Introduction
Pancreatic cancer (PC) has the worst prognosis of all cancers and its worldwide incidence is continuously rising [
1]. Pancreatic ductal adenocarcinoma (PDAC) and pancreatic neuroendocrine tumours (PNET) are the two major PC subtypes with PDAC being the more aggressive subtype and accounting for 90% of all PC cases [
2,
3]. PDAC has no specific symptoms and is consequently diagnosed late and only 24% of PDAC patients survive for one year and 9% for 5 years after diagnosis [
4]. At present, surgery is the only curative option for PDAC, however, only 15−20% of patients present with resectable disease, and of these a large proportion are not eligible for immediate surgical resection due to immense vascular involvement [
5]. The remaining 80% of patients present with either locally advanced or metastatic disease [
1]. Clinical care for these patients is through systemic chemotherapy with the first lines of therapy being Gemcitabine (GEM) alone, GEM administered with a nanoparticle-bound paclitaxel (nab-paclitaxel), or a combination of 5-fluorouracil (5-FU), leucovorin, irinotecan and oxaliplatin (FOLFIRINOX) [
6]. However, these treatment modalities do not significantly impact patient survival due to drug resistance, tumour recurrence, and their high toxicity profiles which lead to debilitating side effects. Over the past decades, efforts to address these drawbacks have been marginally successful [
7] and limited by the drug development pipeline being time-consuming, risky, and expensive [
8]. Drug repurposing, which identifies new indications for existing and approved drugs, has therefore gained traction as an alternative approach to anti-cancer drug discovery [
9]. The rationale for this is that the candidate drugs have well-documented and established pharmacokinetic, pharmacodynamic, dosing, and toxicity profiles which accelerate their clinical development [
8].
Pyrvinium pamoate (PP) is a small, fluorescent, and lipophilic compound that was first described in the 1940s for use as a dye, fluorescent probe, and antihelminthic drug [
10]. Although the development of more effective anti-parasitic agents halted its use as an anthelminthic, PP remained clinically relevant due to its potential to target other disease-causing organisms as well as its ability to reduce toxicity, promote wound repair, and inhibit fibrotic tissue development [
11]. Of late, PP has gained significant attention in cancer research. Indeed, several studies have reported on its anti-cancer activities which involve its ability to inhibit the Wnt signalling pathway and mitochondrial function [
10]. In the context of PC, Esumi et al. (2004) showed that PP is highly toxic to serum-starved PANC-1 cells and can clear PANC-1 xenografts
in vivo [
12]. Tomitsuka et al. (2012) compared its effects under hypoxic-hypoglycemic conditions, which mimics the tumour microenvironment (TME), and normoxia-normoglycemic and found that in PDAC, PP inhibits mitochondrial energy metabolism through inhibition of the NADH-fumarate reductase (NADH-FR) system [
13]. More recently, a study by Schultz et al. (2021) showed that PP does not only function by specific inhibition of NADH-FR but through a more global inhibition of mitochondrial function in PDAC [
14]. Collectively, these studies revealed the potential of PP as a TME-specific anti-cancer drug. Since the efficacy of the drugs currently used to treat PDAC, including GEM, is negatively affected by the dense and desmoplastic PDAC TME [
15], combining PP and GEM may be an effective option for treating PDAC.
This study showed that under normal serum conditions, PP inhibited the viability and survival of PDAC 2D cell cultures and 3D spheroids through the inhibition of the PI3K/AKT cell survival pathway and induction of apoptotic and autophagic cell death. PP also inhibited epithelial-to-mesenchymal transition (EMT), cell migration, and invasion in 2D and 3D PDAC cell culture models. Importantly, low concentrations of PP and GEM acted synergistically and their combination was more effective at exhibiting anti-pancreatic cancer activities than when used as single agents. Overall, this study extends our understanding of the anti-cancer activities of PP and provides additional evidence that PP is effective as a single agent and in combination with GEM for the treatment of pancreatic cancer.
Method and Materials
Cell Culture
The human PANC-1 and CFPAC-1 pancreatic ductal adenocarcinoma (PDAC) cell lines were purchased from the American Type Culture Collections (ATCC) and maintained in Dulbecco’s Modified Eagle Medium (DMEM) and Iscove's Modified Dulbecco's Medium (IMDM) (Gibco, Life Technologies/Thermo Fisher Scientific, USA), respectively. Media were supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 1% penicillin and streptomycin (pen/strep) (Gibco, USA). Cells were maintained at 37°C in a 95% air and 5% CO2 humidified incubator. Media were replaced every 2 – 3 days and the cells were routinely tested for mycoplasma infections to ensure that only mycoplasma-negative cells were used.
Treatments
The experimental drug PP (P0027) and a positive control drug GEM were purchased from Sigma Aldrich (Missouri, USA), dissolved in dimethyl sulfoxide (DMSO) (Sigma Aldrich, USA) to a final concentration of 5 mM, and stored at -20˚ C with limited freeze/thaw cycles. For cell culture treatments, the drugs were diluted in supplemented media to achieve the desired final concentration(s) and the percentage of DMSO in the highest drug concentration was used as a vehicle control. Unless stated, cells were treated at a confluency of 60%. To inhibit autophagy, cells were treated with 10 nM Bafilomycin A1 (B1793; Sigma Aldrich, USA) for 1h before treatment with PP or GEM. For cell migration experiments, cells were treated with 10 µM Mitomycin C (M4287; Sigma Aldrich, USA) to inhibit cell proliferation.
Cell Viability Assay
The effects of PP and GEM on the viability of PANC-1 and CFPAC-1 cells were assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltrazolium bromide (MTT) assay (11465007001; Sigma Aldrich, USA) following the manufacturer’s instructions. Briefly, cells were seeded in 96-well plates at a density of 8 x 103 cells/well overnight then treated with vehicle (0.1% DMSO) or increasing concentrations (2 – 10 µM) of PP or GEM. After 72h, cells were incubated with 10 μL of 5 mg/mL MTT for 4h in the dark, after which the wells were emptied followed by the addition of 100 μL DMSO to dissolve the formazan crystals. Absorbance was read at 600 nm using the GloMax® plate reader (Promega, USA), and mean cell viability was calculated and expressed as a percentage of the vehicle control. Half maximum inhibitory concentrations (IC50) were determined using the GraphPad Prism version 8.0 software (GraphPad Prism software, USA) from sigmoidal plots of data obtained from three independent experiments performed in quadruplicate.
Clonogenic Assays
Cells were plated in 6-well plates at a density of 3 x 105 cells/well overnight then treated with vehicle or ½IC50 or IC50 of PP or IC50 of GEM. After 48h, cells were harvested, counted, and replated in 35mm dishes at a low density (500 cells/dish) in duplicate and incubated in drug-free media for 14 days to allow for colony formation. The colonies were then fixed for 10 mins with 3:1 methanol: acetic acid, stained for 15 mins with 0.5% (w/v) crystal violet (Sigma Aldrich, USA) in 100% methanol, imaged and quantified using the ColonyArea plugin of the ImageJ software. Colony areas were determined for each treatment condition and expressed as a percentage of vehicle-treated control.
Cell Cycle Analyses
Cells treated with vehicle, or ½IC50 or IC50 of PP or IC50 GEM for 72h were collected, washed with 1x PBS, counted, diluted to 1 x 106 cells/mL, and permeabilised with 70% ethanol at -20°C overnight. Cells were then pelleted, incubated for 15 mins with 50 µg/mL RNase (Fermentas, Massachusetts, USA) in 1 X PBS at 37°C, transferred into FACS tubes, and stained with propidium iodide. A minimum of 5 x 104 cells/sample were analysed using a Becton Dickinson FACS Calibur flow cytometer (Becton Dickinson, New Jersey, USA) with a 488nm coherent laser. Data were acquired using CellQuest Pro version 5.2.1. software (Becton Dickinson, New Jersey, USA) and analysed using ModFit version 2.0. software (Verity Software House Inc, Maine, USA).
Immunofluorescence
Cells were plated on coverslips in a 12-well plate at a density of 1.5 x 10
5 cells/well overnight, treated with vehicle or ½IC
50 or IC
50 of PP or IC
50 of GEM for 72h and immunofluorescence was performed as previously described [
16]. Briefly, the treated cells were washed three times with 1x PBS, fixed for 5 mins with 100% methanol at -20˚ C, permeabilised for 10 mins with 0.2% (v/v) Triton X-100 in PBS, blocked for 1h with 1% (w/v) BSA in PBS/Tween at RT and incubated overnight with rabbit monoclonal anti- Phospho-Histone H2A.X (#2577; 1:100 dilution) or LC3 (#2775; 1:200 dilution) from Cell Signaling Technology (Massachusetts, USA) at 4˚ C. After incubation, the cells were washed as above and incubated with a donkey anti-rabbit Alexa 488 conjugated secondary antibody (1:500 dilution; Jackson Immuno Research Laboratories Inc., Pennsylvania, USA) for 2 hours in the dark. Both primary and secondary antibodies were diluted in 1% (w/v) BSA in PBS/Tween. Cells were subsequently stained for 10 mins with 1 µg/mL DAPI in PBS in the dark before being visualised using a Zeiss immunofluorescence microscope at 400× magnification or an LSM510 Meta confocal microscope (Zeiss, Oberkochen, Germany).
Western Blotting
Total proteins were harvested from cells treated for 72h with vehicle or ½ IC
50 or IC
50 PP or IC
50 GEM and western blotting was performed as previously described [
17]. Briefly, the proteins were resolved on 8 – 15% gels using sodium dodecyl sulfate/polyacrylamide gel electrophoresis (SDS/PAGE) until a desired separation was obtained and transferred to Hybond Enhanced chemiluminescence (ECL) membranes (Amersham Biosciences, UK) for 1 – 2h. The ECL membranes were blocked with 5% fat-free milk in PBS/TBS containing 1% (v/v) Tween 20 for 1h and incubated with primary antibodies in blocking buffer (PBS/TBS-Tween 20/Milk) overnight at 4˚ C. Unless stated, the following primary antibodies were used at a 1:1000 dilution: rabbit polyclonal antibodies to Phospho-Histone H2A.X (Ser139) (#2577), PARP (#9542), Caspase-9 (#9502), LC3 (#2775), Vimentin (R28) (#3932); rabbit monoclonal antibody to Cleaved Caspase-7 (Asp198) (D6H1) (#8438) and β-catenin (D10A8) (#8480); mouse monoclonal antibodies to Cyclin B1 (V152) (#4135), Caspase-8 (1C12) (#9746), SQSTM1/p62 (D5L7G) (#88588), E-cadherin (4A2) (#14472), N-cadherin (13A9) (#14215) from Cell Signaling Technology (Massachusetts, USA). Mouse monoclonal antibodies to Cyclin A (H-432) (sc-751) and β-actin (1:3000; sc-47778) were purchased from Santa Cruz Biotechnology (California, USA). Secondary antibodies used were: horseradish peroxidase (HRP)-conjugated goat anti-rabbit (Biorad, USA), and goat anti-mouse (Biorad, USA) antibodies at 1:3000 dilution for 1h in a blocking buffer. β-actin was used as a loading control and the band intensities were measured using the ImageJ software. Densitometric readings were calculated as a ratio of the protein of interest/β-actin and normalised to either the vehicle control or first lane with a band.
Apoptosis Assay
Cells were plated on coverslips in a 12-well plate at a density of 1.5 x 105 cells/well overnight and treated with vehicle or ½IC50 or IC50 of PP or IC50 of GEM. After 72h, the cells were washed three times with 1X PBS, fixed for 10 mins with 3:1 methanol: acetic acid, and stained with 1 µg/ml of ethidium bromide and 1 µg/ml of acridine orange for 10 mins in the dark. After washing as above, the cells were mounted onto microscope slides, dried, and imaged under a fluorescence microscope (EVOS M5000 Imaging System, Thermo Fisher Scientific, USA).
Transwell Invasion Assay
Cells were plated in 6-well plates at a density of 3 x 105 cells/well overnight and then treated with vehicle or IC50 of PP or IC50 of GEM for 24h. The cells were then harvested and counted and 1 x 105 cells were resuspended in 200 µL of media with 1% FBS and replated in the upper chambers of the transwell inserts that were coated with 0.3 µg/ml of Matrigel (CLS354234; Merk, USA). The inserts were then placed in 12-well plates with media supplemented with 10% FBS as a chemoattractant and incubated for 20 h for cell invasion to occur. The non-invaded cells were removed using cotton swaps and invaded cells were fixed for 10 mins with 3.7% (w/v) paraformaldehyde and stained with 0.5% (w/v) crystal violet (Sigma Aldrich, USA). After 15 mins, the inserts were washed three times with 1x PBS to remove excess crystal violet, air-dried, and imaged under an inverted light microscope (EVOS M5000 Imaging System, Thermo Fisher Scientific, USA). The invasive cells were quantified by ImageJ and expressed as a percentage of the vehicle control.
In Vitro 2D Scratch Motility Assays
Cells were plated in 24-well plates at a density of 1.5 x 105 cells/well and incubated for 2 days to form a 100% confluent cell monolayer. A vertical wound was created on the cell monolayers using a 20 µL sterile pipette tip. The wounded cells were then treated with vehicle or ½IC50 or IC50 of PP or IC50 of GEM and 10 µM mitomycin C (M4287; Sigma Aldrich, USA) to inhibit cell proliferation and imaged at 0, 3, 6, 9, 12, and 24h. Marks were made on the plates to ensure that the cells were imaged in the same area at all time points. The wound areas were measured using ImageJ and the total migrated areas were calculated by subtracting the area for each treatment condition at a specific time point from the area measured at 0h.
Spheroid Growth Assays
Cells were plated at a density of 5000 cells/well in 96-well plates coated with 70 µL of 1.2% (w/v) agarose (SeaKem LE Agarose 50004, Lonza, USA) to prevent cell adhesion and incubated for 6 days for compact spheroid formation. Once formed, the spheroids were imaged and treated with vehicle or IC50 or 2x IC50 of PP or IC50 of GEM for 6 days (on day 0 and day 3). To avoid disrupting the spheroids during treatment, 50 µL of the media was removed and replaced with 50 µL of a doubled concentration of vehicle or drugs. After treatment, the spheroids were imaged and their areas were measured using ImageJ. A minimum of four spheroids per treatment condition were analyzed and the spheroid growth rates were calculated by dividing the areas of the spheroids on day 6 by their respective areas on day 0 and expressed relative to the vehicle-treated spheroids.
Spheroid Viability Assay
The effects of PP and GEM on spheroid viability were assessed by Calcein-AM staining. Briefly, a 2x staining solution was prepared by mixing 2 μM Calcein-AM (C1430; Invitrogen, USA) which stains viable cells, and 20 μg/mL of 4,6-diamidino-2-phenylindole (DAPI) (Thermo Fisher Scientific, USA) as a counterstain. To stain the spheroids, 50 µL of the media with/without treatment was removed and replaced with 50 µL of the 2x staining solution (which was diluted to 1x, i.e., 1 μM Calcein-AM and 10 μg/mL DAPI by the remaining 50 µL in each well) and incubated at 37°C and 5% CO2 for 1h in the dark. The spheroids were imaged under a fluorescence microscope (EVOS M5000 Imaging System, Thermo Fisher Scientific, USA) and the calcein-AM fluorescence intensity was measured using ImageJ, normalized to the spheroid areas, and expressed relative to the vehicle-treated spheroids.
Spheroid Invasion Assay
The spheroids generated as above were transferred to a new 96-well plate where they were embedded in 70 µL of 1.5 mg/ml collagen I rat tail matrix (Gibco, A1048301, Thermo Fisher Scientific, USA), imaged under a light microscope (EVOS M5000 Imaging System, Thermo Fisher Scientific, USA), and treated with 100 µL of vehicle or IC50 or 2xIC50 of PP or IC50 of GEM for 72h. A minimum of four spheroids per treatment condition were analysed per treatment condition.
Analyses of Drug Combination
Cells were treated for 72h with vehicle or ⅛ IC
50, ⅟
4 IC
50, or ½ IC
50 of PP or GEM as single agents and in combination and subjected to MTT cell viability assays as described above. The MTT assay data were analysed using the highest single agent (HSA) model synergy and antagonism model by the Combenefit software (Cancer Research, Cambridge, UK) and the CompuSyn version 1.0 software (ComboSyn, Inc., Paramus, USA) according to instructions [
18,
19].
Statistical Analysis
Unless stated, data were obtained from at least three independent experimental repeats and analysed by a parametric unpaired t-test using the GraphPad Prism version 8.0 Software (GraphPad Prism software, USA). Error bars represent the standard error of the mean (SEM) and significance was accepted at *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
Discussion
Pancreatic cancer, commonly referred to as PDAC, is a fatal disease with an incredibly poor prognosis and a 5-year survival rate of less than 10% [
32]. Drug repurposing has shown great promise in the rapid identification of effective anti-PDAC drugs and several FDA-approved non-cancer drugs are currently being explored for the treatment of this lethal neoplasm [
8]. The antihelminthic drug pyrvinium pamoate (PP) was of interest to this study because it was shown to inhibit mitochondrial function under conditions that mimic the PDAC TME, but details of its anti-PDAC activites and the mechanism(s) involved are not known [
12,
13,
14]. Here we showed using 2D and 3D PDAC cell culture models that PP induces double-strand DNA breaks, cell cycle arrests, apoptotic and autophagic cell deaths and inhibits the PI3K/AKT survival pathway. Furthermore, we provided important data that show that PP can enhance the anti-cancer effects of GEM in these cell culture models.
Systemic chemotherapies used to treat PDAC do not significantly improve patient survival due to their high toxicity profiles, which lead to adverse side effects, as well as tumour drug resistance and their failure to inhibit metastatic spread [
6]. Here we provide several lines of evidence that PP may be an effective drug that can be repurposed to treat PDAC patients because it exerts cytoxocity in PDAC cells while exerting minimal cytotoxicity to normal cells, inhibits PDAC long-term survival and recurrence as well as metastasis. Indeed, we showed, using 2D and 3D cell culture models, that PP was cytotoxic in PDAC cells. Our findings together with that of others who showed that PP induced cytoxicity in PDAC cells while sparing nonmalignant human pancreatic epithelial cells suggest that it is selective for PDAC cells and may therefore be associated with limited side effects [
33,
34,
35]. Using clonogenic assays we showed that PP prevented the colony forming ability of PDAC cells and hence inhibited their long-term survival and recurrence. These results are significant because post-treatment, tumour cells can retain their clonogenicity which ultimately leads to tumour recurrence and relapse and the clonogenic assay is therefore an effective tool for predicting a patient's long-term sensitivity to anti-cancer drugs [
36]. Furthermore, our findings that PP inhibited PDAC spheroid growth, viability and invasion are promising, because compared to 2D cell cultures, 3D spheroids give a more accurate drug response because they closely resemble PDAC tumours in terms of their structure and their increased chemoresistance [
37]. Furthermore, 3 in 4 PDAC patients develop recurrence within 2 years after resection which suggests that even the small proportion of patients that undergo surgical resection harbor micro-metastasis [
6]. Indeed, PDAC cells acquire pro-metastatic traits which enable them to disseminate and spread before primary tumour formation, and as a result, PDAC patients present with liver (76–80%), peritoneum (48%), or lung (45%) metastasis at diagnosis [
38,
39]. Among the key regulators of PDAC metastasis is the canonical Wnt/β-catenin pathway which, upon activation, leads to the translocation of β-catenin into the nucleus where it facilitates the transcription of genes that induce EMT, invasion, and migration to distant sites [
40,
41]. PP is a well-known inhibitor of the Wnt/β-catenin pathway and it is therefore tempting to speculate that it may have in part exerted its anti-metastatic effects in our PDAC cells by inhibiting this pathway. Future studies should further explore this.
An understanding of the mechanism(s) by which anti-cancer drugs function may provide insight into the potential mechanisms of tumour drug resistance [
42]. This study provided evidence that the anti-cancer effects of PP occur through mechanisms that may enable it to escape resistance by PDAC cells. Indeed, we show for the first time in PDAC that PP induced double-strand DNA breaks, an S-phase cell cycle arrest, inhibited the PI3K/AKT cell survival pathway, and induced intrinsic and extrinsic apoptosis as well as autophagic cell death. Our findings that PP triggers apoptotic and autophagic cell death is important because anti-cancer agents that induce more than one form of programmed cell death are more efficacious and less prone to resistance [
43]. Furthermore, its ability to activate intrinsic and extrinsic apoptotic pathways is significant because the inactivation of the intrinsic apopotic pathway is one of the mechanisms by which PDAC cells become resistant to GEM [
43,
44]. In addition, our observations that PP induced autophagic cell death is of interest because autophagy is elevated in the late stages of PDAC where it fuels tumour metabolism to promote tumour growth and drug resistance [
45]. Consistent with our data, another antihelminthic drug niclosamide inhibited PARP cleavage in the presence of a late inhibitor of autophagy, Bafilomycin A1, in COLO 357 and SW1990 PDAC cells [
46]. Finally, the PI3K/AKT pathway contributes to the mechanisms by which PDAC cells develop drug resistance and here we showed that PP inhibited this pathway [
47]. Interestingly, inhibiting the PI3K/AKT pathway with wortmannin and LY294002 sensitised PDAC cells to GEM [
48] and inhibition of phosphatidylinositide 3-kinase-protein kinase B (PKB)/AKT by wortmannin was shown to promote the anti-cancer activities of GEM in PDAC mouse models [
49]. Together, these studies suggests that when combined with GEM, inhibitors of the AKT pathway such as PP may be effective in the treatment of PDAC.
Combination therapies have proven to be an effective strategy for the management of PDAC. Indeed combining low doses of drugs with dinstict mechanisms of action may overcome drug resistance and lead to an increased efficacy without or with minimal side effects [
29]. Most anti-PDAC combination regimens are centered on GEM because it is the only FDA-approved monotherapy and several of these including the FDA-approved GEM plus nab-paclitaxel have improved patient survival [
50]. However, these combinations fail to achieve complete remission and they are associated with high toxicities and a high degree of intrinsic or acquired resistance due to the PDAC TME [
51]. Indeed, the PDAC TME limits delivery of GEM to tumours by downregulating the concentrative and equilibrative nucleoside transporters and enzymes that are involved in GEM transport and metabolism, respectively [
52]. Therefore, less cytotoxic combinations that target both PDAC cells and the TME are urgently needed to combat PDAC. PP was previously shown to be effective under conditions that mimic the PDAC TME [
12,
13,
14] and we therefore investigated whether GEM and PP acted synergistically in PDAC. Using 2D cell cultures and 3D spheroids, we showed for the first time that PP can improve the anti-cancer activities of GEM in PDAC. It is therefore tempting to speculate that combining PP with GEM may lead to significant improvements in PDAC treatment. In this regard, it is worth noting that there is an ongoing Phase I clinical trial (NCT05055323) which is investigating the safety and tolerability of pyrvinium pamoate in early-stage PDAC patients [
53] and based on our findings we recommend that this be extended to include its combination with gemcitabine.
Figure 1.
Pyrvinium pamoate exerts short- and long-term cytotoxicity in PDAC cells. (A) Chemical structure of pyrvinium pamoate (PP). (B) MTT cell viability assays of PANC-1 and CFPAC-1 cells post 72h treatment with PP or gemcitabine (GEM). The IC50 values were determined using GraphPad Prism version 8.0 software (GraphPad Prism software, USA) from sigmoidal plots of data obtained from three independent experiments performed in quadruplicate. (C) Representative images and quantification of clonogenic assays of PANC-1 and CFPAC-1 cells treated for 48h with ½ IC50 or IC50 PP or IC50 GEM and replated at low densities and left for 14 days in drug-free medium to form colonies. Colonies were stained with crystal violet and images from three independent experiments were quantified using the ImageJ Colony Area plugin. The graph represents the mean colony area ± SEM of each treatment condition expressed as a percentage of the vehicle control. Data are represented as mean ± SEM and ***p < 0.001 and **** < 0.0001.
Figure 1.
Pyrvinium pamoate exerts short- and long-term cytotoxicity in PDAC cells. (A) Chemical structure of pyrvinium pamoate (PP). (B) MTT cell viability assays of PANC-1 and CFPAC-1 cells post 72h treatment with PP or gemcitabine (GEM). The IC50 values were determined using GraphPad Prism version 8.0 software (GraphPad Prism software, USA) from sigmoidal plots of data obtained from three independent experiments performed in quadruplicate. (C) Representative images and quantification of clonogenic assays of PANC-1 and CFPAC-1 cells treated for 48h with ½ IC50 or IC50 PP or IC50 GEM and replated at low densities and left for 14 days in drug-free medium to form colonies. Colonies were stained with crystal violet and images from three independent experiments were quantified using the ImageJ Colony Area plugin. The graph represents the mean colony area ± SEM of each treatment condition expressed as a percentage of the vehicle control. Data are represented as mean ± SEM and ***p < 0.001 and **** < 0.0001.
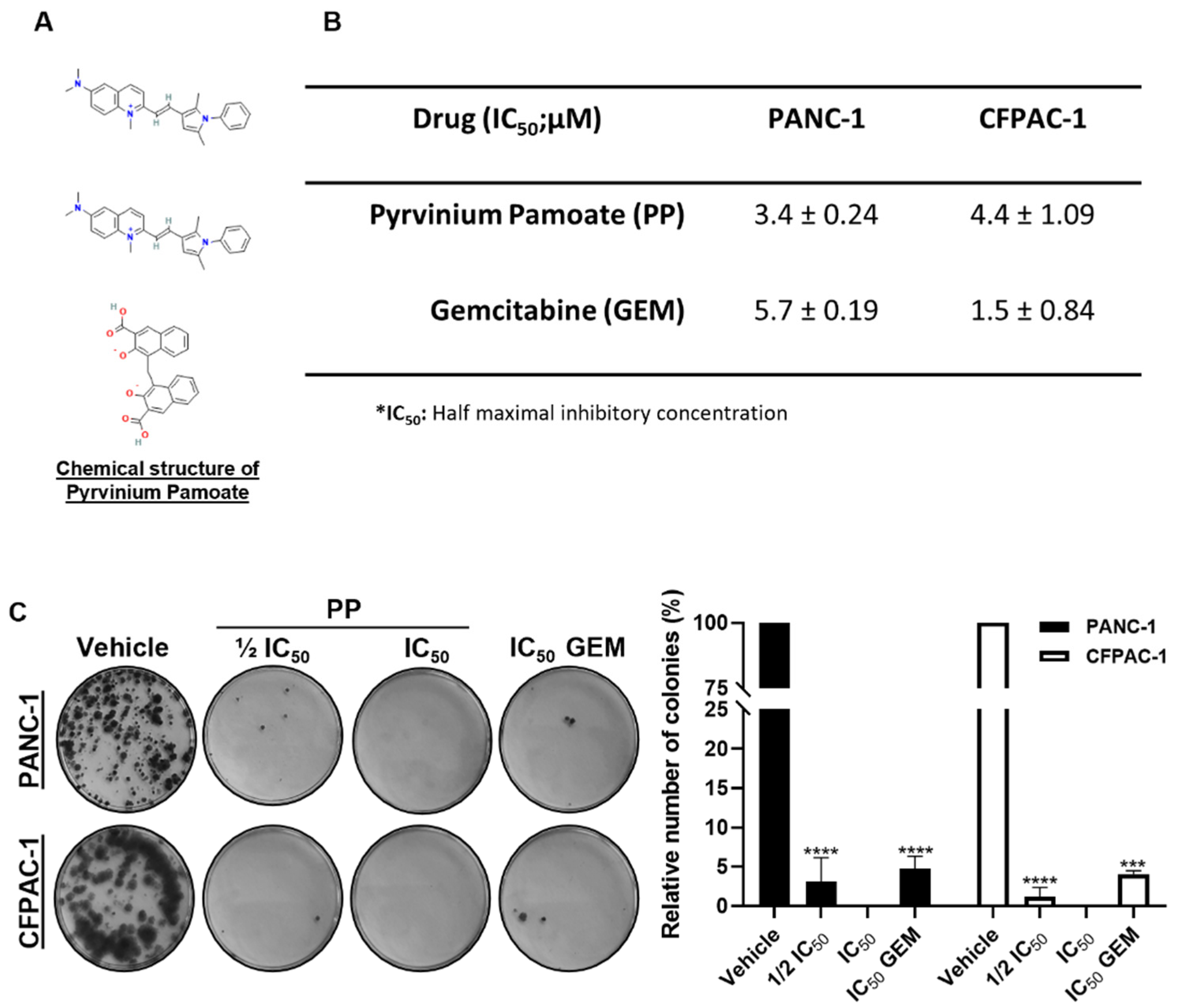
Figure 2.
: DNA damage induction and cell cycle arrest by pyrvinium pamoate in PDAC cells. (A) Western blot showing the levels of γH2AX in PANC-1 and CFPAC-1 cells post 72h treatment with indicated concentrations of PP or GEM. (B) Representative confocal microscope images (×630; Carl Zeiss LSM 510; Scale bar = 20 μM) and quantitation of γH2AX foci in PANC-1 and CFPAC-1 cells treated for 72h with IC50 PP or GEM. γH2AX was detected using a fluorophore-conjugated Alexa-488 secondary antibody and DAPI was used to stain the nuclei. (C) Flow cytometry analysis of cells treated for 72h with IC50 PP or GEM and stained with propidium iodide for cell cycle analysis. Graphs represent the mean proportion of cells in each phase of the cell cycle. (D) Western blot analyses of protein harvested from cells treated as indicated and incubated with antibodies against cell cycle markers cyclin A and cyclin B1. The blots shown are representative of three independent experiments where β-actin was used as a loading control. Band intensities used for densitometry readings were obtained using ImageJ and protein levels are represented as a ratio of protein of interest/β-actin normalized to the vehicle control (or first lane with a band). Data are represented as mean ± SEM and *, p < 0.05; **, p < 0.01.
Figure 2.
: DNA damage induction and cell cycle arrest by pyrvinium pamoate in PDAC cells. (A) Western blot showing the levels of γH2AX in PANC-1 and CFPAC-1 cells post 72h treatment with indicated concentrations of PP or GEM. (B) Representative confocal microscope images (×630; Carl Zeiss LSM 510; Scale bar = 20 μM) and quantitation of γH2AX foci in PANC-1 and CFPAC-1 cells treated for 72h with IC50 PP or GEM. γH2AX was detected using a fluorophore-conjugated Alexa-488 secondary antibody and DAPI was used to stain the nuclei. (C) Flow cytometry analysis of cells treated for 72h with IC50 PP or GEM and stained with propidium iodide for cell cycle analysis. Graphs represent the mean proportion of cells in each phase of the cell cycle. (D) Western blot analyses of protein harvested from cells treated as indicated and incubated with antibodies against cell cycle markers cyclin A and cyclin B1. The blots shown are representative of three independent experiments where β-actin was used as a loading control. Band intensities used for densitometry readings were obtained using ImageJ and protein levels are represented as a ratio of protein of interest/β-actin normalized to the vehicle control (or first lane with a band). Data are represented as mean ± SEM and *, p < 0.05; **, p < 0.01.
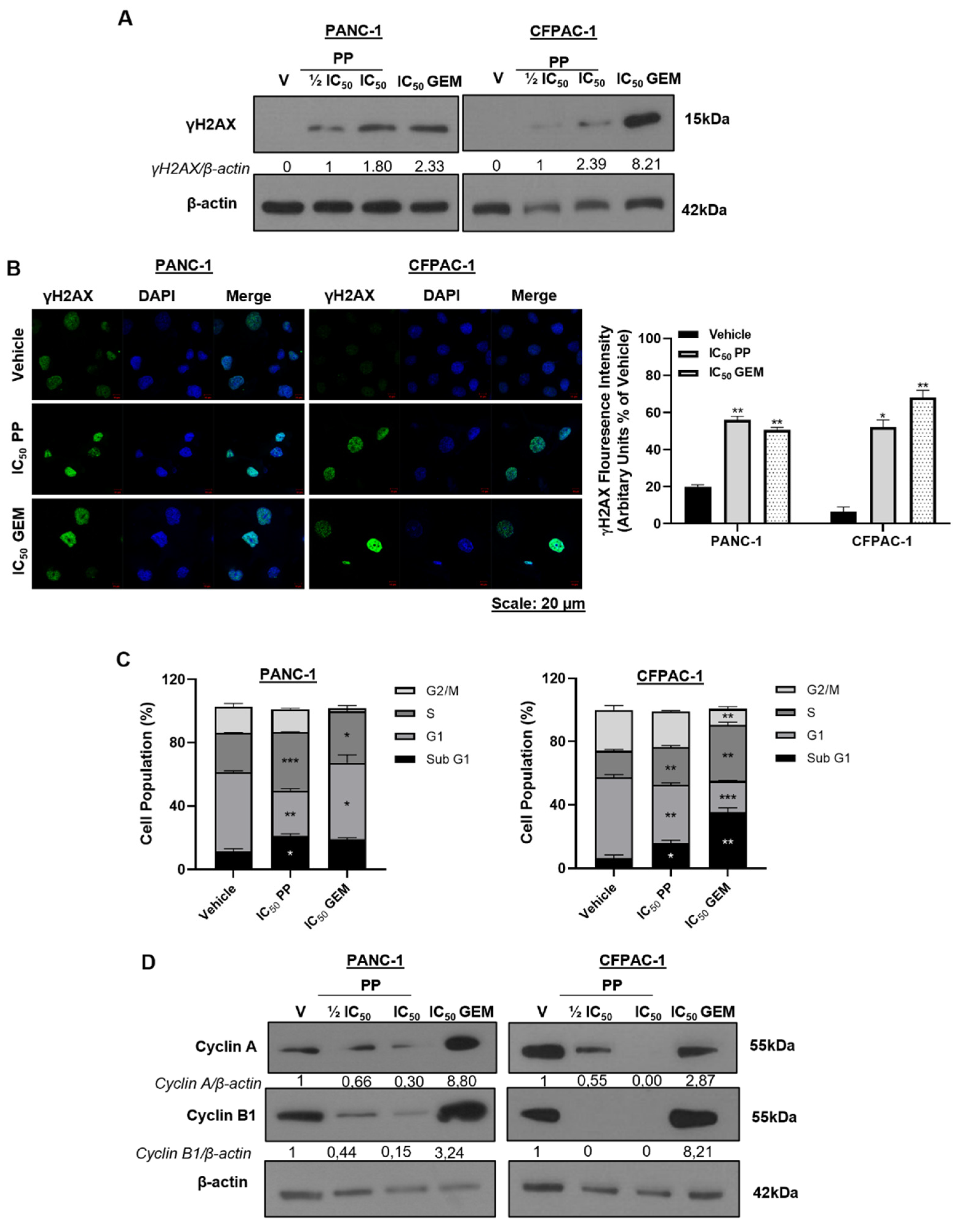
Figure 3.
Apoptosis induction by pyrvinium pamoate in PDAC cells. (A) Representative light microscopy images (×200; EVOS XL AMEX1000 Core Imaging System) showing the impact of PP or GEM on the morphology of PANC-1 and CFPAC-1 cells. The coloured circles correspond to the magnified images on the right and indicate morphological features of apoptosis (Red: cell shrinking; yellow: membrane blebbing; and black: apoptotic bodies). (B) Representative fluorescence microscope images (10X; EVOS M5000 Imaging System; scale bars = 250 μm) showing the effects of PPP or GEM on biochemical features of apoptosis post 72h of treatment PP or IC50 of GEM as indicated. Cells were stained with acridine orange and ethidium bromide, and the arrows indicate cells in different stages of apoptosis (white arrows: viable cells; red arrows: early apoptotic cells, and green arrows: late apoptotic cells). (C) Western blot analysis of molecular markers of apoptosis caspase-8, caspase-9, cleaved caspase-7, and PARP in cells treated as indicated. The blots shown are representative of three independent experiments where β-actin was used as a loading control. Band intensities used for densitometry readings were obtained using ImageJ and protein levels are represented as a ratio of protein of interest/β-actin normalized to the vehicle control (or first lane with a band).
Figure 3.
Apoptosis induction by pyrvinium pamoate in PDAC cells. (A) Representative light microscopy images (×200; EVOS XL AMEX1000 Core Imaging System) showing the impact of PP or GEM on the morphology of PANC-1 and CFPAC-1 cells. The coloured circles correspond to the magnified images on the right and indicate morphological features of apoptosis (Red: cell shrinking; yellow: membrane blebbing; and black: apoptotic bodies). (B) Representative fluorescence microscope images (10X; EVOS M5000 Imaging System; scale bars = 250 μm) showing the effects of PPP or GEM on biochemical features of apoptosis post 72h of treatment PP or IC50 of GEM as indicated. Cells were stained with acridine orange and ethidium bromide, and the arrows indicate cells in different stages of apoptosis (white arrows: viable cells; red arrows: early apoptotic cells, and green arrows: late apoptotic cells). (C) Western blot analysis of molecular markers of apoptosis caspase-8, caspase-9, cleaved caspase-7, and PARP in cells treated as indicated. The blots shown are representative of three independent experiments where β-actin was used as a loading control. Band intensities used for densitometry readings were obtained using ImageJ and protein levels are represented as a ratio of protein of interest/β-actin normalized to the vehicle control (or first lane with a band).
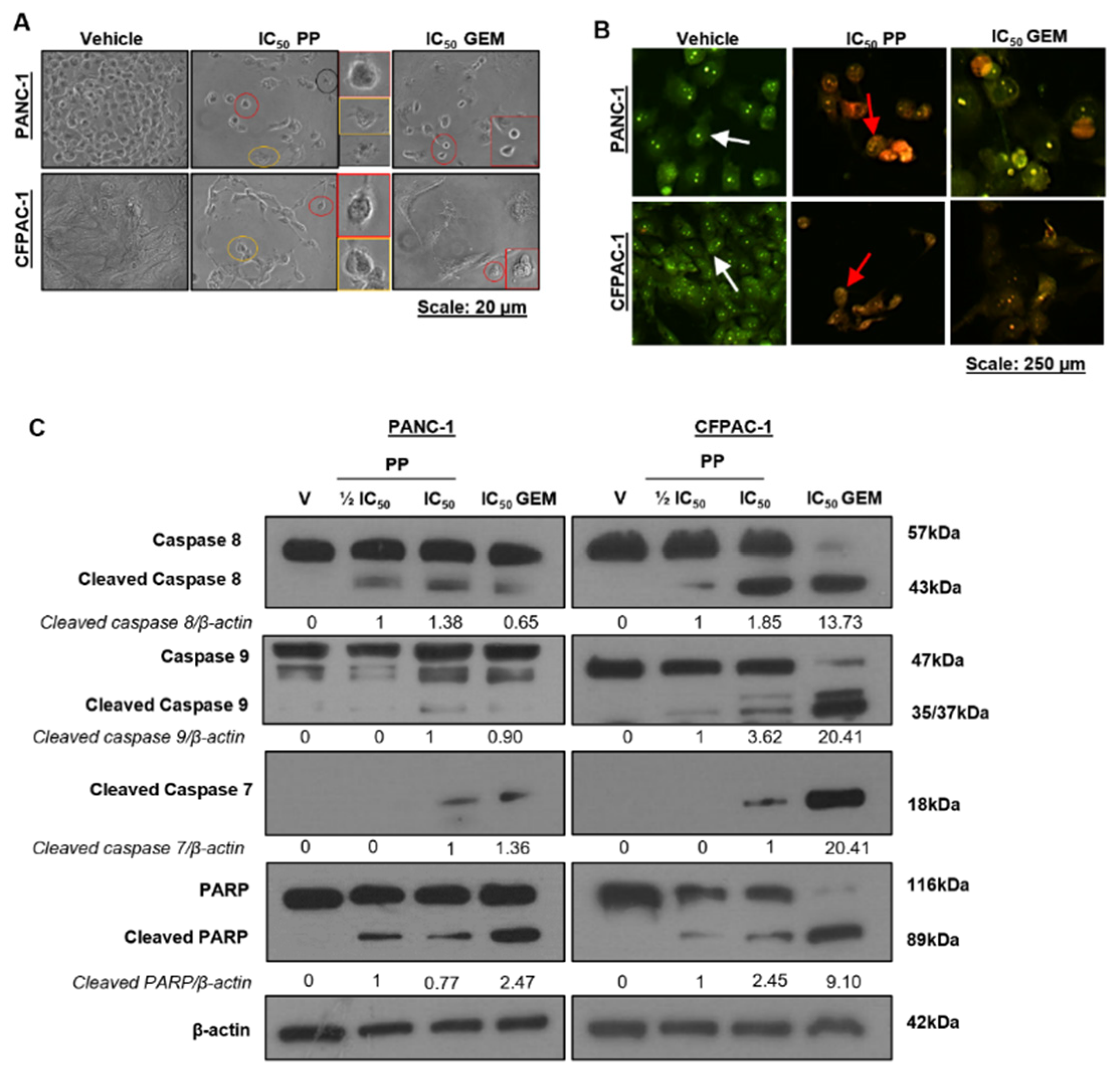
Figure 4.
Pyrvinium pamoate induces autophagic cell death and inhibits the PI3K/AKT pathway in PDAC cells. (A) Western analysis of molecular markers of autophagy LC3I/II and p62/SQSTM1 in PANC-1 and CFPAC-1 cells post 72h treatment as indicated. (B) Representative confocal microscope images (×630; Carl Zeiss LSM 510; Scale bar = 20 μM) showing LC3 puncta in cells treated as in (A). LC3 was detected using a fluorophore-conjugated Alexa-488 secondary antibody and DAPI was used to stain the nuclei. (C) Western blot analysis of LC3I/II and PARP in PANC-1 cells treated for 2h with 10 nM Bafilomycin A1 followed by 72h treatment with IC50 PP or GEM. (D) Western blots show levels of AKT, AKT1, and pAKT in PANC-1 and CFPAC-1 cells treated for 72h as indicated. The blots shown represent three independent experiments where β-actin was used as a loading control and the band intensities used for densitometry readings were obtained using ImageJ. Protein levels are represented as a ratio of protein of interest/β-actin normalized to the vehicle control (or first lane with a band).
Figure 4.
Pyrvinium pamoate induces autophagic cell death and inhibits the PI3K/AKT pathway in PDAC cells. (A) Western analysis of molecular markers of autophagy LC3I/II and p62/SQSTM1 in PANC-1 and CFPAC-1 cells post 72h treatment as indicated. (B) Representative confocal microscope images (×630; Carl Zeiss LSM 510; Scale bar = 20 μM) showing LC3 puncta in cells treated as in (A). LC3 was detected using a fluorophore-conjugated Alexa-488 secondary antibody and DAPI was used to stain the nuclei. (C) Western blot analysis of LC3I/II and PARP in PANC-1 cells treated for 2h with 10 nM Bafilomycin A1 followed by 72h treatment with IC50 PP or GEM. (D) Western blots show levels of AKT, AKT1, and pAKT in PANC-1 and CFPAC-1 cells treated for 72h as indicated. The blots shown represent three independent experiments where β-actin was used as a loading control and the band intensities used for densitometry readings were obtained using ImageJ. Protein levels are represented as a ratio of protein of interest/β-actin normalized to the vehicle control (or first lane with a band).
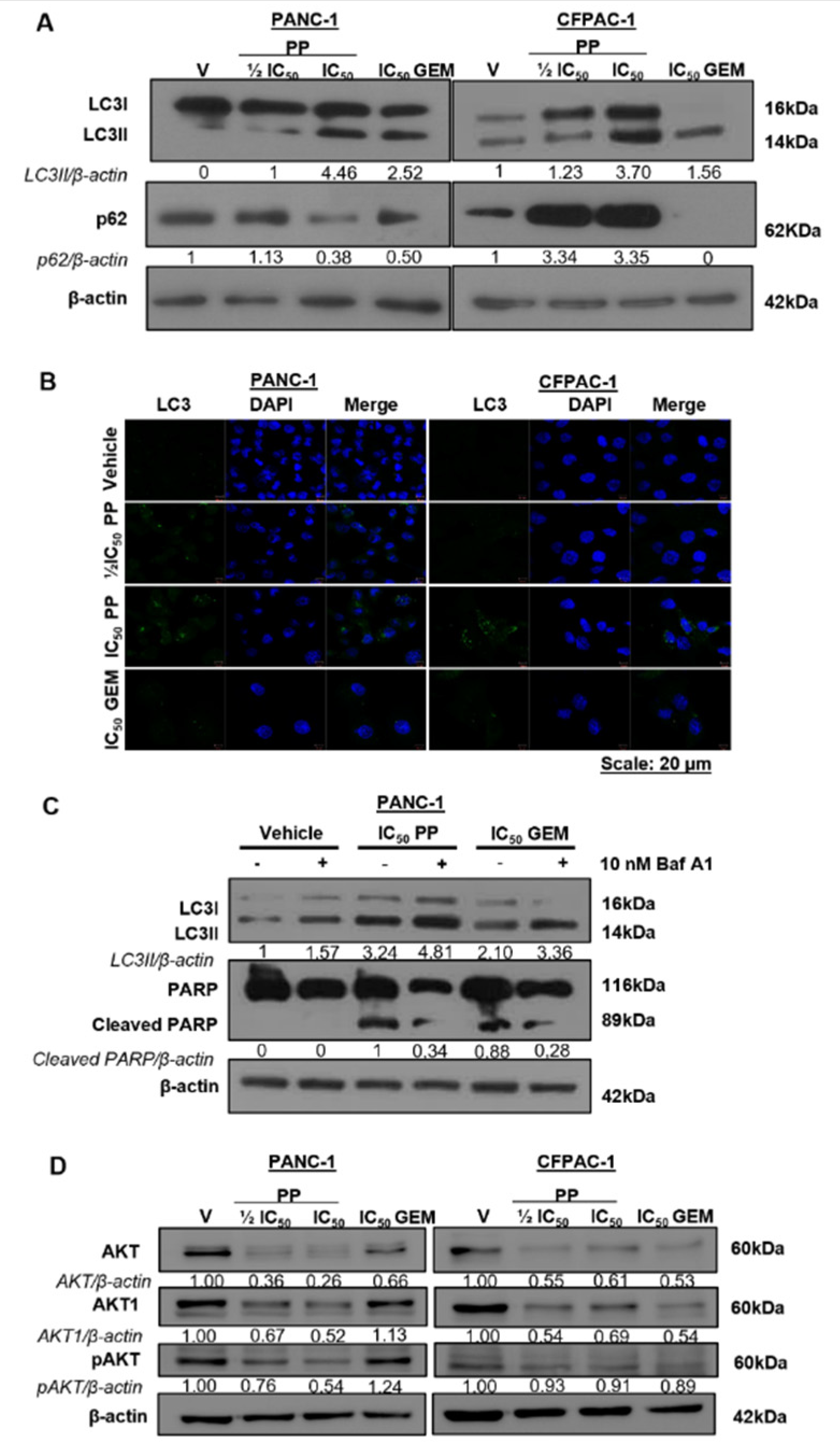
Figure 5.
Inhibition of PDAC cell invasion and migration by pyrvinium pamoate. (A) Representative light microscope images (10X; EVOS M5000 Imaging System; scale bars = 250 μm) and quantification of Transwell invasion assays where PANC-1 and CFPAC-1 cells were treated for 24h with IC50 PP or GEM and replated in the upper chamber of the Matrigel-coated Transwell insert with medium + 1% FBS for 20 h. Invasive cells were stained with crystal violet and images from three independent experiments were quantified using the ImageJ software. The graph represents the mean number of invasive cells ± SEM of each treatment condition expressed as a percentage of the vehicle control. (B) Representative light microscope images (4X; EVOS M5000 Imaging System; scale bars = 600 μm) of scratch motility assays where PANC-1 and CFPAC-1 cells were grown to 100% confluency, wounded using a sterile 20 µL pipette tip and treated as indicated in the presence of 10 µM Mitomycin C. The wound areas were measured using the ImageJ software and cell migration was calculated by subtracting the wound area at each time point from the initial wound area at 0 h and expressed as a percentage of the vehicle control. Accompanying graphs represent the mean percentage of cell migration ± SEM pooled from three independent experiments. (C) Western blot analysis of proteins harvested from cells in (B) showing the effect of PP or GEM on EMT markers, E-cadherin, N-cadherin, β-catenin, and vimentin. The blots shown are representative of three independent experiments where β-actin was used as a loading control. Band intensities used for densitometry readings were obtained using ImageJ and protein levels are represented as a ratio of protein of interest/β-actin normalized to the vehicle control (or first lane with a band). Data are represented as mean ± SEM and p < 0.05, **p < 0.01 ***p < 0.001, and **** < 0.0001. .
Figure 5.
Inhibition of PDAC cell invasion and migration by pyrvinium pamoate. (A) Representative light microscope images (10X; EVOS M5000 Imaging System; scale bars = 250 μm) and quantification of Transwell invasion assays where PANC-1 and CFPAC-1 cells were treated for 24h with IC50 PP or GEM and replated in the upper chamber of the Matrigel-coated Transwell insert with medium + 1% FBS for 20 h. Invasive cells were stained with crystal violet and images from three independent experiments were quantified using the ImageJ software. The graph represents the mean number of invasive cells ± SEM of each treatment condition expressed as a percentage of the vehicle control. (B) Representative light microscope images (4X; EVOS M5000 Imaging System; scale bars = 600 μm) of scratch motility assays where PANC-1 and CFPAC-1 cells were grown to 100% confluency, wounded using a sterile 20 µL pipette tip and treated as indicated in the presence of 10 µM Mitomycin C. The wound areas were measured using the ImageJ software and cell migration was calculated by subtracting the wound area at each time point from the initial wound area at 0 h and expressed as a percentage of the vehicle control. Accompanying graphs represent the mean percentage of cell migration ± SEM pooled from three independent experiments. (C) Western blot analysis of proteins harvested from cells in (B) showing the effect of PP or GEM on EMT markers, E-cadherin, N-cadherin, β-catenin, and vimentin. The blots shown are representative of three independent experiments where β-actin was used as a loading control. Band intensities used for densitometry readings were obtained using ImageJ and protein levels are represented as a ratio of protein of interest/β-actin normalized to the vehicle control (or first lane with a band). Data are represented as mean ± SEM and p < 0.05, **p < 0.01 ***p < 0.001, and **** < 0.0001. .
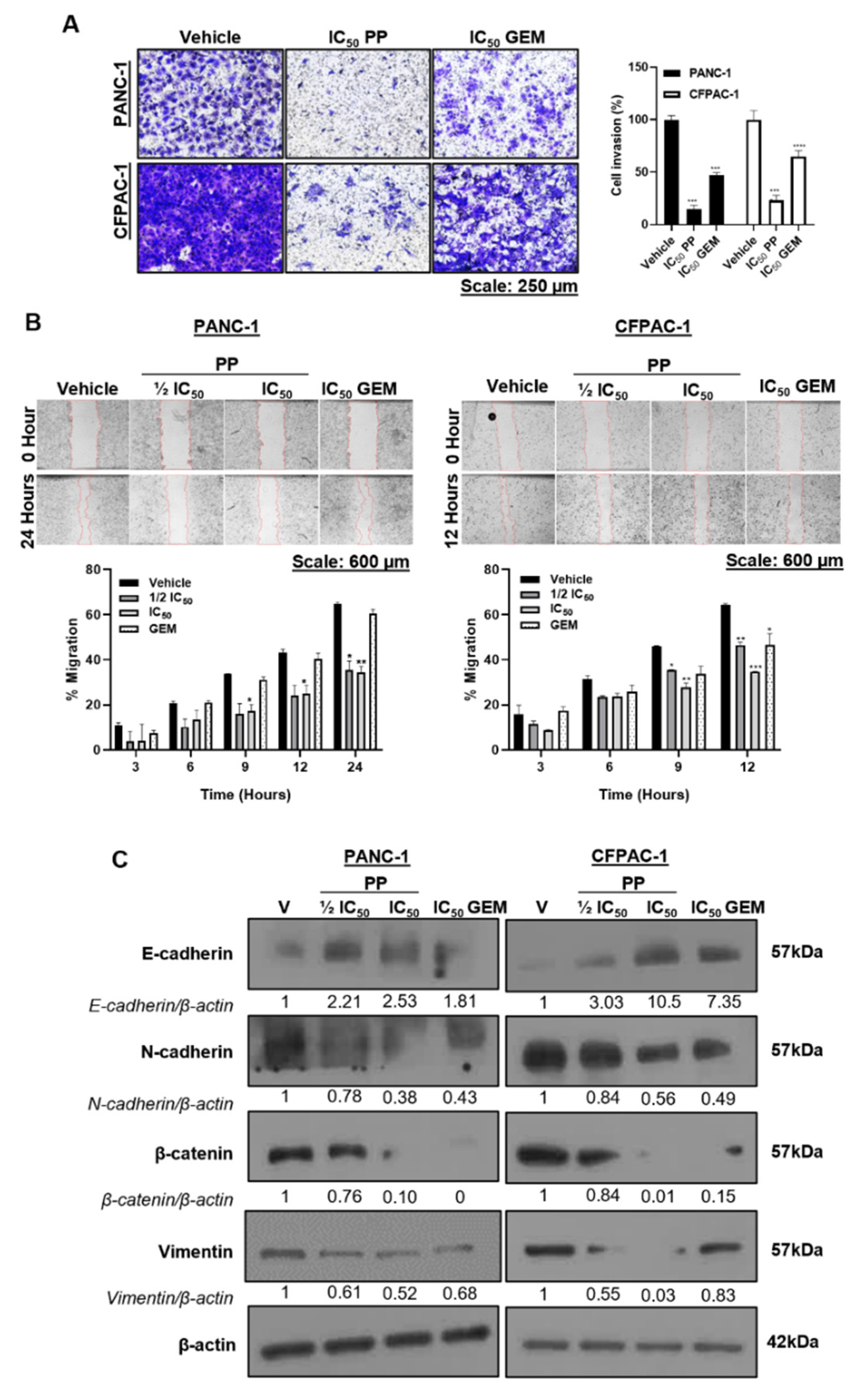
Figure 6.
Pyrvinium Pamoate inhibits PDAC spheroid growth, viability, and invasiveness. (A) Representative light microscope images (10X; EVOS M5000 Imaging System; scale bars = 250 μm) of 3D spheroids that were established by plating PANC-1 cells on agarose-coated plates to prevent cell adhesion and treated for 6 days with PP or GEM. (B) Quantitation of spheroid growth rate calculated by dividing each spheroid area on day 6 by its respective area on day 0. The graph represents the mean spheroid growth rate ± SEM pooled from three independent experiments performed in hexaplicate. (C) Representative fluorescence microscope images (10X; EVOS M5000 Imaging System; scale bars = 250 μm) of the spheroids in (A) stained with Calcein-AM on day 6. The accompanying graph show the mean calcein-AM fluorescence intensity measured using the ImageJ software and expressed as the percentage of the vehicle control. (D) Representative light microscope images (4X; EVOS M5000 Imaging System; scale bars = 600 μm) of PANC-1 spheroids that were established as in (A), embedded in collagen I, and treated for 72h as indicated. The red circles indicate invasion into the collagen matrix and corresponds to the magnified views on the bottom conners. Data are represented as mean ± SEM and **p < 0.01 ***p < 0.001, and **** < 0.0001.
Figure 6.
Pyrvinium Pamoate inhibits PDAC spheroid growth, viability, and invasiveness. (A) Representative light microscope images (10X; EVOS M5000 Imaging System; scale bars = 250 μm) of 3D spheroids that were established by plating PANC-1 cells on agarose-coated plates to prevent cell adhesion and treated for 6 days with PP or GEM. (B) Quantitation of spheroid growth rate calculated by dividing each spheroid area on day 6 by its respective area on day 0. The graph represents the mean spheroid growth rate ± SEM pooled from three independent experiments performed in hexaplicate. (C) Representative fluorescence microscope images (10X; EVOS M5000 Imaging System; scale bars = 250 μm) of the spheroids in (A) stained with Calcein-AM on day 6. The accompanying graph show the mean calcein-AM fluorescence intensity measured using the ImageJ software and expressed as the percentage of the vehicle control. (D) Representative light microscope images (4X; EVOS M5000 Imaging System; scale bars = 600 μm) of PANC-1 spheroids that were established as in (A), embedded in collagen I, and treated for 72h as indicated. The red circles indicate invasion into the collagen matrix and corresponds to the magnified views on the bottom conners. Data are represented as mean ± SEM and **p < 0.01 ***p < 0.001, and **** < 0.0001.
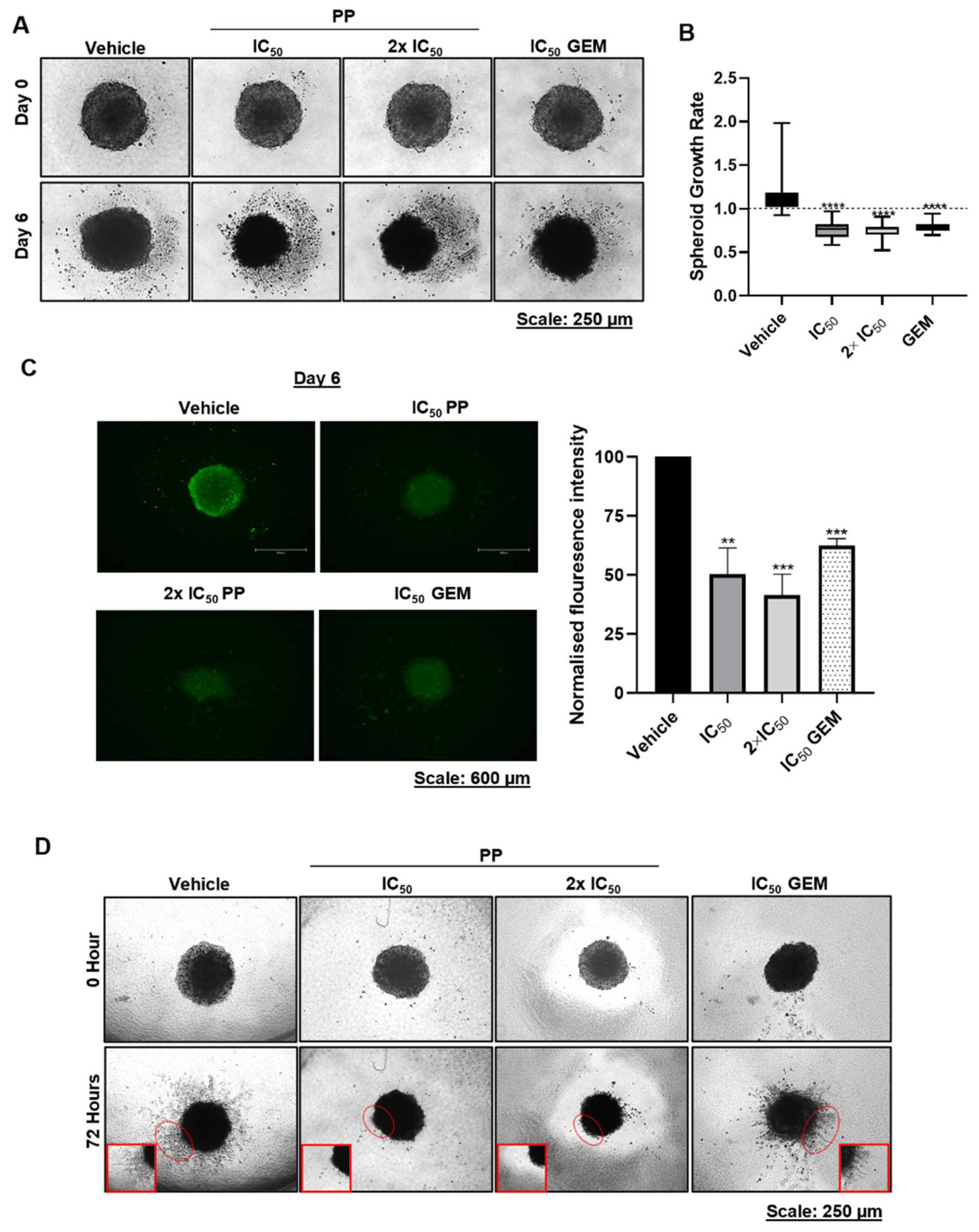
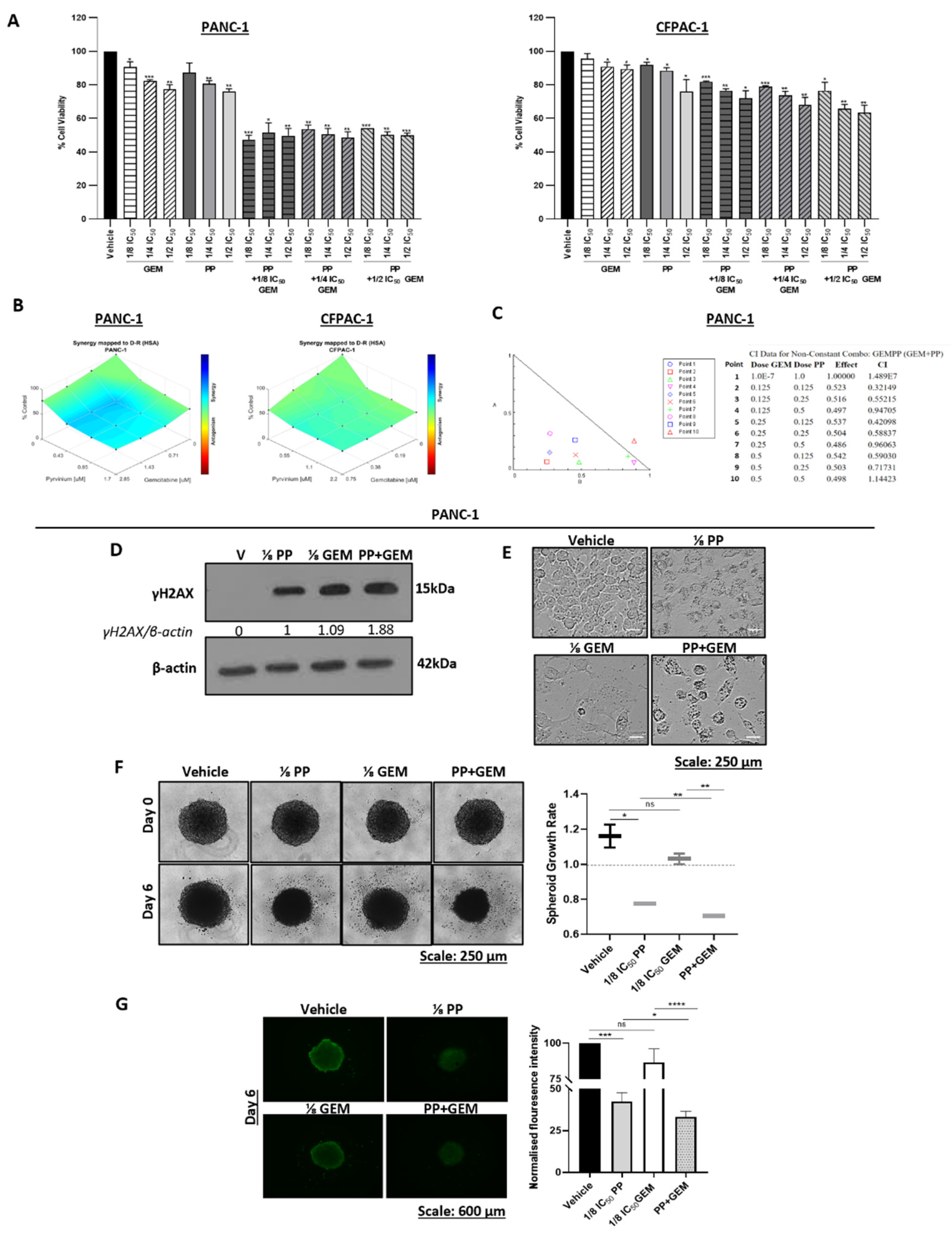
Figure 7.
Synergistic effects of pyrvinium pamoate and gemcitabine on PDAC cell viability, DNA damage and PDAC spheroid size/viability. (A) MTT assays of PANC-1 and CFPAC-1 cells post 72h treatment with ⅛ IC50, ⅟4 IC50, and ½ IC50 of PP or GEM and their combinations. Experiments were performed in quadruplicate and the graphs show mean cell viability ± SEM for each treatment condition. (B) Highest single agent (HSA) model synergy and antagonism surface maps generated using the Combenefit software by analysing the MTT cell viability assay results in (A). (C) Combination index (CI) plot and values generated using the CompuSyn software based on the results from (A). (D) Western blot analysis of γH2AX levels in PANC-1 treated for 72h with ⅛ IC50 PP or ⅛ IC50 GEM or their combination (PP+GEM). β-actin was used as a loading control and band intensities used for densitometry readings were obtained using ImageJ the software. Protein levels are represented as a ratio of the protein of interest/β-actin normalised to the first lane with a band. (E) Representative light microscopy images (10X; EVOS M5000 Imaging System; scale bars = 250 μm) showing the impact of ⅛ IC50 PP or ⅛ IC50 GEM or PP+GEM on the morphology of PANC-1 cells. (F) Representative light microscope images (10X; EVOS M5000 Imaging System; scale bars = 250 μm) of PANC-1 spheroids treated for 6 days with as indicated. The graph represents the mean spheroid growth rate ± SEM calculated by dividing each spheroid area on day 6 and by its respective area on day 0 pooled from three independent experiments performed in hexaplicate. (G) Representative fluorescence microscope images (4X; EVOS M5000 Imaging System; scale bars = 600 μm) of the spheroids in (F) stained with Calcein-AM on day 6. The accompanying graph show the mean calcein-AM fluorescence intensity measured using the ImageJ software and expressed as the percentage of the vehicle control. Data are represented as mean ± SEM; n=3. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001.
Figure 7.
Synergistic effects of pyrvinium pamoate and gemcitabine on PDAC cell viability, DNA damage and PDAC spheroid size/viability. (A) MTT assays of PANC-1 and CFPAC-1 cells post 72h treatment with ⅛ IC50, ⅟4 IC50, and ½ IC50 of PP or GEM and their combinations. Experiments were performed in quadruplicate and the graphs show mean cell viability ± SEM for each treatment condition. (B) Highest single agent (HSA) model synergy and antagonism surface maps generated using the Combenefit software by analysing the MTT cell viability assay results in (A). (C) Combination index (CI) plot and values generated using the CompuSyn software based on the results from (A). (D) Western blot analysis of γH2AX levels in PANC-1 treated for 72h with ⅛ IC50 PP or ⅛ IC50 GEM or their combination (PP+GEM). β-actin was used as a loading control and band intensities used for densitometry readings were obtained using ImageJ the software. Protein levels are represented as a ratio of the protein of interest/β-actin normalised to the first lane with a band. (E) Representative light microscopy images (10X; EVOS M5000 Imaging System; scale bars = 250 μm) showing the impact of ⅛ IC50 PP or ⅛ IC50 GEM or PP+GEM on the morphology of PANC-1 cells. (F) Representative light microscope images (10X; EVOS M5000 Imaging System; scale bars = 250 μm) of PANC-1 spheroids treated for 6 days with as indicated. The graph represents the mean spheroid growth rate ± SEM calculated by dividing each spheroid area on day 6 and by its respective area on day 0 pooled from three independent experiments performed in hexaplicate. (G) Representative fluorescence microscope images (4X; EVOS M5000 Imaging System; scale bars = 600 μm) of the spheroids in (F) stained with Calcein-AM on day 6. The accompanying graph show the mean calcein-AM fluorescence intensity measured using the ImageJ software and expressed as the percentage of the vehicle control. Data are represented as mean ± SEM; n=3. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001.