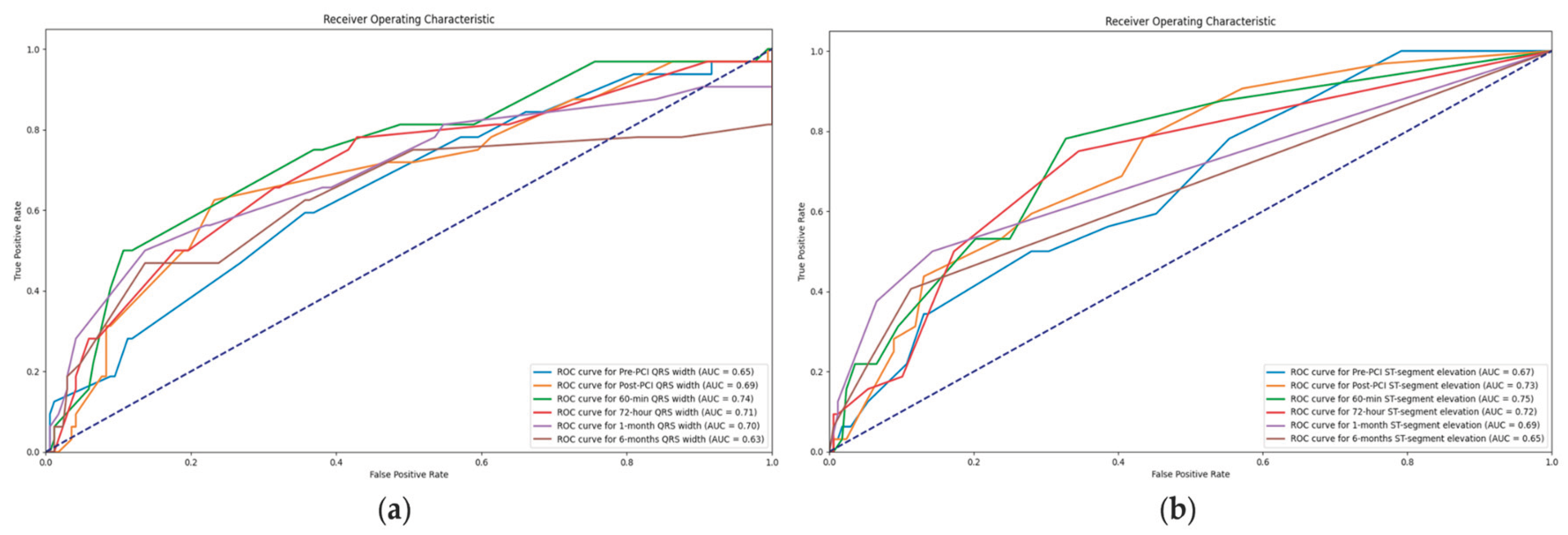
3.4.1. Value of QRS complex width in association with MACE
3.4.2. Value of ST-segment elevation in association with MACE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Liu, M.; Pan, J.; Meng, K.; Wang, Y.; Sun, X.; Ma, L.; Yu, X. Triglyceride-Glucose Body Mass Index Predicts Prognosis in Patients with ST-Elevation Myocardial Infarction. Sci Rep 2024, 14 (1), 976. [CrossRef]
- He, J.; Kong, L.; An, D.; Chen, B.; Zhao, C.; Li, Z.; Yang, F.; Dong, J.; Wei, L.; Shan, P.; Chen, Y.; Wu, L.; Xu, J.; Ge, H.; Pu, J. Prognostic Value of Segmental Strain After ST-Elevation Myocardial Infarction: Insights From the EARLY Assessment of MYOcardial Tissue Characteristics by Cardiac Magnetic Resonance (EARLY-MYO-CMR) Study. Journal of Magnetic Resonance Imaging n/a (n/a). [CrossRef]
- Giustino, G.; Mehran, R.; Dangas, G. D.; Kirtane, A. J.; Redfors, B.; Généreux, P.; Brener, S. J.; Prats, J.; Pocock, S. J.; Deliargyris, E. N.; Stone, G. W. Characterization of the Average Daily Ischemic and Bleeding Risk After Primary PCI for STEMI. Journal of the American College of Cardiology 2017, 70 (15), 1846–1857. [CrossRef]
- Tsai, I.-T.; Wang, C.-P.; Lu, Y.-C.; Hung, W.-C.; Wu, C.-C.; Lu, L.-F.; Chung, F.-M.; Hsu, C.-C.; Lee, Y.-J.; Yu, T.-H. The Burden of Major Adverse Cardiac Events in Patients with Coronary Artery Disease. BMC Cardiovasc Disord 2017, 17 (1), 1. [CrossRef]
- Poudel, I.; Tejpal, C.; Rashid, H.; Jahan, N. Major Adverse Cardiovascular Events: An Inevitable Outcome of ST-Elevation Myocardial Infarction? A Literature Review. Cureus 2019. [CrossRef]
- Miao, B.; Hernandez, A. V.; Alberts, M. J.; Mangiafico, N.; Roman, Y. M.; Coleman, C. I. Incidence and Predictors of Major Adverse Cardiovascular Events in Patients With Established Atherosclerotic Disease or Multiple Risk Factors. Journal of the American Heart Association 2020, 9 (2), e014402. [CrossRef]
- Pedersen, F.; Butrymovich, V.; Kelb, æk H.; Wachtell, K.; Helqvist, S.; Kastrup, J.; Holmvang, L.; Clemmensen, P.; Engstr, øm T.; Grande, P.; Saunam, äki K.; J, ørgensen E. Short- and Long-Term Cause of Death in Patients Treated With Primary PCI for STEMI. Journal of the American College of Cardiology 2014, 64 (20), 2101–2108. [CrossRef]
- Tanriverdi, Z.; Dursun, H.; Simsek, M. A.; Unal, B.; Kozan, O.; Kaya, D. The Predictive Value of Fragmented QRS and QRS Distortion for High-Risk Patients with STEMI and for the Reperfusion Success. Annals of Noninvasive Electrocardiology 2015, 20 (6), 578–585. [CrossRef]
- Savonitto, S.; Ardissino, D.; Granger, C. B.; Morando, G.; Prando, M. D.; Mafrici, A.; Cavallini, C.; Melandri, G.; Thompson, T. D.; Vahanian, A.; Ohman, E. M.; Califf, R. M.; Van de Werf, F.; Topol, E. J. Prognostic Value of the Admission Electrocardiogram in Acute Coronary Syndromes. JAMA 1999, 281 (8), 707–713. [CrossRef]
- Rafie, N.; Kashou, A. H.; Noseworthy, P. A. ECG Interpretation: Clinical Relevance, Challenges, and Advances. Hearts 2021, 2 (4), 505–513. [CrossRef]
- Alhamaydeh, M.; Gregg, R.; Ahmad, A.; Faramand, Z.; Saba, S.; Al-Zaiti, S. Identifying the Most Important ECG Predictors of Reduced Ejection Fraction in Patients with Suspected Acute Coronary Syndrome. Journal of Electrocardiology 2020, 61, 81–85. [CrossRef]
- López-Castillo, M.; Aceña, Á.; Pello-Lázaro, A. M.; Viegas, V.; Merchán Muñoz, B.; Carda, R.; Franco-Peláez, J.; Martín-Mariscal, M. L.; Briongos-Figuero, S.; Tuñón, J. Prognostic Value of Initial QRS Analysis in Anterior STEMI: Correlation with Left Ventricular Systolic Dysfunction, Serum Biomarkers, and Cardiac Outcomes. Annals of Noninvasive Electrocardiology 2021, 26 (1), e12791. [CrossRef]
- BUSZMAN, P.; SZAFRANEK, A.; KALARUS, Z.; GASIOR, M. Use of Changes in ST Segment Elevation for Prediction of Infarct Artery Recanalization in Acute Myocardial Infarction. European Heart Journal 1995, 16 (9), 1207–1214. [CrossRef]
- Matetzky, S.; Novikov, M.; Gruberg, L.; Freimark, D.; Feinberg, M.; Elian, D.; Novikov, I.; Di, S. E.; Agranat, O.; Har, -Zahav Yedael; Rabinowitz, B.; Kaplinsky, E.; Hod, H. The Significance of Persistent ST Elevation versus Early Resolution of ST Segment Elevation after Primary PTCA. Journal of the American College of Cardiology 1999, 34 (7), 1932–1938. [CrossRef]
- Resting Heart Rate and Long-Term Outcomes in Patients with Percutaneous Coronary Intervention: Results from a 10-Year Follow-Up of the CORFCHD-PCI Study. https://www.hindawi.com/journals/crp/2019/5432076/ (accessed 2024-02-28).
- Noman, A.; Balasubramaniam, K.; Das, R.; Ang, D.; Kunadian, V.; Ivanauskiene, T.; Zaman, A. G. Admission Heart Rate Predicts Mortality Following Primary Percutaneous Coronary Intervention for ST-Elevation Myocardial Infarction: An Observational Study. Cardiovascular Therapeutics 2013, 31 (6), 363–369. [CrossRef]
- Parodi, G.; Bellandi, B.; Valenti, R.; Memisha, G.; Giuliani, G.; Velluzzi, S.; Migliorini, A.; Carrabba, N.; Antoniucci, D. Heart Rate as an Independent Prognostic Risk Factor in Patients with Acute Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. Atherosclerosis 2010, 211 (1), 255–259. [CrossRef]
- Wah, W.; Pek, P. P.; Ho, A. F. W.; Fook-Chong, S.; Zheng, H.; Loy, E. Y.; Chua, T. S. J.; Koh, T. H.; Chow, K. Y.; Earnest, A.; Pang, J.; Ong, M. E. H. Symptom-to-Door Delay among Patients with ST-Segment Elevation Myocardial Infarction in Singapore. Emergency Medicine Australasia 2017, 29 (1), 24–32. [CrossRef]
- Hafiz, A. M.; Naidu, S. S.; DeLeon, J.; Islam, S.; Alkhatib, B.; Lorenz, M.; D’Elia, A.; Rosenthal, B.; Marzo, K. Impact of First Contact on Symptom Onset–to-Door Time in Patients Presenting for Primary Percutaneous Coronary Intervention. The American Journal of Emergency Medicine 2013, 31 (6), 922–927. [CrossRef]
- De Luca, G.; Parodi, G.; Sciagrà, R.; Venditti, F.; Bellandi, B.; Vergara, R.; Migliorini, A.; Valenti, R.; Antoniucci, D. Time-to-Treatment and Infarct Size in STEMI Patients Undergoing Primary Angioplasty. International Journal of Cardiology 2013, 167 (4), 1508–1513. [CrossRef]
- Symptom onset-to-balloon time and mortality in the first seven years after STEMI treated with primary percutaneous coronary intervention | Heart. https://heart.bmj.com/content/98/23/1738.short (accessed 2024-02-28).
- Birdal, O.; Pay, L.; Aksakal, E.; Yumurtaş, A. Ç.; Çinier, G.; Yücel, E.; Tanboğa, İ. H.; Karagöz, A.; Oduncu, V. Naples Prognostic Score and Prediction of Left Ventricular Ejection Fraction in STEMI Patients. Angiology 2024, 75 (1), 36–43. [CrossRef]
- Lazăr, M.-A.; Ionac, I.; Luca, C.-T.; Petrescu, L.; Vacarescu, C.; Crisan, S.; Gaiță, D.; Cozma, D.; Sosdean, R.; Arnăutu, D.-A.; Cozlac, A.-R.; Luca, S.-A.; Gurgu, A.; Totorean, C.; Mornos, C. Reduced Left Ventricular Twist Early after Acute ST-Segment Elevation Myocardial Infarction as a Predictor of Left Ventricular Adverse Remodelling. Diagnostics 2023, 13 (18), 2896. [CrossRef]
- Evaluation of a QRS scoring system for estimating myocardial infarct size. I. Specificity and observer agreement. | Circulation. https://www.ahajournals.org/doi/abs/10.1161/01.CIR.65.2.342 (accessed 2024-02-28).
- QRS Score at Presentation Electrocardiogram Is Correlated With Infarct Size and Mortality in ST-Segment Elevation Myocardial Infarction Patients Undergoing Primary Percutaneous Coronary Intervention. https://www.jstage.jst.go.jp/article/circj/81/8/81_CJ-16-1255/_article/-char/ja/ (accessed 2024-02-28).
- Das, M. K.; Michael, M. A.; Suradi, H.; Peng, J.; Sinha, A.; Shen, C.; Mahenthiran, J.; Kovacs, R. J. Usefulness of Fragmented QRS on a 12-Lead Electrocardiogram in Acute Coronary Syndrome for Predicting Mortality. The American Journal of Cardiology 2009, 104 (12), 1631–1637. [CrossRef]
- Liu, Q.; Zhang, Y.; Zhang, P.; Zhang, J.; Cao, X.; He, S.; Yang, D. Both Baseline Selvester QRS Score and Change in QRS Score Predict Prognosis in Patients with Acute ST-Segment Elevation Myocardial Infarction after Percutaneous Coronary Intervention. Coron Artery Dis 2020, 31 (5), 403–410. [CrossRef]
- Rodríguez-Palomares, J. F.; Figueras-Bellot, J.; Descalzo, M.; Moral, S.; Otaegui, I.; Pineda, V.; del Blanco, B. G.; González-Alujas, M. T.; Evangelista Masip, A.; García-Dorado, D. Relation of ST-Segment Elevation Before and After Percutaneous Transluminal Coronary Angioplasty to Left Ventricular Area at Risk, Myocardial Infarct Size, and Systolic Function. The American Journal of Cardiology 2014, 113 (4), 593–600. [CrossRef]
- Weaver, J. C.; Ramsay, D. D.; Rees, D.; Binnekamp, M. F.; Prasan, A. M.; McCrohon, J. A. Dynamic Changes in ST Segment Resolution After Myocardial Infarction and the Association with Microvascular Injury on Cardiac Magnetic Resonance Imaging. Heart, Lung and Circulation 2011, 20 (2), 111–118. [CrossRef]
- Dizon, J. M.; Brener, S. J.; Maehara, A.; Witzenbichler, B.; Biviano, A.; Godlewski, J.; Parise, H.; Dambrink, J.-H.; Mehran, R.; Gibson, C. M.; Stone, G. W. Relationship between ST-Segment Resolution and Anterior Infarct Size after Primary Percutaneous Coronary Intervention: Analysis from the INFUSE-AMI Trial. European Heart Journal. Acute Cardiovascular Care 2014, 3 (1), 78–83. [CrossRef]
- Prognostic Impact of Early ST-Segment Resolution in Acute ST-Elevation Myocardial Infarction | Circulation. https://www.ahajournals.org/doi/full/10.1161/01.CIR.0000147778.05979.E6 (accessed 2024-02-28).
- Rakowski, T.; Dziewierz, A.; Siudak, Z.; Mielecki, W.; Brzozowska-Czarnek, A.; Legutko, J.; Rzeszutko, L.; Urbanik, A.; Dubiel, J. S.; Dudek, D. ST-Segment Resolution Assessed Immediately after Primary Percutaneous Coronary Intervention Correlates with Infarct Size and Left Ventricular Function in Cardiac Magnetic Resonance at 1-Year Follow-Up. Journal of Electrocardiology 2009, 42 (2), 152–156. [CrossRef]
- Haeck, J. D. E.; Verouden, N. J. W.; Kuijt, W. J.; Koch, K. T.; Majidi, M.; Hirsch, A.; Tijssen, J. G. P.; Krucoff, M. W.; De Winter, R. J. Impact of Early, Late, and No ST-Segment Resolution Measured by Continuous ST Holter Monitoring on Left Ventricular Ejection Fraction and Infarct Size as Determined by Cardiovascular Magnetic Resonance Imaging. Journal of Electrocardiology 2011, 44 (1), 36–41. [CrossRef]
- Røsand, Ø.; Høydal, M. A. Cardiac Exosomes in Ischemic Heart Disease—A Narrative Review. Diagnostics 2021, 11 (2), 269. [CrossRef]
- Fröhlich, G. M.; Meier, P.; White, S. K.; Yellon, D. M.; Hausenloy, D. J. Myocardial Reperfusion Injury: Looking beyond Primary PCI. European Heart Journal 2013, 34 (23), 1714–1722. [CrossRef]
- Stone, G. W.; Selker, H. P.; Thiele, H.; Patel, M. R.; Udelson, J. E.; Ohman, E. M.; Maehara, A.; Eitel, I.; Granger, C. B.; Jenkins, P. L.; Nichols, M.; Ben-Yehuda, O. Relationship Between Infarct Size and Outcomes Following Primary PCI: Patient-Level Analysis From 10 Randomized Trials. J Am Coll Cardiol 2016, 67 (14), 1674–1683. [CrossRef]
- de Waha, S.; Patel, M. R.; Granger, C. B.; Ohman, E. M.; Maehara, A.; Eitel, I.; Ben-Yehuda, O.; Jenkins, P.; Thiele, H.; Stone, G. W. Relationship between Microvascular Obstruction and Adverse Events Following Primary Percutaneous Coronary Intervention for ST-Segment Elevation Myocardial Infarction: An Individual Patient Data Pooled Analysis from Seven Randomized Trials. Eur Heart J 2017, 38 (47), 3502–3510. [CrossRef]
- Byrne, R. A.; Rossello, X.; Coughlan, J. J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M. J.; Dan, G.-A.; Dweck, M. R.; Galbraith, M.; Gilard, M.; Hinterbuchner, L.; Jankowska, E. A.; Jüni, P.; Kimura, T.; Kunadian, V.; Leosdottir, M.; Lorusso, R.; Pedretti, R. F. E.; Rigopoulos, A. G.; Rubini Gimenez, M.; Thiele, H.; Vranckx, P.; Wassmann, S.; Wenger, N. K.; Ibanez, B.; ESC Scientific Document Group. 2023 ESC Guidelines for the Management of Acute Coronary Syndromes. Eur Heart J 2023, 44 (38), 3720–3826. [CrossRef]
| Variables | Whole sample (N = 200) | Symptoms duration of less than 6 hours (N = 100) | Symptoms duration of more than 6 hours (N = 100) | P value |
|---|---|---|---|---|
| Sex | 1,000 | |||
| Female | 58 (29%) | 29 | 29 | |
| Male | 142 (71%) | 71 | 71 | |
| Age (years) | 60,6 | 58.5 [52.0 - 67.0] | 60.0 [52.0 - 74.0] | 0,154 |
| Height (cm) | 173,1 | 175.0 [166.75 - 180.0] | 175.0 [165.0 - 180.0] | 0,463 |
| Weight (kg) | 82,6 | 82.0 [75.0 - 90.0] | 80.0 [70.0 - 90.0] | 0,299 |
| Body mass index (kg/m2) | 27,5 | 26.8 [25.24 - 29.55] | 26.45 [24.35 - 29.4] | 0,426 |
| Body surface area (m2) | 2,0 | 2.0 [1.87 - 2.08] | 1.98 [1.83 - 2.12] | 0,301 |
| Heart rate (beats/min) | 78,5 | 77.5 [65.0 - 85.0] | 79.0 [70.0 - 86.25] | 0,035 |
| Systolic blood pressure (mmHg) | 141,0 | 140.0 [120.0 - 150.0] | 140.0 [130.0 - 160.0] | 0,131 |
| Diastolic blood pressure (mmHg) | 82,4 | 80.0 [70.0 - 90.0] | 80.0 [80.0 - 90.0] | 0,437 |
| Vascular risk factors and comorbidities | ||||
| Hypertension | 126 (63%) | 65 | 61 | 0,660 |
| Diabetes mellitus | 36 (18%) | 17 | 19 | 0,854 |
| Family history | 63 (32%) | 32 | 31 | 1,000 |
| Kidney function | ||||
| Creatinine (micromol/l) | 96,6 | 93.0 [79.0 - 108.25] | 94.0 [81.75 - 106.25] | 0,829 |
| Clearance (ml/min) | ||||
| >90 | 89 (45%) | 50 | 39 | 0,155 |
| 60-90 | 80 (40%) | 40 | 40 | 1,000 |
| 45-59 | 23 (12%) | 7 | 16 | 0,076 |
| 30-44 | 4 (2%) | 2 | 2 | 1,000 |
| 15-29 | 4 (2%) | 1 | 3 | 0,614 |
| Duration of the symptoms (min) | 304,3 | 120.0 [90.0 - 180.0] | 420.0 [360.0 - 600.0] | 0,000 |
| Killip | ||||
| 1 | 128 (64%) | 72 | 56 | 0,027 |
| 1/2 | 4 (2%) | 1 | 3 | 0,614 |
| 2 | 56 (28%) | 24 | 32 | 0,270 |
| 2/3 | 6 (3%) | 1 | 5 | 0,214 |
| 3 | 4 (2%) | 0 | 4 | 0,130 |
| 4 | 2 (1%) | 2 | 0 | 0,477 |
| Post MI NYHA status | ||||
| 1 | 175 (88%) | 89 | 86 | 0,669 |
| 2 | 18 (9%) | 11 | 7 | 0,459 |
| 2/3 | 2 (1%) | 0 | 2 | 0,477 |
| 3 | 1 (0.5%) | 0 | 1 | 1,000 |
| 4 | 4 (2%) | 0 | 4 | 0,130 |
| Post MI CCS | ||||
| 0 | 188 (94%) | 95 | 93 | 0,766 |
| 1 | 9 (5%) | 4 | 5 | 1,000 |
| 2 | 1 (1%) | 0 | 1 | 1,000 |
| 3 | 1 (1%) | 0 | 1 | 1,000 |
| 4 | 1 (1%) | 1 | 0 | 1,000 |
| Myocardial injury assessment | ||||
| Creatine Kinase isoenzyme MB (CKMB) | ||||
| Pre-PCI | 61,5 | 27.5 [21.0 - 35.25] | 59.0 [36.0 - 110.0] | 0,000 |
| 6-hour | 285,1 | 244.0 [122.25 - 376.25] | 345.5 [168.0 - 500.0] | 0,007 |
| 24-hour | 148,1 | 107.0 [72.25 - 188.0] | 139.5 [81.0 - 194.25] | 0,324 |
| 72-hour | 38,7 | 30.0 [21.75 - 38.0] | 32.0 [25.0 - 45.0] | 0,040 |
| Troponin I (TnI) | ||||
| Pre-PCI | 1,4 | 0.04 [0.01 - 0.2] | 1.29 [0.37 - 3.46] | 0,000 |
| 6-hour | 17,7 | 11.43 [5.26 - 29.2] | 25.0 [10.1 - 30.0] | 0,000 |
| 24-hour | 12,9 | 7.65 [3.3 - 14.89] | 10.42 [5.5 - 16.9] | 0,575 |
| 72-hour | 3,4 | 2.21 [1.04 - 3.64] | 3.1 [1.2 - 4.77] | 0,074 |
| In-hospital stay length (days) | 6,2 | 6.0 [5.0 - 7.0] | 5.0 [5.0 - 7.0] | 0,265 |
| Intrahospital complications | 0 (0%) | 0 | 0 | 1,000 |
| Without | 176 (88%) | 88 | 88 | 1,000 |
| Ventricular thrombus | 4 (2%) | 2 | 2 | 1,000 |
| Pericardial effusion | 5 (3%) | 1 | 4 | 0,365 |
| Ventricular fibrillation | 11 (6%) | 8 | 3 | 0,215 |
| Pulseless electrical activity | 1 (1%) | 1 | 0 | 1,000 |
| Cardiac tamponade | 1 (1%) | 0 | 1 | 1,000 |
| Stroke | 1 (1%) | 0 | 1 | 1,000 |
| In-hospital death | 1 (1%) | 0 | 1 | 1,000 |
| Echocardiographic assessment | ||||
| In-hospital FSLV | 35,0 | 34.7 [29.55 - 38.42] | 35.95 [32.6 - 40.15] | 0,254 |
| In-hospital EFLV | 48,9 | 50.0 [46.0 - 55.0] | 48.0 [42.0 - 55.0] | 0,024 |
| In-hospital LVIDs | 3,2 | 3.2 [2.9 - 3.5] | 3.15 [2.8 - 3.5] | 0,647 |
| In-hospital LVIDd | 4,9 | 4.9 [4.6 - 5.2] | 4.9 [4.6 - 5.22] | 0,823 |
| In-hospital LVEDV | 102,0 | 94.0 [84.0 - 115.25] | 97.0 [78.5 - 121.5] | 0,787 |
| In-hospital LVESV | 52,6 | 48.0 [40.88 - 60.0] | 50.0 [36.25 - 65.0] | 0,727 |
| In-hospital MADd | 3,0 | 3.0 [2.9 - 3.2] | 3.0 [2.9 - 3.1] | 0,374 |
| In-hospital mitral regurgitation | ||||
| 0 | 59 (30%) | 26 | 33 | 0,352 |
| 0/1 | 5 (3%) | 4 | 1 | 0,365 |
| 1 | 64 (32%) | 38 | 26 | 0,095 |
| 1/2 | 31 (16%) | 11 | 20 | 0,118 |
| 2 | 34 (17%) | 19 | 15 | 0,572 |
| 2/3 | 7 (4%) | 2 | 5 | 0,442 |
| Variables | Whole sample (N = 200) | Symptoms duration of less than 6 hours (N = 100) | Symptoms duration of more than 6 hours (N = 100) | P value |
|---|---|---|---|---|
| Dynamics of the QRS complex | ||||
| Pre-PCI QRS width | 100,5 | 100.0 [90.0 - 110.0] | 100.0 [93.5 - 110.0] | 0,463 |
| Post-PCI QRS width | 98,8 | 95.0 [85.0 - 100.0] | 100.0 [90.0 - 110.0] | 0,111 |
| 60-min QRS width | 94,7 | 86.5 [80.0 - 100.0] | 100.0 [87.25 - 100.0] | 0,008 |
| 72-hour QRS width | 89,8 | 81.0 [80.0 - 90.5] | 95.0 [85.0 - 100.0] | 0,000 |
| 1-month QRS width | 87,9 | 80.0 [80.0 - 90.0] | 90.0 [85.0 - 100.0] | 0,000 |
| 6-months QRS width | 87,7 | 80.0 [80.0 - 90.0] | 90.0 [80.0 - 100.0] | 0,000 |
| ST-segment elevation (mm) | ||||
| Pre-PCI | 3,75 | 3.5 [2.5 - 5.0] | 3.0 [2.0 - 5.0] | 0,064 |
| Post-PCI | 1,89 | 1.0 [0.0 - 2.5] | 2.0 [0.5 - 3.0] | 0,051 |
| 60-min | 1,02 | 0.5 [0.0 - 1.0] | 1.0 [0.0 - 2.0] | 0,005 |
| 72-hour | 0,47 | 0.0 [0.0 - 0.5] | 0.5 [0.0 - 1.0] | 0,000 |
| 1-month | 0,18 | 0.0 [0.0 - 0.0] | 0.0 [0.0 - 0.5] | 0,003 |
| 6-months | 0,14 | 0.0 [0.0 - 0.0] | 0.0 [0.0 - 0.0] | 0,013 |
| ST-segment resolution (>50%) | ||||
| Post-PCI | 121 (61%) | 72 | 49 | 0,001 |
| 60-min | 98 (49%) | 48 | 50 | 0,888 |
| 72-hour | 82 (41%) | 39 | 43 | 0,666 |
| 1-month | 52 (26%) | 23 | 29 | 0,420 |
| 6-months | 16 (8%) | 7 | 9 | 0,794 |
| Newly formed branch block | ||||
| No | 175 (88%) | 87 | 88 | 1,000 |
| Transitory RBBB | 2 (1%) | 1 | 1 | 1,000 |
| Transitory LBBB | 9 (5%) | 7 | 2 | 0,172 |
| Transitory LAHB | 4 (2%) | 2 | 2 | 1,000 |
| Permanent LAHB | 5 (3%) | 1 | 4 | 0,365 |
| Permanent ILBBB | 2 (1%) | 0 | 2 | 0,477 |
| Permanent RBBB | 2 (1%) | 2 | 0 | 0,477 |
| Variables | Whole sample (N = 200) | Symptoms duration of less than 6 hours (N = 100) | Symptoms duration of more than 6 hours (N = 100) | P value |
|---|---|---|---|---|
| 6-months follow-up data | ||||
| In-hospital death | 1 (1%) | 0 | 1 | 1,000 |
| Out-of-hospital death | 5 (3%) | 1 | 4 | 0,365 |
| Coronary event-related death | 6 (3%) | 1 | 5 | 0,214 |
| Reinfarction of the treated vessel | 1 (1%) | 1 | 0 | 1,000 |
| Manifested HF | 28 (14%) | 6 | 22 | 0,002 |
| Stent thrombosis | 4 (2%) | 2 | 2 | 1,000 |
| Clinically manifested restenosis | 2 (1%) | 0 | 2 | 0,477 |
| Ventricular thrombus | 1 (1%) | 1 | 0 | 1,000 |
| MACE | 32 (16%) | 9 | 23 | 0,012 |
| Dual Antiplatelet Treatment | ||||
| Acetylsalicylic acid + Ticagrelor | 79 (40%) | 27 | 52 | 0,001 |
| Acetylsalicylic acid + Clopidogrel | 109 (55%) | 69 | 40 | 0,000 |
| Acetylsalicylic acid + Ticagrelor + Oral anticoagulant | 2 (1%) | 2 | 0 | 0,477 |
| Acetylsalicylic acid + Clopidogrel + Oral anticoagulant | 9 (5%) | 2 | 7 | 0,172 |
| Acetylsalicylic acid + Clopidogrel + Direct oral anticoagulant | 1 (1%) | 0 | 1 | 1,000 |
| 6-months NYHA | ||||
| 1 | 171 (86%) | 94 | 77 | 0,001 |
| 1/2 | 1 (1%) | 1 | 0 | 1,000 |
| 2 | 19 (10%) | 4 | 15 | 0,016 |
| 2/3 | 1 (1%) | 0 | 1 | 1,000 |
| 3 | 2 (1%) | 0 | 2 | 0,477 |
| 3/4 | 1 (1%) | 0 | 1 | 1,000 |
| 6-months CCS | ||||
| 0 | 174 (87%) | 86 | 88 | 0,833 |
| 1 | 13 (7%) | 8 | 5 | 0,566 |
| 2 | 6 (3%) | 4 | 2 | 0,678 |
| 3 | 2 (1%) | 1 | 1 | 1,000 |
| Echocardiographic assessment | ||||
| 6-months FSLV | 35,7 | 35.8 [33.3 - 40.0] | 36.0 [32.65 - 39.25] | 0,572 |
| 6-months EFLV | 51,8 | 55.0 [50.0 - 57.5] | 50.0 [45.0 - 55.0] | 0,000 |
| 6-months LVIDs | 3,2 | 3.1 [2.8 - 3.45] | 3.2 [2.9 - 3.5] | 0,368 |
| 6-months LVIDd | 5,0 | 5.0 [4.65 - 5.3] | 5.0 [4.5 - 5.35] | 0,502 |
| 6-months LVEDV | 104,0 | 95.0 [80.0 - 115.0] | 101.0 [80.5 - 124.0] | 0,251 |
| 6-months LVESV | 52,3 | 47.0 [37.0 - 52.5] | 50.0 [40.0 - 67.0] | 0,023 |
| 6-months MADd | 3,1 | 3.1 [3.0 - 3.2] | 3.0 [2.9 - 3.2] | 0,183 |
| 6-months mitral regurgitation | ||||
| 0 | 54 (27%) | 28 | 26 | 0,873 |
| 0/1 | 7 (4%) | 7 | 0 | 0,021 |
| 1 | 66 (33%) | 36 | 30 | 0,452 |
| 1/2 | 29 (15%) | 9 | 20 | 0,045 |
| 2 | 25 (13%) | 13 | 12 | 1,000 |
| 2/3 | 10 (5%) | 5 | 5 | 1,000 |
| 3 | 1 (1%) | 0 | 1 | 1,000 |
| 3/4 | 2 (1%) | 1 | 1 | 1,000 |
| 6-years follow-up data | ||||
| All-cause mortality | 22 (11%) | 7 | 15 | 0,114 |
| Cardiovascular death | 14 (7%) | 6 | 8 | 0,782 |
| Non-cardiovascular death | 8 (4%) | 1 | 7 | 0,071 |
| Hospitalization due to HF | 5 (3%) | 3 | 2 | 1,000 |
| Reinfarction | 7 (4%) | 6 | 1 | 0,124 |
| Stroke | 4 (2%) | 3 | 1 | 0,614 |
| Stent restenosis | 4 (2%) | 2 | 2 | 1,000 |
| Re-PCI of the non-culprit vessel | 10 (5%) | 5 | 5 | 1,000 |
| Number of days until the first MACE | 1745 | 2287.0 [1874.0 - 2476.0] | 1854.0 [615.0 - 2208.5] | 0,000 |
| Number of days until death | 2063 | 2361.0 [2272.5 - 2484.0] | 1942.0 [1601.75 - 2260.5] | 0,000 |
| QRS complex | MACE | Width (mm) | P value | OR (95% CI) | P value |
|---|---|---|---|---|---|
| Pre-PCI | No | 100 (IQR 90-105) | 0.026 | 1.028 (1.003-1.053) | 0.029 |
| Yes | 102.5 (IQR 90-110) | ||||
| Right after the procedure | No | 95 (IQR 85-100) | 0.010 | 1.026 (1.004-1.048) | 0.020 |
| Yes | 100 (IQR 90-110) | ||||
| 1-hour after the procedure | No | 94 (IQR 85-100) | 0.013 | 1.027 (1.003-1.052) | 0.025 |
| Yes | 95 (IQR 85-105) | ||||
| 72 hours after the procedure | No | 85 (IQR 80-95) | 0.020 | 1.031 (1.002-1.060) | 0.034 |
| Yes | 89 (IQR 80-100) | ||||
| 1 month | No | 85 (IQR 80-90) | 0.034 | 1.042 (1.012-1.074) | 0.007 |
| Yes | 85 (IQR 80-100) | ||||
| 6 months | No | 85 (IQR 80-90) | 0.041 | 1.038 (1.009-1.069) | 0.011 |
| Yes | 87.5 (IQR 80-100) |
| ST-segment | MACE | Elevation (mm) | P value | OR (95% CI) | P value |
|---|---|---|---|---|---|
| Pre-PCI | No | 3 (IQR 2-5) | 0.018 | 1.61 (1.024-1.317) | 0.020 |
| Yes | 3 (IQR 2.5-5) | ||||
| Right after the procedure | No | 1 (IQR 0-2.5) | 0.000 | 1.296 (1.103-1.523) | 0.002 |
| Yes | 2 (IQR 0.75-3) | ||||
| 1-hour after the procedure | No | 0 (IQR 0-1) | 0.000 | 1.391 (1.111-1.741) | 0.004 |
| Yes | 0.5 (IQR 0.5-2) | ||||
| 72 hours after the procedure | No | 0 (IQR 0-5) | 0.004 | 1.500 (1.037-2.170) | 0.031 |
| Yes | 0 (IQR 0-0.75) | ||||
| 1 month | No | 0 (IQR 0-0) | 0.000 | 3.256 (1.572-6.747) | 0.001 |
| Yes | 0 (IQR 0-0.5) | ||||
| 6 months | No | 0 (IQR 0-0) | 0.003 | 2.972 (1.268-6.965) | 0.012 |
| Yes | 0 (IQR 0-0.25) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





