Submitted:
28 February 2024
Posted:
29 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Main Acidity Tests for Cables in the European Union (EU) and the Application Field
1.2. Main Acidity Tests for Cables in the EU
1.3. pH and Conductivity Measures and Their Incongruences
1.4. Thermal Decomposition and Combustion of PVC Compounds
1.5. Scope of the article
2. Materials and Methods
2.1. Materials
| Raw Materials | Trade name | F50.0 [phr] |
F50.1 [phr] |
F50.2 [phr] |
F50.3 [phr] |
F50.4 [phr] |
F50.5 [phr] |
|---|---|---|---|---|---|---|---|
| PVC | Inovyn 271 PC | 100 | 100 | 100 | 100 | 100 | 100 |
| DINP | Diplast N | 50 | 50 | 50 | 50 | 50 | 50 |
| ESBO | Reaflex EP/6 | 2 | 2 | 2 | 2 | 2 | 2 |
| Antioxidant | Arenox A10 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| COS | RPK B-CV/ 3037 | 3 | 3 | 3 | 3 | 3 | 3 |
| CaCO3 | Riochim | 90 | 0 | 0 | 0 | 0 | 0 |
| Al(OH)3 | Apyral 40 CD | 0 | 90 | 0 | 0 | 0 | 0 |
| Mg(OH)2 | Ecopyren 3.5 | 0 | 0 | 90 | 0 | 0 | 0 |
| PCC | Winnofl S | 0 | 0 | 0 | 90 | 0 | 0 |
| HTAS 1 | AS-1B | 0 | 0 | 0 | 0 | 90 | 0 |
| HTAS 2 | AS-6B | 0 | 0 | 0 | 0 | 0 | 90 |
| Raw Materials | Trade name | REA1 [phr] |
REA2 [phr] |
REA3 [phr] |
REA4 [phr] |
REA5 [phr] |
REA6 [phr] |
REA7 [phr] |
REA8 [phr] |
REA9 [phr] |
REA10 [phr] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PVC | Inovyn 271 PC | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| DINP | Diplast N | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 |
| ESBO | Reaflex EP/6 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Antioxidant | Arenox A10 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| COS | RPK B-CV/ 3037 | 5 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Antimony Trioxide | RI004 | 0 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| CaCO3 standard | Riochim | 0 | 0 | 90 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CaCO3 fine | Omya 95 T | 0 | 0 | 0 | 90 | 0 | 0 | 0 | 0 | 0 | 0 |
| PCC | Winnofil S | 0 | 0 | 0 | 0 | 90 | 0 | 0 | 0 | 90 | 90 |
| HTAS 2 | AS-6B | 0 | 0 | 0 | 0 | 0 | 90 | 0 | 0 | 0 | 0 |
| Al(OH)3 | Apyral 40 CD | 0 | 0 | 0 | 0 | 0 | 0 | 90 | 0 | 30 | 0 |
| Mg(OH)2 | Ecopyren 3.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 90 | 0 | 30 |
2.2. Test Apparatus
2.3. Sample Preparation
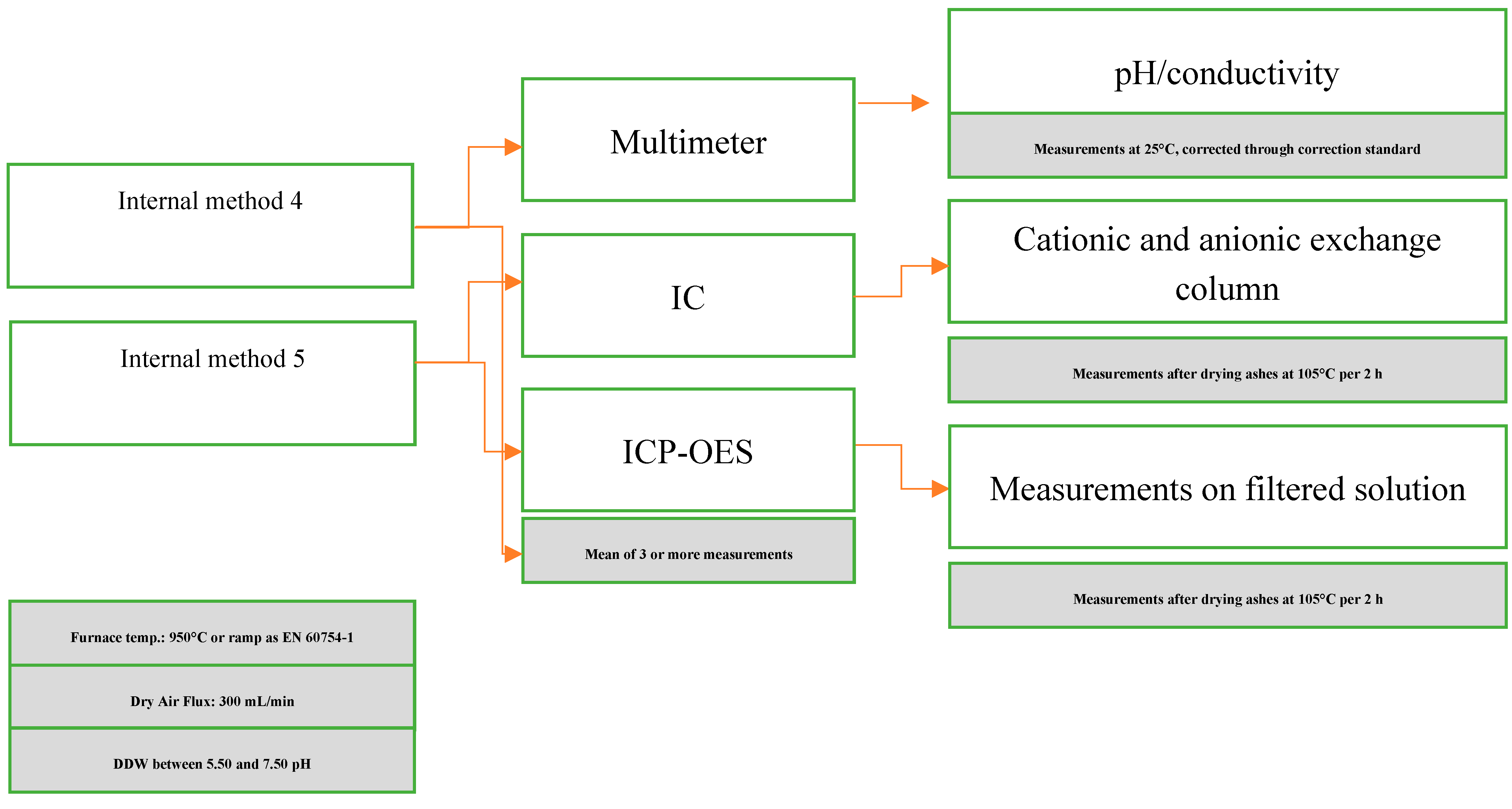
2.4. Internal methods for evaluating the species in solutions
| Technical standard | Measurement | Temperature [°C] | Note |
|---|---|---|---|
| Internal method 4 | Multimeter pH and Conductivity |
Isothermal at 950 °C | DDW, pH, and Conductivity. The general method, according to the 2014 version. |
| IC Anions and cations |
Isothermal at 950 °C | Li+, Na+, NH4+, K+, Mg2+, Ca2+, Cl-, F-, NO2-, Br-, NO3-, PO43-, SO42- | |
| ICP-OES Elements |
Isothermal at 950 °C | Mg, Al, Ca, Zn, Sb | |
| Internal method 5 | Multimeter pH and Conductivity |
Thermal profile of EN 60754-11 | DDW, pH, and Conductivity. The general method, according to the 2014 version. |
| IC Anions and cations |
Thermal profile of EN 60754-11 | Li+, Na+, NH4+, K+, Mg2+, Ca2+, Cl-, F-, NO2-, Br-, NO3-, PO43-, SO42- | |
| ICP-OES Elements |
Thermal profile of EN 60754-11 | Mg, Al, Ca, Zn, Sb |
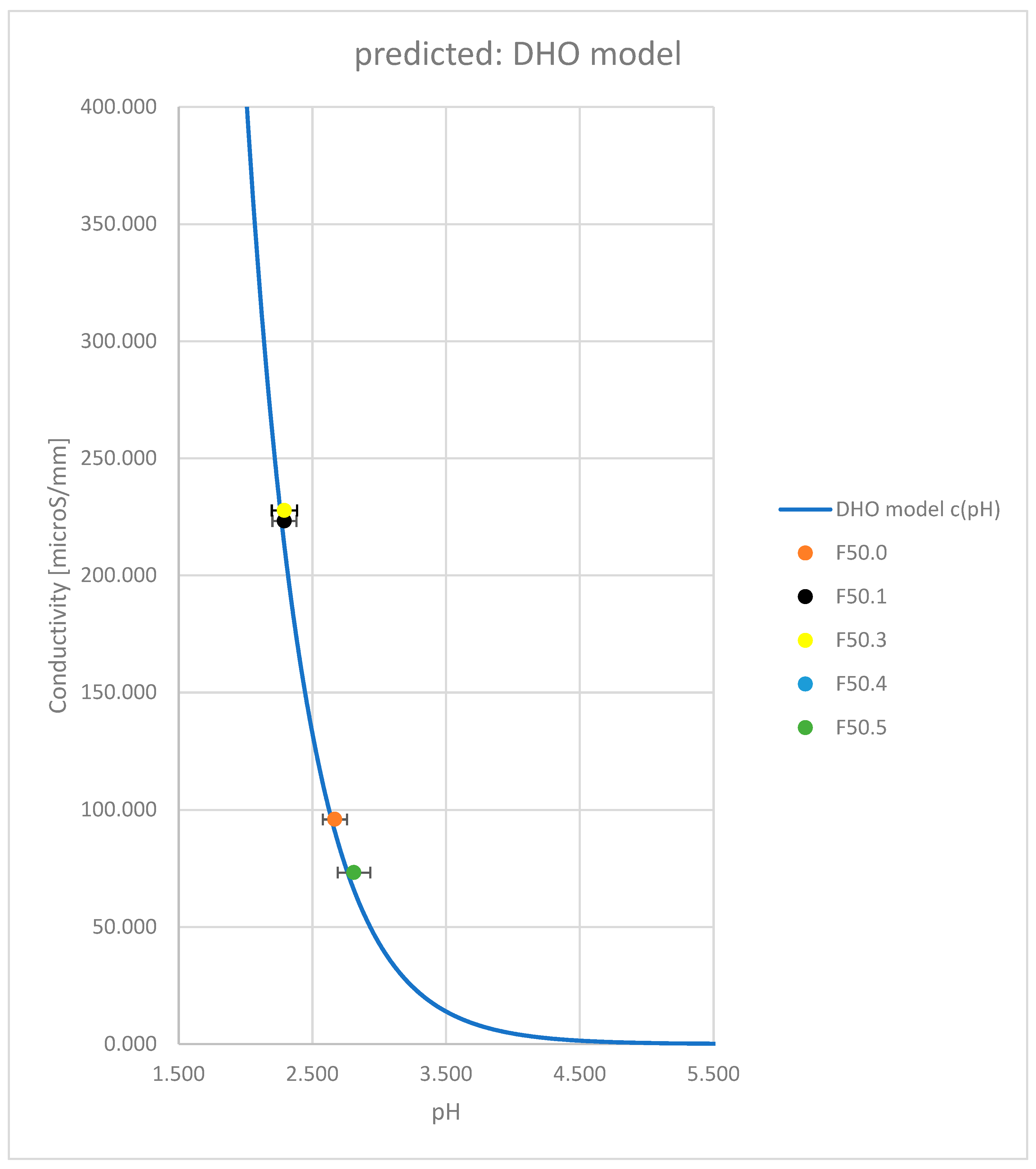
3. Results
| Formulation → | F50.0 | F50.1 | F50.2 | F50.3 | F50.4 | F50.5 |
|---|---|---|---|---|---|---|
| pH | 2.668 | 2.291 | 2.291 | 2.715 | 2.748 | 2.810 |
| SDpH | 0.090 | 0.112 | 0.095 | 0.030 | 0.125 | 0.122 |
| CVpH [%] | 3.4 | 4.9 | 4.1 | 1.1 | 4.5 | 4.3 |
| Conductivity [mS/mm] | 95.9 | 223.2 | 222.7 | 77.0 | 72.1 | 73.2 |
| SDc | 3.2 | 8.8 | 10.2 | 4.2 | 3.2 | 3.1 |
| CVc [%] | 3.3 | 3.9 | 4.6 | 5.5 | 4.4 | 4.2 |
| Accuracy [%] from DHO model | 5.7 | 5.1 | 4.8 | -5.6 | -4.9 | 11.1 |
| pH (1st run) | 2.62 | 2.27 | 2.27 | 2.74 | 2.89 | 2.79 |
| Conductivity [mS/mm] (1st run) [16] | 97.3 | 221.5 | 224.3 | 74 | 70.1 | 70.1 |
| Conductivity [mS/mm] (DHO) | 90.7 | 212.4 | 212.4 | 81.6 | 75.8 | 65.9 |
| Sample | [Cl-] mg/L | SD [Cl-] |
[F-] mg/L |
[NO2-] mg/L |
[Br-] mg/L |
[NO3-] mg/L |
[PO43-] mg/L |
[SO42-] mg/L |
|---|---|---|---|---|---|---|---|---|
| REA3 | 98.70 | 1.18 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| REA4 | 70.94 | 1.20 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| REA5 | 97.07 | 2.78 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| REA6 | 64.05 | 1.30 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| REA7 | 206.06 | 6.23 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| REA8 | 197.12 | 8.23 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| REA9 | 42.81 | 2.41 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| REA10 | 62.05 | 1.23 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| Sample | [Cl-] mg/L | SD [Cl-] |
[F-] mg/L |
[NO2-] mg/L |
[Br-] mg/L |
[NO3-] mg/L |
[PO43-] mg/L |
[SO42-] mg/L |
|---|---|---|---|---|---|---|---|---|
| REA3 | 64.47 | 1.93 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| REA4 | 60.81 | 2.43 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| REA5 | 23.44 | 1.23 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| REA6 | 2.98 | 0.45 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| REA7 | 173.64 | 2.45 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| REA8 | 192.44 | 3.25 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| REA9 | 10.33 | 1.00 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| REA10 | 25.00 | 1.25 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| Sample | [Ca] mg/L | [Mg] mg/L | [Al] mg/L | [Sb] mg/L | [Zn] mg/L |
|---|---|---|---|---|---|
| REA3 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| REA4 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| REA5 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| REA6 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| REA7 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| REA8 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| REA9 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| REA10 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| Sample | [Al] mg/L | [Sb] mg/L | [Zn] mg/L |
|---|---|---|---|
| REA3 | < LOQ | < LOQ | < LOQ |
| REA4 | < LOQ | < LOQ | < LOQ |
| REA5 | < LOQ | < LOQ | < LOQ |
| REA6 | < LOQ | < LOQ | < LOQ |
| REA7 | < LOQ | < LOQ | < LOQ |
| REA8 | < LOQ | < LOQ | < LOQ |
| REA9 | < LOQ | < LOQ | < LOQ |
| REA10 | < LOQ | < LOQ | < LOQ |
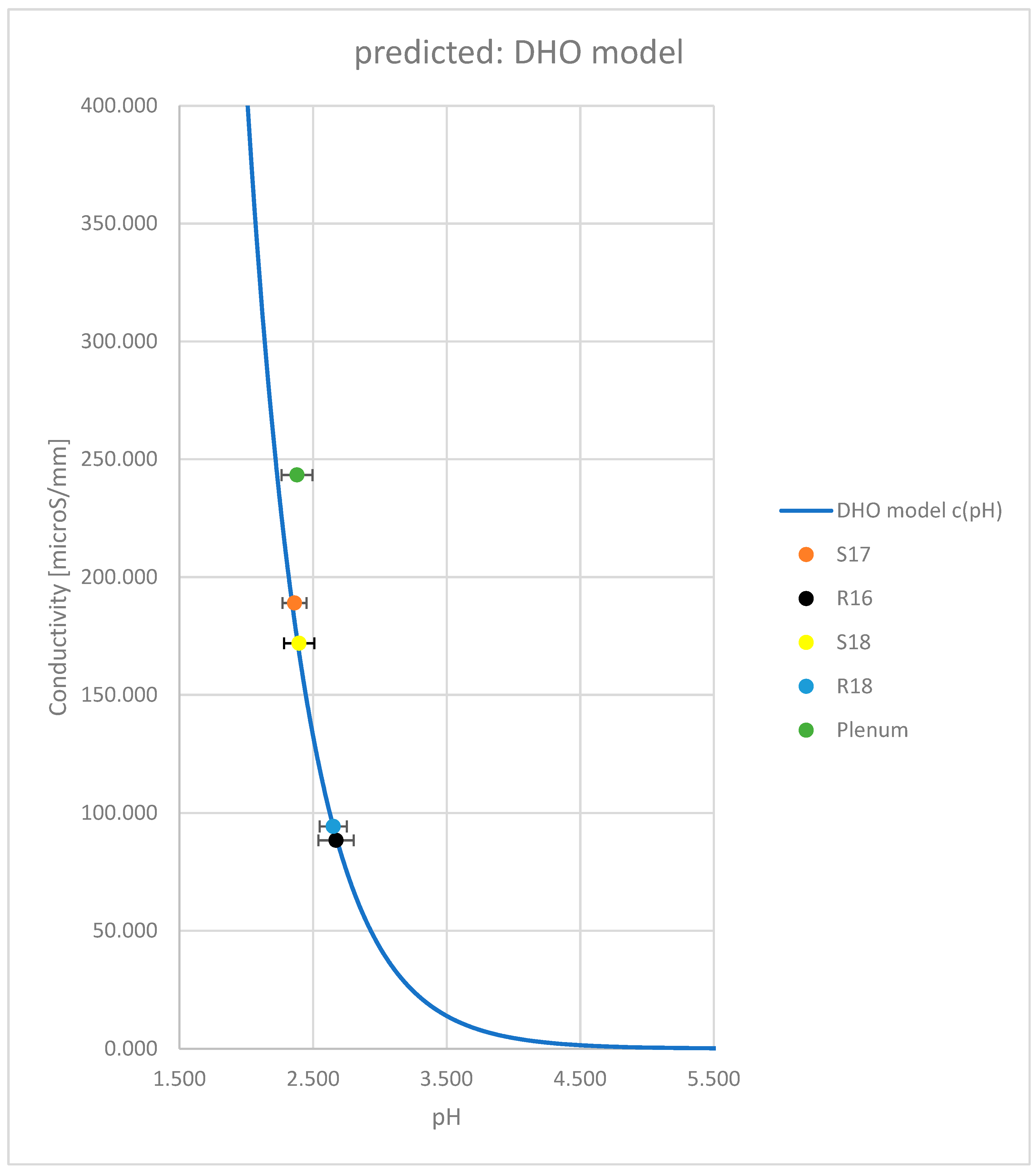
| Formulation → | R16 | S18 | R18 | S17 | PVC Plenum | TPU-FR |
|---|---|---|---|---|---|---|
| pH | 2.673 | 2.396 | 2.651 | 2.361 | 2.38 | 8.20 |
| SDpH | 0.132 | 0.114 | 0.1024 | 0.09 | 0.115 | 0.22 |
| CVpH [%] | 4.9 | 4.8 | 3.9 | 3.8 | 4.8 | 2.7 |
| Conductivity [mS/mm] | 88.4 | 171.9 | 94.3 | 189 | 243.4 | 32.2 |
| SDc | 1.2 | 5.4 | 4.2 | 9.0 | 10.0 | 1.3 |
| CVc [%] | 1.4 | 3.1 | 4.5 | 4.8 | 4.1 | 4.0 |
| Conductivity [mS/mm] (DHO) | 89.7 | 167.6 | 92.3 | 181.3 | 173.8 | n.a. |
| Accuracy [%] | -1.45 | 2.57 | 2.17 | 4.25 | 40.0 | n.a. |
4. Discussion
4.1. Species Found in Solutions
4.2. Statistical Approach
4.3. The Idiosyncrasy of EN 60754-2, EN 50525-1 and EN 50620
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PVC—Poly(vinyl chloride); |
| EU—European Union; |
| CPR—Construction Product Regulation; |
| DDW—Double Deionized Water; |
| LOQ – Limit of Quantification; |
| HCl—Hydrogen chloride; |
| GPP – General Purpose Plasticizer; |
| ATO – Antimony Trioxide; |
| ATH – Aluminum Tri Hydroxide; |
| MDH – Magnesium Di Hydroxide; |
| PCC—Precipitated Calcium Carbonate; |
| GCC—Ground Calcium Carbonate; |
| Phr—Part per Hundred Resin; |
| DINP—Di Iso Nonyl Phthalate; |
| ESBO—Epoxidized Soy Bean Oil; |
| COS—Calcium Organic Stabilizer; |
| IC – Ion Chromatography; |
| ICP-OES — Inductively Coupled Plasma – Optical Emission Spectroscopy; |
| SD—Standard Deviation; |
| CV—Coefficient of variation; |
| DHO – Debye-Hückel-Onsager; |
| AOM – Ammonium Octa Molybdate. |
Appendix-A. A schematic diagram of the sample preparation and testing process

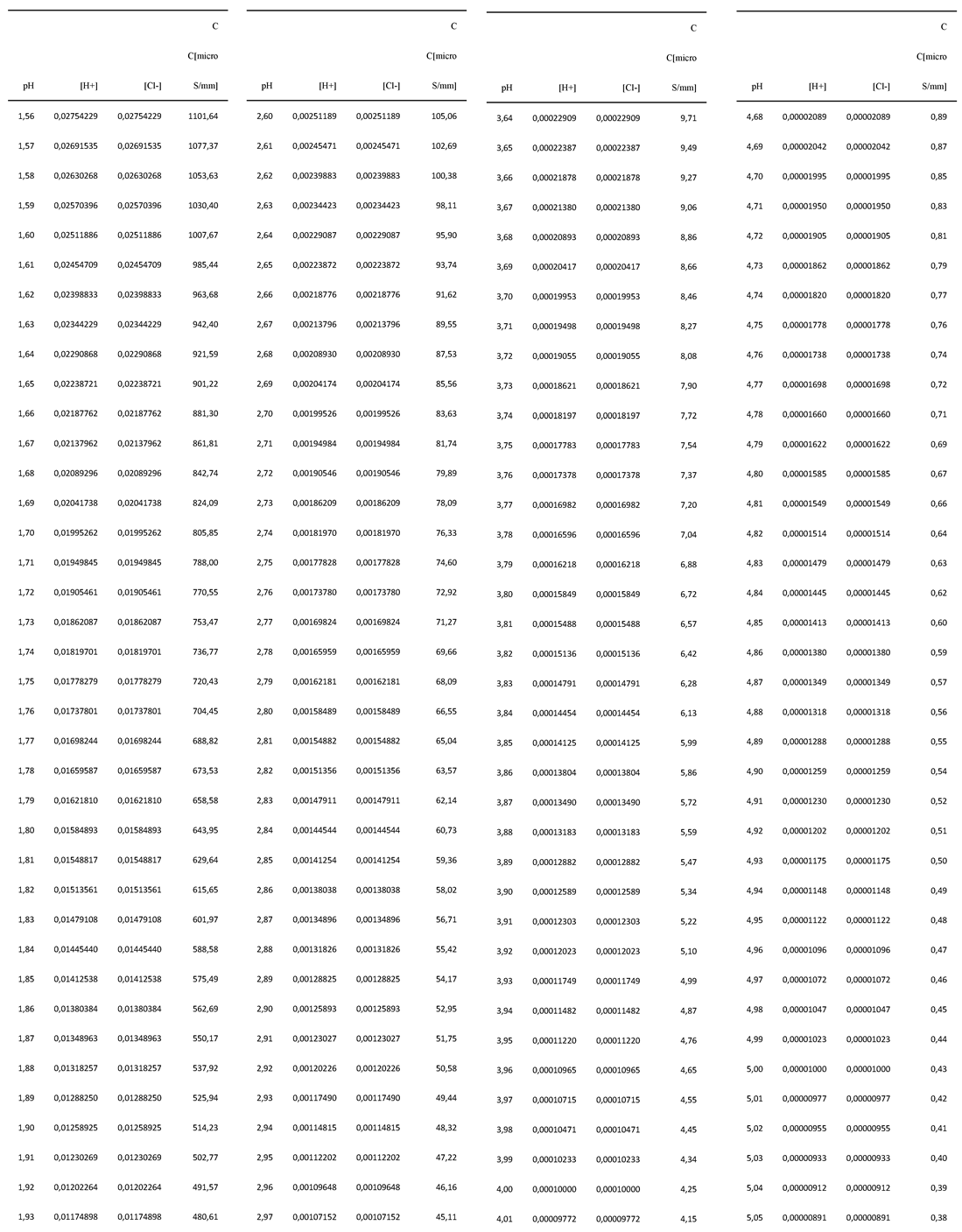
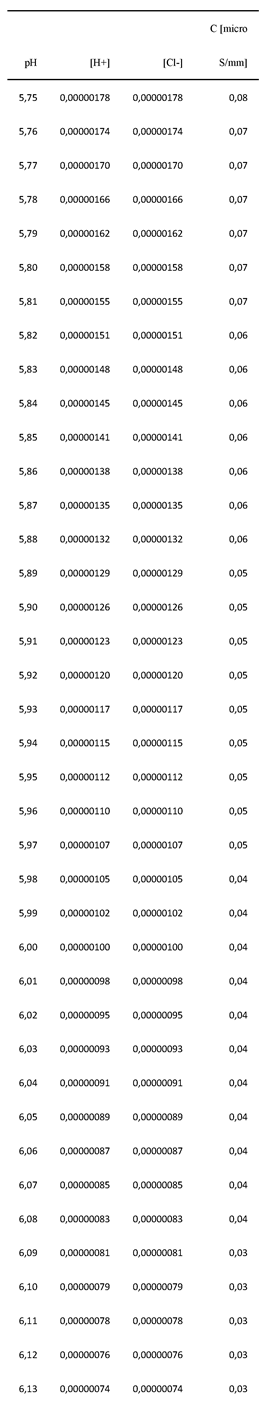
Appendix-B. Statistical approach: the mathematical relationship between C and pH for standard solutions of HCl
| a | b | |
|---|---|---|
| Samples | 39117.15 | 2.2772 |
| Standard solutions HCl | 40476.18 | 2.2897 |
| DHO model | 37262.15 | 2.2555 |
Appendix-C. Conductivity and pH relationship according to the DHO equation
| 1 | EN 60754-2/A1:2020 [2] has a new way to evaluate the data not considered in this paper. |
References
- EN 60754-1/A1:2020; Test on gases evolved during combustion of materials from cables - Part 1: Determination of the halogen acid gas content. CENELEC; Brussels, Belgium, 2020. Available online: https://mycatalogo.ceinorme.it/cei/item/0010018242 (accessed on 01 February 2024).
- EN 60754-2:2014/A1:2020; Test on gases evolved during combustion of materials from cables - Part 2: Determination of acidity (by pH measurement) and Conductivity. CENELEC; Brussels, Belgium, 2020. Available online: https://mycatalogo.ceinorme.it/cei/item/0010018243 (accessed on 01 February 2024).
- IEC EN 60754-3:2019; Test on gases evolved during combustion of materials from cables - Part 3: Measurement of low level of halogen content by ion chromatography. CENELEC; Brussels, Belgium, 2019. Available online: https://mycatalogo.ceinorme.it/cei/item/0000017364 (accessed on 01 February 2024).
- EN 50267-2-1:1998; Common test methods for cables under fire conditions - Tests on gases evolved during combustion of materials from cables - Part 2-1: Procedures - Determination of the amount of halogen acid gas. CENELEC; Brussels, Belgium, 1998. Available online: https://mycatalogo.ceinorme.it/cei/item/000005326 (accessed on 1 February 2024).
- EN 50267-2-2:1998; Common test methods for cables under fire conditions - Tests on gases evolved during combustion of materials from cables - Part 2-2: Procedures - Determination of degree of acidity of gases for materials by measuring pH and Conductivity. CENELEC; Brussels, Belgium, 1998. Available online: https://mycatalogo.ceinorme.it/cei/item/000005327 (accessed on 1 February 2024).
- IEC 60684-2:2011; Flexible insulating sleeving - Part 2: Methods of test. IEC; Geneva, Switzerland, 2011. Available online: https://webstore.iec.ch/publication/2873 (accessed on 1 February 2024).
- EN 50267-2-3:1998; Common test methods for cables under fire conditions - Tests on gases evolved during combustion of materials from cables - Part 2-3: Procedures - Determination of degree of acidity of gases for cables by determination of the weighted average of pH and Conductivity. CENELEC; Brussels, Belgium, 1998. Available online: https://mycatalogo.ceinorme.it/cei/item/000005327 (accessed on 1 February 2024).
- EN 50525-1:2011+A1:2022; Electric cables - Low voltage energy cables of rated voltages up to and including 450/750 V (U0/U)- Part 1: General requirements. CENELEC; Brussels, Belgium, 2011. Available online: https:// mycatalogo.ceinorme.it/cei/item/0000019411 (accessed on 1 February 2024).
- EN 50620:2017+A1:2021; Electric cables - Charging cables for electric vehicles. CENELEC; Brussels, Belgium, 2021. Available online: https://mycatalogo.ceinorme.it/cei/item/0000015830 (accessed on 1 February 2024).
- EN 50618:2017; Electric cables for photovoltaic systems. CENELEC; Brussels, Belgium, 2014. Available online: https://mycatalogo.ceinorme.it/cei/item/0000014504 (accessed on 1 February 2024).
- EN 50575:2014 +A1: 2016; Power, control and communication cables. Cables for general applications in construction works subject to reaction to fire requirements. CENELEC:; Brussels, Belgium, 2016. Available online: https:// mycatalogo.ceinorme.it/cei/item/0000015059 (accessed on 1 February 2024).
- EN 50525-1:2011; Electric cables - Low voltage energy cables of rated voltages up to and including 450/750 V (U0/U)- Part 1: General requirements. CENELEC:; Brussels, Belgium, 2011. Available online: https:// mycatalogo.ceinorme.it/cei/item/0000011661 (accessed on 1 February 2024).
- Regulation (EU) No 305/2011 of the European Parliament and of the Council of 9 March 2011 Laying Down Harmonised Conditions for the Marketing of Construction Products and Repealing Council Directive 89/106/EEC. Consolidate Version. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02011R0305-20210716 (accessed on 2 February 2024).
- EN 13501-6:2018 + A1:2022; Fire classification of construction products and building elements - Part 6: Classification using data from reaction to fire tests on power, control and communication cables. CENELEC:; Brussels, Belgium, 2022. Available online: http://www.store.uni.com/uni-en-13501-6-2023 (accessed on 01 February 2024).
- UNI EN ISO 10304-1:2009; Qualità dell'acqua - Determinazione di anioni disciolti mediante cromatografia ionica in fase liquida - Parte 1: Determinazione di bromuri, cloruri, fluoruri, nitrati, nitriti, fosfati e solfati, 2009; ISO: Geneva, Switzerland. Available online: https://store.uni.com/uni-en-iso-10304-1-2009 (accessed on 1 February 2024).
- Bassi, I.; Delchiaro, F.; Bandinelli, C.; Mazzocchetti, L.; Salatelli, E.; Sarti, G. A New Perspective on Hydrogen Chloride Scavenging at High Temperatures for Reducing the Smoke Acidity of PVC Cables in Fires, IV: The Impact of Acid Scavengers at High Temperatures on Flame Retardance and Smoke Emission. Fire 2023, 6, 259. [Google Scholar] [CrossRef]
- Starnes, W.H.; Wescott, L.D.; Reents, W.D.; Cais, R.E.; Villacorta, G.M.; Plitz, I.M.; Anthony, L.J. Mechanism of poly(vinyl chloride) fire retardance by molybdenum(vi) oxide. Further evidence in favor of the Lewis acid theory. In Polymer Additives. Polymer Science and Technology; Kresta, J.E., Ed.; Springer: Boston, MA, USA, 2007; Volume 26, pp. 237–248. [Google Scholar] [CrossRef]
- Montaudo, G.; Puglisi, C. Evolution of aromatics in the thermal degradation of poly(vinyl chloride): A mechanistic study. Polym. Degrad. Stab. 1991, 33, 229–262. [Google Scholar] [CrossRef]
- Wu, C.H.; Wu, C.H.; Chang, C.Y.; Hor, J.L.; Shih, S.M.; Chen, L.W.; Chang, F.W. Two-stage pyrolysis model of PVC. Can. J. Chem. Eng. 1994, 72, 644–650. [Google Scholar] [CrossRef]
- Anthony, G.M. Kinetic and Chemical Studies of Polymer Crosslinking Using Thermal Gravimetry and Hyphenated Methods. Degradation of Polyvinylchloride. Polym. Degrad. Stab. 1999, 64, 353–357. [Google Scholar] [CrossRef]
- O’Mara, M.M. Combustion of PVC. Pure Appl. Chem. 1977, 49, 649–660. [Google Scholar] [CrossRef]
- Sarti, G.; Piana, M. PVC in cables for building and construction. Can the “European approach” be considered a good example for other countries? Acad. Lett. 2022, 5453. [Google Scholar] [CrossRef]
- Commercial GCC Purchased by Umbria Filler. Available online: https://elastomeri-polimeri.hu/pdf/umbria/riochim.pdf (accessed on 1 February 2024).
- Commercial GCC Purchased by Omya. Available online: https://polymer-additives.specialchem.com/product/a-omya-hydrocarb-95-t (accessed on 1 February 2024).
- Commercial PCC Purchased by Imerys. Available online: https://www.imcdus.com/en-us/products/winnofil-s#product-wizard (accessed on 1 February 2024).
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. Rep. 2009, 63, 100–125 [Google Scholar] [CrossRef]. [Google Scholar] [CrossRef]
- Sarti, G. A New Perspective on Hydrogen Chloride Scavenging at High Temperatures for Reducing the Smoke Acidity of PVC Cables in Fires. I: An Overview of the Theory, Test Methods, and the European Union Regulatory Status. Fire 2022, 5, 127. [Google Scholar] [CrossRef]
- Sarti, G. A New Perspective on Hydrogen Chloride Scavenging at High Temperatures for Reducing the Smoke Acidity of PVC Cables in Fires. II: Some Examples of Acid Scavengers at High Temperatures in the Condensed Phase. Fire 2022, 5, 142. [Google Scholar] [CrossRef]
- ISO 14911:1998; Water quality Determination of dissolved Li+, Na+, NH4+, K+, Mn2+, Ca2+, Mg2+, Sr2+ and Ba2+ using ion chromatography, 1998. ISO: Geneva, Switzerland. Available online: https://store.uni.com/uni-en-iso-10304-1-2009 (accessed on 1 February 2024).
- ISO 11885:2007; Determination of 33 elements by inductively coupled plasma atomic emission spectroscopy, 2007. ISO: Geneva, Switzerland. Available online: https:// store.uni.com/uni-en-iso-11885-2009 (accessed on 1 February 2024).
- Kipouros, G.J.; Sadoway, D.R. A thermochemical analysis of the production of anhydrous MgCl2. Journal of Light Metals 2001, 1, 111–117. [Google Scholar] [CrossRef]
- Galwey, A.K.; Laverty, G.M. The thermal decomposition of magnesium chloride dihydrate. Thermochimica Acta 1989, 138, 115–127. [Google Scholar] [CrossRef]
- Bassi, I.; Bandinelli, C.; Delchiaro, F.; Piana, M.; Sarti, G. A New Perspective on Hydrogen Chloride Scavenging at High Temperatures for Reducing the Smoke Acidity of PVC Cables in Fires V: Comparison between EN 60754-1 and EN 60754-2. Fire 2023, 6, 326. [Google Scholar] [CrossRef]
- Chandler, L.A.; Hirschler Smith, G.F. A heated tube furnace test for the emission of acid gas from PVC wire coating materials: effects of experimental procedures and mechanistic considerations. European Polymer Journal. 1987, 23, 51–61. [Google Scholar] [CrossRef]
- Wright, M.R. An Introduction to Aqueous Electrolyte Solutions, John Wiley & Sons Ltd, The Atrium, Southern Gate, Chichester, West Sussex PO19 8SQ, England, 2007, Chapter 12.
- Vanysek, P. Equivalent Conductivities of Electrolytes in Aqueous Solution, in CRC Handbook of Chemistry and Physics, Internet Version 2006, David, R. Lide, ed., Taylor and Francis, Boca Raton, FL, 2006, p. 5-75.
- Wright, M.R. An Introduction to Aqueous Electrolyte Solutions, John Wiley & Sons Ltd, The Atrium, Southern Gate, Chichester, West Sussex PO19 8SQ, England, 2007, Chapter 11, 429-474.
- Vanysek, P. Ionic Conductivity and Diffusion at Infinite Dilution, in CRC Handbook of Chemistry and Physics, Internet Version 2006, David, R. Lide, ed., Taylor and Francis, Boca Raton, FL, 2006, p. 5-76.
| EU Standard | Use | Product Standard | Current Status | Test Apparatus |
|---|---|---|---|---|
| EN 60754-1:2014 + A1:2020 [1] | Assessment of halogens1 | EN 50525-1:2011/A1:2022 Annex B [8], EN 50620 [9], EN 50618 [10] | Active | Furnace tube, titration |
| EN 60754-2:2014 + A1:2020 [2] | Assessment of halogens 1 | EN 50525-1:2011/A1:2022 Annex B, EN 50620, EN 50618 | Active | Furnace tube, pH, conductivity |
| EN 60754-2:2014 + A1:2020 | Additional classification for acidity | EN 50575:2014+A1:2016 [11] | Active | Furnace tube, pH, conductivity |
| EN IEC 60754-3:2019 [3] | Additional classification for acidity | EN 50575:2014+A1:2016 | Under evaluation | Furnace tube, Ion Chromatography |
| EN 50267-2-1 [4] | Assessment of halogens 1 | EN 50525-1:2011 [12] Annex B | Withdrawn in 20221 | Furnace tube, titration |
| EN 50267-2-2 [5] | Assessment of halogens 1 | EN 50525-1:2011 Annex B | Withdrawn in 20221 | Furnace tube, pH, conductivity |
| IEC 60684-2 [6] | Assessment of halogens 1 | EN 50525-1:2011/A1:2022 Annex B | Active | F- Selective Electrode |
| EN 50267-2-3 [7] | Additional classification for acidity before 2014 | EN 50575:2014 | Withdrawn in 20221 | Furnace tube, pH, conductivity |
| IEC standard | EN standard | Amendments | Amendments |
|---|---|---|---|
| IEC 60754-1:2011 | EN 60754-1:2014 | EN 60754-1+A1:2019 | EN 60754-1+A1:2020 |
| IEC 60754-2:2011 | EN 60754-2:2014 | EN 60754-2+A1:2019 | EN 60754-2+A1:2020 |
| IEC 60754-3:2011 | EN IEC 60754-3:2019 |
| Additional classification for acidity | pH | Conductivity [mS/mm] |
|---|---|---|
| a1 | > 4.3 | < 2.5 |
| a2 | > 4.3 | < 10 |
| a3 | ≤ 4.3 | ≥ 10 |
| Test apparatus | Producer | model | Additional Info's |
|---|---|---|---|
| Plasticorder | Brabender | Plastograph EC | 50 CC, chamber |
| Halogen Acid Gas test apparatus | SA Associates | Standard model | Porcelain combustion boats |
| Multimeter | Mettler Toledo | S213 standard kit | |
| Conductivity electrode | Mettler Toledo | S213 standard kit | Reference thermocouple adjusting temperature fluctuation. |
| pH electrode | Mettler Toledo | S213 standard kit | Reference thermocouple adjusting temperature fluctuation. |
| Ion Chromatography System | Thermo | Dionex IonPacTM AS22 4, x 250 mm | |
| Anion exchange column | Thermo | Dionex IonPacTM CS12A 4, x 250 mm | |
| Cation exchange column | Thermo | Aqueon | |
| ICP-OES | Thermo | iCAP 7000 series | |
| Sample | pH | [H+] Mol/L | [Cl-] Mol/L | [Cl-] g/L | [Cl-] mg/L | [Cl-] IC [mg/L] | Delta |
|---|---|---|---|---|---|---|---|
| F50.0 | 2.668 | 0.0021503 | 0.0021503 | 0.0762348 | 76.2 | 78.7 | -3.26 |
| F50.1 | 2.291 | 0.0051227 | 0.0051227 | 0.1816155 | 181.6 | 177.0 | 2.54 |
| F50.2 | 2.291 | 0.0051227 | 0.0051227 | 0.1816155 | 181.6 | 175.8 | 3.21 |
| F50.3 | 2.715 | 0.0019297 | 0.0019297 | 0.0684153 | 68.4 | 69.1 | -1.02 |
| F50.4 | 2.748 | 0.0017865 | 0.0017865 | 0.0633363 | 63.3 | 64.6 | -2.04 |
| F50.5 | 2.810 | 0.0015506 | 0.0015506 | 0.0549734 | 55.0 | 57.0 | -3.67 |
| Sample | [Cl-] mg/L | SD [Cl-] mg/L |
[F-] mg/L |
[NO2-] mg/L |
[Br-] mg/L |
[NO3-] mg/L | [PO43-] mg/L |
[SO42-] mg/L |
|---|---|---|---|---|---|---|---|---|
| F50.0 | 78.72 | 2.91 | 0.10 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| F50.1 | 177.00 | 5.50 | 0.11 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| F50.2 | 175.78 | 6.76 | 0.11 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| F50.3 | 69.11 | 2.44 | 0.11 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| F50.4 | 64.63 | 2.50 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| F50.5 | 56.99 | 1.89 | 0.10 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| Sample | [Li+] mg/L | [Na+] mg/L | [NH4+] mg/L | [K+] mg/L | [Mg2+] mg/L | [Ca2+] mg/L |
|---|---|---|---|---|---|---|
| F50.0 | 0.01 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| F50.1 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| F50.2 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| F50.3 | 0.01 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| F50.4 | 0.01 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| F50.5 | 0.01 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| Sample | [Al] mg/L | [Sb] mg/L | [Zn] mg/L |
|---|---|---|---|
| F50.0 | < LOQ | < LOQ | < LOQ |
| F50.1 | < LOQ | < LOQ | < LOQ |
| F50.2 | < LOQ | < LOQ | < LOQ |
| F50.3 | < LOQ | < LOQ | < LOQ |
| F50.4 | < LOQ | < LOQ | < LOQ |
| F50.5 | < LOQ | < LOQ | < LOQ |
| Formulation → | F50.0 | F50.1 | F50.2 | F50.3 | F50.4 | F50.5 |
|---|---|---|---|---|---|---|
| pH | 2.515 | 2.293 | 2.380 | 3.198 | 3.556 | 3.273 |
| SDpH | 0.100 | 0.110 | 0.080 | 0.081 | 0.087 | 0.102 |
| CVpH [%] | 4.0 | 4.8 | 3.4 | 2.5 | 2.4 | 3.1 |
| Conductivity [mS/mm] | 135.7 | 219.7 | 174.2 | 30.7 | 11.6 | 24.9 |
| SDc | 6.3 | 5.4 | 8.7 | 2.5 | 0.2 | 1.2 |
| CVc [%] | 4.6 | 2.5 | 5.0 | 8.1 | 1.7 | 4.8 |
| Accuracy [%] from DHO model | 5.9 | 3.9 | 0.2 | 11.6 | -4.9 | 7.3 |
| pH (1st run) | 2.63 | 2.3 | 2.29 | 3.26 | 3.52 | 3.2 |
| Conductivity [mS/mm] (1st run) [16] | 100.4 | 206.4 | 208.9 | 23.7 | 13.5 | 25.7 |
| Conductivity [mS/mm] (DHO) | 128.1 | 211.4 | 173.8 | 27.5 | 12.2 | 23.2 |
| Sample | pH | [H+] Mol/L | [Cl-] Mol/L | [Cl-] g/L | [Cl-] mg/L | [Cl-] IC [mg/L] | Delta |
|---|---|---|---|---|---|---|---|
| F50.0 | 2.515 | 0.0030584 | 0.0030584 | 0.1084309 | 108.43 | 113.82 | -4.97 |
| F50.1 | 2.293 | 0.0050933 | 0.0050933 | 0.1805731 | 180.57 | 176.10 | 2.47 |
| F50.2 | 2.380 | 0.0041687 | 0.0041687 | 0.1477927 | 147.79 | 147.25 | 0.37 |
| F50.3 | 3.198 | 0.0006339 | 0.0006339 | 0.0224726 | 22.47 | 21.23 | 5.55 |
| F50.4 | 3.556 | 0.0002780 | 0.0002780 | 0.0098549 | 9.85 | 7.42 | 24.67 |
| F50.5 | 3.273 | 0.0005339 | 0.0005339 | 0.0189301 | 18.93 | 17.41 | 8.03 |
| Sample | [Cl-] mg/L | SD [Cl-] |
[F-] mg/L |
[NO2-] mg/L |
[Br-] mg/L |
[NO3-] mg/L |
[PO43-] mg/L |
[SO42-] mg/L |
|---|---|---|---|---|---|---|---|---|
| F50.0 | 113.82 | 2.32 | 0.10 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| F50.1 | 176.10 | 4.23 | 0.11 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| F50.2 | 147.25 | 2.66 | 0.11 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| F50.3 | 21.23 | 1.21 | 0.11 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| F50.4 | 7.42 | 0.57 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| F50.5 | 17.41 | 1.00 | 0.10 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| Sample | [Li+] mg/L | [Na+] mg/L | [NH4+] mg/L | [K+] mg/L | [Mg2+] mg/L | [Ca2+] mg/L |
|---|---|---|---|---|---|---|
| F50.0 | 0.01 | 0.10 | < LOQ | 0.10 | < LOQ | < LOQ |
| F50.1 | < LOQ | 0.10 | 0.12 | 0.10 | < LOQ | < LOQ |
| F50.2 | < LOQ | 0.10 | 0.19 | < LOQ | < LOQ | < LOQ |
| F50.3 | 0.01 | 0.13 | < LOQ | 0.19 | < LOQ | < LOQ |
| F50.4 | 0.01 | 0.10 | < LOQ | 0.19 | < LOQ | < LOQ |
| F50.5 | 0.01 | 0.17 | < LOQ | 0.33 | < LOQ | < LOQ |
| Sample | [Al] mg/L | [Sb] mg/L | [Zn] mg/L |
|---|---|---|---|
| F50.0 | < LOQ | < LOQ | < LOQ |
| F50.1 | < LOQ | < LOQ | < LOQ |
| F50.2 | < LOQ | < LOQ | < LOQ |
| F50.3 | < LOQ | < LOQ | < LOQ |
| F50.4 | < LOQ | < LOQ | < LOQ |
| F50.5 | < LOQ | < LOQ | < LOQ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




